Abstract
The production of hydraulic binders, representing the essential constituent part of concrete and mortar, can be associated with high energy consumption and huge CO2 emissions (at least 2.4 billion tons in 2022). Without appropriate measures, the situation will only worsen. The global annual output of cement stood at 4.4 billion tons of cement, whereas the annual production has been increasing at a rate of ca 5%. In order to significantly reduce CO2 emissions, the following solutions are most widely used in the world: clinker additives; unconventional fuels; decreased energy-related expenses; and technological innovations. However, these are not sufficient to cut down on greenhouse gas emissions and bring them close to zero. Therefore, the utilization and development of alternative binders denoted by a reduced CO2 footprint in comparison to that of conventional cement are among the main objectives of building materials manufacturers as well as researchers. This paper reviews obstacles, solutions and alternatives for the fabrication of hydraulic cementitious materials, along with the general principles of the carbonization of binders, such as natural processes and intensified processes, the impact of various parameters on the chemical and physical transformations, as well as the mechanism of interaction of OPC, belite, and blended cement with CO2. The production of low-lime binders, along with time-optimized carbonation, can significantly improve carbon footprint values. However, due to the huge variety of blended cements, their hardening process by mineral carbonation needs to be investigated extensively and systematically, as it is emphatically dependent on many numerical values and criteria. Environmentally and economically acceptable production can only be achieved on the grounds of the optimized parameters of the entire process.
1. Introduction
Concrete is the number two item used worldwide by demand after water in terms of overall consumption. Concrete based on Ordinary Portland cement (OPC) represents the most extensively produced building material in the world [1]; it was manufactured at an estimated rate of 4.3 billion tons in 2023 [2,3]. This type of binder material is the most commonly employed in concrete for construction due to its longevity, efficient usage of natural resources, inherent strength, and relatively low cost. Because of the extensive, as well as ever-increasing demand, for building materials, the cement industry constitutes approximately 5–7% of human-triggered greenhouse gas emissions worldwide; when producing a single ton of cement, roughly the same amount of carbon dioxide is emitted into the atmosphere. The Global Cement and Concrete Association GCCA 2050 Cement and Concrete Industry Roadmap for Net Zero Concrete sets out a net-zero pathway to help limit global warming to 1.5 °C. The sector is committed to producing net-zero concrete by 2050 and is committed to acting now. It outlines a proportionate reduction in CO2 emissions of 25% associated with concrete by 2030 from 2020 as a key milestone on the way to achieving full decarbonization by the mid-century [4]. All stages of OPC manufacturing have an adverse effect on the environment, particularly in terms of high energy consumption and air pollution. The carbon footprint of cement plants totals 5–7% of global anthropogenic CO2 emissions, while ~0.8–1 t of CO2 is emitted in the course of producing one ton of cement [5,6]. Furthermore, in terms of electricity, ca. 110 kWh is required to obtain a ton of cement. The lion’s share of this overwhelming amount of energy pertains to the thermal treatment of the clinker [7]. Although cement production technology has been systematically and prominently improving to conform to cement quality and quantity requirements, to enhance the fuel efficiency and mitigate the extent of pollution, it is still important to pursue alternatives that allow us to diminish the perilous impact of OPC manufacturing in order to pursue the objective of sustainable development [8]. Therefore, academics and cement manufacturers, are pursuing innovative technologies and strategies that could involve, as follows: the usage of industrial waste as a source material in the manufacturing of Portland cement and the substitution of conventional fuels with alternative options [9]; the creation and implementation of advanced engineering solutions; and the development of alternative cementitious products. In this context, the scientific community has been struggling to find an efficient solution for greenhouse gas mitigation and take (over)due steps towards reducing the devastating effects of cement manufacturing. Although many solutions have recently been offered with the objective of mitigating the perilous effects of cement production on our planet, the most recent research has demonstrated that directions and solutions, such as clinker substitutions, non-fossil fuels, and/or enhanced energetic efficiency, on their own cannot help to achieve the required carbon footprint reductions. Therefore, the development of innovative cementitious materials featuring a reduced CO2 contribution compared to that of ordinary cement is among the most fundamental and urgent targets for the construction materials producers as well as for scholars, innovators and visionaries in this field.
Many scientists around the world are working to accelerate and scale up the practical implementation of carbon capture and utilization technologies through their fundamental and applied research. These efforts are summarized in a number of review articles published over the past five years. Cavalett et al. [10] assessed 15 decarbonization options for the European cement industry under current and future conditions. Solutions, such as alternative fuels or technological innovations, reduce the climate impact by up to 30%, while additional measures could reduce CO2 emissions by around 50% by 2050. Only rapid and large-scale implementation of carbon capture and storage can bring the industry closer to climate neutrality. Van Roijen et al. [11] present both the global magnitude of cement carbonation and its dynamic impact and show that slow carbonation rates cause climate change that is about 60% less than the benefits obtained. They examine the sensitivity of conventional decarbonization strategies, such as the use of supplementary cementitious materials (SCMs), to the carbonation process, and emphasize the importance of the timing of emissions and their disposal. Hu et al. [12] reviewed the historical development of, and presented some perspectives on, carbonate binders. Their properties are strongly influenced by crystalline polymorphs (calcite, aragonite and vaterite) and the degree of polymerization of amorphous silica gel. The authors discussed the physical, chemical, and biological methods proposed over the past 4 years to control the polymorphic content of CaCO3 and concluded that it is urgent to increase the degree of carbonation and regulate the composition and structure of binders to improve the properties of carbonized construction products. According to Von Greve-Dierfeld et al. [13], the carbonation of concrete with SCMs differs from the carbonation of concrete based solely on OPC. This is a consequence of differences in hydrate phase assembly and pore solution chemistry, as well as pore structure and transport properties, when the composition, age, and curing conditions of the concrete binder are changed. Their main conclusion is that blended cement is the most suitable means to reduce CO2 emissions associated with concrete production. Zajac et al. [14] focused their review on the application of carbonation technologies to evaluate the following industrial material streams: recycled concrete paste; steel slag; and fly ash. They discussed various carbonation technologies together with the related reaction mechanisms of the respective phase sets. They showed that carbonated materials, as pozzolanic additives, are well suited for the production of construction products. Forced carbonization of industrial products can be performed at ambient temperatures and pressures while achieving a high degree of CO2 sequestration. It is also very important to choose an appropriate and reliable methodology. According to Merah et al. [15], the accuracy of accelerated carbonation tests is affected by a variety of factors, including CO2 concentration, humidity, temperature, cement and aggregate type, setting time, and the duration of CO2 exposure. These factors must be taken into account to reliably assess the durability of concrete and to prevent corrosion. Karimi et al. [16] categorized the factors influencing cement carbonation and examined the mathematical models of this process proposed by different scientists, aiming to investigate the most important parameters and the accuracy of their predictions. You et al. [17] reviewed the known empirical, thermodynamic, kinetic and numerical models that describe the carbonization process of cementitious materials, and Wang et al. [18] provides a comprehensive review of existing models for predicting the depth of carbonation in concrete. Medvedev et al. [19] reviewed information on the course of the carbonation process under irradiation conditions, as well as the causes, dynamics, and mechanisms of carbonization and corrosion processes occurring in the reinforced concrete used in nuclear power plants.
On the grounds of the literature analysis data, it is evident that OPC production inflicts a major damaging effect on the environment; critical changes need to be implemented to reduce this effect. Even though abundant suggestions have been presented as to how to achieve lower greenhouse gas emissions, only a small fraction of these have an actual significant ability to achieve the desired outcomes. The list of possible solutions includes the manufacturing of blended cement and low-carbon cement binder materials, which, together with accelerated CO2 carbonation, can dramatically cut down on the generated carbon footprint and thus reduce the adverse effects of OPC manufacturing on the environment. However, due to the huge variety of blended cements, their hardening process by mineral carbonation demands careful and systematic investigation, as the process itself highly relies on an extensive list of factors and parameters, all of which need to be considered to obtain both ecologically and economically viable manufacturing processes.
Among the highly successful alternatives to OPC, blended cements can be singled out [20,21]. The production processes of these cements are more sustainable and their use improves the performance of concrete. More blended cements are already produced worldwide (by volume) than OPC; however, the mechanism of accelerated hardening of this binder in a CO2 environment, and the characteristics of the obtained products, have been less studied in the literature.
The aim of this work is to examine and summarize the studies conducted in recent decades on the carbonization mechanism of calcium silicate-based binders, to discuss and evaluate the main factors that influence the kinetics of this process (reactivity, diffusivity and pressure of CO2; relative humidity and temperature of the environment) and to discuss the carbonation features of the main types of calcium silicate cements.
2. Blended Cements: Main Types, Advantages, Solutions and Alternatives
2.1. Main Types and Their Advantages
The global blended cement market size was USD 306,870 million in 2022. Judging by the current trends, an increase of nearly 40 percent should be expected by 2030, with a year-on-year increase of 3.9% within the discussed timeframe [22].
Categories of blended cement are singled out by the type of blending material in cement. The main types of blended cement are:
- Binary cements. Derived by mixing OPC clinker with one supplementary cementitious material (SCM), e.g., fly ash, slag, microsilica [23,24], or geopolymers [25]. The addition of additives not only reduces the required amount of clinker in the mixture, it enhances the robustness and mechanical properties of the obtained concrete [26]. The main sub-types include:
- ○
- Portland–slag cement. Blends with up to 70% slag cement serve well in the field of general construction;
- ○
- Portland–pozzolan cement is beneficial in general construction. This amalgam may constitute up to 50% pozzolan;
- ○
- Portland–limestone cement. It features between 5% and 15% percent inter-ground limestone.
- Ternary blended cement. Ternary cements are blends of two supplementary cementitious materials such as fly ash, slag cement, or silica fume [27]. For example, by adding fly ash and microsilica to OPC clinker, we boost the compressive strength of the material and reduce its permeability [28,29].
- Alkali-activated cements. These cements are produced by activating aluminosilicate materials with alkalis [30,31].
A number of advantages of blended cements can be listed:
- Reduced CO2 emissions. The replacement of part of Portland cement clinker in the mix with cementitious supplements potentially offers a significant reduction in carbon dioxide emissions. Waste from cement production is also recycled, which is a highly beneficial bonus [28].
- Increased durability of concrete. Blended cements increase the durability of concrete, as the SCM in cements reduces shrinkage and permeability while increasing chemical resistance. During pozzolanic reactions, these materials contribute to the formation of calcium silicate hydrate (CSH). This strengthens the concrete matrix. This property is particularly important in marine or aggressive chemical industrial environments [32].
- Economic benefits are obtained. Replacing part of the Portland cement clinker with supplementary cementitious materials yields a reduction in the required amount of clinker in the mix while maintaining or even improving the properties of the concrete. In addition, the employment of manufacturing waste is aligned with the principles of the circular economy [33].
However, disadvantages of blended cements must be noted:
- Inconsistency in the properties of supplementary cementitious materials. The variability of the characteristics of the additional materials in use significantly impacts the properties of blended cements. The particle size, chemical composition, and reactivity determine the hydration and strength of the cement. Therefore, no SCM deviations are possible and strict standards must be followed [34].
- Hydration. Different materials are denoted by different degrees of hydration, making it quite difficult to achieve the desired setting time and strength gain [35].
2.2. Solutions and Alternatives
The main methods of reducing CO2 emissions currently used in the cement industry were described in detail by Ige, Von Kallon and Desai in their review published in 2024 (Table 1) [36].

Table 1.
The main methods of reducing CO2 emissions.
The reuse of manufacturing waste is perceived as one feasible solution to scale down the ever-increasing amounts of manufacturing by-products. Due to their inert nature, an extensive list of waste materials can be introduced into the concrete mixture as SCM [37]. Such types of waste include: granulated ground blast furnace slag [38]; fly ash [39]; silica fume [40,41]; palm oil fuel ash [42,43,44]; foundry waste [45,46,47], fibers from pre- and post-consumer wastes [42,48]; and agricultural wastes (including rice husk ash, wheat straw ash, sugarcane bagasse ash, hazel nutshell ashes, etc. [49]). Up to 30% of raw materials can be replaced with waste without a significant negative influence on the properties of concrete [50,51]. Industrial waste utilization in concrete offers sustainable, green, and environmentally friendly construction materials while also reducing the price of the components.
