Studying Land Cover Changes in a Malaria-Endemic Cambodian District: Considerations and Constraints
Abstract
1. Introduction
1.1. Foreword
1.2. Background
1.3. Malaria and Deforestation
1.4. Issues When Studying the Relationship between Deforestation and Malaria
1.5. Situation in Cambodia
1.6. Objectives
2. Materials and Methods
2.1. Considerations for Methodological Choices
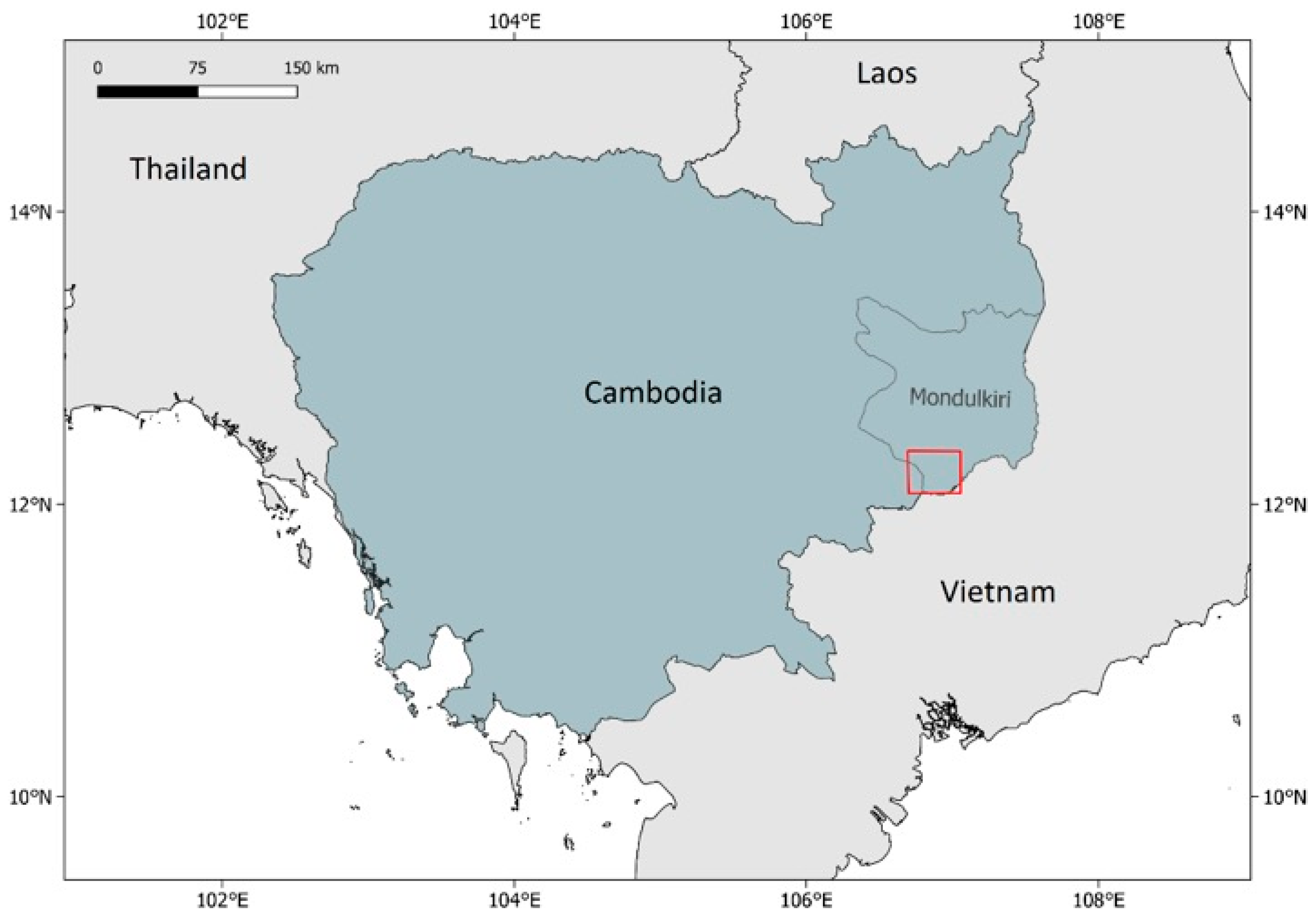
2.2. Study Site
2.3. Malaria Data
2.4. Satellite Imagery and Processing
2.5. Ground Truthing and Accuracy Measurement
2.6. Comparison between Maps and Landscape Metrics
3. Results
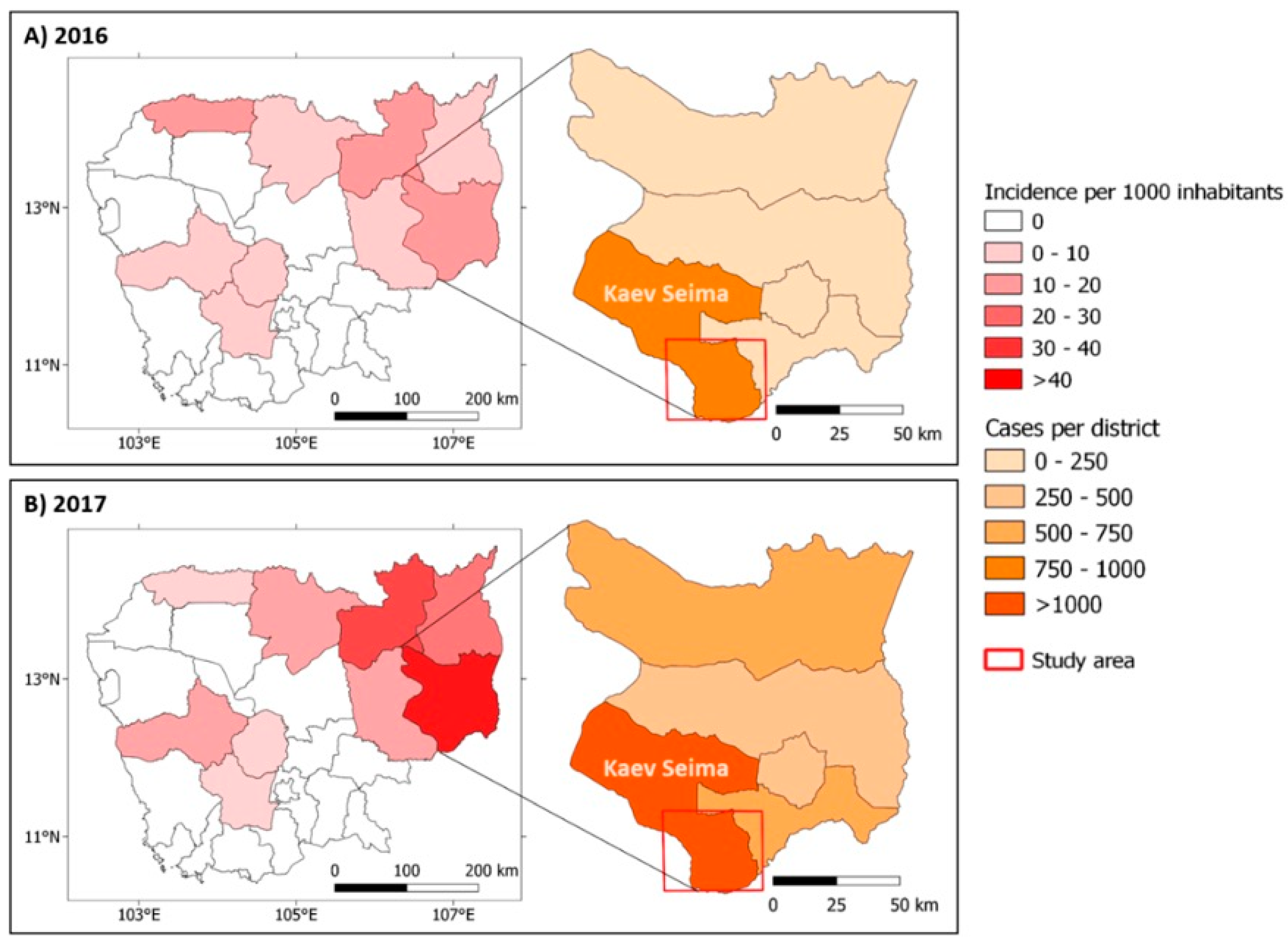
3.1. Malaria Situation in Mondulkiri Province
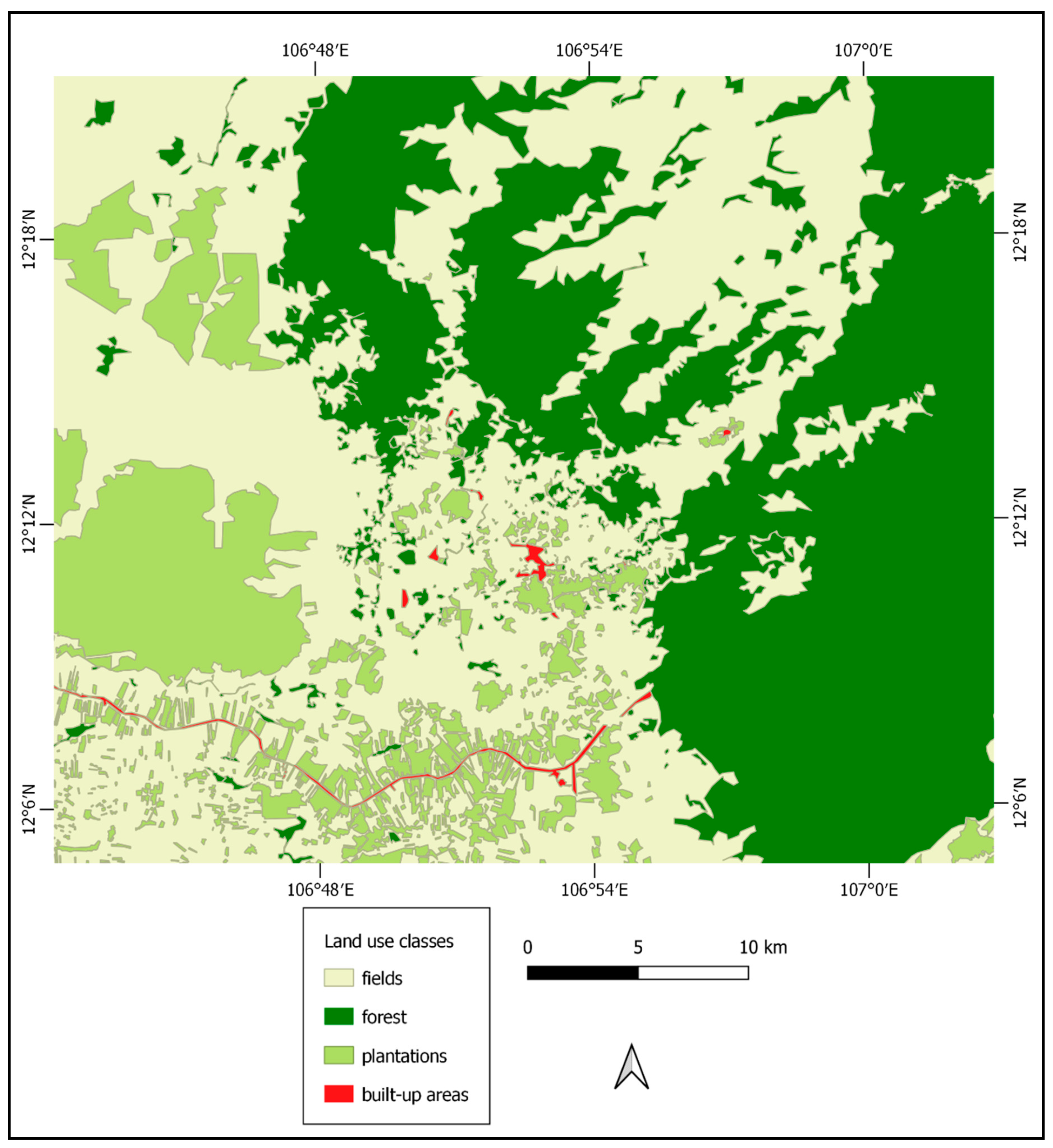
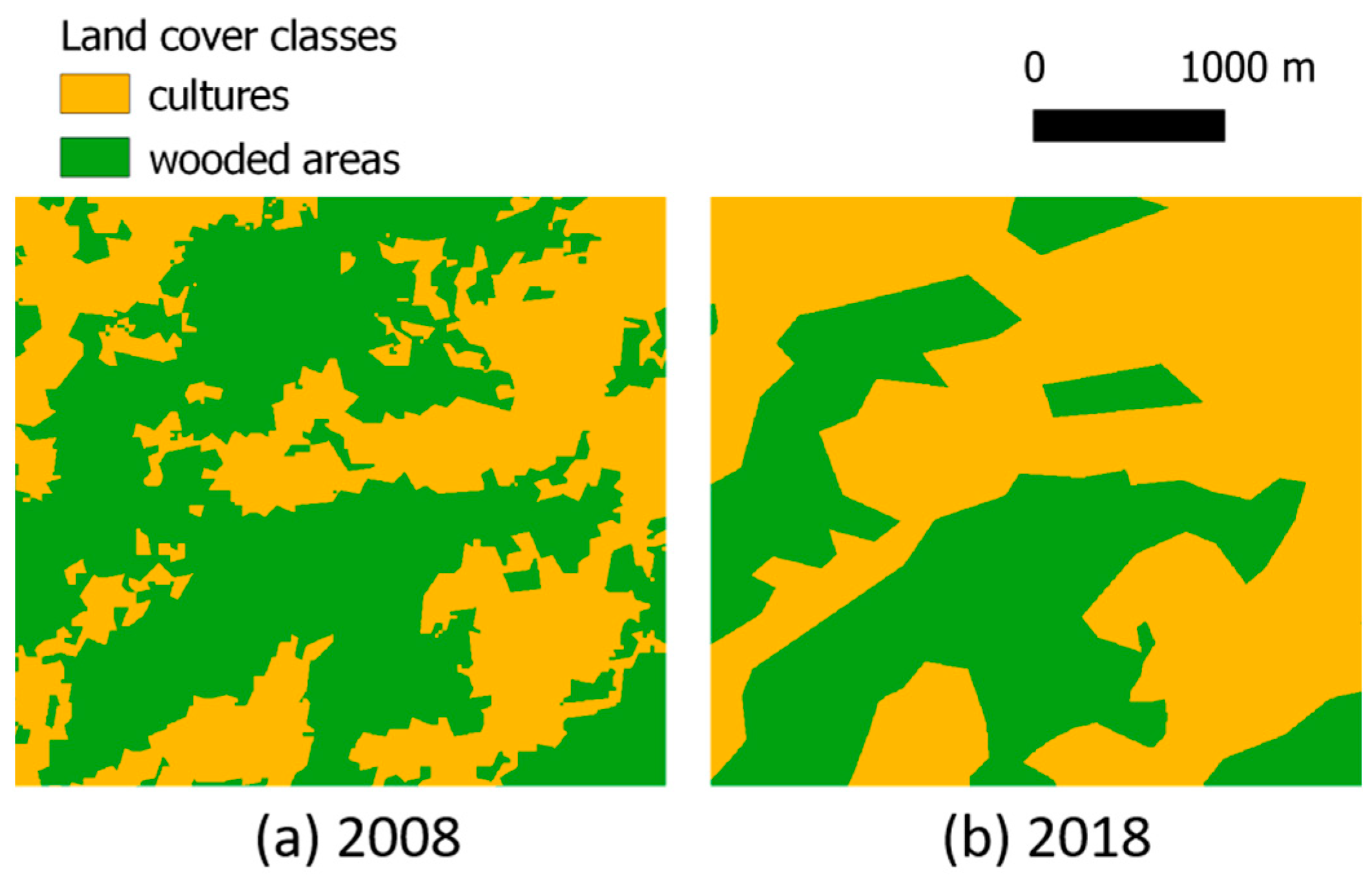
3.2. Land Use/Land Cover Classification
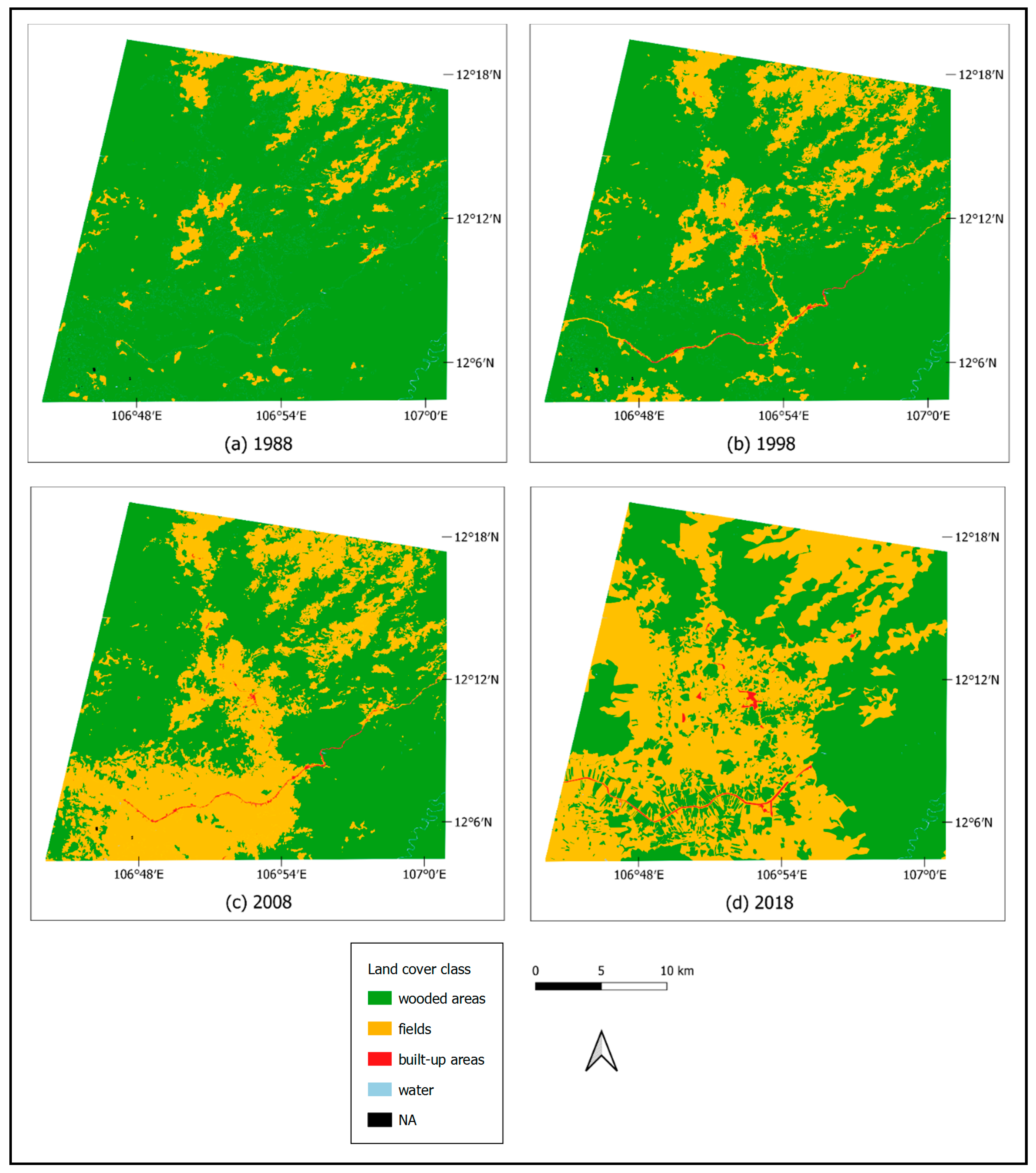
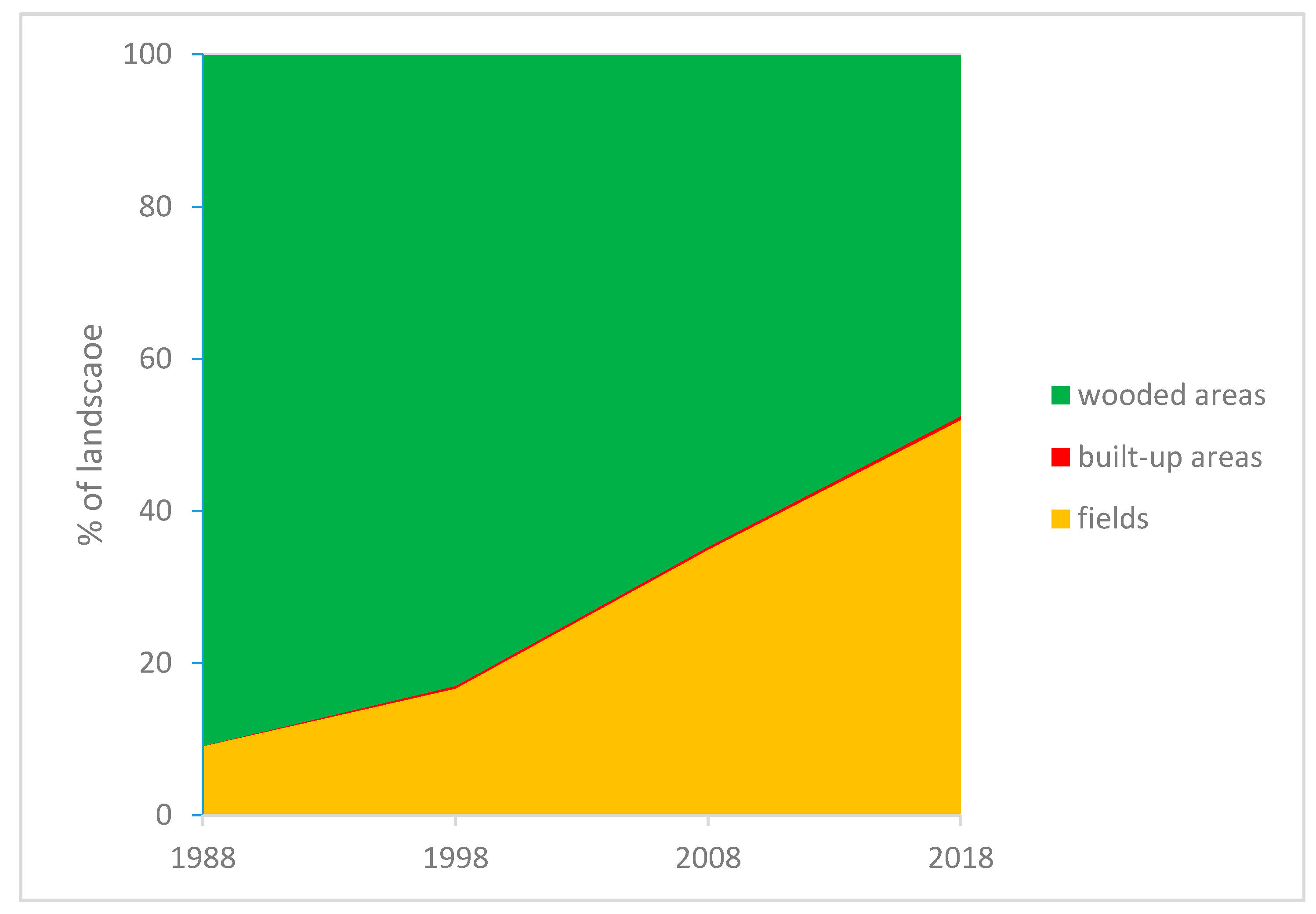
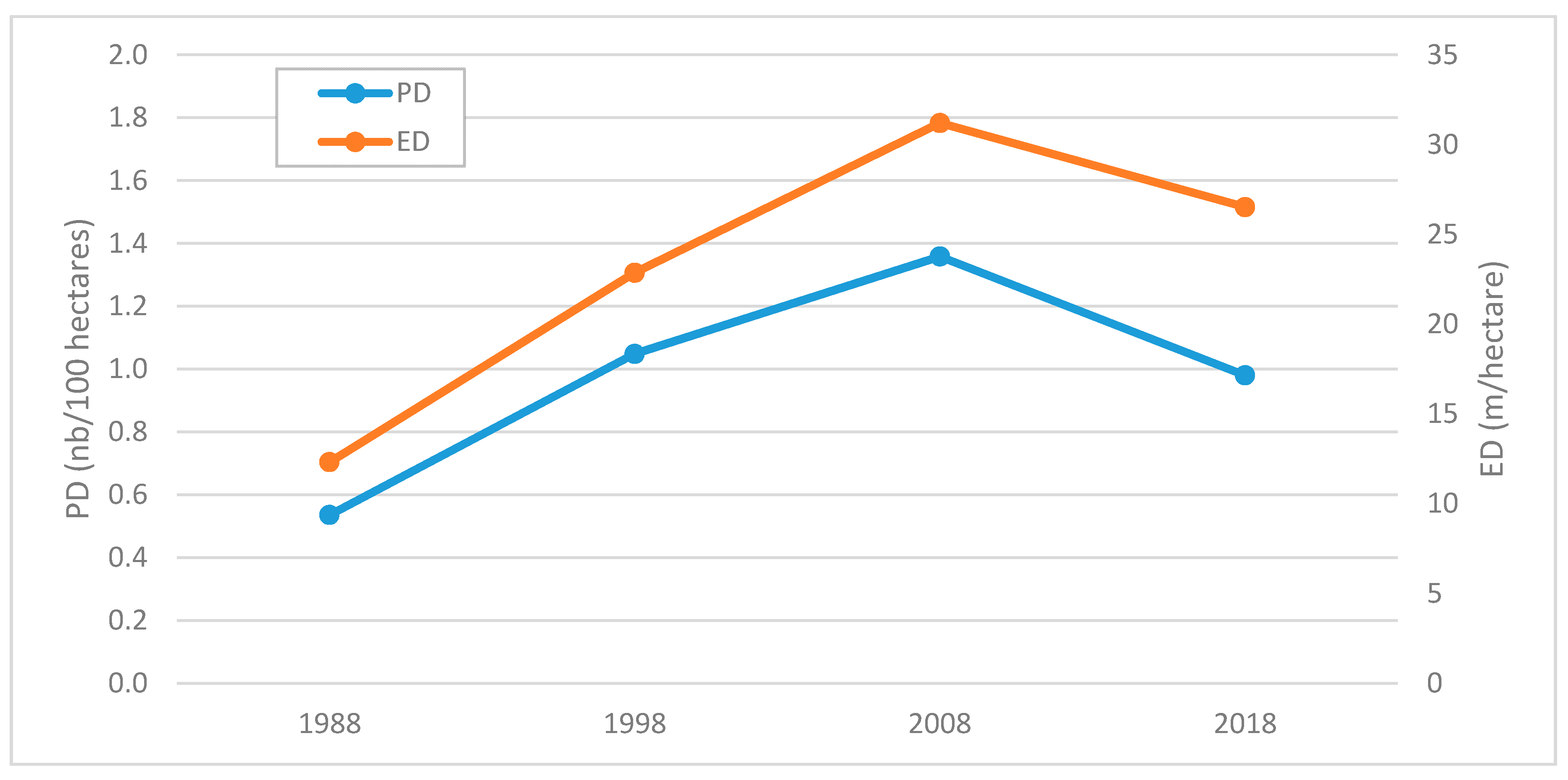
3.3. Comparison between Maps
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burkett-Cadena, N.D.; Vittor, A.Y. Deforestation and vector-borne disease: Forest conversion favors important mosquito vectors of human pathogens. Basic Appl. Ecol. 2018, 26, 101–110. [Google Scholar] [CrossRef]
- Austin, K.F.; Bellinger, M.O.; Rana, P. Anthropogenic forest loss and malaria prevalence: A comparative examination of the causes and disease consequences of deforestation in developing nations. AIMS Environ. Sci. 2017, 4, 217. [Google Scholar] [CrossRef]
- Tucker Lima, J.M.; Vittor, A.; Rifai, S.; Valle, D. Does deforestation promote or inhibit malaria transmission in the Amazon? A systematic literature review and critical appraisal of current evidence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.L.; Maslin, M.A. Defining the Anthropocene. Nature 2015, 519, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Norris, D.E. Mosquito-borne Diseases as a Consequence of Land Use Change. EcoHealth 2004, 1, 19–24. [Google Scholar] [CrossRef]
- Takken, W.; Vilarinhos, P.D.T.R.; Schneider, P.; Santos, F.D. Effects of environmental change on malaria in the Amazon region of Brazil. Frontis 2005, 9, 113–123. [Google Scholar]
- Yasuoka, J.; Levins, R. Impact of deforestation and agricultural development on anopheline ecology and malaria epidemiology. Am. J. Trop. Med. Hyg. 2007, 76, 450–460. [Google Scholar] [CrossRef]
