Cultivar-Specific Responses of Spinach to Root-Zone Cooling in Hydroponic Systems in a Greenhouse Under Warm Climates
Abstract
1. Introduction
2. Materials and Methods
2.1. Seedling Propagation
2.2. Experimental Design and Treatments
2.3. Data Collection
2.3.1. Growth and Morphology
2.3.2. Plant Physiological Responses and Phytochemical and Biochemical Analyses
Chlorophyll Fluorescence and Pigments
Phytochemicals
2.3.3. Stress Indicators
2.4. Statistical Analyses
3. Results
3.1. Growth and Morphological Parameters
3.2. Physiological Attributes
3.3. Stress Indicators
4. Discussion
4.1. Root-Zone Cooling Can Improve the Growth and Morphology of Spinach in Warm Climates, but the Effectiveness Varies with Cultivar and Air Temperature
4.2. Root-Zone Cooling Has the Potential to Improve Spinach Physiology, Reduce Stress, and Protect Phytochemical Properties Under Warm Climate
4.3. Optimal Root-Zone Cooling Temperature with Consideration of Electricity Consumption and Plant Performance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, S. Global Leafy Greens Market Overview. 2025. Available online: https://www.marketresearchfuture.com/reports/leafy-greens-market-21536 (accessed on 14 January 2025).
- Lomnitski, L.; Bergman, M.; Nyska, A.; Ben-Shaul, V.; Grossman, S. Composition, efficacy, and safety of spinach extracts. Nutr. Cancer 2003, 46, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Arru, L.; Mussi, F.; Forti, L.; Buschini, A. Biological effect of different spinach extracts in comparison with the individual components of the phytocomplex. Foods 2021, 10, 382. [Google Scholar] [CrossRef] [PubMed]
- Masanetz, C.; Guth, H.; Grosch, W. Fishy and hay-like off-flavours of dry spinach. Z. Leb. Und-Forsch. A 1998, 206, 108–113. [Google Scholar] [CrossRef]
- Rajaseger, G.; Chan, K.L.; Tan, K.Y.; Ramasamy, S.; Khin, M.C.; Amaladoss, A.; Haribhai, P.K. Hydroponics: Current trends in sustainable crop production. Bioinformation 2023, 19, 925–938. [Google Scholar] [CrossRef]
