Using Time Series of High-Resolution Planet Satellite Images to Monitor Grapevine Stem Water Potential in Commercial Vineyards
Abstract
:1. Introduction
2. Materials and Methods
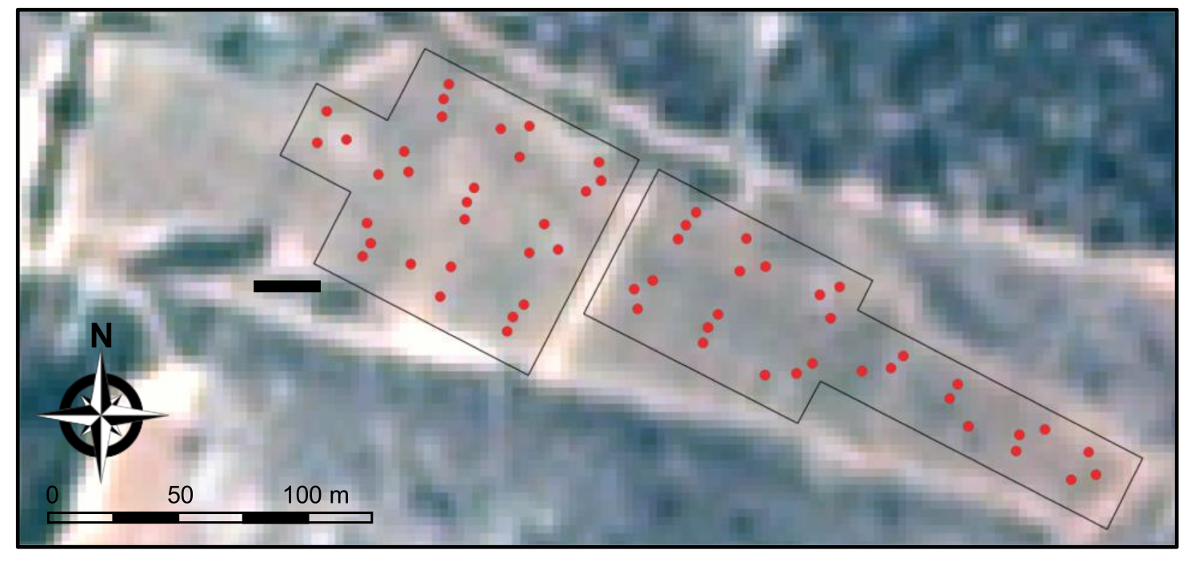
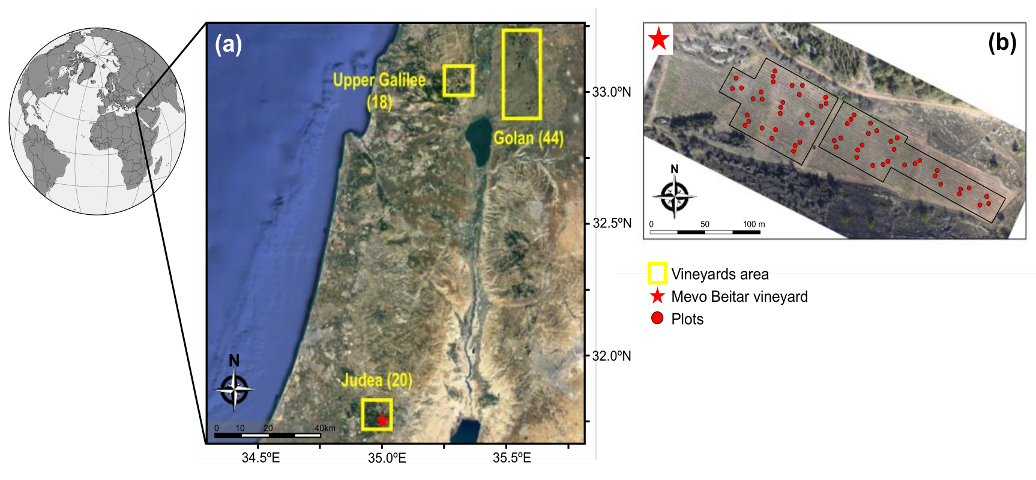
2.1. The Study Region
2.2. Characteristics of the Vineyards and Irrigation Strategy
2.3. Measurements of Midday Stem Water Potential in Vineyards
2.4. Vegetation Indices
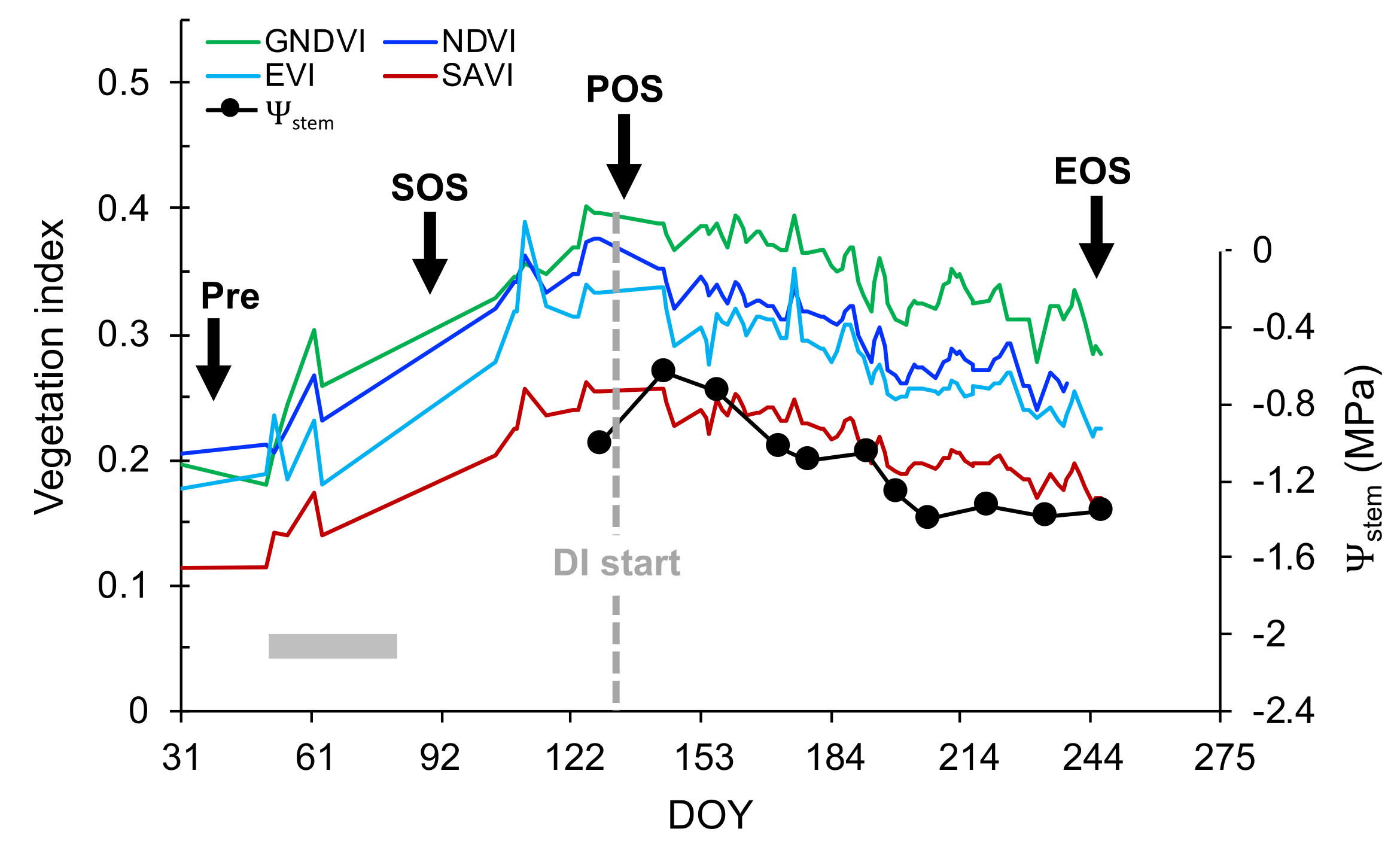
2.5. Phenological Stages
2.6. Satellite Data
2.6.1. Planet Satellites
2.6.2. Building Time Series of Planet’s Vegetation Indices in Google Earth Engine
2.6.3. Time Series Analysis
2.7. Statistical Analysis
- A multivariable linear model with five variables (VI avg, VI max, VI min, ΔVI and day of year) was used to predict weekly Ψstem (one model per week) in Mevo Beitar vineyard (hereafter, MB-Mult model).
