Periodic Relations between Terrestrial Vegetation and Climate Factors across the Globe
Abstract
1. Introduction
2. Materials and Methods
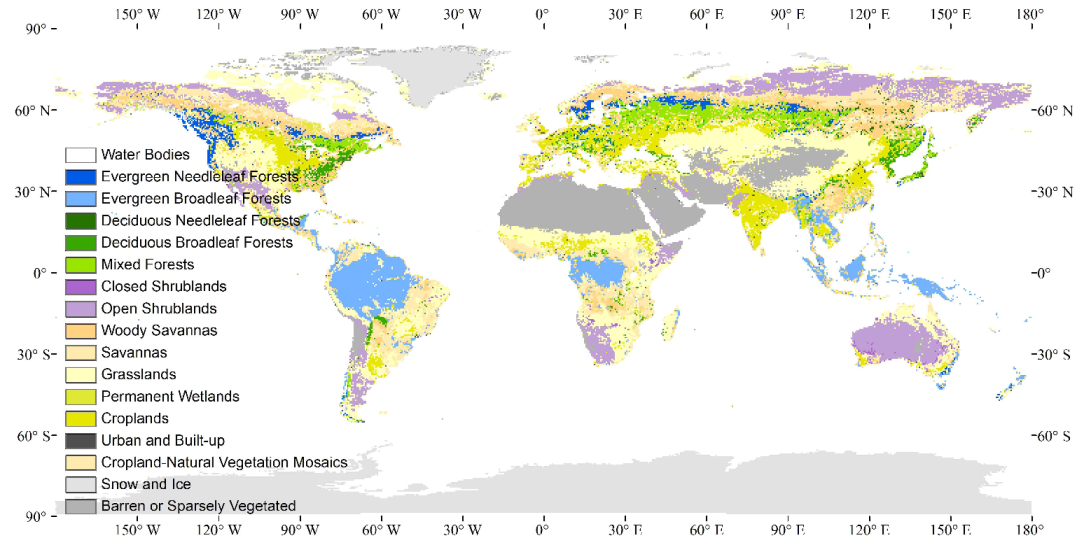
2.1. Global Vegetation Classification
2.2. LAI Data
2.3. Climate Data
2.4. Cross-Spectrum Analysis
2.5. Categorizing Phase
- The driver (climate variable) and the phenology (LAI) are “in phase”: the phase value is statistically indistinguishable (given the phase error) from 0° ± 30° (or 0.0 ± 1.0 months);
- The driver “leads” the phenology: a positive phase that is between + 30° (or +1.0 month) and +150° (or +5.0 months);
- The driver and phenology are “in antiphase”: a phase value that is statistically indistinguishable from 180° ± 30° (or 6.0 ± 1.0 months);
- The driver “lags” the phenology: a negative phase between −30° (or −1.0 months) and −150° (or −5.0 months).
3. Results
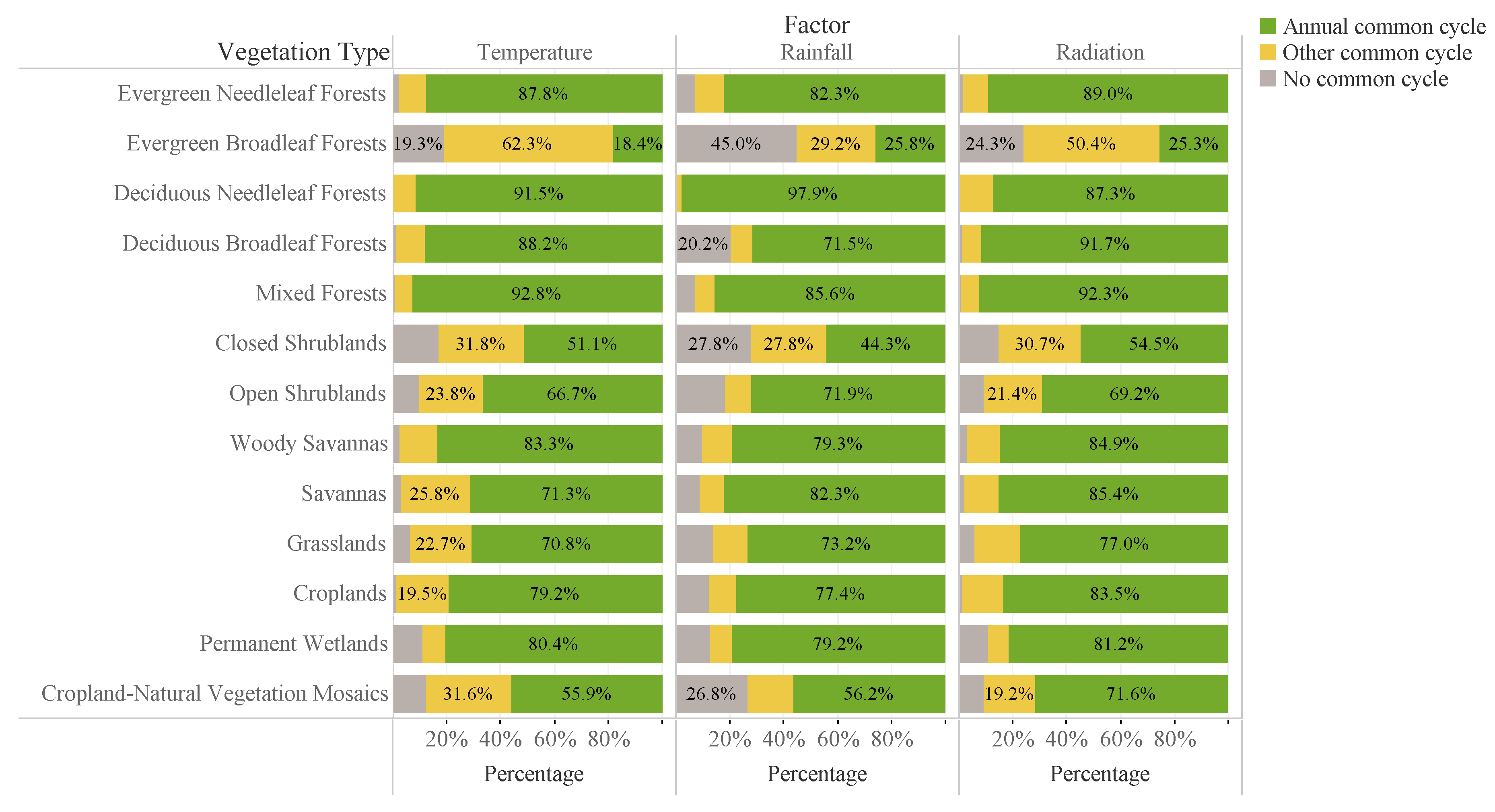
3.1. Common Cycles of LAI and Climate Data
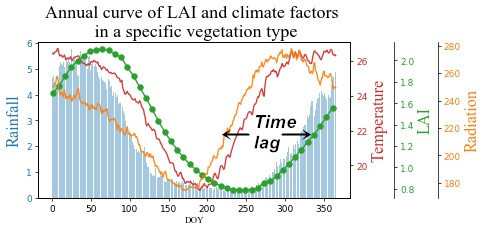
3.2. Time Lag
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, T.; de Jeu, R.A.M.; Liu, Y.Y.; van der Werf, G.R.; Dolman, A.J. Using satellite based soil moisture to quantify the water driven variability in NDVI: A case study over mainland Australia. Remote Sens. Environ. 2014, 140, 330–338. [Google Scholar] [CrossRef]
- Churkina, G.; Running, S.W. Contrasting climatic controls on the estimated productivity of global terrestrial biomes. Ecosystems 1998, 1, 206–215. [Google Scholar] [CrossRef]
- Nemani, R.R.; Keeling, C.D.; Hashimoto, H.; Jolly, W.M.; Piper, S.C.; Tucker, C.J.; Myneni, R.B.; Running, S.W. Climate-driven increases in global terrestrial net primary production from 1982 to 1999. Science 2003, 300, 1560–1563. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, N.L. Climatic control of vegetation distribution: The role of the water balance. Am. Nat. 1990, 135, 649–670. [Google Scholar] [CrossRef]
- Friedman, J.M.; Roelle, J.E.; Cade, B.S. Genetic and environmental influences on leaf phenology and cold hardiness of native and introduced riparian trees. Int. J. Biometeorol. 2011, 55, 775–787. [Google Scholar] [CrossRef]
- Laskin, D.N.; McDermid, G.J. Evaluating the level of agreement between human and time-lapse camera observations of understory plant phenology at multiple scales. Ecol. Inform. 2016, 33, 1–9. [Google Scholar] [CrossRef]
- Liang, B.; Liu, S.; Qu, Y.; Qu, Y. Estimating fractional vegetation cover using the hand-held laser range finder: Method and validation. Remote Sens. Lett. 2015, 6, 20–28. [Google Scholar] [CrossRef]
