Utilization of Phosphogypsum as Sustainable Adsorbent for Removal of Crystal Violet Dye from Wastewater: Kinetics, Thermodynamics, and Applications in Textile Effluent Treatment
Abstract
1. Introduction
2. Materials
2.1. Phosphogypsum Sample
2.2. Chemicals
3. Methods
3.1. X-Ray Diffraction (XRD)
3.2. Particle Size and Zeta Potential Measurements
3.3. Adsorption Experiments
4. Results and Discussion
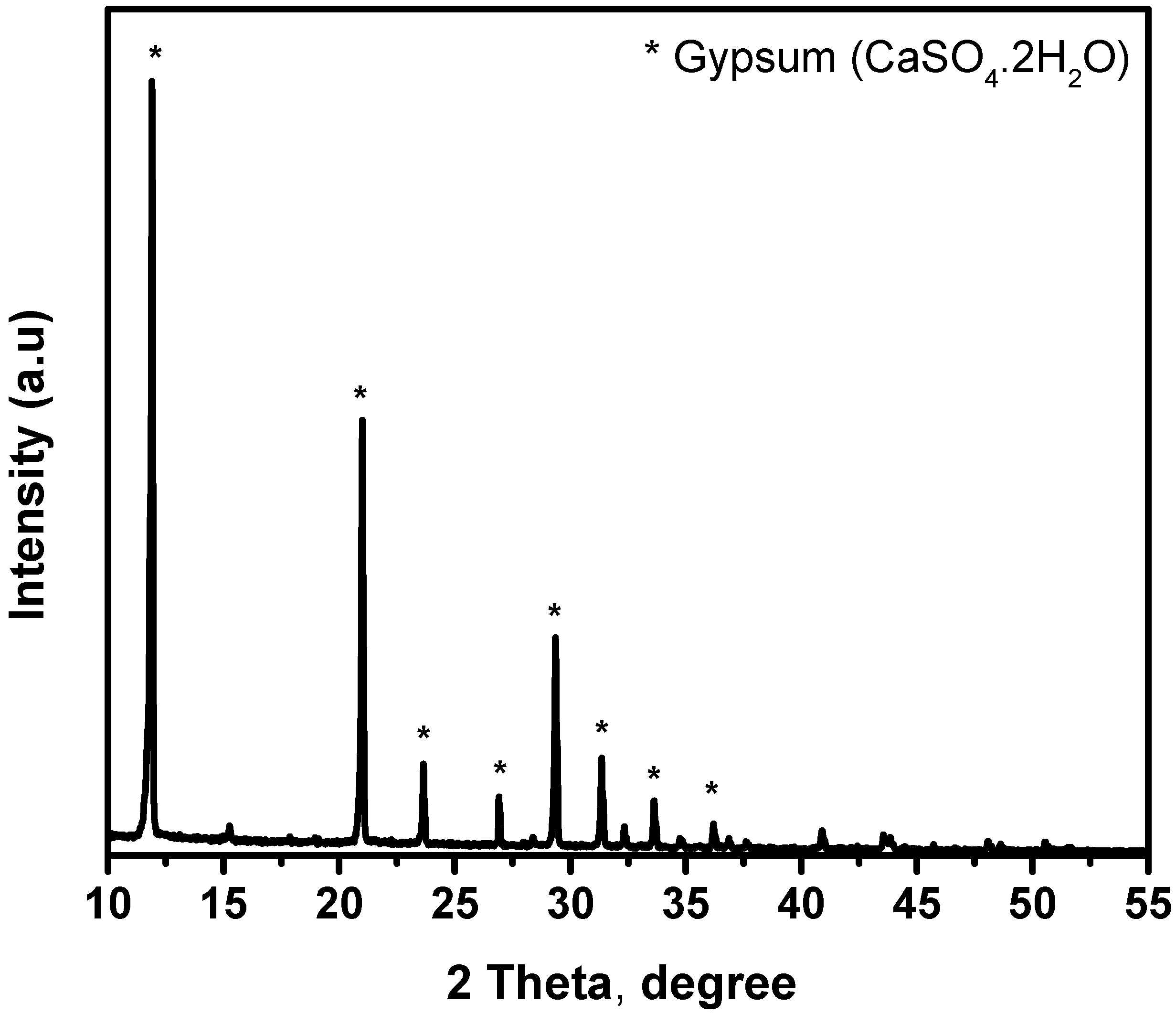
4.1. XRD and XRF Analysis
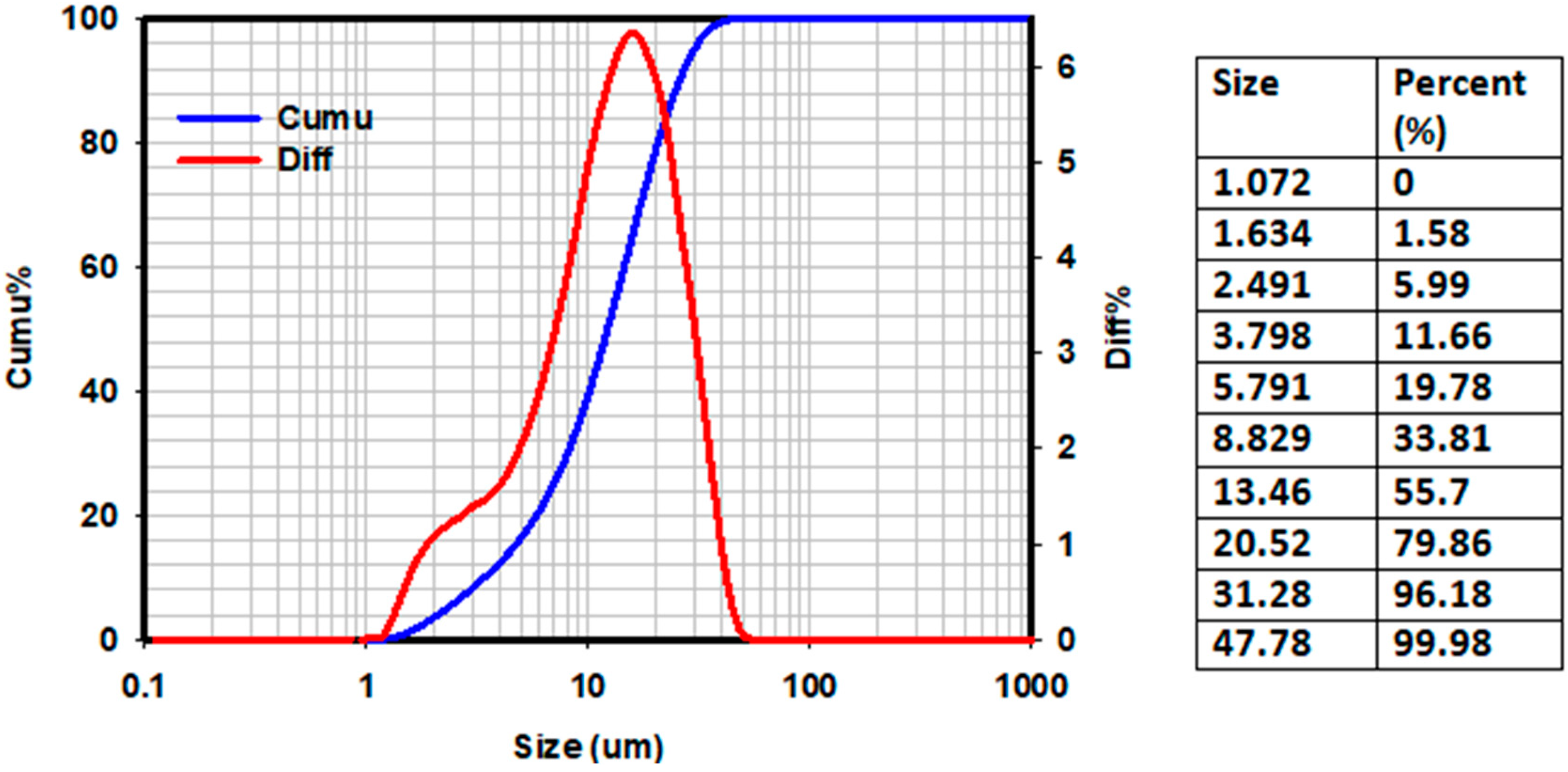
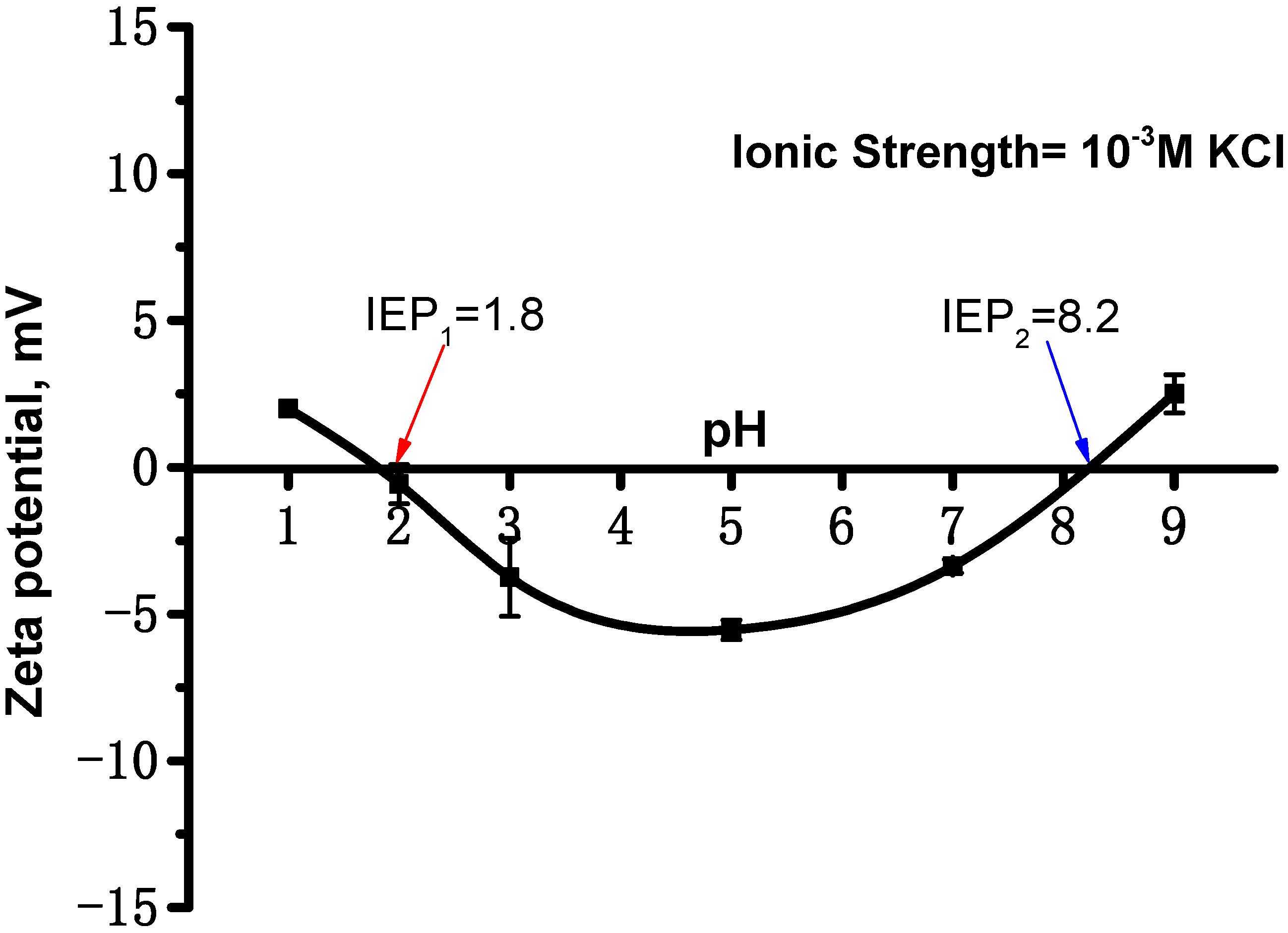
4.2. Particle Size and Zeta Potential
4.3. Adsorption Study
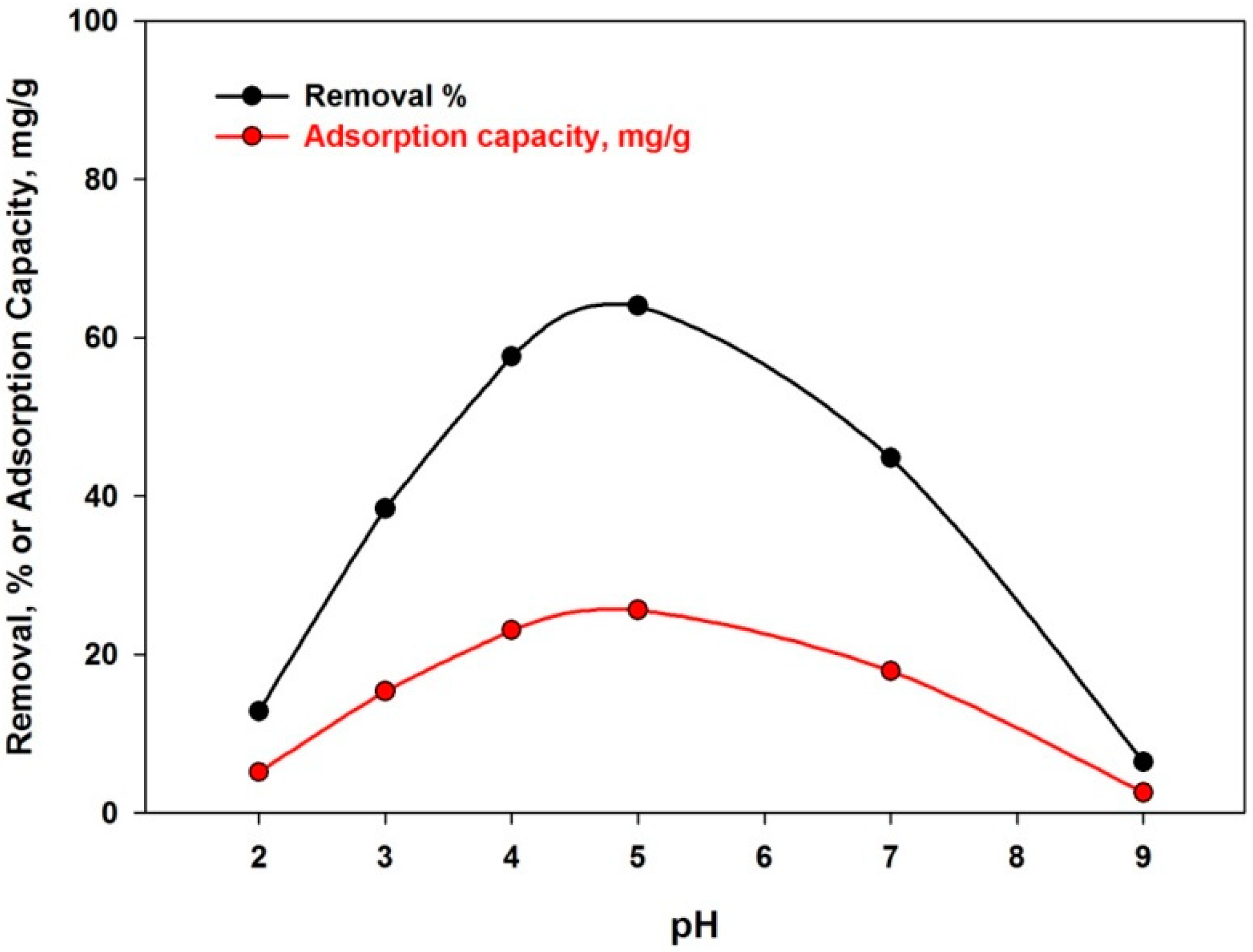
4.3.1. Effect of pH
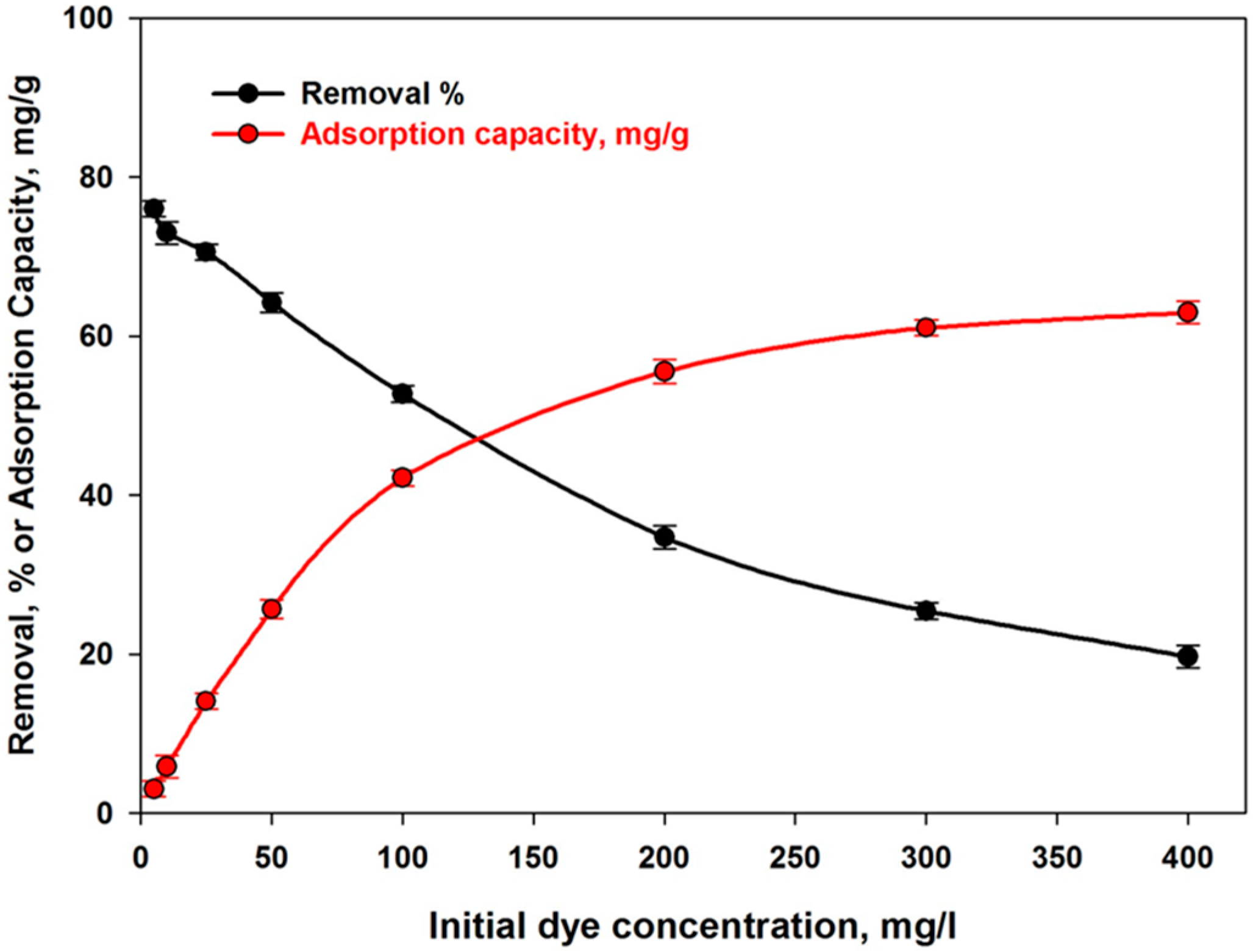
4.3.2. Effect of Initial Concentration
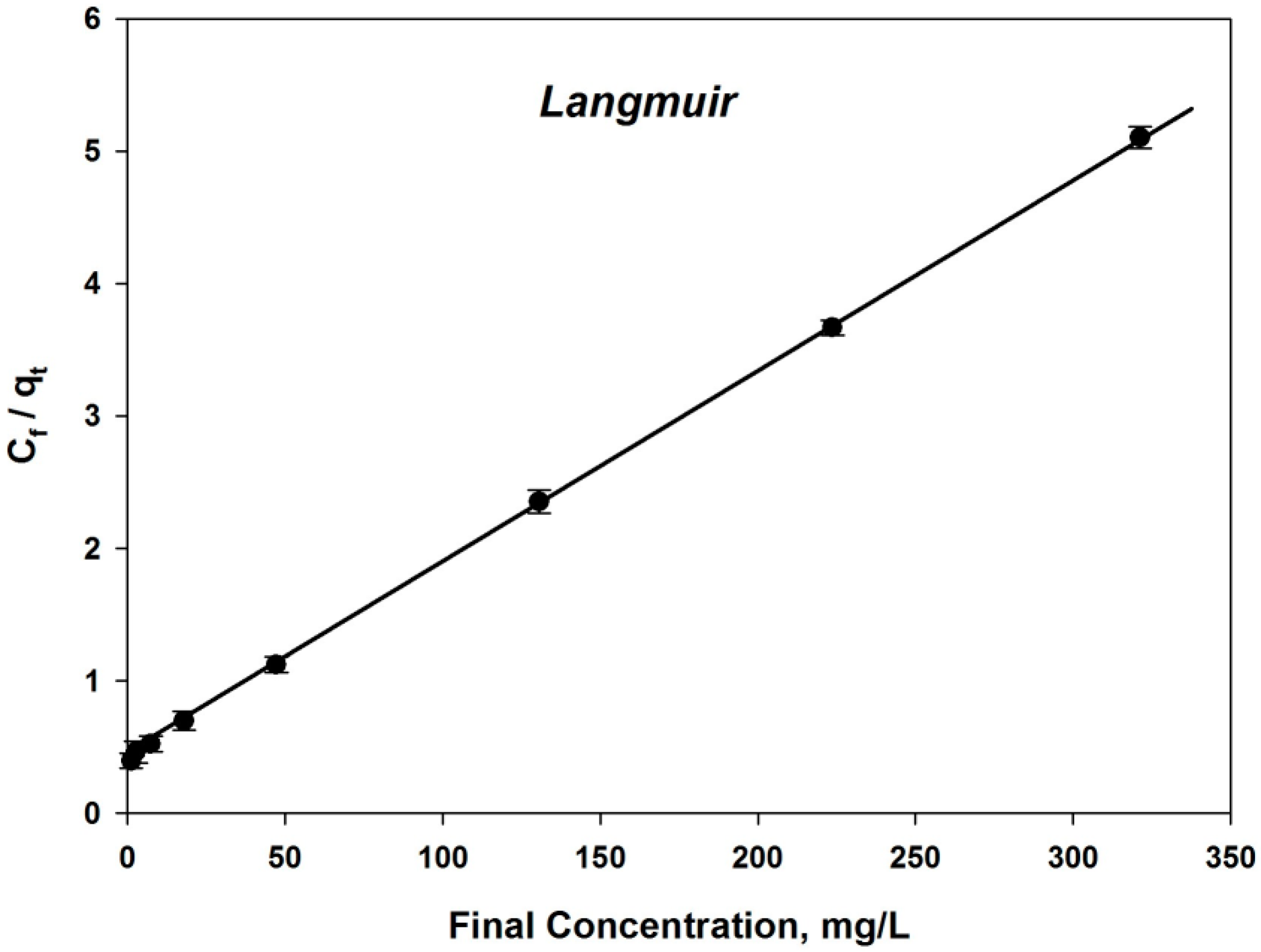
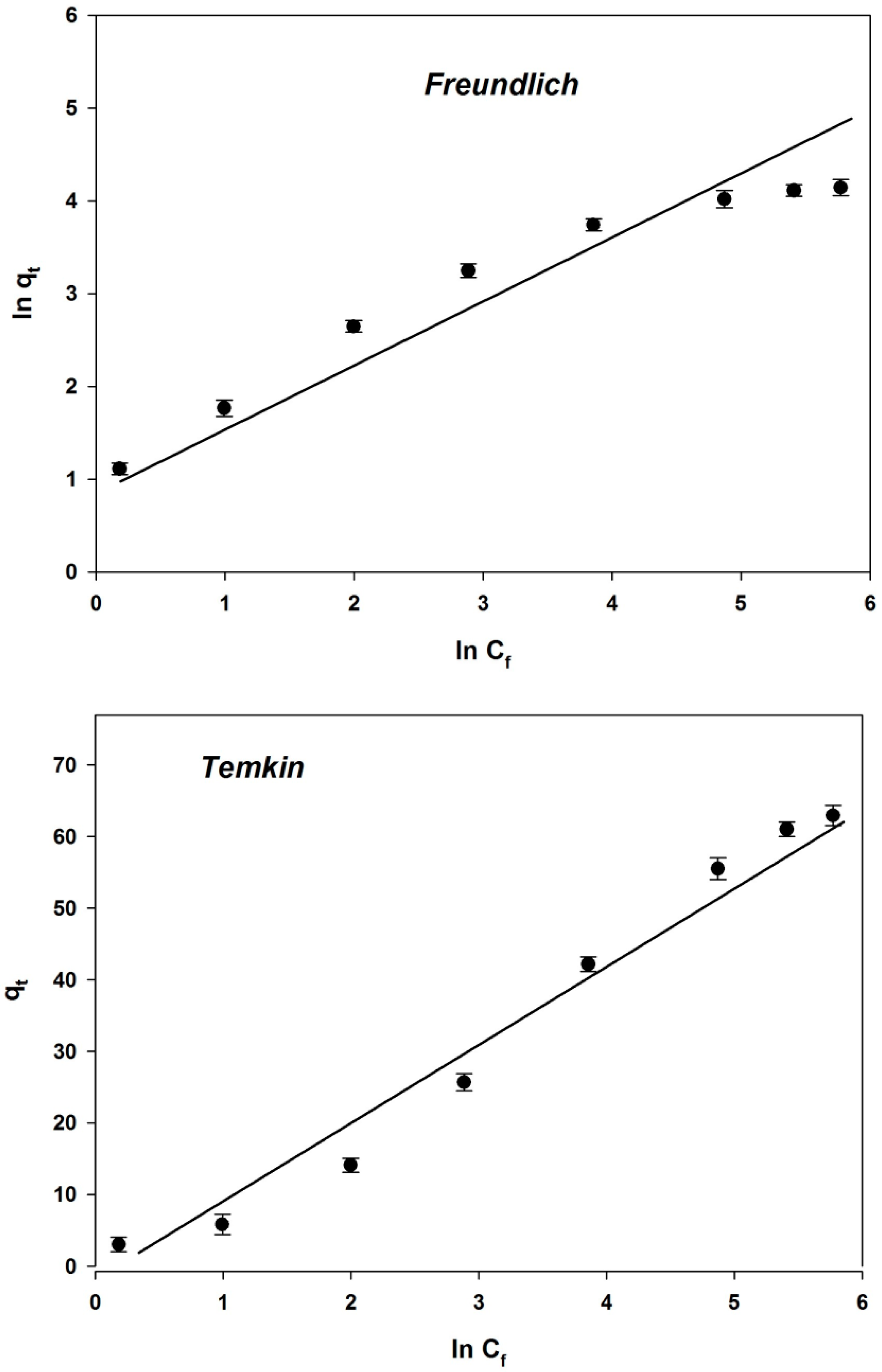
4.3.3. Analysis of Isotherm Models
4.3.4. Effect of Contact Time
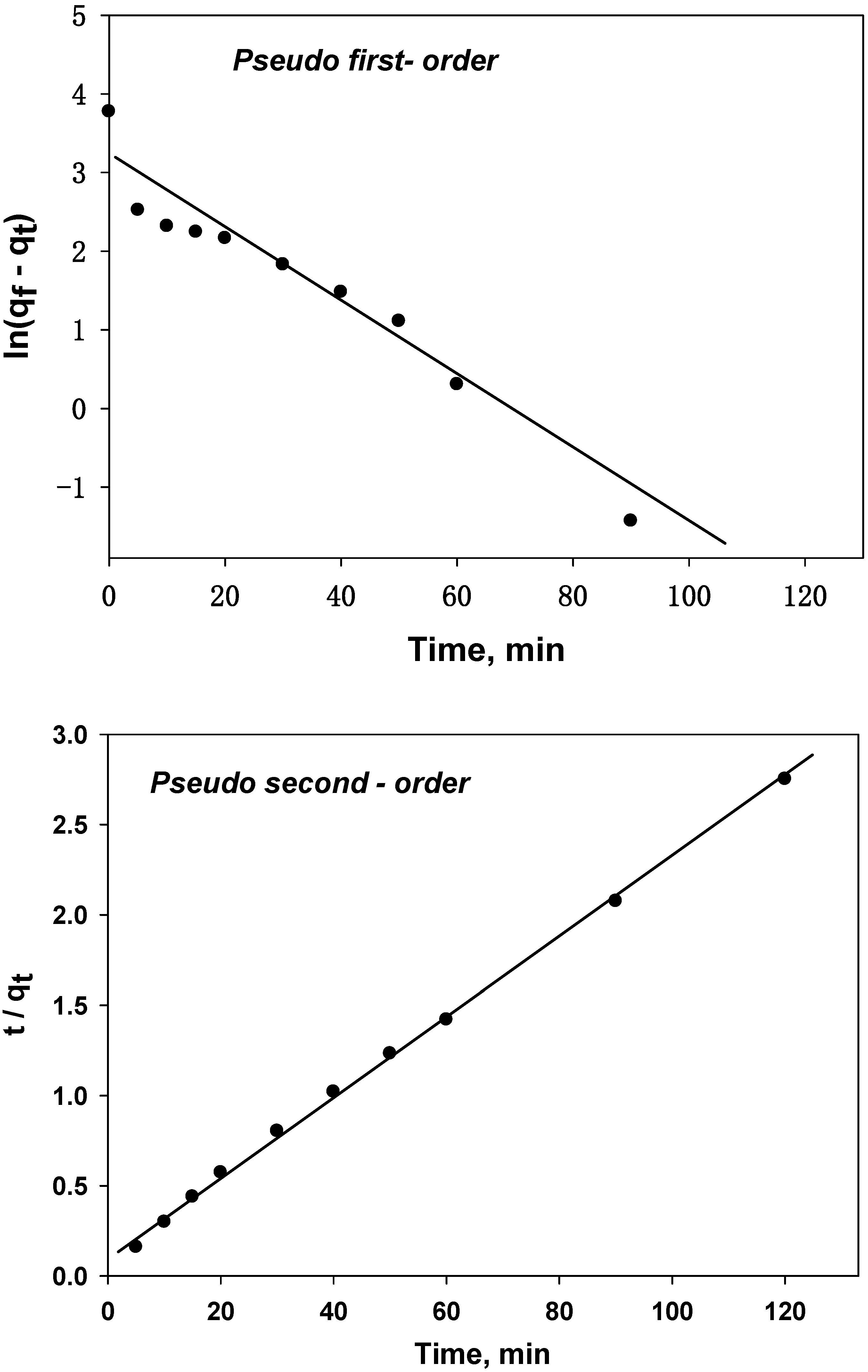
4.3.5. Sorption Kinetics
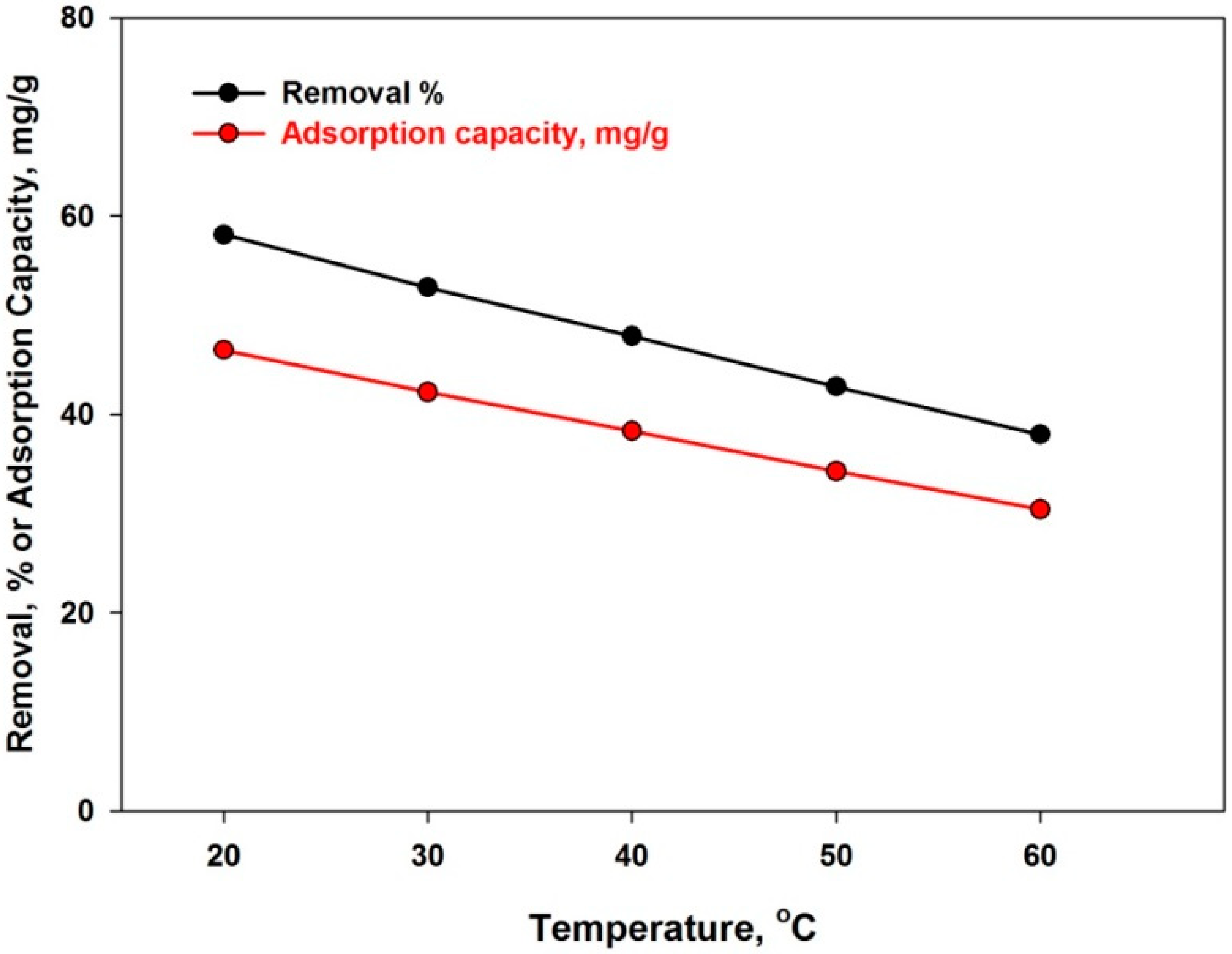
4.3.6. Effect of Temperature
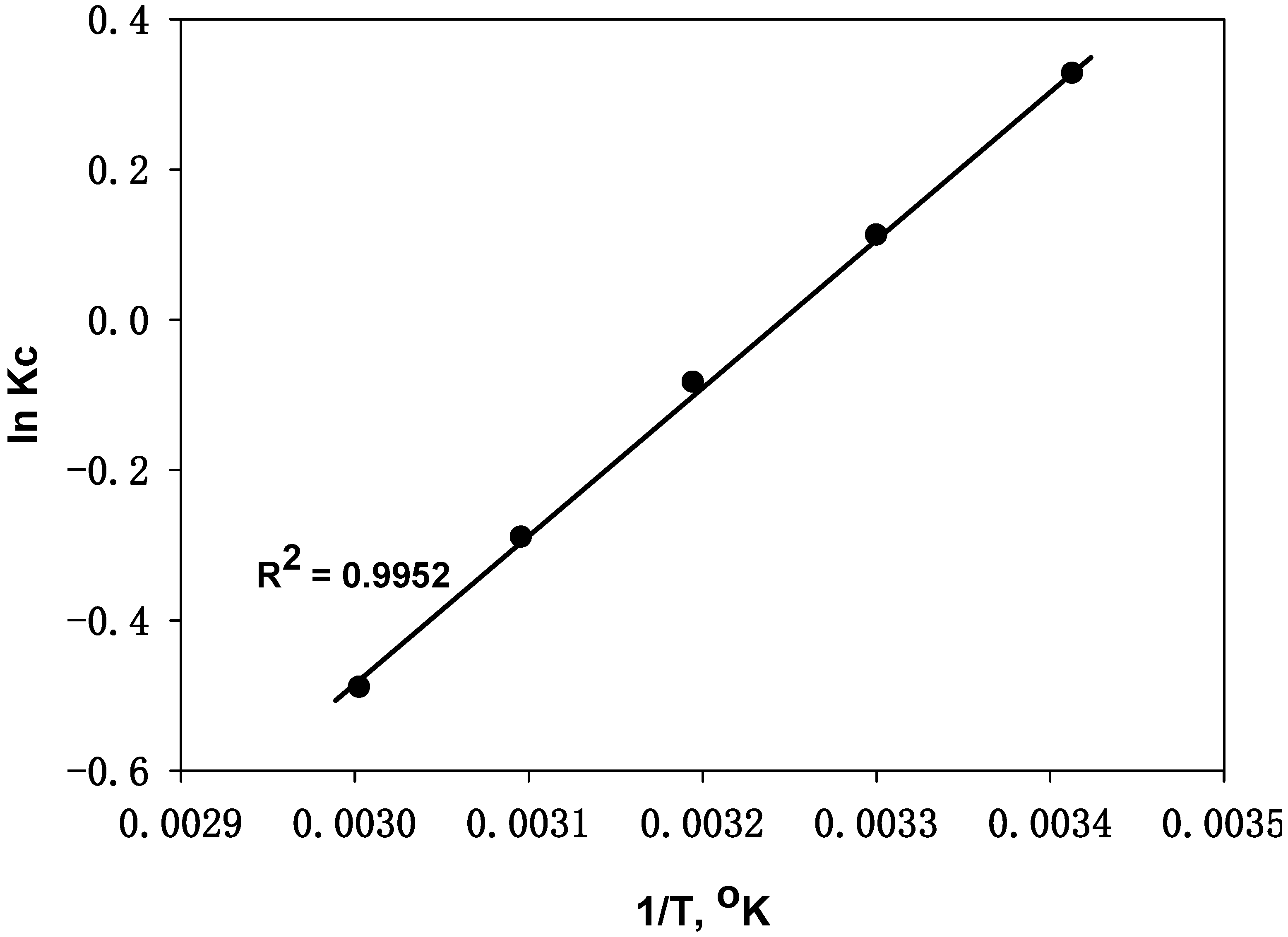
4.3.7. Thermodynamic Study
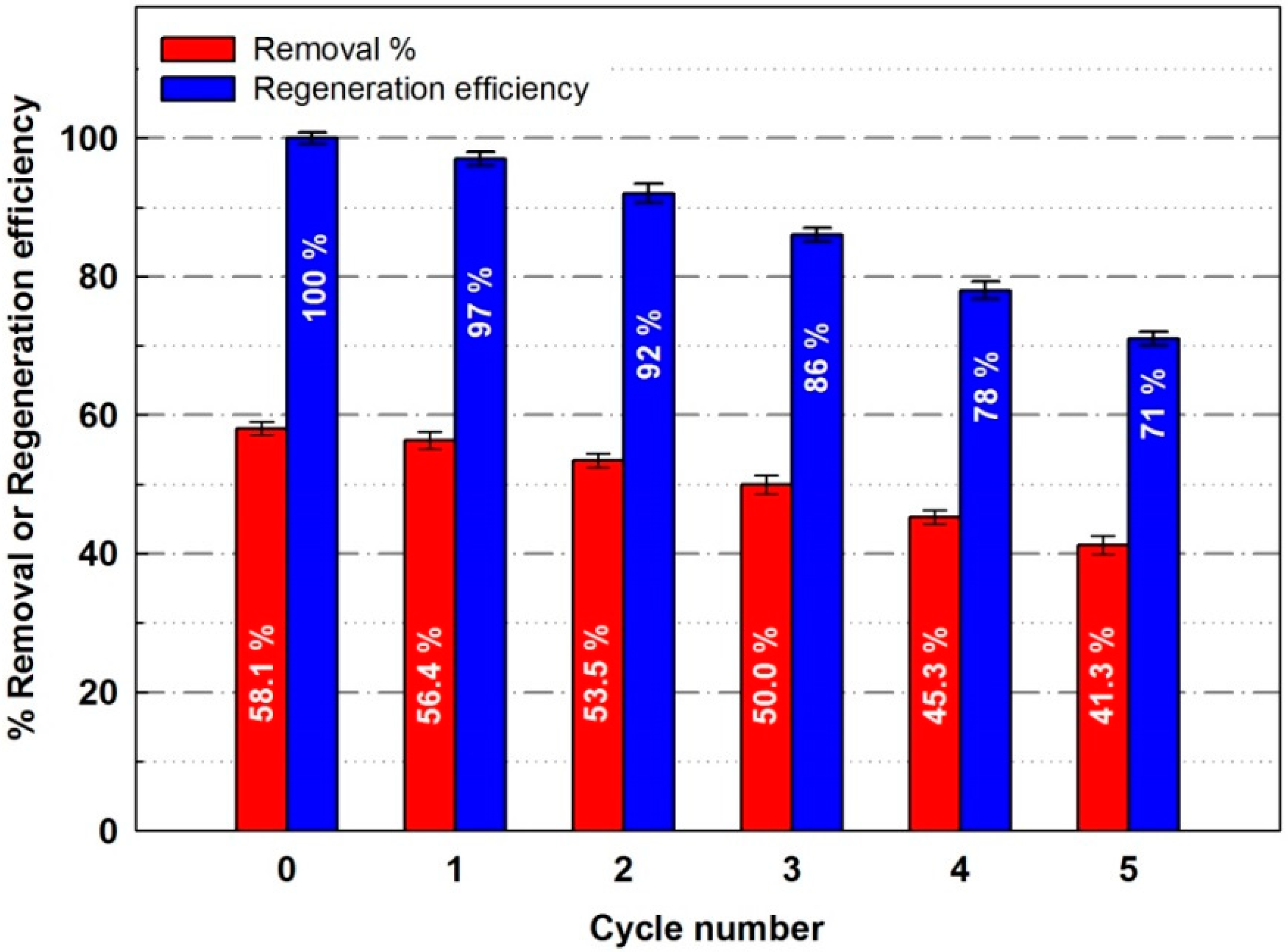
4.3.8. Regeneration
4.3.9. Application to Real Sample
4.3.10. Comparison Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kishor, R.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Bilal, M.; Chandra, R.; Bharagava, R.N. Ecotoxicological and Health Concerns of Persistent Coloring Pollutants of Textile Industry Wastewater and Treatment Approaches for Environmental Safety. J. Environ. Chem. Eng. 2020, 9, 105012. [Google Scholar] [CrossRef]
- Sudarshan, S.; Harikrishnan, S.; RathiBhuvaneswari, G.; Alamelu, V.; Aanand, S.; Rajasekar, A.; Govarthanan, M. Impact of Textile Dyes on Human Health and Bioremediation of Textile Industry Effluent Using Microorganisms: Current Status and Future Prospects. J. Appl. Microbiol. 2023, 134, lxac064. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.; Ahmad, R. An Efficient Sequestration of Toxic Crystal Violet Dye from Aqueous Solution by Alginate/Pectin Nanocomposite: A Novel and Ecofriendly Adsorbent. Groundw. Sustain. Dev. 2020, 11, 100373. [Google Scholar] [CrossRef]
