Abstract
In recent years, a significant increase in the market availability of products with a phytostimulant effect on plants has occurred. However, these products are not always low-cost, and their effects on crops are not always reproducible. In this study, an alternative use of lavender, already known for its antimicrobial activity, is proposed: an aqueous extract from self-produced lavender (Lavandula angustifolia Mill., var. Hidcote) flowering tops was tested for its phytostimulant activity on lettuce (Lactuca sativa L., var. Bionda d’estate) cultivated under organic farming management. Lettuce plants were planted in an open field on a private farm (in the Lazio region, Italy): lettuce plants were treated weekly for two months with lavender aqueous extracts while control plants were sprayed with water. Results showed that treatment with lavender extract enhanced fresh edible production and dry biomass (12.08% and 15.09%, respectively) in lettuce plants, as well as leaf area index (28.01%) and photosynthetic efficiency (increased SPAD). At the same time, an increase in mineral content was observed: compared to the control, a 30.46% increase was observed for N, 31.10%, 35.52%, 36.19%, 47.51%, 48.11%, and 91.44% for K, Ca, Mg, P, Mn, and Fe, respectively. All these factors contribute to enhancing the commercial and nutritional quality of lettuce, as well as strengthening its self-defense and extending its shelf life. Results of this study showed that lavender aqueous extract exhibits phytostimulant activity and could be a useful product for obtaining higher yield and better nutritional quality of lettuce in organic farming.
1. Introduction
The excessive use of synthetic substances, such as pesticides and fertilizers, in agricultural practices has led to soil degradation and environmental contamination. These practices can also have adverse effects on non-target organisms and on the well-being of farm workers and consumers [1]. The necessity to limit soil degradation has led to the enhancement of agricultural crop sustainability, thereby propelling research toward the development of innovative solutions. Such solutions include natural substances, such as essential oils, derived from steam distillation, and medicinal aqueous plant extracts, derived from solvent extraction, which are utilized as an alternative to plant protection products and synthetic fertilizers [2]. Directive 2009/128/EC [3] on the sustainable use of pesticides promotes the use of alternative methods, such as organic and integrated farming, to combat pathogens and parasites. In addition, EU Regulation 2019/1009 [4] on fertilizers provides for the harmonization of safety standards for biostimulants on the European market and regulates the permissible limits for the toxic contaminants they may contain. The importance of these measures concerns not only the protection of the soil and the environment but, above all, the prevention of human health risks, especially for agricultural workers. Numerous studies have been carried out on the use of medicinal plants and their derivatives in agriculture as an alternative to synthetic products, including lavender and its essential oil [5]. Lavender, belonging to the genus Lavandula (Lamiaceae family), is widespread throughout the world (Mediterranean, Europe, North Africa, Southwest Asia, and Southeast India) [6]. To date, the most common species include Lavandula angustifolia Mill. (Lavender), Lavandula latifolia Medik. (Broadleaf Lavender), and Lavandula x intermedia Emeric ex Loisel (Lavandin) [7]. From time immemorial, lavender has been utilized for its aromatic properties in the preservation of foodstuffs, the treatment of ailments and lesions, and the management of microbial and viral infections. It has also been employed as a sedative and antispasmodic agent [6]. From lavender, an essential oil can be extracted with a very complex composition. It contains various terpene alcohols, the most important of which is linalool, and their esters, which are responsible for the therapeutic properties of the oil. In the different species of Lavandula, these components can vary, which determines the differences in quality between the essential oils [8,9,10]. Numerous studies have described the chemical composition and major constituents of lavender essential oils, such as monoterpenoids (linalool, linalyl acetate, 1,8-cineol, β-ocimene, terpinen-4-ol, and camphor), sesquiterpenoids (β-caryophyllene and nerolidol), and other terpenoids (perillyl alcohol, etc.) [6]. The antimicrobial activity of lavender has been confirmed by scientific research. In fact, lavender essential oils have a wide range of biological activities (antiseptic, antimicrobial, antifungal, antioxidant, and anti-inflammatory) [7,11,12,13]. In particular, the antibacterial effect, which has been demonstrated in vitro, can be bacteriostatic. At higher concentrations, it can also be bactericidal. Currently, particular attention has been gained by antimicrobial peptides because of their multiple activities, which makes them less susceptible to bacterial resistance [14]. In recent years, several studies have highlighted their effectiveness against parasites as well [7]. However, further studies are needed to define the extent of efficacy, mechanisms of action, optimal doses, long-term safety, and potential side effects of lavender [6]. In agriculture, lavender essential oil can be used as a herbicide [15], but like all essential oils, it can be toxic even in moderately high doses. Alternatively, the aqueous extract of lavender can be used, which is equally effective but does not show any particular toxicity [16]. Plant extracts are made up of a combination of several compounds (phytocomplexes) that act synergistically. This means that these mixtures have a higher activity than the single active ingredient, resulting in a more effective response. This phenomenon, called synergism, was first observed in essential oils [17], where mixtures produce better and longer-lasting results than simple additive effects of individual components. The activity of plant extracts is based on the presence of allelopathic compounds, which, when in contact with a plant, are able to modify the functionality of some enzymes and hormones and influence their metabolic processes. The extracts are made from medicinal herbs and maintain the original properties of the specific plant and/or part of the plant from which they are derived. Plant extracts are known for their protective effect on cultivated plants due to their herbicidal, bactericidal, or fungicidal properties, depending on their composition [18]. In fact, they effectively promote the self-defense of crops against fungal and bacterial attacks in agriculture [19]. In a preliminary study carried out in 2014 [20] on zucchini plants treated with 12 aqueous extracts at 1% (garlic, burdock, horsetail, St. John’s wort, lavender, lantana, pomegranate, mint, oregano, sage, tansy, and thyme), it was observed that the use of the extracts, in addition to protecting the plants from possible adversities (fungi, insects, etc.), induced greater vegetative vigor, better chlorophyll content in the leaves, better flowering induction, and higher fruit production, compared to the untreated plants. In particular, extracts of garlic, thyme, tansy, and lavender have been shown to have a phytostimulant effect [20].
Given the well-established knowledge of lavender phytocomplex as an antimicrobial [21,22,23], we chose to investigate another of its possible uses in the agricultural sector as a phytostimulant and fertilizer. The phytostimulant effects are due to the compounds that enhance plant nutrition, stress tolerance, and/or metabolic processes, thereby optimizing crop quality. Phytostimulants can be composed of different substances and are generally classified according to their content and origin [7]. It has been demonstrated that plant extracts possess phytostimulant properties, enhancing and stimulating the vital processes of plants through mechanisms that diverge from those of fertilizers or phytohormones. The induction of these effects has been attributed to the interaction of plant extracts with plant-signaling cascades, leading to a reduction in negative reactions to stress [7]. Specifically, the aqueous extract of lavender has the potential to be utilized in the enhancement of plants cultivated through an integrated pest management strategy subsequent to a thorough and comprehensive characterization and validation. In a study dating back to 2005 [24], it was observed that lavender (Lavandula spp.) proved to be highly phytotoxic to annual ryegrass (Lolium rigidum, ARG), one of the main weeds affecting winter wheat crops in Australia, while in subsequent work, the same authors [25] reported coumarin as the most phytotoxic and largely responsible compound for the observed phytotoxicity of the lavender aqueous extract.
To the best of our knowledge, no published papers were found on the use of lavender aqueous extract as a phytostimulant in agriculture, other than that reported by Casorri et al. in 2014 [20]. On the contrary, many works have been reported in the literature regarding the use of phytostimulants or fertilizers to improve the quality parameters of lettuce [26,27,28,29,30,31,32]. The aim of this study is, therefore, to evaluate the phytostimulant effect of lavender aqueous extract on lettuce cultivated under organic farming conditions. For this purpose, a fine chemical characterization of the lavender aqueous extract was also provided using advanced analytical platforms.
