1. Introduction
Water pollution caused by chemical, microbial, and physical contamination is a worldwide health issue [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14]. While microbes cause acute diseases (e.g., cholera, diarrhea, typhoid fever), chemicals mainly cause chronic diseases including cancer [
4,
9,
11,
14,
15,
16,
17,
18,
19]. Physical contamination (e.g., color, suspended solids) is generally easy to remove. There are many classifications for chemical contaminants (e.g., (i) organic, inorganic, heavy metal, radioactive, (ii) conventional micro-pollutants vs. emerging contaminants like pesticides, pharmaceuticals, and personal care products (PPCPs)), among which water-soluble species can be collectively classified into three main groups: anionic, cationic, and neutral (nonionic). The next universal classification criterion is the size of the soluble species (small/medium/large). The presence of any contaminant in drinking water is a potential cause for concern as it might be toxic or be transformed into toxic species [
4,
19,
20]. Therefore, the development of efficient and affordable technologies for water treatment in developing countries, and specifically under remote and marginal living conditions, is urgently required.
Providing universal access to reliable, chemical- and pathogen-free water supplies is the ideal solution to water-borne illness [
1,
2,
4,
6,
8,
9,
17,
18,
20,
21,
22,
23,
24,
25]. This objective has not been achieved by previous efforts, including the Millennium Development Goals (MDGs; 2000–2015) [
20]. In September 2015, the countries of the world identified goals and set targets to substantially improve the human condition by 2030. This was done by adopting the United Nations (UN) Sustainable Development Goals (SDGs). Goal 6 (one of the 17 SDGs) focuses explicitly on freshwater: “
Ensure availability and sustainable management of water and sanitation for all” [
26]. Accordingly, Goal 6 calls for improving water quality as well as protecting and restoring water-related ecosystems [
10]. However, the goal does not explicitly include universal access to safe drinking water. This view gains importance, considering that in 13 years (by 2030) the countries of the world will evaluate the extent to which the UN SDGs have been achieved and set new goals/targets. This communication reiterates that universal access to safe drinking water is possible and feasible within one or two decades [
27]. Thus, existing knowledge from the science of aqueous iron corrosion (Corrosion Science) needs to be effectively translated into practical solutions [
27,
28,
29,
30] by designing efficient filtration systems based on metallic iron (Fe
0 filters) for safe drinking water provision. This includes the use of established and recommended efficient slow sand filters (SSFs) and biosand filters (BSFs) [
31,
32], which can be optimized by amendment with Fe
0 [
30,
33]. Based on these studies, the long-lasting need for an appropriate, demand-based, affordable, efficient, and sustainable water treatment technology, which is additionally centered on local communities (not only in the developing world) has been scientifically resolved [
26,
27,
28,
29,
30,
33]. Means of universal, practical implementation are presented herein. An overview of recent achievements in using Fe
0 for decentralized safe drinking water provision is given first.
3. The Affordability of Fe0-Amended Sand Filters
One major argument against universal access to safe drinking water in the short term is the high capital cost of piped supply systems, which are still regarded as the default option [
4,
8,
9,
95]. Current cost estimations are based on universal safe piped water for many developing regions. Accordingly, household water treatment and safe storage (HWTS) practices like boiling, chlorination, or filtration are collectively regarded as an interim solution [
95,
96,
97]. Ojomo et al. [
8] reported on controversies surrounding the question of whether HWTS practices yield improvements in drinking water quality and reductions in diarrheal disease [
97,
98,
99]. In particular, it was argued that studies claiming the efficiency of HWTS practices were assessed over too short a duration [
99]. However, these reports are not based on the instrumental analysis of treated water, making this discussion questionable, as only biological and chemical water analyses should be used to determine the water quality: the presence, level, and nature of contamination [
11,
27].
The success of HWTS practices in treating water (e.g., eliminating pollution) has been randomly interchanged with “the success in preventing diarrheal disease,” mostly for children under five. This oversimplification is no longer acceptable [
28]. Diseases are potentially caused by many other factors including sudden changes in the diet or the natural growth process [
100]. On the other hand, the most common HWTS practice (disinfection by boiling or chlorination) addresses only biological contamination [
33]. In other words, a child drinking water polluted with As, F, or U (the three ‘natural inorganic killers’) will not suffer from any diarrheal disease, but the water s/he is drinking is not safe. Therefore, relating the efficacy of HWTS methods to the frequency of diarrheal diseases is misleading. Additionally, HWTS definitively has the potential to improve water safety, but does not address water accessibility [
101,
102,
103]. Nevertheless, because affordable methods for water accessibility are increasingly available (rainwater harvesting, solar pump), HWTS is not just a “partial and interim solution to unsafe water” [
8], but a potentially reliable stand-alone solution for sustainable, safe drinking water [
17,
26,
27,
28,
30,
103].
The affordability of Fe
0 filters results from the evidence that they rely on two universally available materials: Fe
0 and sand. As stated in
Section 1, amending conventional BSFs with Fe
0 reactive layers will make them efficient at removing (i) pathogens in the BSF part and (ii) ‘excess’ pathogens and micro-pollutants in the reactive layers. Moreover, the BFS should precede Fe
0 layers and acts as an O
2-scavenger to enable the operation of Fe
0 layers under subsurface-like anoxic conditions (
Section 2). Conventionally, the Fe
0 reactive layer is made up of sand and Fe
0, wherein the volumetric proportion of sand should exceed 50% [
80,
81]. In practice, sand can be partly or totally replaced by other available and affordable natural minerals like anthracite, MnO
2, or pumice [
104,
105,
106,
107,
108]. These inert (e.g., anthracite, pumice) or reactive, but non-expansive (MnO
2) materials primarily serve as a storage surface for in situ generated corrosion products (in situ coating) [
109], and thus as an adsorptive surface for inflowing contaminants. This is the fundamental mechanism of contaminant removal in Fe
0/H
2O systems [
33]. The role of MnO
2 in sustaining the efficiency of Fe
0 filters was explained as follows [
105,
106,
107,
108,
109,
110]: MnO
2 uses Fe
2+ from Fe
0 oxidative dissolution by water (Equation (1)) for its reductive dissolution (Equation (2)):
MnO
2 works as an Fe
2+ scavenger and thus a sustaining reaction after Equation (1) according to the Lechatelier Principle. Despite stoichiometric disadvantage, small amounts of MnO
2 will act as a catalyst because MnOOH is permanently recycled into MnO
2 [
110]. This catalytic aspect has been put forward to rationalize the sustainability of SONO arsenic filters [
17,
25,
53].