- Kweka, E.J.; Zhou, G.; Gilbreath, T.M.; Afrane, Y.; Nyindo, M.; Githeko, A.K.; Yan, G. Predation efficiency of Anopheles gambiae larvae by aquatic predators in western Kenya highlands. Parasit. Vectors 2011, 4, 128. [Google Scholar] [CrossRef]
- Yanoviak, S.P.; Paredes, J.E.R.; Lounibos, L.P.; Weaver, S.C. Deforestation alters phytotelm habitat availability and mosquito production in the Peruvian Amazon. Ecol. Appl. 2006, 16, 1854–1864. [Google Scholar] [CrossRef]
- Afrane, Y.A.; Zhou, G.; Lawson, B.W.; Githeko, A.K.; Yan, G. Life-table analysis of Anopheles arabiensis in western Kenya highlands: Effects of land covers on larval and adult survivorship. Am. J. Trop. Med. Hyg. 2007, 77, 660–666. [Google Scholar] [CrossRef]
- Guerra, C.A.; Snow, R.W.; Hay, S.I. A global assessment of closed forests, deforestation and malaria risk. Ann. Trop. Med. Parasitol. 2006, 100, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Lindenmayer, D.B. Landscape modification and habitat fragmentation: A synthesis. Glob. Ecol. Biogeogr. 2007, 16, 265–280. [Google Scholar] [CrossRef]
- Taubert, F.; Fischer, R.; Groeneveld, J.; Lehmann, S.; Müller, M.S.; Rödig, E.; Wiegand, T.; Huth, A. Global patterns of tropical forest fragmentation. Nature 2018, 554, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Blanchet, F.G.; Koper, N. Measuring habitat fragmentation: An evaluation of landscape pattern metrics. Methods Ecol. Evol. 2014, 5, 634–646. [Google Scholar] [CrossRef]
- Hughes, A.C. Understanding the drivers of Southeast Asian biodiversity loss. Ecosphere 2017, 8, e01624. [Google Scholar] [CrossRef]
- Davidson, G.; Chua, T.H.; Cook, A.; Speldewinde, P.; Weinstein, P. The Role of Ecological Linkage Mechanisms in Plasmodium knowlesi Transmission and Spread. Ecohealth 2019. [Google Scholar] [CrossRef]
- Morand, S.; Jittapalapong, S.; Suputtamongkol, Y.; Abdullah, M.T.; Huan, T.B. Infectious diseases and their outbreaks in Asia-Pacific: Biodiversity and its regulation loss matter. PLoS ONE 2014, 9, e90032. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef]
- Ostfeld, R.S. A Candide response to Panglossian accusations by Randolph and Dobson: Biodiversity buffers disease. Parasitology 2013, 140, 1196–1198. [Google Scholar] [CrossRef]
- Laporta, G.Z.; Lopez de Prado, P.I.K.; Kraenkel, R.A.; Coutinho, R.M.; Sallum, M.A.M. Biodiversity can help prevent malaria outbreaks in tropical forests. PLoS Negl. Trop. Dis. 2013, 7, e2139. [Google Scholar] [CrossRef]