- Liang, B.; Liu, S. Measurement of vegetation parameters and error analysis based on Monte Carlo method. J. Geogr. Sci. 2018, 28, 819–832. [Google Scholar] [CrossRef]
- Liang, B.; Dahlsjö, C.A.; Maguire-Rajpaul, V.; Malhi, Y.; Liu, S. Modelling error evaluation of ground observed vegetation parameters. IEEE Trans. Instrum. Meas. 2019. [Google Scholar] [CrossRef]
- Ma, M.; Veroustraete, F. Reconstructing pathfinder AVHRR land NDVI time-series data for the Northwest of China. Adv. Space Res. 2006, 37, 835–840. [Google Scholar] [CrossRef]
- Morton, D.C.; DeFries, R.S.; Shimabukuro, Y.E.; Anderson, L.O.; Arai, E.; del Bon Espirito-Santo, F.; Freitas, R.; Morisette, J. Cropland expansion changes deforestation dynamics in the southern Brazilian Amazon. Proc. Natl. Acad. Sci. USA 2006, 103, 14637–14641. [Google Scholar] [CrossRef] [PubMed]
- Menenti, M.; Azzali, S.; Verhoef, W.; Van Swol, R. Mapping agroecological zones and time lag in vegetation growth by means of Fourier analysis of time series of NDVI images. Adv. Space Res. 1993, 13, 233–237. [Google Scholar] [CrossRef]
- Verger, A.; Filella, I.; Baret, F.; Peñuelas, J. Vegetation baseline phenology from kilometric global LAI satellite products. Remote Sens. Environ. 2016, 178, 1–14. [Google Scholar] [CrossRef]
- Vrieling, A.; De Leeuw, J.; Said, M.Y. Length of growing period over Africa: Variability and trends from 30 years of NDVI time series. Remote Sens. 2013, 5, 982–1000. [Google Scholar] [CrossRef]
- Julien, Y.; Sobrino, J. Global land surface phenology trends from GIMMS database. Int. J. Remote Sens. 2009, 30, 3495–3513. [Google Scholar] [CrossRef]
- Nackaerts, K.; Coppin, P.; Muys, B.; Hermy, M. Sampling methodology for LAI measurements with LAI-2000 in small forest stands. Agric. For. Meteorol. 2000, 101, 247–250. [Google Scholar] [CrossRef]
- Running, S.W.; Thornton, P.E.; Nemani, R.; Glassy, J.M. Global terrestrial gross and net primary productivity from the Earth Observing System. In Methods in Ecosystem Science; Springer: Berlin/Heidelberg, Germany, 2000; pp. 44–57. [Google Scholar]
- Wu, D.; Zhao, X.; Liang, S.; Zhou, T.; Huang, K.; Tang, B.; Zhao, W. Time-lag effects of global vegetation responses to climate change. Glob. Chang. Biol. 2015, 21, 3520–3531. [Google Scholar] [CrossRef]
- Bunting, E.L.; Munson, S.M.; Villarreal, M.L. Climate legacy and lag effects on dryland plant communities in the southwestern U.S. Ecol. Indic. 2017, 74, 216–229. [Google Scholar] [CrossRef]