Roughly 50 percent of the carbon footprint triggered by OPC clinker burning stems from fossil fuel combustion. The availability of a low-carbon fuel denoted by an elevated hydrogen-to-carbon (H/C) ratio as a replacement for traditional carbon-based fuels offers a remarkable reduction in the rate of CO2 emissions [52]. Potential alternative materials include: vegetable compounds or natural products (oil shale, peat, tree bark, sawdust, waste wood and other wood-processing waste, etc.); synthetic products (discarded tires, rubber waste, waste plastics, etc.); and other sources (parts of shredded cars, fuels derived from rejects, household garbage, etc.) [53]. The blending of different fuels with fossil fuels offers a plethora of economic, environmental, and chemical benefits. However, there are many factors to be considered before opting for alternative fuels. Such factors include: the share of moisture and ash; the calorific value; the volatile content; reactivity; potential threats of ambient air contamination; slagging and fouling; and corrosion [54,55]. A reduction in fuel and energy use constitutes a valid path towards cutting down on the rate of depletion of non-renewable fuel resources.
With regards to engineering solutions, three major directions in cement manufacturing are being practiced worldwide: wet; semi-wet; and dry processes. The wet process uses raw materials typically containing 30–40% of moisture. Meanwhile, the semi-wet pathway seeks to eliminate 10–20% of H2O before the feeding of raw materials to the kiln by preheating them on a conveyor. Savings of 50% of the currently required amount of energy may potentially be achieved; by shifting to the dry process, a reduction in CO2 emissions can be targeted [4]. Other solutions to reduce energy losses include the application of insulation layers, proper sealing methods, heat recovery from exhaust streams, and the implementation of innovative design(s) for pyro-processing units in cement factories. In the latter case, decomposition reactions are separated from other reactions. Consequently, pure CO2 is obtained. As a result of such modifications, this technological innovation may lower CO2 emissions by as much as 66%; in addition, a 2.3% reduction in energy requirements is achieved compared to the conventional process, which, in absolute numbers, represents a positive impact of immense proportions [56]. In terms of heat recovery, up to 20% of the fuel can be saved; consequently, an 8% reduction in CO2 emissions can be achieved. Even though the OPC clinker production technology is rather well developed, there is still some scope for further technological improvements of the current state-of-the-art practices and hardware. Synthesis reactions are boosted with increased contact surfaces of the substances participating in the reaction. Compaction also has an impact on the free lime content, which subsequently exerts a major impact on the clinker produced. Free CaO content within a range of 1–1.5% leads to optimized energy consumption while maintaining maximum kiln reactivity, improved fuel consumption; this ultimately leads to a decrease in CO2 emissions [57].
An additional direction towards achieving lower CO2 emissions and energy consumption is the reduction of the lime saturation factor of the raw mixture. This may lead to an increase in the belite amount and a decrease in the alite phase content in the clinker. When the raw mixture CaCO3 content is reduced, the energy requirements fall by 15–20% as well [58]; CO2 emissions are also lowered by 20–30% [59]. However, in the initial hardening stage, the mechanical strength of belite cement is emphatically low because the belite phase is hydrated very slowly. The weakness is avoidable by stabilizing hydraulically reactive forms of belite and its chemical activation [59,60,61].
Another way to decrease the cement CaO/SiO2 (C/S) molar ratio is the use of low-lime calcium silicates such as rankinite (3CaO·2SiO2 or C3S2) [62,63,64,65,66,67,68], wollastonite, pseudo-wollastonite (CaO·SiO2 or CS) [62,68,69,70,71,72], α-C2SH, and kilhoanite [73,74]. However, it should be noted that the synthesis of these calcium silicates from natural rocks requires careful investigation in each particular case, as impurities in the raw materials can change the course of the synthesis. The mineral composition and properties of the resulting products must also be carefully considered [75].
These calcium silicates require only more than half the amount of limestone is needed compared to OPC; as a result, the reduced calcination requirements lead to the curtailing of CO2 emissions during the process of calcination [76]. However, unlike conventional hydraulic calcium silicates, the aforementioned silicates are non-hydraulic binders; therefore, carbonation curing can serve fairly well to readily activate and harden them. A notable innovation contributing to this carbonated binder system is a cement commonly referred to as “calcium silicate-based cement (CSC)”, which has been commercialized for roughly a decade [63,76].
Owing to this, a highly relevant alternative that can significantly reduce CO2 emissions is the capture, storage, and sequestration of CO2 emissions in concrete materials [77]. Among the methods of storage that have been considered as feasible, geological storage, ocean storage, and CO2 mineralization (i.e., mineral carbonation) [78,79,80,81] should be mentioned. The method with the greatest potential for the cement industry is mineral carbonation, a process in which CO2 is chemically stored in solid inorganic carbonates by carbonation reactions of alkaline materials containing calcium oxides and silicates [79]. Both natural and accelerated processes take place in this scheme. Among the major consequences of the carbonation reaction of concrete is the lowering of the pH value in the pore solution [82]. Therefore, conventionally, the carbonation reaction of concrete is perceived as unfavorable as it compromises the robustness of the materials [83]. Moreover, this process is also prohibitively slow due to the low CO2 concentration in the atmosphere. Nevertheless, concrete carbonation contributes positively to the mechanical properties of the material. It is known that concrete can be treated with carbonation curing at an early age for accelerated strength gain and an enhanced robustness [84,85]. OPC concrete carbonation has received significant attention by theoretical and field researchers [86,87,88,89,90,91,92,93,94].
Even though low CO2 carbonated binders have been gaining major interest in recent years, only a few have already reached the manufacturing stage and become commercially available, including: Solidia cement [63]; a carbonated concrete named CO2-SUICOM (which, notably, can achieve CO2 emissions levels below zero [95]); Calera calcium carbonate [96]; and Novacem magnesium silicate [97].
3. General Principles of Carbonation of Cementitious Materials
3.1. Natural vs. Accelerated
Concrete and other cementitious materials absorb CO2 in the course of a natural process, commonly referred to as mineral carbonation, which leads to permanent CO2 sequestration in these materials. A fraction (under one-fifth) of CO2 generated during the manufacturing of cement is reabsorbed by concrete in the course of its lifecycle. However, natural carbonation in concrete is impractically slow—ca. 1 mm per year—because the atmospheric CO2 concentration is inherently low (400 ppm, or 0.04%) [83]. Moreover, in this case, the mass of CO2 dissolved in the pore water is minor due to physical restrictions imposed by the poor solubility of atmospheric CO2 in water [98,99] as well as the slowly proceeding diffusion of CO2 into the structure of mortar [83].
Carbonation is a diffusion process either by CO2 or by carbonate ions. It is controlled by water saturation in the capillary system [89]. Over time, the carbonation rate decreases since CO2 faces the requirement of crossing a thickening layer of its alteration products. The transportation tempo is principally determined by concentration gradients, porosity, and thickness of the previously formed layer of carbonation products [89]. On the other hand, the carbonation rates of sped-up carbonation are significantly higher than those of natural carbonation. The elevated rates are achieved by a higher CO2 concentration and controlled environment; in this context, humidity is a key criterion contributing to the progress of the carbonation front as well as to the mass of CO2 combined.
Naturally, concrete is carbonated when atmospheric carbon dioxide reacts with the alkaline components of concrete, primarily, portlandite, and/or CSH gel; as a result, CaCO3 is obtained. As a result of this reaction, the pH value of the aqueous phase of the concrete pores generally decreases, i.e., it transitions from elevated alkaline values above 12 to moderate values not exceeding 8 [69]. If the reaction occurs at the depth of the steel reinforcement, its passive layer may be compromised, thereby exposing the steel surface to corrosion. Therefore, specifically regarding concrete, carbonation is largely perceived as a process leading to undesirable deterioration. The perilous effect of atmospheric CO2 on modern concretes and mortars is known as ‘weathering carbonation’ [100]. Another important consequence of carbonation is a change in the effective permeability due to volume changes and microcracking caused by the chemical reactions. The permeability change is twofold: it is either an increase, for example, when concrete contains blast furnace slag or fly ash, or else a decrease, as in OPC [101]. Accelerated carbonation, on the other hand, may serve to ensure an accelerated strength gain and the improved durability of the concrete products. Among other differences, researchers highlight that the reaction products formed during natural and accelerated carbonation emphatically differ due to the dependence of the alkali–carbonate phase equilibrium on the CO2 concentration [102]. Goni et al. [103] compared CaCO3 polymorphs formed naturally and with 100% carbonation and detected that in naturally carbonated calcite, vaterite and aragonite were formed, whereas at 100% CO2, the only polymorph present was calcite.
Accelerated carbonation curing offers a number of tangible advantages compared to moisture curing [99]. Accelerated carbonation curing can deliver enhanced early age strength along with an intense strength gain due to the more densified crystalline structure. As established by research, carbonation curing augments robustness by decreasing the capillary water uptake, chloride resistance, and also by providing superior sulphate resistance [104].
3.2. Parameters Influencing the Carbonation Process
Although carbonation seems to be quite straightforward theoretically, in real life it is denoted by complexity as there are many and various parameters determining the outcomes. The carbonation reaction of hydrated OPC in air is generally a slow process that is dependent on the relative humidity of the environment, temperature, permeability of the concrete, and the concentration of CO2 available. In accelerated carbonation, the pressure value is an additional variable. As stated by Bertos et al. [87], the carbonation process is primarily affected by factors such as the diffusivity and reactivity of carbon dioxide. Carbonation-dependent factors are presented in a systemized way in the Figure 1 below.
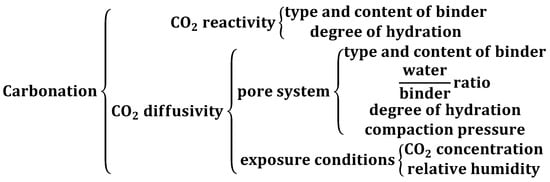
Figure 1.
Factors on which the carbonation process depends.
Prior to using carbonated calcium silicate as an alternative binder, essentially, we first need to explore the mechanical performance of this kind of system as well as the dependency of the mechanical strength on the entire range of process parameters and conditions, such as CO2 pressure, length of exposure, and temperature, along with the qualities of the sample such as the binder/sand ratio, w/c ratio, and compaction.
3.2.1. Reactivity of CO2
To make CO2 reactive and ensure effective carbonation, the solid material needs to be denoted by certain chemical properties. First, the substances must be inorganic in nature. Calcium and/or silicon salts must be constituent parts, or there may be hydraulic, pozzolanic, lime-bearing or other CO2-reactive calcium-containing material heavy metals [87]. A higher concentration of Ca leads to a better carbonation result; therefore, the C/S molar ratio increases, and the carbonation degree is increased as well.
The amount water is also significant as it is needed to promote the carbonation reaction by dissolving CO2 and releasing CO32− ions. CO2 in the gaseous form cannot react directly with the cementitious material as CO2 gas must primarily be dissolved in water to form carbonate ions (carbonic acid). Subsequently, these ions react with the calcium ions of pore water. However, excessive water may compromise the rate of carbonation due to the clogging of pores and reduced carbon dioxide diffusion. In this case, a faster accumulation of precipitations on the surface is observed. At low water-to-cement (w/c) ratios, the gas permeability increases and diffusion of CO2 into the material is effectively achieved. For this reason, as has already been determined, the optimum water-to-solids (w/s) ratio is in the range of 0.06 to 0.20 [105]. However, the same degree of carbonation may require a different water content for various types of cement.
3.2.2. Diffusivity of CO2
The diffusion efficiency of carbon dioxide gas is profoundly linked with the structure, i.e., the porosity, of the material. It follows that compaction of the material prior to its carbonation exerts an impact on the final product as well. An increase in compaction leads to a decrease in the structural porosity and permeability; as a result of this, a greater strength can be achieved. However, under conditions of high compaction, the reaction surface becomes smaller, and the water layer in pores becomes thicker. Since the diffusion of CO2 in water is slower than that in air, the early-age carbonation can be impeded. However, water evaporation can become difficult and this can provide moisture for carbonation at a later stage; thus, it can improve the total efficiency. Lower compaction provides a higher reaction surface since the structure is then looser. However, it should be considered that water evaporation during the carbonation process in looser systems may speed up. It thus follows that there is an optimum pressure value that allows for the maximized compressive strength in the samples to be achieved as long as sufficient porosity is ensured for easy gas penetration and access to moisture.
The physical properties of solids constrain the diffusivity of CO2 [87]. A higher surface area of the material contributes to a greater degree of carbonation, whereas enhanced compaction results in lower porosity, and, therefore, in superior strength. On the other hand, it may prevent CO2 diffusion. The diffusion rate of carbon dioxide controls the carbonation of cement-based materials to a great extent through the microstructure if hardening has already been achieved. Therefore, the higher microporosity of the hydration products results in better-carbonated materials [87].
Shao et al. [106] suggest that, theoretically, with a carbonation efficiency of 100% being achieved, one ton of cement has the potential to absorb 0.5 tons of CO2 to form 1.5 tons of solid calcium carbonates and silica gels. In real life, however, strength and diffusivity aspects must be taken into consideration; values of CO2 uptake within a range of 9–20% by cement mass represent state-of-the art technologies so far [107].