- Halsey, S. Defuse the dilution effect debate. Nat. Ecol. Evol. 2019, 3, 145. [Google Scholar] [CrossRef] [PubMed]
- Laurance, W.F.; Camargo, J.L.C.; Fearnside, P.M.; Lovejoy, T.E.; Williamson, G.B.; Mesquita, R.C.G.; Meyer, C.F.J.; Bobrowiec, P.E.D.; Laurance, S.G.W. An Amazonian rainforest and its fragments as a laboratory of global change. Biol. Rev. 2018, 93, 223–247. [Google Scholar] [CrossRef] [PubMed]
- Vittor, A.Y.; Gilman, R.H.; Tielsch, J.; Glass, G.; Shields, T.; Lozano, W.S.; Pinedo-Cancino, V.; Patz, J.A. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of Falciparum malaria in the Peruvian Amazon. Am. J. Trop. Med. Hyg. 2006, 74, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Valle, D.; Clark, J. Conservation Efforts May Increase Malaria Burden in the Brazilian Amazon. PLoS ONE 2013, 8, e57519. [Google Scholar] [CrossRef]
- Hahn, M.B.; Olson, S.H.; Vittor, A.Y.; Barcellos, C.; Patz, J.A.; Pan, W. Conservation Efforts and Malaria in the Brazilian Amazon. Am. J. Trop. Med. Hyg. 2014, 90, 591–594. [Google Scholar] [CrossRef][Green Version]
- Overgaard, J.H.; Ekbom, B.; Suwonkerd, W.; Takagi, M. Effect of landscape structure on anopheline mosquito density and diversity in northern Thailand: Implications for malaria transmission and control. Landsc. Ecol. 2003, 18, 605–619. [Google Scholar] [CrossRef]
- Piantadosi, S.; Byar, D.P.; Green, S.B. The ecological fallacy. Am. J. Epidemiol. 1988, 127, 893–904. [Google Scholar] [CrossRef]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-Resolution Global Maps of 21st-Century Forest Cover Change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef]
- Saah, D.; Tenneson, K.; Matin, M.; Uddin, K.; Cutter, P.; Poortinga, A.; Nguyen, Q.H.; Patterson, M.; Johnson, G.; Markert, K.; et al. Land Cover Mapping in Data Scarce Environments: Challenges and Opportunities. Front. Environ. Sci. 2019, 7. [Google Scholar] [CrossRef]
- Palaniyandi, M. The role of remote sensing and GIS for spatial prediction of vector-borne diseases transmission: A systematic review. J. Vector Borne Dis. 2012, 49, 197–204. [Google Scholar]
- Kalluri, S.; Gilruth, P.; Rogers, D.; Szczur, M. Surveillance of arthropod vector-borne infectious diseases using remote sensing techniques: A review. PLoS Pathog. 2007, 3, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Durnez, L.; Mao, S.; Denis, L.; Roelants, P.; Sochantha, T.; Coosemans, M. Outdoor malaria transmission in forested villages of Cambodia. Malar. J. 2013, 12, 329. [Google Scholar] [CrossRef] [PubMed]