Some existing efficient Fe
0 filters have been built by local populations without any particular skills and are maintained by them [
56,
111,
112,
113]. As an example, the community-scale Fe
0 arsenic filter developed at the Indian Institute of Technology Bombay (IITB filter) [
56,
113] uses commercial iron nails. Each community filter contains some 10 kg of iron nails and should work for about five years. Fe
0 filters use the same construction materials as any other filtration systems (e.g., biochar, biosand, activated carbon). As an example, Kearns [
114] used commercially available 200-liter high-density polyethylene (HDPE) drums to build biochar-based filtration systems in Thailand. The same drums could be used for Fe
0 filters. For IITB-like filters, 10 kg of iron nails are needed; 10 kg of iron nails every five years is certainly affordable. However, ‘iron nails’ is not a well-characterized class of Fe
0 materials [
92,
115,
116]. This makes the transferability of results achieved with IITB filters difficult.
4. The Efficiency of Fe0 Filters
The removal of many contaminants from natural water to meet drinking water standards is difficult as a result of their high solubility in water. Common decentralized water treatment technologies such as adsorptive filtration, boiling (pasteurizing), chlorination, ceramic filtration, membrane filtration (e.g., reverse osmosis (RO), biosand filtration (BSF), and solar water disinfection (SODIS)) are either energy-intensive (RO) or restrictive under marginal living conditions due to high complexity (chlorination), the possible production of toxic byproducts (chlorination), and the small spectrum of contaminants addressed (boiling, BSF, chlorination, SODIS) [
1,
117]. Adsorptive filtration is considered the most affordable, reliable, and effective means for decentralized safe drinking water production [
17,
118]. Further arguments for adsorptive filtration include its simplicity, ease of operation, economic feasibility, recyclability of adsorbents, and the availability of a wide range of adsorbents such as activated carbon, metal oxides, and zeolites. Despite the large spectrum of available adsorbents, it is still challenging to find efficient, readily available, economically feasible, and high-adsorption capacity materials for field applications. In recent years, Fe
0 has been established as an in situ generator of hydroxides and oxides for water treatment (
Section 2) [
3,
21,
26,
119,
120].
The in situ generation of metal oxides for the removal of aqueous biological and chemical contaminants was known prior to the era of Fe
0 filters [
121,
122,
123,
124,
125,
126,
127,
128,
129,
130]. Despite their inherently engineered nature (fabrication costs), Fe
0-based materials are abundantly available and are sometimes low in cost [
130,
131]. Fe
0 in the forms of granulated iron (chips, fillings, nails, plates), iron powder, nanoscale iron (nano-Fe
0), sponge iron, steel wool, etc. has been widely used to remove aqueous contaminants. At pH values of natural waters (6.0–9.0), Fe
0 filters are basically ion-selective as the surface of iron (hydr)oxides (iron corrosion products, FeCPs) shielding Fe
0 is positively charged [
132,
133,
134]. Accordingly, Fe
0 filters are more suitable for the removal of negatively charged species (e.g., fluoride and arsenates/AsV). However, regardless of their surface charge and molecular size, contaminants are (i) physically sequestrated or enmeshed during the precipitation of FeCPs (co-precipitation) [
125,
126,
127,
135,
136,
137], and/or (ii) removed from the aqueous phase by size exclusion. Size exclusion is mediated by the volumetric expansive nature of iron corrosion (V
oxide > V
iron) [
80,
81,
138,
139]. This implies that contaminant removal by size exclusion is constantly improved during the filter lifespan (provided the system does not get clogged). Therefore, well-designed Fe
0 filters efficiently remove all classes of aqueous contaminants in a single-stage process by a synergy between adsorption, co-precipitation, and size-exclusion [
26,
27,
28,
29,
30,
33,
140].
A major challenge of the Fe
0 filtration technology is to select the appropriate material capable of efficiently treating polluted water within an appropriate packed-bed. None of the tested/used Fe
0 material classes (e.g., iron fillings, iron nails, steel wool) is homogeneous in term of intrinsic reactivity or efficiency for contaminant removal. Accordingly, despite 27 years of extensive research, information is lacking to confidently select Fe
0 materials to be used in filters for safe drinking water provision [
44,
92,
93,
94]. This evidence makes the design of Fe
0 filters challenging.
5. Designing Efficient Fe0 Filters
A critical understanding of past design efforts made in the arena of Fe
0 filters is a fundamental prerequisite for future research. Considerable information on contaminant removal by Fe
0 filters is available (
Section 2 and
Section 3), but randomly scattered in the literature [
3,
141,
142,
143,
144,
145,
146]. Three concise review articles have been recently presented to catalyze further advancements [
26,
27,
52]. Hence, this section focuses on conceptual aspects.
5.1. General Aspects
Fe
0 fixed beds are a reactive filtration technology. Like adsorptive filtration, it is expected to be an extremely versatile technology. Adsorptive filtration has proved to be the least expensive treatment option for many water treatment applications [
16,
52,
147,
148]. Research over the past three decades has demonstrated the suitability of Fe
0 filters to quantitatively remove a wide variety of pathogens and toxic chemicals [
17,
25,
53,
71,
116,
119,
142,
144,
145,
146,
149]. Its suitability on a specific application depends on costs as they relate to the amount of Fe
0 consumed.