- A single linear regression model was used in Mevo Beitar to predict Ψstem from VIs for the entire season using the VI time series (hereafter, MB-Reg model).
- A single ‘global’ multivariable linear model with the same variables as in MB-Mult was used to predict seasonal Ψstem from VI time series of the 81 commercial vineyards (hereafter, Global-Mult).
3. Results
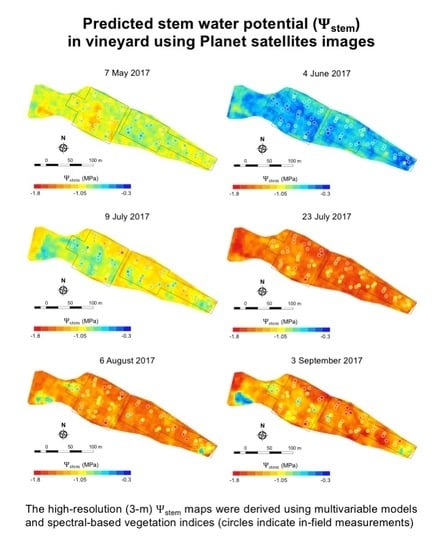
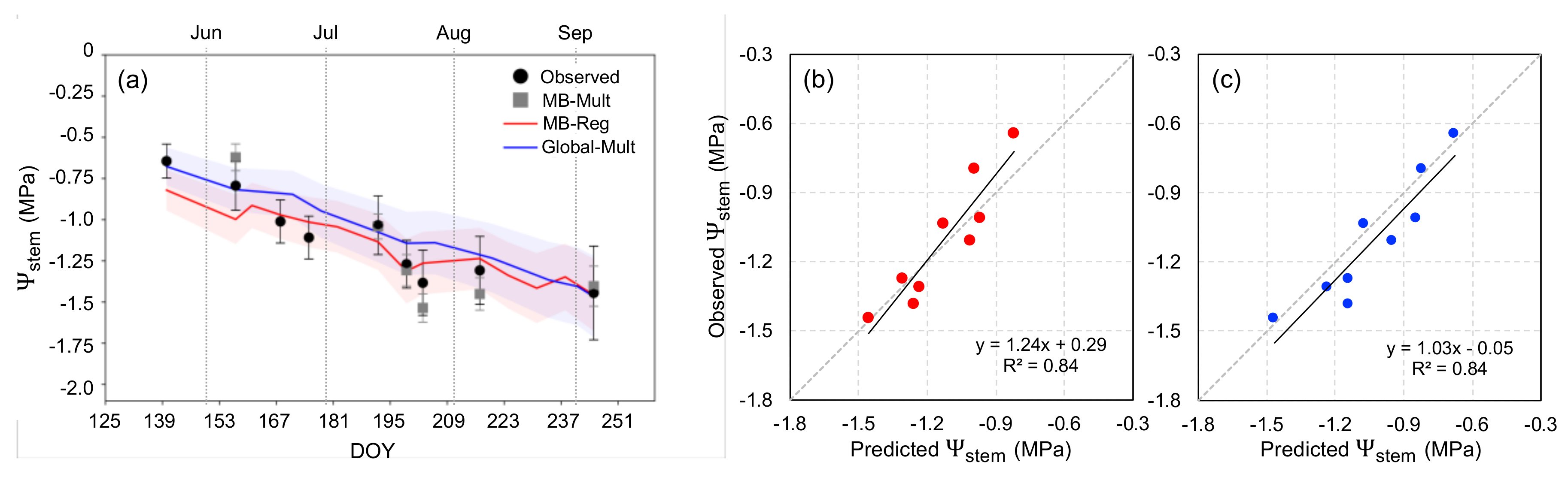
3.1. Deriving Midday Stem Water Potential for Mevo Beitar Vineyard
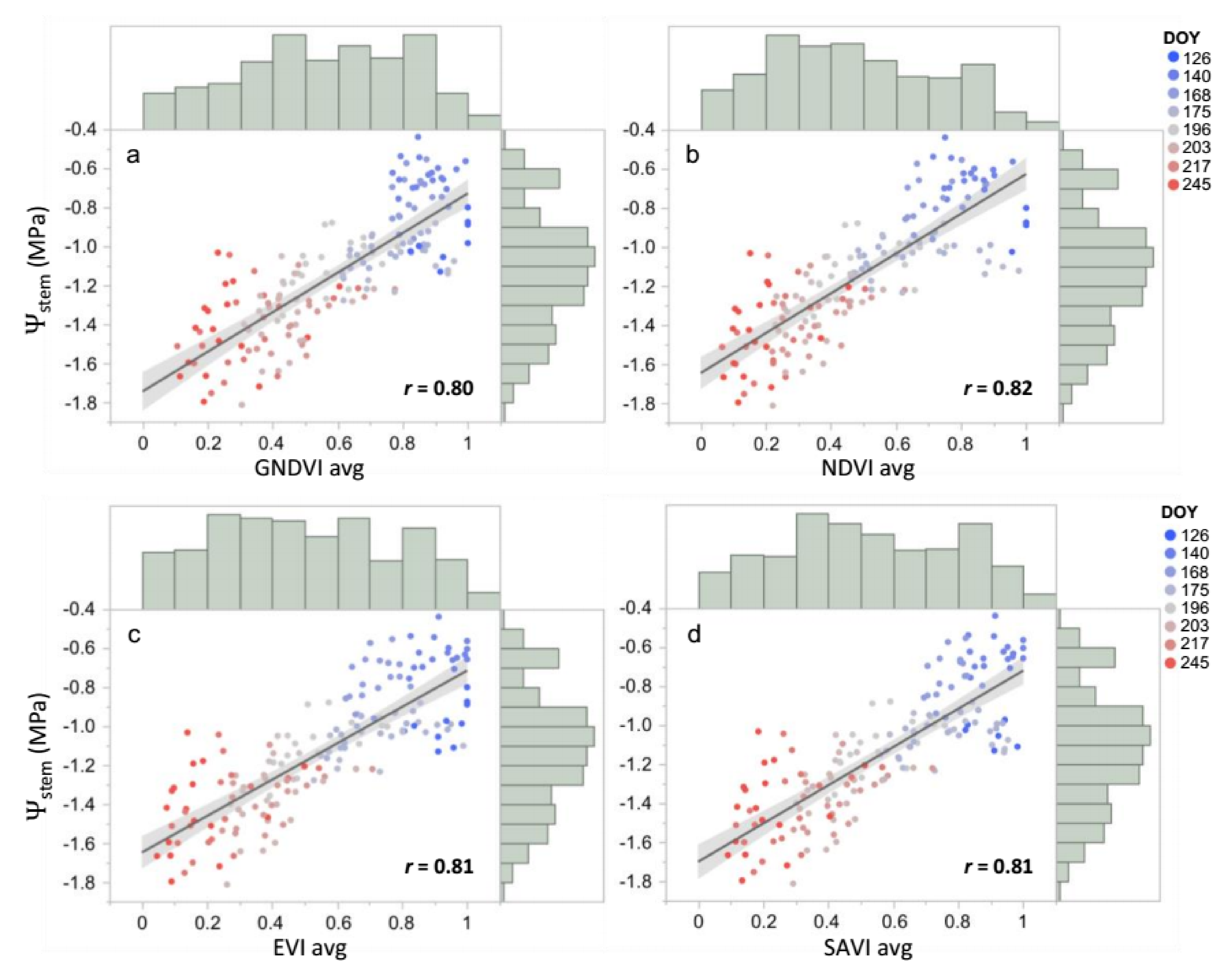
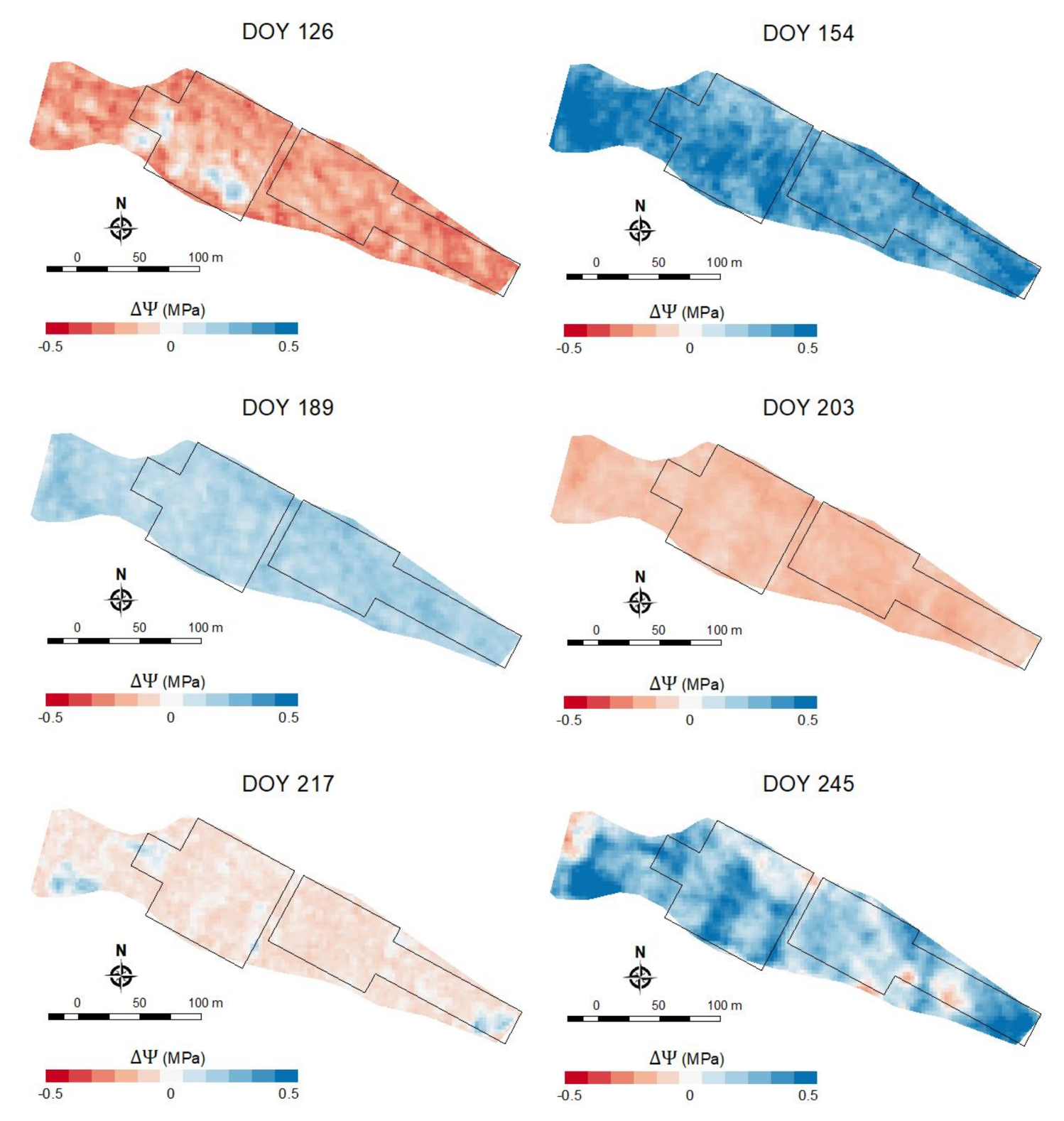
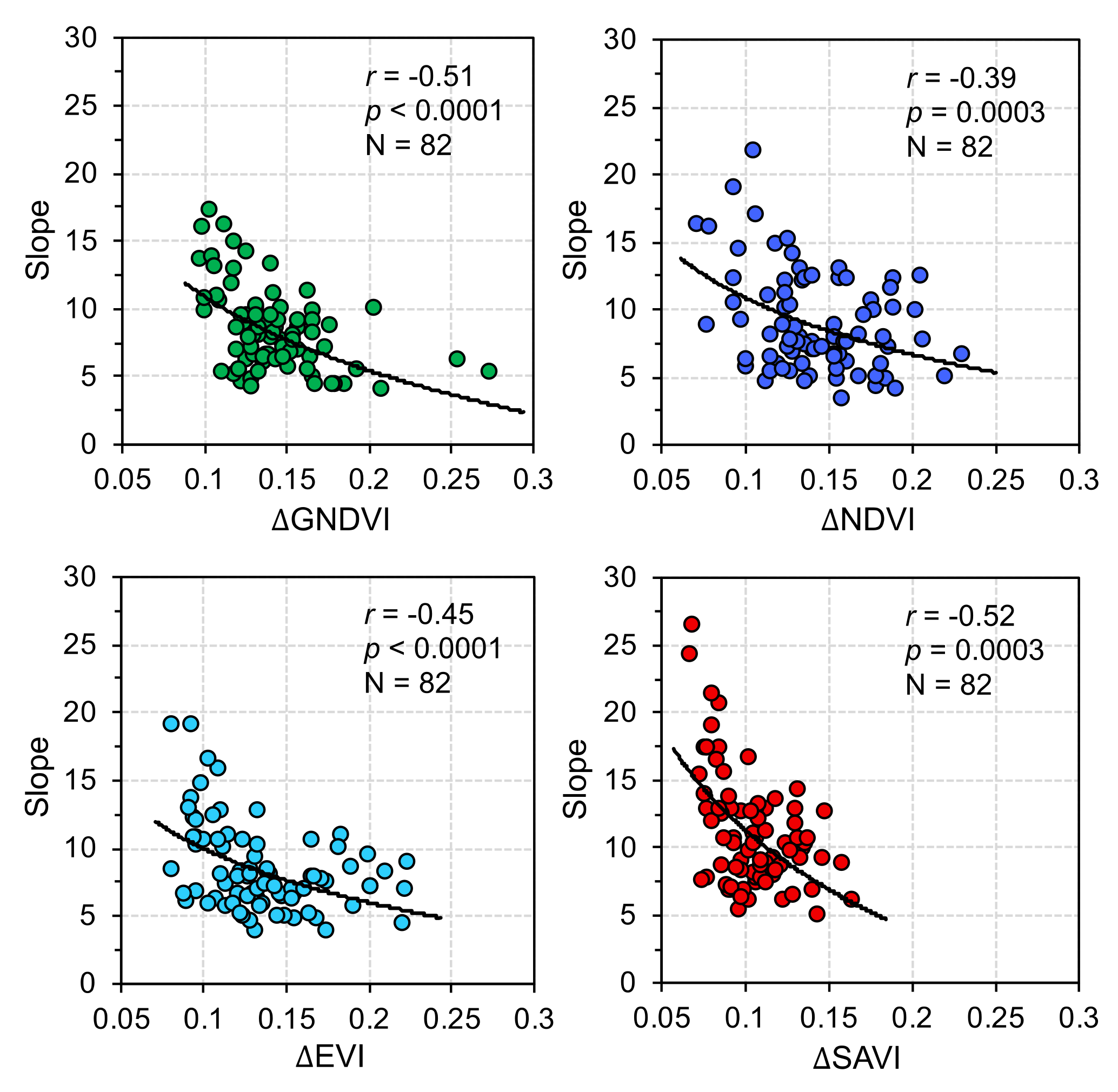
3.2. Vegetation Indices and Stem Water Potential in Vineyards across Rainfall Gradient
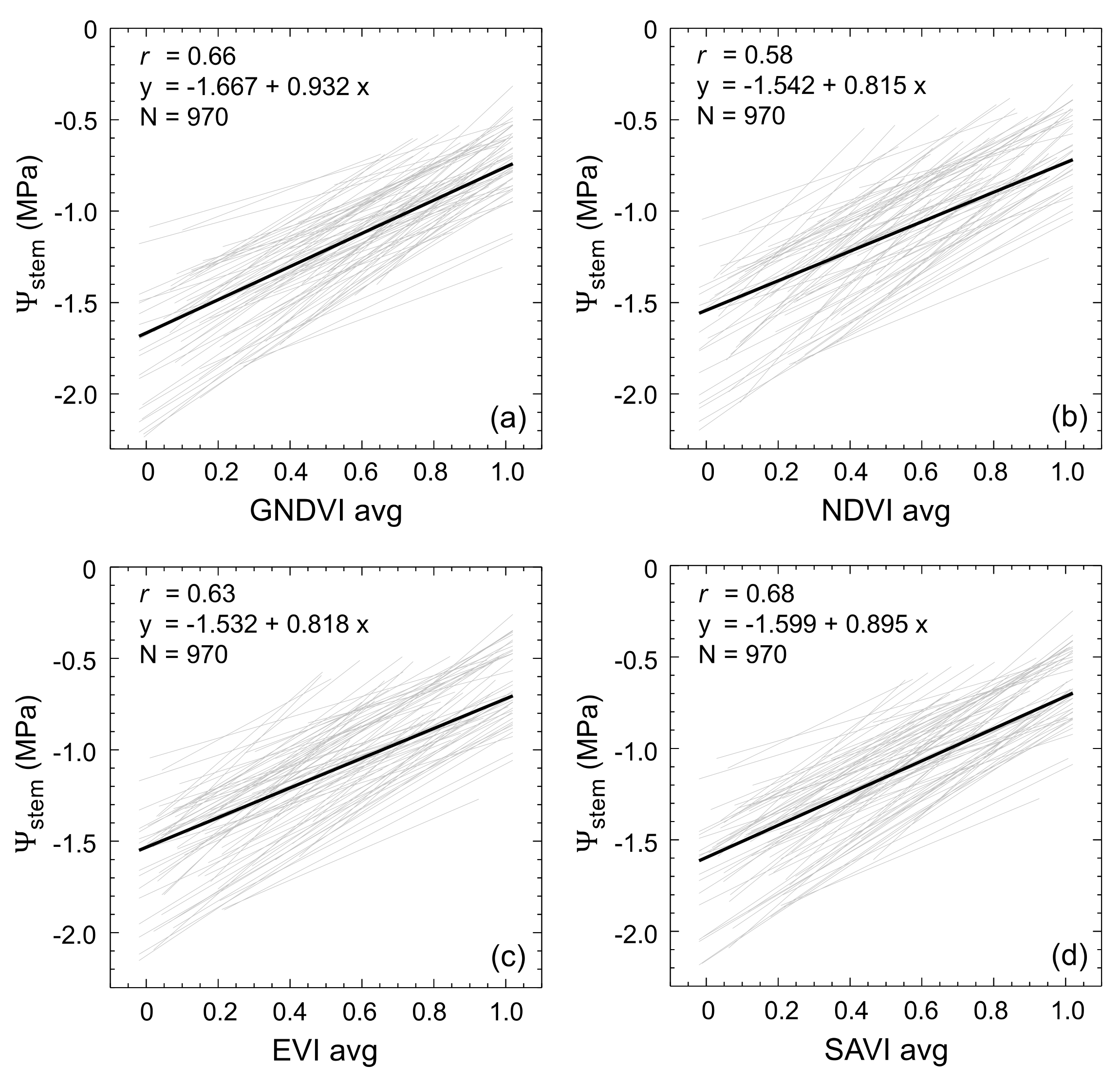
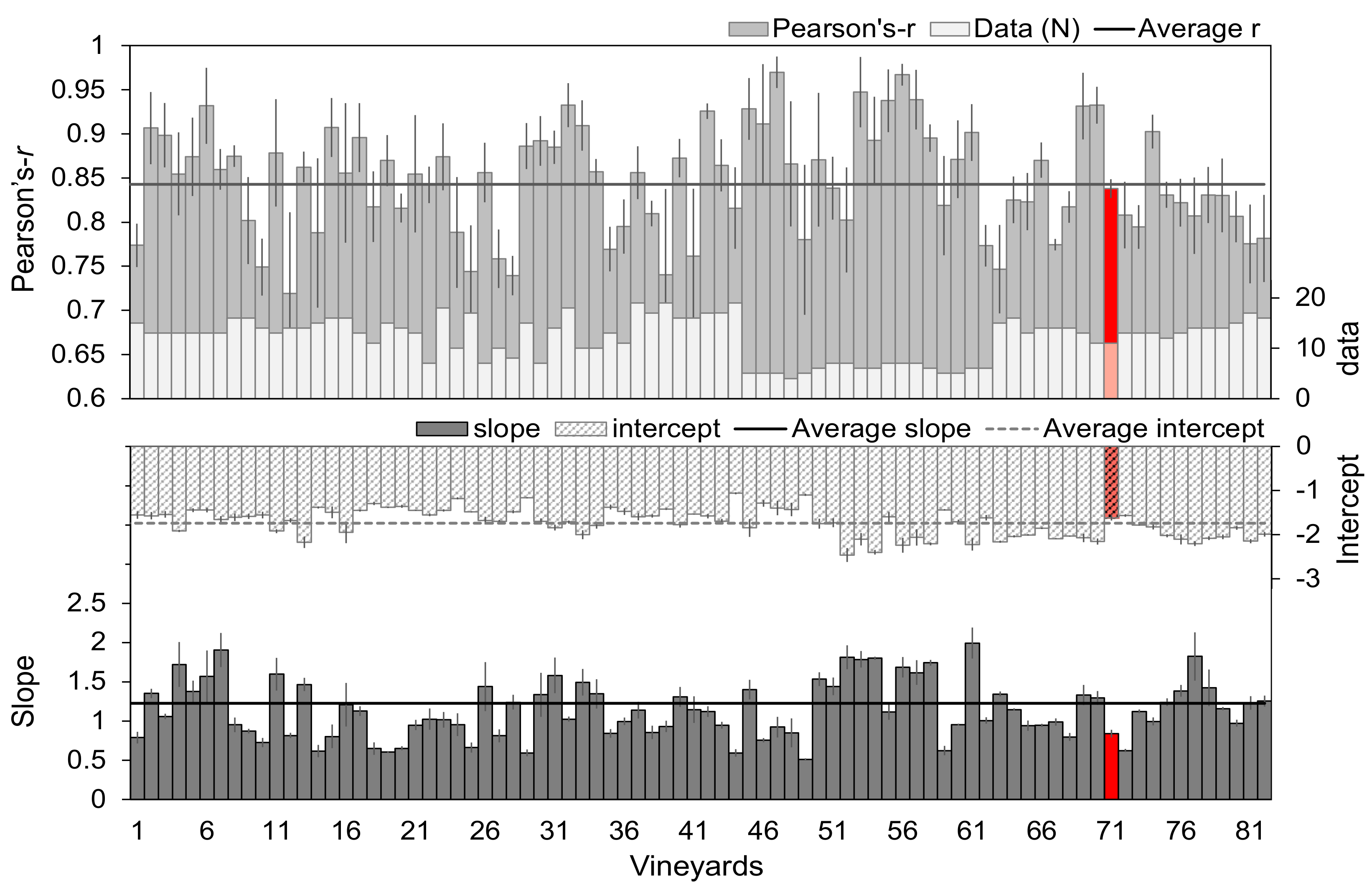
3.3. Predicting Stem Water Potential at Mevo Beitar Vineyard Using Single ‘Global’ Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| # | Region | Size (ha) | Species | Red/White | Ψstem (MPa) | DI Start | EOS |
|---|---|---|---|---|---|---|---|
| 1 | Golan | 1.74 | Cabernet Franc | red | −1.376 | 14-May | 27-Aug |
| 2 | Golan | 1.34 | Chardonnay | white | −1.721 | 28-May | 10-Sep |
| 3 | Golan | 0.95 | Chardonnay | white | −1.518 | 28-May | 10-Sep |
| 4 | Golan | 1.30 | Cabernet Sauvignon | red | −1.806 | 28-May | 10-Sep |
| 5 | Golan | 0.85 | Cabernet Sauvignon | red | −1.500 | 28-May | 10-Sep |
| 6 | Golan | 0.70 | Cabernet Sauvignon | red | −1.427 | 28-May | 10-Sep |
| 7 | Golan | 1.16 | Cabernet Sauvignon | red | −1.743 | 28-May | 10-Sep |
| 8 | Golan | 1.00 | Cabernet Sauvignon | red | −1.543 | 14-May | 05-Sep |
| 9 | Golan | 1.42 | Cabernet Sauvignon | red | −1.533 | 14-May | 05-Sep |
| 10 | Golan | 2.07 | Merlot | red | −1.432 | 14-May | 14-Aug |
| 11 | Golan | 1.30 | Petit Verdot | red | −1.729 | 28-May | 10-Sep |
| 12 | Golan | 1.81 | Syra | red | −1.583 | 14-May | 13-Aug |
| 13 | Golan | 1.13 | Syra | red | −1.445 | 14-May | 14-Aug |
| 14 | Golan | 1.30 | Chardonnay | white | −1.340 | 29-May | 11-Sep |
| 15 | Golan | 1.21 | Chardonnay | white | −1.338 | 29-May | 11-Sep |
| 16 | Golan | 2.13 | Chardonnay | white | −1.556 | 29-May | 11-Sep |
| 17 | Golan | 3.13 | Chardonnay | white | −1.485 | 29-May | 11-Sep |
| 18 | Golan | 0.84 | Cabernet Sauvignon | red | −1.504 | 05-Jun | 03-Sep |
| 19 | Golan | 1.47 | Cabernet Sauvignon | red | −1.366 | 29-May | 11-Sep |
| 20 | Golan | 1.77 | Cabernet Sauvignon | red | −1.550 | 29-May | 11-Sep |
| 21 | Golan | 1.36 | Pinot Gris | white | −1.463 | 29-May | 04-Sep |
| 22 | Golan | 1.98 | Cabernet Sauvignon | red | −1.486 | 22-May | 07-Sep |
| 23 | Golan | 2.42 | Cabernet Sauvignon | red | −1.545 | 23-May | 18-Sep |
| 24 | Golan | 2.11 | Cabernet Sauvignon | red | −1.161 | 05-Jun | 07-Sep |
| 25 | Golan | 2.05 | Merlot | red | −1.638 | 29-May | 18-Sep |
| 26 | Golan | 1.58 | Merlot | red | −1.410 | 25-Jun | 10-Sep |