- French, T.D.; Campbell, L.M.; Jackson, D.A.; Casselman, J.M.; Scheider, W.A.; Hayton, A. Long-term changes in legacy trace organic contaminants and mercury in Lake Ontario salmon in relation to source controls, trophodynamics, and climatic variability. Limnol. Oceanogr. 2006, 51, 2794–2807. [Google Scholar] [CrossRef]
- Legay, N.; Piton, G.; Arnoldi, C.; Bernard, L.; Binet, M.-N.; Mouhamadou, B.; Pommier, T.; Lavorel, S.; Foulquier, A.; Clément, J.-C. Soil legacy effects of climatic stress, management and plant functional composition on microbial communities influence the response of Lolium perenne to a new drought event. Plant. Soil 2018, 424, 233–254. [Google Scholar] [CrossRef]
- Pederson, N.; Dyer, J.M.; McEwan, R.W.; Hessl, A.E.; Mock, C.J.; Orwig, D.A.; Rieder, H.E.; Cook, B.I. The legacy of episodic climatic events in shaping temperate, broadleaf forests. Ecol. Monogr. 2014, 84, 599–620. [Google Scholar] [CrossRef]
- Sala, O.; Gherardi, L.; Reichmann, L.; Jobbágy, E.; Peters, D. Legacies of precipitation fluctuations on primary production: Theory and data synthesis, Philos. Ecol. Appl. 2012, 22, 2065–2077. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y.; Gavrichkova, O. Time lag between photosynthesis and CO2 efflux from soil. EGU Gen. Assem. 2009, 11, 7184. [Google Scholar]
- Saatchi, S.; Asefi-Najafabady, S.; Malhi, Y.; Aragao, L.E.O.C.; Anderson, L.O.; Myneni, R.B.; Nemani, R. Persistent effects of a severe drought on Amazonian forest canopy. Proc. Natl. Acad. Sci. USA 2013, 110, 565–570. [Google Scholar] [CrossRef]
- Vicente-Serrano, S.M.; Gouveia, C.; Camarero, J.J.; Begueria, S.; Trigo, R.; Lopez-Moreno, J.I.; Azorin-Molina, C.; Pasho, E.; Lorenzo-Lacruz, J.; Revuelto, J. Response of vegetation to drought time-scales across global land biomes. Proc. Natl. Acad. Sci. USA 2013, 110, 52–57. [Google Scholar] [CrossRef]
- Gu, Z.; Duan, X.; Shi, Y.; Li, Y.; Pan, X. Spatiotemporal variation in vegetation coverage and its response to climatic factors in the Red River Basin, China. Ecol. Indic. 2018, 93, 54–64. [Google Scholar] [CrossRef]
- Justice, C.O.; Holben, B.N.; Gwynne, M.D. Monitoring East African vegetation using AVHRR data. Int. J. Remote Sens. 1986, 7, 22. [Google Scholar] [CrossRef]
- Richard, Y.; Poccard, I. A statistical study of NDVI sensitivity to seasonal and interannual rainfall variations in Southern Africa. Int. J. Remote Sens. 1998, 19, 2907–2920. [Google Scholar] [CrossRef]
- Tei, S.; Sugimoto, A. Time lag and negative responses of forest greenness and tree growth to warming over circumboreal forests. Glob. Chang. Biol. 2018, 24, 4225–4237. [Google Scholar] [CrossRef]
