Foliar Spectra and Traits of Bog Plants across Nitrogen Deposition Gradients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Sampling Design
2.3. Environmental Gradient in Atmospheric N Deposition
2.4. Leaf Collection and Preparation
2.5. Spectral Measurements
2.6. Leaf Chemical Analyses
2.7. Statistical Analyses
3. Results
3.1. Foliar Functional Traits
3.2. Variation in Foliar Spectra
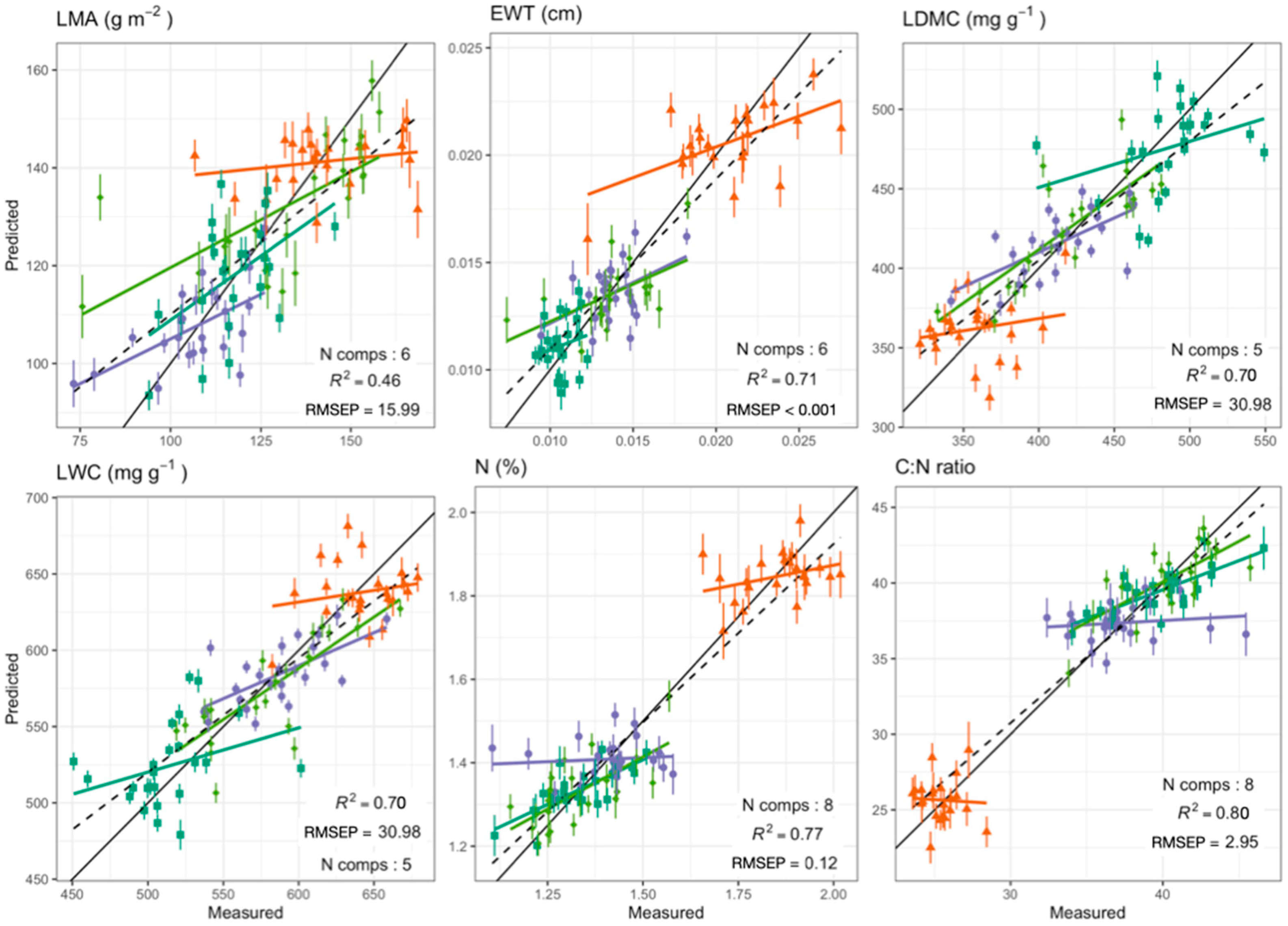
Foliar Spectra in Relation to Functional Traits
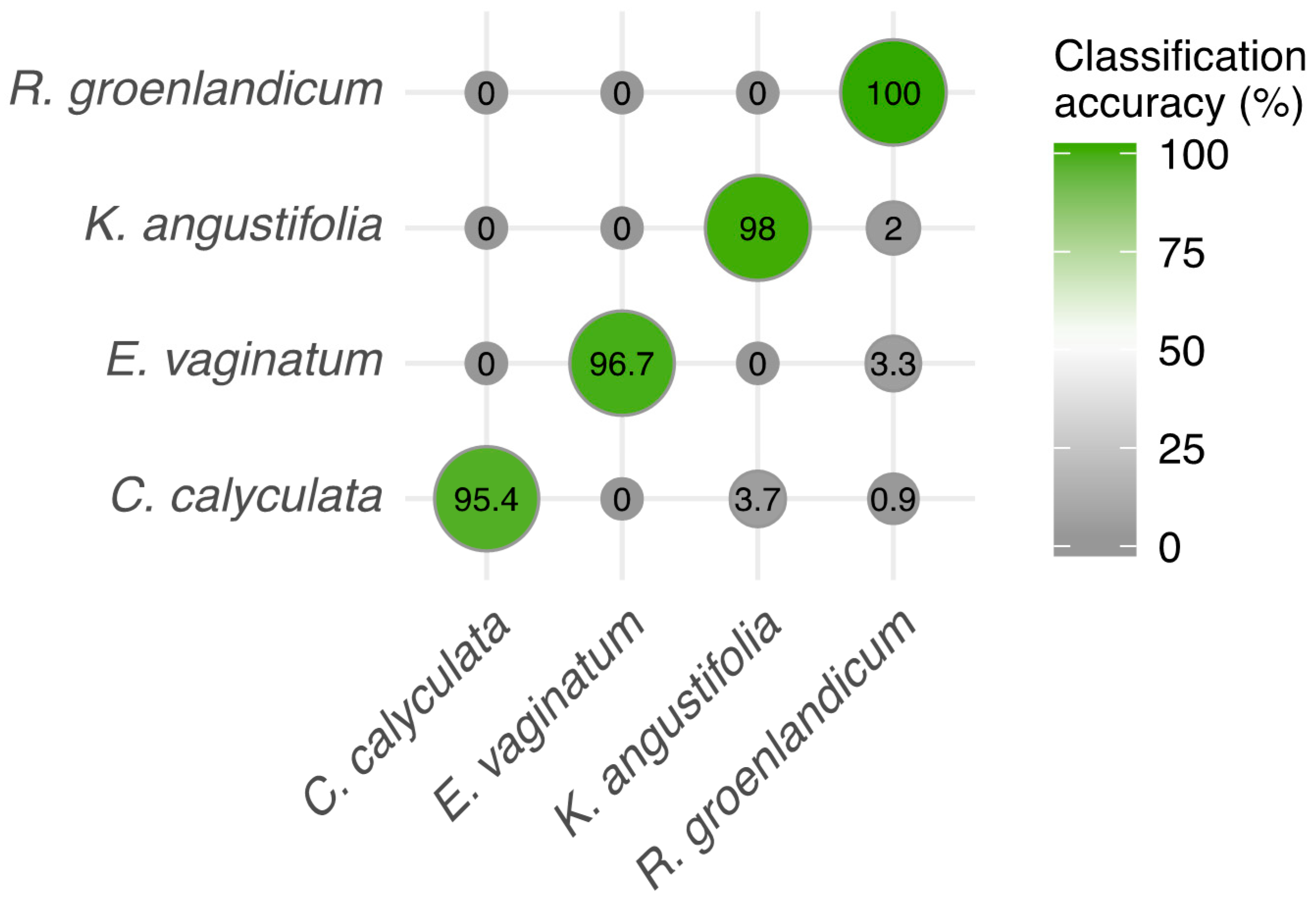
3.3. Species Discrimination Using Spectra
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Van Breemen, N. How Sphagnum bogs down other plants. Trends Ecol. Evol. 1995, 10, 270–275. [Google Scholar] [CrossRef]
- Gorham, E. Northern peatlands: Role in the carbon cycle and probable responses to climatic warming. Ecol. Appl. 1991, 1, 182–195. [Google Scholar] [CrossRef]
- Tarnocai, C.; Kettles, I.; Lacelle, B. Peatlands of Canada. In Geological Survey of Canada, Open File 6551; Natural Resources Canada: Ottawa, ON, Canada, 2011; Volume 10. [Google Scholar]
- Vitt, D.H.; Wieder, K.; Halsey, L.A.; Turetsky, M. Response of Sphagnum fuscum to nitrogen deposition: A case study of ombrogenous peatlands in Alberta, Canada. Bryologist 2003, 106, 235–246. [Google Scholar] [CrossRef]
- Turunen, J.; Roulet, N.T.; Moore, T.R.; Richard, P.J.H. Nitrogen deposition and increased carbon accumulation in ombrotrophic peatlands in eastern Canada. Glob. Biogeochem. Cycles 2004, 18, GB3002. [Google Scholar] [CrossRef]
- Moore, T.R.; Knorr, K.-H.; Thompson, L.; Roy, C.; Bubier, J.L. The effect of long-term fertilization on peat in an ombrotrophic bog. Geoderma 2019, 343, 176–186. [Google Scholar] [CrossRef]
- Bragazza, L.; Freeman, C.; Jones, T.; Rydin, H.; Limpens, J.; Fenner, N.; Ellis, T.; Gerdol, R.; Hájek, M.; Hájek, T. Atmospheric nitrogen deposition promotes carbon loss from peat bogs. Proc. Natl. Acad. Sci. USA 2006, 103, 19386–19389. [Google Scholar] [CrossRef]
- Bubier, J.L.; Moore, T.R.; Crosby, G. Fine-scale vegetation distribution in a cool temperate peatland. Botany 2006, 84, 910–923. [Google Scholar] [CrossRef]
- Bubier, J.L.; Moore, T.R.; Bledzki, L.A. Effects of nutrient addition on vegetation and carbon cycling in an ombrotrophic bog. Glob. Chang. Biol. 2007, 13, 1168–1186. [Google Scholar] [CrossRef]
- Kalacska, M.; Lalonde, M.; Moore, T.R. Estimation of foliar chlorophyll and nitrogen content in an ombrotrophic bog from hyperspectral data: Scaling from leaf to image. Remote Sens. Environ. 2015, 169, 270–279. [Google Scholar] [CrossRef]
- Arroyo-Mora, J.P.; Kalacska, M.; Inamdar, D.; Soffer, R.; Lucanus, O.; Gorman, J.; Naprstek, T.; Schaaf, E.S.; Ifimov, G.; Elmer, K. Implementation of a UAV–hyperspectral pushbroom imager for ecological monitoring. Drones 2019, 3, 12. [Google Scholar] [CrossRef]