- Bauhoff, S.; Busch, J. Does deforestation increase malaria prevalence? Evidence from satellite data and health surveys. World Dev. 2020, 127, 104734. [Google Scholar] [CrossRef]
- Busch, J.; Ferretti-Gallon, K. What Drives Deforestation and What Stops It? A Meta-Analysis. Rev. Environ. Econ. Policy 2017, 11, 3–23. [Google Scholar] [CrossRef]
- Singer, B.; de Castro, M.C. Enhancement and suppression of malaria in the Amazon. Am. J. Trop. Med. Hyg. 2006, 74, 1–2. [Google Scholar] [CrossRef][Green Version]
- De Castro, M.C.; Monte-Mór, R.L.; Sawyer, D.O.; Singer, B.H. Malaria risk on the Amazon frontier. Proc. Natl. Acad. Sci. USA 2006, 103, 2452–2457. [Google Scholar] [CrossRef]
- WHO. World Malaria Report 2019; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-156572-1. [Google Scholar]
- Noedl, H.; Se, Y.; Schaecher, K.; Smith, B.L.; Socheat, D.; Fukuda, M.M. Artemisinin Resistance in Cambodia 1 (ARC1) Study Consortium Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 2008, 359, 2619–2620. [Google Scholar] [CrossRef]
- Ariey, F.; Witkowski, B.; Amaratunga, C.; Beghain, J.; Langlois, A.-C.; Khim, N.; Kim, S.; Duru, V.; Bouchier, C.; Ma, L.; et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 2014, 505, 50–55. [Google Scholar] [CrossRef]
- Leang, R.; Taylor, W.R.J.; Bouth, D.M.; Song, L.; Tarning, J.; Char, M.C.; Kim, S.; Witkowski, B.; Duru, V.; Domergue, A.; et al. Evidence of Plasmodium falciparum Malaria Multidrug Resistance to Artemisinin and Piperaquine in Western Cambodia: Dihydroartemisinin-Piperaquine Open-Label Multicenter Clinical Assessment. Antimicrob. Agents Chemother. 2015, 59, 4719–4726. [Google Scholar] [CrossRef]
- Gryseels, C.; Kuijpers, L.M.F.; Jacobs, J.; Grietens, K.P. When ‘substandard’ is the standard, who decides what is appropriate? Exploring healthcare provision in Cambodia. Crit. Public Health 2019, 29, 460–472. [Google Scholar] [CrossRef]
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Chareonviriyaphap, T.; Patil, A.P.; Temperley, W.H.; Gething, P.W.; Elyazar, I.R.; Kabaria, C.W.; Harbach, R.E.; et al. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: Occurrence data, distribution maps and bionomic précis. Parasites Vectors 2011, 4, 89. [Google Scholar] [CrossRef] [PubMed]