- Wang, J.; Rich, P.M.; Price, K.P. Temporal responses of NDVI to precipitation and temperature in the central Great Plains, USA. Int. J. Remote Sens. 2003, 24, 2345–2364. [Google Scholar] [CrossRef]
- Friedl, M.A.; Sulla-Menashe, D.; Tan, B.; Schneider, A.; Ramankutty, N.; Sibley, A.; Huang, X. MODIS Collection 5 global land cover: Algorithm refinements and characterization of new datasets. Remote Sens. Environ. 2010, 114, 168–182. [Google Scholar] [CrossRef]
- Sulla-Menashe, D.; Friedl, M.A. User Guide to Collection 6 MODIS Land Cover (MCD12Q1 and MCD12C1) Product. USGS Reston VA USA 2018, 1–18. [Google Scholar]
- Liang, S.; Zhao, X.; Liu, S.; Yuan, W.; Cheng, X.; Xiao, Z.; Zhang, X.; Liu, Q.; Cheng, J.; Tang, H. A long-term Global LAnd Surface Satellite (GLASS) data-set for environmental studies. Int. J. Digit. Earth 2013, 6, 5–33. [Google Scholar] [CrossRef]
- Xiao, Z.; Liang, S.; Wang, J.; Chen, P.; Yin, X.; Zhang, L.; Song, J. Use of general regression neural networks for generating the GLASS leaf area index product from time-series MODIS surface reflectance. IEEE Trans. Geosci. Remote Sens. 2014, 52, 209–223. [Google Scholar] [CrossRef]
- Tang, H.; Yu, K.; Geng, X.; Zhao, Y.; Jiang, K.; Liang, S. A time series method for cloud detection applied to MODIS surface reflectance images. Int. J. Digit. Earth 2013, 6, 157–171. [Google Scholar] [CrossRef]
- Derroire, G.; Lagrange, A.; Tassin, J. Flowering and fruiting phenology in maquis of New Caledonia. Acta Bot. Gallica 2008, 155, 263–275. [Google Scholar] [CrossRef][Green Version]
- Zalamea, M.; González, G. Leaffall phenology in a subtropical wet forest in Puerto Rico: From species to community patterns. Biotropica 2008, 40, 295–304. [Google Scholar] [CrossRef]
- Zhou, L.; Tucker, C.J.; Kaufmann, R.K.; Slayback, D.; Shabanov, N.V.; Myneni, R.B. Variations in northern vegetation activity inferred from satellite data of vegetation index during 1981 to 1999. J. Geophys. Res. Space Phys. 2001, 106, 20069–20083. [Google Scholar] [CrossRef]
- Ren, S.; Chen, X.; Lang, W.; Schwartz, M.D. Climatic controls of the spatial patterns of vegetation phenology in mid-latitude grasslands of the Northern Hemisphere. J. Geophys. Res. Biogeosci. 2018, 123, 2323–2336. [Google Scholar] [CrossRef]
- Weedon, G.P.; Balsamo, G.; Bellouin, N.; Gomes, S.; Best, M.J.; Viterbo, P. The WFDEI meteorological forcing data set: WATCH Forcing Data methodology applied to ERA-Interim reanalysis data. Water Resour. Res. 2014, 50, 7505–7514. [Google Scholar] [CrossRef]
- Kay, S.M.; Marple, S.L. Spectrum analysis—A modern perspective. Proc. IEEE 1981, 69, 1380–1419. [Google Scholar] [CrossRef]