- Arroyo-Mora, J.; Kalacska, M.; Soffer, R.; Ifimov, G.; Leblanc, G.; Schaaf, E.; Lucanus, O. Evaluation of phenospectral dynamics with Sentinel-2A using a bottom-up approach in a northern ombrotrophic peatland. Remote Sens. Environ. 2018, 216, 544–560. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E. Spectral and chemical analysis of tropical forests: Scaling from leaf to canopy levels. Remote Sens. Environ. 2008, 112, 3958–3970. [Google Scholar] [CrossRef]
- Yang, X.; Tang, J.; Mustard, J.F.; Wu, J.; Zhao, K.; Serbin, S.; Lee, J.-E. Seasonal variability of multiple leaf traits captured by leaf spectroscopy at two temperate deciduous forests. Remote Sens. Environ. 2016, 179, 1–12. [Google Scholar] [CrossRef]
- Schweiger, A.K.; Cavender-Bares, J.; Townsend, P.A.; Hobbie, S.E.; Madritch, M.D.; Wang, R.; Tilman, D.; Gamon, J.A. Plant spectral diversity integrates functional and phylogenetic components of biodiversity and predicts ecosystem function. Nat. Ecol. Evol. 2018, 2, 976–982. [Google Scholar] [CrossRef] [PubMed]
- McManus, K.M.; Asner, G.P.; Martin, R.E.; Dexter, K.G.; Kress, W.J.; Field, C.B. Phylogenetic structure of foliar spectral traits in tropical forest canopies. Remote Sens. 2016, 8, 196. [Google Scholar] [CrossRef]
- Meireles, J.E.; Cavender-Bares, J.; Townsend, P.A.; Ustin, S.L.; Gamon, J.A.; Schweiger, A.K.; Schaepman, M.E.; Asner, G.P.; Martin, R.E.; Singh, A.; et al. Leaf reflectance spectra capture the evolutionary history of seed plants. New Phytol. 2020. [Google Scholar] [CrossRef]
- Middleton, M.; Närhi, P.; Arkimaa, H.; Hyvönen, E.; Kuosmanen, V.; Treitz, P.; Sutinen, R. Ordination and hyperspectral remote sensing approach to classify peatland biotopes along soil moisture and fertility gradients. Remote Sens. Environ. 2012, 124, 596–609. [Google Scholar] [CrossRef]
- Thomas, V.; Treitz, P.; Jelinski, D.; Miller, J.; Lafleur, P.; McCaughey, J.H. Image classification of a northern peatland complex using spectral and plant community data. Remote Sens. Environ. 2003, 84, 83–99. [Google Scholar] [CrossRef]
- Schmidtlein, S.; Zimmermann, P.; Schüpferling, R.; Weiss, C. Mapping the floristic continuum: Ordination space position estimated from imaging spectroscopy. J. Veg. Sci. 2007, 18, 131–140. [Google Scholar] [CrossRef]
- Harris, A.; Charnock, R.; Lucas, R.M. Hyperspectral remote sensing of peatland floristic gradients. Remote Sens. Environ. 2015, 162, 99–111. [Google Scholar] [CrossRef]
- Cole, B.; McMorrow, J.; Evans, M. Empirical modelling of vegetation abundance from airborne hyperspectral data for upland peatland restoration monitoring. Remote Sens. 2014, 6, 716–739. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E. Airborne spectranomics: Mapping canopy chemical and taxonomic diversity in tropical forests. Front. Ecol. Environ. 2009, 7, 269–276. [Google Scholar] [CrossRef]