| 27 | Golan | 0.96 | Merlot | red | −1.568 | 25-Jun | 07-Sep |
| 28 | Golan | 3.88 | Merlot | red | −1.621 | 12-Jun | 10-Sep |
| 29 | Golan | 1.17 | Petit Verdot | red | −1.258 | 13-Jun | 18-Sep |
| 30 | Golan | 2.39 | Sauvignon Blanc | white | −1.511 | 19-Jun | 23-Aug |
| 31 | Golan | 0.87 | Syra | red | −1.807 | 06-Jun | 05-Sep |
| 32 | Golan | 0.96 | Syra | red | −1.765 | 23-May | 18-Sep |
| 33 | Golan | 1.22 | Syra | red | −1.807 | 05-Jun | 31-Aug |
| 34 | Golan | 3.60 | Syra | red | −1.554 | 12-Jun | 16-Aug |
| 35 | Golan | 2.51 | Viognier | white | −1.368 | 15-May | 31-Aug |
| 36 | Golan | 2.36 | Viognier | white | −1.520 | 15-May | 07-Sep |
| 37 | Golan | 0.76 | Cabernet Sauvignon | red | −1.479 | 08-May | 11-Sep |
| 38 | Golan | 2.60 | Cabernet Sauvignon | red | −1.540 | 19-May | 11-Sep |
| 39 | Golan | 1.56 | Malbec | red | −1.469 | 08-May | 11-Sep |
| 40 | Golan | 1.98 | Merlot | red | −1.601 | 19-May | 11-Sep |
| 41 | Golan | 2.77 | Merlot | red | −1.419 | 19-May | 11-Sep |
| 42 | Golan | 3.98 | Sangiovese | red | −1.581 | 19-May | 11-Sep |
| 43 | Golan | 1.52 | Syra | red | −1.712 | 19-May | 11-Sep |
| 44 | Golan | 1.00 | Tinta Cao | red | −1.104 | 08-May | 11-Sep |
| 45 | Galilee | 2.97 | Cabernet Franc | red | −1.270 | 10-May | 18-Jul |
| 46 | Galilee | 1.35 | Petit Syra | red | −1.009 | 10-May | 18-Jul |
| 47 | Galilee | 2.95 | Viognier | white | −1.073 | 10-May | 18-Jul |
| 48 | Galilee | 1.81 | Malbec | red | −1.151 | 10-May | 18-Jul |
| 49 | Galilee | 2.99 | Chardonnay | white | −0.869 | 10-May | 18-Jul |
| 50 | Galilee | 2.54 | Chardonnay | white | −1.195 | 02-May | 29-Aug |
| 51 | Galilee | 2.64 | Muscat Caneli | white | −1.486 | 02-May | 29-Aug |
| 52 | Galilee | 3.84 | Merlot | red | −1.431 | 02-May | 29-Aug |
| 53 | Galilee | 2.87 | Viognier | white | −1.356 | 02-May | 15-Aug |
| 54 | Galilee | 3.59 | Chardonnay | white | −1.441 | 02-May | 15-Aug |
| 55 | Galilee | 4.81 | Roussanne | white | −1.196 | 06-Jun | 29-Aug |
| 56 | Galilee | 3.67 | Cabernet Sauvignon | red | −1.594 | 06-Jun | 29-Aug |
| 57 | Galilee | 4.26 | Cabernet Sauvignon | red | −1.536 | 06-Jun | 29-Aug |
| 58 | Galilee | 3.35 | Gewurztraminer | white | −1.246 | 02-Jun | 15-Aug |
| 59 | Galilee | 3.65 | Pinot Noir | red | −1.243 | 24-May | 16-Aug |
| 60 | Galilee | 3.06 | Tannat | red | −1.528 | 24-May | 16-Aug |
| 61 | Galilee | 5.46 | Cabernet Sauvignon | red | −1.770 | 24-May | 30-Aug |
| 62 | Galilee | 2.43 | Cabernet Sauvignon | red | −1.611 | 24-May | 30-Aug |
| 63 | Judea | 1.43 | Merlot | red | −1.989 | 15-May | 11-Sep |
| 64 | Judea | 1.28 | Cabernet Sauvignon | red | −1.961 | 29-May | 11-Sep |
| 65 | Judea | 1.83 | Cabernet Sauvignon | red | −1.758 | 14-May | 20-Aug |
| 66 | Judea | 1.08 | Petit Verdot | red | −1.624 | 14-May | 20-Aug |
| 67 | Judea | 1.23 | Syra | red | −1.826 | 14-May | 20-Aug |
| 68 | Judea | 1.13 | Syra | red | −1.799 | 14-May | 20-Aug |