- Priestley, M.B. Spectral Analysis and Time Series; Academic Press: Cambridge, MA, USA, 1981; Volume 1. [Google Scholar]
- Aragao, L.E.O.; Malhi, Y.; Barbier, N.; Lima, A.; Shimabukuro, Y.; Anderson, L.; Saatchi, S. Interactions between rainfall, deforestation and fires during recent years in the Brazilian Amazonia. Philos. Trans. R. Soc. B Boil. Sci. 2008, 363, 1779–1785. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Huete, A.; Yu, Q.; Coupe, N.R.; Davies, K.; Broich, M.; Ratana, P.; Beringer, J.; Hutley, L.B.; Cleverly, J. Spatial patterns and temporal dynamics in savanna vegetation phenology across the North Australian Tropical Transect. Remote Sens. Environ. 2013, 139, 97–115. [Google Scholar] [CrossRef]
- Bale, S.; Kellogg, P.; Mozer, F.; Horbury, T.; Reme, H. Measurement of the electric fluctuation spectrum of magnetohydrodynamic turbulence. Phys. Rev. Lett. 2005, 94, 215002. [Google Scholar] [CrossRef]
- Denman, K.L.; Abbott, M.R. Time scales of pattern evolution from cross-spectrum analysis of advanced very high resolution radiometer and coastal zone color scanner imagery. J. Geophys. Res. Ocean. 1994, 99, 7433–7442. [Google Scholar] [CrossRef]
- Granger, C.W. Investigating causal relations by econometric models and cross-spectral methods. Econom. J. Econom. Soc. 1969, 424–438. [Google Scholar] [CrossRef]
- Bradley, A.V.; Gerard, F.F.; Barbier, N.; Weedon, G.P.; Anderson, L.O.; Huntingford, C.; Aragao, L.E.; Zelazowski, P.; Arai, E. Relationships between phenology, radiation and precipitation in the Amazon region. Glob. Chang. Boil. 2011, 17, 2245–2260. [Google Scholar] [CrossRef]
- Bradley, A.; Gerard, F.; Barbier, N.; Weedon, G.; Huntingford, C.; Zelazowski, P.; Anderson, L.; De Aragao, L.; Kaduk, J. Template Phenology For Vegetation Models. In Proceedings of the Geoscience & Remote Sensing Symposium, Cape Town, South Africa, 12–17 July 2009. [Google Scholar]
- Borchert, R. Phenology and control of flowering in tropical trees. Biotropica 1983, 15, 81–89. [Google Scholar] [CrossRef]
- Bullock, S.H.; Solis-Magallanes, J.A. Phenology of canopy trees of a tropical deciduous forest in Mexico. Biotropica 1990, 22, 22–35. [Google Scholar] [CrossRef]
- Wright, S.J.; Van Schaik, C.P. Light and the phenology of tropical trees. Am. Nat. 1994, 143, 192–199. [Google Scholar] [CrossRef]
- Espy, P.; Stegman, J. Trends and variability of mesospheric temperature at high-latitudes. Phys. Chem. Earth Parts A/B/C 2002, 27, 543–553. [Google Scholar] [CrossRef]