- Sinka, M.E.; Bangs, M.J.; Manguin, S.; Rubio-Palis, Y.; Chareonviriyaphap, T.; Coetzee, M.; Mbogo, C.M.; Hemingway, J.; Patil, A.P.; Temperley, W.H.; et al. A global map of dominant malaria vectors. Parasites Vectors 2012, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Hii, J.; Rueda, L.M. Malaria vectors in the Greater Mekong Subregion: Overview of malaria vectors and remaining challenges. Southeast Asian J. Trop. Med. Public Health 2013, 44 (Suppl. 1), 73–165. [Google Scholar] [PubMed]
- Incardona, S.; Vong, S.; Chiv, L.; Lim, P.; Nhem, S.; Sem, R.; Khim, N.; Doung, S.; Mercereau-Puijalon, O.; Fandeur, T. Large-scale malaria survey in Cambodia: Novel insights on species distribution and risk factors. Malar. J. 2007, 6, 37. [Google Scholar] [CrossRef]
- Kar, N.P.; Kumar, A.; Singh, O.P.; Carlton, J.M.; Nanda, N. A review of malaria transmission dynamics in forest ecosystems. Parasit. Vectors 2014, 7, 265. [Google Scholar] [CrossRef]
- Sluydts, V.; Heng, S.; Coosemans, M.; Van Roey, K.; Gryseels, C.; Canier, L.; Kim, S.; Khim, N.; Siv, S.; Mean, V.; et al. Spatial clustering and risk factors of malaria infections in Ratanakiri Province, Cambodia. Malar. J. 2014, 13, 387. [Google Scholar] [CrossRef]
- Peeters Grietens, K.; Gryseels, C.; Dierickx, S.; Bannister-Tyrrell, M.; Trienekens, S.; Uk, S.; Phoeuk, P.; Suon, S.; Set, S.; Gerrets, R.; et al. Characterizing Types of Human Mobility to Inform Differential and Targeted Malaria Elimination Strategies in Northeast Cambodia. Sci. Rep. 2015, 5, 16837. [Google Scholar] [CrossRef]
- Kiernan, B. The Pol Pot Regime: Race, Power, and Genocide in Cambodia under the Khmer Rouge, 1975–1979, 3rd ed.; Yale University Press: New Haven, CT, USA, 2014; Volume 1, ISBN 978-0-300-14434-5. [Google Scholar]
- Fu, C.; Lopes, S.; Mellor, S.; Aryal, S.; Sovannaroth, S.; Roca-Feltrer, A. Experiences from Developing and Upgrading a Web-Based Surveillance System for Malaria Elimination in Cambodia. JMIR Public Health Surveill. 2017, 3. [Google Scholar] [CrossRef]
- Cox, J.; Sovannaroth, S.; Dy Soley, L.; Ngor, P.; Mellor, S.; Roca-Feltrer, A. Novel approaches to risk stratification to support malaria elimination: An example from Cambodia. Malar. J. 2014, 13, 371. [Google Scholar] [CrossRef][Green Version]
- Davis, K.; Yu, K.; Rulli, M.C.; Pichdara, L.; D’Odorico, P. Accelerated deforestation driven by large-scale land acquisitions in Cambodia. Nat. Geosci. 2015, 8, 772–775. [Google Scholar] [CrossRef]