| 69 | Judea | 0.49 | Merlot | red | −1.928 | 15-May | 28-Aug |
| 70 | Judea | 0.73 | Merlot | red | −1.928 | 15-May | 28-Aug |
| 71 | Judea | 2.40 | Cabernet Sauvignon | red | −1.400 | 10-May | 05-Sep |
| 72 | Judea | 1.27 | Cabernet Sauvignon | red | −1.514 | 14-May | 27-Aug |
| 73 | Judea | 1.27 | Cabernet Sauvignon | red | −1.566 | 14-May | 27-Aug |
| 74 | Judea | 1.18 | Cabernet Sauvignon | red | −1.593 | 14-May | 27-Aug |
| 75 | Judea | 0.73 | Merlot | red | −1.675 | 14-May | 13-Aug |
| 76 | Judea | 0.55 | Malbec | red | −1.773 | 21-May | 27-Aug |
| 77 | Judea | 0.63 | Merlot | red | −2.232 | 15-May | 28-Aug |
| 78 | Judea | 0.89 | Merlot | red | −1.840 | 15-May | 28-Aug |
| 79 | Judea | 1.55 | Merlot | red | −1.926 | 15-May | 28-Aug |
| 80 | Judea | 2.01 | Cabernet Sauvignon | red | −1.792 | 15-May | 11-Sep |
| 81 | Judea | 1.26 | Cabernet Sauvignon | red | −2.021 | 22-May | 11-Sep |
| 82 | Judea | 1.39 | Cabernet Sauvignon | red | −1.928 | 15-May | 11-Sep |
References
- Ruiz-Sanchez, M.C.; Domingo, R.; Castel, J.R. Deficit irrigation in fruit trees and vines in Spain. Span. J. Agric. Res. 2010, 8, 5–20. [Google Scholar] [CrossRef]
- Munitz, S.; Netzer, Y.; Schwartz, A. Sustained and regulated deficit irrigation of field-grown Merlot grapevines. Aust. J. Grape Wine Res. 2017, 23, 87–94. [Google Scholar] [CrossRef]
- Myburgh, P.; Cornelissen, M.; Southey, T. Interpretation of Stem Water Potential Measurements. WineLand. 2016, pp. 78–80. Available online: http://www.wineland.co.za/interpretation-of-stem-water-potential-measurements/ (accessed on 15 July 2018).
- Mulla, D.J. Twenty five years of remote sensing in precision agriculture: Key advances and remaining knowledge gaps. Biosyst. Eng. 2013, 114, 358–371. [Google Scholar] [CrossRef]
- Gebbers, R.; Adamchuk, V.I. Precision Agriculture and Food Security. Science 2010, 327, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Matese, A.; Baraldi, R.; Berton, A.; Cesaraccio, C.; Di Gennaro, F.S.; Duce, P.; Facini, O.; Mameli, G.M.; Piga, A.; Zaldei, A. Estimation of Water Stress in Grapevines Using Proximal and Remote Sensing Methods. Remote Sens. 2018, 10, 114. [Google Scholar] [CrossRef]
- Meron, M.; Tsipris, J.; Orlov, V.; Alchanatis, V.; Cohen, Y. Crop water stress mapping for site-specific irrigation by thermal imagery and artificial reference surfaces. Precis. Agric. 2010, 11, 148–162. [Google Scholar] [CrossRef]
- Helman, D.; Lensky, I.M.; Tessler, N.; Osem, Y. A phenology-based method for monitoring woody and herbaceous vegetation in mediterranean forests from NDVI time series. Remote Sens. 2015, 7, 12314–12335. [Google Scholar] [CrossRef]
- Helman, D.; Lensky, I.M.; Osem, Y.; Rohatyn, S.; Rotenberg, E.; Yakir, D. A biophysical approach using water deficit factor for daily estimations of evapotranspiration and CO2 uptake in Mediterranean environments. Biogeosciences 2017, 14, 3909–3926. [Google Scholar] [CrossRef]