- Adak, A.; Bandyopadhyay, M.; Pal, A. Removal of Crystal Violet Dye from Wastewater by Surfactant-Modified Alumina. Sep. Purif. Technol. 2005, 44, 139–144. [Google Scholar] [CrossRef]
- Gupta, V.K.; Suhas. Application of Low-Cost Adsorbents for Dye Removal—A Review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Akhtar, M.; Sarfraz, M.; Ahmad, M.; Raza, N.; Zhang, L. Use of Low-Cost Adsorbent for Wastewater Treatment: Recent Progress, New Trend, and Future Perspectives. Desalination Water Treat. 2025, 321, 100914. [Google Scholar] [CrossRef]
- Sanakousar, M.F.; Vidyasagar, C.C.; Jiménez-Pérez, V.M.; Jayanna, B.K.; Mounesh; Shridhar, A.H.; Prakash, K. Efficient Photocatalytic Degradation of Crystal Violet Dye and Electrochemical Performance of Modified MWCNTs/Cd-ZnO Nanoparticles with Quantum Chemical Calculations. J. Hazard. Mater. Adv. 2021, 2, 100004. [Google Scholar] [CrossRef]
- Chen, J.; Liu, M.; Zhang, J.; Ying, X.; Jin, L. Photocatalytic Degradation of Organic Wastes by Electrochemically Assisted TiO2 Photocatalytic System. J. Environ. Manag. 2004, 70, 43–47. [Google Scholar]
- Sifat, M.; Shin, E.; Schevon, A.; Ramos, H.; Pophali, A.; Jung, H.-J.; Halada, G.; Meng, Y.; Olynik, N.; Sprouster, D.J.; et al. Photocatalytic Degradation of Crystal Violet (CV) Dye over Metal Oxide (MOx) Catalysts. Catalysts 2024, 14, 377. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, F.; Liu, R.; Sun, C.; Fan, H. Crystal Violet Degradation in the Ozone/Persulfate/Ferroferric Oxide System: A Heterogeneous Catalytic Process for Simultaneous Catalysis of Ozone and Persulfate. J. Clean. Prod. 2024, 434, 139937. [Google Scholar] [CrossRef]
