Abstract
The relationship between stand cumulative production at harvesting age and carbon stock for different soil types in forest plantations is critical for sustainable forest management and climate change mitigation. This study evaluated carbon stocks in Pinus radiata D. Don on granitic and metamorphic soils in central Chile. We selected 10 plantations and established three 1000 m2 stands per plantation to quantify the carbon stock of total biomass using allometric equations and in situ carbon assessments of the forest floor and mineral soil (up to 1 m deep). A strong positive correlation was observed between stand cumulative production at harvesting age and total carbon stock (r2 = 0.767), regardless of the soil type. Metamorphic and granitic soils demonstrated a high carbon stock capacity, particularly in deeper soil layers (40–100 cm), with soil contributing over 40% of the total soil carbon stock. Soil bulk density and carbon concentration were strongly correlated (r2 = 0.74), emphasizing the role of soil physical properties in carbon storage at deep soil horizons. These findings highlight the critical role of subsoils as carbon reservoirs. Predictive linear models developed from this study offer a useful and simple approach for estimating carbon stocks, contributing to national carbon neutrality goals and sustainable forest management.
1. Introduction
The increase in greenhouse gas concentrations over the past century, mainly due to anthropogenic activities, has significantly contributed to global warming and changes in global temperature and precipitation patterns [1,2,3]. Forests play a critical role in mitigating climate change by acting as carbon sinks, offsetting the effects of rising greenhouse gases. Global forest carbon stocks are estimated to be about 861 Pg C., representing 29% of the carbon in major terrestrial and oceanic reservoirs [3]. Of this forest carbon, 44% is stored in soils, 42% in above and below-ground biomass, 8% in deadwood, and 5% in litter [4,5]. This distribution highlights the importance of all components of forest ecosystems in the global carbon cycle. The soil organic matter (SOM) is a key component of soil, which contains more organic carbon than global vegetation and the atmosphere combined [6]. Also, factors such as stand age [7,8,9], species composition [10,11,12,13], climate, site conditions, and soil type [13,14,15] significantly influence carbon accumulation, with more productive sites having greater carbon storage potential in both biomass and soil [13,16].
Currently, native forests and forestry plantations coexist in the ecosystems, necessitating a balanced approach that leverages the strengths of both systems to maximize carbon storage and enhance ecosystem resilience against climate change [17]. Afforestation and reforestation in managed forests are important strategies for mitigating climate change, especially in degraded or non-forested areas [18,19]. These practices not only enhance CO₂ sequestration but also improve ecosystem services, biodiversity, and soil quality [20,21,22,23]. Developing knowledge about the carbon stock capacity of plantations, along with implementing sustainable management practices, is essential to ensure that these plantations continue to provide valuable goods and services while minimizing their environmental impacts [24,25,26,27,28].
In Chile, the forestry sector is a key component of the national economy, with Pinus radiata D. Don being the predominant non-native species covering approximately 1,299,451 hectares, which constitutes about 55.9% of the total forest plantation area [29]. This species is recognized for its rapid growth and high biomass production that depends on advanced genotypes, silviculture, and environmental conditions [30,31,32]. Numerous studies worldwide have explored carbon stock in Pinus radiata plantations [13,33,34,35,36], highlighting the global interest and efforts in understanding and optimizing carbon stock in these ecosystems. Understanding the carbon stock capacity of Pinus radiata plantations is essential; however, most existing studies have predominantly focused on carbon stocks in above-ground biomass, with limited attention given to soils, especially deep horizons, as significant carbon reservoirs [4,33,37]. This gap underscores the importance of assessing carbon stocks at greater soil depths to fully capture the sequestration potential of different soil types [36,38]. Soil types such as sandy, recent volcanic ash, metamorphic, and granitic exhibit distinct physicochemical and biological characteristics that influence their capacity to stock carbon. These differences are primarily driven by variations in organic matter dynamics, nutrient availability, and microbial activity, which collectively govern soil carbon storage and stabilization [39,40].
Sandy soils, characterized by coarse texture and low water and nutrient retention, generally exhibit limited carbon storage potential due to reduced organic matter accumulation and microbial activity [36,41,42]. In contrast, recent volcanic ash soils initially provide high nutrient availability that supports rapid plant growth and carbon input; however, their long-term sequestration potential depends on factors such as soil stabilization and sustained organic matter inputs [36,43,44]. Metamorphic soils, with their complex mineral compositions, are conducive to stabilizing organic matter and reducing decomposition rates, making them effective for long-term carbon storage [45,46]. Granitic soils, while limited by coarse texture and low nutrient-holding capacity, can enhance their carbon sequestration potential through management interventions, such as fertilization, that improve vegetation growth and organic matter accumulation [47,48]. Recent analyses of Pinus radiata plantations in Chile reveal significant variability in soil carbon stocks across parent materials. The highest total stocks were observed in soils from marine sediments and recent volcanic ash (445.4 and 473.2 Mg·ha−1), while the lowest were in lacustrine soils (144.4 Mg·ha−1). Within the 0–30 cm depth, soils from recent volcanic ash over metamorphic rock had the highest carbon values (171.3–172.6 Mg·ha−1), followed by old volcanic ash over marine sediments. Lacustrine soils consistently showed the lowest values, with just 37.6 Mg·ha−1 in this layer [13,36].
This study evaluated the relationship between total carbon stock in Pinus radiata plantations and stand cumulative production at harvesting age. A model was developed integrating information on carbon stock reservoirs in biomass, forest floor, and soil up to 1 m in depth, based on field data from plantations established on granitic and metamorphic soils—two predominant soil types in Chilean forestry [13]. The objective is to provide a predictive model that enhances the understanding of carbon stocks under different productive conditions. Our findings could serve as a practical tool for landowners to assess how their management practices align with national carbon neutrality goals, contributing to climate change mitigation.