- Dupuy, S.; Herbreteau, V.; Feyfant, T.; Morand, S.; Tran, A. Land-Cover Dynamics in Southeast Asia: Contribution of Object-Oriented Techniques for Change Detection. In Proceedings of the 4th International Conference on GEographic Object-Based Image Analysis (GEOBIA), Rio de Janeiro, Brazil, 7–9 May 2012; pp. 217–222. [Google Scholar]
- Singh, M.; Evans, D.; Chevance, J.-B.; Tan, B.S.; Wiggins, N.; Kong, L.; Sakhoeun, S. Evaluating the ability of community-protected forests in Cambodia to prevent deforestation and degradation using temporal remote sensing data. Ecol. Evol. 2018, 8, 10175–10191. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Tokola, T.; Hou, Z.; Notarnicola, C. Remote sensing-based landscape indicators for the evaluation of threatened-bird habitats in a tropical forest. Ecol. Evol. 2017, 7, 4552–4567. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.; Sochea, P.; Sarath, M.; MacDonald, A.; Pratt, A.; Poyer, S.; Allen, H.; Kunthy, S.; Chamroeun, S.; Daro, K.; et al. Rubber plantations and drug resistant malaria: A cross-sectional survey in Cambodia. Malar. J. 2019, 18, 379. [Google Scholar] [CrossRef] [PubMed]
- Chiang, G.L.; Loong, K.P.; Chan, S.T.; Eng, K.L.; Yap, H.H. Capture-recapture studies with Anopheles maculatus Theobald (Diptera: Culicidae) the vector of malaria in peninsular Malaysia. Southeast Asian J. Trop. Med. Public Health 1991, 22, 643–647. [Google Scholar] [PubMed]
- Foody, G.M. Status of land cover classification accuracy assessment. Remote Sens. Environ. 2002, 80, 185–201. [Google Scholar] [CrossRef]
- Shannon, C.; Weaver, W. The Mathematical Theory of Communication. ACM Sigmobile Mobile Comput. Commun. Rev. 1949, 1, 131. [Google Scholar]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- McGarigal, K.; Marks, B.J. FRAGSTATS: Spatial Pattern Analysis Program for Quantifying Landscape Structure; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 1995.
- Wilcove, D.S.; Giam, X.; Edwards, D.P.; Fisher, B.; Koh, L.P. Navjot’s nightmare revisited: Logging, agriculture, and biodiversity in Southeast Asia. Trends Ecol. Evol. 2013, 28, 531–540. [Google Scholar] [CrossRef]
- Wiens, J.A. Spatial Scaling in Ecology. Funct. Ecol. 1989, 3, 385–397. [Google Scholar] [CrossRef]
- Olson, S.H.; Gangnon, R.; Silveira, G.A.; Patz, J.A. Deforestation and malaria in Mâncio Lima County, Brazil. Emerg. Infect. Dis. 2010, 16, 1108–1115. [Google Scholar] [CrossRef]
