Leaf–Air Temperature Difference as a Nondestructive Indicator of Waterlogging Tolerance in Cassava Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Plant Materials
2.2. Experimental Design
2.3. Data Collection
2.3.1. Microclimate Data
2.3.2. Leaf Gas Exchange Parameters
2.3.3. Chlorophyll Fluorescence (Fv/Fm) Measurement
2.3.4. Soil–Plant Analysis Development (SPAD)
2.3.5. Morphological Data
2.4. Statistical Analyses
3. Results
3.1. Growth Environments, Average Daily Temperature, Daily Percentage of Relative Humidity, Soil Temperature, Daily Soil Moisture Content, and Average Daily Light Intensity
3.2. Effect of Waterlogging Treatment on Leaf Temperature and ΔT
3.3. Effect of Waterlogging on Photosynthetic Traits (Pn, E, gs, and PWUE)
3.4. Effect of WL Treatment on Photosynthetic Traits (SPAD and Fv/Fm)
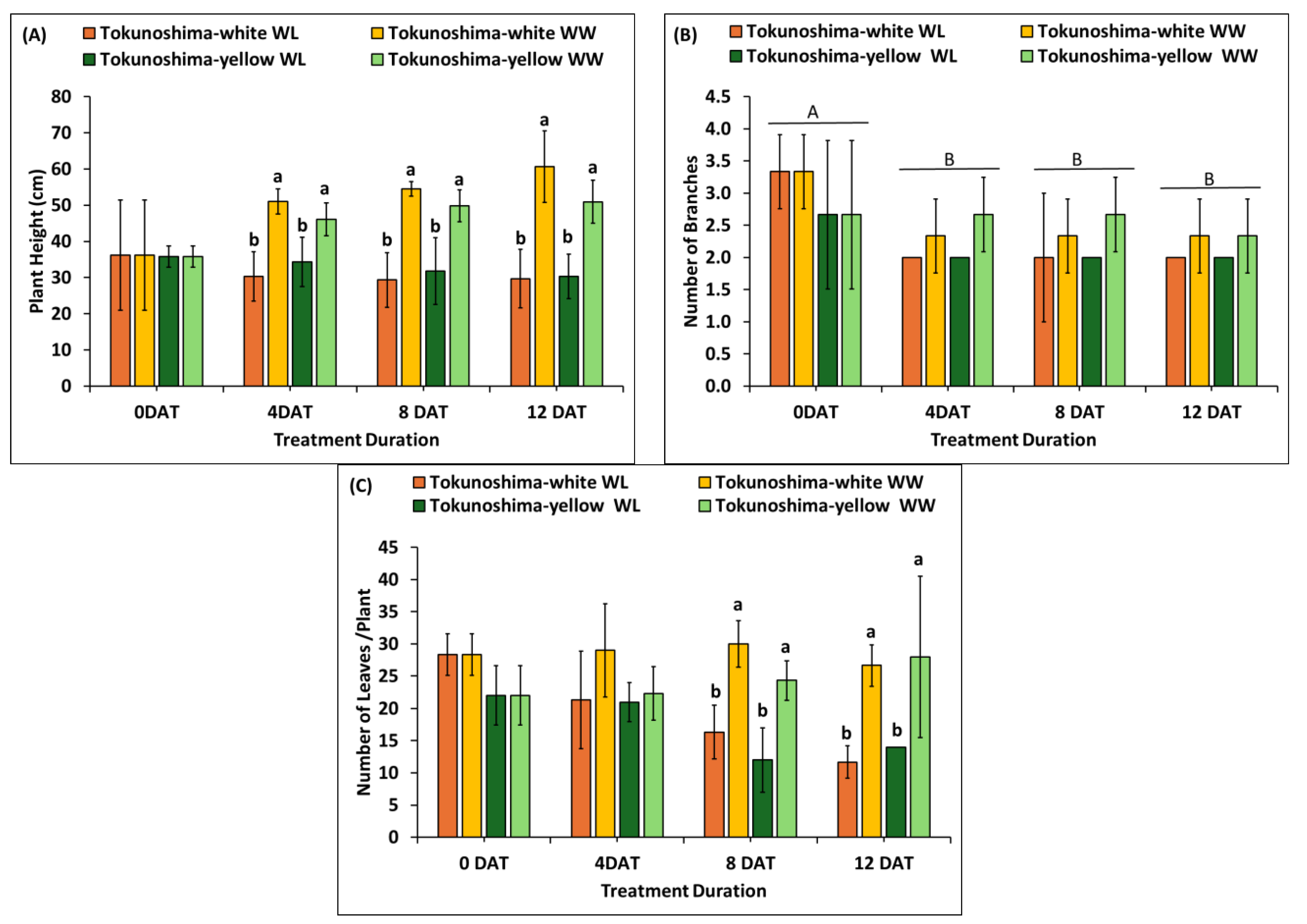
3.5. Effect of WL Treatment on Morphological Traits (Plant Height, Number of Branches, and Number of Leaves)
3.6. Relationships Between Morphological Parameters, Leaf Gas Exchange, and Photosynthetic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stromquist, N.P. World Development Report 2019: The Changing Nature of Work: By the World Bank; World Bank: Washington, DC, USA, 2019; 151p, ISBN 978-1-4648-1342-9. [Google Scholar] [CrossRef]
- Nguyen, T.L.T.; Gheewala, S.H.; Garivait, S. Full Chain Energy Analysis of Fuel Ethanol from Cassava in Thailand. Environ. Sci. Technol. 2007, 41, 4135–4142. [Google Scholar] [CrossRef] [PubMed]
- Carsky, R.; Walker, P.; Hauser, S.; Dashiell, K.; Dixon, A. Response of Selected Crop Associations to Groundwater Table Depth in an Inland Valley. Field Crop. Res. 1993, 34, 1–13. [Google Scholar] [CrossRef]
- Andriesse, W.; Fresco, L.O.; Dujvenbooden, N.V.; Windeijer, A.P.N. Multiscale Characterization of Inland Valley Agro-Ecosystems in West Africa. Neth. J. Agric. Sci. 1994, 42, 159–179. [Google Scholar]
- Ekanayake, I.J.; Dixon, A.G.O.; Asiedu, R.; Izac, A.-M.N. Improved-Cassava-for-Inland-Valley-Agro-Ecosystems. In Proceedings of the 5th Symposium of the International Society for Tropical Root Crops-African Branch (ISTR-AB), Kampala, Uganda, 22–28 November 1992. [Google Scholar]
- More, S.J.; Ravi, V.; Raju, S. The Quest for high Yielding Drought Tolerant Cassava Variety. J. Pharmacogn. Phytochem. 2020, SP6, 433–439. [Google Scholar]
- Zhai, P.; Pörtner, H.; Roberts, D.; Skea, J.; Shukla, P.; Pirani, A. Global Warming of 1.5 C. An IPCC Special Report on the impacts of global warming of 1.5 C above pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty. In Sustainable Development, and Efforts to Eradicate Poverty; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2018; p. 32. [Google Scholar]