2. Materials and Methods
2.1. Study Area and Experimental Design
The analysis was carried out across ten Pinus radiata plantations, selected to represent a stand cumulative production at harvesting age gradient (18–24 years old, the age at which radiata pine plantations are harvested in Chile). The plantations are located in central Chile, spanning the Maule (latitude 35°25′ S) to Araucanía (latitude 38°54′ S) administrative regions. This area features a Mediterranean climate with significant variation in environmental conditions. Mean annual precipitation ranges from 950 mm in the northern areas to 2500 mm in the southern regions, while mean annual temperature decreases progressively from 19 °C in the north to 11 °C in the south (Figure 1). All selected sites correspond to afforestation areas, meaning that plantations were established on previously highly degraded agricultural soils. All plantations followed conventional soil preparation practices, including ripping to a depth of 0.6 cm and disking. Standard operational fertilization was applied, with NPK 15–13.2–12.5 (100–150 g tree−1) and an additional 2 to 3 g of boron per plant, administered after planting. Annual weed control was conducted, followed by banded herbicide applications for two years after plantation establishment. Each plantation was pruned to a minimum height of 5.5 m and underwent one or two commercial thinnings from below, the first to 700 trees per hectare and the second to the final stocking density ranging between 400 and 550 trees per hectare, depending on the specific silvicultural regime of the stand.
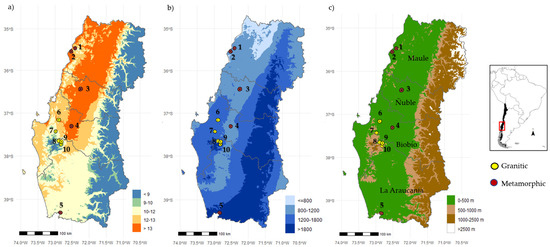
Figure 1.
Location of Pinus radiata plantations evaluated per soil type and climate variability. (a) Mean annual temperature (°C); (b) Mean annual precipitation (mm) and (c) elevation (m a.s.l.) and administrative regions. Red points represent plantations on metamorphic soils, while yellow points represent plantations on granitic soils. Plantation code: 1–SPC402, 2–EP530, 3–HH252, 4–LQ381, 5–SF690, 6–SM401, 7–HO669, 8–PM437, 9–LD397 and 10–LN574.
Field measurements were conducted between May and November 2023 in plantations under intensive management. The experimental design includes 5 plantations per soil type, metamorphic and granitic [49,50], with 3 independent stands of 1000 m2 established at each plantation, resulting in a total of 30 independent stands across all evaluated plantations. The selected plantations were planted between 1998 and 2004, each consisting of a single cohort of trees, meaning that all trees within a plantation were of the same age. In each stand, the diameter at breast height (DBH; measured at 1.3 m) and total height (H) were measured for all trees. Additionally, 10 forest floor and 15 soil samples were collected within each stand according to a zig-zag sample design.
2.2. Stand Cumulative Production at Harvesting Age Estimation
Stand cumulative production at harvesting age (Sp, m3 ha−1) refers to the current volume of the stand at the time of harvest, excluding the volume removed during thinning. To estimate the Sp, the volume for individual trees over bank (Vtree, m3 tree−1) was calculated using the allometric Equation (1) established by CMPC Forestal Mininco (1995) [51,52]:
where the sum of all Vtree per stand was multiplied by 10 to scale the volume on a per-hectare basis, yielding the stand’s cumulative production at harvesting age (Sp). Finally, the plantation cumulative production at harvesting age (Plp) was then calculated as the mean of the three Sp estimates (m3 ha−1) in each plantation evaluated.
Vtree = −0.00214 + 0.0000295 ∗ DBH2 + 0.001349 ∗ H + 0.00002486 ∗ DBH2 ∗ H
2.3. Carbon Stock in Above and Belowground Biomass
The aboveground biomass of each tree was calculated using the following published allometric Equations (2)–(5) developed for Pinus radiata plantations [53]:
where AGBs is the steam biomass, AGBb is the bark biomass, AGBbr is the branch, and AGBn is the needle biomass, all in kg tree−1. The total aboveground biomass (AGBtotal) was then calculated as the sum of all components as Equation (6):
AGBs = 0.02389 ∗ (D2 ∗ H)0.93216
AGBb = 0.00127 ∗ (D2 ∗ H)0.99646
AGBbr = 0.00431 ∗ (D2 ∗ H)0.92709
AGBn = 0.19428 ∗ (D2 ∗ H)0.48666
AGBtotal = AGBs + AGBb + AGBbr + AGBn
The belowground biomass (BGB, kg tree−1). of each tree was calculated using Equation (7), which estimates the total root biomass as follows [53,54]:
BGB = exp (0.902 ∗ ln(AGBtotal) − 0.7368)
The carbon stock of each individual tree was estimated by multiplying AGBtotal and BGB by a carbon factor (CF), representing the carbon fraction in each biomass component. For this calculation, a CF of 0.48 was used, as recommended by the IPCC (2013) [55]. The carbon stocks of all trees within each stand were summed, and the mean value across the three stands of each plantation was calculated. This value was then scaled to a per hectare basis to estimate the aboveground biomass carbon stock (CAGB, Mg·ha−1) and belowground biomass carbon stock (CBGB, Mg·ha−1) for each plantation. The sum of both estimates provided the aboveground and belowground carbon stocks (CTGB).
