Multitemporal Remote Sensing Based on an FVC Reference Period Using Sentinel-2 for Monitoring Eichhornia crassipes on a Mediterranean River
Abstract
1. Introduction
2. Materials and Methods
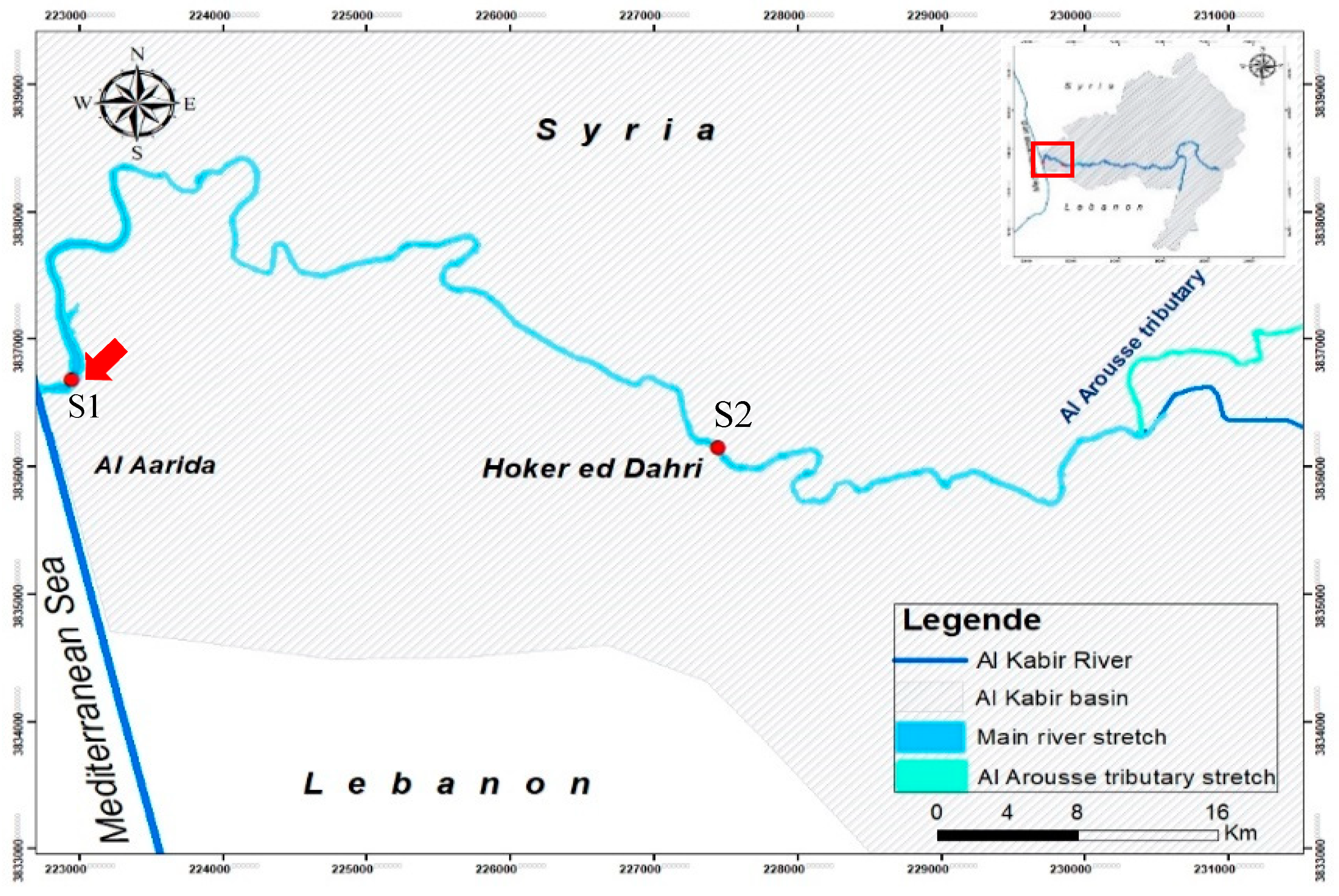
2.1. Study Area
2.2. Distinguishing Riparian and Water Hyacinth Cycles: Building the Reference Image
2.2.1. Estimating Vegetation Cover
- Vegetation Indices
- Choice of Fractional Vegetation Cover
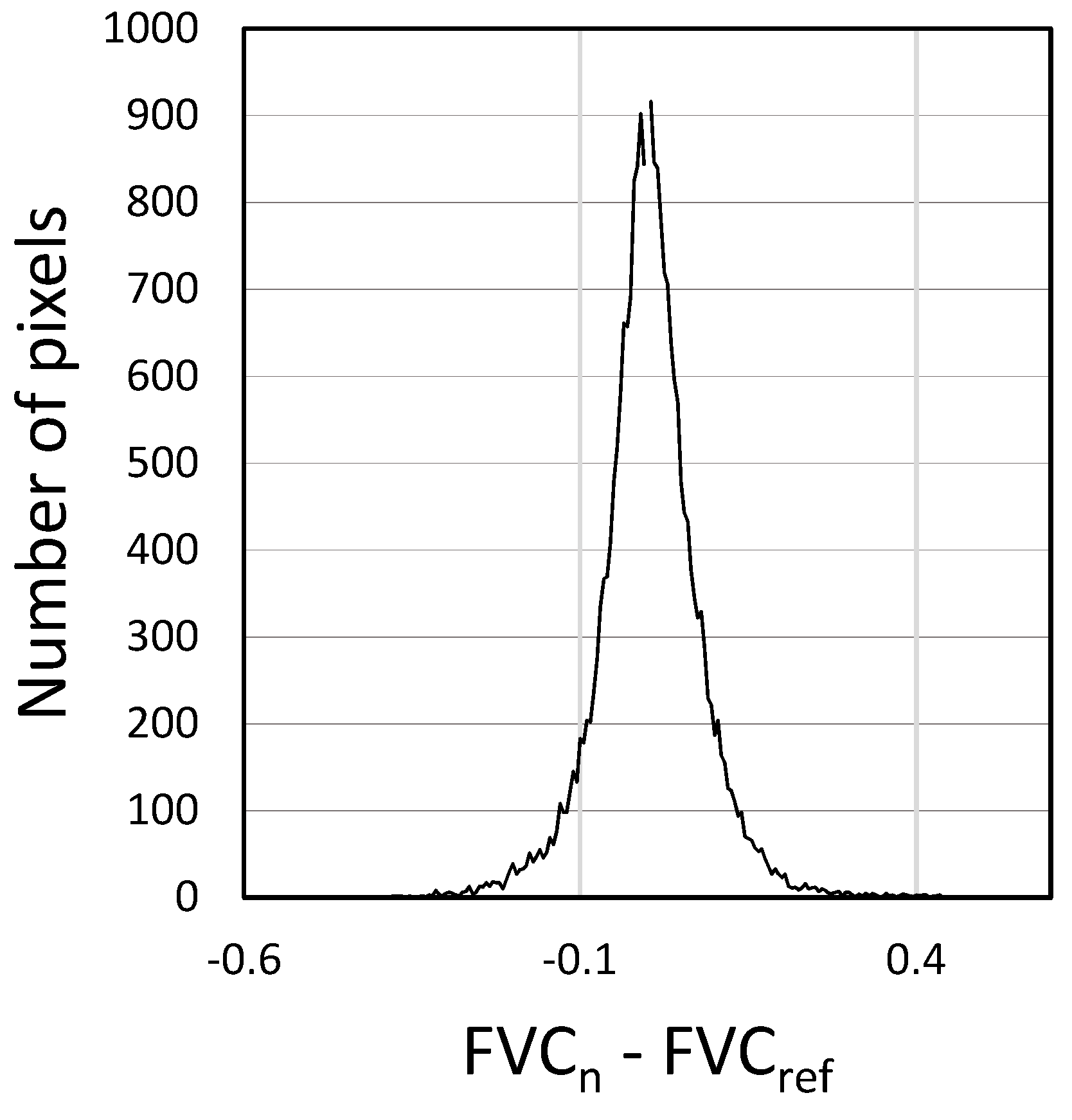
2.2.2. Validation of the Partitioning of Vegetative Cycles
2.3. Data Processing
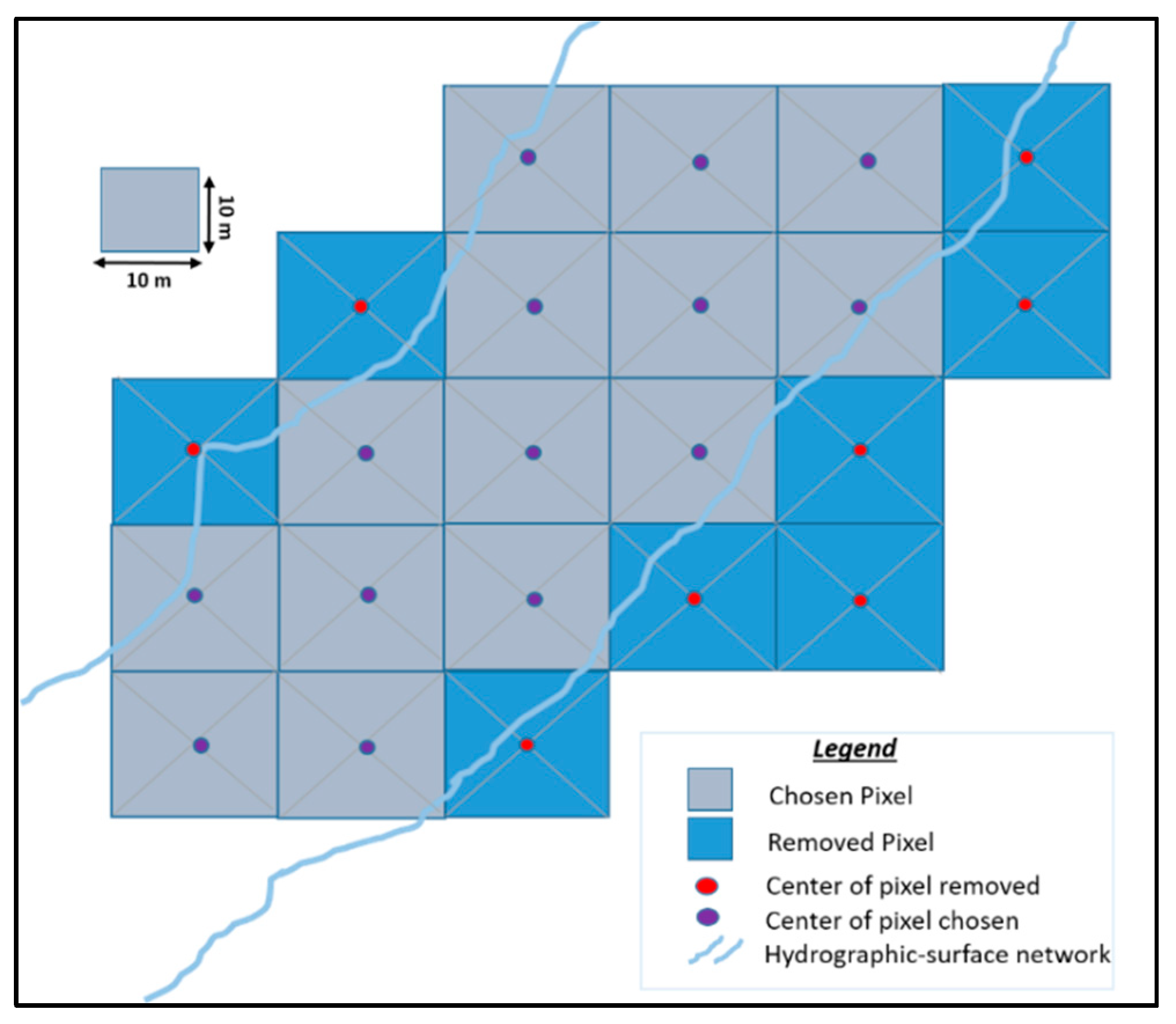
2.3.1. Geospatial Data: the Hydrographic Surface Network
2.3.2. Remotely-Sensed Data
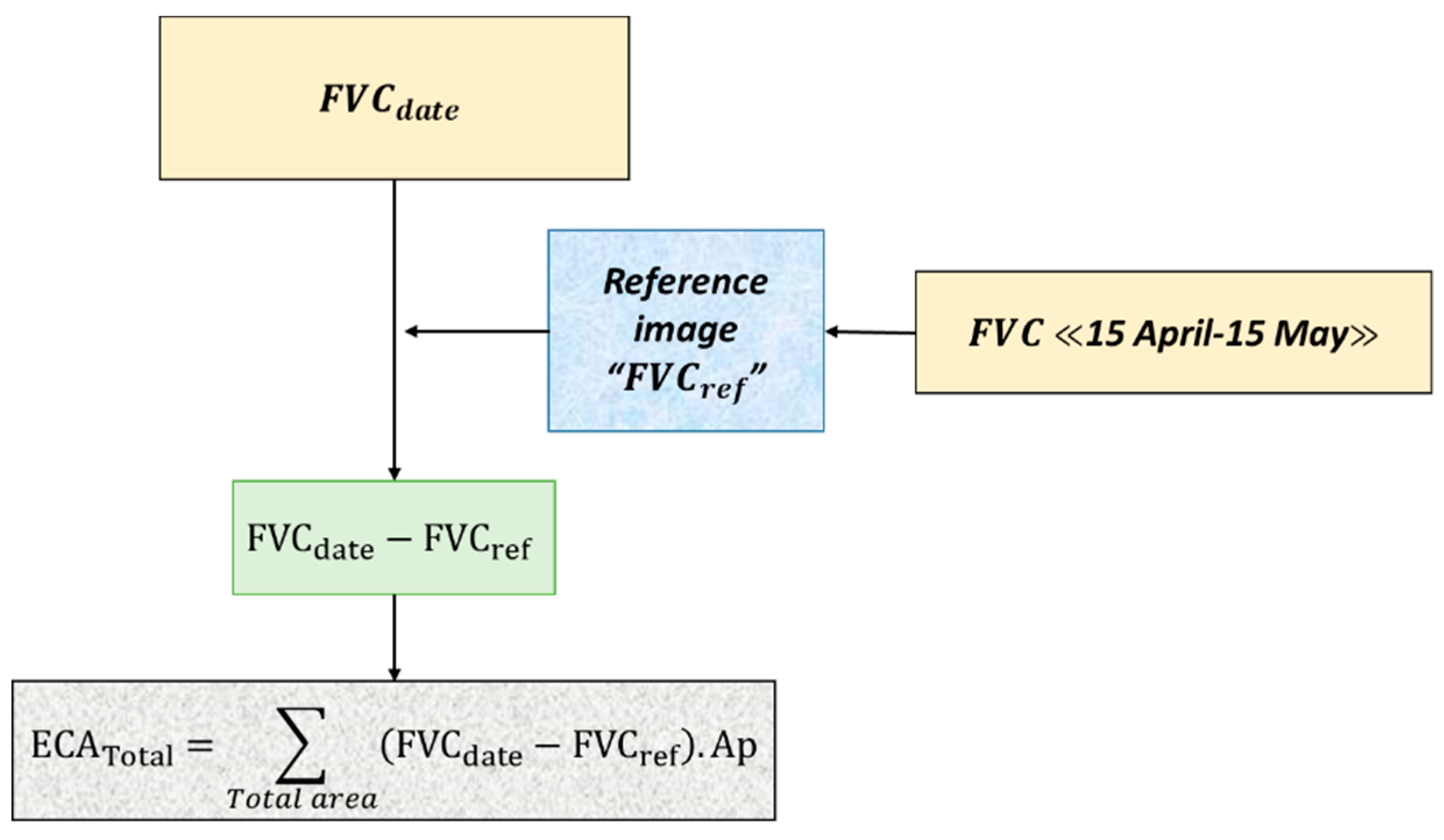
2.3.3. Estimation of the Fractional Vegetation Cover
- Fractional vegetation cover calculation
- Reference image
- Area colonized by water hyacinth
- ECATotal: Total Eichhornia crassipes area on the river
- FVCn: Pixel Fractional Vegetation Cover value at the date n
- FVCref: Pixel Fractional Vegetation Cover of the reference image
- Ap: pixel area (100 m2)
- Validation of the water hyacinth surface area estimation model
3. Results
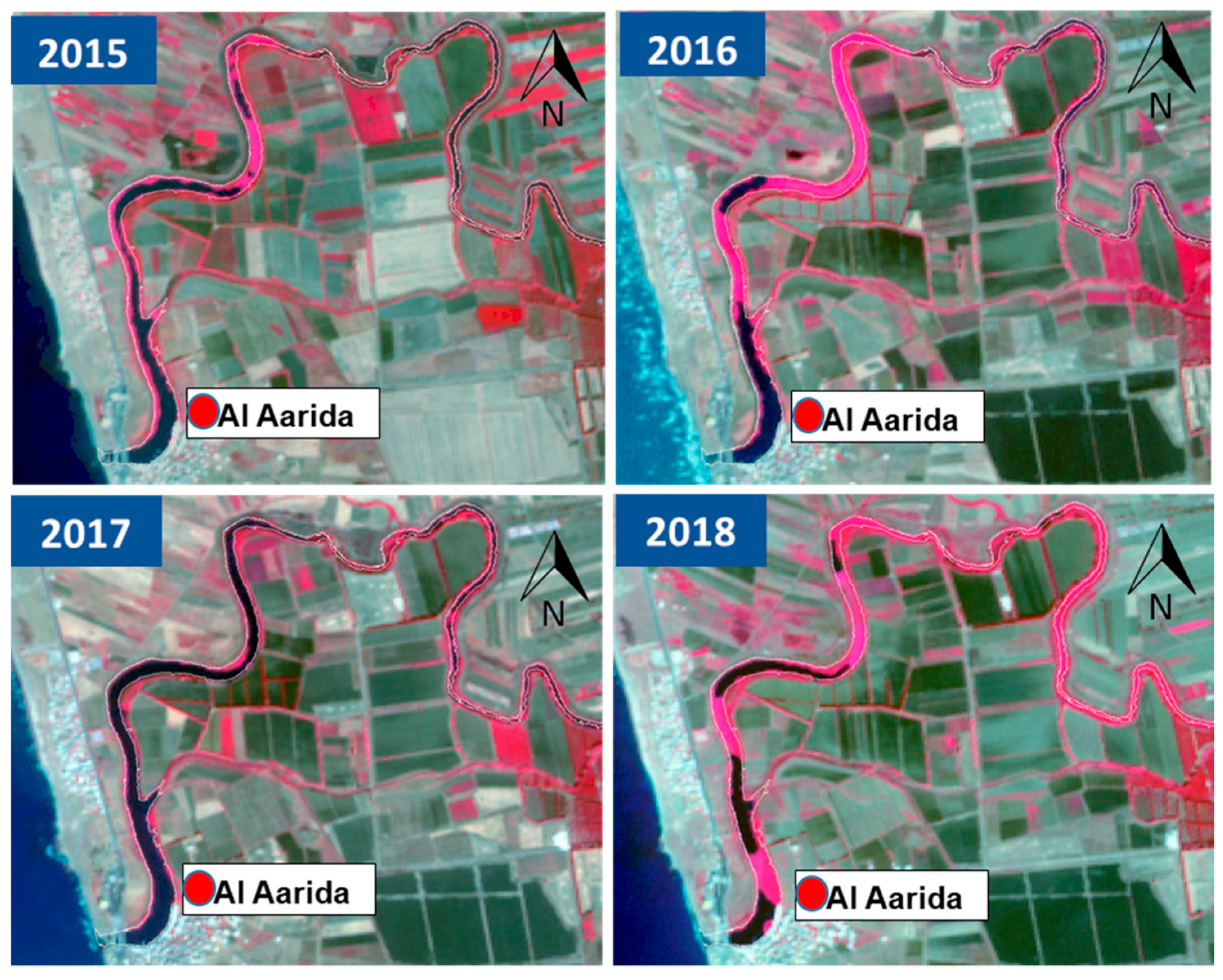
3.1. Vegetation of Al Kabir River
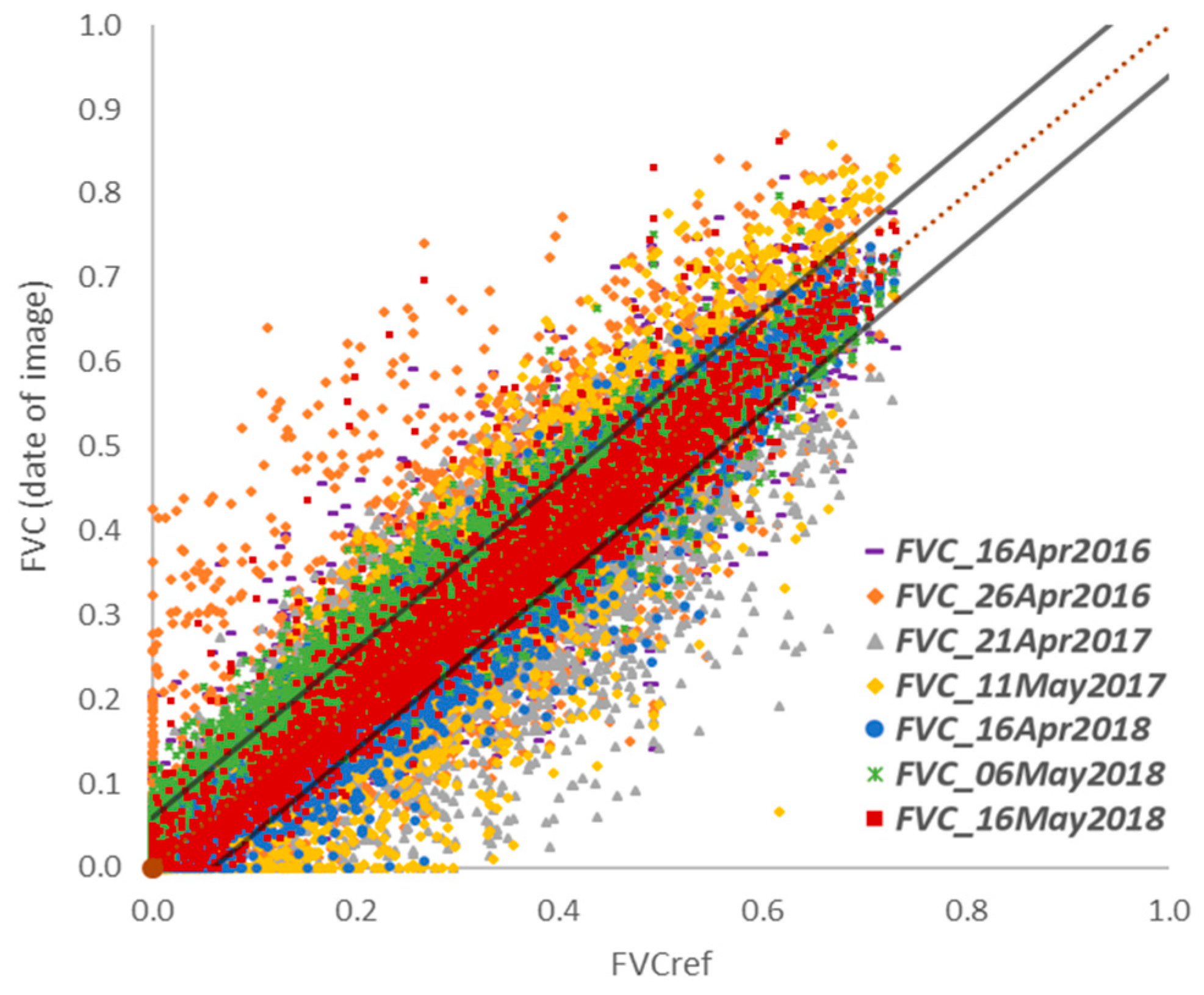
3.2. Effectiveness of the Reference image and Validation of Vegetative Cycle Separation
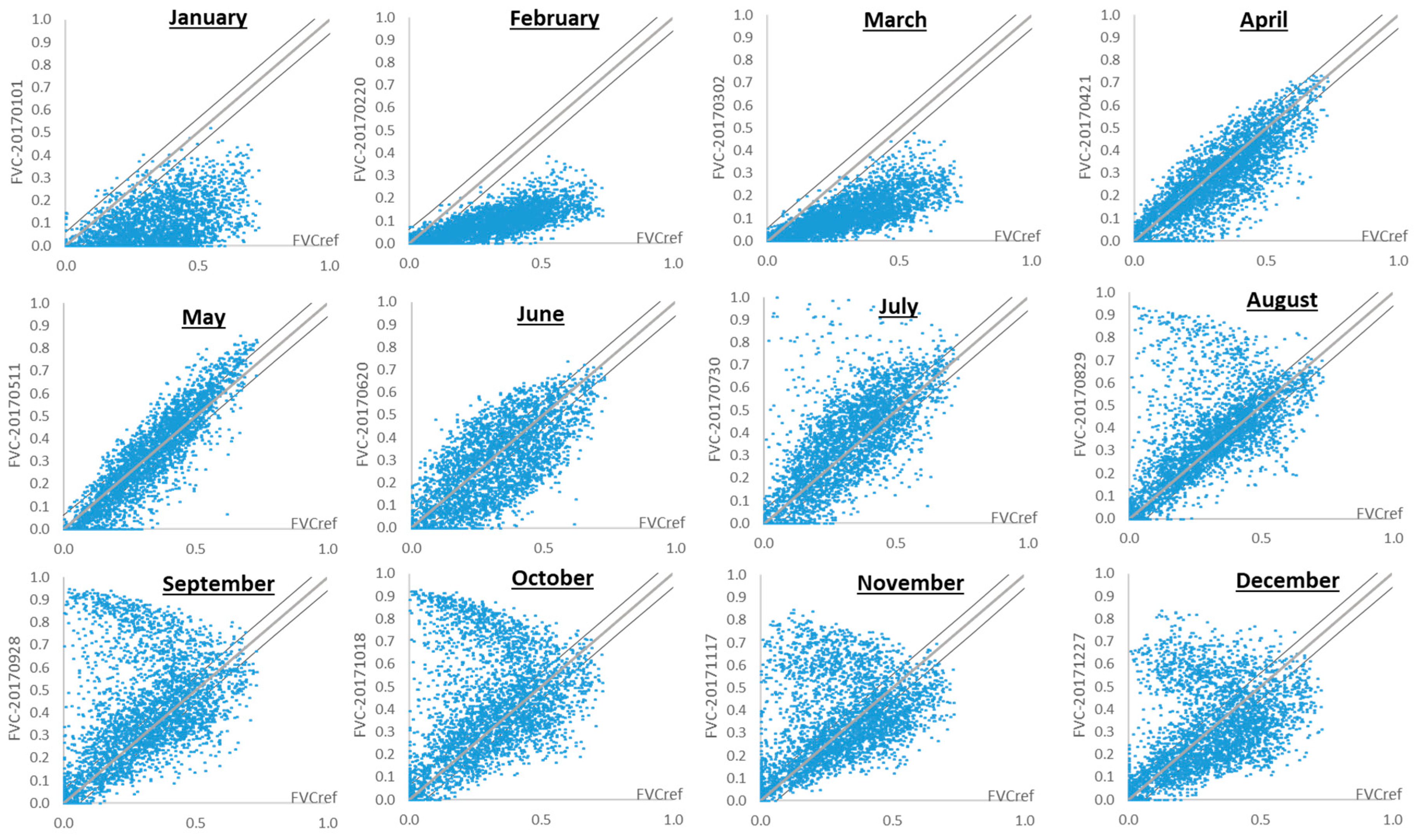
3.3. Monthly Dynamics of Fractional Vegetation Cover: 2017 Case Study
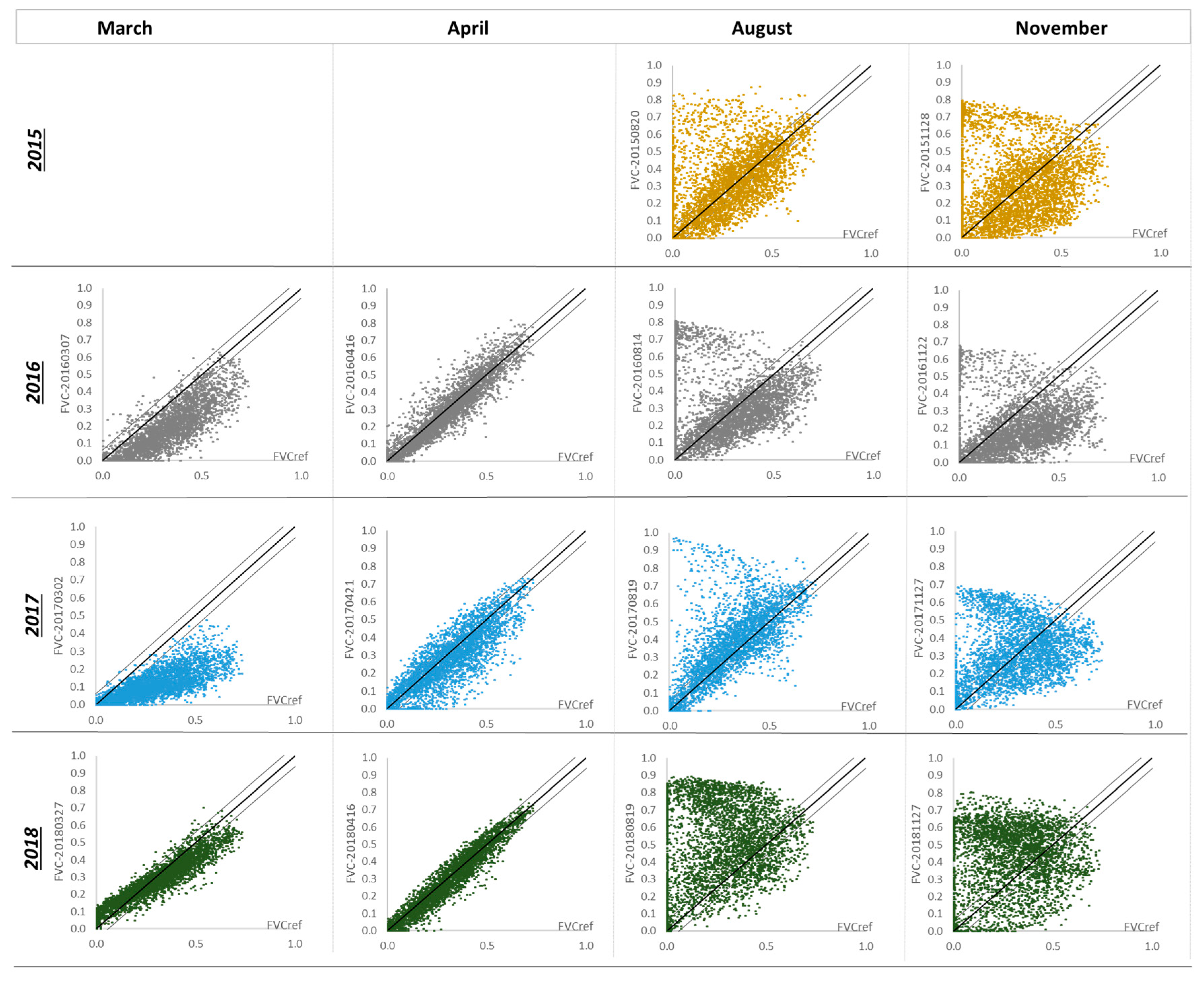
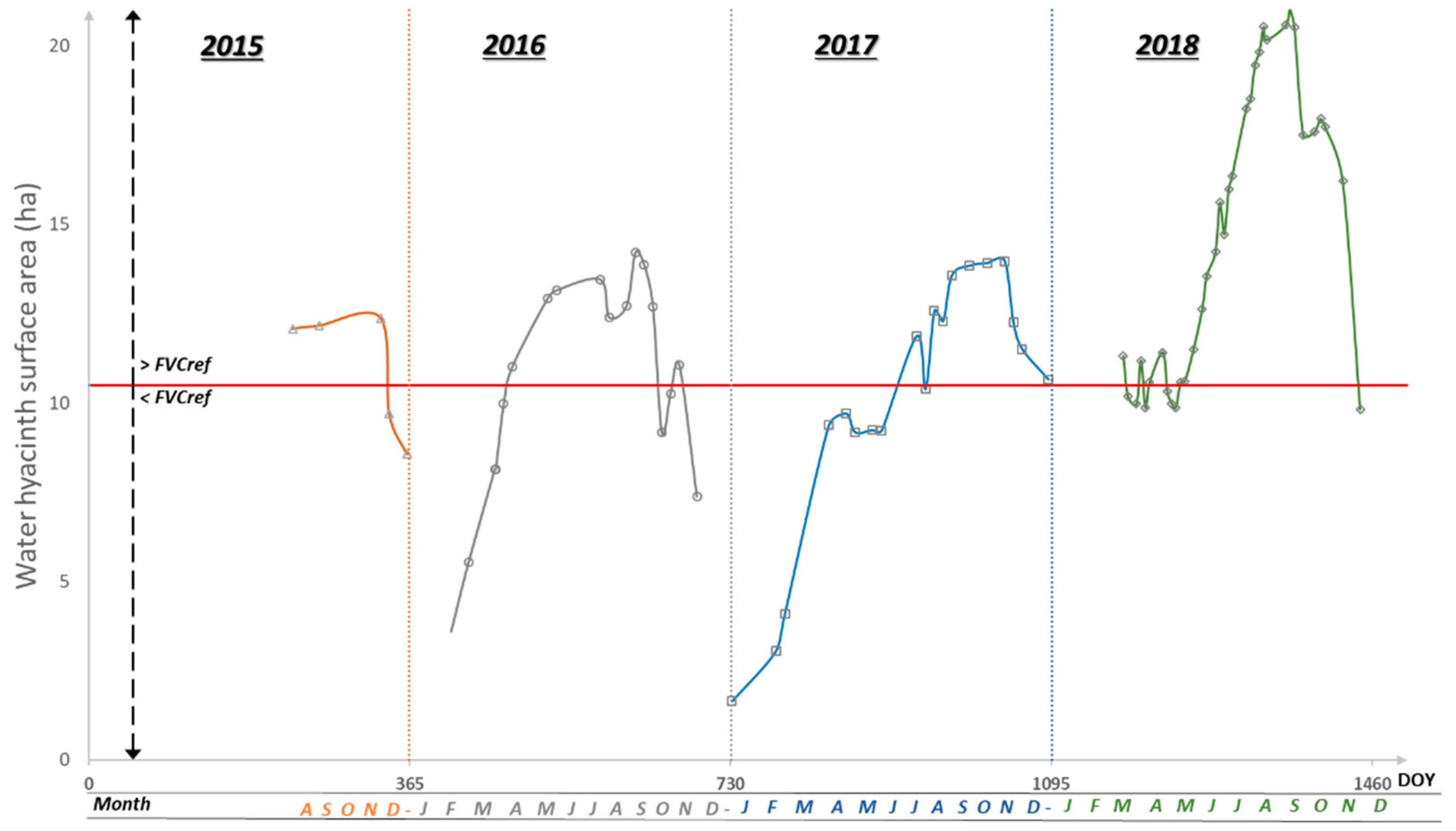
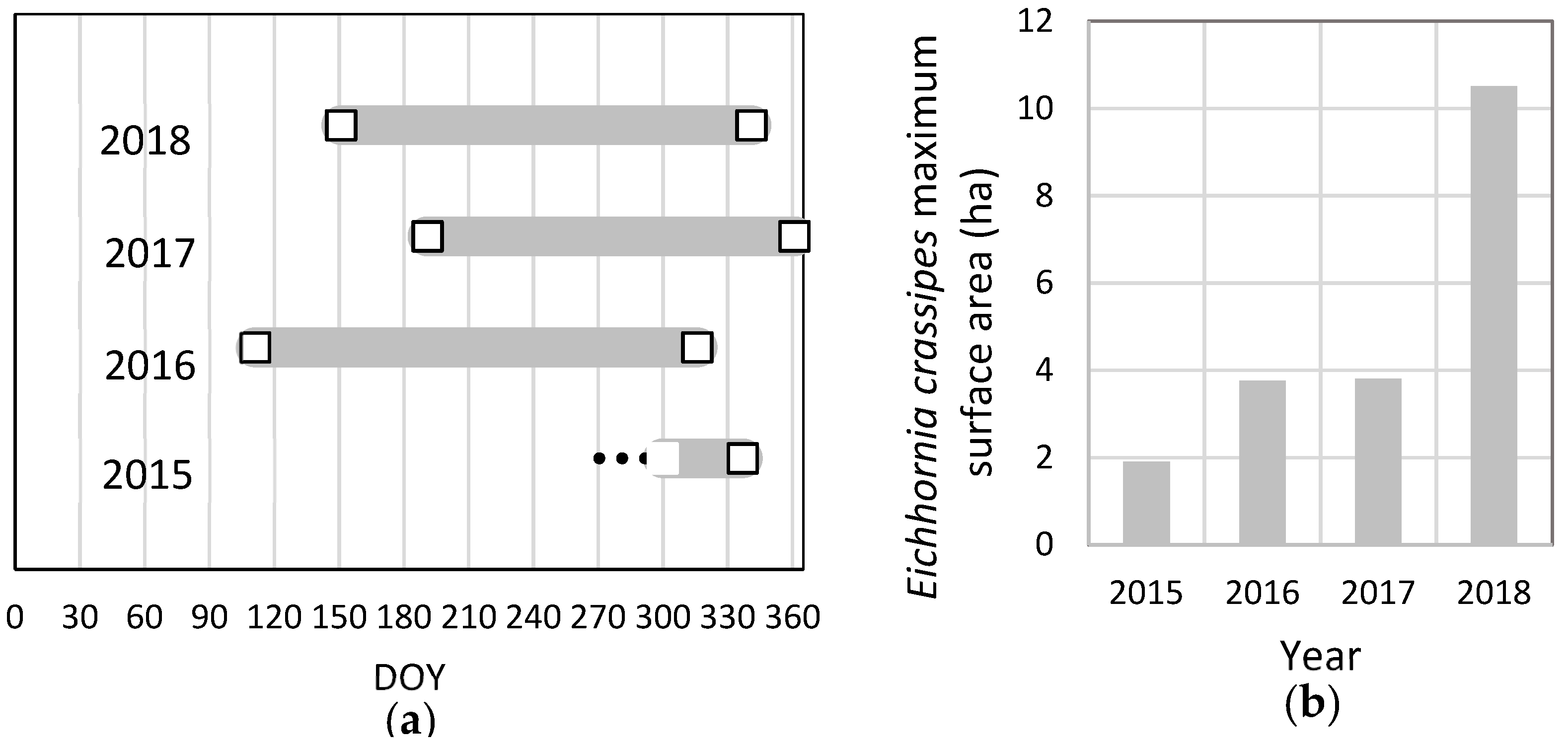
3.4. Inter-Year Comparison of the Fractional Vegetation Cover (2015–2018)
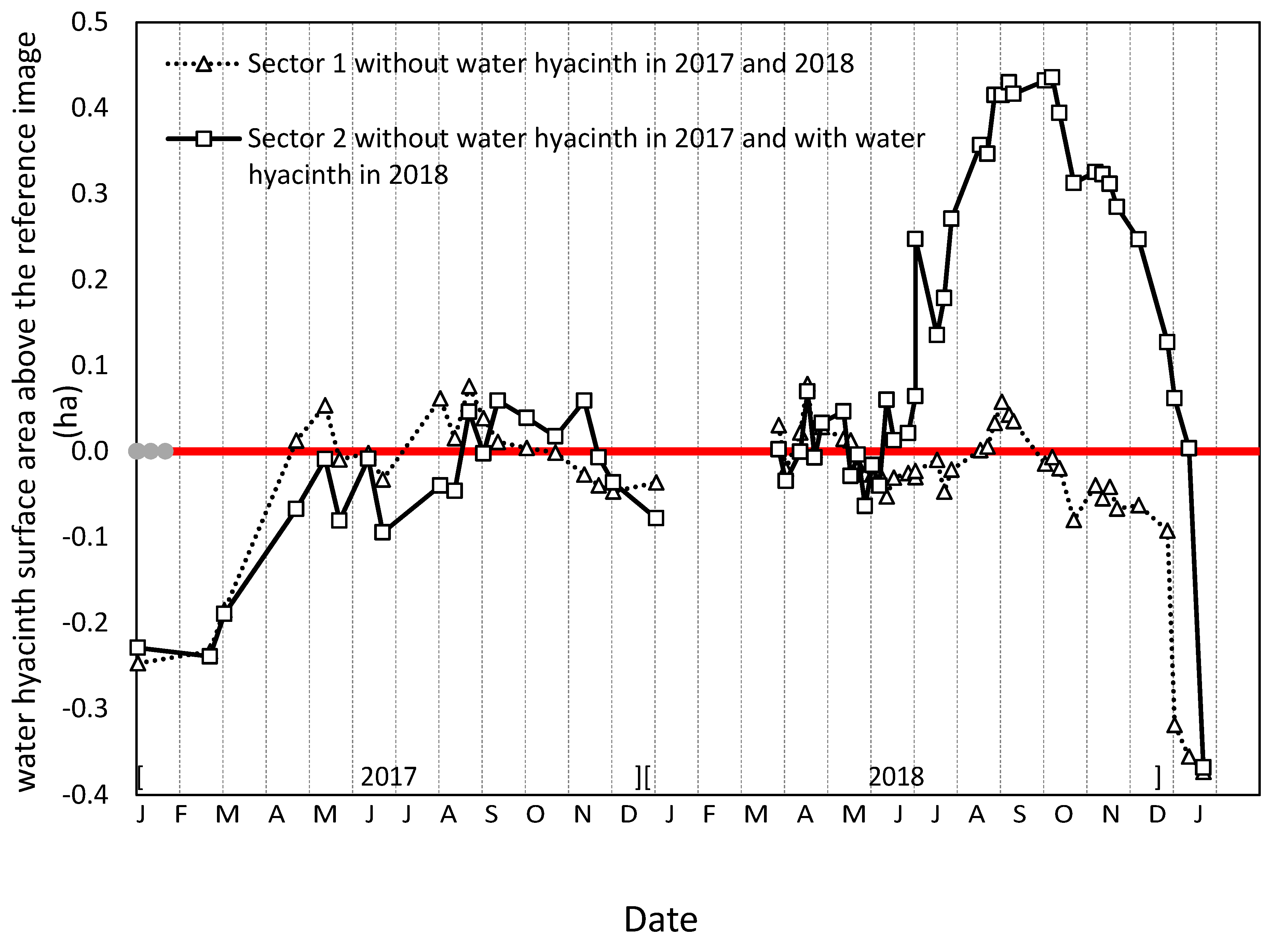
3.5. Dynamics of Water Hyacinth Area in the River
3.6. Validation of Water Hyacinth Surface Area Estimation Model
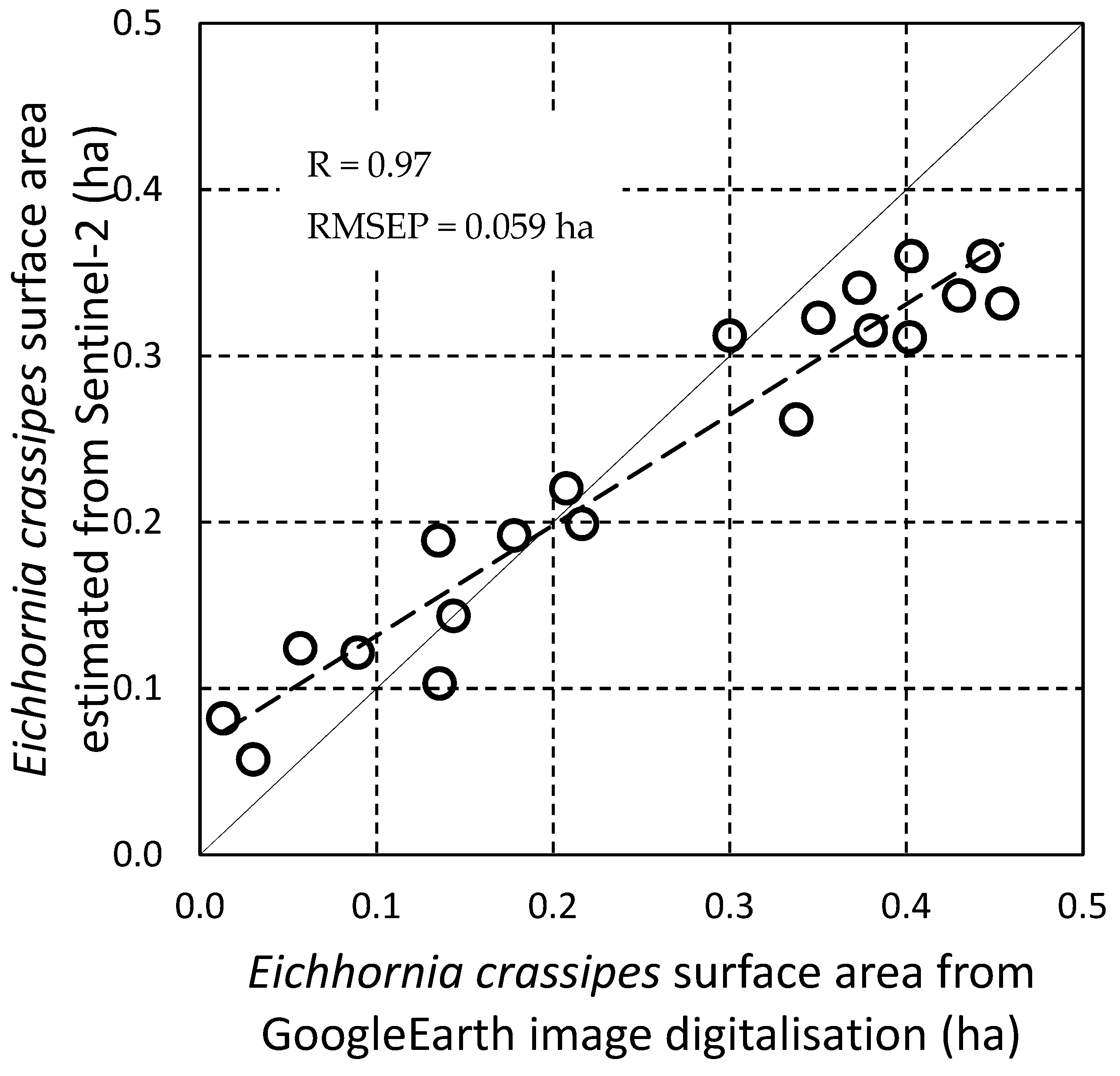
3.6.1. Comparison of the Estimated Water Hyacinth Surface Area Values with Manually Digitized Observations on Google Earth
3.6.2. Estimation of FVC from in Situ Spot Observations
4. Discussion
4.1. Fluvial Corridor Geomorphology
4.2. Reference Period