- Larmola, T.; Bubier, J.L.; Kobyljanec, C.; Basiliko, N.; Juutinen, S.; Humphreys, E.; Preston, M.; Moore, T.R. Vegetation feedbacks of nutrient addition lead to a weaker carbon sink in an ombrotrophic bog. Glob. Chang. Biol. 2013, 19, 3729–3739. [Google Scholar] [CrossRef] [PubMed]
- Ouimet, R. Programme de Surveillance de la Qualité des Précipitation du Ministère des Forêts, de la Faune et des Parcs du Québec; Ministère des Forêts, de la Faune et des Parcs: Québec, QC, Canada, 2018. [Google Scholar]
- Pinsonneault, A.J.; Moore, T.R.; Roulet, N.T. Effects of long-term fertilization on peat stoichiometry and associated microbial enzyme activity in an ombrotrophic bog. Biogeochemistry 2016, 129, 149–164. [Google Scholar] [CrossRef]
- Government of Canada. Canadian Climate Normals 1981–2010; Government of Canada: Ottawa, ON, Canada, 2011.
- Goud, E.M.; Moore, T.R.; Roulet, N.T. Predicting peatland carbon fluxes from non-destructive plant traits. Funct. Ecol. 2017, 31, 1824–1833. [Google Scholar] [CrossRef]
- Larocque, M.; Colpron-Tremblay, J.; Lavoie, M.; Tremblay, L. Écohydrologie de la Grande Tourbière de Villeroy; Rapport d’activités; Ministère du Développement durable, de l’Environnement, de la Faune et des Parcs: Montréal, QC, Canada, 2013. [Google Scholar]
- Lavoie, M.; Colpron-Tremblay, J.; Robert, É.C. Développement d’une vaste tourbière ombrotrophe non perturbée en contexte périurbain au Québec méridional. Ecoscience 2012, 19, 285–297. [Google Scholar] [CrossRef]
- Bubier, J.L.; Smith, R.; Juutinen, S.; Moore, T.R.; Minocha, R.; Long, S.; Minocha, S. Effects of nutrient addition on leaf chemistry, morphology, and photosynthetic capacity of three bog shrubs. Oecologia 2011, 167, 355–368. [Google Scholar] [CrossRef]
- Malmer, N.; Wallén, B. The dynamics of peat accumulation on bogs: Mass balance of hummocks and hollows and its variation throughout a millennium. Ecography 1999, 22, 736–750. [Google Scholar] [CrossRef]
- Kalacska, M.; Arroyo-Mora, J.; Soffer, R.; Roulet, N.; Moore, T.; Humphreys, E.; Leblanc, G.; Lucanus, O.; Inamdar, D. Estimating peatland water table depth and net ecosystem exchange: A comparison between satellite and airborne imagery. Remote Sens. 2018, 10, 687. [Google Scholar] [CrossRef]
- Foley, S.; Rivard, B.; Sanchez-Azofeifa, G.A.; Calvo, J. Foliar spectral properties following leaf clipping and implications for handling techniques. Remote Sens. Environ. 2006, 103, 265–275. [Google Scholar] [CrossRef]
- Laliberté, E. Measuring Specific Leaf Area and Water Content V.1. Available online: https://www.protocols.io/view/measuring-specific-leaf-area-and-water-content-p3tdqnn (accessed on 16 June 2019).
