Preparation of Coal Gangue-Based Artificial Soil and Investigation of the Mechanism of Aggregate Structure Formation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Experimental Design
2.3. Methods of Analysis
2.3.1. Instrumental Characterization
2.3.2. Indicator Testing
2.3.3. Statistical Analysis
3. Results and Discussion
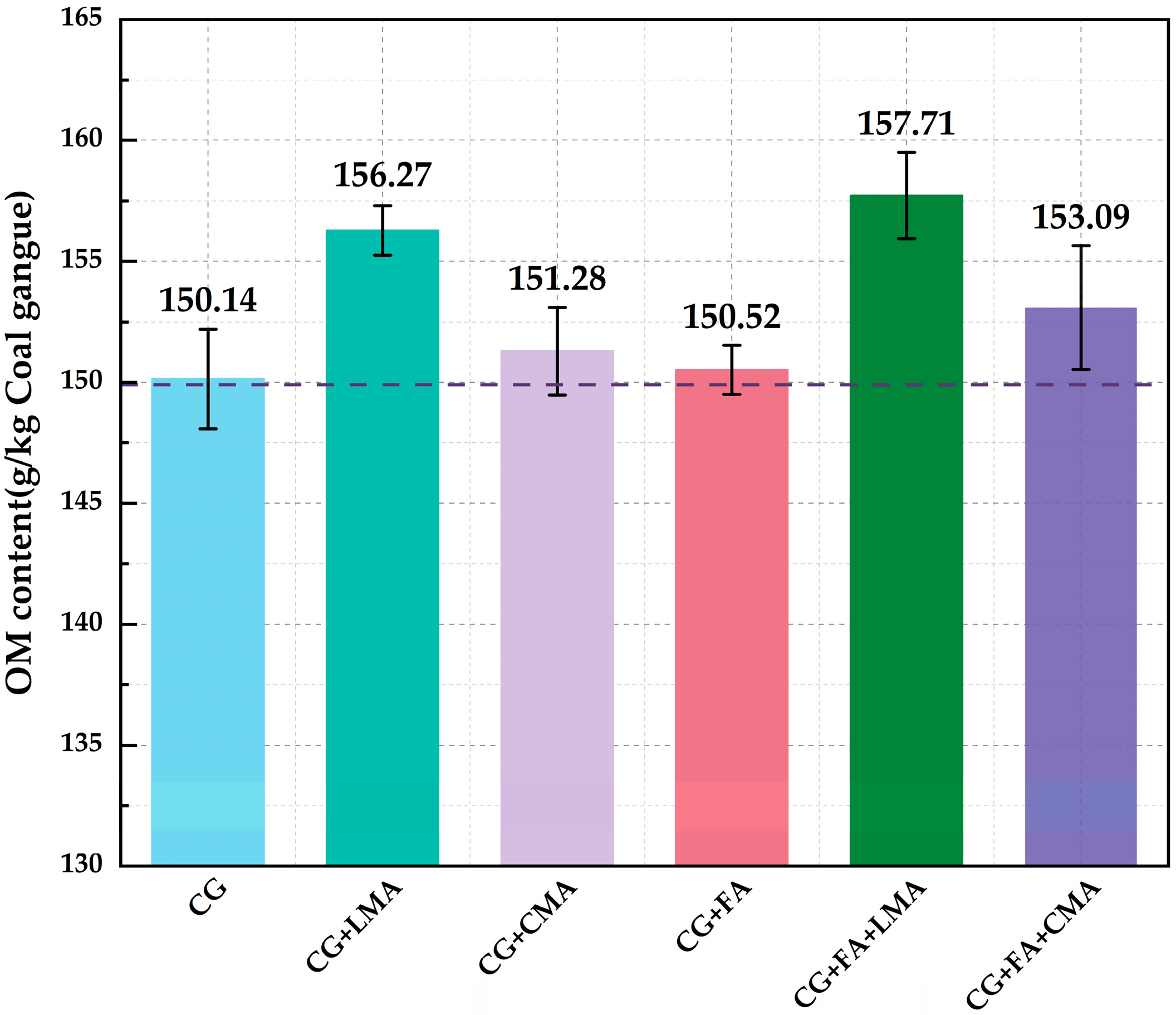
3.1. Comparison of OM Content per Unit Mass of CG
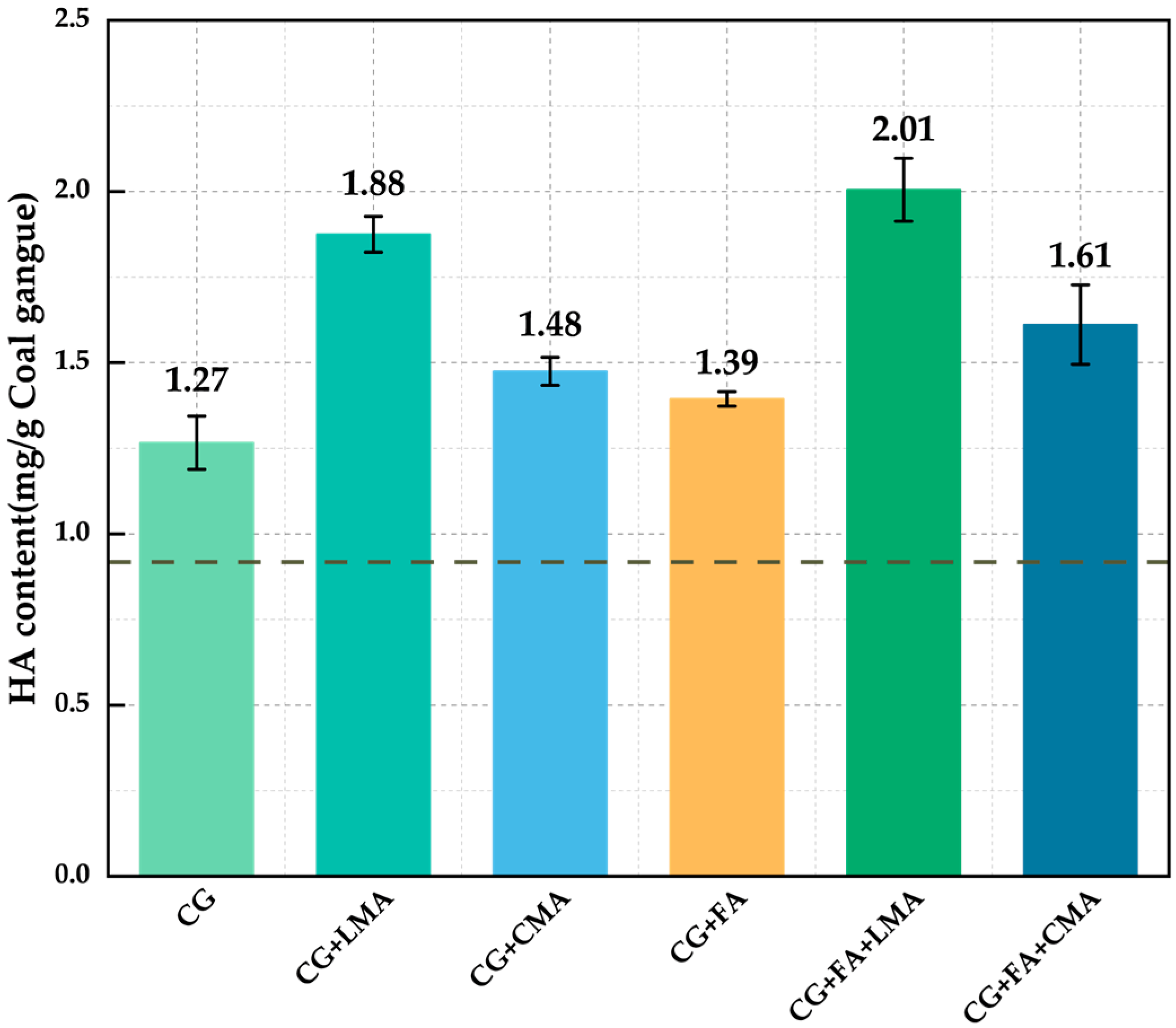