2. Materials and Methods
2.1. Preparation of the Aqueous Extracts
Extracts were prepared using the farm’s self-produced lavender (Lavandula angustifolia Mill., var. Hidcote) flowering tops that have been freeze-dried (Lio 5PDGT, 5Pascal, Trezzano sul Naviglio, Italy) for 35 min at −54 °C, vacuum-packed for approximately one year, and ground for 30 s at 800 rpm, at concentrations of 1% w/v (10 g L−1), using distilled water at room temperature. The extraction procedure lasted 24 h to favor the maximum extraction, and then the extracts were filtered with Whatman® filter paper No. 4 (Merck Life Science Limited, Maharashtra, India). The filtered aqueous extract can be sprayed immediately or stored for 6–7 days at +4 °C; alternatively, it last about a few hours at 20 °C. The extract was prepared at a concentration of 10 g L−1 for saturating the solution [19]; this concentration is generally used in open-field experiments without toxic effects, as reported in previous works [19,33,34]. The cost of the entire procedure was limited to 60 min of total labor and 115 Wh of energy demand for freeze-drying and grinding.
2.2. Chemical Characterization of the Aqueous Extracts
Evaluation of the mineral content and the presence of other metabolites in lavender extract was performed using ICP-OES and NMR techniques.
2.2.1. Elemental Analysis with ICP-OES and Kjeldahl Method
The chemical means used as analytical-quality reagents and elemental standard solutions were produced by Sigma-Aldrich Co.® (St. Louis, MO, USA). All solutions were prepared using high-purity water (Millipore®, Molsheim, France). After filtration, macronutrients (P, Mg, Ca, Na, and K) and micronutrients (Fe, Mn, Cu, Zn, B, and Mo) content were quantified with an Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES) ICAP 6100 (Thermo-Scientific®, Waltham, MA, USA). The instrument setting included power 1.29 kW, nebulizer flow rate 0.81 L min−1, plasma flow 14.8 L min−1, and secondary gas flow 2.1 L min−1. The ICP-OES was calibrated with a blank and four multi-element reference solutions. After digestion, total N was measured by means of the Kjeldahl method [35].
2.2.2. NMR Analysis
About 100 mL of aqueous extracts were placed in a standard 5 mm NMR tube and were dissolved by adding 0.600 mL of D2O (99.8% deuterated) containing 0.05% (v/v) TMS. NMR experiments were carried out at the University of Perugia by using a Bruker AVANCE 600 MHz spectrometer (Bruker Biospin Gmbh, Rheinstetten, Germany) equipped with a 5 mm with a smartprobe (600 MHz for 1H) with a z gradient coil. The deuterium signal of D2O was used for locking and shimming of the B0 magnetic field, and the spectra were recorded at room temperature (298 K). 1H-NMR and 13C-NMR spectra signals were referenced to the TMS signal. 1D- and 2D-NMR spectra were recorded according to standard conditions by using a pulse program already loaded on the instrument database. All pulse sequences contained a pre-saturation of the water signal in order to cancel out its strong signal. 1H spectral width was set equal to 12.0 ppm, accumulating 32 scans with an acquisition time of 2.93 s, each with 64 K datapoints. 13C spectra were recorded over a 220 ppm window, accumulating 256 scans.
1H-1H-TOCSY experiments were collected in the phase-sensitive mode using Time Proportional Phase Incrementation (TPPI) for quadrature detection in the direct dimension, with a 4807 Hz spectral width in both dimensions, 100 ms of spin-lock time, 2K data points in f2, and 1K increments in f1, each with 32 scans. The water signal was suppressed. 1H-13C-HMQC spectra were acquired in TPPI phase-sensitive mode, with a 4807 Hz spectral width in f2 dimension and a 15,083 Hz spectral width in f1. 1K data points in f2 and 256 increments in f1, each with 32 scans, were used. HMQC was preferred to HSQC since the latter is more sensible for the optimization of the acquisition parameters.
2.3. Field Experiment
The phytostimulant effect of the lavender extracts was evaluated on lettuce cultivation (Lactuca sativa L., var. Bionda d’estate) in an open-field trial. By means of analyses performed according to official methods [36], the soil was characterized as having a clayey-silty texture, a bulk density of 1.23 Mg m−3, an available water content of 35.62%, a high content of total organic carbon (4.7%), a sub-alkaline pH (7.4), and a significant cation exchange activity (35.86 meq 100 g−1). The soil also contained 0.28% N, available P (044.73 mg kg−1), and exchangeable K (441.31 mg kg−1). Lettuce plants were planted in an open field on a private farm located in the Lazio region (Poggio Mirteto—60 km North of Rome) (42.281833 N, 12.657924 E), in the period 7 April 2022–9 June 2022 and cultivated under organic farming management. The thirty-year average thermo-pluviometric values of the experimental area are 1003 mm of annual rainfall, primarily concentrated in the autumn, winter, and spring, and 13.82 °C of average temperature. Meteorological data influencing the effectiveness of foliar sprays during the trial period (temperature, rainfall, relative humidity, solar radiation, wind speed) came from the MADIA gridded dataset, as dekadal (10-days) series (cell centroid: 42.25 N, 12.75 E) [37], and shown in Figure 1.
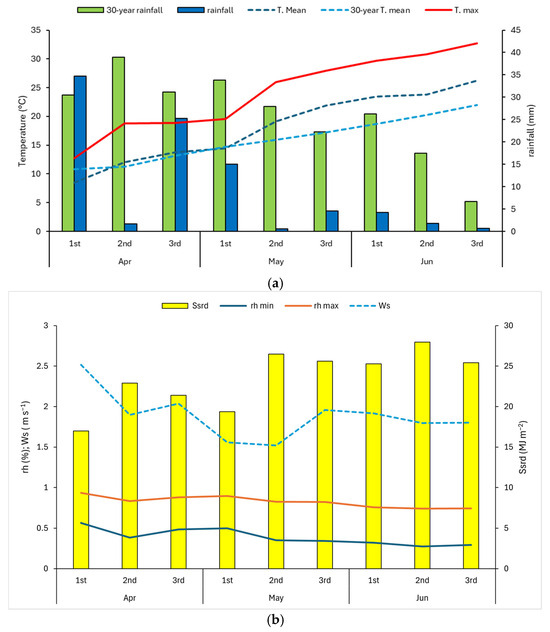
Figure 1.
(a) Thermo-pluviometric dekadal data (10 days) of period of trial (7 April 2022–9 June 2022) in the experimental area (Rainfall in mm, T. mean and T. max in °C). 1st, 2nd, and 3rd are dekads; 30-year rainfall and 30-year T. mean are, respectively, the long-term (1991–2020) mean of annual rainfall sum and the mean dekadal temperature. (b) Mean dekadal data of surface solar radiation downwards (Ssrd in MJ m−2), minimum and maximum relative humidity (rh min and rh max in % × 100), and wind speed (Ws in m s−1).
In Figure 1a, it is observed that the rainfall during the test period was considerably lower than the thirty-year average value. Furthermore, the average temperatures during the test reached values higher than the thirty-year average values, especially in the second half of the vegetative cycle, when the maximum temperatures also recorded very high values. Regarding the values of solar radiation, relative humidity, and wind speed, these parameters recorded optimal values for carrying out foliar treatments in the early morning with solar radiation between 20 and 25 MJ m−2, relative humidity greater than 70%, and wind speed lower than 2 m s−1 (Figure 1b). At the transplant, a single operation of local fertilization was carried out, distributing 50 g per plant of self-produced green compost (N 1.3%, P 0.2%, K 0.8%). Compost was analyzed for its macronutrient concentration through an accredited private laboratory according to Regulation (EC) 2019/1009 [4] and Legislative Decree 29 April 2010 n. 75 [38] and subsequent amendments (Italian Laws) [39]. The plants were divided into 6 plots of 20 plants (planting distance 0.8 × 0.4 m) with three replicates in a randomized block design; each block consisted of a treated plot and a control block without treatment. In each treated plot, the plants were treated weekly with 2 L of extract per plot, regardless of the phenological phase, for two months, with a total of 8 applications, while the plants of the control plots were sprayed with water. No pathogen attack was detected either on treated or non-treated plots during the entire vegetative cycle.