- Hooks, T.; Sun, L.; Kong, Y.; Masabni, J.; Niu, G. Effect of nutrient solution cooling in summer and heating in winter on the performance of baby leafy vegetables in deep-water hydroponic systems. Horticulturae 2022, 8, 749. [Google Scholar] [CrossRef]
- Barbosa, G.; Gadelha, F.; Kublik, N.; Proctor, A.; Reichelm, L.; Weissinger, E.; Wohlleb, G.; Halden, R. Comparison of Land, Water, and Energy Requirements of Lettuce Grown Using Hydroponic vs. Conventional Agricultural Methods. Int. J. Environ. Res. Public Health 2015, 12, 6879–6891. [Google Scholar] [CrossRef]
- Yamori, W.; Noguchi, K.O.; Terashima, I. Temperature acclimation of photosynthesis in spinach leaves: Analyses of photosynthetic components and temperature dependencies of photosynthetic partial reactions. Plant Cell Environ. 2005, 28, 536–547. [Google Scholar] [CrossRef]
- Agrawal, D.; Jajoo, A. Study of high temperature stress induced damage and recovery in photosystem II (PSII) and photosystem I (PSI) in Spinach leaves (Spinacia oleracea). J. Plant Biochem. Biotechnol. 2021, 30, 532–544. [Google Scholar] [CrossRef]
- Zaman, Q.U.; Abbasi, A.; Tabassum, S.; Ashraf, K.; Ahmad, Z.; Siddiqui, M.H.; Alamri, S.; Maqsood, S.; Sultan, K. Calcium induced growth, physio-biochemical, antioxidant, osmolyte adjustments and phytoconstituent status in spinach under heat stress. S. Afr. J. Bot. 2022, 149, 701–711. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.D.; Barman, M.; Mitra, A. Photosynthetic apparatus plays a central role in photosensitive physiological acclimations affecting spinach (Spinacia oleracea L.) growth in response to blue and red photon flux ratios. Environ. Exp. Bot. 2018, 156, 170–182. [Google Scholar] [CrossRef]
- Wang, X.; Altaf, M.A.; Hao, Y.; Wang, Z.; Zhu, G. Effect of heat stress on root architecture, photosynthesis, and antioxidant profile of water spinach (Ipomoea aquatica Forsk) seedlings. Horticulturae 2023, 9, 923. [Google Scholar] [CrossRef]