3.2. Comparison of HA Content per Unit Mass of CG
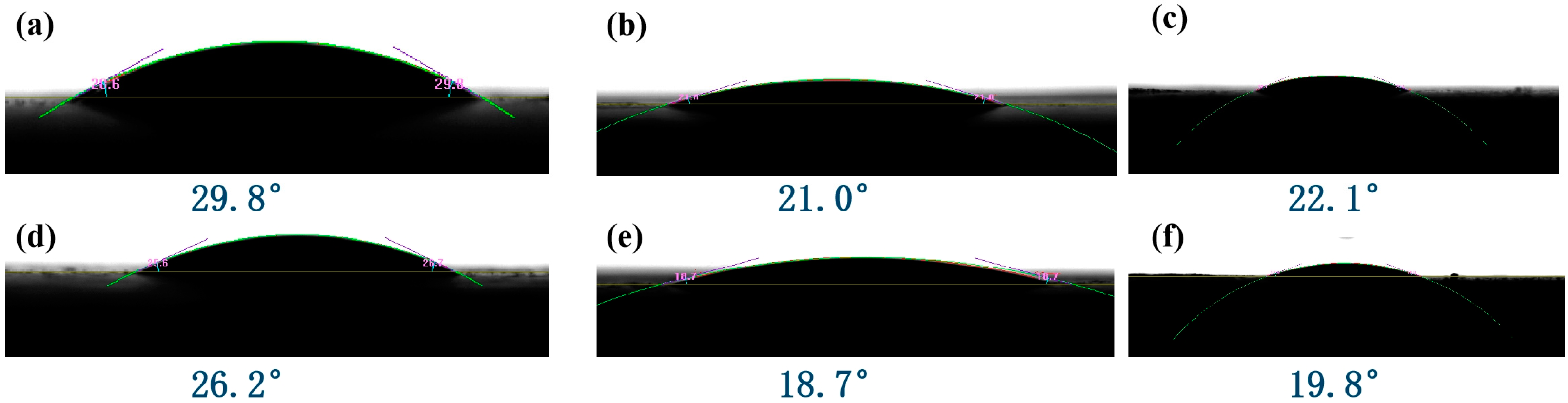
3.3. Water-Holding Capacity (WHC) Test for Artificial Soils