- Helman, D.; Osem, Y.; Yakir, D.; Lensky, I.M. Relationships between climate, topography, water use and productivity in two key Mediterranean forest types with different water-use strategies. Agric. For. Meteorol. 2017, 232, 319–330. [Google Scholar] [CrossRef]
- Helman, D.; Lensky, I.M.; Yakir, D.; Osem, Y. Forests growing under dry conditions have higher hydrological resilience to drought than do more humid forests. Glob. Chang. Biol. 2017, 23, 2801–2817. [Google Scholar] [CrossRef] [PubMed]
- Rotbart, N.; Schmilovitch, Z.; Cohen, Y.; Alchanatis, V.; Erel, R.; Ignat, T.; Shenderey, C.; Dag, A.; Yermiyahu, U. Estimating olive leaf nitrogen concentration using visible and near-infrared spectral reflectance. Biosyst. Eng. 2013, 114, 426–434. [Google Scholar] [CrossRef]
- Nigon, T.J.; Mulla, D.J.; Rosen, C.J.; Cohen, Y.; Alchanatis, V.; Knight, J.; Rud, R. Hyperspectral aerial imagery for detecting nitrogen stress in two potato cultivars. Comput. Electron. Agric. 2015, 112, 36–46. [Google Scholar] [CrossRef]
- Di Gennaro, S.F.; Battiston, E.; Di Marco, S.; Facini, O.; Matese, A.; Nocentini, M.; Palliotti, A.; Mugnai, L. Unmanned Aerial Vehicle (UAV)-based remote sensing to monitor grapevine leaf stripe disease within a vineyard affected by esca complex. Phytopathol. Mediterr. 2016, 55, 262–275. [Google Scholar]
- Mahlein, A.-K. Plant Disease Detection by Imaging Sensors—Parallels and Specific Demands for Precision Agriculture and Plant Phenotyping. Plant Dis. 2015, 100, 241–251. [Google Scholar] [CrossRef]
- Bonfil, D.J. Wheat phenomics in the field by RapidScan: NDVI vs. NDRE. Isr. J. Plant Sci. 2017, 9978, 1–14. [Google Scholar] [CrossRef]
- Manfreda, S.; McCabe, M.; Miller, P.; Lucas, R.; Madrigal, V.P.; Mallinis, G.; Dor, E.B.; Helman, D.; Estes, L.; Ciraolo, G.; et al. On the use of Unmanned Aerial Systems for environmental monitoring. Remote Sens. 2018, 10, 641. [Google Scholar] [CrossRef]
- Khanal, S.; Fulton, J.; Shearer, S. An overview of current and potential applications of thermal remote sensing in precision agriculture. Comput. Electron. Agric. 2017, 139, 22–32. [Google Scholar] [CrossRef]
- Cohen, Y.; Alchanatis, V.; Saranga, Y.; Rosenberg, O.; Sela, E.; Bosak, A. Mapping water status based on aerial thermal imagery: Comparison of methodologies for upscaling from a single leaf to commercial fields. Precis. Agric. 2017, 18, 801–822. [Google Scholar] [CrossRef]
- Rud, R.; Cohen, Y.; Alchanatis, V.; Levi, A.; Brikman, R.; Shenderey, C.; Heuer, B.; Markovitch, T.; Dar, Z.; Rosen, C.; et al. Crop water stress index derived from multi-year ground and aerial thermal images as an indicator of potato water status. Precis. Agric. 2014, 15, 273–289. [Google Scholar] [CrossRef]
- Jackson, R.D.; Idso, S.B.; Reginato, J.R.; Pinter, J.P. Canopy temperature as a crop water stress indicator. Water Resour. Res. 1981, 17, 1133–1138. [Google Scholar] [CrossRef]