- Zhang, X.; Friedl, M.A.; Schaaf, C.B.; Strahler, A.H.; Hodges, J.C.; Gao, F. Using MODIS Data to Study the Relation Between Climatic Spatial Variability and Vegetation Phenology in Northern High Latitudes. In Proceedings of the IEEE International Geoscience and Remote Sensing Symposium, Toronto, ON, Canada, 24–28 June 2002; pp. 1149–1151. [Google Scholar]
- de Azevedo, I.F.P.; Nunes, Y.R.F.; de Ávila, M.A.; da Silva, D.L.; Fernandes, G.W.; Veloso, R.B. Phenology of riparian tree species in a transitional region in southeastern Brazil. Braz. J. Bot. 2014, 37, 47–59. [Google Scholar] [CrossRef]
- Silva, F.B.; Shimabukuro, Y.E.; Aragao, L.E.; Anderson, L.O.; Pereira, G.; Cardozo, F.; Arai, E. Large-scale heterogeneity of Amazonian phenology revealed from 26-year long AVHRR/NDVI time-series. Environ. Res. Lett. 2013, 8, 024011. [Google Scholar] [CrossRef]
- Vernon, A.J. The Description of Yield Curves of Multi-Harvest Crops. J. Pomol. Hortic. Sci. 2015, 44, 13–25. [Google Scholar] [CrossRef]
- Faveri, J.D.; Verbyla, A.P.; Pitchford, W.S.; Venkatanagappa, S.; Cullis, B.R. Statistical methods for analysis of multi-harvest data from perennial pasture variety selection trials. Crop. Pasture Sci. 2015, 66, 947–962. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, S.K.; Singh, P.; Bajpai, P.; Gupta, M.M.; Singh, D.; Gupta, A.K.; Ram, G.; Shasany, A.K.; Sharma, S. High yields of artemisinin by multi-harvest of Artemisia annua crops. Ind. Crop. Prod. 2004, 19, 77–90. [Google Scholar] [CrossRef]
- Palmer, A.R.; Fuentes, S.; Taylor, D.; Macinnis-Ng, C.; Zeppel, M.; Yunusa, I.; February, E.; Eamus, D. The use of pre-dawn leaf water potential and MODIS LAI to explore seasonal trends in the phenology of Australian and southern African woodlands and savannas. Aust. J. Bot. 2008, 56, 557–563. [Google Scholar] [CrossRef]
- Sankaran, M.; Hanan, N.P.; Scholes, R.J.; Ratnam, J.; Augustine, D.J.; Cade, B.S.; Gignoux, J.; Higgins, S.I.; Le Roux, X.; Ludwig, F. Determinants of woody cover in African savannas. Nature 2005, 438, 846. [Google Scholar] [CrossRef]
- Seghieri, J.; Floret, C.; Pontanier, R. Plant phenology in relation to water availability: Herbaceous and woody species in the savannas of northern Cameroon. J. Trop. Ecol. 1995, 11, 237–254. [Google Scholar] [CrossRef]
- Higgins, S.I.; Delgado-Cartay, M.D.; February, E.C.; Combrink, H.J. Is there a temporal niche separation in the leaf phenology of savanna trees and grasses? J. Biogeogr. 2011, 38, 2165–2175. [Google Scholar] [CrossRef]
- Notaro, M.; Liu, Z.; Williams, J.W. Observed Vegetation-Climate Feedbacks in the United States. J. Clim. 2006, 19, 0260. [Google Scholar] [CrossRef]