- Ponnamperuma, F.N. Effects of Flooding on Soils. In Flooding and Plant Growth; Kozlowski, T.T., Ed.; Academic Press: New York, NY, USA, 1984; pp. 9–45. [Google Scholar]
- Koslowski, T.T.; Pallardy, S.G. Effects of Flooding on Water, Carbohydrate and Mineral Relations. In Flooding and Plant Growth; Koslowski, T.T., Ed.; Academic Press Inc.: Orlando, FL, USA, 1984. [Google Scholar]
- Voesenek, L.A.C.J.; Blom, C.W.P.M. Blom, Growth Responses of Rumex Species in Relation to Submergence and Ethylene. Plant Cell Environ. 1989, 12, 433–439. [Google Scholar] [CrossRef]
- Setter, T.L.; Waters, I.; Sharma, S.K.; Singh, K.N.; Kulshreshtha, N.; Yaduvanshi, N.P.S.; Ram, P.C.; Singh, B.N.; Rane, J.; McDonald, G.; et al. Review of Wheat Improvement for Waterlogging Tolerance in Australia and India: The Importance of Anaerobiosis and Element Toxicities Associated with Different Soils. Ann. Bot. 2009, 103, 221–235. [Google Scholar] [CrossRef]
- Popescu, M.; Popescu, G.C. Diurnal Changes in Leaf Photosynthesis and Relative Water Content of Grapevine. Curr. Trends Nat. Sci. 2014, 3, 74–81. [Google Scholar]
- Zhang, R.; Zhou, Y.; Yue, Z.; Chen, X.; Cao, X.; Ai, X.; Jiang, B.; Xing, Y. The Leaf-Air Temperature Difference Reflects the Variation in Water Status and Photosynthesis of Sorghum under Waterlogged Conditions. PLoS ONE 2019, 14, e0219209. [Google Scholar] [CrossRef]
- Feng, X.; Zhou, G. Relationship of Leaf Water Content with Photosynthesis and Soil Water Content in Summer Maize. Acta Ecol. Sin. 2018, 38, 177–185. [Google Scholar] [CrossRef]
- Giorio, P.; Sorrentino, G.; D’andria, R. Stomatal Behaviour, Leaf Water Status and Photosynthetic Response in Field-Grown Olive Trees Under Water Deficit. Environ. Exp. Bot. 1999, 42, 95–104. [Google Scholar] [CrossRef]
- Egilla, J.N.; Davies, F.T., Jr.; Boutton, T.W. Drought Stress Influences Leaf Water Content, Photosynthesis, and Water-Use Efficiency of Hibiscus Rosa-Sinensis at Three Potassium Concentrations. Photosynthetica 2005, 43, 135–140. [Google Scholar] [CrossRef]
- Aquilino, L.; Nayatami, K.L.; Tamu, A.; Soe, I.; Sakagami, J.I. Photosynthetic Function Analysis under Rhizosphere Anaerobic Conditions in Early-Stage Cassava. Photosynth. Res. 2025, 163, 42. [Google Scholar] [CrossRef]
- Blum, A.; Mayer, J.; Gozlan, G. Infrared Thermal Sensing of Plant Canopies as a Screening Technique for Dehydration Avoidance in Wheat. Field Crop. Res. 1982, 5, 137–146. [Google Scholar] [CrossRef]
- Brahmesh, R.B.; Kiran, B.O.; Somanagouda, B.P.; Ashwathama, V.H. Canopy Temperature in Sorghum under Drought Stress: Influence of Gas-Exchange Parameters. J. Cereal Res. 2022, 14, 81–85. [Google Scholar] [CrossRef]