4.3. Estimation of Fractional Vegetation Cover
4.3.1. Before the Reference Period: Winter and Early Spring
4.3.2. After the Reference Period: Late Spring to Autumn
4.4. Inter-Annual Comparison
4.5. Further Developments
4.6. Use for Management
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alvarez-Taboada, F.; Paredes, C.; Julián-Pelaz, J. Mapping of the invasive species Hakea sericea using Unmanned Aerial Vehicle (UAV) and worldview-2 imagery and an object-oriented approach. Remote Sens. 2017, 9, 913. [Google Scholar] [CrossRef]
- Gopal, B. Ecology and Management of Aquatic Vegetation in the Indian Subcontinent; Springer Science & Business Media: Berlin, Germany, 1987. [Google Scholar]
- Thamaga, K.H.; Dube, T. Remote sensing of invasive water hyacinth (Eichhornia crassipes): A review on applications and challenges. Remote Sens. Appl. Soc. Environ. 2018, 10, 36–46. [Google Scholar] [CrossRef]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species. From A Selection Global. The Database, Invasive Species; ISCG: Rome, Italy, 2004. [Google Scholar]
- Kriticos, D.J.; Brunel, S. Assessing and Managing the Current and Future Pest Risk from Water Hyacinth, (Eichhornia crassipes), an Invasive Aquatic Plant Threatening the Environment and Water Security. PLoS ONE 2016, 11, e0120054. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.R.; Holst, N.; Rees, M. Determinants and patterns of population growth in water hyacinth. Aquat. Bot. 2005, 81, 51–67. [Google Scholar] [CrossRef]
- Téllez, T.; López, E.; Granado, G.; Pérez, E.; López, R.; Guzmán, J. The Water Hyacinth, Eichhornia crassipes: An invasive plant in the Guadiana River Basin (Spain). Aquat. Invasions 2008, 3, 42–53. [Google Scholar] [CrossRef]
- Timmer, C.E.; Weldon, L.W. Evapotranspiration and pollution of water by water hyacinth. Hyacinth Control J. 1966, 6, 34–37. [Google Scholar]
- Mozaffarian, V.; Yaghoubi, B. New record of Eichhornia crassipes (Water Hyacinth) from north of Iran. Rostaniha Agric. Res. Educ. Ext. Organ. Res. Assist. Prof. 2015, 16, 208–211. [Google Scholar]