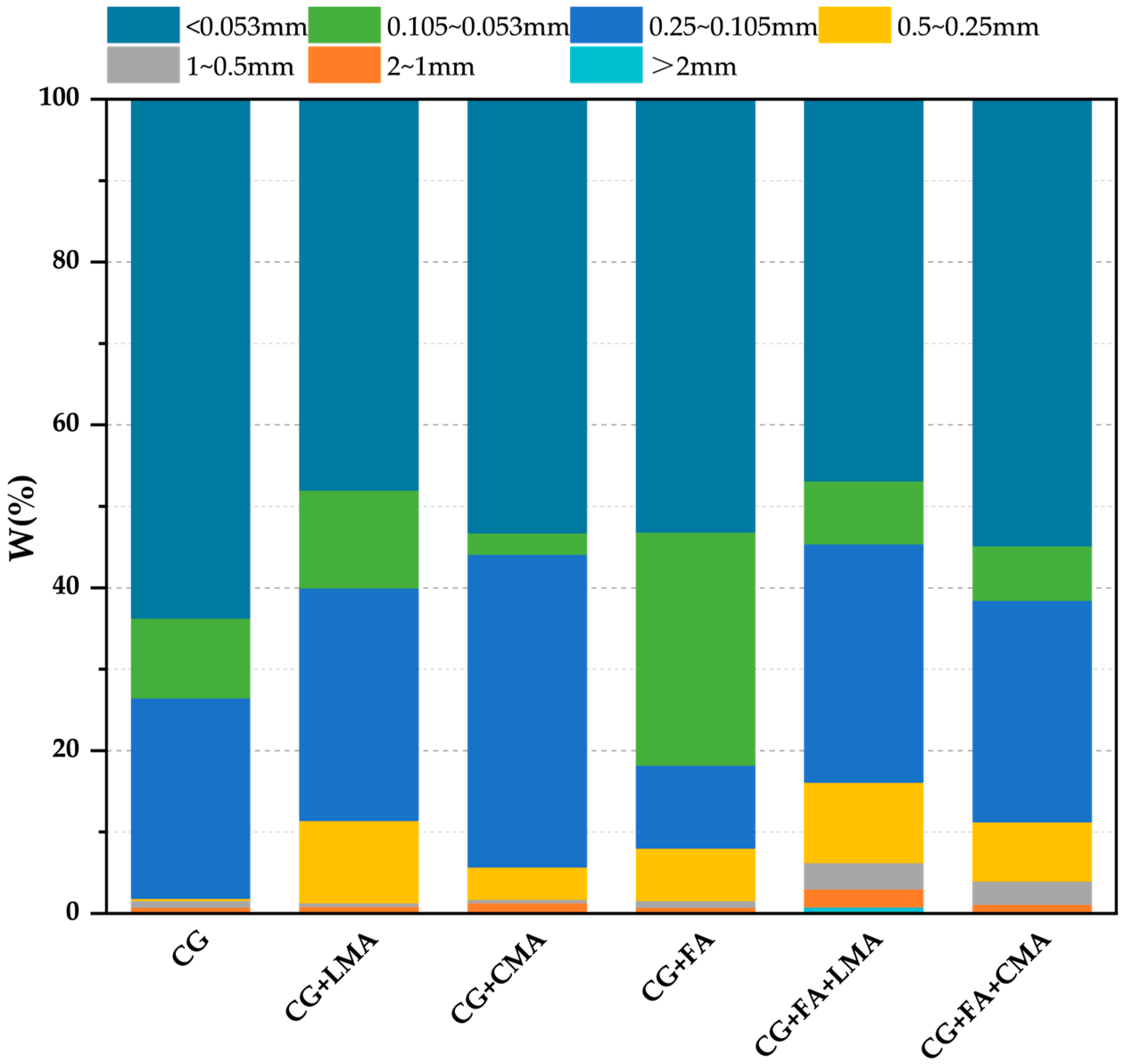
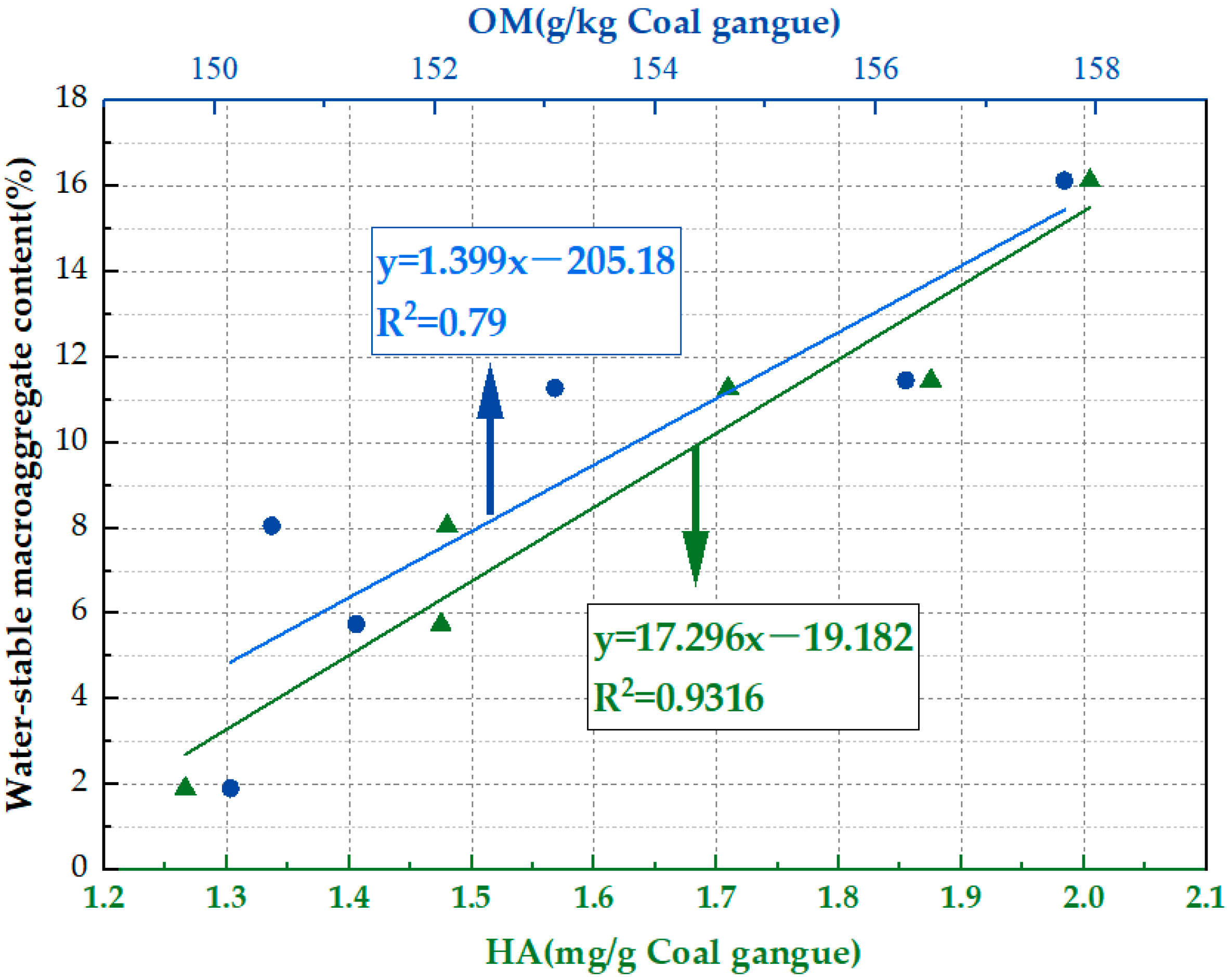
3.4. Evaluation of Water-Stable Macroaggregates in Artificial Soils