- Oliveira-Ferreira, J.; Lacerda, M.V.; Brasil, P.; Ladislau, J.L.; Tauil, P.L.; Daniel-Ribeiro, C.T. Malaria in Brazil: An overview. Malar. J. 2010, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.B.; Gangnon, R.E.; Barcellos, C.; Asner, G.P.; Patz, J.A. Influence of Deforestation, Logging, and Fire on Malaria in the Brazilian Amazon. PLoS ONE 2014, 9, e85725. [Google Scholar] [CrossRef] [PubMed]
- Bhumiratana, A.; Sorosjinda-Nunthawarasilp, P.; Kaewwaen, W.; Maneekan, P.; Pimnon, S. Malaria-associated rubber plantations in Thailand. Travel Med. Infect. Dis. 2013, 11, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Gryseels, C.; Bannister-Tyrrell, M.; Uk, S.; Set, S.; Sokha, S.; Gerrets, R.; Peeters Grietens, K. A Critical Enquiry into Variability of Insecticidal Net Use in Cambodia: Implications for Assessing Appropriateness of Malaria Elimination Interventions. Am. J. Trop. Med. Hyg. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sriwichai, P.; Karl, S.; Samung, Y.; Sumruayphol, S.; Kiattibutr, K.; Payakkapol, A.; Mueller, I.; Yan, G.; Cui, L.; Sattabongkot, J. Evaluation of CDC light traps for mosquito surveillance in a malaria endemic area on the Thai-Myanmar border. Parasit. Vectors 2015, 8, 636. [Google Scholar] [CrossRef]
- Drame, P.M.; Poinsignon, A.; Besnard, P.; Cornelie, S.; Mire, J.L.; Toto, J.-C.; Foumane, V.; Dos-Santos, M.A.; Sembène, M.; Fortes, F.; et al. Human Antibody Responses to the Anopheles Salivary gSG6-P1 Peptide: A Novel Tool for Evaluating the Efficacy of ITNs in Malaria Vector Control. PLoS ONE 2010, 5, e15596. [Google Scholar] [CrossRef]
- Fornace, K.M.; Alexander, N.; Abidin, T.R.; Brock, P.M.; Chua, T.H.; Vythilingam, I.; Ferguson, H.M.; Manin, B.O.; Wong, M.L.; Ng, S.H.; et al. Local human movement patterns and land use impact exposure to zoonotic malaria in Malaysian Borneo. eLife 2019, 8, e47602. [Google Scholar] [CrossRef]
- Hast, M.; Searle, K.M.; Chaponda, M.; Lupiya, J.; Lubinda, J.; Sikalima, J.; Kobayashi, T.; Shields, T.; Mulenga, M.; Lessler, J.; et al. The use of GPS data loggers to describe the impact of spatio-temporal movement patterns on malaria control in a high-transmission area of northern Zambia. Int. J. Health Geogr. 2019, 18, 19. [Google Scholar] [CrossRef]
- Gottdenker, N.L.; Streicker, D.G.; Faust, C.L.; Carroll, C.R. Anthropogenic Land Use Change and Infectious Diseases: A Review of the Evidence. EcoHealth 2014, 11, 619–632. [Google Scholar] [CrossRef]