- Leopold, A.C.; Sun, W.Q.; Bernal-Lugo, I. The Glassy State in Seeds: Analysis and Function. Seed Sci. Res. 1994, 4, 267–274. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, S.; Pan, Y.; Zhang, L.; Lin, S.; Xue, Y. Diurnal Variation in Leaf-air Temperature Difference and Leaf Temperature Difference and the Hybrid Difference in Maize under Different Drought Stress. J. China Agric. Univ. 2014, 19, 13–21. [Google Scholar] [CrossRef]
- Dhıllon, R.; Rojo, F.; Roach, J.; Upadhyaya, S.; Delwıche, M.A. A Continuous Leaf Monitoring System for Precision Irrigation Management in Orchard Crops. Tarım Makinaları Bilim. Derg. 2014, 10, 267–272. [Google Scholar]
- Rajarajan, K.; Ganesamurthy, K.; Raveendran, M.; Jeyakumar, P.; Yuvaraja, A.; Sampath, P.; Prathima, P.T.; Senthilraja, C. Differential Responses of Sorghum Genotypes to Drought Stress Revealed by Physio-Chemical and Transcriptional Analysis. Mol. Biol. Rep. 2021, 48, 2453–2462. [Google Scholar] [CrossRef] [PubMed]
- Pineda, M.; Barón, M.; Pérez-Bueno, M.-L. Thermal Imaging for Plant Stress Detection and Phenotyping. Remote. Sens. 2020, 13, 68. [Google Scholar] [CrossRef]
- Jones, H.G. Use of Thermography for Quantitative Studies of Spatial and Temporal Variation of Stomatal Conductance over Leaf Surfaces. Plant Cell Environ. 1999, 22, 1043–1055. [Google Scholar] [CrossRef]
- Anderegg, J.; Aasen, H.; Perich, G.; Roth, L.; Walter, A.; Hund, A. Temporal Trends in Canopy Temperature and Greenness are Potential Indicators of Late-Season Drought Avoidance and Functional Stay-Green in Wheat. Field Crop. Res. 2021, 274, 108311. [Google Scholar] [CrossRef]
- Pradhan, A.; Aher, L.; Hegde, V.; Jangid, K.K.; Rane, J. Cooler Canopy Leverages Sorghum Adaptation to Drought and Heat Stress. Sci. Rep. 2022, 12, 4603. [Google Scholar] [CrossRef] [PubMed]
- Balota, M.; Payne, W.A.; Evett, S.R.; Peters, T.R. Morphological and Physiological Traits Associated with Canopy Temperature Depression in Three Closely Related Wheat Lines. Crop. Sci. 2008, 48, 1897–1910. [Google Scholar] [CrossRef]
- Ginkel, M.V.; Reynolds, M.P.; Trethowan, R.; Hernandez, E. (Eds.) Complementing the Breeder’s Eye with Canopy Temperature Measurements. In International Symposium on Wheat Yield Potential: Challenges to International Wheat Breeding; International Maize and Wheat Improvement Center: El Batán, Mexico, 2006. [Google Scholar]
- Yabuta, S.; Fukuta, T.; Tamaru, S.; Goto, K. The Productivity of Cassava (Manihot esculenta Crantz) in Kagoshima, Japan, Which Belongs to the Temperate Zone. Agronomy 2021, 11, 2021. [Google Scholar] [CrossRef]
- Jones, H.G. Use of infrared thermometry for estimation of stomatal conductance as apossible aid to irrigation scheduling. Agric. For. Meteorol. 1999, 95, 139–149. [Google Scholar] [CrossRef]