- Baluja, J.; Diago, M.P.; Balda, P.; Zorer, R.; Meggio, F.; Morales, F.; Tardaguila, J. Assessment of vineyard water status variability by thermal and multispectral imagery using an unmanned aerial vehicle (UAV). Irrig. Sci. 2012, 30, 511–522. [Google Scholar] [CrossRef] [Green Version]
- Bellvert, J.; Zarco-Tejada, P.J.; Girona, J.; Fereres, E. Mapping crop water stress index in a ‘Pinot-noir’ vineyard: Comparing ground measurements with thermal remote sensing imagery from an unmanned aerial vehicle. Precis. Agric. 2014, 15, 361–376. [Google Scholar] [CrossRef]
- Santesteban, L.G.; Di Gennaro, S.F.; Herrero-Langreo, A.; Miranda, C.; Royo, J.B.; Matese, A. High-resolution UAV-based thermal imaging to estimate the instantaneous and seasonal variability of plant water status within a vineyard. Agric. Water Manag. 2017, 183, 49–59. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Diago, M.P.; Fernández-Novales, J.; Tardaguila, J. Vineyard water status assessment using on-the-go thermal imaging and machine learning. PLoS ONE 2018, 13, e0192037. [Google Scholar] [CrossRef] [PubMed]
- Möller, M.; Alchanatis, V.; Cohen, Y.; Meron, M.; Tsipris, J.; Naor, A.; Ostrovsky, V.; Sprintsin, M.; Cohen, S. Use of thermal and visible imagery for estimating crop water status of irrigated grapevine. J. Exp. Bot. 2007, 58, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Dugo, V.; Zarco-Tejada, P.; Nicolás, E.; Nortes, P. A.; Alarcón, J. J.; Intrigliolo, D. S.; Fereres, E. Using high resolution UAV thermal imagery to assess the variability in the water status of five fruit tree species within a commercial orchard. Precis. Agric. 2013, 14, 660–678. [Google Scholar] [CrossRef]
- Espinoza, C.Z.; Khot, L.R.; Sankaran, S.; Jacoby, P.W. High Resolution Multispectral and Thermal Remote Sensing-Based Water Stress Assessment in Subsurface Irrigated Grapevines. Remote Sens. 2017, 9, 961. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; González-Dugo, V.; Williams, L.E.; Suárez, L.; Berni, J.A.J.; Goldhamer, D.; Fereres, E. A PRI-based water stress index combining structural and chlorophyll effects: Assessment using diurnal narrow-band airborne imagery and the CWSI thermal index. Remote Sens. Environ. 2013, 138, 38–50. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Pérez, J.R.; Riaño, D.; Carlisle, E.; Ustin, S.; Smart, D.R. Evaluation of hyperspectral reflectance indexes to detect grapevine water status in vineyards. Am. J. Enol. Vitic. 2007, 58, 302–317. [Google Scholar]
- Maimaitiyiming, M.; Ghulam, A.; Bozzolo, A.; Wilkins, J.L.; Kwasniewski, M.T. Early Detection of Plant Physiological Responses to Different Levels of Water Stress Using Reflectance Spectroscopy. Remote Sens. 2017, 9, 745. [Google Scholar] [CrossRef]
- Helman, D. Land surface phenology: What do we really ‘see’ from space? Sci. Total Environ. 2018, 618, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Houborg, R.; McCabe, F.M. High-Resolution NDVI from Planet’s Constellation of Earth Observing Nano-Satellites: A New Data Source for Precision Agriculture. Remote Sens. 2016, 8, 768. [Google Scholar] [CrossRef]