2.4. Field and Laboratory Measurements on Plants
To assess the photosynthetic efficiency of each plant, the in vivo chlorophyll content was assessed using the SPAD 5200 portable fluorimeter (Konica Minolta® Business Solution Italia S.p.A., Milan, Italy). Field measurements with SPAD were carried out before the harvest at five points in the central leaf portion of the plants and subsequently collected for the other analyses. Six plants from the middle of each plot were collected at the end of the month of June, then weighed and measured (fresh and dry mass, height). The leaves of the fresh plants were used to determine the Leaf Area Index (LAI). The shape of the leaves was monitored by taking digital images of the surface. Image pixels of leaves were measured using Matlab v.7 software (The MathWorks Inc., Natick, MA, USA), and then the total leaf area of each plant was obtained. Macronutrients (P, K, Ca, and Mg) and micronutrients (Fe, Mn, Cu, Zn, B, and Mo) concentration were determined in dried leaves after wet digestion, as described above for the aqueous extract. The standard reference BCR-679 (white cabbage, IRMM, Geel, Belgium) was analyzed under the same conditions used for the sample analysis to ensure method accuracy and precision. Total N was determined using Kjeldahl digestion [35]. Plant elemental uptake was calculated by multiplying every element concentration by the dry weight of each plant.
2.5. Statistical Analysis
All data collected were preliminarily submitted to descriptive statistics and graphic analysis (box plot) to identify any outliers. Univariate analysis of variance (ANOVA) was performed when the homogeneity of variance by Levene’s test was found. In variables where homogeneity of variance was not verified, the non-parametric Kruskal–Wallis Test was performed.
To better clarify the interactions of biometric and photosynthetic parameters and the relative importance between element concentrations in lettuce plants, we used the advanced multivariate statistical procedure of principal component analysis (PCA), and the results were summarized in the related loadings plot.
All measurements and analytical determinations performed on the six plants per plot were used in the analysis. All statistical elaborations were performed using JASP statistical software version 0.19.3 (JASP Team, Amsterdam, The Netherlands) [40].
3. Results and Discussion
3.1. Elemental Composition of the Lavender Aqueous Extract
The elemental content of lavender aqueous extract is shown in Table 1.

Table 1.
Concentration (mg L−1) of nutritive elements of the aqueous extract of lavender.
The main macronutrients present are K (196.02 mg L−1) and N (146.10 mg L−1). K is essential for carbohydrate metabolism, cell multiplication, and water balance regulation. Its absorption by plants is influenced by the presence of other cations, such as Ca, Mg, and ammonium ions [41]. N is an important component of proteins and enzymes; it promotes the assimilation of nutrients in the leaves, the emission of inflorescences, and the growth of roots, stems, and leaves [42]. Ca and Mg are also present in medium–high concentrations (51.06 and 18.34 mg L−1, respectively). Ca is essential for the growth of meristematic tissues, the induction of mechanical resistance of tissues, and the activation of numerous enzymatic complexes, while Mg is the main component of chlorophyll, and it has the function of enzymatic activator of the complexes involved in the conversion and transport of energy systems [43]. Among the macroelements, P is the least present in the aqueous extract of lavender (9.13 mg L−1). This element contributes to the synthesis of many enzymes that regulate the development and growth of plants, photosynthesis, nutrient transport, and energy accumulation in plant tissues [44]. As for the microelements, Mn, Fe, and Zn are present in the extract (0.32, 0.25, and 0.12 mg L−1, respectively). These trace elements are all coenzymes that activate different enzymatic complexes responsible for photosynthesis, protein synthesis, cellular respiration, and resistance to diseases [45]. Cu, B, and Mo were found to be below the quantification limit of the ICP-OES spectrometer (0.05 mg kg−1).
3.2. NMR Analysis of the Lavender Aqueous Extract
The 1H-NMR spectrum of the lavender aqueous extract is reported in Figure 2.
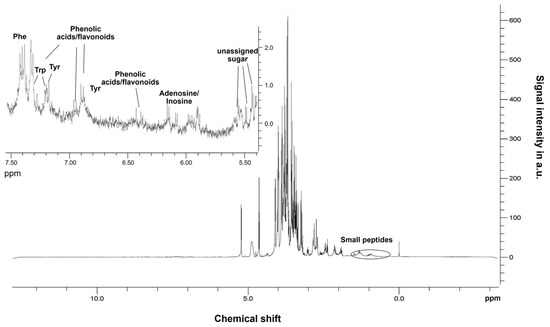
Figure 2.
1H-NMR spectrum of the aqueous lavender extract reporting the signals related to the presence of peptides. In the upper left part, the spectrum enlargement of the aromatic region (from 5.50 to 7.50 ppm) contains the signals of polyphenol compounds.
It shows the entire spectrum, with the enlargement from 5.50 to 7.50 ppm. The latter contains very weak signals that correspond to aromatic molecules, such as amino acids (namely phenylalanine, tyrosine, and tryptophan), unidentified sugar fragments, and some compounds belonging to the class of polyphenols (particularly phenolic acids and flavonoids). Their content is very limited; nevertheless, the NMR spectroscopy was able to identify their presence. The region between 3.00 and 4.50 ppm is the most intense and accounts for the presence of amino acids: asparagine, glutamine, arginine, threonine, etc., and sugars, predominantly b- and a-Glucose. Finally, the region around 1.5 ppm shows the presence of small traces of some fatty acids, such as saturated C18 and C16 acids (stearic and palmitic acids, respectively), as well as unsaturated fatty acids (C18:1, oleic acid, and C18:2, linoleic acid). In the same spectral region, some broad bands are also visible: they can be attributed to molecules with high molecular weight, such as small peptides. Amino acids are known to contribute to the synthesis of numerous non-protein nitrogenous substances in plants, such as nitrogen bases, vitamins, pigments, and coenzymes [46]. On the other side, bioactive peptides may exert hormone-like regulatory action (similar to auxins and gibberellins), promoting shoot and root growth, enhancing the crop’s ability to absorb nutrients (particularly N and Fe), and increasing yields [31]. Polyphenols, generally known for their antioxidant activity, include a large number of chemical compounds, such as phenolic acids and flavonoids. They show antifungal, antibacterial, and antiviral activities together with protection against UV rays [47]. Also, flavonoids are a large family of compounds with several functions in plants: they have antimicrobial effects on plant pests and anti-repellent effects regarding insects, animals, etc, protect against reactive oxygen species, and are also visual attractors for pollinating insects [48,49]. In agriculture, flavonoids can be used as biopesticides against certain weed species [50].
3.3. Biometric and Vegetative Characteristics of Lettuce
Weekly treatments with lavender extract positively influenced, with statistical significance, fresh edible production and dry biomass (Figure 3a,b), with percentage increments of 12.08% and 15.09%, respectively.
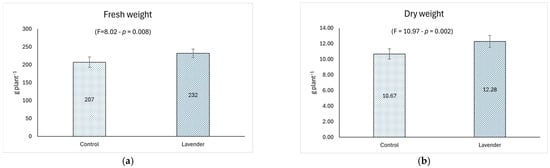
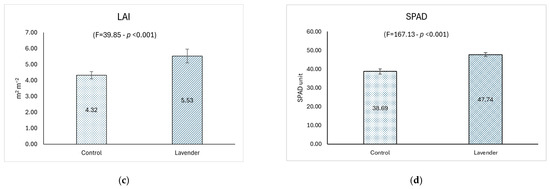
Figure 3.
Biometric and vegetative data of lettuce treated with foliar spray applications: lavender extract (10 g L−1) vs. control (distilled water). Data of lettuce samples was recorded for (a) fresh weight (g plant−1); (b) dry weight (g plant−1); (c) Leaf Area Index (LAI) (m2 m−2); and (d) SPAD unit. The ANOVA F-test and the respective p value are reported in brackets. Error bars represent ±95% Confidence Interval for mean (n = 18).