- Abdi, M.; Balagabri, M.; Karimi, H.; Hossini, H.; Rastegar, S.O. Degradation of Crystal Violet (CV) from Aqueous Solutions Using Ozone, Peroxone, Electroperoxone, and Electrolysis Processes: A Comparison Study. Appl. Water Sci. 2020, 10, 168. [Google Scholar] [CrossRef]
- Roy, D.C.; Biswas, S.K.; Saha, A.K.; Sikdar, B.; Rahman, M.; Roy, A.K.; Prodhan, Z.H.; Tang, S.-S. Biodegradation of Crystal Violet Dye by Bacteria Isolated from Textile Industry Effluents. PeerJ 2018, 6, e5015. [Google Scholar] [CrossRef] [PubMed]
- Zahmatkesh Anbarani, M.; Nourbakhsh, S.; Toolabi, A.; Bonyadi, Z. Biodegradation of Crystal Violet Dye by Saccharomyces cerevisiae in Aqueous Medium. Heliyon 2023, 9, e19460. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, G.; Shanthakumar, S. Removal of Crystal Violet Dye in Textile Effluent by Coagulation Using Algal Alginate from Brown Algae Sargassum sp. Desalination Water Treat. 2020, 196, 402–408. [Google Scholar] [CrossRef]
- Fosso-Kankeu, E.; Webster, A.; Ntwampe, I.O.; Waanders, F.B. Coagulation/Flocculation Potential of Polyaluminium Chloride and Bentonite Clay Tested in the Removal of Methyl Red and Crystal Violet. Arab. J. Sci. Eng. 2017, 42, 1389–1397. [Google Scholar] [CrossRef]
- Sadoq, M.; Atlas, H.; Imame, S.; Kali, A.; Amar, A.; Loulidi, I.; Jabri, M.; Sadoq, B.-E.; Ouchabi, M.; Abdullah, P.S.; et al. Elimination of Crystal Violet from Aqueous Solution by Adsorption on Natural Polysaccharide: Kinetic, Isotherm, Thermodynamic Studies, and Mechanism Analysis. Arab. J. Chem. 2024, 17, 105453. [Google Scholar]
- Abbas, S.; Javeed, T.; Zafar, S.; Taj, M.B.; Ashraf, A.R.; Din, M.I. Adsorption of Crystal Violet Dye by Using a Low-Cost Adsorbent—Peanut Husk. Desalination Water Treat. 2021, 233, 387–398. [Google Scholar] [CrossRef]
- Espantaleón, A.G.; Nieto, J.A.; Fernández, M.; Marsal, A. Use of Activated Clays in the Removal of Dyes and Surfactants from Tannery Wastewaters. Appl. Clay Sci. 2003, 24, 105–110. [Google Scholar]