- Laliberté, E.; Soffer, R. Measuring Spectral Reflectance and Transmittance (350–2500 nm) of Small and/or Narrow Leaves Using the Spectra Vista Corporation (SVC) DC-R/T Integrating Sphere V.3. Available online: https://www.protocols.io/view/measuring-spectral-reflectance-and-transmittance-3-q56dy9e (accessed on 3 July 2019).
- Noda, H.M.; Motohka, T.; Murakami, K.; Muraoka, H.; Nasahara, K.N. Accurate measurement of optical properties of narrow leaves and conifer needles with a typical integrating sphere and spectroradiometer. Plant Cell Environ. 2013, 36, 1903–1909. [Google Scholar] [CrossRef]
- Warren, C.R. Rapid measurement of chlorophylls with a microplate reader. J. Plant Nutr. 2008, 31, 1321–1332. [Google Scholar] [CrossRef]
- Ritchie, R.J. Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth. Res. 2006, 89, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Jocelyne Ayotte, E.L. Measuring Leaf Carbon Fractions with the ANKOM2000 Fiber Analyzer. 2019. Available online: https://www.protocols.io/view/measuring-leaf-carbon-fractions-with-the-ankom2000-yinfude (accessed on 20 June 2019). [CrossRef]
- Ayotte, J.; Guilbeault-Mayers, X.; Laliberté, E. Measuring CN Content in Leaf Samples Using Elementar Vario MICRO Cube. 2019. Available online: https://www.protocols.io/view/measuring-cn-content-in-leaf-samples-using-element-udces2w (accessed on 20 June 2019). [CrossRef]
- Van Der Heijden, F.; Duin, R.P.; De Ridder, D.; Tax, D.M. Classification, Parameter Estimation and State Estimation: An Engineering Approach Using MATLAB; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Castro-Esau, K.L.; Sánchez-Azofeifa, G.A.; Rivard, B.; Wright, S.J.; Quesada, M. Variability in leaf optical properties of Mesoamerican trees and the potential for species classification. Am. J. Bot. 2006, 93, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Castro-Esau, K.L.; Sánchez-Azofeifa, G.; Caelli, T. Discrimination of lianas and trees with leaf-level hyperspectral data. Remote Sens. Environ. 2004, 90, 353–372. [Google Scholar] [CrossRef]
- Wold, S.; Ruhe, A.; Wold, H.; Dunn, I.I.I. The collinearity problem in linear regression. The partial least squares (PLS) approach to generalized inverses. SIAM J. Sci. Stat. Comput. 1984, 5, 735–743. [Google Scholar] [CrossRef]
- Curran, P.J. Remote sensing of foliar chemistry. Remote Sens. Environ. 1989, 30, 271–278. [Google Scholar] [CrossRef]
- Garden, D.M. Physical and physiological properties of plants. In Remote Sensing with Special Reference to Agriculture and Forestry: With Special Reference to Agriculture and Forestry; National Academy of Sciences: Washington, DC, USA, 1970; Volume 224. [Google Scholar]
- Asner, G.P.; Martin, R.E.; Tupayachi, R.; Emerson, R.; Martinez, P.; Sinca, F.; Powell, G.V.N.; Wright, S.J.; Lugo, A.E. Taxonomy and remote sensing of leaf mass per area (LMA) in humid tropical forests. Ecol. Appl. 2011, 21, 85–98. [Google Scholar] [CrossRef]
- Asner, G.P.; Martin, R.E.; Carranza-Jiménez, L.; Sinca, F.; Tupayachi, R.; Anderson, C.B.; Martinez, P. Functional and biological diversity of foliar spectra in tree canopies throughout the Andes to Amazon region. New Phytol. 2014, 204, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, A.K.; Cavender-Bares, J.; Townsend, P.A.; Hobbie, S.E.; Madritch, M.D.; Kothari, S.; Grossman, J.J.; Gholizadeh, H.; Wang, R.; Gamon, J.A. Spectral niches reveal taxonomic identity and complementarity in plant communities. bioRxiv 2020. [Google Scholar] [CrossRef]
- Feilhauer, H.; Asner, G.P.; Martin, R.E.; Schmidtlein, S. Brightness-normalized partial least squares regression for hyperspectral data. J. Quant. Spectrosc. Radiat. Transf. 2010, 111, 1947–1957. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M. The vegan package. Community Ecol. Package 2007, 10, 631–637. [Google Scholar]
- Williams, C.K.; Engelhardt, A.; Cooper, T.; Mayer, Z.; Ziem, A.; Scrucca, L.; Tang, Y.; Candan, C.; Hunt, T.; Kuhn, M.M. Package ‘Caret’. 2019. Available online: https://pbil.univ-lyon1.fr/CRAN/web/packages/caret/caret.pdf (accessed on 11 August 2019).
- Lenth, R. Emmeans: Estimated Marginal Means, aka Least-Squares Means; R Package Version 1.4. 2019. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 13 October 2019).
- Meireles, J.E.; Schweiger, A.K.; Cavender-Bares, J. Spectrolab: Class and Methods for Hyperspectral Data. R Package Version 0.0.8. 2018. Available online: https://cran.r-project.org/web/packages/spectrolab/index.html (accessed on 10 October 2019).
- Developers, S. Signal: Signal Processing. 2013. Available online: http://r-forge.r-project.org/projects/signal (accessed on 16 November 2019).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-140. 2019. Available online: https://cran.r-project.org/web/packages/nlme/index.html (accessed on 16 November 2019).