3.2.3. Pressure
As a process, carbonation is limited by diffusion. Increased pressure, combined with extended duration, increases CO2 penetration into the porous—yet relatively dense—material. Nevertheless, over-pressurization can lead to the blockage of the pores, thus hindering the entrance of gas into the inner parts of the material. Therefore, it is important to achieve a combined parameter balance.
CO2 partial pressure can control the carbonation process and can be implemented within a spectrum of pressure values, ranging between atmospheric and super-critical CO2 (scCO2). CO2 pressure, in combination with other relevant parameters, dictates the way in which the reaction proceeds. Depending on the specific system, individual fine-tuning of the full set of parameters should also be considered. By boosting pressure and temperature values, an increase in the rate of carbonation in concrete is certainly achievable.
Shi and Wu [108] state that, in the course of reactions between CO2 and cement minerals, acceleration is observed mainly within the first 15 min of curing, independently from the CO2 pressure and the pre-conditioning environment. Furthermore, Shi and Wu demonstrated that an enhanced CO2 pressure promotes elevated CO2 consumption without exerting any significant impact on concrete strength.
Some authors have taken into account the carbonation process using scCO2. Supercritical CO2 is a fluid state of CO2 as it is stored at or above its critical temperature and pressure values [109]. The critical point of carbon dioxide is 31.1 °C (i.e., ca. 304 K) and 7.39 MPa (equivalent to ca. 74 bar). scCO2 features a liquid-like density while manifesting gas-like transport properties. All of this, in combination with the absence of surface tension, enables its penetration into the finest pores of cement paste [88]. Under natural conditions of carbonation, the poor solubility of atmospheric CO2 in water limits the amount of CO2 dissolved in pore water. The high pressure helps to increase the amount of CO2 molecules dissolved into the pore water; therefore, CO2 is able to penetrate even small pores with ease, thus providing continuous availability of fresh CO2 [110]. Therefore, the carbonation of cement under supercritical conditions is not controlled by diffusion [111].
3.2.4. Relative Humidity
The highest rate of carbonation reaction is observed when a relative humidity (RH) value is within the range of 50–70%; it decreases at both higher and lower relative humidity levels [111]. The somewhat narrow range of RH is related to the progress of carbonation reaching its maximum at ca. 60% RH [99]. At RH >70%, capillary condensation seems to be pore size-dependent; thus, the cement-specific influence on carbonation resistance is maintained [112].
Galan et al. [113] determined that, at the lowest RH values, the amount of water in the pores is insufficient to dissolve the phases involved; as a result, their reaction becomes impossible. Meanwhile, intermediate RH values help to ensure the optimal conditions for carbonation, which include sufficient water for the reaction to take place, as well as sufficient space for the carbon dioxide to be diffused. Starting with a specific value of RH, saturation of the pores with water is initiated, thus complicating the diffusion.
3.2.5. Temperature
As carbonation curing is a process of hardening taking place in a gas environment, it is highly dependent on the diffusivity of the working gas. Therefore, the temperature significantly affects any attempts to reach higher degrees of carbonation and to enhance the mechanical properties of the system. A more elevated temperature increases CO2 diffusivity and promotes ion leaching, which boosts the chemical reaction. On the other hand, CO2 solubility in water can decrease, thereby negatively impacting the carbonation rate. In this context, striking the optimal balance between all the involved limitations is the way forward.
Temperature is typically denoted by the complicated nature of its effect. An elevated temperature can enhance ion leaching and stimulate the chemical reaction; however, it can reduce carbon dioxide solubility. At low(er) temperatures, superficial carbonation temperatures are preferable, as carbonate shell formation over the particles’ surfaces is promoted. At higher temperatures, diffusion processes are optimized, and carbonation can proceed through the bulk material; thus, with carbonation ongoing at an elevated temperature, CO2 can quickly cure and be stored in the concrete structure permanently. Considering the transfer of this concept to an industrial scale, high temperatures preliminarily seem to represent a more feasible solution than high-pressure environments [90].
According to Bertos et al. [87], CO2 uptake increases when the temperature is increased up to 60 °C (at atmospheric pressure). This is most likely due to the leaching of Ca2+ ions from the solid material’s particles. Temperatures above this value reduce CO2 solubility in water, thus lowering the carbonation rate. However, as the carbonation reaction is exothermic, the reaction-generated heat stimulates the formation of meta-stable polymorphs of CaCO3. The desired stable polymorph (i.e., calcite) can be optimally obtained when the process is maintained at highly reduced temperatures—i.e., in the interval from 0 to 10 °C. Asavapisit et al. [114] demonstrated that more calcite is formed in the environment where cold carbonic acid is used. Meanwhile, Wang et al. [110] established that, by raising the temperature to 40 °C, a prominent increase in the starting rate of the reaction as well as the total CO2 uptake capacity can be achieved. However, when this peak has been passed, a further augmentation of the temperature to 60 °C affects the reaction rate negatively (at 15 bar and w/c of 0.16). Contrarily, Liu et al. [115] produced data showing that a temperature augmentation up to 60 °C results in a higher CO2 content in the solidified material, while an increase of the reaction temperature above 60 °C delivers a decreased CO2 content due to the decreased CO2 solubility in water at elevated temperatures.
Mazzella et al. [80] determined that an upward shift of the temperature from 25 to 45 °C resulted in a doubled CO2 uptake at 2.5 bar and nearly tripled the uptake at 7.5 bar CO2 pressure; therefore, it is evident that temperature plays an important role in the carbonation process.
Due to the staggering complexity and abundance of parameters exerting impacts on carbonation, the key point is to determine and investigate the coexistence and interaction of these factors upon the reaction course as well as its kinetics. As the carbonation process is largely based on diffusion, some criteria have a positive impact on the reaction path while other criteria are disruptive. Thus, the ultimate challenge for researchers is to determine an optimized combination of values to ensure the perfect parameter synergy.
3.3. Ordinary Portland Cement Carbonation
The carbonation of cementitious materials proceeds naturally due to the presence of atmospheric CO2. According to studies in the literature [116,117,118,119,120,121,122,123,124,125,126], carbonation is an outcome of the neutralization reaction between the basic compounds of calcium silicates and H2CO3 producing thermodynamically stable carbonates and water, which leads to a drop in pH values. Concrete carbonation can be described as a physicochemical process involving a series of chemical reactions. CO2 dissolves in the aqueous pore solution, thus yielding carbonic acid (H2CO3). A diffusion mechanism induces the penetration of concrete by CO2. This is a slow process from the surface inwards, with the depth of the carbonated cement concrete front increasing with time. The rate of carbonation depends on many factors such as the moisture content, the permeability of concrete, the fraction of CO2 in the environment, as well as relative humidity. From the theoretical point of view, as hydration compounds are inherently unstable in environments containing CO2, over time, absorption of the mass of CO2 nearly equal to that which was chemically released during the reverse reaction of limestone calcination in the cement kiln can be achieved [127]. In addition, when CO2 reacts with calcium-bearing phases in the cement paste, chemical–mechanical changes can be induced regarding the microstructure and porosity [98]. These changes are usually considered as ‘passive’ since, when the cement paste reacts with CO2, these changes are spontaneous; that is why, even despite the improvement in such features, e.g., the compressive strength manifested during carbonation, they are generally perceived as deterioration effects. Contrarily, an active carbonation process stems from a procedure designed to deliberately benefit from the ability of calcium-bearing phases of the cement paste to react with carbon dioxide for a broad range of applications. Active or accelerated carbonation curing may serve as a strategy towards the capture and storage of excess CO2 contained in the air. The presently discussed strategy is based on the exposure of the freshly cast cement paste or concrete to a pure or CO2-rich environment. With fresh pastes and high concentrations of CO2, the hydration process of OPC is greatly accelerated as well. There are a number of major benefits of accelerated carbonation curing, such as an augmented strength at an early age and an intense gaining of strength. This is due to pore-filling by CaCO3, and also because of modifications in the CSH microstructure [128].
The process of OPC carbonation is strongly exothermic and controlled by diffusion. When carbon dioxide dissolves in water, carbonic acid is produced; this reaction yields a significant amount of heat, specifically, 669.9 × 103 J/mol [87]. CO2 gas diffuses into the solid. This results in a growing front of carbonated material encircling the inner zone of non-carbonated material. The penetration of carbonation can be defined by referring to the following sequence of steps that are generally part of the reactive-transport mechanism [87]:
- Diffusion of CO2 in air;
- Permeation of CO2 through the solid;
- Solvation of CO2(g) in water to CO2(aq);
- Hydration of CO2(aq) to H2CO3;
- Ionization of H2CO3 to H+, HCO3- and CO32-. During this stage, the pH typically drops from 11 to 8;
- Dissolution of cementitious phases C3S and C2S, which occurs swiftly and extensively. Calcium silicate grains are covered with a loose layer of calcium silicate hydrate gel. The gel promptly dissolves and Ca2+ and SiO44- ions are released;
- The nucleation of CaCO3 and CSH, for which minutely higher temperatures are required in addition to the presence of finely derived material acting like heterogeneous nuclei;
- Precipitation of solid phases. Initially, vaterite and aragonite can be formed. At a later stage, these polymorphs of CaCO3 are reverted to calcite. The final product may still contain some amorphous calcium carbonate;
- Secondary carbonation. CSH gel is formed and gradually decalcifies; after conversion, the final products are silica gel and CaCO3.
During the carbonation reaction of calcium silicates, the primary product is CaCO3. The principal chemical reaction governing the carbonation curing of cement-based materials is as follows:
Ca(OH)2 + CO2 → CaCO3 + H2O
As calcium is the most common element among the OPC hydrates and Ca(OH)2 offers the highest water solubility compared to other calcium compounds, the reaction with CO2 duly proceeds. Even though this reaction is between a gas and a solid, water is significantly involved as the ion dissolution of calcium hydroxide and CO2 dissolved in pore water is a prerequisite, without which there is no carbonation. Ca(OH)2 dissolved in the pore solution can react with carbonic acid, which yields calcium carbonate [77,129,130,131,132,133,134,135,136]:
Ca(OH)2 + H2CO3 → CaCO3 + 2H2O
The reactions forming both carbonic acid and crystalline CaCO3 are exothermic and 160 kcal and 288 kcal of heat per mole are released, respectively [137]. During the course of the reaction, the fraction of the calcium hydroxide content in the cement pastes is gradually reduced, and the production of calcium carbonate is intensified; ultimately, the pH value of the hardened paste is reduced.
The hydration reaction of cement clinker minerals proceeds over the course of time. Unreacted C3S and C2S are involved in a carbonation reaction where, although portlandite and CSH can be produced at an early stage, calcite and silica gel are ultimately produced instead:
3CaO · SiO2 + 3CO2 + nH2O → SiO2·nH2O + 3CaCO3
2CaO · SiO2 + 2CO2 + nH2O → SiO2·nH2O + 2CaCO3
Belite phases can be present as β-C2S and γ-C2S, with the CO2 uptake capacity of γ-C2S higher than that of β-C2S, and the formation of various CaCO3 polymorphs is observed [129].
Figure 2 shows a specific area of the carbonated sample at different magnifications. A visible pore that is around 2 μm wide and 5 μm long is observed that is surrounded by cubic, small-scale (<1 μm) crystals attributed to calcite [69]. These clusters of crystals were found to be forming inside and around pores, which indicates that the first layer surrounding the unreacted binder particle is silica gel, after which a layer of calcium carbonates fills the pores, thereby densifying the microstructure of the material. Ultimately, the layers of the carbonation reaction products surrounding the unreacted binder particles become excessively thick and the CO2 is obstructed from further diffusion to the unreacted particles. Further carbonation is therefore compromised and the material is prevented from gaining additional strength.

Figure 2.
SEM images of carbonated (48 h, 45 °C) blended cement CEM II/A-LL 42.5R sample microstructure at different magnifications [138].
CSH phase carbonation leads to the formation of a silica gel network; a considerable decrease in porosity and a prominent increase in compressive strength is thus achieved. CSH carbonation starts when a sufficient degree of carbonation has been reached, i.e., it is only observed when most of the calcium hydroxide has already been used up. After lowering the content of Ca2+ in the pore solution due to carbonation, the content of Ca2+ is augmented again because of its release from CSH. This modifies the structure of CSH and results in a lower C/S ratio. When the ratio falls below 1 and the pH value is around 10, CSH fully decalcifies and finally converts into CaCO3 and a highly polymerized silica gel that is acid-stable and sustains a morphology similar to that of the initial hydrate. As we still lack knowledge regarding the exact stoichiometry of CSH, the carbonation reaction of CSH gel can be assumed to proceed as outlined below [99,132,133]:
xCaO·ySiO2·zH2O + xCO2 → xCaCO3 + y(SiO2 · nH2O) + (z-nt)H2O
Ettringite and aluminates are also effectively carbonates at low partial CO2 pressures. Ettringite decomposes when involved in a reaction with CO2, which yields gypsum and alumina gel [134], as follows:
3CaO·Al2O3·3CaSO4·32H2O + 3CO2 → 3CaCO3 + 3(CaSO4·2H2O) + Al2O3·xH2O + (26 − x)H2O
The released sulphate ions can either precipitate as gypsum or diffuse inwards and react with aluminate ions from the decomposed monosulphate, thus forming new ettringite. This procedure is determined and triggered by diminishing pH values. However, the end result is that a sizable share of Ca2+ ions from the aluminate phases will yield carbonate; the aluminate and ferrite phases will produce stable metal hydroxides [121].