5.2. Fe0 Characteristics: Form, Size, and Intrinsic Reactivity
Determining the Fe
0 amount to be used in an application is a challenging task for at least two reasons: (i) Fe
0 is not the contaminant-removing agent but rather the generator of contaminant collectors, (ii) contaminant collectors are in situ generated and further transformed in a highly dynamic process. Reason 1 implies that, unlike inert adsorbents (e.g., granular activated carbons), the determination of the “specific adsorption capacity” for Fe
0 materials is not easy and cannot be derived from short-term adsorption isotherms [
52,
147]. The ‘adsorption capacity’ gives the maximum amount of each contaminant that can be removed per unit weight (usually in grams) of adsorbing material (Fe
0 is not one such). When additionally considering that the initial reaction products (Fe
II and H/H
2 species) are further transformed to a variety of hydroxides and oxides (Reason 2) and that each Fe
0 reacts with its own reaction kinetics (intrinsic reactivity), it becomes evident that selecting the appropriate Fe
0 for a specific application is a challenging task [
52,
86,
93,
150,
151,
152]. Tested and used reactive Fe
0 exists in several sizes and forms, including iron fillings, iron nails, scrap iron, sponge iron, and steel wool. Materials of each class have been positively tested for contaminant removal without any effort to link individual materials to specific applications. Moreover, a common tool to characterize the intrinsic reactivity of Fe
0 materials is still missing [
45,
92,
93].
5.3. Characteristics of the Admixing Aggregates
The next important feature for the design of Fe
0 filters arises from the evidence that pure Fe
0 systems (100% Fe
0) are not sustainable [
28,
29,
80,
81,
82]. In fact, iron corrosion is a volumetric expansive process as each iron corrosion product (hydroxide, oxide) is at least 2.1 times larger than the parent atom (V
oxide > V
iron) [
80,
81,
138,
139,
153]. Therefore, Fe
0 should be mixed with at least one non-expansive aggregate (e.g., gravel, pumice, sand) [
80,
81,
152]. The nature and proportion of the appropriate aggregate is an important operational parameter for the design of Fe
0 filters. Each aggregate has adsorptive affinity to individual contaminants but another criterion for their selection is the high surface area to volume ratio (porosity) as the accessible porous system may enable the accumulation of in situ generated corrosion products and thus delay permeability loss [
80,
81,
150].
To summarize, from a purely material perspective, the efficiency of a Fe0 filter depends on (i) the intrinsic reactivity of the used Fe0, (ii) the form and size of used Fe0, (iii) the nature of the admixing aggregate, and (iv) the Fe0:aggregate ratio. It appears that material selection is crucial for the functionality and sustainability of Fe0 filters. Regardless of the Fe0 type used, it is applied for the mitigation of the extent of contamination from polluted water. Accordingly, besides the nature of the contaminant, the characteristics of the polluted water (solution chemistry) should be considered as well.
5.4. Impact of Solution Chemistry
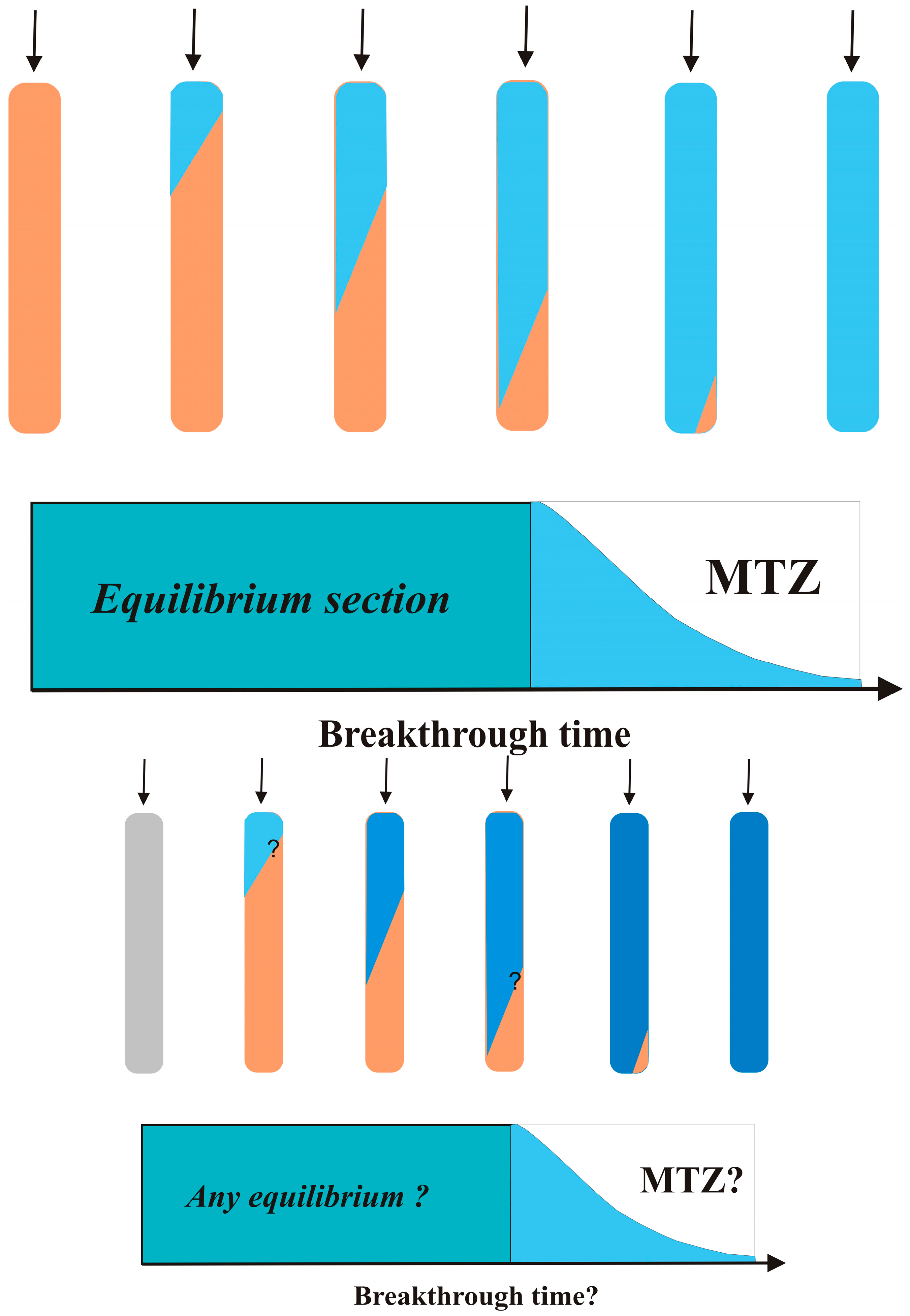
As a polluted stream passes through a Fe
0 filter, a dynamic condition develops that should produce a decrease in the contaminant concentration from the initial to the final level, ideally lower than the maximum contamination level (MCL). When the contamination level starts off higher than MCL, there is a “breakthrough.” In adsorptive filtration, the breakthrough corresponds to the exhaustion of the capacity of used adsorbent (breakthrough capacity) [
147,
148]. For Fe
0 filters, however, breakthrough may/should be observed before Fe
0 is exhausted. This is because there is no real ‘removal front’ and Fe
0 is oxidized in all parts of the filter, even in the absence of contaminants and dissolved O
2. For this reason, all parameters influencing aqueous water corrosion must be considered and their impact of the decontamination process discussed. In essence, such parameters influence the dissolution of Fe
0, the solubility of iron, the formation of the oxide scale on Fe
0, and the permeability of the oxide scale [
154,
155].