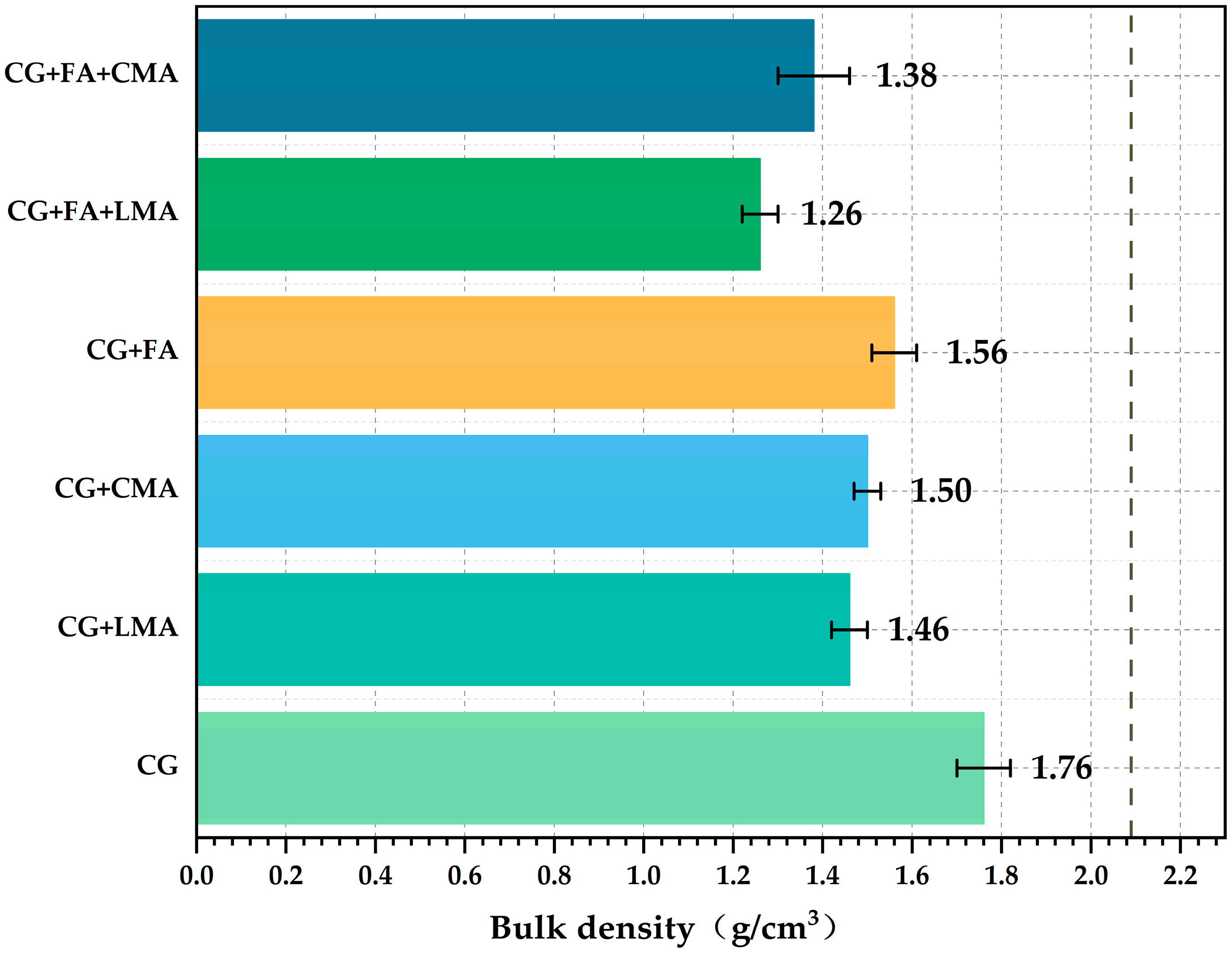
3.5. Artificial Soil Bulk Weight
4. Analysis of Aggregate Formation Mechanisms
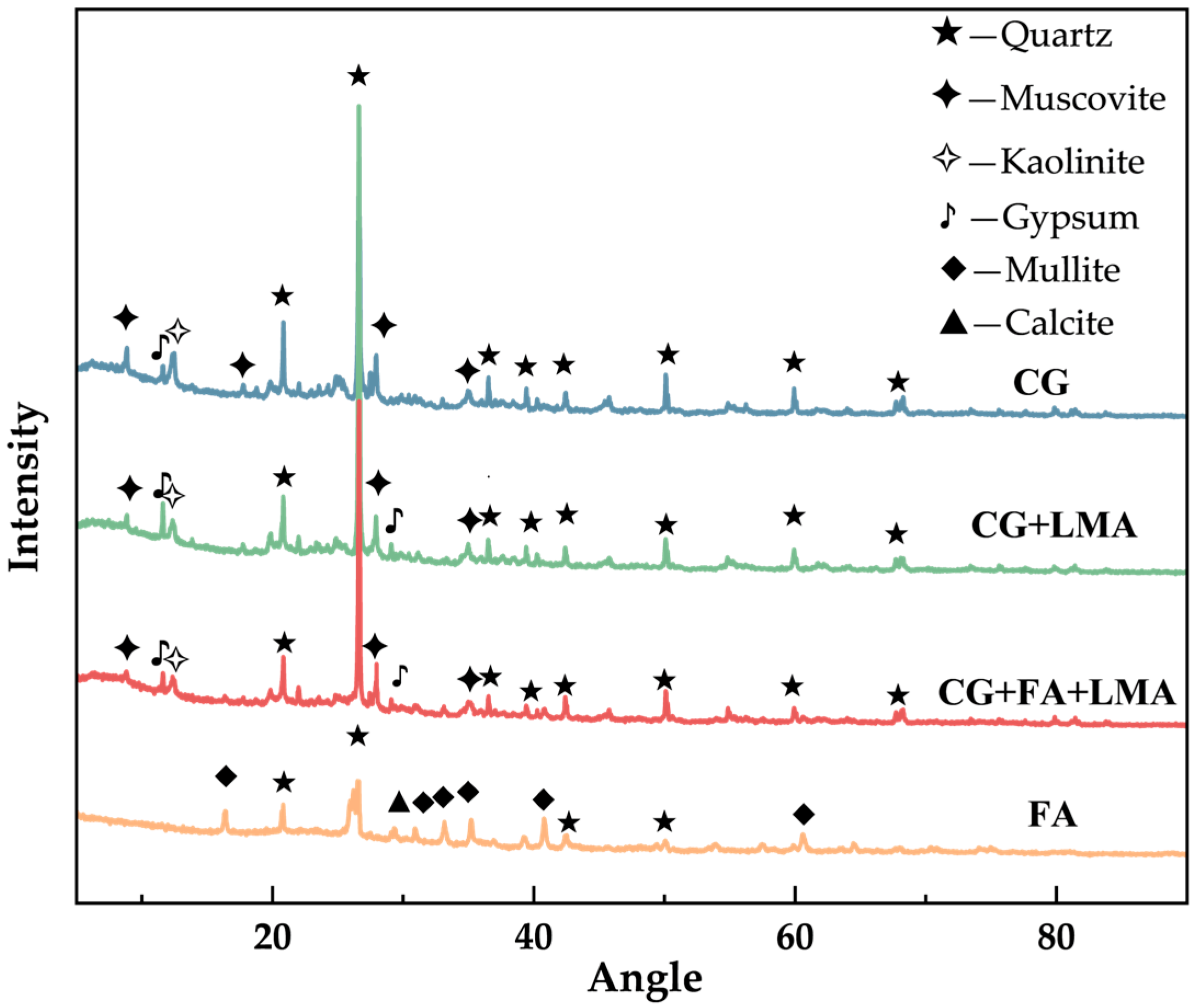