2.4. Forest Floor Sampling and Carbon Estimations
Forest floor samples were collected from all plantations. Ten sample points of litter (L) and coarse woody debris (W) were taken in each stand using a circular cutting frame (25 cm diameter). In total, 600 forest floor samples (10 sample points × 2 sample types (L and W) × 3 stands × 10 plantations) were obtained. Each floor sample was oven-dried at 65 °C for 48 h and weighed. The dried samples for each forest floor type were composited, homogenized, and ground using a 250 µm sieve blade mill for each stand. A 5 g subsample was obtained to analyze the carbon concentration (C) in litter and coarse woody debris of each stand. The values obtained were averaged to provide a value per plantation (FFL and FFW). The forest floor carbon stock for each sample type per plantation (FFCL and FFCW) was determined by multiplying the dry biomass weight by the carbon concentration (C) and then scaled to a per hectare basis. Finally, the total forest floor carbon stock per plantation (FFCT) was calculated by summing the FFCL and FFCW values. All analyses were conducted at the Soil, Water, and Forest Research Laboratory (LISAB, by its Spanish acronym) Faculty of Forest Sciences, University of Concepción, Chile.
2.5. Soil Sampling and Carbon Estimations
Soil samples were collected from 10 plantations at three depths (0–20, 20–40, and 40–100 cm) using an auger, following a zigzag sampling design. Fifteen samples were taken per depth within each stand and then pooled into a bucket per depth. After completing the sampling for each stand, the contents of each bucket were thoroughly mixed on a tarp to create a composite sample of approximately 250 g per depth. In total, 90 composite soil samples (3 depths × 3 stands × 10 plantations) were obtained. The samples were air-dried and sieved through a 2 mm mesh to remove roots and plant debris. A 10 g subsample of each composite sample was oven-dried at 65 °C for 24 h, and a 5 g subsample was subsequently analyzed for organic carbon (Cd) and total nitrogen (Nd) concentrations using an Infrared Mass Spectrometer (IRMS, SERCON).
Additional soil samples were collected from each stand at three depths (0–20, 20–40, and 40–100 cm) using a metal cylinder with a volume of 100 cm3. These samples were oven-dried at 65 °C for 24 h to determine their dry mass. Bulk density (BDd, g/cm3) was calculated as the ratio of the oven-dried mass to the cylinder volume, and soil texture was determined via the Bouyoucos hydrometer method. All analyses were conducted at the Soil, Water, and Forest Research Laboratory (LISAB, by its Spanish acronym) Faculty of Forest Sciences, University of Concepción, Chile.
Soil organic carbon stock for each depth (SOCd, Mg·ha−1) was calculated by using Equation (8) [56]:
where Cd is the soil organic carbon concentration, BD is the soil bulk density, d is the depth (cm), and δ is the proportion of rock fragments (>2 mm).
SOCd = Cd ∗ BD ∗ d ∗ (1 − δ)
The mean value for each depth per plantation was calculated, and the total soil organic carbon stock (SOCT) until 1 m of depth per plantation was determined by summing the mean SOCd estimates at each depth.
2.6. Statistical Analysis
The software R version 4.3.3 [57] was used for all statistical analyses. We tested the data for independence of residuals, equality of variance (homoscedasticity), and normality of residuals using diagnostic plots and Shapiro–Wilk and Levene’s tests. Analyses were performed without data transformation. An analysis of variance (ANOVA) was conducted for each carbon reservoir, total carbon stock, and all stand inventory estimates to evaluate mean differences between soil types. Regression models were used to determine the relationship between stand cumulative production at harvesting age and total carbon stock with the function lm () in R. The models were selected based on their relevance, i.e., adjusted coefficient of determination r2 closer to 1 and lower p-value. Additionally, predictive models were developed to estimate carbon stock based on the factors considered in the analysis. These predictions were generated using the total carbon stock per plantation (C_Total), which was calculated as the sum of carbon stocks estimated in the reservoirs, including soil carbon (SOCT), forest floor carbon (FFCT), and biomass carbon (CTGB). The predictive models were developed to estimate C_Total based on Sp and soil type (SoilType, categorical: granitic or metamorphic). The selected model included an interaction term to account for potential differences in the relationship between Sp and C_Total across soil types:
C_Total = β0 + β1 (Sp) + β (SoilType) + β3 (Sp × SoilType) + ϵ
Predictions were generated by creating a sequence of Sp values and combining them with the two soil type categories. Using the fitted model, we applied the predict() function in R to estimate C_Total under different combinations of predictor variables.
3. Results
3.1. Plantation Cumulative Production at Harvesting Age
No significant differences were observed on average for diameter at breast height (DBH, p = 0.96), total height (H, p = 0.68), number of trees (Ntrees, p = 0.33), and plantation cumulative production at harvesting age (Plp, p = 0.7) estimates between metamorphic and granitic soils (Table 1). Results showed the existence of a gradient of cumulative production at harvesting age among the plantations for both soil types.

Table 1.
Mean stand characteristics for each plantation.
3.2. Soil Horizon Characteristics
We evaluated bulk density, C/N ratio, carbon concentration, and the percentage of clay, sand, and silt in three soil horizons from forest plantations with varying Plp levels using soil pits (Table 2). Linear regression analyses for each soil type showed a significant relationship between carbon concentration and bulk density, in which higher carbon concentrations were associated with lower bulk density values. High Plp, such as SF-690 (690.4 m3·ha−1) and HO-669 (669.8 m3·ha−1), are associated with surface horizons (0–20 cm) exhibiting higher total carbon content (4.33% and 5.78%, respectively) and lower bulk density (1.16 g·cm3 and 0.71 g·cm3, respectively). The relationships were stronger in metamorphic soils (r2 = 0.74, p-value < 0.0001) compared to granitic soil (r2 = 0.5, p-value < 0.0002).

Table 2.
Soil horizon characteristics based on pit descriptions.
3.3. Carbon Stock
No significant difference was observed for aboveground and belowground carbon stock estimates between soil types (p-values of 0.46 and 0.41, respectively) (Table 3). The linear regression model analyzing CTGB as a function of stand cumulative production at harvesting age (Sp), combining data from both soils, showed an overall good fit (r2 = 0.99, p-value = 1.92 × 10−41). When analyzed separately by soil type, the regression lines showed almost identical intercepts (13.06) and slopes (0.318). This indicates that soil type does not significantly alter the relationship between Sp and CTGB, emphasizing the dominant role of cumulative production at harvesting age in determining biomass carbon storage (Figure 2a).