3.4. Belite and Blended Cement Carbonation
Another approach towards more sustainable cement production is the carbonation curing of belite-rich cement, which not only has the capacity to modify belite cement but can also sequestrate CO2 into chemically stable carbonates in the long run. Jang and Lee [135] determined that a higher share of belite in cement increases the CO2 uptake during carbonation curing, thus encouraging microstructural densification. Belite phases can manifest themselves as β-C2S and γ-C2S [136], with γ-C2S offering a higher CO2 uptake capacity than β-C2S. The mechanical strength of belite-rich cement mortar cured by carbonation for 28 days was found to have dramatically improved compared to normally cured cement for an equal period of time, with diminished pore connectivity and more instances of pore closure, which resulted in a complex microstructure. CO2 uptake capacity increases with an increasing belite content, leading to a higher amount of calcite formed, which directly corresponds to increased strength. Fang and Chang [137] also investigated the hardening behavior of β-C2S by accelerated carbonation, demonstrating a carbonation degree of 21.6% and a compressive strength of 85.7 MPa after 6 h of carbonation in a 99.9% CO2 concentration.
Moreover, not only can hydraulically active forms of belite be carbonated, the activation and hardening of γ-C2S by a carbonation reaction is considered a feasible approach towards the production of low-lime cement [95]. Guan et al. [139] produced mortars based on γ-C2S binder that had an equal or even higher strength than OPC and could fully harden within 24 h by using accelerated carbonation. The CO2 footprint related to the manufacturing and employment of a γ-C2S binder can be reduced by as much as 53%, compared with that of OPC. Chang et al. [131] compared the carbonation of β-C2S and γ-C2S to examine strength development in the accelerated carbonation process and the microstructure alterations caused by carbonation. The authors determined that β-C2S absorbs less CO2 than γ-C2S while offering a superior compressive strength, as well as different CaCO3 polymorphs formed in the carbonated systems. Notably, a few authors [140,141,142] have stated that, by adding γ-C2S to the OPC system, we obtain not only densified surface layers, a lower porosity and higher compressive strengths, but also an increased resistance to leaching after accelerated carbonation curing.
The carbonation of blended cements becomes increasingly important in the context of the sustainable construction sector [143]. Blended cements containing supplementary cementitious materials, e.g., burnt clay, fly ash, blast furnace slag [144,145], opoka [146] (a detailed description of this Ca-Si sedimentary rock is provided in [147]) and limestone/calcined clay [148] are particularly important because of a significant reduction in carbon dioxide emissions. The use of SCM not only helps to reduce high CO2 emissions but also improves the overall strength of the concrete. The key advantage of blended cements is the reduced clinker content in the mix. Ostvari et al. discovered that by using blended cements, the content of clinker in the mix can be reduced by up to 10%, resulting in a 50% reduction in carbon dioxide emissions compared to that of OPC [149]. The use of SCM materials not only helps to reduce greenhouse gas emissions, but also eliminates by-products generated in the industry, thus promoting the development of the circular economy [150].
Other positive factors include the CO absorption capacity, which increases when using SCM [151], and the mechanical strength of carbonized concrete products. The same trends are characteristic of blended calcium sulfoaluminate cements [152].
3.5. Carbonation of Low-Lime Cement
A highly promising approach is the preference given to the manufacturing of low-lime calcium silicate cement (CSC). This type of binding material not only requires smaller amounts of limestone but is denoted by a lower production temperature, which leads to dramatically reduced CO2 emissions. In addition, such binders are environmentally favorable not just because of their reduced CO2 emissions but also due to their capacity to permanently store CO2 in the concrete structure in their carbonation hardening process. The adoption of such well-functioning and capable carbonation technologies offers perspectives towards cementitious materials that can serve as one of the core CO2 sequestration segments worldwide. Rankinite (Ca3Si2O7 or C3S2) and wollastonite (CaSiO3 or CS) are the main low-lime calcium silicates that can practically serve as alternative binders.
Rankinite carbonation. The process of rankinite carbonation is comparable to that of OPC carbonation. During the initial stage, the dissolution of CO2 gas into water yields carbonic acid, which subsequently ionizes H+, HCO−3, and CO32-. As the H+ concentration significantly increases, the pH correspondingly drops; the leaching of Ca2+ from the C3S2 structure is thus induced. This leads to immediate precipitation or the formation of insoluble CaCO3, which is considered as a rate-limiting step of the carbonation reaction. Calcite occurs as a compact, continuous coating of small crystals, having a major effect on the carbonation extent [69]. The carbonation reaction products of the rankinite binder system include calcium carbonate, silica gel (or else Ca-modified silica gel), and a composite phase featuring both formerly mentioned phases (intermixed) [107,153,154]. At this point, the most accurate reaction path for the rankinite carbonation system has been proposed by Ashraf et al. [62]:
5(3CaO·2SiO2) + 11CO2 + 2.5H2O → 11CaCO3 + 4CaO·10SiO2·2.5H2O
As the solvation of Ca2+ ions from C3S2 structure occurs, the polymerization of the remaining silicon tetrahedral monomers (H2SiO4) simultaneously produces highly polymerized silica gels [155,156]. The stiffness of the produced polymerized silica gel phase has been shown to be greater than the stiffness of CSH gel, which is the primary binding phase of the hydrated OPC system [76]. The concept of the derivation of a Ca-modified silica gel was proposed by Ashraf and Olek [157]. The authors state that this type of silica gel phase can be plausibly perceived as containing semi-spherical clusters of silica gel with silanol (Si–OH) bonds located primarily on their surfaces [147]. It has been suggested that calcium is potentially present inside the gel clusters that moisture cannot reach. Due to this inaccessibility, calcium is not removed from the Ca-modified silica gel during the carbonation process, nor does it convert to CaCO3. In their previous study [158], the same authors observed that, during the carbonation reaction of C3S2, the initial gel phase is CSH, which ultimately decomposes, thus forming a highly polymerized Ca-modified silica gel.
The carbonation of calcium silicates involves two clearly defined stages: (1) an initial fast carbonation reaction that is controlled by chemical kinetics; and (2) a slower reaction then takes place that is controlled by product-layer diffusion [158]. As diffusion is the major limiting process in the course of carbonation curing, it is controlled by the pore system and by pore saturation; thus, it has a major impact on the extent of carbonation. The carbonation of calcium silicates triggers the loss of pore connectivity and shifts the pore size distribution curve in the direction of diminished pore diameters since changes in porosity are generally caused by the dissolution of cementitious phases. Carbonation dramatically impacts the transport properties by virtue of modifying and densifying the concrete microstructure. Concrete porosity drops with an increase in CO2 pressure, as the pores, which previously were large and open, become filled with calcium carbonate and silica gel. Ultimately, they are of a higher molar volume compared to the primary components [159,160]. Due to the fact that the coating of CaCO3 on the binder particle becomes thicker, the whole structure is surrounded by a densified layer of reaction products, preventing further permeation and diffusion of CO2 into the unreacted clinker, thus restricting the extent of rankinite carbonation [69].
The main advantages of manufacturing rankinite binder over OPC are presented in Reference [63] and Figure 3 and Figure 4. The CaO/SiO2 molar ratio in cement is 2.7; in rankinite it is 1.5, i.e., 1.8 times lower. This reduction in lime content causes a 30% decrease in the enthalpy of the decarbonation of CaCO3 (from 2.138 to 1.514 GJ). In both cements, the enthalpies of the reactions of the clay mineral decomposition (0.063 and 0.075 GJ) and cement phase formation (-0.377 and -0.538 GJ) are close. Thus, the enthalpy of the formation of rankinite clinker is ~40% lower than that of OPC. In addition, rankinite clinker production can reduce CO2 emissions by as much as 30% (from 810 kg/1-ton OPC to 565 kg/1-ton rankinite) [63].
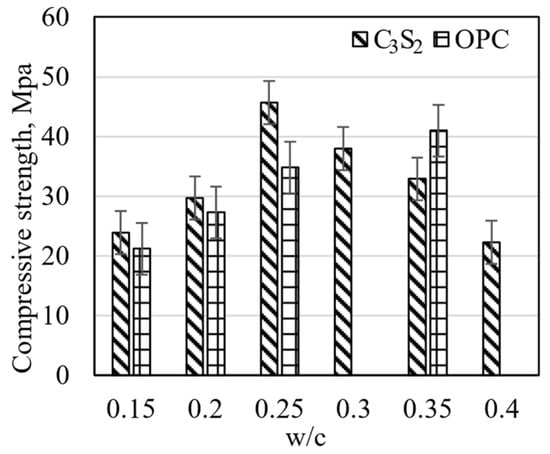
Figure 3.
Compressive strength of the carbonated rankinite binder and OPC mortars with different w/c ratios [138].
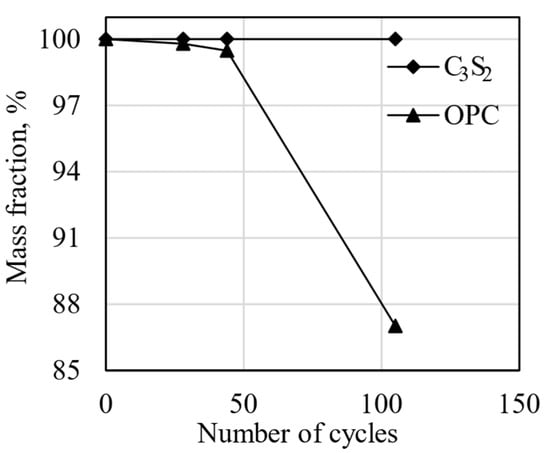
Figure 4.
Freeze–thaw mass loss of the carbonated rankinite binder and cement samples [138].
The water-to-binder ratio has a strong impact on the strength of the samples (Figure 2). Increasing the w/c ratio from 0.15 to 0.25 resulted in the compressive strength of the rankinite binder mortars (binder/sand 1:3) also increasing, reaching the highest value of more than 45 MPa. However, further increases in the w/c ratio up to 0.4 led to a decrease in the compressive strength value. OPC samples showed a continuous increase in the compressive strength value with increasing water content. This may be due to the fact that OPC is a hydraulic material that initially forms Ca(OH)2 and CSH; the reactivity to CO2 in this might be higher than the initial calcium silicates.
The durability of the rankinite and OPC samples was assessed by freeze–thaw resistance using de-icing salt. The obtained results are provided in Figure 4, where it can be seen that rankinite binder samples showed significantly better performance compared to the OPC samples. Even after more than 100 freeze–thaw cycles, the mass of the scaled rankinite binder samples was less than 1 g/m2; for the OPC samples, the mass was significantly higher [138].
Wollastonite carbonation. In terms of Ca-bearing silicates, wollastonite is among the most reactive minerals [71]. Its carbonation is exothermic (ΔHr = −87 kJ/mol) [161], which can potentially reduce the total consumption of energy—and, inherently, the cost—of carbon sequestration. The carbonation of Ca-bearing silicates takes place in a gas–solid–water slurry, which dramatically speeds up the reaction rate in comparison to direct gas–solid carbonation [162].
Wollastonite carbonates at a high rate, with a peak conversion rate of 70% in 15 min under comparatively mild conditions (d < 38 μm, T = 200 °C and pCO2 = 20 bar) [163]. Daval et al. [71] researched wollastonite carbonation kinetics and used kinetic modelling. They proved that the rate-limiting step of the general reaction is wollastonite dissolution. However, Min et al. [164] researched CS carbonation in water-bearing supercritical CO2 and determined that the carbonation reaction does not experience any impact from the kinetics of the surface reactions. Rather, it is affected by the diffusion of water-bearing scCO2 through the surface layer. Therefore, the state and medium in which CO2 approaches the material is fundamentally important. Wollastonite carbonation is intensified by the increasing water content, temperature, and pressure. According to Miller et al. [165], it is possible to achieve almost 50% wollastonite conversion to carbonate at 70 °C, 160 bar, with no less than 24 h carbonation. A similar carbonation degree (~50%) was achieved by Daval et al. [70] at 250 bar and 90 °C.