- Barbieri, A.F.; Sawyer, I.O.; Soares-Filho, B.S. Population and Land Use Effects on Malaria Prevalence in the Southern Brazilian Amazon. Hum. Ecol. 2005, 33, 847–874. [Google Scholar] [CrossRef]
- Bui, T.Q.; Pham, H.M. Web-based GIS for spatial pattern detection: Application to malaria incidence in Vietnam. Springerplus 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Souris, M.; Marcombe, S.; Laforet, J.; Brey, P.T.; Corbel, V.; Overgaard, H.J. Modeling spatial variation in risk of presence and insecticide resistance for malaria vectors in Laos. PLoS ONE 2017, 12, e0177274. [Google Scholar] [CrossRef] [PubMed]



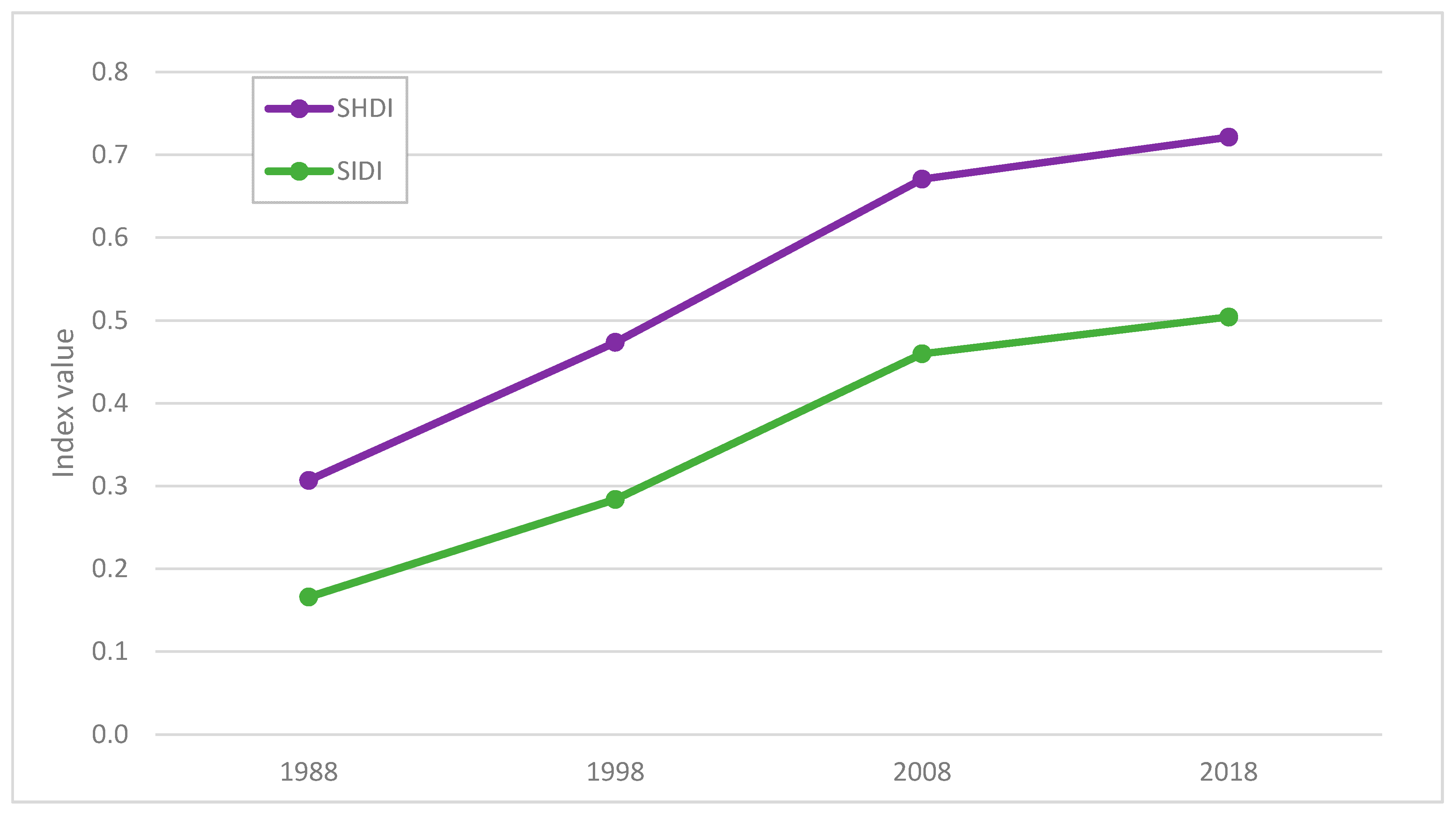
| Reference Test Accuracy | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fields | Forests | Plantations | Built-Up Areas | Total | User’s Accuracy | Relative Omission Error | ||
| Remote sensing Classification | Fields | 37 | 3 | 2 | 1 | 43 | 0.86 | 0.14 |
| Forests | 11 | 17 | 2 | 2 | 32 | 0.53 | 0.47 | |
| Plantations | 8 | 1 | 29 | 0 | 38 | 0.76 | 0.24 | |
| Built-up Areas | 7 | 0 | 1 | 28 | 36 | 0.78 | 0.22 | |
| Total | 63 | 21 | 34 | 31 | 149 | |||
| Producer’s Accuracy | 0.59 | 0.81 | 0.85 | 0.90 | ||||
| Relative Commission Error | 0.41 | 0.19 | 0.15 | 0.10 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pepey, A.; Souris, M.; Vantaux, A.; Morand, S.; Lek, D.; Mueller, I.; Witkowski, B.; Herbreteau, V. Studying Land Cover Changes in a Malaria-Endemic Cambodian District: Considerations and Constraints. Remote Sens. 2020, 12, 2972. https://doi.org/10.3390/rs12182972
Pepey A, Souris M, Vantaux A, Morand S, Lek D, Mueller I, Witkowski B, Herbreteau V. Studying Land Cover Changes in a Malaria-Endemic Cambodian District: Considerations and Constraints. Remote Sensing. 2020; 12(18):2972. https://doi.org/10.3390/rs12182972
Chicago/Turabian StylePepey, Anaïs, Marc Souris, Amélie Vantaux, Serge Morand, Dysoley Lek, Ivo Mueller, Benoit Witkowski, and Vincent Herbreteau. 2020. "Studying Land Cover Changes in a Malaria-Endemic Cambodian District: Considerations and Constraints" Remote Sensing 12, no. 18: 2972. https://doi.org/10.3390/rs12182972
APA StylePepey, A., Souris, M., Vantaux, A., Morand, S., Lek, D., Mueller, I., Witkowski, B., & Herbreteau, V. (2020). Studying Land Cover Changes in a Malaria-Endemic Cambodian District: Considerations and Constraints. Remote Sensing, 12(18), 2972. https://doi.org/10.3390/rs12182972