- Janos, P.; Buchtová, H.; Rýznarová, M. Sorption of Dyes from Aqueous Solutions onto Fly Ash. Water Res. 2003, 37, 4938–4944. [Google Scholar]
- Rathi, B.S.; Kumar, P.S. Application of Adsorption Process for Effective Removal of Emerging Contaminants from Water and Wastewater. Environ. Pollut. 2021, 280, 116995. [Google Scholar] [CrossRef] [PubMed]
- Tayibi, H.; Choura, M.; López, F.A.; Alguacil, F.J.; López-Delgado, A. Environmental Impact and Management of Phosphogypsum. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef]
- Balkaya, N.; Cesur, H. Adsorption of Cadmium from Aqueous Solution by Phosphogypsum. Chem. Eng. J. 2008, 140, 247–254. [Google Scholar] [CrossRef]
- Es-Said, A.; Nafai, H.; El Hamdaoui, L.; Bouhaouss, A.; Bchitou, R. Adsorptivity and Selectivity of Heavy Metals Cd(II), Cu(II), and Zn(II) toward Phosphogypsum. Desalination Water Treat. 2020, 197, 291–299. [Google Scholar] [CrossRef]
- Panda, A.; Upadhyaya, A.; Kumar, R.; Acooli, A.; Banerjee, S.; Mishra, A.; Khan, M.A.; Chowdhury, S.; Jeon, B.-H.; Chakrabortty, S.; et al. Chemical Activation of Phosphogypsum Exhibits Enhanced Adsorption of Malachite Green from Aqueous Solution due to Porosity Refinement. Front. Chem. Sci. Eng. 2024, 18, 124. [Google Scholar] [CrossRef]
- Chouaybi, I.; Moujahid, E.M.; Bettach, M. From Waste to Clean Water: Effective Removal of Acid Red 97 Dye Using Green Synthesized Hydrocalumite from Phosphogypsum and Aluminum Foils. Inorg. Chem. Commun. 2023, 158, 111653. [Google Scholar] [CrossRef]
- Sakurai, K.; Ohdate, Y.; Kyuma, K. Factors Affecting Zero Point of Charge (Zpc) of Variable Charge Soils. Soil Sci. Plant Nutr. 1989, 35, 21–31. [Google Scholar] [CrossRef]
- Abdel-Khalek, M.A.; Abdel Rahman, M.K.; Francis, A.A. Exploring the Adsorption Behavior of Cationic and Anionic Dyes on Industrial Waste Shells of Egg. J. Environ. Chem. Eng. 2017, 5, 319–327. [Google Scholar]