Wang et al. [110] researched the impact of a CS addition to OPC system carbonation and determined that the reaction rates, along with CO2 uptake capacity, were significantly enhanced. The additive CS content encouraged the carbonation conversion of OPC components throughout the process of curing and acted mainly on the gaseous diffusion properties, thus enhancing gas permeability during the reaction.
Highly relevant topics of recent research are the binding substances of LC3 (limestone-calcined clay cements). Their properties are close to those of PC; however, they can reduce CO2 emissions to 30%. The process of their carbonization has been recently described in detail in two studies [49,50].
3.6. Carbonation Effect on Reinforced Concrete
Carbonation in concrete structures not only exerts a positive environmental impact, it also contributes a significant negative impact on reinforced concrete structures as well. During carbonation, atmospheric CO2 interacts with hydrated products in cement and yields carbonates that significantly reduce the alkalinity of the concrete. This is crucial for the protection of steel reinforcement. This issue was recently examined in detail in a review article by A.F.A. Fuhaid and A. Niaz [166].
We would like to mention a few observations by other authors. The alkaline environment in concrete protects steel from corrosion; therefore, when alkalinity decreases, corrosion processes begin. Babalola et al. demonstrated that the corrosion caused by reinforcement reduces its load-bearing capacity and can seriously damage the robustness as well as the longevity of concrete structures [167]. Ikumapayi et al. observed that the corrosion of reinforcing bars due to carbonization is a common phenomenon responsible for structural failures, including even the complete collapse of buildings [168]. In addition to the chemical processes caused by carbonation, as discussed above, erosion is also manifested in concrete. Research conducted by Yin et al. highlights the likelihood of greater material losses in structures that can potentially be caused by the ongoing carbonation process; in the long run, this can affect the strength and, ultimately, the integrity of these structures [169]. The resulting erosion of structures can lead to the appearance of cracks and the spalling of concrete surfaces, which can then be followed by the complete destruction of the coating layer protecting the steel. Studies have highlighted the economic consequences of carbonization-induced reinforcement corrosion. Structural deterioration caused by the carbonization process has significant financial implications due to the restoration measures required, both through retrofitting and preventative strategies [170].
The influence of chlorides on the corrosion of steel reinforcing bars is a significant challenge to the durability of reinforced concrete structures. It has been shown that carbonation in concrete affects the diffusion of chlorides and accelerates the corrosion of reinforcement [171]. All problems and their solutions are described in detail in [172].
These problems do not arise when using basalt-fiber reinforced concrete. This product is relatively a new type of fiber-reinforced concrete and has demonstrated good mechanical performance. Basalt fibers are considered superior to other widely used types of fibers, because of their comparable mechanical strength, higher durability than glass fibers, lower cost compared to carbon fibers, the sustainability manifested by the abundance of the raw material used, and a production process denoted by its environmental friendliness. The advantages and future prospects of this concrete are examined in great detail in a recently published review [173].
In summary, the negative effects of carbonization evidently impose a strong impact on steel-reinforced concrete structures. This process results in the corrosion of steel reinforcement, and reduces the durability of structures, which inevitably results in devastating economic consequences.
To summarize the present study, it is safe to assume that not only ordinary Portland cement, but also low-lime calcium silicate binder materials are applicable in carbonation curing and offer potential to be used in the production of carbonated construction materials. In comparison to OPC, their negative impacts on the environment are less severe. The raw mixture contains a lower amount of CaO. The lower calcinating temperature reduces fuel and energy consumption. Carbon dioxide is sequestrated in the concrete structure in the form of stable carbonates. However, each of the blended cement hardening processes using mineral carbonation requires systematic and profound investigation because the process is greatly impacted by a broad range of factors and parameters.
It is evident that curing in a CO2 environment accelerates the formation of calcium silicate cement phases. However, additional research is required to establish. beyond reasonable doubt. the effect of carbonation on the hardening of low-lime binders. Therefore, a further extension of the present study may be directed towards the synthesis of new cementitious materials, the study of their hardening mechanisms, and modifications in the mineralogical composition of the products obtained. It is essential to realize that although experimental research will create environmentally friendly, durable, and mechanically strong concretes, the practical introduction of this technology at an industrial scale is still denoted by major complexity. For this reason, the large-scale production of concrete cured in a CO2 environment is a challenge due to the operational and chemical composition considerations in real-life implementations.
4. Conclusions
The OPC used in the OC industry is a key component of concrete and mortar, is associated with high CO2 emissions, and is on the “blacklist” of factors that most affect climate change. Recent studies have shown that measures, such as traditional clinker additives, recovered solid fuels, more efficient energy use and engineering solutions, are not enough to achieve CO2 reduction targets. The implementation of new cementitious materials is one of the main goals of the academic community and cement and concrete manufacturers. One way to reduce the negative impact of OPC production on climate change is to use blended cements, i.e., with 10–30% additions of various additives and then harden the products in a CO2 environment. This improves the properties of these products, especially their durability. It is also very important that SCMs can be mixed with OPC by concrete manufacturers. Such a binder requires a smaller amount of limestone, which significantly reduces CO2 emissions. In addition, two more goals are achieved: (1) reduced emissions make such binders more environmentally friendly; and (2) CO2 is utilized in the product structure during carbonization. If these technologies are widely implemented in industry, the production and use of cementitious materials would likely become one of the most important means of CO2 utilization in the world.
The main factors influencing the carbonation kinetics of calcium silicate-based binders are as follows:
- Reactivity of CO2. To make CO2 achieve effective carbonation, the solid inorganic material must possess certain chemical properties. The higher the concentration of Ca in the material (CaO/SiO2 ratio), the higher the degree of carbonation obtained. The water content in the material is also of major importance since it is required to promote the carbonation reaction by releasing CO32− ions;
- Diffusivity of CO2. The higher the surface area of the material, the greater the degree of carbonation. A high degree of compaction leads to a lower porosity and, therefore, to a higher strength. However, this may prevent CO2 diffusion;
- Pressure. CO2 partial pressure, along with other process parameters, determines the course of the reaction, and, depending on the individual system, all the parameters should be set individually as well. By increasing the temperature and pressure, it is possible to increase the carbonation rate;
- Relative humidity. The carbonation rate is the highest within a relative humidity range (RH) of 50–70%. At a low RH value, there is not enough water in the pores to dissolve the phases involved. From a certain value of RH, the pores start to be saturated with water, complicating the diffusion;
- Temperature. The effect of temperature is usually complicated. At low temperatures, the carbonate shell forms more easily on the particle surface. At higher temperatures, diffusion processes are more effective and carbonation can proceed through the bulk material. Considering the application on an industrial scale, it may be more convenient to operate at higher temperatures rather than at a high pressure.
Author Contributions
This paper was written using the contributions of all authors. R.S.: conceptualization, supervision, writing—review and editing. A.S.: selection and analysis of literary sources, data overview, validation, writing and editing; N.A.: selection and analysis of literary sources, methodology, validation, writing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by a grant (No. S-ST-24-20) provided by the Research Council of Lithuania.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gao, T.; Shen, L.; Shen, M.; Liu, L.; Chen, F. Analysis of material flow and consumption in cement production. J. Clean. Prod. 2016, 112, 553–565. [Google Scholar] [CrossRef]
- Statista. Available online: https://www.statista.com/statistics/1087115/global-cement-production-volume/ (accessed on 14 February 2025).
- World Economic Forum. Nature Positive: Role of the Cement and Concrete Sector. 2023. Available online: https://www3.weforum.org/docs/WEF_Nature_Positive_Role_of_the_Cement_and_Concrete_Sector_2023.pdf (accessed on 15 July 2024).
- GCCA. Concrete Future: The GCCA 2050 Cement and Concrete Industry Roadmap for Net Zero. Available online: https://gccassociation.org/concretefuture/wp-content/uploads/2022/10/GCCA-Concrete-Future-Roadmap-Document-AW-2022.pdf (accessed on 19 March 2025).
- Benhelal, E.; Zahedi, G.; Shamsaei, E.; Bahadori, A. Global strategies and potentials to curb CO2 emissions in cement industry. J. Clean. Prod. 2012, 51, 142–161. [Google Scholar] [CrossRef]
- Ige, O.E.; Olanrewaju, O.A. Comparative Life Cycle Assessment of Different Portland Cement Types in South Africa. Clean Technol. 2023, 5, 901–920. [Google Scholar] [CrossRef]
- Oda, J.; Akimoto, K.; Tomoda, T.; Nagashima, M.; Wada, K.; Sano, F. International comparisons of energy efficiency in power, steel, and cement industries. Energy Policy 2012, 44, 118–129. [Google Scholar] [CrossRef]
- Supriya; Chaudhury, R.; Sharma, U.; Thapliyal, P.C.; Singh, L.P. Low-CO2 emission strategies to achieve net zero target in the cement sector. J. Clean. Prod. 2023, 417, 137466. [Google Scholar] [CrossRef]
- Beguedou, E.; Narra, S.; Armoo, E.A.; Agboka, K.; Damgou, M.K. Alternative fuels substitution in cement industries for improved energy efficiency and sustainability. Energies 2023, 16, 3533. [Google Scholar] [CrossRef]
- Cavalett, O.; Watanabe, M.D.B.; Voldsund, M.; Roussanaly, S.; Cherubini, F. Paving the way for sustainable decarbonization of the European cement industry. Nat. Sustain. 2024, 7, 568–580. [Google Scholar] [CrossRef]
- Van Roijen, E.; Sethares, K.; Kendall, A.; Miller, S.A. The climate benefits from cement carbonation are being overestimated. Nat. Commun. 2024, 15, 4848. [Google Scholar] [CrossRef]
- Hu, X.; He, P.; Shi, C. Carbonate binders: Historic developments and perspectives. Cem. Concr. Res. 2024, 175, 107352. [Google Scholar] [CrossRef]
- Von Greve-Dierfeld, S.; Lothenbach, B.; Vollpracht, A.; Wu, B.; Huet, B.; Andrade, C.; Medina, C.; Thiel, C.; Gruyaert, E.; Vanoutrive, H.; et al. Understanding the carbonation of concrete with supplementary cementitious materials: A critical review by RILEM TC 281-CCC. Mater. Struct. 2020, 53, 136. [Google Scholar] [CrossRef]
- Zajac, M.; Maruyama, I.; Iizuka, A.; Skibsted, J. Enforced carbonation of cementitious materials. Cem. Concr. Res. 2023, 174, 107285. [Google Scholar] [CrossRef]
- Merah, A. Methods of concrete accelerated carbonation test: A review. Discov. Civ. Eng. 2024, 1, 53. [Google Scholar] [CrossRef]
- Karimi, A.; Ghanooni-Bagha, M.; Ramezani, E.; Javid, A.A.S.; Samani, M.Z. Influential factors on concrete carbonation: A review. Mag. Concr. Res. 2023, 75, 1212–1242. [Google Scholar] [CrossRef]
- You, X.; Hu, X. A review on the modelling of carbonation of hardened and fresh cement-based materials. Cem. Concr. Comp. 2022, 125, 104315. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Q.; Peng, X.; Qin, F. A Review of Concrete Carbonation Depth Evaluation Models. Coatings 2024, 14, 386. [Google Scholar] [CrossRef]
- Medvedev, V.; Pustovgar, A. A Review of Concrete Carbonation and Approaches to Its Research under Irradiation. Buildings 2023, 13, 1998. [Google Scholar] [CrossRef]
- Global Cement and Concrete Association. Blended Cement—Green, Durable & Sustainable; Global Cement and Concrete Association: India, Mumbai, 2022. [Google Scholar]
- Rhaouti, Y.; Taha, Y.; Benzaazoua, M. Assessment of the environmental performance of blended cements from a life cycle perspective: A systematic review. Sustain. Prod. Consum. 2023, 36, 32–48. [Google Scholar] [CrossRef]
- Cognitive Market Research. Blended Cement Market Report 2025 (Global Edition). Available online: https://www.cognitivemarketresearch.com/blended-cement-market-report (accessed on 5 March 2025).