- Dagno, K.; Lahlali, R.; Friel, D.; Bajji, M.; Haïssam Jijakli, M. Synthèse bibliographique: Problématique de la jacinthe d’eau, Eichhornia crassipes, dans les régions tropicales et subtropicales du monde, notamment son éradication par la lutte biologique au moyen des phytopathogènes. Biotechnol. Agron. Soc. Environ. 2007, 11, 144–150. [Google Scholar]
- Everitt, J.H.; Yang, C.; Escobar, D.E.; Webster, C.F.; Lonard, R.I.; Davis, M.R. Using remote sensing and spatial information technologies to detect and map two aquatic macrophytes. J. Aquat. Plant Manag. 1999, 37, 71–80. [Google Scholar]
- Hussner, A.; Stiers, I.; Verhofstad, M.J.J.M.; Bakker, E.S.; Grutters, B.M.C.; Haury, J.; Van Valkenburg, J.L.C.H.; Brundu, G.; Newman, J.; Clayton, J.S.; et al. Management and control methods of invasive alien freshwater aquatic plants: A review. Aquat. Bot. 2017, 136, 112–137. [Google Scholar] [CrossRef]
- Hestir, E.L.; Khanna, S.; Andrew, M.E.; Santos, M.J.; Viers, J.H.; Greenberg, J.A.; Rajapakse, S.S.; Ustin, S.L. Identification of invasive vegetation using hyperspectral remote sensing in the California Delta ecosystem. Remote Sens. Environ. 2008, 112, 4034–4047. [Google Scholar] [CrossRef]
- Barati, S.; Rayegani, B.; Saati, M.; Sharifi, A.; Nasri, M. Comparison the accuracies of different spectral indices for estimation of vegetation cover fraction in sparse vegetated areas. Egypt. J. Remote Sens. Space Sci. 2011, 14, 49–56. [Google Scholar] [CrossRef]
- Albright, T.P.; Moorhouse, T.G.; Mcnabb, T.J. The Rise and Fall of Water Hyacinth in Lake Victoria and the Kagera River Basin, 1989–2001. J. Aquat. Plant Manag. 2004, 42, 73–84. [Google Scholar]
- Mund, J.; Murach, D.; Parplies, A. Monitoring and Quantification of Floating Biomass on Tropical Water Bodies. Geospatial Innov. Soc. 2014, 67–76. [Google Scholar] [CrossRef]
- Ogamba, E.N.; Izah, S.C.; Oribu, T. Water quality and proximate analysis of Eichhornia crassipes from River Nun, Amassoma Axis, Nigeria. Res. J. Phytomed. 2015, 1, 43–48. [Google Scholar]
- Rommens, W.; Maes, J.; Dekeza, N.; Inghelbrecht, P.; Nhiwatiwa, T.; Holsters, E.; Ollevier, F.; Marshall, B.; Brendonck, L. The impact of water hyacinth (Eichhornia crassipes) in a eutrophic subtropical impoundment (Lake Chivero, Zimbabwe). I. Water quality. Archiv für Hydrobiologie 2003, 158, 373–388. [Google Scholar] [CrossRef]