- Abdel-Khalek, M.A.; Mahmoud, G.A.; Shoukry, E.M.; Amin, M.; Abdulghany, A.H. Adsorptive Removal of Nitrate Ions from Aqueous Solution Using Modified Biodegradable-Based Hydrogel. Desalination Water Treat. 2019, 155, 390–401. [Google Scholar] [CrossRef]
- Mahmoud, G.; Abdel-Khalek, M.; Shoukry, E.; Amin, M.; Abdulghany, A. Removal of Phosphate Ions from Wastewater by Treated Hydrogel Based on Chitosan. Egypt. J. Chem. 2019, 62, 1537–1549. [Google Scholar] [CrossRef]
- Hałas, P.; Kołodyńska, D.; Płaza, A.; Gęca, M.; Hubicki, Z.; Rudziński, W.; Tarasevich, Y.I. Modified Fly Ash and Zeolites as an Effective Adsorbent for Metal Ions from Aqueous Solution. Adsorpt. Sci. Technol. 2017, 35, 519–533. [Google Scholar] [CrossRef]
- Chen, X.J.; Guo, Y.X.; Cheng, F.Q.; Song, H.P.; Zheng, N.; Wang, X.M. Application of Modified Coal Fly Ash as an Absorbent for Ammonia-Nitrogen Wastewater Treatment. Adv. Mater. Res. 2012, 518–523, 2380–2384. [Google Scholar] [CrossRef]
- Visa, M. Synthesis and Characterization of New Zeolite Materials Obtained from Fly Ash for Heavy Metals Removal in Advanced Wastewater Treatment. Powder Technol. 2016, 294, 338–347. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Chen, H.-R. Adsorption Kinetics of Herbicide Paraquat in Aqueous Solution onto a Low-Cost Adsorbent, Swine-Manure-Derived Biochar. Int. J. Environ. Sci. Technol. 2013, 10, 1349–1356. Available online: http://www.bioline.org.br/abstract?id=st13131 (accessed on 25 January 2025). [CrossRef]
- Shehab, A.; Abdelbasir, S.M.; Khalek, M.; Soliman, M.; Elgemeie, G. Dye Removal from Aqueous Solution by Regenerated Spent Bleaching Earth. World Acad. Sci. Eng. Technol. Int. J. Chem. Mater. Eng. 2019, 13, 452–461. [Google Scholar]
- Goldberg, S. Equations and Models Describing Adsorption Processes in Soils. In Chemical Processes in Soils; Wiley Online Library: Hoboken, NJ, USA, 2018; pp. 489–517. [Google Scholar] [CrossRef]