The decontamination with Fe0 is affected by various interrelated chemical and physical characteristics of the water. These parameters include: (i) pH value, (ii) nature and extent of contamination, (iii) nature and extent of co-solutes (e.g., NO3−, PO43−), (iv) presence of organic matter, (v) availability of dissolved O2, and (vi) availability of CO2. From the relevant parameters, the pH value and the availability of dissolved O2 will be commented on in some detail. The nature of the contamination is a site-specific issue. However, for a concept paper, the four most common contaminants can be considered: arsenic, bacteria, fluoride, and uranium.
5.4.1. Impact of pH Value
Fe
0 oxidative dissolution rates are faster at lower pH values (abundance of H
+—Equation (1)) than at higher pH values. For safe drinking water provision, especially in the developing country, the water source should have a pH > 5.0. Because Fe
0 is the source of contaminant collectors, the real contaminant scavengers are low-soluble iron corrosion products and their solubility is low at pH > 4.5 [
156]. The surface of iron oxides and hydroxides is positively charged in the pH range of interest, suggesting that negatively charged contaminants will be more efficiently removed. In other words, because of the ion-selective nature of the Fe
0/H
2O system at pH > 5.0, the most important impact of the pH value is its impact on the contaminant speciation. The large majority of available studies have not properly discussed this aspect (
Section 2 and
Section 3).
5.4.2. Impact of O2 Level
O
2 accelerates the initial kinetics of Fe
0 oxidative dissolution but its most important impact is indirect as it cannot quantitatively reach the shielded O
2 surface. The O
2 level might favors the formation of a thick oxide scale on Fe
0 (lowering the oxidation kinetics) as well as the cementation of Fe
0 particles and other aggregates (yielding permeability loss). It appears that the effect of O
2 level is situation-dependent, even within the same system. Accordingly, it is not surprising that controversial reports have been published (
Section 2).
A certain impact of O
2 is on the nature of in situ generated iron hydroxides and oxides. When O
2 is abundantly available, more voluminous compounds are generated (Fe
III oxides/hydroxides). The net result is a rapid porosity loss yielding system clogging (permeability loss). When the system is clogged, it become useless despite the remaining Fe
0 amount. For this reason, a sure way to sustain Fe
0 filters is to operate at low O
2 levels (e.g., [O
2] < 2.0 mg/L) [
33]. Fortunately, such conditions are achieved in biosand filters (BSF). This means that using a BSF as pre-treatment unit for a Fe
0 filters is advantageous [
26,
27,
28,
29,
30].
5.5. Design Considerations
The oxidative dissolution of Fe
0 and the associated reactions are not instantaneous. In other words, the generation of contaminant collectors needs time. Fe
0 filters should be designed such that contaminant removal is completed within the bed for the selected flow velocity [
26,
27,
28,
29,
30,
122,
123,
124,
125,
126]. As the rate of aqueous Fe
0 corrosion is not a constant function of the time, and each Fe
0 has its own intrinsic reactivity, the prediction of the efficiency of each filter goes through pilot testing. Another key factor is the expected change of the hydraulic properties (permeability) of Fe
0 filters during their operation. The question arises: which testing approach would enable the achievement of reliable results that may be regarded as system-independent?
The efficiency of a Fe
0 filter for As removal has illustrated the complexity of the issue (
Section 2). The efficiency depends on the following five parameters: (i) the pH value determining the ion selectivity of the filter, (ii) the redox speciation of As (As
III, As
V or As
III/As
V ratio), (iii) the As concentration and the concentrations of all other species interfering with As adsorption by competing for adsorption sites, modifying surface charges, or modifying As and Fe solubility, (iv) porosity and pore size distribution of iron precipitates (and aggregates), and (v) the hydraulic conductivity of the filter as influenced by in situ generated contaminant collectors.
5.6. Approach for the RSM Modeling
The presentation herein has reiterated the availability of limited guidance for the Fe
0 selection for designing efficient Fe
0 filters. However, Fe
0 is the heart of the system and the efficiency of each system depends on the (i) initial kinetics of Fe
0 corrosion (corrosion rate) and (ii) its time-dependent change, which is not a linear function ([
28] and refs. cited therein). Accordingly, the determination of the corrosion rate of various Fe
0 materials under relevant field conditions is urgently needed [
89]. The next section (
Section 6) will present a protocol for the characterization of steel wool as Fe
0 material for drinking water production at a community level. Steel wool is selected for its worldwide availability [
25,
131] and the rapid kinetics of its corrosion [
127,
128].
The illustrative RSM model herein will discuss the effects of Fe0 type (four samples), the nature of the contaminants (As, bacteria, F, U) (four species), flow rate (0.2 to 2.4 mL/min) (five values), bed height (10 to 70 cm) (5 values), and initial fluoride concentration (2 to 15 mg/L) (five values) on the efficiency of Fe0 filters.