This result had positive repercussions on the gross saleable production and on the post-harvest shelf life of lettuce due to the greater amount of dry matter in the treated plants. Weight loss, which is related to plant water content and dry biomass, seems to be the most appropriate quality index for lettuce at storage temperature [51]. The observed increase in production is similar to that found in research that involved the use of synthetic or organic fertilizers or other phytostimulants. In particular, Aguiar et al. in 2024 [52], when testing two products based on commercial phytostimulants, a leonardite and a seaweed extract, on the quality parameters of romaine lettuce, observed an increase in dry matter yield only with leonardite application and only in the first growing cycle, with results in the same order of magnitude as this study (11.5 and 13.5 g plant−1, in control and leonardite treated lettuce, respectively). Although seaweed extracts currently represent the wide group of plant phytostimulants used in agriculture, their positive effects on plants have not always been observed [53,54] or come mainly under environmental stresses such as salinity, drought, or heat [55,56,57]. The optimal growth conditions during the experimental trials maintained for lettuce plants by the authors [52] could justify the no-observed results concerning seaweed extract application. An increase in fresh and dry weight, as well as leaf area and photosynthetic activity, was also observed by Helmy et al. in 2024 [58] in crisphead lettuce (Lactuca sativa L.) following combined treatments of organic fertilizers and melatonin under water stress conditions. Similar results were reported by Atero-Calvo et al. [59] applying the phytostimulant Green Leaves (based on Macrocystis algae extract and containing a mixture of amino acids, corn steep liquor extract, Ca, and glycine betaine) on lettuce plants: the authors attributed the greater growth of lettuce plants to the amino acids exogenously applied through foliar applications by Green Leaves, which are used by the plant in its primary metabolism functions. The high content of amino acids observed in the lavender aqueous extract could have promoted the greater growth of lettuce treated with the same extract observed in this study. Increased leaf area and chlorophyll content were also reported by Martins Filho et al. in 2019 [60] on Lactuca sativa L. after soil inoculation with Trichoderma and Pseudomonas microorganisms combined with cattle manure: these effects were due to the ability of Trichoderma and Pseudomonas genera to improve nutrient absorption and promote plant growth [61,62]. Another microorganism (Methylobacterium symbioticum), contained in a commercial plant biostimulant and known for its N-fixing capacity, was tested by Arrobas et al. in 2024 [63] in lettuce cultivation: the authors observed poor results both in terms of dry matter yield (increase in one out of the four growing cycles) and N concentration in lettuce tissues (N level higher in the plants treated with the bacterium after the second vegetative cycle, and lower in the second vegetative cycle). However, increased levels of lettuce dry matter yield with increasing doses of N, up to the maximum absorption, were observed by the same authors [63]. Interestingly, Franzoni and Ferrante in 2024 [64] observed different behaviors of two lettuce cultivars (red variety (Lactuca sativa L. cv. Codex RZ) vs. green variety (Lactuca sativa L. cv. Expertise RZ)) after the application of a plant-derived phytostimulant, obtained from aqueous maceration of borage (Borago officinalis L.) flowers: fresh biomass and yield were negatively affected by extract application in the red cultivar, whereas they did not change in the green lettuce. This confirms that the genetic characteristics of different species and cultivars led crops to exhibit different behaviors after the application of the same phytostimulant [64]. The production results obtained in this study are in line with those concerning LAI, evidenced by the highly significant increase (p < 0.001) of the leaf area of the treated lettuce (28.01%), which improves the relative commercial category. In turn, these increments induced a greater photosynthetic efficiency of the plants, highlighted by the significant increase in SPAD levels (p < 0.001; 23.26%), together with the increase in leaf area. These results also confirm that the use of natural extracts can be considered an alternative to the use of synthetic technical means or other phytostimulants. An increase in chlorophyll content in Lactuca sativa L. (green variety) was also observed by Franzoni and Ferrante [64] after application of an aqueous extract of borage flowers; however, the same result was not observed by the authors in the red variety (Lactuca sativa L. cv. Codex RZ). Dudaš et al. in 2016 [65] related the increase in chlorophyll content observed in Lactuca sativa (var. capitata L.) after treatment with Megagreen (a commercial Ca fertilizer with micronutrients for foliar application) also to the high content of Mg provided by the fertilizer (2.2% MgO), since Mg2+ represents the core of each chlorophyll molecule. The lavender aqueous extract used in this study is a good source of Mg; therefore, it could have contributed to the observed increase in chlorophyll content of lettuce. In a study conducted under greenhouse conditions, the effects of the Herasim microbial fertilizer integrated with mineral fertilizer on yield and some quality characteristics of lettuce were investigated. The best results were obtained for the integrated fertilization in terms of lettuce growth, color, and chlorophyll (SPAD) content [66]. The increase in chlorophyll, associated with SPAD’s positive variation, can be considered a factor in extending the commercial shelf life of a leafy vegetable [67]. Furthermore, chlorophyll seems to play an important role in anticancer and antimutagenic activities as a cancer-preventing agent [68].
3.4. Elemental Concentrations
The concentration of nutrients in the lettuce plants is reported in Table 2.

Table 2.
Nutritive elements concentration and uptake in the lettuce plant—significance (p value) of ANOVA F-test or Kruskal–Wallis Test.
All the elements present in the lavender extract increased significantly in the treated plants, in a highly statistically significant way, except for Zn, which decreased significantly. Cu, B, and Mo did not undergo significant variations, in line with their contents found in the extract, where their level was lower than the limit of quantification. The low amount of these elements in lettuce plants could also have been affected by the high content of total organic carbon measured in the soil of the experimental field: metal availability in soil, in fact, is known to be strongly affected by the amount and the quality of organic matter, which can form complexes and chelates with metals [69].
The relationships between biometric and photosynthetic parameters and nutrient concentrations were investigated using PCA (Figure 4). The first two extracted components explain 49.7% of the total variance. The first component explains that 38.3% of the variance is probably related to the treatment effect. The second component probably and weakly associates the variability related to the transfer ratios of nutrients from the soil-plant system. Worthy of attention are the results relating to macro and mesoelements involved in photosynthesis (SPAD), cell division, and meristem growth (plant height, LAI, and fresh weight), such as N, K, Ca, and Mg, confirming the results discussed above in ANOVA table regarding the photosynthetic activity and productivity of the treated plants, which are related to the first component of PCA. Nutrient availability is known to be closely related to chlorophyll synthesis [70]. Furthermore, a positive impact of plant compost application in enhancing nutrient availability and uptake, as well as growth parameters in Lactuca sativa L., previously reported by Helmy et al. [58], could be hypothesized in this study. Increased levels of Ca and Mg were also observed by Franzoni and Ferrante [64] after application of an aqueous extract of borage flowers, both in green (Expertise) and red (Codex) lettuce leaves, while a significant increase in total N was reported only in the red leaves. Carillo et al. in 2022 [31] reported an increase in Ca and K content, while no significant differences were observed for N and Mg in lettuce treated with a legume-derived phytostimulant, but the authors observed a treatment dose-dependent response. Since increased potassium uptake and accumulation in crops have been related to root growth, cell expansion, auxin homeostasis, and cell signaling [71], the significant increase in K observed in plants treated with lavender aqueous extracts could also be responsible for the increase in lettuce fresh weight observed in this study. According to Dudaš et al. [65], if a phytostimulant contains amino acids, an increase in the concentration of N in lettuce leaves after phytostimulant treatment is expected. However, the same authors did not observe this outcome after treatment of Lactuca sativa (var. capitata L.) with Bio-algeen S-90 (an organic phytostimulant derived from marine alga Ascophyllum nodosum): they ascribed this phenomenon to the limited root absorption of amino acids from the soil and/or retention of amino acids in the lettuce root. The amino acid content and/or the small peptides of the aqueous lavender extract could have contributed to the increase in nitrogen observed for lettuce leaves in this study. Despite the low extract concentrations, P exhibits a particular behavior, showing a highly significant increase (27.90%, p < 0.001 of ANOVA). Regarding the PCA, P was also linked to the first component of PCA, seen as the element’s role in energy transport and growth. It can be assumed that the general stimulating effect due to the elemental composition of the extract may have also increased the assimilation capacity of the root system of the treated plants with respect to the available forms of phosphorus. Carillo et al. [31] also reported an increase in P level in lettuce after treatment with a legume-derived phytostimulant. The microelements Mn and Fe, which are involved in the metabolic processes mentioned above, follow the same trend; in particular, it is noteworthy that Fe increased by 66.83% in a highly significant manner (p < 0.001 of ANOVA), as confirmed by PCA. Increased levels of Fe and Mn in lettuce plants treated with a biostimulant (leonardite) were also observed by Aguiar et al. [52], who attributed this phenomenon to soil acidification, which generally increases the solubility of these metals. However, their results contrasted with previous similar studies reported on leonardite application [72,73]. The only microelement that showed a significant decrease (p < 0.05 of ANOVA) in the concentration of the treated plants was Zn, which was probably subjected to the competitive effect of alkaline cations (Ca, Mg) and other metals (Fe, Mn) present in the aqueous extract, as confirmed by the PCA [74,75].