- Féret, J.-B.; Asner, G.P. Spectroscopic classification of tropical forest species using radiative transfer modeling. Remote Sens. Environ. 2011, 115, 2415–2422. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Meireles, J.E.; Couture, J.J.; Kaproth, M.A.; Kingdon, C.C.; Singh, A.; Serbin, S.P.; Center, A.; Zuniga, E.; Pilz, G. Associations of leaf spectra with genetic and phylogenetic variation in oaks: Prospects for remote detection of biodiversity. Remote Sens. 2016, 8, 221. [Google Scholar] [CrossRef]
- Hestir, E.L.; Khanna, S.; Andrew, M.E.; Santos, M.J.; Viers, J.H.; Greenberg, J.A.; Rajapakse, S.S.; Ustin, S.L. Identification of invasive vegetation using hyperspectral remote sensing in the California Delta ecosystem. Remote Sens. Environ. 2008, 112, 4034–4047. [Google Scholar] [CrossRef]
- Lin, W.-S.; Yang, C.-M.; Kuo, B.-J. Classifying cultivars of rice (Oryza sativa L.) based on corrected canopy reflectance spectra data using the orthogonal projections to latent structures (O-PLS) method. Chemom. Intell. Lab. Syst. 2012, 115, 25–36. [Google Scholar] [CrossRef]
- Madritch, M.D.; Kingdon, C.C.; Singh, A.; Mock, K.E.; Lindroth, R.L.; Townsend, P.A. Imaging spectroscopy links aspen genotype with below-ground processes at landscape scales. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130194. [Google Scholar] [CrossRef]
- Govender, M.; Govender, P.; Weiersbye, I.; Witkowski, E.; Ahmed, F. Review of commonly used remote sensing and ground-based technologies to measure plant water stress. Water SA 2009, 35, 741–772. [Google Scholar] [CrossRef]