- Ali, I.; Al-Othman, Z.A.; Alwarthan, A. Synthesis of Composite Iron Nano Adsorbent and Removal of Ibuprofen Drug Residue from Water. J. Mol. Liq. 2016, 219, 858–864. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Duan, J.; Xu, H.; Zhou, Z.; Zhang, J.; Han, L. HF-Free Synthesis of Nanoscale Metal–Organic Framework NMIL-100(Fe) as an Efficient Dye Adsorbent. J. Mater. Chem. A 2016, 4, 1512–1519. [Google Scholar] [CrossRef]
- Nazarzadeh Zare, E.; Mansour Lakouraj, M.; Ramezani, A. Efficient Sorption of Pb(II) from an Aqueous Solution Using a Poly(Aniline-co-3-Aminobenzoic Acid)-Based Magnetic Core–Shell Nanocomposite. New J. Chem. 2016, 40, 2521–2529. [Google Scholar] [CrossRef]
- Plaza, L.; Castellote, M.; Nevshupa, R.; Jimenez-Relinque, E. Correction to: High-Capacity Adsorbents from Stainless Steel Slag for the Control of Dye Pollutants in Water. Environ. Sci. Pollut. Res. 2021, 28, 23911. [Google Scholar] [CrossRef]
- Karmaker, S.; Sintaha, F.; Saha, T.K.; Karmaker, S.; Sintaha, F.; Saha, T.K. Kinetics, Isotherm and Thermodynamic Studies of the Adsorption of Reactive Red 239 Dye from Aqueous Solution by Chitosan 8B. Adv. Biol. Chem. 2019, 9, 89691. [Google Scholar] [CrossRef]
- Adeogun, A.I.; Ofudje, E.A.; Idowu, M.A.; Kareem, S.O.; Vahidhabanu, S.; Babu, B.R. Biowaste-Derived Hydroxyapatite for Effective Removal of Reactive Yellow 4 Dye: Equilibrium, Kinetic, and Thermodynamic Studies. ACS Omega 2018, 3, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, H.; Wang, Y.; Zhao, Y.; Li, X. Efficient Removal of Methyl Blue from Aqueous Solution by Using Poly(4-Vinylpyridine)-Graphene Oxide-Fe₃O₄ Magnetic Nanocomposites. J. Mater. Sci. 2019, 54, 7603–7616. [Google Scholar] [CrossRef]
- Filho, A.C.D.; Mazzocato, A.C.; Dotto, G.L.; Thue, P.S.; Pavan, F.A. Eragrostis Plana Nees as a Novel Eco-Friendly Adsorbent for Removal of Crystal Violet from Aqueous Solutions. Environ. Sci. Pollut. Res. 2017, 24, 19909–19919. [Google Scholar] [CrossRef]