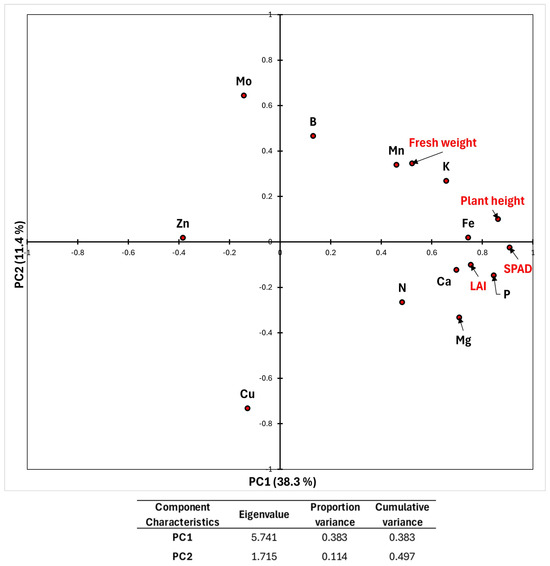
Figure 4.
Loadings plot of the first two components by multivariate PCA analysis of biometric, photosynthetic parameters (red labels), and nutritive elements concentration (black labels) of the aerial biomass of lettuce plants. A summary of the main characteristics of the first two extracted components is shown in the lower part of the plot. No axis rotation method was applied.
3.5. Elemental Uptake
Regarding the uptake, biofortification of all the elements examined was observed, even in cases where the concentration decreased due to the increase in biomass (dilution effect). The improvements are also confirmed by the high statistical significance of most of the elements (N, P, K, Ca, Mg, Mn, and Fe). Less relevant effects were observed in Cu, Zn, B, and Mo. In this context, the strong improvement in the nutritional aspect should be highlighted, with the production of lettuce with high quality from the point of view of food integration [76]. In terms of percentage increases, a 30.46% enrichment was observed for nitrogen in lettuce treated with aqueous lavender extract. Phytostimulants containing a wide range of chemical components, including bioactive molecules (such as vitamins, amino acids, polysaccharides, etc.), as well as micro and macronutrients, such as seaweed extracts [77,78], have been shown to exert a positive impact on plants by modifying the root architecture and improving the nutrient use efficiency, particularly nitrogen [59]. The complexity of the aqueous lavender extract used in this study could explain the increased nitrogen content in the treated lettuce plants. P also showed significant increases of 47.51%, despite the low concentration in the lavender aqueous extract. This result is particularly relevant for the lettuce’s nutritional value, especially in terms of energy transport in cells and tissues, which is important for the diet of athletes or workers who engage in strenuous physical work. K was enriched by 31.10%, and, in this case, it can be hypothesized that it may be used in cases where electrolytic and osmotic rebalancing is necessary in particular patients. For the alkaline bivalent cations, Ca and Mg, significant increases were observed, equal to 35.52% and 36.19%, respectively. Ca primarily serves a structural function in the skeleton, being essential for bone growth, but it plays a regulatory role in many other functions in the human body, such as muscle contraction, neurotransmitter secretion, digestion, and blood coagulation [79]. Mg is vital for the health of the nervous system, muscles, and bones [80]. Although lettuce is typically poor in Ca and Mg, the increased levels observed for these essential minerals in response to the application of lavender extract may indicate an increase in the quality of the final product. It is also worth noting that calcium deficiency in lettuce causes a physiological disorder, better known as tipburn [81], which generally occurs in indoor cultivation using artificial lighting and can result in necrosis of the leaf marginal apex [64]. The higher concentration of Ca in lettuce leaves following the application of the lavender extract, besides having a positive impact on the nutritional value of the product, also lowers the probability of developing tipburn. Mn showed a significant increase of 48.11%, making the fortified food particularly beneficial for its antioxidant effects and contribution to the synthesis of digestive enzymes. The element that has benefited most from the fortification of the lettuce is Fe, with an increase of 91.44% in uptake. This remarkable result is especially important from a nutritional perspective because iron is involved in several metabolic processes in the human body, including oxygen transport, deoxyribonucleic acid (DNA) synthesis, and electron transport. Iron deficiency can lead to anemia and other neurodegenerative diseases [82].
4. Conclusions
In this study, an aqueous extract of lavender was tested for the first time for its phytostimulant activity in lettuce cultivated in organic farming conditions. The most consistent results were enhanced growth of lettuce plants (in terms of fresh and dry weight, as well as LAI) and more efficient photosynthetic activity (higher SPAD values). All these factors contribute to enhancing the commercial quality of lettuce and to extending its commercial shelf life. Furthermore, the application of the lavender extract led to higher levels of key minerals, particularly N, P, K, Ca, Mg, Mn, and Fe, in the treated lettuce compared to the control. This enhancement not only improves the physiological state of the plants but also boosts the nutritional quality of lettuce.
The promising results obtained in this study support the use of aqueous lavender extract as a phytostimulant, offering an alternative to traditional fertilizers or other organic phytostimulants. The latter often presents several disadvantages due to the complexity of the treatment of matrixes, which includes higher costs of extraction process and distribution. On-farm aqueous extraction of bioactive substances by plants appears to be a simple and cost-effective practice from the perspective of the circular economy and offers both economic and environmental advantages for organic farms.
However, further experiments are needed to evaluate the effects of different doses of this extract on different crops, soils, and habitats to determine the optimal dose of phytostimulant that can enhance crops growth and quality parameters. Additionally, the nutritional quality of crops affected by lavender extract should be more thoroughly investigated.
Author Contributions
Conceptualization, C.B., L.C. and M.R.; methodology, C.B., L.C. and M.R.; investigation, C.B., L.C., M.R., U.N., E.M., M.D.L. and M.V.; data curation, U.N. and M.V.; writing—reviewing and editing M.R., U.N., C.B., E.M., M.D.L., M.V. and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are original and come from the research described in the paper. The data are available upon request from the authors.
Acknowledgments
We thank A. Macchioni, Department of Chemistry, Biology and Biotechnology, University of Perugia, 06123 Perugia, Italy, for the kind use of the NMR spectrometer.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kostina-Bednarz, M.; Płonka, J.; Barchanska, H. Allelopathy as a Source of Bioherbicides: Challenges and Prospects for Sustainable Agriculture. Rev. Environ. Sci. Biotechnol. 2023, 22, 471–504. [Google Scholar] [CrossRef]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Asgari Lajayer, B.; Hadian, J.; Astatkie, T. Applications of Essential Oils and Plant Extracts in Different Industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef] [PubMed]
- Directive-2009/128-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/dir/2009/128/oj/eng (accessed on 23 January 2025).
- Regulation-2019/1009-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2019/1009/oj/eng (accessed on 28 January 2025).