- Laliberté, E.; Schweiger, A.K.; Legendre, P. Partitioning plant spectral diversity into alpha and beta components. Ecol. Lett. 2020, 23, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Kellner, J.R.; Albert, L.P.; Burley, J.T.; Cushman, K.C. The case for remote sensing of individual plants. Am. J. Bot. 2019, 106, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Gamon, J.A.; Cavender-Bares, J.; Townsend, P.A.; Zygielbaum, A.I. The spatial sensitivity of the spectral diversity–biodiversity relationship: An experimental test in a prairie grassland. Ecol. Appl. 2018, 28, 541–556. [Google Scholar] [CrossRef] [PubMed]
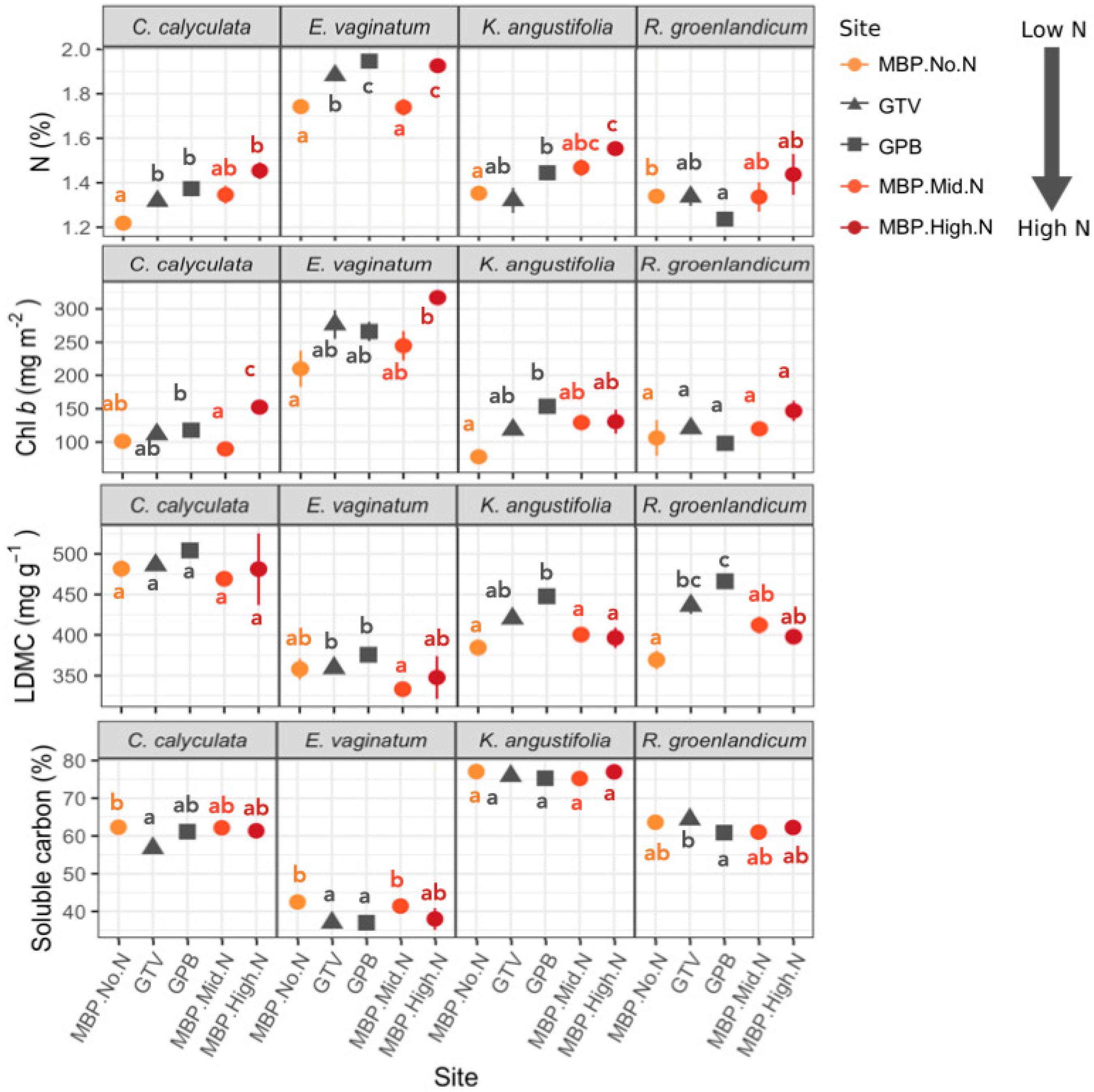
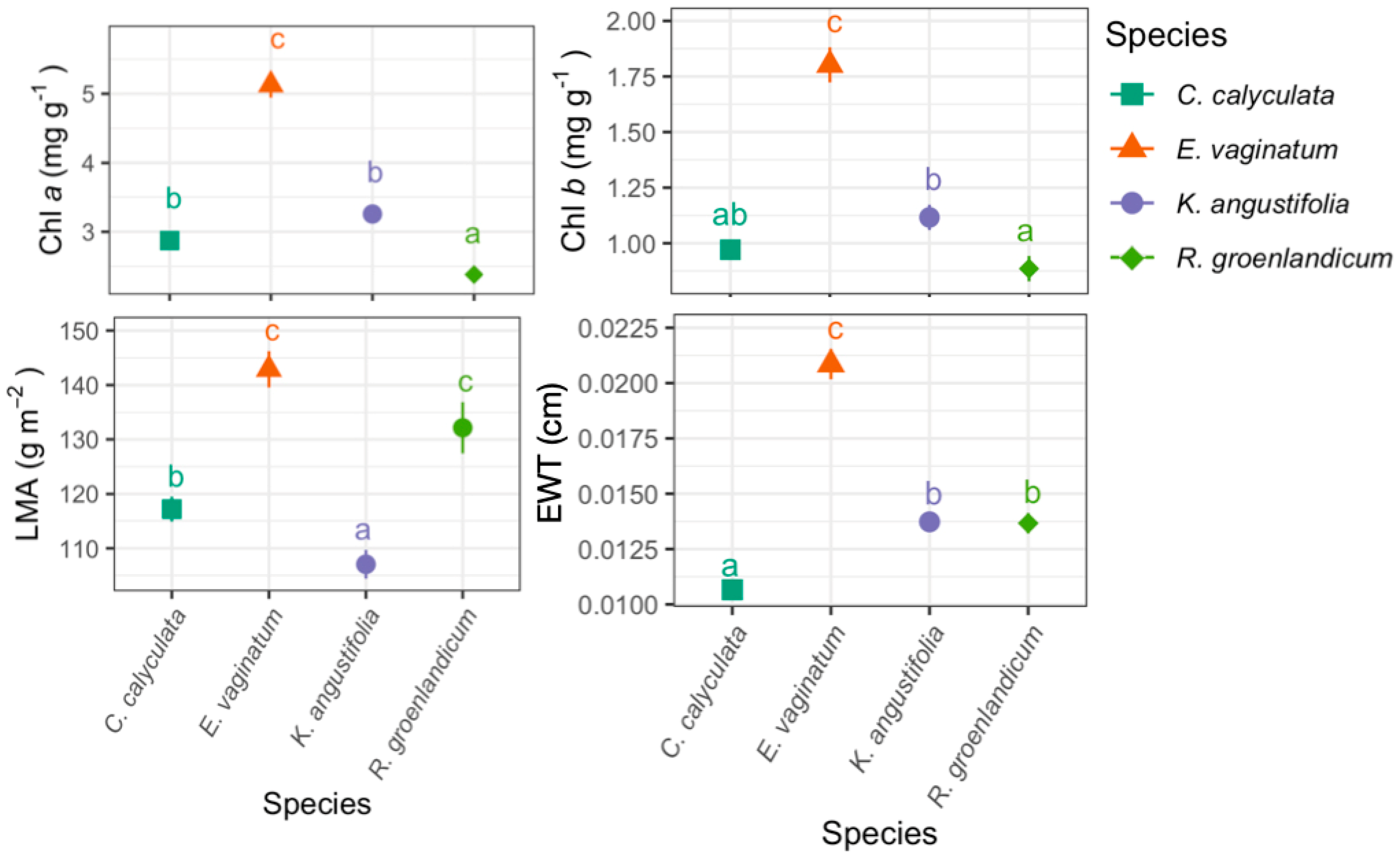
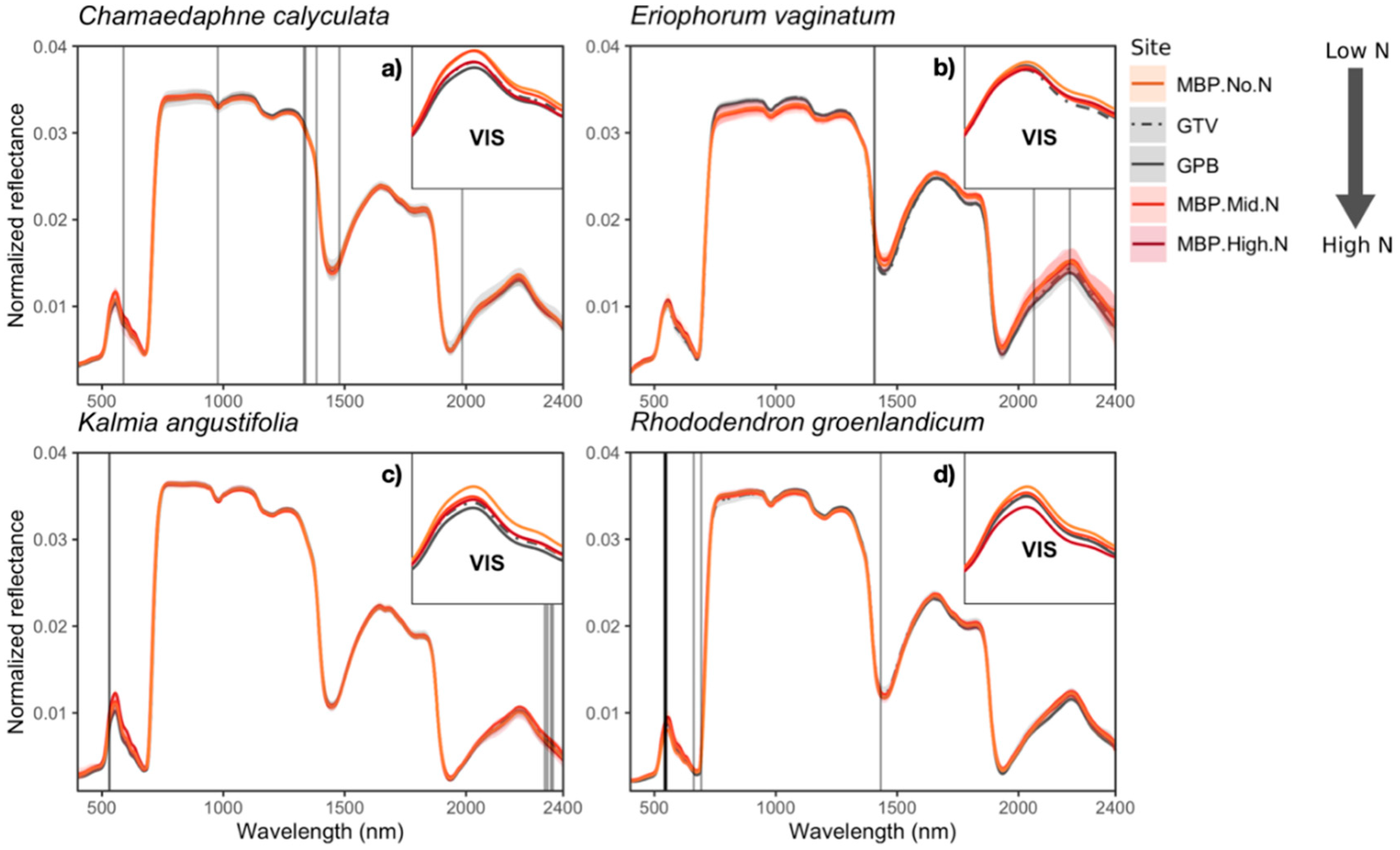

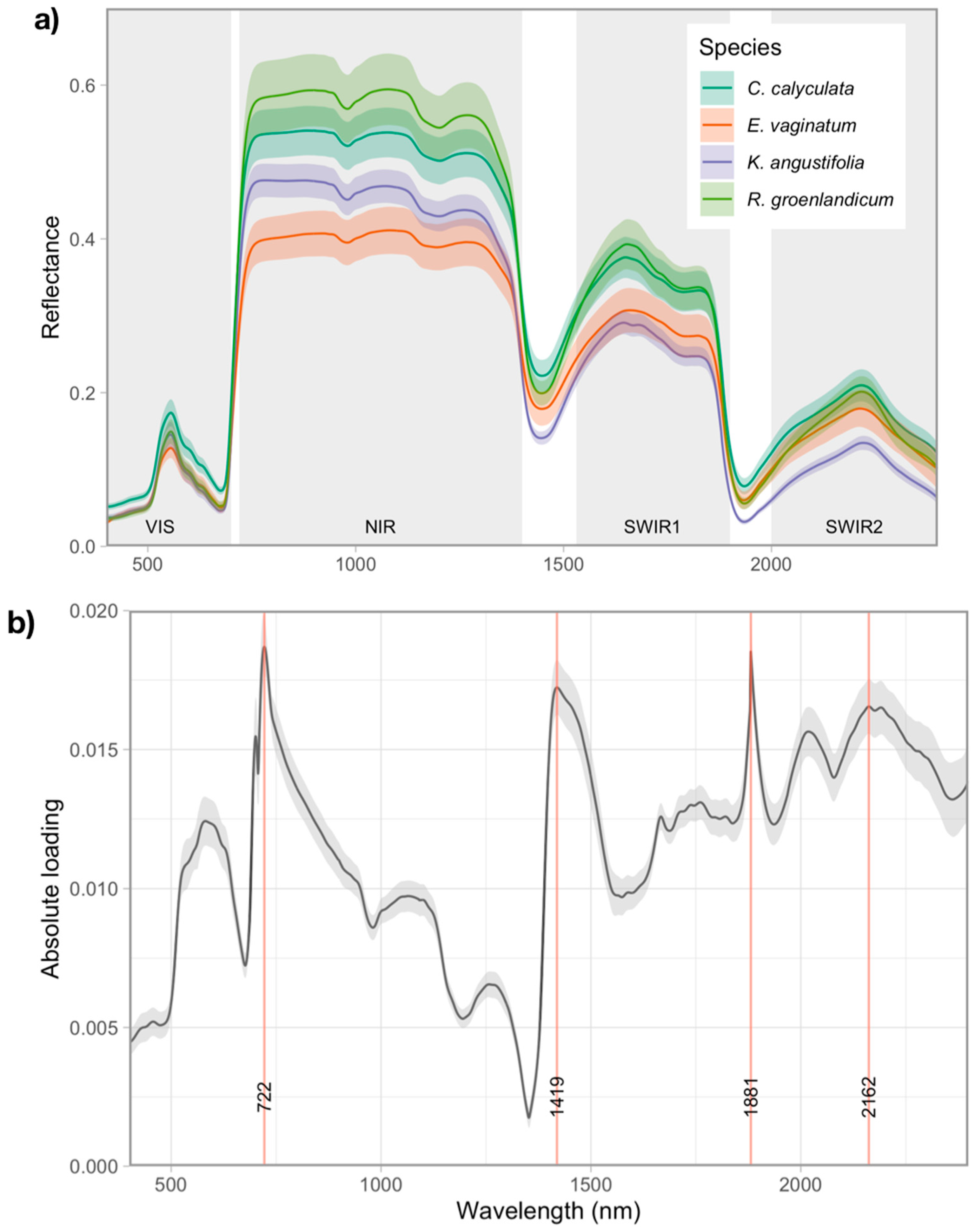
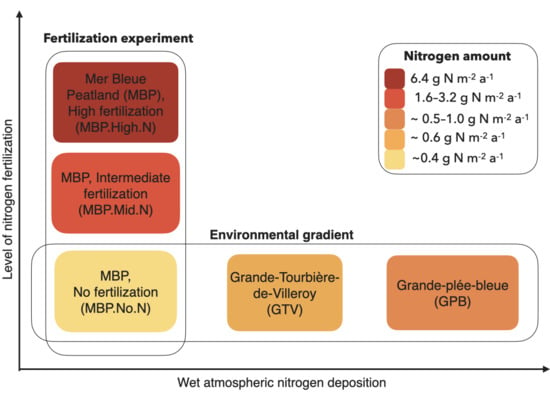
| Site | Replicates | Treatment Name | Sampled Species | Grouping Name | Starting Year of the Experiment | Nitrogen Content of Treatment |
|---|---|---|---|---|---|---|
| MBP (Mer Bleue Peatland) | 6 | — (background deposition only) | all | MBP.No.N | 2005 | 0 g m−2 a−1 |
| 3 | 5N | all except Eriophorum vaginatum | MBP.Mid.N | 2001 | 1.6 g m−2 a−1 | |
| 3 | 10N | E. vaginatum | 2005 | 3.2 g m−2 a−1 | ||
| 3 | 20N | all | MBP.High.N | 2005 | 6.4 g m−2 a−1 |
| Site | Replicates | Peat NO3 (mg kg−1) | Peat NH4 (mg kg−1) | Peat N (%) | Water pH | Water Corrected EC (µS cm−1) | Water Table Depth (cm) | Nitrogen Atmospheric Depositions (g m−2 a−1) |
|---|---|---|---|---|---|---|---|---|