- Pan, X.; Villamagna, A.M.; Li, B.; Villamagna, A.M.; Li, B. Eichhornia crassipes Mart. (Solms-Laubach) (Water Hyacinth). In A Handbook of Global Freshwater Invasive Species; Routledge: London, UK, 2012. [Google Scholar]
- Patel, S. Threats, management and envisaged utilizations of aquatic weed Eichhornia crassipes: An overview. Rev. Environ. Sci. Biotechnol. 2012, 11, 249–259. [Google Scholar] [CrossRef]
- Venugopal, G. Monitoring the effects of biological control of water hyacinths using remotly sensed data: A case study of Bangalore, India. Singap. J. Trop. Geogr. 1998, 19, 92–105. [Google Scholar] [CrossRef]
- Uremis, I.; Uludag, A.; Arslan, Z.F.; Abaci, O. A new record for the flora of Turkey: Eichhornia crassipes (Mart.) Solms (Pontederiaceae). EPPO Bull. 2014, 44, 83–86. [Google Scholar] [CrossRef]
- EPPO. EPPO A1 and A2 Lists of Pests Recommended for Regulation as Quarantine Pests. EPPO Bull. 2008, 5, 205–207. [Google Scholar]
- UN-ESCWA; BGR. Naher El Kabir basin. In Inventory of Shared Water Resources in Western Asia; United Nations Economic and Social Commission for Western Asia (UN-ESCWA) and the German Federal Institute for Geosciences and Natural Resources (BGR): New York, NY, USA, 2013. [Google Scholar]
- García-Berthou, E.; Alcaraz, C.; Pou-Rovira, Q.; Zamora, L.; Coenders, G.; Feo, C. Introduction pathways and establishment rates of invasive aquatic species in Europe. Can. J. Fish. Aquat. Sci. 2005, 62, 453–463. [Google Scholar] [CrossRef]
- Hussner, A. Alien aquatic plant species in European countries. Weed Res. 2012, 52, 297–306. [Google Scholar] [CrossRef]
- Mazza, G.; Aquiloni, L.; Inghilesi, A.F.; Giuliani, C.; Lazzaro, L.; Ferretti, G.; Lastrucci, L.; Foggi, B.; Tricarico, E. Aliens just a click away: The online aquarium trade in Italy. Manag. Biol. Invasions 2015, 6, 253–261. [Google Scholar] [CrossRef]