- Hernández-Bolaños, E.; Sánchez-Retuerta, V.; Matías-Hernández, L.; Cuyas, L. Promising Applications on the Use of Medicinal and Aromatic Plants in Agriculture. Discov. Agric. 2025, 3, 36. [Google Scholar] [CrossRef]
- Salehi, B.; Mnayer, D.; Özçelik, B.; Altin, G.; Kasapoğlu, K.N.; Daskaya-Dikmen, C.; Sharifi-Rad, M.; Selamoglu, Z.; Acharya, K.; Sen, S.; et al. Plants of the Genus Lavandula: From Farm to Pharmacy. Nat. Prod. Commun. 2018, 13, 1934578X1801301037. [Google Scholar] [CrossRef]
- Truzzi, E.; Benvenuti, S.; Bertelli, D.; Francia, E.; Ronga, D. Effects of Biostimulants on the Chemical Composition of Essential Oil and Hydrosol of Lavandin (Lavandula x intermedia Emeric Ex Loisel.) Cultivated in Tuscan-Emilian Apennines. Molecules 2021, 26, 6157. [Google Scholar] [CrossRef]
- Danh, L.T.; Triet, N.D.A.; Han, L.T.N.; Zhao, J.; Mammucari, R.; Foster, N. Antioxidant Activity, Yield and Chemical Composition of Lavender Essential Oil Extracted by Supercritical CO2. J. Supercrit. Fluids 2012, 70, 27–34. [Google Scholar] [CrossRef]
- Białoń, M.; Krzyśko-Łupicka, T.; Nowakowska-Bogdan, E.; Wieczorek, P.P. Chemical Composition of Two Different Lavender Essential Oils and Their Effect on Facial Skin Microbiota. Molecules 2019, 24, 3270. [Google Scholar] [CrossRef]
- Kozuharova, E.; Simeonov, V.; Batovska, D.; Stoycheva, C.; Valchev, H.; Benbassat, N. Chemical Composition and Comparative Analysis of Lavender Essential Oil Samples from Bulgaria in Relation to the Pharmacological Effects. Pharmacia 2023, 70, 395–403. [Google Scholar] [CrossRef]
- Soulaimani, B.; Abbad, I.; Amssayef, A. Seasonal Variation in the Chemical Composition and Antimicrobial Activity of Essential Oil Obtained from Moroccan Lavender Lavandula maroccana Murb. Collected from the Wild. Nat. Prod. Commun. 2024, 19, 1934578X241297990. [Google Scholar] [CrossRef]
- Ciocarlan, A.; Lupascu, L.; Aricu, A.; Dragalin, I.; Popescu, V.; Geana, E.-I.; Ionete, R.E.; Vornicu, N.; Duliu, O.G.; Hristozova, G.; et al. Chemical Composition and Assessment of Antimicrobial Activity of Lavender Essential Oil and Some By-Products. Plants 2021, 10, 1829. [Google Scholar] [CrossRef]
- Speranza, B.; Guerrieri, A.; Racioppo, A.; Bevilacqua, A.; Campaniello, D.; Corbo, M.R. Sage and Lavender Essential Oils as Potential Antimicrobial Agents for Foods. Microbiol. Res. 2023, 14, 1089–1113. [Google Scholar] [CrossRef]
- Hernández-Jiménez, M.O.; Loa, J.D.A.; Rojas-Avelizapa, N.G. Agroindustrial Plant Wastes: Novel Source of Antimicrobial Peptides. Circ. Econ. Sust. 2025, 1–35. [Google Scholar] [CrossRef]
- Sturchio, E.; Donnarumma, L.; Annesi, T.; Milano, F.; Casorri, L.; Masciarelli, E.; Zanellato, M.; Meconi, C.; Boccia, P. Essential Oils: An Alternative Approach to Management of Powdery Mildew Diseases. Phytopathol. Mediterr. 2014, 53, 385–395. [Google Scholar] [CrossRef]
- Prashar, A.; Locke, I.C.; Evans, C.S. Cytotoxicity of Lavender Oil and Its Major Components to Human Skin Cells. Cell Prolif. 2004, 37, 221–229. [Google Scholar] [CrossRef]
- Hummelbrunner, L.A.; Isman, M.B. Acute, Sublethal, Antifeedant, and Synergistic Effects of Monoterpenoid Essential Oil Compounds on the Tobacco Cutworm, Spodoptera Litura (Lep., Noctuidae). Available online: https://pubs.acs.org/doi/abs/10.1021/jf000749t (accessed on 28 January 2025).
- Koul, O.; Walia, S.; Dhaliwal, G.S.; Nagar, P. Essential Oils as Green Pesticides: Potential and Constraints. Available online: https://www.semanticscholar.org/paper/Essential-Oils-as-Green-Pesticides-%3A-Potential-and-Koul-Walia/c2cb6d3ec38480c1519ebc1cbcc028217e2758ad (accessed on 23 January 2025).
- Beni, C.; Casorri, L.; Masciarelli, E.; Ficociello, B.; Masetti, O.; Rinaldi, S.; Neri, U.; Papetti, P.; Cichelli, A. Characterization of Garlic (Allium sativum L.) Aqueous Extract and Its Hypothetical Role as Biostimulant in Crop Protection. J. Food Agric. Environ. 2018, 16, 38–44. [Google Scholar]
- Casorri, L.; Masciarelli, E.; Ficociello, B.; Beni, C. L’importanza dell’agricoltura sostenibile nel recupero della biodiversità. In Proceedings of the Atti del X Convegno Nazionale sulla Biodiversità, Roma, Italy, 3–5 September 2014; pp. 405–410. [Google Scholar]
- Boutahiri, S.; Eto, B.; Bouhrim, M.; Mechchate, H.; Saleh, A.; Al Kamaly, O.; Drioiche, A.; Remok, F.; Samaillie, J.; Neut, C.; et al. Lavandula Pedunculata (Mill.) Cav. Aqueous Extract Antibacterial Activity Improved by the Addition of Salvia Rosmarinus Spenn., Salvia Lavandulifolia Vahl and Origanum Compactum Benth. Life 2022, 12, 328. [Google Scholar] [CrossRef]
- Dostálová, L.; Detvanová, L.; Kalhotka, L. Antimicrobial Activity of Aqueous Herbal Extracts. 2014. Available online: https://www.semanticscholar.org/paper/Antimicrobial-Activity-of-Aqueous-Herbal-Extracts-Dost%C3%A1lov%C3%A1-Detvanov%C3%A1/4d39dfb4ecdd2e6b64b9cdecbae6926d49b98d6e (accessed on 27 February 2025).