- Aji, G.K.; Hatou, K.; Morimoto, T. Modeling the dynamic response of plant growth to root zone temperature in hydroponic chili pepper plants using neural networks. Agriculture 2020, 10, 234. [Google Scholar] [CrossRef]
- Moccio, M.; Dunn, B.L.; Kaur, A.; Fontanier, C.; Zhang, L. Effects of root zone temperature of hydroponic lettuce affects plant growth, nutrient uptake, and vitamin a content. HortScience 2024, 59, 255–257. [Google Scholar] [CrossRef]
- Yasuba, K.I.; Yashiro, M.; Matsuo, K. Effect of cooling the root zone with a duct of microporous film on the cultivation of spinach. J. Jpn. Soc. Hortic. Sci. 2006, 75, 109–115. [Google Scholar] [CrossRef]
- Sumarni, E.; Suhardiyanto, H.; Seminar, K.B.; Saptomo, S.K. Seed potato production using aeroponics system with zone cooling in wet tropical lowlands. In Proceedings of the II Asia Pacific Symposium on Postharvest Research Education and Extension: APS2012, Yogyakarta, Indonesia, 18–20 September 2012; Volume 1011, pp. 141–145. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Matsuo, S.; Suzuki, K.; Kanayama, Y.; Kanahama, K. Root-zone cooling at high air temperatures enhances physiological activities and internal structures of roots in young tomato plants. J. Jpn. Soc. Hortic. Sci. 2013, 82, 322–327. [Google Scholar] [CrossRef]
- He, J.; See, X.E.; Qin, L.; Choong, T.W. Effects of root-zone temperature on photosynthesis, productivity and nutritional quality of aeroponically grown salad rocket (Eruca sativa) vegetable. Am. J. Plant Sci. 2016, 7, 1993–2005. [Google Scholar] [CrossRef]
- Al-Rawahy, M.S.; Al-Rawahy, S.A.; Al-Mulla, Y.A.; Nadaf, S.K. Effect of cooling root-zone temperature on growth, yield and nutrient uptake in cucumber grown in hydroponic system during summer season in cooled greenhouse. J. Agric. Sci. 2018, 11, 47. [Google Scholar] [CrossRef]