- Planet. Planet Satellite Imagery Products. 2018. Available online: https://www.planet.com/docs/spec-sheets/sat-imagery/ (accessed on 15 July 2018).
- Munitz, S.; Netzer, Y.; Shetin, I.; Schwartz, A. Water availability dynamics have long-term effects on mature stem structure in Vitis vinifera. Am. J. Bot. 2018, 105. [Google Scholar] [CrossRef] [PubMed]
- Netzer, Y.; Yao, C.; Shenker, M.; Bravdo, B.-A.; Schwartz, A. Water use and the development of seasonal crop coefficients for Superior Seedless grapevines trained to an open-gable trellis system. Irrig. Sci. 2009, 27, 109–120. [Google Scholar] [CrossRef]
- Munitz, S.; Schwartz, A.; Netzer, Y. Evaluation of Seasonal Water Use and Crop Coefficients for Cabernet Sauvignon Grapevines as the Base for Skilled regulated irrigation. Acta Hortic. 2016, IV, 33–40. [Google Scholar] [CrossRef]
- Boyer, J.S. Measuring the Water Status of Plants and Soils; Academic Press, Inc.: San Diego, CA, USA, 1995. [Google Scholar]
- Helman, D.; Givati, A.; Lensky, I.M. Annual evapotranspiration retrieved from satellite vegetation indices for the eastern Mediterranean at 250 m spatial resolution. Atmos. Chem. Phys. 2015, 15, 12567–12579. [Google Scholar] [CrossRef]
- Candiago, S.; Remondino, F.; De Giglio, M.; Dubbini, M.; Gattelli, M. Evaluating Multispectral Images and Vegetation Indices for Precision Farming Applications from UAV Images. Remote Sens. 2015, 7, 4026–4047. [Google Scholar] [CrossRef] [Green Version]
- Gnyp, M.L.; Miao, Y.; Yuan, F.; Ustin, S.L.; Yu, K.; Yao, Y.; Huang, S.; Bareth, G. Hyperspectral canopy sensing of paddy rice aboveground biomass at different growth stages. Field Crops Res. 2014, 155, 42–55. [Google Scholar] [CrossRef]
- Odi-Lara, M.; Campos, I.; Neale, M.C.; Ortega-Farías, S.; Poblete-Echeverría, C.; Balbontín, C.; Calera, A. Estimating Evapotranspiration of an Apple Orchard Using a Remote Sensing-Based Soil Water Balance. Remote Sens. 2016, 8, 253. [Google Scholar] [CrossRef]
- Huete, A.; Didan, K.; Miura, T.; Rodriguez, E.P.; Gao, X.; Ferreira, L.G. Overview of the radiometric and biophysical performance of the MODIS vegetation indices. Remote Sens. Environ. 2002, 83, 195–213. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Buschmann, C.; Lichtenthaler, H.K. Leaf chlorophyll fluorescence corrected for re-absorption by means of absorption and reflectance measurements. J. Plant Physiol. 1998, 152, 283–296. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote estimation of chlorophyll content in higher plant leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.W.; Schell, J.A.; Deering, D.H.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation (Greenwave Effect) of Natural Vegetation; NASA/GSFC: Greenbelt, MD, USA, 1974.