- Alnamer, R.; Alaoui, K.; Doudach, L.; Bouidida, E.H.; AL-Sobarry, M.; Benjouad, A.; Cherrah, Y. Investigation of Methanolic and Aqueous Extract of Lavandula Officinalis for Toxicity and Antibacterial Activity. World J. Pharm. Res. 2012, 1, 1223–1233. [Google Scholar]
- Haig, T.; Pratley, J.; An, M.; Haig, T.; Hildebrand, S. Using Allelopathy to Search for New Natural Herbicides from Plants. In Proceedings of the Allelopathy, Establishing the Scientific Base, Gosford, Australia, 21–26 August 2005; The Regional Institute: Gosford, Australia, 2005; pp. 1–5. [Google Scholar]
- Haig, T.J.; Haig, T.J.; Seal, A.N.; Pratley, J.E.; An, M.; Wu, H. Lavender as a Source of Novel Plant Compounds for the Development of a Natural Herbicide. J. Chem. Ecol. 2009, 35, 1129–1136. [Google Scholar] [CrossRef]
- Amanda, A.; Ferrante, A.; Valagussa, M.; Piaggesi, A. Effect of Biostimulants on Quality of Baby Leaf Lettuce Grown under Plastic Tunnel. Acta Hortic. 2009, 807, 407–412. [Google Scholar] [CrossRef]
- Bulgari, R.; Podetta, N.; Cocetta, G.; Piaggesi, A.; Ferrante, A. The Effect of a Complete Fertilizer for Leafy Vegetables Production in Family and Urban Gardens. Bulg. J. Agric. Sci. 2014, 20, 1361–1367. [Google Scholar]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The Effect of a Plant-Derived Biostimulant on Metabolic Profiling and Crop Performance of Lettuce Grown under Saline Conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Shehata, S.M.; Schmidhalter, U.; Valšíková, M.; Junge, H. Effect of Bio-Stimulants on Yield and Quality of Head Lettuce Grown Under Two Sources of Nitrogen. Gesunde Pflanz. 2016, 68, 33–39. [Google Scholar] [CrossRef]
- Ottaiano, L.; Di Mola, I.; Cozzolino, E.; El-Nakhel, C.; Rouphael, Y.; Mori, M. Biostimulant Application under Different Nitrogen Fertilization Levels: Assessment of Yield, Leaf Quality, and Nitrogen Metabolism of Tunnel-Grown Lettuce. Agronomy 2021, 11, 1613. [Google Scholar] [CrossRef]
- Carillo, P.; De Micco, V.; Ciriello, M.; Formisano, L.; El-Nakhel, C.; Giordano, M.; Colla, G.; Rouphael, Y. Morpho-Anatomical, Physiological, and Mineral Composition Responses Induced by a Vegetal-Based Biostimulant at Three Rates of Foliar Application in Greenhouse Lettuce. Plants 2022, 11, 2030. [Google Scholar] [CrossRef]
- Yaseen, A.A.; Takacs-Hajos, M. The Effect of Plant Biostimulants on the Macronutrient Content and Ion Ratio of Several Lettuce (Lactuca sativa L.) Cultivars Grown in a Plastic House. S. Afr. J. Bot. 2022, 147, 223–230. [Google Scholar] [CrossRef]
- Nashwa, S.M.A.; Abo-Elyousr, K.A.M. Evaluation of Various Plant Extracts against the Early Blight Disease of Tomato Plants under Greenhouse and Field Conditions. Plant Prot. Sci. 2012, 48, 74–79. [Google Scholar] [CrossRef]
- Beni, C.; Casorri, L.; Masciarelli, E.; Ficociello, B.; Masetti, O.; Neri, U.; Aromolo, R.; Rinaldi, S.; Papetti, P.; Cichelli, A. Characterization of Thyme and Tansy Extracts Used as Basic Substances in Zucchini Crop Protection. J. Agric. Stud. 2020, 8, 95–110. [Google Scholar] [CrossRef]
- Jones, J. Kjeldahl Method for Nitrogen Determination. Available online: https://www.semanticscholar.org/paper/Kjeldahl-method-for-nitrogen-determination.-Jones/26868024bcfc756a71f905e65c081e9f11545fdf (accessed on 23 January 2025).
- MIPAF—Ministero Politiche Agricole e Forestali (Italy). Metodi Ufficiali di Analisi Chimica del Suolo. Decreto Ministeriale del 13/09/1999; Gazzetta Ufficiale della Repubblica Italiana, n. 248, 21/10/1999, Supplemento Ordinario n. 185. Available online: https://www.gazzettaufficiale.it/eli/gu/1999/10/21/248/so/185/sg/pdf (accessed on 31 October 2024). (In Italian).
- Parisse, B.; Alilla, R.; Pepe, A.G.; De Natale, F. MADIA-Meteorological Variables for Agriculture: A Dataset for the Italian Area. Data Brief 2023, 46, 108843. [Google Scholar] [CrossRef]
- Decreto Legislativo 29 Aprile 2010 n. 75. Riordino e Revisione Della Disciplina in Materia Di Fertilizzanti. Gazzetta Ufficiale Della Repubblica Italiana Serie Generale n. 121, 26/05/2010. Available online: https://www.gazzettaufficiale.it/eli/gu/2010/05/26/121/so/106/sg/pdf (accessed on 20 January 2025). (In Italian).
- Decreto 10 Luglio Decree 10 July 2013. Aggiornamento Degli Allegati Del Decreto Legislativo 29 Aprile 2010, n. 75, Concernente Il Riordino e La Revisione Della Disciplina in Materia Di Fertilizzanti. (13A07510) (GU Serie Generale n.218 Del 17-09-2013). Gazzetta Ufficiale Della Repubblica Italiana General Series n. 218 of 17/09/2013. Available online: https://www.gazzettaufficiale.it/eli/id/2013/09/17/13a07510/sg (accessed on 20 January 2025). (In Italian).
- JASP Team. JASP, version 0.19.3. Computer software. JASP Team: Amsterdam, The Netherlands, 2025.
- Wakeel, A.; Ishfaq, M. Potash Use and Dynamics in Agriculture; Springer: Singapore, 2022; ISBN 9789811668821. [Google Scholar]
- De Mello Prado, R. Nitrogen. In Mineral Nutrition of Tropical Plants; Springer International Publishing: Cham, Switzerland, 2021; pp. 69–98. ISBN 978-3-030-71261-7. [Google Scholar]
- Tang, R.-J.; Luan, S. Regulation of Calcium and Magnesium Homeostasis in Plants: From Transporters to Signaling Network. Curr. Opin. Plant Biol. 2017, 39, 97–105. [Google Scholar] [CrossRef]
- Anjum, N.A.; Masood, A.; Umar, S.; Khan, N.A. Phosphorus in Soils and Plants; IntechOpen: London, UK, 2024; ISBN 978-1-83769-035-0. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-0-429-19203-6. [Google Scholar]
- Francesca, S.; Arena, C.; Hay Mele, B.; Schettini, C.; Ambrosino, P.; Barone, A.; Rigano, M.M. The Use of a Plant-Based Biostimulant Improves Plant Performances and Fruit Quality in Tomato Plants Grown at Elevated Temperatures. Agronomy 2020, 10, 363. [Google Scholar] [CrossRef]
- Foss, K.; Przybyłowicz, K.E.; Sawicki, T. Antioxidant Activity and Profile of Phenolic Compounds in Selected Herbal Plants. Plant Foods Hum. Nutr. 2022, 77, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Yu, O.; Jez, J.M. Nature’s Assembly Line: Biosynthesis of Simple Phenylpropanoids and Polyketides. Plant J. 2008, 54, 750–762. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Yu, O. Metabolic Engineering of Flavonoids in Plants and Microorganisms. Appl. Microbiol. Biotechnol. 2011, 91, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.L.; Maia, B.H.L.N.S.; Ferriani, A.P.; Teixeira, S.D. Flavonoids: Classification, Biosynthesis and Chemical Ecology. In Flavonoids-from Biosynthesis to Human Health; Justino, G.C., Ed.; InTech: Houston, TX, USA, 2017; ISBN 978-953-51-3423-7. [Google Scholar]
- Belisle, C.E.; Sargent, S.A.; Brecht, J.K.; Sandoya, G.V.; Sims, C.A. Accelerated Shelf-Life Testing to Predict Quality Loss in Romaine-Type Lettuce. HortTechnology 2021, 31, 490–499. [Google Scholar] [CrossRef]
- Aguiar, P.; Corrêa, G.M.G.; Rodrigues, M.Â.; Arrobas, M. Reduced Effect of Commercial Leonardite and Seaweed Extract on Lettuce Growth under Mineral, Organic, and No Fertilization Regimes. Agronomy 2024, 14, 1939. [Google Scholar] [CrossRef]