Table 3.
Mean carbon stock across soil types and plantations.

Figure 2.
(a) Relationship between stand cumulative production at harvesting age (Sp) and biomass carbon stock (CTGB), and (b) relationship between stand cumulative production at harvesting age (Sp) and forest floor carbon stock (FFCT). The black circles represent data points (mean stand observations) from granitic soils, while the white triangles correspond to metamorphic soils. The solid black line represents the overall linear regression model, which includes both soil types. The dashed line represents the regression for metamorphic soils, and the dotted line represents the regression for granitic soils. The p-value, r2, and equation of the regression line for all three models are shown on the graph to provide insight into the statistical significance and fit of the models.
For forest floor carbon stock (FFCT), the mean values per plantation ranged from 5.6 Mg·ha−1 in SPC-402 to 26.8 Mg·ha−1 in SF-690 (Table 3). There was no significant difference in average FFCT between soil types (p-value = 0.056). The overall linear regression model for FFCT explained 41.4% of the variance (r2 = 0.41; p-value = 0.000523) with a slope of 0.026 (Figure 2b). The ANCOVA analysis for the linear regressions by soil type showed that slopes (p-value = 0.19) and intercepts (p-value = 0.06) were statistically similar for both soil types, despite granitic soils having a slightly steeper slope than metamorphic soils.
For soil organic carbon stock (SOCT), the mean values per plantation ranged from 138 Mg·ha−1 in HH-252 to 829 Mg·ha−1 in SF-690 (Table 3). There was a significant difference in average SOCT between soil types (p-value = 0.032). The overall linear regression model explained 69% of the variance (r2 = 0.69) and showed a significant positive effect of Sp (p-value = 7.63 × 106), with a slope of 0.376. The ANCOVA showed statistical differences in intercepts (p-value = 0.000187) and slopes (p-value = 0.00899) between soil types. The linear regression analysis for the relationship between Sp and SOCT showed a higher determination coefficient for metamorphic soils than for granitic soils (0.68 vs. 0.27). This suggests that metamorphic soils have a greater capacity to accumulate carbon in response to increased Sp. Notably, SF-690, a metamorphic soil site with the highest Sp (690.4 m3·ha−1), recorded the highest SOCT value (829 Mg·ha−1), significantly exceeding the overall model predictions. This very high SOCT value may be attributed to higher soil organic matter concentrations, which enhance carbon accumulation. Such deviations highlight the potential influence of local factors, such as organic matter enrichment or site-specific conditions, on the relationship between Sp and soil carbon stocks (Figure 3a).
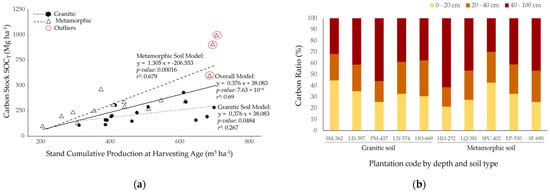
Figure 3.
(a) Relationship between stand cumulative production at harvesting age (Sp) and soil carbon stock (SOCT), and (b) average plantation carbon ratio by depth and soil type. The black circles represent data points (mean stand observations) from granitic soils, while the white triangles correspond to metamorphic soils. The solid black line represents the overall linear regression model, which includes both soil types. The dashed line represents the regression for metamorphic soils, and the dotted line represents the regression for granitic soils. The red circles highlight outlier values that deviate significantly from the predicted trends, indicating sites with unusual soil carbon stocks relative to their Sp. The p-value, r2, and equation of the regression line for all three models are shown on the graph to provide insight into the statistical significance and fit of the models.
There was no effect of sampling depth on the distribution of soil organic carbon stock (SOCT) (p-value = 0.28), with values ranging from 25% to 44% (Figure 3b).
For total carbon stock (CTotal), the mean values per plantation ranged from 245.8 Mg·ha−1 in HH-252 to 1085.8 Mg·ha−1 in SF-690 (Table 3). There was no significant difference in average CTotal between soil types (p-value = 0.1). The overall linear regression model explained 76.7% of the variance (r2 = 0.767; p-value = 3.14 × 10−8) with a slope of 0.737. The ANCOVA for the linear regressions detected significant differences in intercepts (p-value = 0.000255) and slopes (p-value = 0.010914) between the two soil types. The regression model showed a stronger relationship between Sp and carbon stock for the metamorphic soil (r2 = 0.768, p-value = 1.83 × 10−5) than the granitic soil (r2 = 0.586, p-value = 0.0008). For metamorphic soils, three outliers were observed at one site where high stand cumulative production at harvest age was associated with unusually high carbon stock values (Figure 4a).

Figure 4.
(a) Relationship between stand cumulative production at harvesting age (Sp) and total carbon stock (CTotal), and (b) Relationship between observed and predicted total carbon stocks. The black circles represent data points (mean stand observations) from granitic soils, while the white triangles correspond to metamorphic soils. The solid black line represents the overall linear regression model, which includes both soil types. The dashed black line represents the regression for metamorphic soils, and the dotted line represents the regression for granitic soils. The dashed red line represents the 1:1 line, indicating perfect agreement between observed and predicted values. The red circles highlight outlier values that deviate significantly from the predicted trends, indicating sites with unusual soil carbon stocks relative to their Sp. The p-value, r2, and equation of the regression line for all three models are shown on the graph to provide insight into the statistical significance and fit of the models.