- Huete, A.R.; Jackson, R.D. Soil and atmosphere influences on the spectra of partial canopies. Remote Sens. Environ. 1988, 25, 89–105. [Google Scholar] [CrossRef]
- Planet Team. Planet Application Program Interface: In Space for Life on Earth; Planet Team: San Francisco, CA, USA, 2018; Available online: https://api.planet.com (accessed on 10 September 2018).
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Verbesselt, J.; Zeileis, A.; Herold, M. Near real-time disturbance detection using satellite image time series. Remote Sens. Environ. 2012, 123, 98–108. [Google Scholar] [CrossRef]
- Arnó, J.; Martínez Casasnovas, J.A.; Ribes Dasi, M.; Rosell, J.R. Research topics, challenges and opportunities in site-specific vineyard management. Span. J. Agric. Res. 2009, 7, 779–790. [Google Scholar] [CrossRef]
- Monaghan, J.M.; Daccache, A.; Vickers, L.H.; Hess, T.M.; Weatherhead, K.E.; Grove, I.G.; Knox, J.W. More ‘crop per drop’: Constraints and opportunities for precision irrigation in European agriculture. J. Sci. Food Agric. 2013, 93, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Gealy, D.V.; McKinley, S.; Guo, M.; Miller, L.; Vougioukas, S.; Viers, J.; Carpin, S.; Goldberg, K. DATE: A handheld co-robotic device for automated tuning of emitters to enable precision irrigation. In Proceedings of the 2016 IEEE International Conference on Automation Science and Engineering (CASE), Fort Worth, TX, USA, 21–25 August 2016; pp. 922–927. [Google Scholar]
- Agam, N.; Segal, E.; Peeters, A.; Levi, A.; Dag, A.; Yermiyahu, U.; Ben-Gal, A. Spatial distribution of water status in irrigated olive orchards by thermal imaging. Precis. Agric. 2014, 15, 346–359. [Google Scholar] [CrossRef]
- Netzer, Y.; Yao, C.; Shenker, M.; Cohen, S.; Bravdo, B.; Schwartz, A. Water consumtion of “superior” grapevines grown in a semiarid region. Acta Hortic. 2005, 689, 399–406. [Google Scholar] [CrossRef]
- Chen, X.; Wang, D.; Chen, J.; Wang, C.; Shen, M. The mixed pixel effect in land surface phenology: A simulation study. Remote Sens. Environ. 2018, 211, 338–344. [Google Scholar] [CrossRef]


| Index | Formulation 1 | Reference |
|---|---|---|
| GNDVI | [45] | |
| NDVI | [47] | |
| EVI 2 | [43] | |
| SAVI 3 | [48] |
| DOY | r | RMSE (MPa) | Ψstem (MPa) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GNDVI | NDVI | EVI | SAVI | GNDVI | NDVI | EVI | SAVI | Average | Std | |
| 126 | 0.45 | 0.56 2 | 0.56 2 | 0.603 | 0.091 | 0.084 | 0.084 | 0.081 | −1.008 | 0.126 |
| 140 | 0.51 1 | 0.48 1 | 0.47 | 0.531 | 0.049 | 0.050 | 0.050 | 0.048 | −0.633 | 0.103 |
| 154 | 0.72 3 | 0.76 3 | 0.76 3 | 0.783 | 0.094 | 0.088 | 0.088 | 0.086 | −0.728 | 0.149 |
| 168 | 0.32 | 0.32 | 0.30 | 0.28 | 0.129 | 0.129 | 0.129 | 0.130 | −0.990 | 0.226 |
| 175 | 0.46 | 0.561 | 0.56 1 | 0.55 1 | 0.065 | 0.061 | 0.061 | 0.061 | −1.089 | 0.130 |
| 189 | 0.783 | 0.76 3 | 0.76 3 | 0.783 | 0.086 | 0.090 | 0.090 | 0.086 | −1.056 | 0.178 |
| 196 | 0.76 2 | 0.77 2 | 0.77 2 | 0.793 | 0.087 | 0.085 | 0.085 | 0.082 | −1.250 | 0.145 |
| 203 | 0.763 | 0.74 3 | 0.73 3 | 0.75 3 | 0.124 | 0.128 | 0.128 | 0.125 | −1.408 | 0.198 |
| 217 | 0.843 | 0.81 3 | 0.81 3 | 0.82 3 | 0.108 | 0.116 | 0.116 | 0.115 | −1.196 | 0.447 |
| 245 | 0.843 | 0.79 3 | 0.79 3 | 0.81 3 | 0.147 | 0.166 | 0.166 | 0.160 | −1.364 | 0.285 |
| Average | 0.64 | 0.65 | 0.65 | 0.67 | 0.098 | 0.099 | 0.100 | 0.097 | −1.072 | 0.199 |
| Variable | Estimate 1 | σ 2 | t-Ratio 3 | LogWorth | p-Value 4 |
|---|---|---|---|---|---|
| DOY | −0.0062 | 0.00045 | −13.59 | 37.906 | <0.0001 |
| ΔNDVI | 2.5164 | 0.37276 | 6.75 | 10.596 | <0.0001 |
| SAVI avg | 0.6570 | 0.09927 | 6.62 | 10.219 | <0.0001 |
| NDVI max | −2.4509 | 0.43914 | −5.58 | 7.508 | <0.0001 |
| NDVI min | −1.0344 | 0.37159 | −2.78 | 2.261 | 0.0055 |
| Intercept | −0.3885 | 0.13803 | −2.81 | 0.0050 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Helman, D.; Bahat, I.; Netzer, Y.; Ben-Gal, A.; Alchanatis, V.; Peeters, A.; Cohen, Y. Using Time Series of High-Resolution Planet Satellite Images to Monitor Grapevine Stem Water Potential in Commercial Vineyards. Remote Sens. 2018, 10, 1615. https://doi.org/10.3390/rs10101615
Helman D, Bahat I, Netzer Y, Ben-Gal A, Alchanatis V, Peeters A, Cohen Y. Using Time Series of High-Resolution Planet Satellite Images to Monitor Grapevine Stem Water Potential in Commercial Vineyards. Remote Sensing. 2018; 10(10):1615. https://doi.org/10.3390/rs10101615
Chicago/Turabian StyleHelman, David, Idan Bahat, Yishai Netzer, Alon Ben-Gal, Victor Alchanatis, Aviva Peeters, and Yafit Cohen. 2018. "Using Time Series of High-Resolution Planet Satellite Images to Monitor Grapevine Stem Water Potential in Commercial Vineyards" Remote Sensing 10, no. 10: 1615. https://doi.org/10.3390/rs10101615
APA StyleHelman, D., Bahat, I., Netzer, Y., Ben-Gal, A., Alchanatis, V., Peeters, A., & Cohen, Y. (2018). Using Time Series of High-Resolution Planet Satellite Images to Monitor Grapevine Stem Water Potential in Commercial Vineyards. Remote Sensing, 10(10), 1615. https://doi.org/10.3390/rs10101615