- Afonso, S.; Dias, M.I.; Ferreira, I.C.F.R.; Arrobas, M.; Cunha, M.; Barros, L.; Rodrigues, M.Â. The Phenolic Composition of Hops (Humulus lupulus L.) Was Highly Influenced by Cultivar and Year and Little by Soil Liming or Foliar Spray Rich in Nutrients or Algae. Horticulturae 2022, 8, 385. [Google Scholar] [CrossRef]
- Amiri, M.E.; Fallahi, E.; Golchin, A. Influence of Foliar and Ground Fertilization on Yield, Fruit Quality, and Soil, Leaf, and Fruit Mineral Nutrients in Apple. J. Plant Nutr. 2008, 31, 515–525. [Google Scholar] [CrossRef]
- Patel, J.S.; Selvaraj, V.; Gunupuru, L.R.; Rathor, P.K.; Prithiviraj, B. Combined Application of Ascophyllum Nodosum Extract and Chitosan Synergistically Activates Host-Defense of Peas against Powdery Mildew. BMC Plant Biol. 2020, 20, 113. [Google Scholar] [CrossRef]
- Gunupuru, L.R.; Patel, J.S.; Sumarah, M.W.; Renaud, J.B.; Mantin, E.G.; Prithiviraj, B. A Plant Biostimulant Made from the Marine Brown Algae Ascophyllum Nodosum and Chitosan Reduce Fusarium Head Blight and Mycotoxin Contamination in Wheat. PLoS ONE 2019, 14, e0220562. [Google Scholar] [CrossRef]
- Di Stasio, E.; Van Oosten, M.J.; Silletti, S.; Raimondi, G.; dell’Aversana, E.; Carillo, P.; Maggio, A. Ascophyllum Nodosum-Based Algal Extracts Act as Enhancers of Growth, Fruit Quality, and Adaptation to Stress in Salinized Tomato Plants. J. Appl. Phycol. 2018, 30, 2675–2686. [Google Scholar] [CrossRef]
- Helmy, A.A.; El-Sherpiny, M.A.; Ghazi, D.A. Organic Fertilization and Melatonin: Improving Crisphead Lettuce Performance in Water-Limited Conditions. Egypt. J. Soil Sci. 2024, 64, 1585–1599. [Google Scholar] [CrossRef]
- Atero-Calvo, S.; Izquierdo-Ramos, M.J.; García-Huertas, C.; Rodríguez-Alcántara, M.; Navarro-Morillo, I.; Navarro-León, E. An Evaluation of the Effectivity of the Green Leaves Biostimulant on Lettuce Growth, Nutritional Quality, and Mineral Element Efficiencies under Optimal Growth Conditions. Plants 2024, 13, 917. [Google Scholar] [CrossRef]
- Martins Filho, A.P.; De Medeiros, E.V.; Barbosa, J.G.; Barbosa, J.M.P.; Kuklinsky-Sobral, J.; Souza-Motta, C. Combined effect of pseudomonas sp. and trichoderma aureoviride on lettuce growth promotion. Biosci. J. 2019, 35, 419–430. [Google Scholar] [CrossRef]
- Ahmad, E.; Khan, M.S.; Zaidi, A. ACC Deaminase Producing Pseudomonas Putida Strain PSE3 and Rhizobium Leguminosarum Strain RP2 in Synergism Improves Growth, Nodulation and Yield of Pea Grown in Alluvial Soils. Symbiosis 2013, 61, 93–104. [Google Scholar] [CrossRef]
- Harman, G.E. Multifunctional Fungal Plant Symbionts: New Tools to Enhance Plant Growth and Productivity. New Phytol. 2011, 189, 647–649. [Google Scholar] [CrossRef]
- Arrobas, M.; Correia, C.M.; Rodrigues, M.Â. Methylobacterium Symbioticum Applied as a Foliar Inoculant Was Little Effective in Enhancing Nitrogen Fixation and Lettuce Dry Matter Yield. Sustainability 2024, 16, 4512. [Google Scholar] [CrossRef]
- Franzoni, G.; Ferrante, A. Plant Extract Improves Quality Traits of Green and Red Lettuce Cultivars. Heliyon 2024, 10, e39224. [Google Scholar] [CrossRef]
- Dudaš, S.; Šola, I.; Sladonja, B.; Erhatić, R.; Ban, D.; Poljuha, D. The Effect of Biostimulant and Fertilizer on “Low Input” Lettuce Production. Acta Bot. Croat. 2016, 75, 253–259. [Google Scholar] [CrossRef]
- Uçan, U.; Demir, H.; Yalçi, H.K. Effects of Microbial Fertisizer on Yield and Quality of Curly Lettuce Grown in Pots. Int. J. Innov. Approaches Agric. Res. 2024, 8, 200–217. [Google Scholar] [CrossRef]
- Chase, K.; Belisle, C.; Ahlawat, Y.; Yu, F.; Sargent, S.; Sandoya, G.; Begcy, K.; Liu, T. Examining Preharvest Genetic and Morphological Factors Contributing to Lettuce (Lactuca sativa L.) Shelf-Life. Sci. Rep. 2024, 14, 6618. [Google Scholar] [CrossRef]
- Shi, M.; Gu, J.; Wu, H.; Rauf, A.; Emran, T.B.; Khan, Z.; Mitra, S.; Aljohani, A.S.M.; Alhumaydhi, F.A.; Al-Awthan, Y.S.; et al. Phytochemicals, Nutrition, Metabolism, Bioavailability, and Health Benefits in Lettuce—A Comprehensive Review. Antioxidants 2022, 11, 1158. [Google Scholar] [CrossRef] [PubMed]
- Margon, A.; Mondini, C.; Valentini, M.; Ritota, M.; Leita, L. Soil Microbial Biomass Influence on Strontium Availability in Mine Soil. Chem. Speciat. Bioavailab. 2013, 25, 119–124. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Bąba, W.; Gediga, K.; Goltsev, V.; Samborska, I.A.; Cetner, M.D.; Dimitrova, S.; Piszcz, U.; Bielecki, K.; Karmowska, K.; et al. Chlorophyll Fluorescence as a Tool for Nutrient Status Identification in Rapeseed Plants. Photosynth. Res. 2018, 136, 329–343. [Google Scholar] [CrossRef]
- Sustr, M.; Soukup, A.; Tylova, E. Potassium in Root Growth and Development. Plants 2019, 8, 435. [Google Scholar] [CrossRef] [PubMed]
- Sugier, D.; Kołodziej, B.; Bielińska, E. The Effect of Leonardite Application on Arnica montana L. Yielding and Chosen Chemical Properties and Enzymatic Activity of the Soil. J. Geochem. Explor. 2013, 129, 76–81. [Google Scholar] [CrossRef]
- Cieschi, M.T.; Lucena, J.J. Leonardite Iron Humate and Synthetic Iron Chelate Mixtures in Glycine max Nutrition. J. Sci. Food Agric. 2021, 101, 4207–4219. [Google Scholar] [CrossRef]
- Palani, V. Synergistic and Antagonistic Interactions of Calcium with Other Nutrients in Soil and Plants. SSRN J. 2019. [Google Scholar] [CrossRef]
- Rai, S.; Singh, P.K.; Mankotia, S.; Swain, J.; Satbhai, S.B. Iron Homeostasis in Plants and Its Crosstalk with Copper, Zinc, and Manganese. Plant Stress 2021, 1, 100008. [Google Scholar] [CrossRef]
- Ibourki, M.; Hallouch, O.; Devkota, K.; Guillaume, D.; Hirich, A.; Gharby, S. Elemental Analysis in Food: An Overview. J. Food Compos. Anal. 2023, 120, 105330. [Google Scholar] [CrossRef]
- El Khattabi, O.; El Hasnaoui, S.; Toura, M.; Henkrar, F.; Collin, B.; Levard, C.; Colin, F.; Merghoub, N.; Smouni, A.; Fahr, M. Seaweed Extracts as Promising Biostimulants for Enhancing Lead Tolerance and Accumulation in Tomato (Solanum lycopersicum). J. Appl. Phycol. 2023, 35, 459–469. [Google Scholar] [CrossRef]
- Mosa, W.F.A.; Sas-Paszt, L.; Głuszek, S.; Górnik, K.; Anjum, M.A.; Saleh, A.A.; Abada, H.S.; Awad, R.M. Effect of Some Biostimulants on the Vegetative Growth, Yield, Fruit Quality Attributes and Nutritional Status of Apple. Horticulturae 2022, 9, 32. [Google Scholar] [CrossRef]
- Theobald, H.E. Dietary Calcium and Health. Nutr. Bull. 2005, 30, 237–277. [Google Scholar] [CrossRef]
- Gupta, U.C.; Gupta, S.C. Sources and Deficiency Diseases of Mineral Nutrients in Human Health and Nutrition: A Review. Pedosphere 2014, 24, 13–38. [Google Scholar] [CrossRef]
- Sago, Y. Effects of Light Intensity and Growth Rate on Tipburn Development and Leaf Calcium Concentration in Butterhead Lettuce. HortScience 2016, 51, 1087–1091. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on Iron and Its Importance for Human Health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).