- Amir, R.; Hussain, S.; Noor-ul-Ain, H.; Hussain, A.; Yun, B.W. ROS mediated plant defense against abiotic stresses. In Plant Biotechnology: Progress in Genomic Era; Springer: Singapore, 2019; pp. 481–515. [Google Scholar] [CrossRef]
- Liu, S.; Yang, R. Regulations of reactive oxygen species in plants abiotic stress: An integrated overview. In Plant Life Under Changing Environment; Academic Press: Cambridge, MA, USA, 2020; pp. 323–353. [Google Scholar] [CrossRef]
- Tariq, A.; Ahmed, A. Plant Phenolics Production: A Strategy for Biotic Stress Management. In Plant Phenolics in Biotic Stress Management; Springer Nature: Singapore, 2024; pp. 441–454. [Google Scholar] [CrossRef]
- Carvajal, M.; Cooke, D.T.; Clarkson, D.T. Plasma membrane fluidity and hydraulic conductance in wheat roots: Interactions between root temperature and nitrate or phosphate deprivation. Plant Cell Environ. 1996, 19, 1110–1114. [Google Scholar] [CrossRef]
- Park, C.J.; Shin, R. Calcium channels and transporters: Roles in response to biotic and abiotic stresses. Front. Plant Sci. 2022, 13, 964059. [Google Scholar] [CrossRef]
- Fortunato, S.; Lasorella, C.; Dipierro, N.; Vita, F.; de Pinto, M.C. Redox signaling in plant heat stress response. Antioxidants 2023, 12, 605. [Google Scholar] [CrossRef]
- Kang, M.; Jang, S.; Kang, I.; Yang, G.; Son, Y.G.; Kim, J.Y.; Goto, E.; Son, K. Root-Zone Cooling Effects on Growth, Physiological, and Biochemical Responses of Taraxacum coreanum Under Hydroponics. J. Plant Growth Regul. 2025. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M. Pre-harvest UV-B radiation and photosynthetic photon flux density interactively affect plant photosynthesis, growth, and secondary metabolites accumulation in basil (Ocimum basilicum) plants. Agronomy 2019, 9, 434. [Google Scholar] [CrossRef]
- Chew, K.K.; Ng, S.Y.; Thoo, Y.Y.; Khoo, M.Z.; Wan Aida, W.M.; Ho, C.W. Effect of Ethanol Concentration, Extraction Time and Extraction Temperature on the Recovery of Phenolic Compounds and Antioxidant Capacity of Centella asiatica Extracts. Int. Food Res. J. 2011, 18, 571. [Google Scholar]