- Celesti-Grapow, L.; Alessandrini, A.; Arrigoni, P.V.; Banfi, E.; Bernardo, L.; Bovio, M.; Brundu, G.; Cagiotti, M.R.; Camarda, I.; Carli, E.; et al. Inventory of the non native flora of Italy. Plant Biosyst. 2009, 143, 386–430. [Google Scholar] [CrossRef]
- Brundu, G.; Azzella, M.M.; Blasi, C.; Camarda, I.; Iberite, M.; Celesti-Grapow, L. The silent invasion of Eichhornia crassipes (Mart.) Solms. in Italy. Plant Biosyst. 2013, 147, 1120–1127. [Google Scholar] [CrossRef]
- Georges, N.; Pax, N. Pistia stratiotes L. et Eichhornia crassipes (Mart) Solms, deux nouvelles hydrophytes dans la vallée de la Moselle. Bull. Liaison Floraine 2002, 28, 3–4. [Google Scholar]
- Perna, C.; Burrows, D. Improved dissolved oxygen status following removal of exotic weed mats in important fish habitat lagoons of the tropical Burdekin River floodplain, Australia. Mar. Pollut. Bull. 2005, 51, 138–148. [Google Scholar] [CrossRef] [PubMed]
- IDRC. Cad Ham; Cadham Hayes Systems Inc: Ottawa, ON, Canada, 2003; p. 108. [Google Scholar]
- Thomas, R.L.; Shaban, A.; Khawlie, M.; Kawass, I.; Nsouli, B. Geochemistry of the sediments of the El-Kabir River and Akkar watershed in Syria and Lebanon. Lakes Reserv. Res. Manag. 2005, 10, 127–134. [Google Scholar] [CrossRef]
- Guyet, T.; Nicolas, H. Long term analysis of time series of satellite images. Pattern Recognit. Lett. 2015, 70, 17–23. [Google Scholar] [CrossRef]
- Schmidt, M. Monitoring aquatic weeds in a river system using SPOT 5 satellite imagery. J. Appl. Remote Sens. 2010, 4, 043528. [Google Scholar] [CrossRef]
- Santos, M.J.; Khanna, S.; Hestir, E.L.; Andrew, M.E.; Rajapakse, S.S.; Greenberg, J.A.; Anderson, L.W.; Ustin, S.L. Use of Hyperspectral Remote Sensing to Evaluate Efficacy of Aquatic Plant Management. Invasive Plant Sci. Manag. 2009, 2, 216–229. [Google Scholar] [CrossRef]
- Jakubauskas, M.E.; Peterson, D.L.; Campbell, S.W.; de Noyelles, F.; Campbell, S.D.; Penny, D. Mapping and monitoring invasive aquatic plant obstructions in navigable waterways using satellite multispectral imagery. In Proceedings of the Pecora 15 Land Satellite Information IV Conference and the ISPRS Commission I Symposium, Denver, CO, USA, 10–15 November 2002. [Google Scholar]