- Wang, L.; Wei, G.; Ma, Y.; Miao, Z. Modeling Regional Vegetation NPP Variations and Their Relationships with Climatic Parameters in Wuhan, China. Earth Interact. 2013, 17, 1–20. [Google Scholar] [CrossRef]
- Huang, S.; Rich, P.M.; Crabtree, R.L.; Potter, C.S.; Fu, P. Modeling monthly near-surface air temperature from solar radiation and lapse rate: Application over complex terrain in Yellowstone National Park. Phys. Geogr. 2008, 29, 158–178. [Google Scholar] [CrossRef]
- Prescott, J.; Collins, J.A. The lag of temperature behind solar radiation. Q. J. R. Meteorol. Soc. 1951, 77, 121–126. [Google Scholar] [CrossRef]
- El-Hussainy, F.; Essa, K. The phase lag of temperature behind global solar radiation over Egypt. Theor. Appl. Clim. 1997, 58, 79–86. [Google Scholar] [CrossRef]
- Mckinnon, K.A.; Stine, A.R.; Huybers, P. The Spatial Structure of the Annual Cycle in Surface Temperature: Amplitude, Phase, and Lagrangian History. J. Clim. 2013, 26, 7852–7862. [Google Scholar] [CrossRef]
- Fisher, J.I.; Mustard, J.F.; Vadeboncoeur, M.A. Green leaf phenology at Landsat resolution: Scaling from the field to the satellite. Remote Sens. Environ. 2006, 100, 265–279. [Google Scholar] [CrossRef]
- Woodcock, C.E.; Strahler, A.H. The factor of scale in remote sensing. Remote Sens. Environ. 1987, 21, 311–332. [Google Scholar] [CrossRef]
- Ogden, J. Dendrochronological studies and the determination of tree ages in the Australian tropics. J. Biogeogr. 1981, 8, 405–420. [Google Scholar] [CrossRef]
- Clark, D.A.; Piper, S.; Keeling, C.; Clark, D.B. Tropical rain forest tree growth and atmospheric carbon dynamics linked to interannual temperature variation during 1984–2000. Proc. Natl. Acad. Sci. USA 2003, 100, 5852–5857. [Google Scholar] [CrossRef]
- Jacoby, G.C. Overview of tree-ring analysis in tropical regions. IAWA J. 1989, 10, 99–108. [Google Scholar] [CrossRef]
- Clark, D.; Clark, D.B. Climate-induced annual variation in canopy tree growth in a Costa Rican tropical rain forest. J. Ecol. 1994, 82, 865–872. [Google Scholar] [CrossRef]
- Worbes, M. Annual growth rings, rainfall-dependent growth and long-term growth patterns of tropical trees from the Caparo Forest Reserve in Venezuela. J. Ecol. 1999, 87, 391–403. [Google Scholar] [CrossRef]
- Rapp, J.M.; Silman, M.R. Diurnal, seasonal, and altitudinal trends in microclimate across a tropical montane cloud forest. Clim. Res. 2012, 55, 17–32. [Google Scholar] [CrossRef]
 | |||
|---|---|---|---|
| Vegetation Types | Temperature | Rainfall | Radiation |