- Homagai, P.L.; Poudel, R.; Poudel, S.; Bhattarai, A. Adsorption and Removal of Crystal Violet Dye from Aqueous Solution by Modified Rice Husk. Heliyon 2022, 8, e09261. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Islam, K.; Hasan, M.A.; Khan, H.M.J.; Khan, M.A.R.; Deb, A.; Raihan, M.A.; Rahman, M.W. Adsorption of Crystal Violet Dye by Coconut Husk Powder: Isotherm, Kinetics, and Thermodynamics Perspectives. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100651. [Google Scholar] [CrossRef]

| Item | CaO | SO3 | SiO2 | P2O5 | LOI | Others | Total |
|---|---|---|---|---|---|---|---|
| % | 36.54 | 34.95 | 3.34 | 4.65 | 19.51 | 4.35 | 100 |
| Langmuir Isotherm Parameters | |
| R2 | 0.9963 |
| qmax (Calculated) | 65.14 |
| qmax (Experimental) | 62.96 |
| b | 0.0189 |
| Freundlich Isotherm Parameters | |
| R2 | 0.8421 |
| n | 1.3268 |
| KF | 0.7507 |
| Temkin Isotherm Parameters | |
| R2 | 0.9307 |
| B | 52.14 |
| AT | 0.316 |
| Pseudo-First-Order Model Parameters | Pseudo-Second-Order Model Parameters | ||
|---|---|---|---|
| R2 | 0.8913 | R2 | 0.9944 |
| K1 | −0.0432 | K2 | 0.0009 |
| Calculated qe | 31.7 | Calculated qe | 46.4 |
| Experimental qe | 43.6 | Experimental qe | 43.6 |
| Temp., °C | ΔH°, KJ·mol−1 | ΔS°, J·k−1 | ΔG°, J·k−1·mol−1 |
|---|---|---|---|
| 20 | −72.7 | −126 | −796 |
| 30 | −282 | ||
| 40 | 218 | ||
| 50 | 778 | ||
| 60 | 1355 |
| Item | Unit | Effluent | Treated | Removal, % |
|---|---|---|---|---|
| pH | 0–14 | 8.1 | -- | -- |
| TDS | mg/L | 2397 | 431 | 82.0 |
| TSS | mg/L | 185 | 28 | 84.9 |
| COD | mg/L | 614 | 129 | 79.0 |
| BOD | mg/L | 93 | 27 | 70.9 |
| Fe | mg/L | 4.1 | 0.65 | 84.1 |
| Zn | mg/L | 1.9 | 0.17 | 91.0 |
| Color | % | 37 | 5 | 86.5 |
| Turbidity | NTU | 246 | 49 | 80.1 |
| Adsorbent | Max Adsorption | Dye | pH | Reference |
|---|---|---|---|---|
| Eragrostis Plana Nees | 76 | Crystal Violet | 8 | [44] |
| Peanut Husk | 21 | 2 | [17] | |