- Snellings, R.; Suraneni, P.; Skibsted, J. Future and emerging supplementary cementitious materials. Cem. Concr. Res. 2023, 171, 107199. [Google Scholar] [CrossRef]
- Song, O.; Guo, M.Z.; Gu, Y.; Ling, T.-C. CO2 curing of SCMs blended cement blocks subject to elevated temperatures. Constr. Build. Mater. 2023, 374, 130907. [Google Scholar] [CrossRef]
- Dadsetan, S.; Siad, H.; Lachemi, M.; Mahmoodi, O.; Şahmaran, M. Geopolymer binders containing construction and demolition waste. In Handbook of Sustainable Concrete and Industrial Waste Management; Pacheco-Torgal, F., Ed.; Woodhead Publishing: Cambridge, UK, 2022; pp. 437–474. [Google Scholar]
- Yazici, S.; Aydin, A. The effects of steel fiber and silica fume on the properties of high-performance concrete. Mater. Sci. Forum. 2012, 730–732, 671–676. [Google Scholar]
- Yuan, Q.; Liu, Z.; Zheng, K.; Ma, C. Inorganic Cementing Materials. In Civil Engineering Materials; Elsevier: Amsterdam, The Netherlands, 2021; pp. 17–57. [Google Scholar]
- Khalaf, F.M.; Al-Halabi, H. Effect of silica fume on the properties of concrete incorporating recycled coarse aggregate. Eng. Technol. J. 2022, 40, 81–92. [Google Scholar]
- Wang, Y.; Lu, B.; Hu, X.; Liu, J.; Zhang, Z.; Pan, X.; Xie, Z.; Chang, J.; Zhang, T.; Nehdi, M.L.; et al. Effect of CO2 surface treatment on penetrability and microstructure of cement-fly ash-slag ternary concrete. Cem. Concr. Compos. 2021, 123, 104194. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, Y.; Liu, S.; Meng, X. Mechanical properties and microstructure of high-performance cement-based materials modified with recycled waste glass powder. Mater. Adv. 2023, 26, 1266–1276. [Google Scholar]
- Alsalman, A.; Assi, L.N.; Kareem, R.S.; Cartetr, K.; Ziehl, P. Energy and CO2 emission assessments of alkali-activated concrete and ordinary Portland cement concrete: A comparative analysis of different grades of concrete. Clean. Environ. Syst. 2021, 3, 100047. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Snellings, R.; Bernal, S.A. Supplementary cementitious materials: New sources, characterization, and performance insights. Cem. Concr. Res. 2019, 122, 257–273. [Google Scholar] [CrossRef]
- Birol, T.; Avcıalp, A. Impact of Macro-Polypropylene Fiber on the Mechanical Properties of Ultra-High-Performance Concrete. Polymers 2025, 17, 1232. [Google Scholar] [CrossRef]
- Senadheera, S.S.; Gupta, S.; Kua, H.W.; Hou, D.; Kim, S.; Tsang, D.C.W.; Ok, Y.S. Application of biochar in concrete—A review. Cem Concr Compos. 2023, 143, 105204. [Google Scholar] [CrossRef]
- Sartika, R.D.; Saputra, E.; Ginting, B. Effect of bamboo charcoal powder as partial replacement of cement on the properties of concrete. IOP Conf. Ser. Earth Environ. Sci. 2022, 1117, 012029. [Google Scholar]
- Ige, O.E.; Von Kallon, D.V.; Desai, D. Carbon emissions mitigation methods for the cement industry using a systems dynamics model. Clean Technol. Environ. Policy 2024, 26, 579–597. [Google Scholar] [CrossRef]
- Juenger, M.; Provis, J.L.; Elsen, J.; Matthes, W.; Hooton, R.D.; Duchesne, J.; Courard, L.; He, H.; Michel, F.; Snellings, R.; et al. Supplementary Cementitious Materials for Concrete: Characterization Needs. Mater. Res. Soc. Symp. Proc. 2012, 1488, 26. [Google Scholar] [CrossRef]
- Qasrawi, H. The use of steel slag aggregate to enhance the mechanical properties of recycled aggregate concrete and retain the environment. Constr. Build. Mater. 2014, 54, 298–304. [Google Scholar] [CrossRef]
- Hassanien, H.; Darweesh, M. A Low Temperature Manufactured Portland Cement Clinker from Pulverized Waste of Fly Ash. Int. J. Mater. Sci. Appl. 2020, 9, 34–39. [Google Scholar] [CrossRef]
- Wonkeo, W.; Thongsanitgarn, P.; Ngamjarurojana, A.; Chaipanich, A. Compressive strength and chloride resistance of self-compacting concrete containing high level fly ash and silica fume. Mater. Des. 2014, 64, 261–269. [Google Scholar] [CrossRef]
- Lilkov, V.; Rostovsky, I.; Petrov, O.; Tzvetanova, Y.; Salvov, P. Long term study of hardened cement pastes containing silica fume and fly ash. Constr. Build. Mater. 2014, 60, 48–56. [Google Scholar] [CrossRef]
- Hamada, H.M.; Jokhio, G.A.; Yahaya, F.M.; Humada, A.M.; Gul, Y. The present state of the use of palm oil fuel ash (POFA) in concrete-review. Constr. Build. Mater. 2018, 175, 26–40. [Google Scholar] [CrossRef]
- Abdul Awal, A.S.M.; Mohammadhosseini, H. Green concrete production incorporating waste carpet fiber and palm oil fuel ash. J. Clean. Prod. 2016, 137, 157–166. [Google Scholar] [CrossRef]
- Mohammad Momeen, U.I.; Jumaat, M.Z. Durability properties of sustainable concrete containing high volume palm oil waste materials. J. Clean. Prod. 2016, 137, 167–177. [Google Scholar]
- Torres, A.; Bartlett, L.; Pilgrim, C. Effect of foundry waste on the mechanical properties of Portland Cement Concrete. Constr. Build. Mater. 2017, 135, 674–681. [Google Scholar] [CrossRef]
- Siddique, R.; de Schutter, G.; Noumowe, A. Effect of used-foundry sand on the mechanical properties of concrete. Constr. Build. Mater. 2009, 23, 976–980. [Google Scholar] [CrossRef]
- Aguiar, I.; Cunha, S.; Aguiar, J. Application of Foundry Wastes in Eco-Efficient Construction Materials: A Review. Appl. Sci. 2025, 15, 10. [Google Scholar] [CrossRef]
- Sengul, O. Mechanical behavior of concretes containing waste steel fibers recovered from scrap tires. Constr. Build. Mater. 2016, 122, 649–658. [Google Scholar] [CrossRef]
- Aprianti, E.S. A huge number of artificial waste material can be supplementary cementitious material (SCM) for concrete production—A review. Part II. J. Clean. Prod. 2017, 4, 4178–4194. [Google Scholar] [CrossRef]
- Letelier, V.; Ortega, J.M.; Munoz, P.; Tarela, E.; Moriconi, G. Influence of Waste Brick Powder in the Mechanical Properties of Recycled Aggregate Concrete. Sustainability 2018, 10, 1037. [Google Scholar] [CrossRef]
- Hanif, A.; Kim, Y.; Lee, K.; Park, C.; Sim, J. Influence of cement and aggregate type on steam-cured concrete-an experimental study. Mag. Concr. Res. 2017, 69, 694–702. [Google Scholar] [CrossRef]
- Chatziaras, N.; Psomopoulus, C.S.; Themelis, N.J. Use of waste derived fuels in cement industry: A review. Manag. Environ. Qual. Int. J. 2016, 27, 178–193. [Google Scholar] [CrossRef]
- Tee, H.T.; Mo, K.H.; Lin, J.; Onn, C.C. An Overview of the Utilization of Common Waste as an Alternative Fuel in the Cement Industry. Hindawi Adv. Civ. Eng. 2023, 7127007. [Google Scholar] [CrossRef]
- Asamany, A.E.; Gibson, M.D.; Pegg, M.J. Evaluating the potential of waste plastics as fuel in cement kilns using bench-scale emissions analysis. Fuel 2017, 193, 178–186. [Google Scholar] [CrossRef]
- Madloon, N.A.; Saidur, R.; Hossain, M.S.; Rahim, N. A Critical Review on Energy Use and Savings in the Cement Industries. Renew. Sustain. Energy Rev. 2011, 15, 2042–2060. [Google Scholar] [CrossRef]
- Benhelal, E.; Zahedi, G.; Hashim, H. A novel design for green and economical cement manufacturing. J. Clean. Prod. 2012, 22, 60–66. [Google Scholar] [CrossRef]
- AZO Materials. Using XRF Determ. Free Lime Content Clinker Cem. Qual. Control. 2011. Available online: https://www.azom.com/article.aspx?ArticleID=5728 (accessed on 12 January 2025).
- Popescu, C.D.; Muntean, M.; Sharp, J.H. Industrial trial production of low energy belite cement. Cem. Concr. Compos. 2003, 25, 689–693. [Google Scholar] [CrossRef]
- Bouzidi, M.A.; Tahakourt, A.; Bouzidi, N.; Merab, D. Synthesis and characterization of belite cement with high hydraulic reactivity and low environmental impact. Arab. J. Sci. Eng. 2014, 39, 8659–8668. [Google Scholar] [CrossRef]
- Stanek, T.; Sulovsky, P. Active low-energy belite cement. Cem. Concr. Res. 2015, 68, 203–210. [Google Scholar] [CrossRef]
- Kacimi, L.; Simon-Masseron, A.; Salem, S.; Ghomari, A.; Derriche, Z. Synthesis of belite cement clinker of high hydraulic reactivity. Cem. Concr. Res. 2009, 39, 559–565. [Google Scholar] [CrossRef]
- Ashraf, W.; Olek, J. Carbonation behavior of hydraulic and non-hydraulic calcium silicates: Potential of utilizing low-lime calcium silicates in cement-based materials. J. Mater. Sci. 2016, 51, 6173–6191. [Google Scholar] [CrossRef]
- Sahu, S.; DeCristofaro, N. Solidia Cement. In Solidia Technol. White Paper; Solidia Cement’s Performance, Properties Detailed in White Paper Series—Cement Optimized. 2013. Available online: https://cementproducts.com/2013/12/26/solidia-cement-s-performance-properties-detailed-in-white-paper-series-2/ (accessed on 13 April 2025).