The relationship between observed and predicted total carbon stocks was evaluated to assess the prediction accuracy of the model (Figure 4b). The predicted values are generally in line with the observed values, with most data points clustering around the 1:1 line. The Root Mean Square Error (RMSE) of the model was calculated to be 114.26 Mg·ha−1.
4. Discussion
This study highlights the close and significant relationship of stand cumulative production at harvesting age (Sp) on carbon stock in Pinus radiata plantations. Our findings demonstrated a strong positive linear relationship between Sp and total carbon stocks (r2 = 0.767, p-value < 0.001). This relationship is useful for sustainable forest management and climate change mitigation, as it suggests that plantation productivity can be used as a practical tool to estimate carbon stock across various sites [36]. These insights are relevant for the carbon market, planning, and monitoring of forest resources [18]. Additionally, they provide an indicator for evaluating how the forestry sector can meet the national carbon neutrality goals set by international agreements for 2050.
The positive and strong relationship (r2 = 0.74, p-value < 0.0001) between soil bulk density and soil carbon concentration indicates that variations in soil physical characteristics, such as compaction or porosity, may play a more critical role in determining carbon availability at deeper soil layers than soil particle size variations. Since bulk density affects the movement of air and water, root growth, microbial activity, and the storage and distribution of organic matter, this property is crucial in determining soil carbon content (Ray, 2019 [59]). This supports the hypothesis that soil structural characteristics significantly influence carbon stabilization and storage at depth.
The importance of soils as a major carbon reservoir in Pinus radiata plantations agrees with Olmedo et al. (2020) [13], who reported the distribution of carbon stock across four pools in Pinus radiata plantations: 196.8 Mg·ha−1 in aboveground biomass, 36.8 Mg·ha−1 in belowground biomass, 10.5 Mg·ha−1 in the forest floor, and 100 Mg·ha−1 in 0–30 cm soil organic carbon. While our results were consistent with their overall observations, the deeper evaluation of soils in our study reveals a significant shift in the relative importance of these pools. In Olmedo et al. (2020) [13], soil accounted for approximately 29% of the total carbon stock. In contrast, by assessing soil carbon stocks down to 1 m depth, we observed that soil contributes over 40% of the total carbon stock. These results agree with Bozo et al. (2024) [36], in which the stock of sequestered carbon is 42.3%. This depth-specific insight aligns with global assessments, such as those by Pan et al. (2011) [4], which underscore the importance of subsoils in long-term carbon storage. Our findings highlight that while aboveground and belowground biomass remain as significant contributors to carbon sequestration, the deeper soil layers serve as a substantial and previously underestimated carbon pool, emphasizing the need to measure carbon sequestration at deep soil layers.
Carbon stocks were not significantly different among sampling depths, confirming that deep soil layers are important contributors to total soil carbon stocks. The high values for carbon stocks below 40 cm depth may be associated with the developmental pattern of roots in commercial plantations of Pinus radiata, as the majority of the roots are typically found within the top 1.5 m of the soil. Therefore, belowground carbon characterization should not be restricted to shallow soil horizons.
Biomass carbon stocks were predominantly driven by cumulative production at harvesting age, with minimal variation attributed to soil type. This suggests that genetic improvements, silvicultural practices, and climatic conditions are the primary determinants of aboveground and belowground biomass carbon. While differences in forest floor carbon stocks were not statistically significant, the higher decomposition rates and nutrient cycling efficiency in metamorphic soils may explain a tendency to show lower forest floor carbon accumulation [44].
For plantations on granitic soils, interventions such as enhanced fertilization and organic matter inputs are crucial to improving carbon stock potential. Conversely, plantations on metamorphic soils may benefit from practices that protect soil integrity and minimize disturbances to improve carbon stock capacity. The observed relationship between productivity and total carbon stock underscores the importance of balancing growth optimization with sustainable management to achieve long-term carbon sequestration goals.
While this study provides valuable insights, some limitations warrant consideration. The relatively short temporal scope may overlook long-term dynamics of carbon stock influenced by soil aging, climatic variability, or changes in management practices. Additionally, local factors such as historical land use or genotypic differences in Pinus radiata were not accounted for and may have contributed to the observed variability. Future research should focus on integrating landscape-level analyses and extending soil assessments to other parent materials to refine predictive models.
The predictive linear models developed in this study offer a practical tool for estimating carbon stocks in forest plantations, providing a scientific basis for decision-making in forest management. Incorporating these models into national strategies for carbon neutrality can help optimize carbon stock while supporting economic and ecological objectives. The predicted values were generally in line with the observed values, with most data points clustering around the 1:1 line. This suggests that the model is effectively capturing the general trends. However, some deviations were observed, particularly in plantations with higher productivity, indicating potential model limitations or the influence of unaccounted for local factors. The Root Mean Square Error (RMSE) of the model was calculated to be 114.26 Mg·ha−1, reflecting the average magnitude of the prediction error. This low level of error suggests that the model performs reasonably well but could benefit from additional predictors or refinement to account for unexplained variance.
5. Conclusions
This study underscores the strong relationship between stand cumulative production at harvesting age and total carbon stock in Pinus radiata plantations, highlighting its potential as a practical proxy for carbon stock estimates. Our findings demonstrate that both granitic and metamorphic soils exhibit similar carbon stock capacity. Notably, deeper soil layers play a critical role in long-term carbon stock, emphasizing the need for comprehensive assessments that extend beyond surface horizons. The predictive models developed provide a scalable tool for estimating carbon stocks, offering a practical application for forest management and climate change mitigation strategies. These insights contribute to optimizing plantation management practices in alignment with national and international carbon neutrality goals. Future research should explore the influence of long-term soil dynamics, land-use history, and genetic variability in Pinus radiata to refine carbon stock estimations and enhance sustainable forestry practices in different soil types.