- Huang, C.; Gao, Y.; Qin, A.; Liu, Z.; Zhao, B.; Ning, D.; Ma, S.; Duan, A.; Liu, Z. Effects of waterlogging at different stages and durations on maize growth and grain yields. Agric. Water Manag. 2022, 261, 107334. [Google Scholar] [CrossRef]
- Alves, A.A.C.; Setter, T.L. Response of cassava leaf area expansion to water deficit: Cell proliferation, cell expansion and delayed development. Ann. Bot. 2004, 94, 605–613. [Google Scholar] [CrossRef]
- Ledent, J. Deficit Hidrico y Crecimiento de las Plantas: Respuestas al Deficit Hidrico: Comportamiento Morfofisiologico/Modelado Del Crecimiento De Las Plantas; Fundacion PROINPA: Cochabamba, Bolivia; Centro Internacional de la Papa (CIP): Lima, Peru; Proyecto Papa Andina: Lima, Peru, 2002; 79p, Available online: https://agris.fao.org/search/en/providers/122619/records/647248dd53aa8c896304ef1b (accessed on 15 November 2008).
- Suárez, L.; Mederos, V. Apuntes sobre el cultivo de la yuca (Manihot esculenta Crantz). Tendencias actuales. Cultiv. Trop. 2011, 32, 27–35. [Google Scholar]
- León, P.R.; Pérez Macias, M.; Gutiérrez Trocel, M.; Rodríguez Izquierdo, A.; Fuenmayor Campos, F.; Marín Rodriguez, C. Caracterización ecofisiológica de cuatro clones de yuca del banco de germoplasma del INIA-CENIAP. Agron. Trop. 2014, 64, 97–105. [Google Scholar]
- Vandegeer, R.; Miller, R.E.; Bain, M.; Gleadow, R.M.; Cavagnaro, T.R. Drought Adversely Affects Tuber Development and Nutritional Quality of the Staple Crop Cassava (Manihot Esculenta Crantz). Funct. Plant Biol. 2012, 40, 195–200. [Google Scholar] [CrossRef]
- Aina, O.O.; Dixon, A.G.O.; Akinrinde, E.A. Effect of Soil Moisture Stress on Growth and Yield of Cassava in Nigeria. Pak. J. Biol. Sci. 2007, 10, 3085–3090. [Google Scholar] [CrossRef]
- Cruz, J.; Mosquim, P.; Pelacani, C.; Araújo, W.; DaMatta, F. Photosynthesis Impairment in Cassava Leaves in Response to Nitrogen Deficiency. Plant Soil 2003, 257, 417–423. [Google Scholar] [CrossRef]
- Long, S.; Humphries, S.; Falkowski, P.G. Photoinhibition of photosynthesis innature. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1994, 45, 633–662. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: A Review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef]
- Chang, W.W.; Huang, L.; Shen, M.; Webster, C.; Burlingame, A.L.; Roberts, J.K. Patterns of Protein Synthesis and Tolerance of Anoxia in Root Tips of Maize Seedlings Acclimated to a Low-Oxygen Environment, and Identification of Proteins by Mass Spectrometry. Plant Physiol. 2000, 122, 295–317. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cao, M.; Zheng, L.; Li, J.; Mao, Y. Transcriptomic Profiling Suggests Candidate Molecular Responses to Waterlogging in Cassava. PLoS ONE 2022, 17, e0261086. [Google Scholar]