- Tan, P.W.; Tan, C.P.; Ho, C.W. Antioxidant Properties: Effects of Solid-to-Solvent Ratio on Antioxidant Compounds and Capacities of Pegaga (Centella asiatica). Int. Food Res. J. 2011, 18, 557–562. [Google Scholar]
- Jessup, W.; Dean, R.T.; Gebicki, J.M. Iodometric determination of hydroperoxides in lipids and proteins. Methods Enzymol. 1994, 233, 289–303. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Vinodh, S.; Kannan, M. Effect of different chemicals on physiological and biochemical parameters of cut stem Lilium cv. Pollyanna during post-harvest period. J. Pharmacogn. Phytochem. 2019, 8, 2270–2274. [Google Scholar]
- Roussev, M.; Lehotay, S.J.; Julius, P. Cryogenic Sample Processing with Liquid Nitrogen for Effective and Efficient Monitoring of Pesticide Residues in Foods and Feeds. J. Agric. Food Chem. 2019, 67, 9203–9209. [Google Scholar] [CrossRef]
- Gent, M.P. Effect of irradiance and temperature on composition of spinach. HortScience 2016, 51, 133–140. [Google Scholar] [CrossRef]
- Cometti, N.N.; Bremenkamp, D.M.; Galon, K.; Hell, L.R.; Zanotelli, M.F. Cooling and concentration of nutrient solution in hydroponic lettuce crop. Hortic. Bras. 2013, 31, 287–292. [Google Scholar] [CrossRef]
- Fazlil Ilahi, W.F.; Ahmad, D.; Husain, M.C. Effects of root zone cooling on butterhead lettuce grown in tropical conditions in a coir-perlite mixture. Hortic. Environ. Biotechnol. 2017, 58, 1–4. [Google Scholar] [CrossRef]
- Mitrichenko, A.N.; Farkhutdinov, R.G.; Teplova, I.P.; Veselov, S.Y.; Kudoyarova, G.R. The effects of temperature on cytokinin levels in shoots and roots of wheat seedlings. Russ. J. Plant Physiol. 1998, 45, 402–404. [Google Scholar]
- Oh, W.; Park, J.H.; Kim, H.K.; Rhie, Y.H.; Chun, C.; Kim, K.S. Root-zone cooling improves growth of Cyclamen persicum under heat stress. Hortic. Environ. Biotechnol. 2007, 48, 68. [Google Scholar]