Author Contributions
Conceptualization, R.R., R.M.A. and J.P.E.; data curation, M.V.A., O.J. and D.B.; formal analysis, M.V.A. and R.R.; funding acquisition, R.R., R.M.A. and J.P.E.; investigation, D.B., R.R., O.J., R.M.A. and J.P.E.; methodology, R.R., R.M.A. and J.P.E.; project administration, R.R., R.M.A. and J.P.E.; resources, R.R., R.M.A., J.P.E. and M.P.; software, M.V.A. and R.R.; supervision, R.R. and R.M.A.; visualization, M.V.A.; writing—original draft, M.V.A.; writing—review and editing, R.R. and D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Forest Productivity Cooperative at Universidad de Concepción and the Chilean National Commission for Scientific and Technological Research (Project Grant ANID BASAL FB210015 “CENAMAD” and Project Grant ANID FOVI220029).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Restrictions apply to the datasets. The datasets presented in this article are not readily available because the data are part of an ongoing study.
Acknowledgments
We acknowledge funding and scientific support from the Forest Productivity Cooperative at Universidad de Concepción and the Chilean National Commission for Scientific and Technological Research (Project Grant ANID BASAL FB210015 “CENAMAD”, Project Grant ANID FOVI220029, ANID-Chile, project ANID-ANILLO ACT210060: FiRING:), and Scholarship Program, DOCTORADO BECAS CHILE/2023-21240118; and we gratefully acknowledge CMPC S.A. (Forestal Mininco) for support to access to forest farms.
Conflicts of Interest
The author M.P. was employed by the Forestal Mininco SpA. company. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest. The author M.P. had the following involvement with the study: conclusions and support to access to forest farms.
References
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Hauck, J.; Olsen, A.; Peters, G.P.; Peters, W.; Pongratz, J.; Sitch, S.; et al. Global Carbon Budget 2020. Earth Syst. Sci. Data 2020, 12, 3269–3340. [Google Scholar] [CrossRef]
- Dellsén, F. Consensus Versus Unanimity: Which Carries More Weight? Br. J. Philos. Sci. 2021. [Google Scholar] [CrossRef]
- IPCC. Global Carbon and Other Biogeochemical Cycles and Feedbacks. In Climate Change 2021—The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Intergovernmental Panel on Climate; Cambridge University Press: Cambridge, UK, 2023; pp. 673–816. [Google Scholar]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Kipping, L.; Goßner, M.M.; Koschorreck, M.; Muszynski, S.; Maurer, F.P.; Weiser, W.W.; Jehmlich, N.; Noll, M. Emission of CO2 and CH4 From 13 Deadwood Tree Species Is Linked to Tree Species Identity and Management Intensity in Forest and Grassland Habitats. Glob. Biogeochem. Cycles 2022, 36, e2021GB007143. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Han, H.; Shi, Z.; Yang, X. Biomass accumulation and carbon sequestration in an age-sequence of Mongolian pine plantations in Horqin sandy land, China. Forests 2019, 10, 197. [Google Scholar] [CrossRef]
- Diao, J.; Liu, J.; Zhu, Z.; Wei, X.; Li, M. Active forest management accelerates carbon storage in plantation forests in Lishui, southern China. For. Ecosyst. 2022, 9, 100004. [Google Scholar] [CrossRef]
- Zha, T.S.; Barr, A.G.; Bernier, P.Y.; Lavigne, M.B.; Trofymow, J.A.; Amiro, B.D.; Arain, M.A.; Bhatti, J.S.; Black, T.A.; Margolis, H.A.; et al. Gross and aboveground net primary production at Canadian forest carbon flux sites. Agric. For. Meteorol. 2013, 174–175, 54–64. [Google Scholar] [CrossRef]
- Díaz Villa, M.V.E.; Cristiano, P.M.; De Diego, M.S.; Rodríguez, S.A.; Efron, S.T.; Bucci, S.J.; Scholz, F.; Goldstein, G. Do selective logging and pine plantations in humid subtropical forests affect aboveground primary productivity as well as carbon and nutrients transfer to soil? For. Ecol. Manag. 2022, 503, 119736. [Google Scholar] [CrossRef]
- López-Senespleda, E.; Calama, R.; Ruiz-Peinado, R. Estimating forest floor carbon stocks in woodland formations in Spain. Sci. Total Environ. 2021, 788, 147734. [Google Scholar] [CrossRef]
- Watt, M.S.; Kimberley, M.O. Spatial comparisons of carbon sequestration for redwood and radiata pine within New Zealand. For. Ecol. Manag. 2022, 513, 120190. [Google Scholar] [CrossRef]
- Olmedo, G.F.; Guevara, M.; Gilabert, H.; Montes, C.R.; Arellano, E.C.; Barría-Knopf, B.; Gárate, F.; Mena-Quijada, P.; Acuña, E.; Bown, H.E.; et al. Baseline of Carbon Stocks in Pinus radiata and Eucalyptus spp. Plantations of Chile. Forests 2020, 11, 1063. [Google Scholar] [CrossRef]
- Mizuta, K.; Grunwald, S.; Bacon, A.R.; Cropper, W.P.; Phillips, M.A.; Moss, C.B.; Gonzalez-Benecke, C.A.; Markewitz, D.; Clingensmith, C.M.; Xiong, X. Holistic aboveground ecological productivity efficiency modeling using data envelopment analysis in the southeastern U.S. Sci. Total Environ. 2022, 824, 153802. [Google Scholar] [CrossRef] [PubMed]