- Everitt, J.H.; Yang, C.H. Using QuickBird satellite imagery to distinguish two aquatic weeds in south Texas. J. Aquat. Plant Manag. 2007, 45, 25–31. [Google Scholar]
- Oyama, Y.; Matsushita, B.; Fukushima, T. Distinguishing surface cyanobacterial blooms and aquatic macrophytes using Landsat/TM and ETM+ shortwave infrared bands. Remote Sens. Environ. 2015, 157, 35–47. [Google Scholar] [CrossRef]
- Fadel, A.; Faour, G.; Slim, K. Assessment of the trophic state and chlorophyll-a concentrations using landsat oli in karaoun reservoir, Lebanon. Leban. Sci. J. 2016, 17, 130–145. [Google Scholar] [CrossRef]
- Dube, T.; Mutanga, O.; Sibanda, M.; Bangamwabo, V.; Shoko, C. Evaluating the performance of the newly-launched Landsat 8 sensor in detecting and mapping the spatial configuration of water hyacinth (Eichhornia crassipes) in inland lakes, Zimbabwe. Phys. Chem. Earth 2017, 100, 101–111. [Google Scholar] [CrossRef]
- Pinardi, M.; Bresciani, M.; Villa, P.; Cazzaniga, I.; Laini, A.; Tóth, V.; Fadel, A.; Austoni, M.; Lami, A.; Giardino, C. Spatial and temporal dynamics of primary producers in shallow lakes as seen from space: Intra-annual observations from Sentinel-2A. Limnologica 2018, 72, 32–43. [Google Scholar] [CrossRef]
- Colombo, R.; Bellingeri, D.; Fasolini, D.; Marino, C.M. Retrieval of leaf area index in different vegetation types using high resolution satellite data. Remote Sens. Environ. 2003, 86, 120–131. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W.; Harlan, J.C. Monitoring the Vernal Advancements and Retrogradation of Natural Vegetation; Remote Sensing Center: College Station, TX, USA, 1974. [Google Scholar]
- Broge, N.H.; Leblanc, E. Comparing prediction power and stability of broadband and hyperspectral vegetation indices for estimation of green leaf area index and canopy chlorophyll density. Remote Sens. Environ. 2000, 76, 156–172. [Google Scholar] [CrossRef]
- Jackson, R.D.; Huete, A.R. Interpreting vegetation indices. Prev. Vet. Med. 1991, 11, 185–200. [Google Scholar] [CrossRef]
- Bronge, L.B.; SwedPower, A.B. Satellite Remote Sensing for Estimating Leaf Area Index, FPAR and Primary Production; Swedish Nuclear Fuel and Waste Management: Stockholm, Sweden, 2004. [Google Scholar]