| GPB | 6 | 1.45 (0.78) | 258.5 (147.24) | 0.93 (0.25) | 3.73 (0.05) | 0.77 (0.99) | 35.5 (2.51) | ~0.5–1.0 [27] |
| GTV | 6 | 2.89 (0.24) | 77.83 (18.63) | 0.81 (0.14) | 3.9 (0.08) | 3.35 (4.43) | 50.17 (4.58) | ~0.6 [27] |
| MBP.No.N | 6 | 2.59 (0.7) | 131.67 (61.76) | 0.79 (0.1) | 4.02 (0.15) | 26.71 (23.64) | 39.33 (2.25) | ~0.4 [26] |
| Functional Trait | Range | Mean (±SD) | Wavelength Range (nm) | Number of Components | R2 | RMSEP | NRMSEP (%) * | ||
|---|---|---|---|---|---|---|---|---|---|
| cal | val | cal | val | ||||||
| Total C (%) | 46.05–56.95 | 51.24 (2.52) | 1200–2400 | 5 | 0.9 | 0.83 | 0.8 | 1.05 | 2.06 |
| LWC (mg g−1) | 450.84–679.03 | 579.49 (55.38) | 800–2400 | 5 | 0.76 | 0.7 | 26.69 | 30.98 | 5.35 |
| Soluble carbon (%) | 34.23–79.24 | 59.96 (13.48) | 1200–2400 | 11 | 0.97 | 0.93 | 2.22 | 3.6 | 6.01 |
| LDMC (mg g−1) | 320.97–549.16 | 420.51 (55.38) | 800–2400 | 5 | 0.76 | 0.7 | 26.69 | 30.98 | 7.37 |
| N (%) | 1.1–2.02 | 1.47 (0.24) | 400–2400 | 8 | 0.87 | 0.77 | 0.08 | 0.12 | 7.99 |
| C:N ratio | 23.57–46.62 | 35.85 (6.47) | 400–2400 | 8 | 0.89 | 0.8 | 2.11 | 2.95 | 8.23 |
| LMA (g m−2) | 73.21–168.53 | 124.55 (21.13) | 800–2400 | 6 | 0.62 | 0.46 | 12.87 | 15.99 | 12.84 |
| Cellulose (%) | 6.45–28.41 | 14.6 (6.83) | 1200–2400 | 7 | 0.97 | 0.92 | 1.25 | 2.02 | 13.85 |
| EWT (cm) | 0.01–0.03 | 0.01 (0) | 800–2400 | 6 | 0.82 | 0.71 | 0.0018 | 0.0025 | 16.66 |
| Hemicellulose (%) | 5.12–33.69 | 13.19 (10.06) | 1200–2400 | 10 | 0.97 | 0.94 | 1.58 | 2.55 | 19.33 |