- Sun, J.; Lu, N.; Xu, H.; Maruo, T.; Guo, S. Root zone cooling and exogenous spermidine root-pretreatment promoting Lactuca sativa L. growth and photosynthesis in the high-temperature season. Front. Plant Sci. 2016, 7, 368. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant. 2015, 153, 284–298. [Google Scholar] [CrossRef]
- Xia, Z.Q.; Si, L.Y.; Jin, Y.; Fu, Y.F.; Wang, Q.; Lu, H.D. Effects of root zone temperature increase on physiological indexes and photosynthesis of different genotype maize seedlings. Russ. J. Plant Physiol. 2021, 68, 169–178. [Google Scholar] [CrossRef]
- Beppu, K.; Iino, M.; Kataoka, I. Effect of root zone cooling on flower development and fruit set of ‘Satohnishiki’sweet cherry. J. Appl. Hortic. 2008, 10, 113–115. [Google Scholar] [CrossRef]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B Biol. 2014, 137, 116–126. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Y. Phenolic compounds: Potential impact on plant growth and stress resistance. Plant Physiol. Biochem. 2017, 119, 178–185. [Google Scholar] [CrossRef]
- Yamagishi, M. Mechanisms by Which High Temperatures Suppress Anthocyanin Coloration in Flowers and Fruits, and Discovery of Floricultural Crops That Exhibit High-Temperature-Tolerant Flower Pigmentation. Hortic. J. 2024, 93, 203–215. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Lu, N.; Kagawa, N.; Kitayama, M.; Takagaki, M. Short-term root-zone temperature treatment enhanced the accumulation of secondary metabolites of hydroponic coriander (Coriandrum sativum L.) grown in a plant factory. Agronomy 2020, 10, 413. [Google Scholar] [CrossRef]
- Wang, P.; Liu, W.C.; Han, C.; Wang, S.; Bai, M.Y.; Song, C.P. Reactive oxygen species: Multidimensional regulators of plant adaptation to abiotic stress and development. J. Integr. Plant Biol. 2024, 66, 330–367. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Franzoni, G.; Petrini, A.; Santoro, P.; Mori, J.; Ferrante, A.; Cocetta, G. Investigating physiological responses of wild rocket subjected to artificial Ultraviolet B irradiation. Sci. Hortic. 2023, 322, 112415. [Google Scholar] [CrossRef]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef]