6. Testing Steel Wool as a Starting Fe0 Material
To illustrate the design practice of Fe
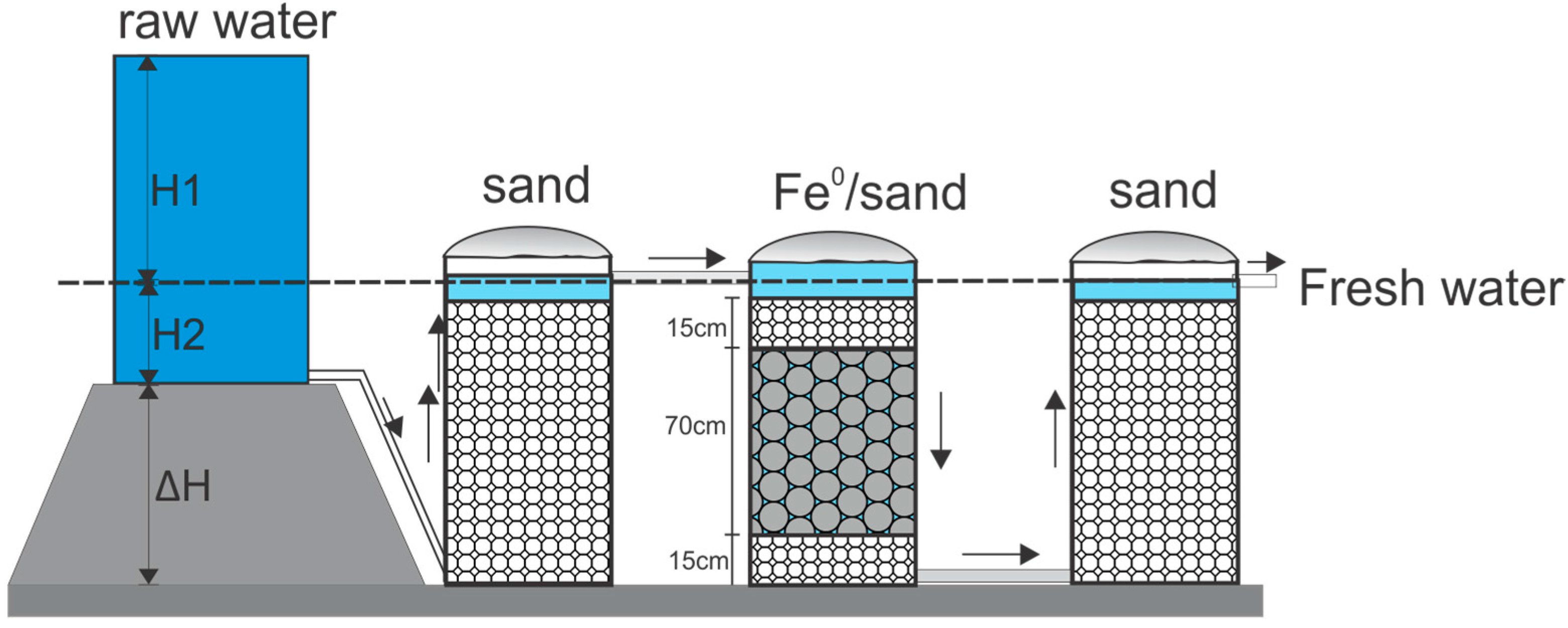
0 filters, a procedure to systematically test a system based on steel wool is discussed in this section. Three cylindrical columns (Column 1 through 3) with a diameter (D) of 15 cm and a height (H) of 100 cm are used (
Figure 2). The bed volume of this filter is 17.7 L (V = π × D
2 × H/4). The first and third columns are filled with fine sand (d < 0.4 mm). The first (Column 1) is basically a conventional BSF, whereas the second (Column 3) fixes dissolved Fe from the Fe
0-based column (in situ coating) to optimize decontamination. Column 2 contains a 70 cm thick reactive layer sandwiched between two layers of fine sand. Accordingly, the volume of the reactive layer is 12.4 L.
Table 4 summarizes the volumes of sand and Fe
0 to be taken to build six different reactive layers. In each case the corresponding masses should be documented as well as the effective depth of the reactive zone. The pure Fe
0 (100%) reactive zone is only to be tested for porous (e.g., foam/sponge) and filamentous (e.g., steel wool) materials.
6.1. Natural Water as a Complex Design Parameter
Natural waters are contaminated and eventually polluted due to three main inherent processes [
5,
20]: (i) atmospheric particle dissolution, (ii) rock weathering, and (iii) soil leaching. Increasing human population, industrialization, and the use of fertilizers and manufactured materials (including metal-based ones) have worsened water pollution due to weathering and leaching. It is necessary that the quality of any water source (e.g., lake, river, well) is checked to establish its (drinking) quality.
Irrespective of the presence of any toxic contamination (pollution), details about conventional physico-chemical parameters should be documented. These parameters include acidity (pH value), alkalinity (HCO3−), chloride (Cl−), color, dissolved organic carbon (DOC), dissolved oxygen (O2), electrical conductivity (EC), hardness (Ca2+, Mg2+), nitrate (NO3−), phosphate (PO43−), potassium (K+), sodium (Na+), sulfate (SO42−), temperature, and turbidity. Although the enumerated parameters are not mutually exclusive, it is seen that up to 14 parameters are necessary to characterize a water source. The relevance of the measurement of individual parameters arises from the evidence that measured EC values give an idea of the salinity of the water but no idea about the actual nature of dissolved ions (co-solutes).
This sub-section recalls that each water body is unique in its nature [
157,
158] and that the water quality is not limited to the nature and extent of contamination. In the context of using Fe
0 for water treatment, a pollutant (e.g., As, F, U) is just one of the operational parameters capable of influencing iron corrosion by (i) enhancing/inhibiting the kinetics of Fe
0 oxidative dissolution, (ii) influencing the process of oxide scale formation, (iii) influencing the ionic conductivity of the oxide scale, and (iv) influencing the porosity/permeability of the oxide scale [
159,
160,
161,
162,
163,
164,
165].