| Evergreen Needleleaf Forests | 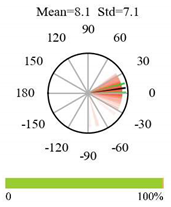 | 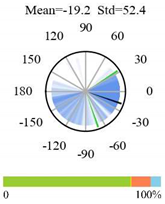 | 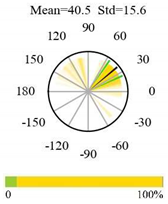 |
| Evergreen Broadleaf Forests | 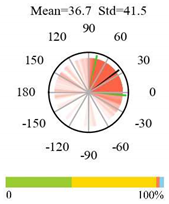 |  | 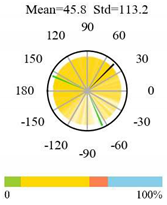 |
| Deciduous Needleleaf Forests | 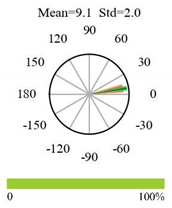 |  | 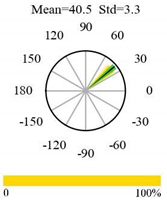 |
| Deciduous Broadleaf Forests |  | 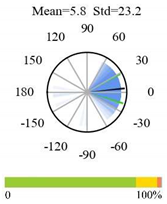 | 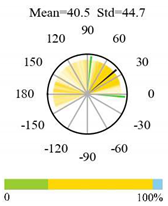 |
| Mixed Forests | 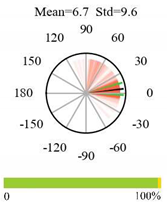 | 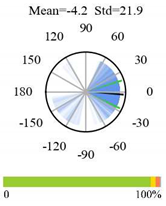 | 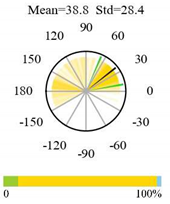 |
| Closed Shrublands | 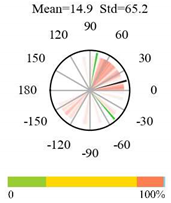 | 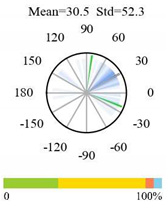 | 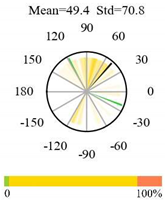 |
| Open Shrublands | 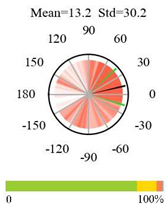 |  | 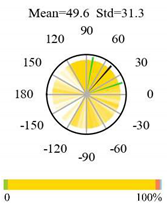 |
| Woody Savannas |  |  | 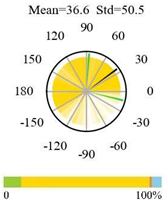 |
| Savannas | 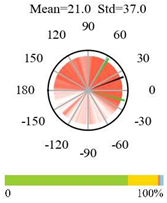 | 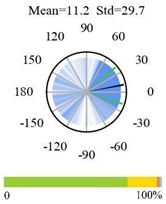 | 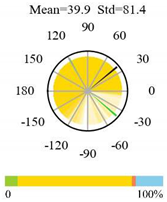 |
| Grasslands | 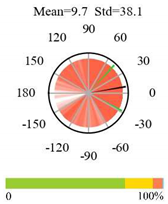 |  | 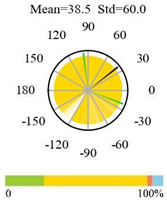 |
| Permanent Wetlands | 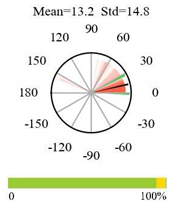 | 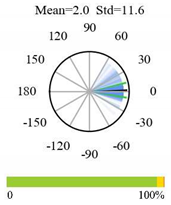 | 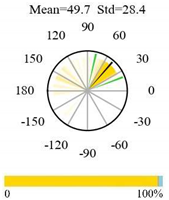 |
| Croplands | 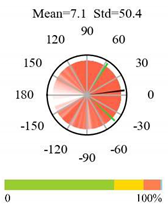 |  |  |
| Cropland-Natural Vegetation Mosaics | 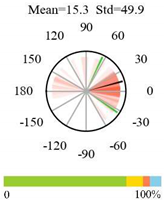 | 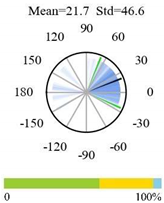 | 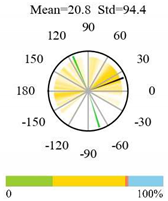 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, B.; Liu, H.; Chen, X.; Zhu, X.; Cressey, E.L.; Quine, T.A. Periodic Relations between Terrestrial Vegetation and Climate Factors across the Globe. Remote Sens. 2020, 12, 1805. https://doi.org/10.3390/rs12111805
Liang B, Liu H, Chen X, Zhu X, Cressey EL, Quine TA. Periodic Relations between Terrestrial Vegetation and Climate Factors across the Globe. Remote Sensing. 2020; 12(11):1805. https://doi.org/10.3390/rs12111805
Chicago/Turabian StyleLiang, Boyi, Hongyan Liu, Xiaoqiu Chen, Xinrong Zhu, Elizabeth L. Cressey, and Timothy A. Quine. 2020. "Periodic Relations between Terrestrial Vegetation and Climate Factors across the Globe" Remote Sensing 12, no. 11: 1805. https://doi.org/10.3390/rs12111805
APA StyleLiang, B., Liu, H., Chen, X., Zhu, X., Cressey, E. L., & Quine, T. A. (2020). Periodic Relations between Terrestrial Vegetation and Climate Factors across the Globe. Remote Sensing, 12(11), 1805. https://doi.org/10.3390/rs12111805