| Charred Rice Husk | 62 | 10 | [45] | |
| Xanthated Rice Husk | 90 | 10 | [45] | |
| Coconut Husk | 54 | 5 | [46] | |
| Naturel Polysaccharide | 32 | 10 | [16] | |
| Phosphogypsum | 63 | 5 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alotaibi, A.M.; Aljabbab, A.A.; Alajmi, M.S.; Qadrouh, A.N.; Farahat, M.; Abdel Khalek, M.A.; Baioumy, H.; Alzahrani, R.Y.; Mana, T.H.; Almutairi, R.S. Utilization of Phosphogypsum as Sustainable Adsorbent for Removal of Crystal Violet Dye from Wastewater: Kinetics, Thermodynamics, and Applications in Textile Effluent Treatment. Sustainability 2025, 17, 3320. https://doi.org/10.3390/su17083320
Alotaibi AM, Aljabbab AA, Alajmi MS, Qadrouh AN, Farahat M, Abdel Khalek MA, Baioumy H, Alzahrani RY, Mana TH, Almutairi RS. Utilization of Phosphogypsum as Sustainable Adsorbent for Removal of Crystal Violet Dye from Wastewater: Kinetics, Thermodynamics, and Applications in Textile Effluent Treatment. Sustainability. 2025; 17(8):3320. https://doi.org/10.3390/su17083320
Chicago/Turabian StyleAlotaibi, Abdulrahman M., Abdulrahman A. Aljabbab, Mamdoh S. Alajmi, Ayman N. Qadrouh, Mohsen Farahat, Mohamed A. Abdel Khalek, Hassan Baioumy, Rashad Y. Alzahrani, Turki H. Mana, and Ramzi S. Almutairi. 2025. "Utilization of Phosphogypsum as Sustainable Adsorbent for Removal of Crystal Violet Dye from Wastewater: Kinetics, Thermodynamics, and Applications in Textile Effluent Treatment" Sustainability 17, no. 8: 3320. https://doi.org/10.3390/su17083320
APA StyleAlotaibi, A. M., Aljabbab, A. A., Alajmi, M. S., Qadrouh, A. N., Farahat, M., Abdel Khalek, M. A., Baioumy, H., Alzahrani, R. Y., Mana, T. H., & Almutairi, R. S. (2025). Utilization of Phosphogypsum as Sustainable Adsorbent for Removal of Crystal Violet Dye from Wastewater: Kinetics, Thermodynamics, and Applications in Textile Effluent Treatment. Sustainability, 17(8), 3320. https://doi.org/10.3390/su17083320