- Dambrauskas, T.; Baltakys, K.; Eisinas, A.; Siauciunas, R. A study on the thermal stability of kilchoanite synthesized under hydrothermal conditions. J. Therm. Anal. Calorim. 2017, 127, 229–238. [Google Scholar] [CrossRef]
- Hillert, M.; Sundman, B.; Wang, X. A reevaluation of the rankinite phase in the CaO-SiO2 system. Calphad 1991, 15, 53–58. [Google Scholar] [CrossRef]
- Wang, M.; Li, C.; Zhai, Y. Sorption-Desorption Behaviour of CO2 on Ca3Si2O7 Absorbent. In Proceedings of the Second China Energy Scientist Forum, Xuzhou, China, 18 October 2010. [Google Scholar]
- Smigelskyte, A.; Siauciunas, R.; Hilbig, H.; Detcker, M.; Urbonas, L.; Skripkiunas, G. Carbonated rankinite binder: Effect of curing parameters on microstructure, strength development, and durability performance. Sci. Rep. 2020, 10, 14462. [Google Scholar] [CrossRef]
- Hou, G.; Chen, J.; Lu, B.; Chetn, S.; Cui, E.; Nalguib, H.M.; Guo, M.-Z.; Zhang, Q. Composition design and pilot study of an advanced energy-saving and low-carbon rankinite clinker. Cem. Concr. Res. 2020, 127, 105926. [Google Scholar] [CrossRef]
- Qian, B.; Li, X.; Shen, X. Preparation and accelerated carbonation of low temperature sintered clinker with low Ca/Si ratio. J. Clean. Prod. 2016, 120, 249–259. [Google Scholar] [CrossRef]
- Daval, D.; Martinez, I.; Guigner, J.M.; Hellmann, R.; Corvisier, J.; Findling, N.; Dominici, C.; Goffé, B.; Guyot, F. Mechanism of wollastonite carbonation deduced from micro- to nanometer length scale observations. Am. Mineral. 2009, 94, 1707–1726. [Google Scholar] [CrossRef]
- Daval, D.; Martinez, I.; Corvisier, J.; Findling, N.; Goffé, B.; Guyot, F. Carbonation of Ca-bearing silicates, the case of wollastonite: Experimental investigations and kinetic modeling. Chem. Geol. 2009, 265, 63–78. [Google Scholar] [CrossRef]
- Seryotkin, Y.V.; Sokol, E.V.; Kokh, S.N. Natural pseudowollastonite: Crystal structure, associated minerals, and geological context. Lithos 2012, 134–135, 75–90. [Google Scholar] [CrossRef]
- Siauciunas, R.; Hilbig, H.; Prichockiene, E.; Smigelskyte, A.; Takulinskas, Z. Accelerated carbonation of C2SH-based dense concrete. Ceram. Int. 2020, 46, 29436–29442. [Google Scholar] [CrossRef]
- Siauciunas, R.; Takulinskas, Ž.; Prichockiene, E.; Setlskiene, A. Hydrothermal synthesis of α-C2SH and kilchoanite mixture and its application in CO2 hardening mortar. Ceram. Int. 2023, 49, 14864–14874. [Google Scholar] [CrossRef]
- Siauciunas, R.; Prichockiene, E.; Valancius, Z. The influence of Mg-impurities in raw materials on the synthesis of rankinite clinker and the strength of mortar hardening in CO2 environment. Materials 2023, 16, 2930. [Google Scholar] [CrossRef]
- Ashraf, W.; Jeong, H.; Atakan, V. Effects of high temperature on carbonated calcium silicate cement (CSC) and ordinary Portland cement (OPC) paste. In Proceedings of the 5th International Conference on Durability of Concrete Structures, Shenzhen, China, 30 June–1 July 2016. [Google Scholar]
- Jang, J.G.; Kim, G.M.; Kim, H.J.; Lete, H. Review on recent advances in CO2 utilization and sequestration. Constr. Build. Mater. 2016, 127, 762–773. [Google Scholar] [CrossRef]
- Kainiemi, L.; Eloneva, S.; Toikka, A.; Letvänen, J.; Järvinen, M. Opportunities and obstacles for CO2 mineralization: CO2 mineralization specific frames in the interviews of Finnish carbon capture and storage (CCS) experts. J. Clean. Prod. 2015, 94, 352–358. [Google Scholar] [CrossRef]
- Xuan, D.; Zhan, B.; Poon, C.S. Development of a new generation of eco-friendly concrete blocks by accelerated mineral carbonation. J. Clean. Prod. 2016, 133, 1235–1241. [Google Scholar] [CrossRef]
- Mazzella, A.; Errico, M.; Spiga, D. CO2 uptake capacity of coal fly ash: Influence of pressure and temperature on direct gas-solid carbonation. J. Environ. Chem. Eng. 2016, 4, 4120–4128. [Google Scholar] [CrossRef]
- Kashef-Haghighi, S.; Ghoshal, S. CO2 sequestration in concrete through accelerated carbonation curing in a flow-through reactor. Ind. Eng. Chem. Res. 2010, 49, 1143–1149. [Google Scholar] [CrossRef]
- García-González, C.A.; El Grouh, N.; Hidalgo, A.; Fraile, J.; López-Periago, A.M.; Andrade, C.; Domingo, C. New insights on the use of supercritical carbon dioxide for the accelerated carbonation of cement pastes. J. Supercrit. Fluids 2008, 43, 500–509. [Google Scholar] [CrossRef]
- Ashraf, W. Carbonation of cement-based materials: Challenges and opportunities. Constr. Build. Mater. 2016, 120, 558–570. [Google Scholar] [CrossRef]
- Rostami, V.; Shao, Y.; Boyd, A.J. Durability of concrete pipes subjected to combined steam and carbonation curing. Constr. Build. Mater. 2011, 25, 3345–3355. [Google Scholar] [CrossRef]
- Urbonas, L.; Heinz, D.; Hilbig, H.; Reger, J. The Effect of Supercritical Carbon Dioxide on the Properties of Hardened Cement Paste. Cem. Int. 2010, 8, 73–81. [Google Scholar]
- Zhang, D.; Shao, Y. Early age carbonation curing for precast reinforced concretes. Constr. Build. Mater. 2016, 113, 134–143. [Google Scholar] [CrossRef]
- Bertos, M.F.; Simons, S.J.R.; Hills, C.D.; Carety, P.J. A review of accelerated carbonation technology in the treatment of cement-based materials and sequestration of CO2. J. Hazard. Mater. 2004, B112, 193–205. [Google Scholar]
- Huet, B.; Tasoti, V.; Khalfallah, I. A review of Portland cement carbonation mechanisms in CO2-rich environment. Energy Procedia 2011, 4, 5275–5282. [Google Scholar] [CrossRef]
- Leemann, A.; Moro, F. Carbonation of concrete: The role of CO2 concentration, relative humidity and CO2 buffer capacity. Mater. Struct. 2017, 50, 30. [Google Scholar] [CrossRef]
- Galan, I.; Andrade, C.; Mora, P.; Sanjuan, M.A. Sequestration of CO2 by concrete carbonation. Environ. Sci. Technol. 2010, 44, 3181–3186. [Google Scholar] [CrossRef]
- Mahoutian, M.; Ghouleh, Z.; Shao, Y. Synthesis of waste-based carbonation cement. Mater. Struct. 2016, 49, 4679–4690. [Google Scholar] [CrossRef]
- Neves Junior, A.; Filho, R.D.T.; Fairbairn, E.M.R.; Dwetck, J. A study of the carbonation profile of cement pastes by thermogravimetry and its effect on the compressive strength. J. Therm. Anal. Calorim. 2014, 116, 69–76. [Google Scholar] [CrossRef]
- Hussain, S.; Bhunia, D.; Singh, S.B. Comparative study of accelerated carbonation of plain cement and fly-ash concrete. J. Build. Eng. 2017, 10, 26–31. [Google Scholar] [CrossRef]
- Wang, X.Y. Modeling of hydration, compressive strength, and carbonation of Portland-limestone cement (PLC) concrete. Materials 2017, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, I.; Obata, D.; Nanjo, H.; Yokozeki, K.; Torichigai, T.; Moriokal, M.; Higuchi, T. New Ecological Concrete That Reduces CO2 Emissions Below Zero Level; New Method for CO2 Capture and Storage. Energy Procedia 2013, 37, 6018–6025. [Google Scholar] [CrossRef]
- Calera. Available online: http://www.calera.com/ (accessed on 17 January 2025).
- Evans, S.M.; Vlasopoulos, N. Novacem—Carbon Negative Cement to Transform the Construction Industry; Energy Futures Lab, Imperial College: London, UK, 2008. [Google Scholar]
- Savija, B.; Mladena, L. Carbonation of cement paste: Understanding, challenges, and opportunities. Constr. Build. Mater. 2016, 117, 285–301. [Google Scholar] [CrossRef]
- Lagerblad, B. Carbon Dioxide Uptake During Concrete Life Cycle—State of the Art; Swedish Cement and Concrete Research Institute: Stockholm, Sweden, 2005. [Google Scholar]
- Zhang, D.; Ghouleh, Z.; Shao, Y. Review on carbonation curing of cement-based materials. J. CO2 Util. 2017, 21, 119–131. [Google Scholar] [CrossRef]
- Johannesson, B.; Utgenannt, P. Microstructural changes caused by carbonation of cement mortar. Cem. Concr. Res. 2001, 31, 925–931. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Brice, D.G.; Kilculletn, A.; Duxson, P.; van Deventer, J.S. Accelerated carbonation testing of alkali-activated binders significantly underestimates service life: The role of pore solution chemistry. Cem. Concr. Res. 2012, 42, 1317–1326. [Google Scholar] [CrossRef]
- Goni, S.; Gaztanaga, M.T.; Guerrero, A. Role of cement type on carbonation attack. J. Mater. Res. 2002, 17, 1834–1842. [Google Scholar] [CrossRef]
- Rostami, V.; Shao, Y.; Boyd, A.J. Carbonation curing versus steam curing for precast concrete production. J. Mater. Civ. Eng. 2012, 24, 1221–1229. [Google Scholar] [CrossRef]
- Klemm, W.A.; Berger, R.L. Accelerated curing of cementitious systems by carbon dioxide: Part I. Portland cement. Cem. Concr. Res. 1972, 2, 567–576. [Google Scholar] [CrossRef]
- Shao, Y.; Mirza, M.S.; Mu, X. CO2 sequestration using calcium-silicate concrete. Can. J. Civ. Eng. 2006, 33, 776–784. [Google Scholar] [CrossRef]
- Ashraf, W.; Olek, J.; Atakan, V. Microscopic features of carbonated calcium silicate based cement paste and mortar. Cem. Concr. Res. 2017, 100, 361–372. [Google Scholar] [CrossRef]
- Shi, C.; Wu, Y. Studies on Some Factors Affecting CO2 Curing of Lightweight Concrete Products. Resour. Conserv. Recycl. 2008, 52, 1087–1092. [Google Scholar] [CrossRef]
- Taylor, C.M.V.; Rubin, J.B.; Carey, J.W.; Jones, R.; Baglin, F.G. Next Generation Enhancement of Cements by the Addition of Industrial Wastes and Subsequent Treatment with Supercritical CO2. In Proceedings of the Green Chemistry and Engineering Conference: Implementing Vision 2020 for the Environment, American Chemical Society, Washington, DC, USA, 23–25 June 1997. [Google Scholar]
- Wang, T.; Huang, H.; Hu, X.; Fang, M.; Luo, Z.; Guo, R. Accelerated Mineral Carbonation Curing of Cement Paste for CO2 Sequestration and Enhanced Properties of Blended Calcium Silicate. Chem. Eng. J. 2017, 323, 320–329. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, C.A.; Hidalgo, A.; Andrade, C.; Alonso, M.C.; Frailet, J.; Lopez-Periago, A.M.; Domingo, C. Modification of Composition and Microstructure of Portland Cement Pastes as a Result of Natural and Supercritical Carbonation Procedures. Ind. Eng. Chem. Res. 2006, 45, 4985–4992. [Google Scholar] [CrossRef]
- Leemann, A.; Nygaard, P.; Kaufmann, J.; Losetr, R. Relation between Carbonation Resistance, Mix Design and Exposure of Mortar and Concrete. Cem. Concr. Compos. 2015, 62, 33–43. [Google Scholar] [CrossRef]
- Galan, I.; Andrade, C.; Castellote, M. Natural and Accelerated CO2 Binding Kinetics in Cement Paste at Different Relative Humidities. Cem. Concr. Res. 2013, 49, 21–28. [Google Scholar] [CrossRef]
- Asavapisit, S.; Fowler, G.; Cheeseman, C.R. Solution Chemistry During Cement Hydration in the Presence of Metal Hydroxide Wastes. Cem. Concr. Res. 1997, 27, 1249–1260. [Google Scholar] [CrossRef]
- Liu, L.; Ha, J.; Hashida, T. Development of a CO2 Solidification Method for Recycling Autoclaved Lightweight Concrete Waste. J. Mater. Sci. Lett. 2001, 20, 1791–1794. [Google Scholar] [CrossRef]
- Van-Loc, T.; Bonnet, S.; Kiesse, T.S.; Ventura, A. A New Meta-Model to Calculate Carbonation Front Depth within Concrete Structures. Constr. Build. Mater. 2016, 129, 172–181. [Google Scholar]
- Silva, R.V.; Neves, R.; de Brito, J.; Dhir, R. Carbonation Behaviour of Recycled Aggregate Concrete. Cem. Concr. Compos. 2015, 62, 22–32. [Google Scholar] [CrossRef]
- Thiery, M.; Dangla, P.; Belin, P.; Habert, G.; Roussel, N. Carbonation Kinetics of a Bed of Recycled Concrete Aggregates: A Laboratory Study on Model Materials. Cem. Concr. Res. 2013, 46, 50–65. [Google Scholar] [CrossRef]
- Duguid, A.; Scherer, G.W. Degradation of Oilwell Cement Due to Exposure to Carbonated Brine. Int. J. Greenh. Gas Control 2010, 4, 546–560. [Google Scholar] [CrossRef]
- Urbonas, L.; Leno, V.; Heinz, D. Effect of carbonation in supercritical CO2 on the properties of hardened cement paste of different alkalinity. Constr. Build. Mater. 2016, 123, 704–711. [Google Scholar] [CrossRef]
- Qian, L.; Jiaxiang, L.; Liqian, Q. Effects of temperature and carbonation curing on the mechanical properties of steel slag-cement binding materials. Constr. Build. Mater. 2016, 124, 999–1006. [Google Scholar]
- Hidalgo, A.; Domingo, C.; Garcia, C.; Petit, S.; Andrade, C.; Alonso, C. Microstructural changes induced in Portland cement-based materials due to natural and supercritical carbonation. J. Mater. Sci. 2008, 43, 3101–3111. [Google Scholar] [CrossRef]
- Short, N.R.; Purnell, P.; Page, C.L. Preliminary investigations into the supercritical carbonation of cement pastes. J. Mater. Sci. 2001, 36, 35–41. [Google Scholar] [CrossRef]
- Chang, C.F.; Chen, J.W. The experimental investigation of concrete carbonation depth. Cem. Concr. Res. 2006, 36, 1760–1767. [Google Scholar] [CrossRef]
- Ahmad, S.; Assaggaf, R.A.; Maslehuddin, M.; Al-Amoudi, O.S.B.; Adetkunle, S.K.; Ali, S.I. Effects of carbonation pressure and duration on strength evolution of concrete subjected to accelerated carbonation curing. Constr. Build. Mater. 2017, 136, 565–573. [Google Scholar] [CrossRef]
- Wigand, M.; Kaszuba, J.P.; Carey, J.W.; Hollis, W.K. Geochemical Effects of CO2 Sequestration on Fractured Wellbore Cement at the Cement/Caprock Interface. Chem. Geol. 2009, 265, 122–133. [Google Scholar] [CrossRef]
- Kjellsen, K.O.; Guimaraes, M.; Nilson, A. The CO2 Balance of Concrete in a Life Cycle Perspective. Danish Technological Institute; Aarhus, Denmark, Nordic Innovation Center: 2005.