Apart from the pH value, it is not yet established whether selected parameters (including the nature and extent of contamination) are more significant than others for the process of contaminant removal in Fe
0/H
2O systems. Accordingly, designing Fe
0 filters based solely on the extent of contaminant removal at selected initial pH values is a highly qualitative task. Natural waters do contain different types of dissolved, suspended, and microbiological contaminants. Suspended contaminants are successfully removed in roughing filters [
22]. To obtain reliable design criteria for Fe
0 filters, the extent to which inherent water contents influence iron corrosion should be established. Only once this task is accomplished can rules of thumb for site-specific design be developed.
6.2. Design Criteria
The principal design criteria for Fe
0 filters are (alphabetically): (i) depth of the reactive zone, (ii) extent (and nature) of water contamination (including dissolved O
2 and co-solutes) (
Section 2), (iii) Fe
0 intrinsic reactivity, (iv) media depth and size (all aggregates including Fe
0; number of columns), (v) proportion of Fe
0 within the reactive zone, (vi) required treatment level, and (vii) water flow velocity (residence time). The performance of the filter is assessed by monitoring changes in the (i) concentrations of relevant species including contaminants and iron, (ii) hydraulic conductivity (permeability), (iii) pH value, and (vi) electrical conductivity.
6.3. Preparing and Implementing Media in Fe0 Filters
Sand is first sieved through a series of sieves to be separated into its different grain sizes. This operation is essential because the filtration rate is influenced by grain size and the grain size distribution. Only the fraction <2.0 mm is used. The fraction 0.2 to 0.4 mm can be used for the BSFs (columns 1 and 3,
Figure 1), the fraction 1.0 to 2.0 to support the reactive zone (column 2,
Figure 1), and the fraction 0.4 to 1.0 mm used in the reactive zone. Sieved sand is then washed to remove fine silt, clay, and other impurities that the media may contain. Store the washed sand in a protected dry area away from possible human or animal contamination.
Fe
0 is also sieved and the fraction lower than 2.0 mm used. Filamentous steel wool is chopped into pieces less than 2 cm in length. It should be ensured that the Fe
0 does not contain grease and/or toxic species (perform appropriate cleaning/washing, if applicable).
Table 5 summarizes the characteristics of commercial steel wool that should be systematically tested in long-term experiments. It is obvious that homogeneously mixing fine grade steel wool with sand will be a difficult task in rural conditions. Accordingly, only coarser materials, for instance with widths >50 μm, will be suggested for testing (four materials: 60, 75, 90, and 100 μm).
6.4. Simulating Testing Practice: RSM Model
In this section, the statistical framework of experiments is simulated to understand the methodology of designing a filter. As the effective parameters influencing filter design according to concerning dilemma may vary from place to place and from one investigator to another, the parameters are simplified according to the following considerations.
Section 6.1 listed 14 parameters relevant for water quality (
Table 6);
Section 6.2 listed seven principal design criteria (see also
Table 7) and three major monitoring parameters.
Section 6.3 suggested four steel wool materials to be tested. From the design criteria, the intrinsic reactivity of Fe
0 (steel wool in
Table 5 or k
EDTA value in
Table 6) [
94], the media depth and size, the proportion of Fe
0 within the reactive zone (
Table 4), the required treatment level (e.g., WHO guidelines), and the initial water flow velocity (mediated by ΔH in
Figure 1) can be considered as fixed. The nature and extent of water contamination are also fixed for any specific case (
Section 6.1).
As shown in
Table 6, many variables as well as their interactions might influence the design of an efficient Fe
0 filter. For this reason, the conditions need to be optimized. Unfortunately, the conventional methods for optimization are “one factor at a time” approaches that frequently fail to identify the variables that give rise to the optimum response because the effects of factor interactions are not taken into account in such procedures. These procedures are time-consuming and require a large number of experiments. They are also incapable of reaching truly optimal conditions due to ignoring such interactions among variables [
166,
167,
168]. In order to overcome these problems, a multivariate statistical design approach could be adopted through RSM, which is a multivariate statistical tool that uses quantitative data from appropriate experiments to solve multivariate equations.
Compared to conventional optimization methods, RSM is introduced as an economic and time-saving instrument because it can provide more information from fewer experiments [
169]. In addition, the estimates of the effects of each factor are more precise, the interaction between factors can be estimated systematically, and there is experimental information in a larger region of the factor space, which improves the prediction of the response in the factor space by reducing the variability of the estimates of the response [
170]. In addition, the main aim of RSM is to find the optimal response, and it has been widely used to describe the interactive and synergistic effects among experimental variables as well as to work on the optimization of operation conditions [
171,
172,
173,
174].
Over the last few decades, many researchers have been using various design of experiment (DoE) techniques in RSM including two-level full factorial design (FFD) [
175], Box–Behnken design (BBD) [
176], and Central Composite Design (CCD) [
177] to predict the ultimate response. CCD was first studied by Box and Wilson in 1951 and is still the most popular design for experiments. CCD has three groups of design points that are codified to summarize the data and ease of statistical calculations: (i) two-level factorial design points (2
k), consists of all possible combinations of the +1 and −1 codified levels of the factors, (ii) axial or star points (2k) codified as (α = (2
k)
1/4) have all of the factors set to 0, the midpoint, except one factor, which has the value ± α., and (iii) center points are points with all levels set to coded level 0 [
178].
The total number of experiments is calculated by the following equation:
where k is the number of factors and n
c the number of central points, which is calculated by the following equation:
where n
F is the number of factorial points and k is calculated by the following equation:
The methodology mainly involves a quadratic model to explain the behavior of the system. This model is flexible and covers all linear, non-linear, and interaction effects between the factors [
179]. The quadratic polynomial equation is as follows:
where y is the predicted response, β
0 is the offset term, β
i is the ith linear coefficient, β
ii is the quadratic coefficient, β
ij is the ijth coefficient, and ε is the error or residual value. Solving this equation and calculating the coefficients are done by using the least squares method. The fitness of the given models can be tested by ANOVA statistics (R
2, adjusted R
2, F-test and t-test) and residuals analysis. As Bezera et al. [
180] introduced RSM as a tool for optimization in analytical chemistry, this kind of design experiment has been widely used by different researchers in order to optimization of water and soil remediation [
166,
173,
181,
182,
183] or designing different types of water filters [
184,
185]. The experimental design and statistical analysis of the data were done by Design-expert10 statistical software.