- Pan, R.; Jiang, W.; Wang, Q.; Xu, L.; Shabala, S.; Zhang, W.Y. Differential Response of Growth and Photosynthesis in Diverse Cotton Genotypes under Hypoxia Stress. Photosynthetica 2019, 57, 772–779. [Google Scholar] [CrossRef]
- Utsumi, Y.; Utsumi, C.; Tanaka, M.; Van Ha, C.; Takahashi, S.; Matsui, A.; Matsunaga, T.M.; Matsunaga, S.; Kanno, Y.; Seo, M.; et al. Acetic Acid Treatment Enhances Drought Avoidance in Cassava (Manihot esculenta Crantz). Front. Plant Sci. 2019, 10, 521. [Google Scholar] [CrossRef]
- Surendar, K.K.; Devi, D.D.; Ravi, I.; Jeyakumar, P.; Velayudham, K. Effect of Water Stress on Leaf Temperature, Transpiration Rate, Stomatal Diffusive Resistance and Yield of Banana. Plant Gene Trait. 2013, 4, 43–47. [Google Scholar] [CrossRef]
- Siddique, M.R.B.; Hamid, A.; Islam, M.S. Drought stress effects on water relations of wheat. Bot. Bull. Acad. Sin. 2000, 41, 35–39. [Google Scholar]
- Nelson, J.; Bugbee, B. Analysis of Environmental Effects on Leaf Temperature under Sunlight, High Pressure Sodium and Light Emitting Diodes. PLoS ONE 2015, 10, e0138930. [Google Scholar] [CrossRef]
- Ahmed, S.; Nawata, E.; Hosokawa, M.; Domae, Y.; Sakuratani, T. Alterations in Photosynthesis and Some Antioxidant Enzymatic Activities of Mungbean Subjected to Waterlogging. Plant Sci. 2002, 163, 117–123. [Google Scholar] [CrossRef]
- Else, M.A.; Tiekstra, A.E.; Croker, S.J.; Davies, W.J.; Jackson, M.B. Stomatal Closure in Flooded Tomato Plants lnvolves Abscisic Acid and a Chemically Unidentified Anti-Transpirant in Xylem Sap. Plant Physiol. 1996, 112, 239–247. [Google Scholar] [CrossRef]
- Zheng, C.; Jiang, D.; Liu, F.; Dai, T.; Jing, Q.; Cao, W. Changes in Photosynthesis, Chloroplast Ultrastructure, and Antioxidant Metabolism in Leaves of Sorghum under Waterlogging Stress. Photosynthetica 2019, 57, 1076–1083. [Google Scholar] [CrossRef]
- Zheng, C.; Jiang, D.; Liu, F.; Dai, T.; Jing, Q.; Cao, W. Effects of Salt and Waterlogging Stresses and their Combination on Leaf Photosynthesis, Chloroplast ATP Synthesis, and Antioxidant Capacity in Wheat. Plant Sci. 2009, 176, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Srivastava, J.P. Effect of Waterlogging on Photosynthetic and Biochemical Parameters in Pigeonpea. Russ. J. Plant Physiol. 2015, 62, 322–327. [Google Scholar] [CrossRef]
- Kawamitsu, Y. Effects of Nitrogen Supply on Growth Characteristics and Leaf Photosynthesis in Sugarcane. Sci Bull. Coll. Agr. Univ. Ryukyus 1999, 46, 1–14. [Google Scholar]
- Djanaguiraman, M.; Prasad, P.V.V.; Boyle, D.L.; Schapaugh, W.T. High-Temperature Stress and Soybean Leaves: Leaf Anatomy and Photosynthesis. Crop. Sci. 2011, 51, 2125–2131. [Google Scholar] [CrossRef]