- Chadirin, Y.; Hidaka, K.; Sago, Y.; Wajima, T.; Kitano, M. Application of temperature stress to root zone of spinach II. Effect of the high temperature pre-treatment on quality. Environ. Control Biol. 2011, 49, 157–164. [Google Scholar] [CrossRef]
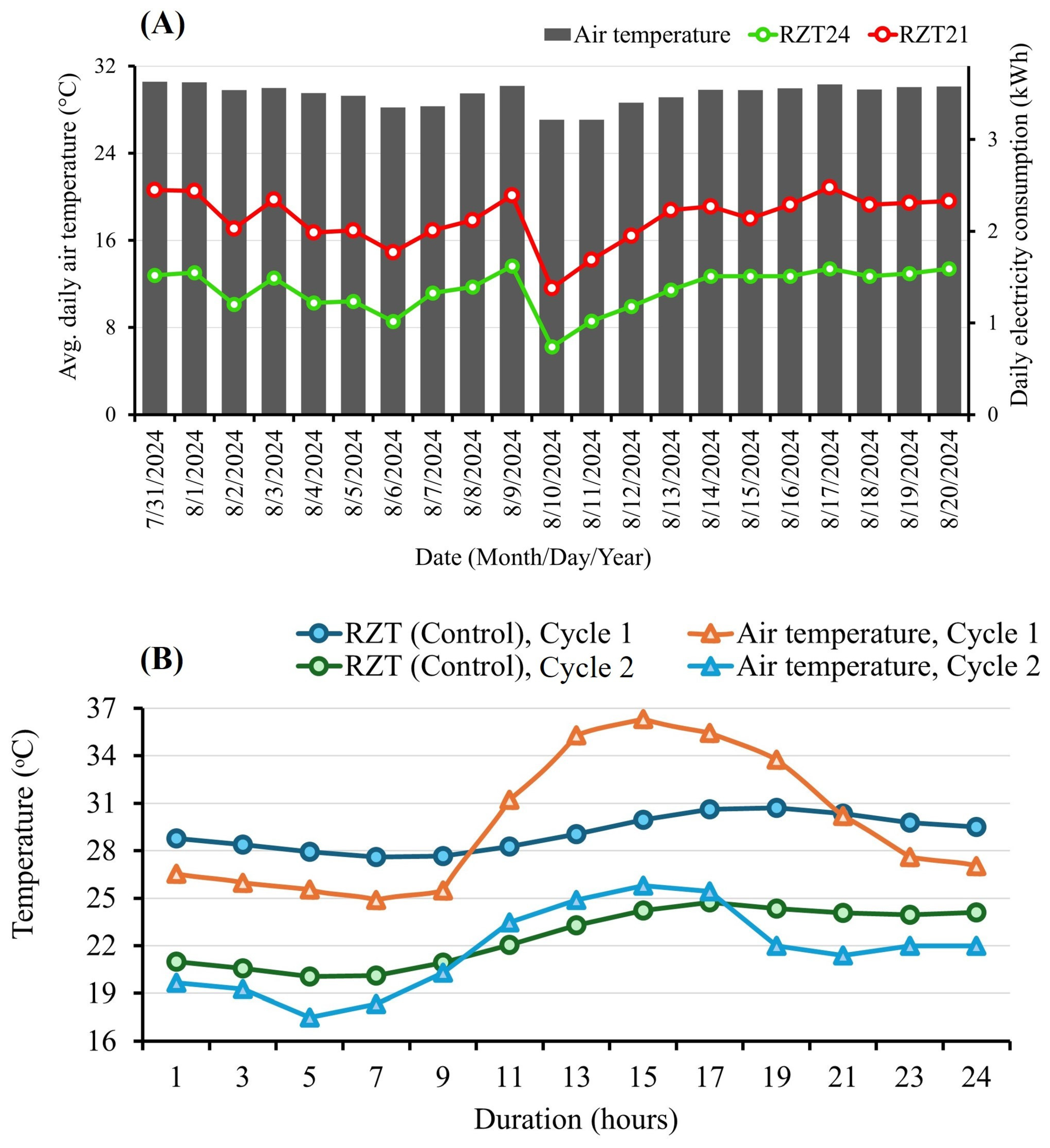
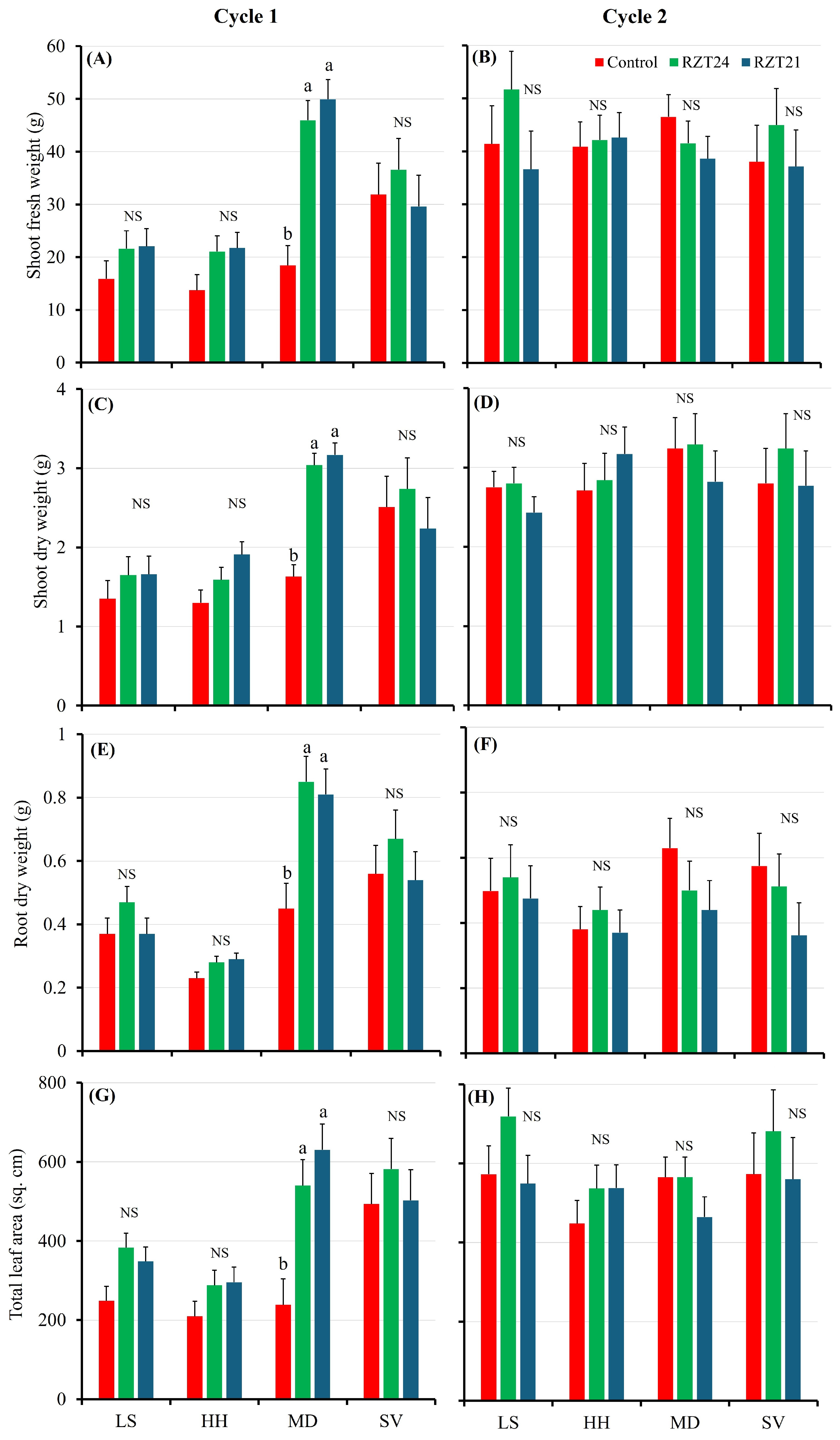
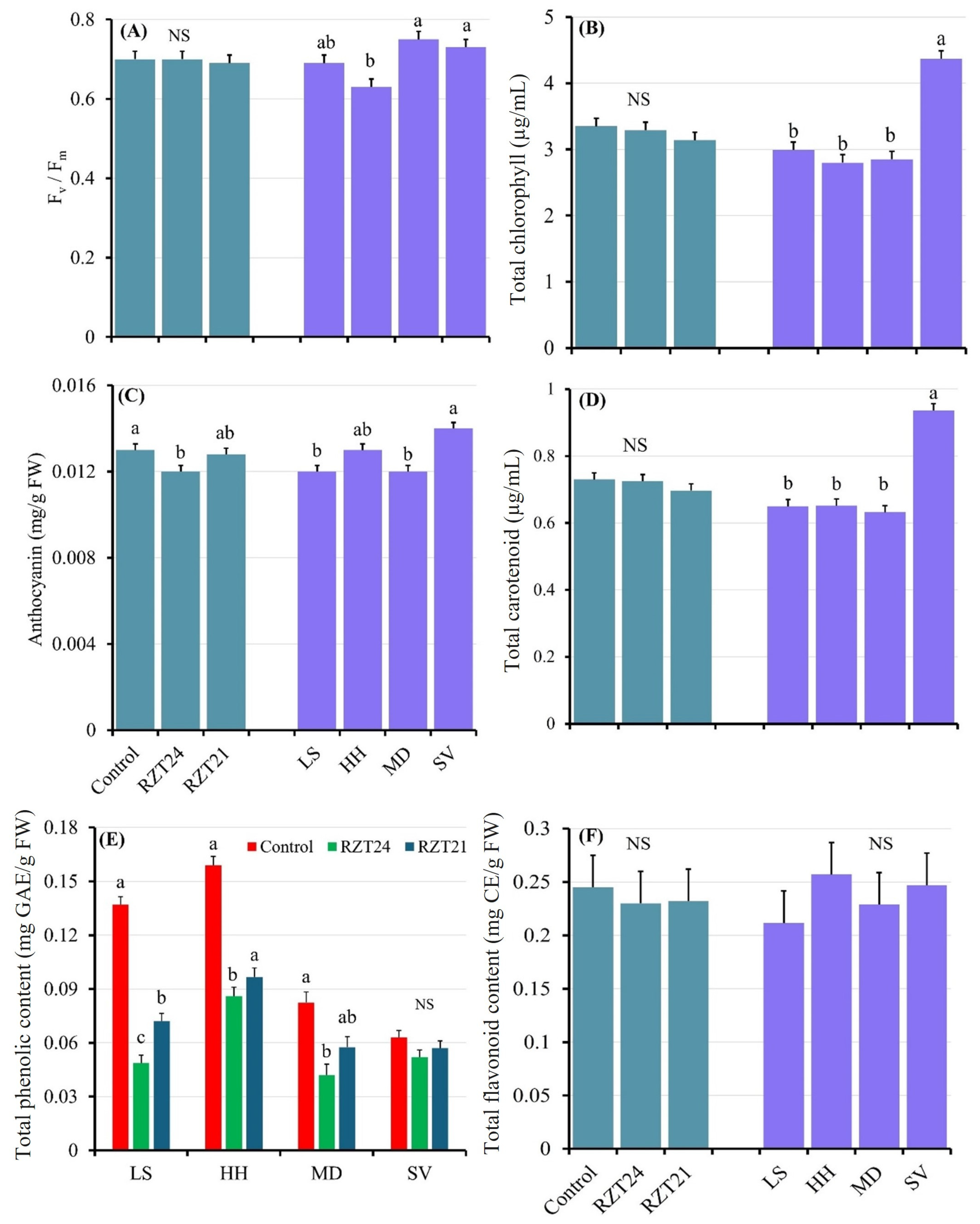
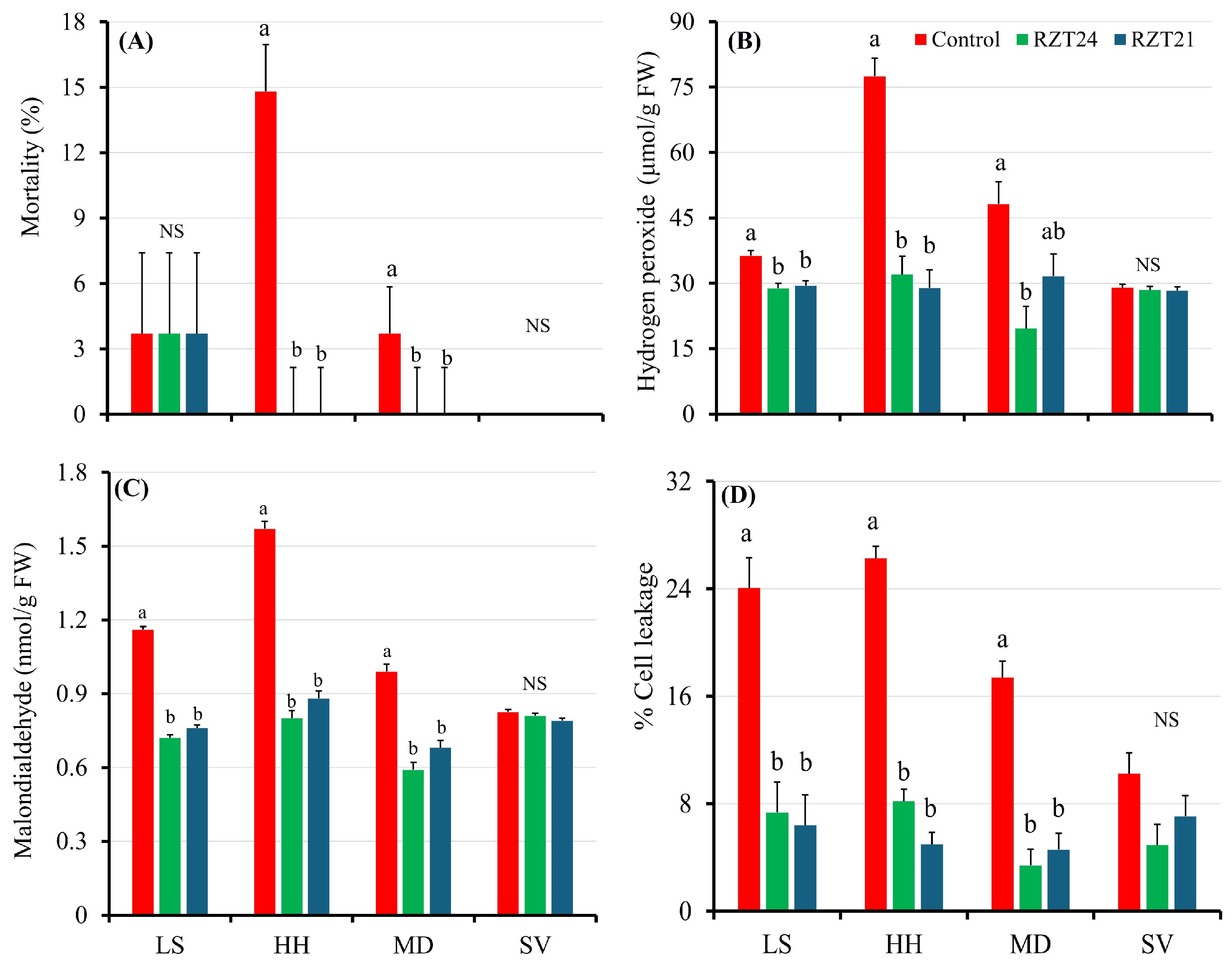
| Cycle | Treatment | Time Frame (Transplanting to Harveting) | Average Root-Zone Temperature (°C) | Average Air Temperature (°C) | Dissolved Oxygen (mg/L) | Daily Light Integral (mol/m2/d) | Range of EC (mS/cm) | Range of pH | Average Electricity Consumption (kWh/day) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Control | 30 July 2024 to 20 August 2024 | 28.0 | 6.28 | 13.74 | 0.99–1.48 | 5.86–6.33 | - | |
| RZT24 | 23.9 | 29.3 | 7.22 | 0.96–1.42 | 6.07–6.68 | 1.23 | |||
| RZT21 | 21.1 | 7.29 | 0.89–1.39 | 6.10–6.57 | 2.67 | ||||
| 2 | Control | 27 August 2024 to 18 September 2024 | 23.9 | 8.02 | 9.86 | 1.02–1.44 | 6.01–6.54 | - | |
| RZT24 | 23.7 | 24.7 | 8.18 | 1.00–1.43 | 6.03–6.49 | 0.83 | |||
| RZT21 | 20.9 | 8.27 | 0.97–1.38 | 6.07–6.66 | 1.84 |
| Growth Data | Physiological Attributes | Stress Indicators | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | Shoot FW | Shoot DW | Root DW | Leaf Area | Fv/Fm | Total Chlorophyll | Total Carotenoids | Anthocyanin | TPC | TFC | Mortality | H2O2 | MDA | Cell Leakage |
| (g) | (g) | (g) | (sq. cm) | (μg/mL) | (μg/mL) | (mg/g FW) | (GAE/g FW) | (CE/g FW) | (%) | (μmol/g FW) | (nmol/g FW) | (%) | ||
| RZT | *** | ** | ** | *** | NS | NS | NS | * | *** | NS | *** | *** | *** | *** |
| CV | *** | *** | *** | *** | * | *** | *** | *** | *** | NS | ** | *** | *** | *** |