In this study, the design of experiments is simulated by considering five factors; the total number of experiments will be 32, which is more economic and time-saving compared to 50 for a full-factorial design (
Table 8).
8. Design Your Fe0 Filter
Fe
0 filters for households and small communities are presented as a further development of the biosand filter (BSF) [
26,
27,
28,
29,
30,
56,
111,
112,
113,
229]. BSF is neither standardized nor patented [
22,
111,
230]. The BSF is considered one of the most promising household water treatment methods but the demonstration of its suitability and efficiency is impaired by two key factors: (i) The quality of treated water is not routinely analytically assessed and (ii) overarching designing or manufacturing and quality control guidelines do not exist. The challenge of accessing equipment for water analysis as a cornerstone for achieving the UN SDGs is obvious [
11,
28]. This section summarizes the efforts achieved in designing consistently high-quality Fe
0 filters. If an open collaboration in designing Fe
0 starts now, it will be easy to identify areas where manufacturing and quality control guidelines are needed. The presentation is limited to three key issues: (i) The nature of used Fe
0 materials, (ii) the design of the treatment units, and (iii) quality control practices.
8.1. Nature of Fe0 Materials
Available studies suggest that improper Fe
0 selection can affect the performance of locally designed Fe
0 filters to the point where their ability to produce safe drinking water is compromised. The historical example of the Kanchan arsenic filter [
111,
112] is well documented [
30]. The variability in the efficiency of designed filters implies that the iron nails used were of different intrinsic reactivity. Smith et al. [
229] recently presented a comparison of two different iron-nail-based Fe
0 filters for arsenic removal in China: (i) the original Kanchan arsenic filter (SONO-style) and (ii) a modification in which the nails are embedded in the upper sand layer (before the fine sand layer). The filter with embedded nails was found to be more efficient. However, this result is only qualitative as different iron nails would show different results and there is no tool to correlate the obtained results to those of Smith et al. [
229]. During the last 20 years, sporadic efforts to characterize the intrinsic reactivity of Fe
0 materials have been presented [
83,
84,
90,
91,
93,
94,
115,
116,
224]. However, only the methods of Reardon [
90] (H
2 evolution) and Noubactep (Fe
0 dissolution in EDTA) [
84] are really simple and affordable. Fe
0 dissolution in EDTA further presents the advantage that it used very small amounts of materials and lasted for just four days. Recently, Btatkeu et al. [
45,
92] further developed the EDTA method and demonstrated an excellent correlation of k
EDTA values and the extent of methylene blue discoloration from aqueous solutions. Accordingly, the routine determination of k
EDTA values is proposed as a tool to characterize the intrinsic reactivity of Fe
0 materials and ease discussion of results from various sources.
8.2. Design of Fe0 Treatment Units
A starting rule of thumb for designing Fe
0 could be: “Design an efficient BSF and add a Fe
0-based unit (new filter or a reactive zone in the BSF) [
30]. Then increase the water flow velocity and control the biological and chemical quality of the filtered water.” Accordingly the reference system is a BSF operating under the same conditions. By altering the current filter design, it will be possible to identify the range of parameters that can be changed while still having an improved BSF filter. It is essential to recall that the experiments should last for at least six months. Results obtained at lab scale demonstrate the viability of the system and could be directly used to design household Fe
0 filters. These results are the cornerstone for the design of pilot tests for scalable community treatment plants. However, lab and current pilot tests are lasting just for a few weeks or months [
67,
231,
232,
233]. This is not acceptable given the non-linearity of the kinetic of iron corrosion [
89].
8.3. Assessing the Efficiency of Fe0 Filters
Designing an efficient Fe0 filter could be regarded as ascertaining whether the flow rate of a BSF could be increased after the addition of a Fe0 unit without sacrificing filter effectiveness in terms of pathogen removal while inducing satisfactory chemical decontamination. Thus, once the specific design variables are identified, the task remaining is to monitor the quality of filtered water as result of the performed alteration.
The research method can be summarized as: “Take a BSF as reference system and create new filter designs by changing one of relevant design variables (
Section 5). Then produce the new designs (e.g., in triplicate along with three control BSF). Finally, pass the model solution or the local natural water through the filters daily, and test the filtrates once a week (or twice a month) for at least six months.” Original and filtrated natural waters should be tested for electrical conductivity, flow rate, iron concentration, major ions (Ca
2+, K
+, Mg
2+, Na
+, Cl
−, HCO
3−, PO
43−, SO
42−) pH value, total coliforms (TC), and turbidity. Additionally, natural waters should be monitored for arsenic, fluoride, and uranium, which have been revealed as global killers.
The large variability in water sources, Fe0 materials, and design options raises concerns about the consistency and quality of locally produced Fe0 filters, especially in the absence of standardized quality control procedures. If the approach presented herein is adopted widely, areas where designing/manufacturing guidelines are needed would soon be identified and this would contribute to consistently high-quality Fe0 filters. The next logical step is to identify areas where further research is needed to refine design recommendations. A significant goal can be to guide the development of a best-practice manual that describes science-based recommendations for quality-controlled Fe0 filters worldwide.