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Almutairi, B.; El, A.; Belaid, M.A.; Musa, N. Comparative Study of SAVI and NDVI Vegetation Indices in Sulaibiya Area (Kuwait) Using Worldview Satellite Imagery. Int. J. Geosci. Geomatics 2013, 1, 50–53. [Google Scholar]
- Zhang, X.; Liao, C.; Li, J.; Sun, Q. Fractional vegetation cover estimation in arid and semi-arid environments using HJ-1 satellite hyperspectral data. Int. J. Appl. Earth Obs. Geoinf. 2012, 21, 7. [Google Scholar] [CrossRef]
- Jiménez-Muñoz, J.C.; Sobrino, J.A.; Plaza, A.; Guanter, L.; Moreno, J.; Martínez, P. Comparison between fractional vegetation cover retrievals from vegetation indices and spectral mixture analysis: Case study of PROBA/CHRIS data over an agricultural area. Sensors 2009, 9, 768–793. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wu, H.; Zhao, X.; Zhou, T.; Tang, B.; Zhao, W.; Jia, K. Evaluation of Spatiotemporal Variations of Global Fractional Vegetation Cover Based on GIMMS NDVI Data from 1982 to 2011. Remote Sens. 2014, 6, 4217–4239. [Google Scholar] [CrossRef]
- Jia, K.; Liang, S.; Liu, S.; Li, Y.; Xiao, Z.; Yao, Y.; Jiang, B.; Zhao, X.; Wang, X.; Xu, S.; et al. Global Land Surface Fractional Vegetation Cover Estimation Using General Regression Neural Networks From MODIS Surface Reflectance. IEEE Trans. Geosci. Remote Sens. 2015, 53, 4787–4796. [Google Scholar] [CrossRef]
- ESA. Sentinel-2 ESA’s Optical High-Resolution Mission for GMES Operational Services. PO Box 299, 2200 AG; ESA Communications ESTEC: Noordwijk, The Netherlands, 2012. [Google Scholar]
- Tello, J.; Gómez-Báguena, R.; Casterad, M.A. Comparison and adjustment in agricultural areas of vegetation indexes derived from Landsat-8 and Sentinel-2. In Proceedings of the Nuevas Plataformas y Sensores Teledetección—XVII Congreso de la Asociación Española de Teledetección, Murcia, Spain, 3–7 October 2017; pp. 81–84. [Google Scholar]
- PEPS. PEPS–Operating platform Sentinel products (CNES). Available online: https://peps.cnes.fr (accessed on 28 June 2019).