- Luan, H.; Guo, B.; Pan, Y.; Lv, C.; Shen, H.; Xu, R. Morpho-Anatomical and Physiological Responses to Waterlogging Stress in Different Barley (Hordeum vulgare L.) Genotypes. Plant Growth Regul. 2018, 85, 399–409. [Google Scholar] [CrossRef]
- Santos, C.M.D.; Endres, L.; Ferreira, V.M.; Silva, J.V.; Rolim, E.V.; Filho, H.C.W. Photosynthetic Capacity and Water Use Efficiency in Ricinus communis (L.) under Drought Stress in Semi-Humid and Semi-Arid Areas. Acad. Bras. Cienc. 2017, 89, 3015–3029. [Google Scholar] [CrossRef] [PubMed]





| Parameters | Cultivars (C) | Treatment (Trt) | Treatment Duration (TrtD) | C × Trt | TrtD × Trt |
|---|---|---|---|---|---|
| DF | 1 | 1 | 3 | 1 | 3 |
| Plant height | 0.66 ns | 47.68 *** | 1.76 ns | 1.84 ns | 6.04 ** |
| Number of leaves | 4.66 * | 28.21 *** | 2.50 ns | 0.52 ns | 5.27 ** |
| Number of branches | 0.5 ns | 3.68 ns | 3.68 * | 0.41 ns | 0.41 ns |
| SPAD Value | 42.74 *** | 4.86 * | 4.55 ** | 0.51 ns | 0.65 ns |
| Fv/Fm Values | 0.91 ns | 20.26 *** | 3.66 * | 0.07 ns | 2.86 * |
| Photosynthetic rate | 0.92 ns | 139.08 *** | 26.27 *** | 0.16 ns | 16.41 *** |
| Stomatal conductance | 0.59 ns | 38.82 *** | 9.24 *** | 0.30 ns | 5.212 *** |
| Transpiration rate | 0.73 ns | 83.89 *** | 15.41 *** | 0.01 ns | 9.61 ** |
| Intercellular CO2 concentration | 0.54 ns | 2.86 ns | 3.57 * | 0.44 ns | 6.42 ** |
| PWUE | 2.56 ns | 1.60 ns | 5.33 ** | 1.64 ns | 7.22 *** |
| Leaf temperature | 0.02 ns | 30.98 *** | 66.13 *** | 0.94 ns | 4.12 ** |
| ΔT | 0.10 ns | 29.09 *** | 43.34 *** | 0.68 ns | 3.97 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Aquilino, L.; Naito, T.; Tamu, A.; Ssenyonga, P.; Chepkoech, R.; Soe, I.; Sakagami, J.-I. Leaf–Air Temperature Difference as a Nondestructive Indicator of Waterlogging Tolerance in Cassava Genotypes. Sustainability 2026, 18, 405. https://doi.org/10.3390/su18010405
Aquilino L, Naito T, Tamu A, Ssenyonga P, Chepkoech R, Soe I, Sakagami J-I. Leaf–Air Temperature Difference as a Nondestructive Indicator of Waterlogging Tolerance in Cassava Genotypes. Sustainability. 2026; 18(1):405. https://doi.org/10.3390/su18010405
Chicago/Turabian StyleAquilino, Lado, Ten Naito, Alex Tamu, Peter Ssenyonga, Rael Chepkoech, Ibrahim Soe, and Jun-Ichi Sakagami. 2026. "Leaf–Air Temperature Difference as a Nondestructive Indicator of Waterlogging Tolerance in Cassava Genotypes" Sustainability 18, no. 1: 405. https://doi.org/10.3390/su18010405
APA StyleAquilino, L., Naito, T., Tamu, A., Ssenyonga, P., Chepkoech, R., Soe, I., & Sakagami, J.-I. (2026). Leaf–Air Temperature Difference as a Nondestructive Indicator of Waterlogging Tolerance in Cassava Genotypes. Sustainability, 18(1), 405. https://doi.org/10.3390/su18010405