8.4. Local Candle Fe0 Filters
8.4.1. General Aspects
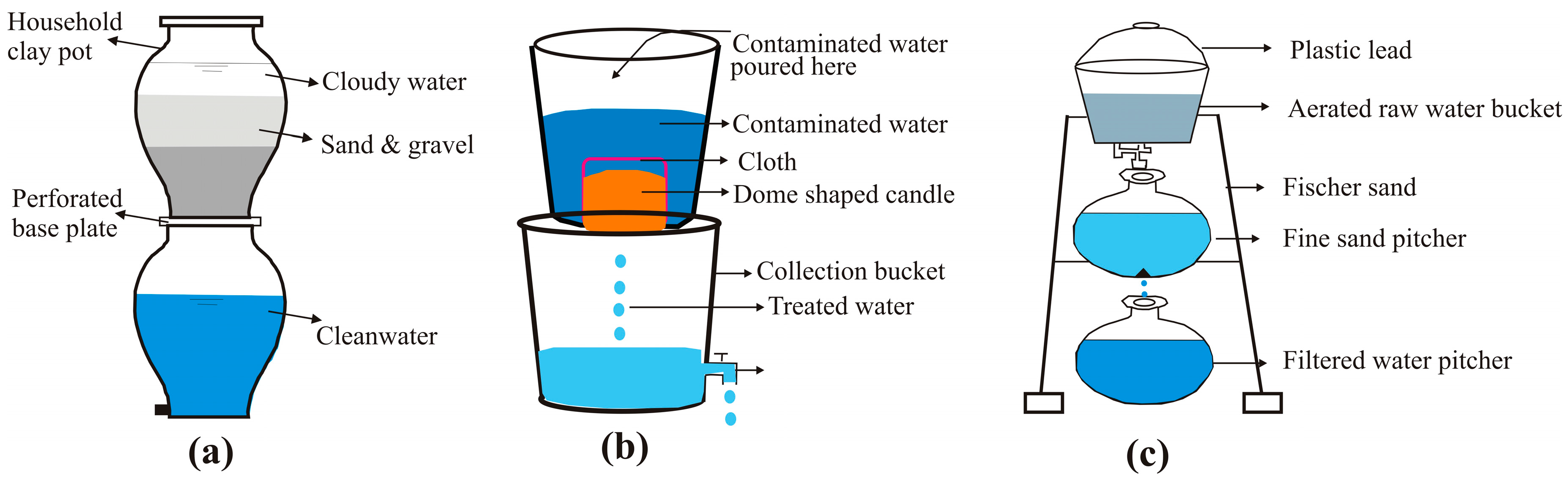
Candle-style Fe
0 filters (
Figure 3) can be locally manufactured, based on local knowledge. Using locally affordable materials would ensure the affordability of the resulted filters. One key benefit is the ability to adjust efficiency by manipulating the number of candles in each filtration system. It is understood that such candle filters may require plastic parts and some type of adhesive that are not yet locally available. Nevertheless, the candle Fe
0 filter would still be an appropriate solution because resulting problems are related to leakage and/or replacing broken parts and not to water treatment. Moreover, the introduction of such candles would galvanize the development of the market for such additional materials or even their local production in the short/long term. Once locally designed and produced candle Fe
0 filters are established, their efficiency would be improved in a locally induced development dynamic. Clearly, the comparison of first-generation local Fe
0 candles with commercial filters does not give a fair sense of their suitability. All that is needed is (i) the readiness to improve own systems with a systematic approach, (ii) an initially efficient system, and (iii) a clear indication for users as to when to replace the candle.
It is essential to recall that ‘old’ systems like BSF or ceramic filters are still in development [
186,
190,
191,
192,
234,
235]. Accordingly, being a ‘complication’ of BSF, many design-related topics are yet to be addressed in further research efforts. Understanding some of the specific causes of Fe
0 filter failures will help to optimize the cost-effectiveness of the Fe
0 filtration technology as a whole [
26]. Long-term studies are needed to warrant sustainable Fe
0 filters. Finally, locally manufactured candle Fe
0 filters could encourage the recycling of filter wastes.
8.4.2. Design Aspects
Using Fe
0 materials in domestic candle-type filters has been experimentally tested but not used in practice for the reasons elucidated herein (
Section 2). In essence, the n-Kolshi systems (
n = 2 or 3), largely tested in Bangladesh and Nepal, are candle Fe
0 filters as they can be regarded as “candles” whose hollow parts (here a Kolshi) are filled with Fe
0. For example, [
73] tested cast-iron filings and steel wool (SW) in domestic candle-type filters. Due to the large difference in density, the tested candle could contain 176 g of fillings and just 47 g of SW. As breakthrough (initial concentration: 300 μg/L) was observed after 6.0 L in the fillings filter and only 1.5 L in the SW filters (per run of intermittent filtration). The authors speculated that using three candles in series would enable the treatment of 18.0 and 4.5 L of water per filtration run, respectively. As demonstrated herein (see also [
56]), this speculation is not acceptable as the kinetics of Fe
0 corrosion is not linear. Moreover, the candles were completely filled with Fe
0 (100%), meaning that even with a supposedly linear rate of iron corrosion, permeability loss will soon occur and at different extents for individual materials [
80,
81]. This example demonstrates that only long-term systematic experiments will determine the feasibility of Fe
0 candle filters for water treatment at the household level. While it is certain that candle filters will be efficient, the main issue to address is the Fe
0 selection and its proportion in the candle. The service life of the candle is to be determined experimentally until enough data is available for the establishment of some correlations (e.g., long-term reactivity). The next section specifies some key issue for sustainable candle Fe
0 filters.
8.4.3. Sustainability Aspects
It is frustrating to observe that even recent reports are not unanimous as to whether candle-type Fe
0 filters are viable or not [
56,
69]. In essence, a newly designed Fe
0 filter can be compared to a highly permeable sand filter, which is characterized by a high level of interconnectivity between the inter-granular voids (pores). Once the corrosion process starts, the pores are progressively filled by iron corrosion products [
80,
81]. Thus, the effective porosity decreases because the proportion of voids in which water flows decreases. In other words, permeability loss in Fe
0 filters is fundamentally due to reduced interconnectivity. Unconnected pores are often called dead-end pores. The non-sustainability of conventional Fe
0 filters is thus due to increased dead-end pores [
28,
30,
80,
81].
Material particle size and shape, Fe
0 ratio, Fe
0 intrinsic reactivity, the height of the reactive zone (Fe
0 layer), and packing arrangements are among the factors that determine the occurrence of dead-end pores (
Section 2). In addition, the porosity of the admixing aggregates (e.g., gravel, MnO
2, pumice) also impacts the water flow. The large array of significant operational parameters impacting the efficiency and sustainability of Fe
0 filters and the absence of a systematic approach to assess the efficiency of such filters explain why, despite 17 years (from 2000 onwards) of intensive research, controversial reports are still published [
56,
69,
236]. To end this frustration, systematic investigations with well-characterized materials are needed [
237,
238].