- Liu, X.; Feng, P.; Cai, Y.; Yu, X.; Yu, C.; Raln, Q. Carbonation behavior of calcium silicate hydrate (C-SH): Its potential for CO2 capture. Chem. Eng. J. 2022, 431, 134243. [Google Scholar] [CrossRef]
- Santra, A.; Sweatman, R. Understanding the Long-Term Chemical and Mechanical Integrity of Cement in a CCS Environment. Energy Procedia 2011, 4, 5243–5250. [Google Scholar] [CrossRef]
- Berger, J.M.; Bukowski, R.L. Reactivity and Strength Development of CO2 Activated Non-hydraulic Calcium Silicates. Cem. Concr. Res. 1979, 9, 57–68. [Google Scholar]
- Chang, J.; Fang, Y.; Shang, X. The Role of β-C2S and γ-C2S in Carbon Capture and Strength Development. Mater. Struct. 2016, 49, 4417–4424. [Google Scholar] [CrossRef]
- Hyvert, N.; Sellier, A.; Duprat, F.; Rougetau, P.; Fralncisco, P. Dependency of C–S–H Carbonation Rate on CO2 Pressure to Explain Transition from Accelerated Tests to Natural Carbonation. Cem. Concr. Res. 2010, 40, 1582–1589. [Google Scholar] [CrossRef]
- Borges, P.H.R.; Costa, J.O.; Milestone, N.B.; Lynsdale, C.J.; Streatfield, R.E. Carbonation of CH and C–S–H in Composite Cement Pastes Containing High Amounts of BFS. Cem. Concr. Res. 2010, 40, 284–292. [Google Scholar] [CrossRef]
- Zhou, Q.; Glasser, F.P. Kinetics and Mechanism of the Carbonation of Ettringite. Adv. Cem. Res. 2000, 12, 131–136. [Google Scholar] [CrossRef]
- Jang, J.G.; Lee, H.K. Microstructural densification and CO2 uptake promoted by the carbonation curing of belite-rich Portland cement. Cem. Concr. Res. 2016, 82, 50–57. [Google Scholar] [CrossRef]
- Shi, C.; Qu, B.; Provis, J.L. Recent progress in low-carbon binders. Cem. Concr. Res. 2019, 122, 227–250. [Google Scholar] [CrossRef]
- Fang, Y.; Chang, J. Rapid hardening β-C2S mineral and microstructure changes activated by accelerated carbonation curing. J. Therm. Anal. Calorim. 2017, 129, 681–689. [Google Scholar] [CrossRef]
- Šmigelskytė, A. Synthesis and Performance of Environmentally Friendly Calcium Silicate Cement. Ph.D. Dissertation, Kaunas University of Technology, Kaunas, Lithuania, 2019. [Google Scholar]
- Guan, X.; Liu, S.; Feng, C.; Qiu, M. The hardening behavior of γ-C2S binder using accelerated carbonation. Constr. Build. Mater. 2016, 114, 204–207. [Google Scholar] [CrossRef]
- Saito, T.; Sakai, E.; Morioka, M.; Qiu, M. Carbonation of γ-Ca2SiO₄ and the mechanism of vaterite formation. J. Adv. Concr. Technol. 2010, 8, 273–280. [Google Scholar] [CrossRef]
- Saito, T.; Khamhou, S.; Yumoto, T.; Otsuki, N. Permeability of sulfate ions in cementitious materials containing c-Ca2SiO₄ after autoclave curing and accelerated carbonation. J. Adv. Concr. Technol. 2011, 9, 223–230. [Google Scholar] [CrossRef]
- Mabudo, G.M.; Lee, S.; Kang, S.; Song, M.; Teng, S.; Li, Q.; Liu, Y.; Conforti, A.; Tiberti, G.; Plizzari, G.A.; et al. Physical properties and carbon dioxide capture of synthetic γ-C2S cement composites in the early days of curing. Mag. Concr. Res. 2016, 68, 1079–1084. [Google Scholar] [CrossRef]
- Boscaro, F.; Palacios, M.; Robert, J. Flatt Formulation of low clinker blended cements and concrete with enhanced fresh and hardened properties. Cem. Concr. Res. 2021, 150, 106605. [Google Scholar] [CrossRef]
- Liu, W.; Lin, S.; Li, Y.; Long, W.; Dong, Z.; Tang, L. Slag Blended Cement Paste Carbonation under Different CO2 Concentrations: Controls on Mineralogy and Morphology of Products. Materials 2020, 13, 3404. [Google Scholar] [CrossRef]
- Kuzielová, E.; Slan, M.; Žemlička, M.; Másilko, J. Accelerated carbonation of oil-well cement blended with pozzolans and latent hydraulic materials. J. Therm. Anal. Calorim. 2023, 148, 9963–9977. [Google Scholar] [CrossRef]
- Siauciunas, R.; Prichockiene, E.; Valancius, Z.; Elsteris, A. Hardening of mortars from blended cement with opoka additive in CO2 environment. Ceramics 2024, 7, 1301–1315. [Google Scholar] [CrossRef]
- Siauciunas, R.; Mikaliunaite, J.; Urbonas, L.; Baltakys, K. Tribochemical and thermal activation of α-C2S hydrate as precursor for cementitious binders. J. Therm. Anal. Calorim. 2014, 118, 817–823. [Google Scholar] [CrossRef]
- Yu, J.; Wu, H.L.; Mishra, D.K.; Li, G.; Letung, C.K. Compressive strength and environmental impact of sustainable blended cement with high-dosage limestone and calcined clay (LC2). J. Clean. Prod. 2021, 278, 123616. [Google Scholar] [CrossRef]
- Ostovari, H.; Müller, L.J.; Skoček, J.; Bardow, A. From unavoidable CO2 source to CO2 sink? A cement industry based on CO2 mineralization. Environ. Sci. Technol. 2021, 55, 5212–5223. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, A.; Kishar, E.A.; Hamed, S.A. Uses of EAFs in production of blended cements. J. Sci. Res. Sci. 2020, 37, 111–124. [Google Scholar] [CrossRef]
- Seo, J.; Amr, I.T.; Park, S.; Bamagain, R.A.; Fadhetl, B.A.; Kim, G.M.; Hunaidy, A.S.; Lee, H.K. CO2 uptake of carbonation-cured cement blended with ground volcanic ash. Materials 2018, 11, 2187. [Google Scholar] [CrossRef]
- Telesca, A.; Ibriş, N.; Marroccoli, M. Use of potabilized water sludge in the production of low-energy blended calcium sulfoaluminate cements. Appl. Sci. 2021, 11, 1679. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Ilić, T.; Ruiz-Agudo, E.; Elert, K. Carbonation mechanisms and kinetics of lime-based binders: An overview. Cem. Concr. Res. 2023, 173, 107301. [Google Scholar] [CrossRef]
- Freitas, A.A.; Santos, R.L.; Colaco, R.; Horta, R.B.; Lopets, J.N.C. From Lime to Silica and Alumina: Systematic Modeling of Cement Clinkers Using a General Force-Field. Phys. Chem. Chem. Phys. 2015, 17, 18477–18494. [Google Scholar] [CrossRef]
- Smigelskyte, A.; Siauciunas, R. Parameter influence on the rankinite binder paste and mortar accelerated carbonation curing. J. Therm. Anal. Calorim. 2019, 138, 2651–2659. [Google Scholar] [CrossRef]
- Wang, K.; Ren, L.; Yang, L. Excellent Carbonation Behavior of Rankinite Prepared by Calcining the C-S-H: Potential Recycling of Waste Concrete Powders for Prefabricated Building Products. Materials 2018, 11, 1474. [Google Scholar] [CrossRef]
- Ashraf, W.; Olek, J. Elucidating the Accelerated Carbonation Products of Calcium Silicates Using Multi-Technique Approach. J. CO2 Util. 2018, 23, 61–74. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, A.; Orlando, A.; Montegrossi, G.; Huet, B.; Virgili, G.; Vaselli, O. Experimental analysis on the carbonation rate of Portland cement at room temperature and CO2 partial pressure from 1 to 51 bar. Cem Concr Compos. 2021, 124, 104271. [Google Scholar] [CrossRef]
- Padmalal, A.; Kulkarni, K.S.; Rawat, P.; Sugandhini, H.K. Efficacy of Accelerated Carbonation Curing and Its Influence on the Strength Development of Concrete. Buildings 2024, 14, 2573. [Google Scholar] [CrossRef]
- Pizzol, V.D.; Mendes, L.M.; Savastano, H., Jr.; Frías, M.; Davila, F.; Cincotto, M.; John, V.; Tonoli, G. Mineralogical and Microstructural Changes Promoted by Accelerated Carbonation and Ageing Cycles of Hybrid Fiber–Cement Composites. Constr. Build. Mater. 2014, 68, 750–756. [Google Scholar] [CrossRef]
- Mo, L.; Panesar, D.K. Accelerated Carbonation—A Potential Approach to Sequester CO2 in Cement Paste Containing Slag and Reactive MgO. Cem. Concr. Compos. 2013, 43, 69–77. [Google Scholar] [CrossRef]
- Lackner, K.S.; Wendt, C.H.; Butt, D.P.; Joycet, E.L., Jr.; Shalrp, D.H. Carbon Dioxide Disposal in Carbonate Minerals. Energy 1995, 20, 1153–1170. [Google Scholar] [CrossRef]
- Ismailov, A.; Merilaita, N.; Levänen, E. Accelerated Carbonation of High-Calcite Wollastonite Tailings. Minerals 2024, 14, 415. [Google Scholar] [CrossRef]
- Min, Y.; Li, Q.; Voltolini, M.; Kneafsey, T.; Jun, Y.-S. Wollastonite Carbonation in Water-Bearing Supercritical CO2: Effects of Particle Size. Environ. Sci. Technol. 2017, 51, 13044–13053. [Google Scholar] [CrossRef]
- Miller, Q.R.S.; Thompson, C.J.; Loring, J.S.; Windisch, C.; Bowden, M.; Hoyt, D.; Hu, J.; Alrey, B.; Rosso, K.; Schaef, H. Insights into Silicate Carbonation Processes in Water-Bearing Supercritical CO2 Fluids. Int. J. Greenh. Gas Control 2013, 15, 104–118. [Google Scholar] [CrossRef]
- Fuhaid, A.F.A.; Niaz, A. Carbonation and Corrosion Problems in Reinforced Concrete Structures. Buildings 2022, 12, 586. [Google Scholar] [CrossRef]
- Babalola, O.E.; Awoyera, P.O.; Le, D.H.; Olalusi, O.B.; Bhagat, S. Anthropogenic Carbon Aerosol Induced Carbonation in Reinforced Concrete: Deterioration Effects on Mechanical Properties. J. Eng. Res. Appl. 2021, 57, 139–148. [Google Scholar] [CrossRef]
- Ikumapayi, C.M.; Adeniji, A.A.; Obisesan, A.A.; Odeyemi, O.; Ajayi, J.A. Effects of Carbonation on the Properties of Concrete. Sci. Rev. 2019, 512, 205–214. [Google Scholar]
- Yin, J.; Zhang, X.C.; Chi, Y.; Pang, Q.L. Analysis of Concrete Performance Used for Non-Ballasted Track Base Plate Based on the Coupling of Effect of Acid and Carbonization. Adv. Mater. Res. 2013, 639–640, 382–389. [Google Scholar]
- Pašek, J.; Vejvara, L. Degradation of the Building Structures Due to Carbonation of Concrete. Adv. Mater. Res. 2014, 1020, 37. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, G.; Ye, H.; Zeng, Q.; Zhang, Z.; Tian, Z.; Jin, X.; Jin, N.; Chen, Z.; Walng, J. Corrosion of Steel Rebar in Concrete Induced by Chloride Ions Under Natural Environments. Constr. Build. Mater. 2023, 369, 130504. [Google Scholar] [CrossRef]
- Ali, M.; Shams, M.A.; Bheel, N.; Almaliki, A.H.; Mahmoud, A.S.; Dodo, Y.A.; Benjeddou, O. A review on chloride induced corrosion in reinforced concrete structures: Lab and in situ investigation. RSC Adv. 2024, 14, 37252–37271. [Google Scholar] [CrossRef]
- Al-Rousan, E.T.; Khalid, H.R.; Rahman, M.K. Fresh, mechanical, and durability properties of basalt fiber-reinforced concrete (BFRC): A review. Dev. Built Environ. 2023, 14, 100155. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).