- Bellassen, V.; Luyssaert, S. Carbon sequestration: Managing forests in uncertain times. Nature 2014, 506, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Kranabetter, J.M. Site carbon storage along productivity gradients of a late-seral southern boreal forest. Can. J. For. Res. 2009, 39, 1053–1060. [Google Scholar] [CrossRef]
- Waring, B.; Neumann, M.; Prentice, I.C.; Adams, M.; Smith, P.; Siegert, M. Forests and Decarbonization—Roles of Natural and Planted Forests. Front. For. Glob. Change 2020, 3, 534891. [Google Scholar] [CrossRef]
- Smith, P.; Bustamante, M.; Ahammad, H.; Clark, H.; Dong, H.; Elsiddig, E.; Haberl, H.; Harper, R.; House, J.; Jafari, M.; et al. Agriculture, forestry and other land use (AFOLU). In Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; pp. 811–922. [Google Scholar]
- McKinley, D.C.; Ryan, M.G.; Birdsey, R.A.; Giardina, C.P.; Harmon, M.E.; Heath, L.S.; Houghton, R.A.; Jackson, R.B.; Morrison, J.F.; Murray, B.C.; et al. A synthesis of current knowledge on forests and carbon storage in the United States. Ecol. Appl. 2011, 21, 1902–1924. [Google Scholar] [CrossRef] [PubMed]
- Štraus, H.; Podvinšek, S.; Klopčić, M. Identifying Optimal Forest Management Maximizing Carbon Sequestration in Mountain Forests Impacted by Natural Disturbances: A Case Study in the Alps. Forests 2023, 14, 947. [Google Scholar] [CrossRef]
- Begotti, R.A.; Pacífico, E.d.S.; Ferraz, S.; Galetti, M. Landscape Context of Plantation Forests in the Conservation of Tropical Mammals. J. Nat. Conserv. 2018, 41, 97–105. [Google Scholar] [CrossRef]
- López-Bedoya, P.A.; Magura, T.; Edwards, F.A.; Edwards, D.P.; Rey-Benayas, J.M.; Löveï, G.L.; Noriega, J.A. What Level of Native Beetle Diversity Can Be Supported by Forestry Plantations? A Global Synthesis. Insect Conserv. Divers. 2021, 14, 736–747. [Google Scholar] [CrossRef]
- Heinrichs, S.; Pauchard, A.; Schall, P. Native Plant Diversity and Composition Across a Pinus radiata D. Don Plantation Landscape in South-Central Chile—The Impact of Plantation Age, Logging Roads and Alien Species. Forests 2018, 9, 567. [Google Scholar] [CrossRef]
- Becerra, P.I.; Simonetti, J.A. Native and exotic plant species diversity in forest fragments and forestry plantations of a coastal landscape of central Chile. Bosque 2020, 41, 125–136. [Google Scholar] [CrossRef]
- Kaipainen, T.; Liski, J.; Pussinen, A.; Karjalainen, T. Managing carbon sinks by changing rotation length in European forests. Environ. Sci. Policy 2004, 7, 205–219. [Google Scholar] [CrossRef]
- Nghiem, N. Optimal rotation age for carbon sequestration and biodiversity conservation in Vietnam. For. Policy Econ. 2014, 38, 56–64. [Google Scholar] [CrossRef]
- López-Bedoya, P.A.; Cardona-Galvis, E.A.; Urbina-Cardona, J.N.; Edwards, F.A.; Edwards, D.P. Impacts of Pastures and Forestry Plantations on Herpetofauna: A Global Meta-analysis. J. Appl. Ecol. 2022, 59, 3038–3048. [Google Scholar] [CrossRef]
- Heilmayr, R.; Echeverría, C.; Lambin, E.F. Impacts of Chilean forest subsidies on forest cover, carbon and biodiversity. Nat. Sustain. 2020, 3, 701–709. [Google Scholar] [CrossRef]
- Escobar-Avello, D.; Ferrer, V.; Bravo-Arrepol, G.; Reyes-Contreras, P.; Elissetche, J.P.; Santos, J.; Fuentealba, C.; Cabrera-Barjas, G. Pretreated Eucalyptus globulus and Pinus radiata Barks: Potential Substrates to Improve Seed Germination for a Sustainable Horticulture. Forests 2023, 14, 991. [Google Scholar] [CrossRef]
- Rubilar, R.; Bozo, D.; Albaugh, T.; Cook, R.; Campoe, O.; Carter, D.; Allen, H.; Lvarez, J.; Pincheira, M.; Zapata, L. Rotation-age effects of subsoiling, fertilization, and weed control on radiata pine growth at sites with contrasting soil physical, nutrient, and water limitations. For. Ecol. Manag. 2023, 544, 121213. [Google Scholar] [CrossRef]
- Ojeda, H.; Rubilar, R.A.; Montes, C.; Cancino, J.; Espinosa, M. Leaf area and growth of Chilean radiata pine plantations after thinning across a water stress gradient. N. Z. J. For. Sci. 2018, 48, 10. [Google Scholar] [CrossRef]
- Alvarez, J.; Allen, H.; Albaugh, T.; Stape, J.; Bullock, B.; Song, C. Factors influencing the growth of radiata pine plantations in Chile. Forestry 2012, 86, 13–26. [Google Scholar] [CrossRef]
- Guo, L.B.; Cowie, A.L.; Montagu, K.D.; Gifford, R.M. Carbon and nitrogen stocks in a native pasture and an adjacent 16-year-old Pinus radiata D. Don. plantation in Australia. Agric. Ecosyst. Environ. 2008, 124, 205–218. [Google Scholar] [CrossRef]
- Guedes, B.S.; Olsson, B.A.; Egnell, G.; Sitoe, A.A.; Karltun, E. Plantations of Pinus and Eucalyptus replacing degraded mountain miombo woodlands in Mozambique significantly increase carbon sequestration. Glob. Ecol. Conserv. 2018, 14, e00401. [Google Scholar] [CrossRef]