- Theia. Theia–The Continental Surface Data and Services Center. Available online: https://theia.cnes.fr (accessed on 28 June 2019).
- Vescovo, L.; Gianelle, D. Using the MIR bands in vegetation indices for the estimation of grassland biophysical parameters from satellite remote sensing in the Alps region of Trentino (Italy). Adv. Space Res. 2008, 41, 1764–1772. [Google Scholar] [CrossRef]
- Eid, E.M.; Shaltout, K.H. Population dynamics of Eichhornia crassipes (C. Mart.) Solms in the Nile Delta, Egypt. Plant Spec. Biol. 2016, 32, 279–291. [Google Scholar] [CrossRef]
- Batanouny, K.H.; El-Fiky, A.M. The water hyacinth (Eichhornia crassipes Solms) in the Nile system, Egypt. Aquat. Bot. 1975, 1, 243–252. [Google Scholar] [CrossRef]
- Yan, S.-H.; Song, W.; Guo, J.-Y. Advances in management and utilization of invasive water hyacinth (Eichhornia crassipes) in aquatic ecosystems—A review. Crit. Rev. Biotechnol. 2017, 37, 218–228. [Google Scholar] [CrossRef]
- Bottner, B.; Noël, C. Repérer les macrophytes depuis le ciel ou sous les eaux, quel appui pour les gestionnaires? Sci. Eaux Territ. 2014, 15, 10–15. [Google Scholar]
- Varray, S.; Haury, J.; Hudin, S. Manuel de Gestion des Espèces Exotiques Envahissantes du Bassin Loire-Bretagne; Fédération des Conservatoires d’Espaces Naturels: Orléans, France, 2018. [Google Scholar]
- Dutartre, A.; Haury, J.; Peltre, M.-C. Plantes Aquatiques d’eau Douce: Biologie, Écologie et Gestion. Cemagref HS Rev. Ingénierie Eau-Agriculture-Territoire; Irstea: Villeurbanne, France, 2008; p. 161. [Google Scholar]
- Joshi, C.; de Leeuw, J.; van Duren, I.C. Remote Sensing and GIS Applications for Mapping and Spatial Modelling of Invasive Species. Geoinf. Sci. 2002, 2, 669–677. [Google Scholar]
- Laranjeira, C.M.; Nadais, G. Eichhornia crassipes control in the largest Portuguese natural freshwater lagoon. EPPO Bull. 2008, 38, 487–495. [Google Scholar] [CrossRef]
- Haury, J.; Damien, J.-P. De nouvelles mauvaises herbes en zones humides: Les formes terrestres des Jussies invasives sur prairies. Sciences Eaux Territoires 2014, 15, 16–21. [Google Scholar]
| Continent | Country | Location | References |
|---|---|---|---|
| North and South America | Bolivia | Dam of the San Jacinto | [10] |
| Colombia | Lagoons outside the Amazon | [10] | |
| Mexico | Chapala and Guadalupe lakes | [10] | |
| USA | Rio Grande River | [11] | |
| USA | Sacramento–San Joaquin River | [12,13] | |
| Africa | Angola | Kwanza River | [10] |
| Egypt | Nile River | [14] | |
| Kenya | Lake Victoria | [15,16] | |
| Mali | Niger River | [10] | |
| Niger | Niger River | [10] | |
| Nigeria | Niger River: Nun River | [10,17] | |
| Zimbabwe | Manyame River:Manyame Lake and Inland Lake | [3,18] | |
| Asia | China | Yangtze River | [10,19] |
| India | Brahmaputra Assam River | [20,21] | |
| Turkey | Asi River | [22] | |
| Syria | Al Kabir River | [23,24] | |
| Lebanon | Al Kabir River | [23,24] | |
| Europe | Spain | Guadiana River | [7,25] |
| Portugal | Sado, Sorraia River, part of Tagus basin | [25,26,27] | |
| Germany | Erft River | [25,26] | |
| Italy | Sardinia and Lazio | [26,27,28,29] | |
| France | Moselle valley | [30] | |
| Australia | Australia | Burdeki River | [31] |
| Stretch | Length (km) | Width (m) | Area (ha) | |
|---|---|---|---|---|
| Max. | Min. | |||
| Main Al Kabir River | 14.21 | 80 | 6 | 39.5 |
| Al Arousse tributary | 2.16 | 50 | 1 | 2.2 |
| Total | 16.37 | 41.7 | ||
| Year | J | F | M | A | M | J | J | A | S | O | N | D | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 1 | 1 | 1 | 2 | 5 | ||||||||
| 2016 | 1 | 1 | 4 | 2 | 2 | 3 | 3 | 2 | 18 | ||||
| 2017 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 3 | 2 | 1 | 3 | 1 | 19 |
| 2018 | 2 | 4 | 6 | 3 | 5 | 5 | 3 | 3 | 2 | 1 | 34 | ||
| Total | 77 | ||||||||||||
| Scientific Name | Site | Type | |
|---|---|---|---|
| Al Aarida | Hoker ed Dahri | ||
| Alternanthera sessilis (L.) R.Br | + | + | Amphibious (Invasive) |
| Ludwigia stolonifera Guill. & Perr. | - | + | Amphibious |
| Lythrum salicaria L. | - | + | Amphibious |
| Paspalum sp. | + | + | Amphibious |
| Paspalum distichum L. | - | + | Amphibious (Invasive) |
| Polygonum salicifolium Wild. | - | + | Amphibious |
| Typha latifolia L. | + | - | Amphibious |
| Eichhornia crassipes (Mart.) Solms | + | + | Aquatic (Invasive) |
| Enteromorpha sp. | + | - | Aquatic algae |
| Lemna minor L. | - | + | Aquatic |
| Myriophyllum spicatum L. | + | - | Aquatic |
| Arundo donax L. | + | + | Terrestrial |
| Bidens tripartite L. | - | + | Terrestrial |
| Calystegia sepium (L.) R. Br | - | + | Terrestrial |
| Chenopodium sp. | + | - | Terrestrial |
| Chrysanthemum sp. | + | - | Terrestrial |
| Foeniculum vulgare Mill. | - | + | Terrestrial |
| Rubus sp. | - | + | Terrestrial |
| Salix spp. | - | + | Terrestrial |
| Senecio sp. | - | + | Terrestrial |
| Sinapis cf nigra (L.) W.D.J. Koch | - | + | Terrestrial |
| Xanthium strumarium L. | + | + | Terrestrial (Invasive) |
| 2017 | 2018 | |
|---|---|---|
| 400 m upstream of the estuary |  |  |
| Photography Date | 25 July 2017 | 31 July 2018 |
| Sentinel-2 acquisition date | 30 July 2017 | 25 July 2018 |
| Estimated FVC | 0 | 0.8 |
| 1 km upstream of the estuary |  |  |
| Photography Date | 13 May 2017 | 4 May 2018 |
| Sentinel-2 acquisition date | 11 May 2017 | 6 May 2018 |
| Estimated FVC | 0.05 | 0.12 |
| 9 km upstream of the estuary |  |  |
| Photography Date | 25 July 2017 | 31 July 2018 |
| Sentinel-2 acquisition date | 30 July 2017 | 25 July 2018 |
| Estimated FVC | 0.42 | 0.53 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghoussein, Y.; Nicolas, H.; Haury, J.; Fadel, A.; Pichelin, P.; Abou Hamdan, H.; Faour, G. Multitemporal Remote Sensing Based on an FVC Reference Period Using Sentinel-2 for Monitoring Eichhornia crassipes on a Mediterranean River. Remote Sens. 2019, 11, 1856. https://doi.org/10.3390/rs11161856
Ghoussein Y, Nicolas H, Haury J, Fadel A, Pichelin P, Abou Hamdan H, Faour G. Multitemporal Remote Sensing Based on an FVC Reference Period Using Sentinel-2 for Monitoring Eichhornia crassipes on a Mediterranean River. Remote Sensing. 2019; 11(16):1856. https://doi.org/10.3390/rs11161856
Chicago/Turabian StyleGhoussein, Youssra, Hervé Nicolas, Jacques Haury, Ali Fadel, Pascal Pichelin, Hussein Abou Hamdan, and Ghaleb Faour. 2019. "Multitemporal Remote Sensing Based on an FVC Reference Period Using Sentinel-2 for Monitoring Eichhornia crassipes on a Mediterranean River" Remote Sensing 11, no. 16: 1856. https://doi.org/10.3390/rs11161856
APA StyleGhoussein, Y., Nicolas, H., Haury, J., Fadel, A., Pichelin, P., Abou Hamdan, H., & Faour, G. (2019). Multitemporal Remote Sensing Based on an FVC Reference Period Using Sentinel-2 for Monitoring Eichhornia crassipes on a Mediterranean River. Remote Sensing, 11(16), 1856. https://doi.org/10.3390/rs11161856