| Chl a (mg g−1) | 1.63–6.42 | 3.4 (1.23) | 400–760 | 6 | 0.76 | 0.68 | 0.6 | 0.72 | 21.19 |
| Lignin (%) | 1.94–23.44 | 10.72 (5.65) | 1200–2400 | 7 | 0.88 | 0.81 | 1.93 | 2.52 | 23.48 |
| Chl a (mg m−2) | 166.2–1024.3 | 429.81 (196.32) | 400–760 | 6 | 0.8 | 0.72 | 87.07 | 109.55 | 25.49 |
| Chl b (mg g−1) | 0.24–2.4 | 1.19 (0.46) | 400–760 | 6 | 0.63 | 0.51 | 0.28 | 0.33 | 28.11 |
| Chl b (mg m−2) | 26.2–364.86 | 150.25 (71.47) | 400–760 | 6 | 0.72 | 0.61 | 37.29 | 46.4 | 30.88 |
| Carotenoids (mg g−1) | 0.12–2.54 | 1.03 (0.42) | 400–760 | 6 | 0.55 | 0.44 | 0.28 | 0.33 | 31.82 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girard, A.; Schweiger, A.K.; Carteron, A.; Kalacska, M.; Laliberté, E. Foliar Spectra and Traits of Bog Plants across Nitrogen Deposition Gradients. Remote Sens. 2020, 12, 2448. https://doi.org/10.3390/rs12152448
Girard A, Schweiger AK, Carteron A, Kalacska M, Laliberté E. Foliar Spectra and Traits of Bog Plants across Nitrogen Deposition Gradients. Remote Sensing. 2020; 12(15):2448. https://doi.org/10.3390/rs12152448
Chicago/Turabian StyleGirard, Alizée, Anna K. Schweiger, Alexis Carteron, Margaret Kalacska, and Etienne Laliberté. 2020. "Foliar Spectra and Traits of Bog Plants across Nitrogen Deposition Gradients" Remote Sensing 12, no. 15: 2448. https://doi.org/10.3390/rs12152448
APA StyleGirard, A., Schweiger, A. K., Carteron, A., Kalacska, M., & Laliberté, E. (2020). Foliar Spectra and Traits of Bog Plants across Nitrogen Deposition Gradients. Remote Sensing, 12(15), 2448. https://doi.org/10.3390/rs12152448