- Balboa-Murias, M.Á.; Rodríguez-Soalleiro, R.; Merino, A.; Álvarez-González, J.G. Temporal variations and distribution of carbon stocks in aboveground biomass of radiata pine and maritime pine pure stands under different silvicultural alternatives. For. Ecol. Manag. 2006, 237, 29–38. [Google Scholar] [CrossRef]
- Bozo, D.; Rubilar, R.; Campoe, O.C.; Alzamora, R.M.; Elissetche, J.P.; Valverde, J.C.; Pizarro, R.; Pincheira, M.; Valencia, J.C.; Sanhueza, C. Soil and Site Productivity Effects on Above- and Belowground Radiata Pine Carbon Pools at Harvesting Age. Plants 2024, 13, 3482. [Google Scholar] [CrossRef] [PubMed]
- Temesgen, H.; Affleck, D.; Poudel, K.; Gray, A.; Sessions, J. A review of the challenges and opportunities in estimating above ground forest biomass using tree-level models. Scand. J. For. Res. 2015, 30, 326–335. [Google Scholar] [CrossRef]
- Pfeiffer, M.; Padarian, J.; Osorio, R.; Bustamante, N.; Olmedo, G.F.; Guevara, M.; Aburto, F.; Albornoz, F.; Antilén, M.; Araya, E.; et al. CHLSOC: The Chilean Soil Organic Carbon database, a multi-institutional collaborative effort. Earth Syst. Sci. Data 2020, 12, 457–468. [Google Scholar] [CrossRef]
- Post, W.M.; Kwon, K.C. Soil carbon sequestration and land-use change: Processes and potential. Glob. Change Biol. 2000, 6, 317–327. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, W.; Sun, X.; Jiang, J.; Li, D.; Tang, G.; Xu, W.; Jia, H. Biochar Aged for Five Years Altered Carbon Fractions and Enzyme Activities of Sandy Soil. Land 2023, 12, 1645. [Google Scholar] [CrossRef]
- Su, Y.Z.; Wang, X.F.; Yang, R.; Lee, J. Effects of Sandy Desertified Land Rehabilitation on Soil Carbon Sequestration and Aggregation in an Arid Region in China. J. Environ. Manag. 2010, 91, 2109–2116. [Google Scholar] [CrossRef]
- Amin, M.N.; Shil, S.C.; Ghosh, R.C.; Shamsuzzoha, M. Influence of Conservation Tillage on Carbon Sequestration Mechanism Related to Aggregation. J. Environ. Sci. Nat. Resour. 2016, 9, 23–27. [Google Scholar] [CrossRef][Green Version]
- Katayama, S.; Omori, T.; Tateno, M. Fresh Litter Acts as a Substantial Phosphorus Source of Plant Species Appearing in Primary Succession on Volcanic Ash Soil. Sci. Rep. 2021, 11, 11497. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.M.; Rodrigo-Comino, J.; Díaz, A. Impact of Lithology and Soil Properties on Abandoned Dryland Terraces During the Early Stages of Soil Erosion by Water in South-East Spain. Hydrol. Process. 2017, 31, 3095–3109. [Google Scholar] [CrossRef]
- Setia, R.; Smith, P.; Marschner, P.; Baldock, J.A.; Chittleborough, D.J.; Smith, J.U. Introducing a Decomposition Rate Modifier in the Rothamsted Carbon Model to Predict Soil Organic Carbon Stocks in Saline Soils. Environ. Sci. Technol. 2011, 45, 6396–6403. [Google Scholar] [CrossRef]
- Zhou, D.; Mao, D.; Ye, M.; Li, S.; Ma, X.-D.; Liu, S. Fractal Features of Soil Grain-Size Distribution in a Typical Tamarix Cones in the Taklimakan Desert, China. Sci. Rep. 2022, 12, 16461. [Google Scholar]
- Vadeboncoeur, M.A.; Hamburg, S.P.; Yanai, R.D.; Blum, J.D. Rates of Sustainable Forest Harvest Depend on Rotation Length and Weathering of Soil Minerals. For. Ecol. Manag. 2014, 318, 194–205. [Google Scholar] [CrossRef]
- CIREN. Descripción de Suelos, Materiales y Símbolos: Estudio Agrológico VIII Región; CIREN: Santiago, Chile, 1999. [Google Scholar]
- Stolpe, N. Descripciones de los Principales Suelos de la VIII Región de Chile; Universidad de Concepción: Concepción, Chile, 2006. [Google Scholar]
- Mininco, F. Compendium of Functions for Species of Interest to Forestal Minin S.A.; Forestal Mininco: Concepción, Chile, 1995. (In Spanish) [Google Scholar]
- Albaugh, T.J.; Alvarez, J.; Rubilar, R.A.; Fox, T.R.; Allen, H.L.; Stape, J.L.; Mardones, O. Long-Term Pinus radiata Productivity Gains from Tillage, Vegetation Control, and Fertilization. For. Sci. 2015, 61, 800–808. [Google Scholar] [CrossRef]
- Sandoval, S.; Montes, C.R.; Olmedo, G.F.; Acuña, E.; Mena-Quijada, P. Modelling above-ground biomass of Pinus radiata trees with explicit multivariate uncertainty. For. Int. J. For. Res. 2022, 95, 380–390. [Google Scholar]
- Zerihun, A.; Montagu, K.D. Belowground to aboveground biomass ratio and vertical root distribution responses of mature Pinus radiata stands to phosphorus fertilization at planting. Can. J. For. Res. 2004, 34, 1883–1894. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Poeplau, C.; Vos, C.; Don, A. Soil organic carbon stocks are systematically overestimated by misuse of the parameters bulk density and rock fragment content. Soil 2017, 3, 61–66. [Google Scholar] [CrossRef]
- R Core Team, R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Staff, S.S. Keys to Soil Taxonomy, 13th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2022. [Google Scholar]
- Ray, M. Relation of Soil Bulk Density with the Soil Carbon in the Tropical Dry Deciduous Forest of Jharkhand, India. Int. J. Sci. Eng. Res. 2019, 3, 1439–1443. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).