| RZT × CV | ** | * | ** | ** | NS | NS | NS | NS | *** | NS | *** | *** | *** | *** |
| Factor | LL (cm) | LW (cm) | LN | SL (cm) | SD (mm) |
|---|---|---|---|---|---|
| Root-zone temperature (RZT) | |||||
| Control | 12.52 ± 1.9 b z | 6.62 ± 1.2 b | 17.45 ± 3.6 | 2.23 ± 0.4 | 4.61 ± 0.9 b |
| RZT24 | 14.79 ± 2.4 a | 8.16 ± 1.1 a | 18.17 ± 3.4 | 2.31 ± 0.7 | 5.15 ± 0.9 a |
| RZT21 | 12.52 ± 2.4 ab | 8.16 ± 1.5 a | 18.26 ± 3.0 | 2.36 ± 0.5 | 5.14 ± 0.8 ab |
| Cultivar (CV) | |||||
| Lakeside | 11.45 ± 1.3 b | 6.65 ± 1.1 c | 16.22 ± 0.9 b | 1.92 ± 0.3 | 4.61 ± 0.6 b |
| Hammerhead | 14.11 ± 2.1 a | 7.21 ± 0.9 bc | 15.69 ± 2.2 b | 2.52 ± 0.6 | 4.03 ± 0.4 b |
| Mandolin | 15.17 ± 2.7 a | 8.12 ± 1.9 ab | 19.85 ± 3.7 a | 2.55 ± 0.6 | 5.48 ± 0.5 a |
| SV2157 | 14.60 ± 1.5 a | 8.61 ± 1.0 a | 20.06 ± 3.1 a | 2.22 ± 0.5 | 5.75 ± 0.6 a |
| ANOVA summary | |||||
| RZT | * | ** | NS | NS | * |
| CV | *** | ** | *** | NS | ** |
| RZT × CV | NS | NS | NS | NS | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.N.E.A.; Masabni, J.; Niu, G. Cultivar-Specific Responses of Spinach to Root-Zone Cooling in Hydroponic Systems in a Greenhouse Under Warm Climates. Sustainability 2025, 17, 3925. https://doi.org/10.3390/su17093925
Khan MNEA, Masabni J, Niu G. Cultivar-Specific Responses of Spinach to Root-Zone Cooling in Hydroponic Systems in a Greenhouse Under Warm Climates. Sustainability. 2025; 17(9):3925. https://doi.org/10.3390/su17093925
Chicago/Turabian StyleKhan, Md Noor E Azam, Joseph Masabni, and Genhua Niu. 2025. "Cultivar-Specific Responses of Spinach to Root-Zone Cooling in Hydroponic Systems in a Greenhouse Under Warm Climates" Sustainability 17, no. 9: 3925. https://doi.org/10.3390/su17093925
APA StyleKhan, M. N. E. A., Masabni, J., & Niu, G. (2025). Cultivar-Specific Responses of Spinach to Root-Zone Cooling in Hydroponic Systems in a Greenhouse Under Warm Climates. Sustainability, 17(9), 3925. https://doi.org/10.3390/su17093925