4.1. Mineral Phase Analysis
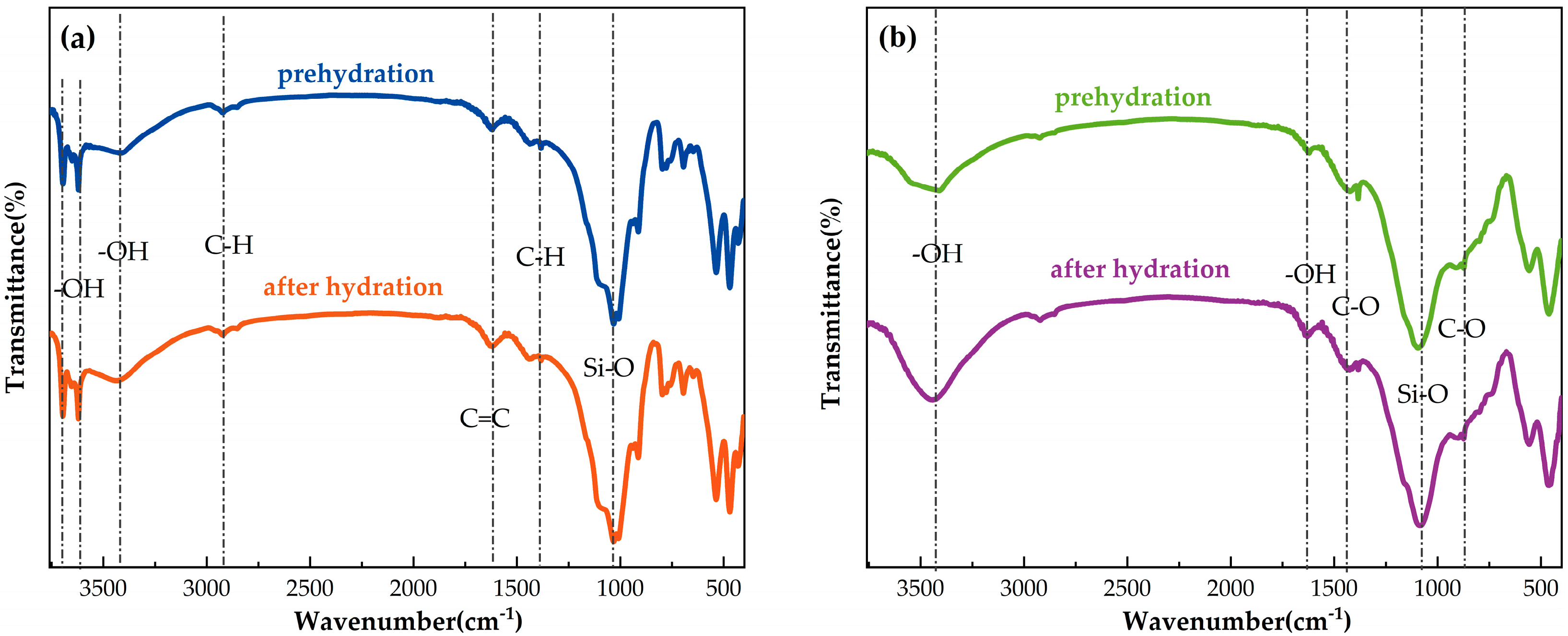
4.2. Surface Functional Group Analysis
4.3. Characterization of Microstructure
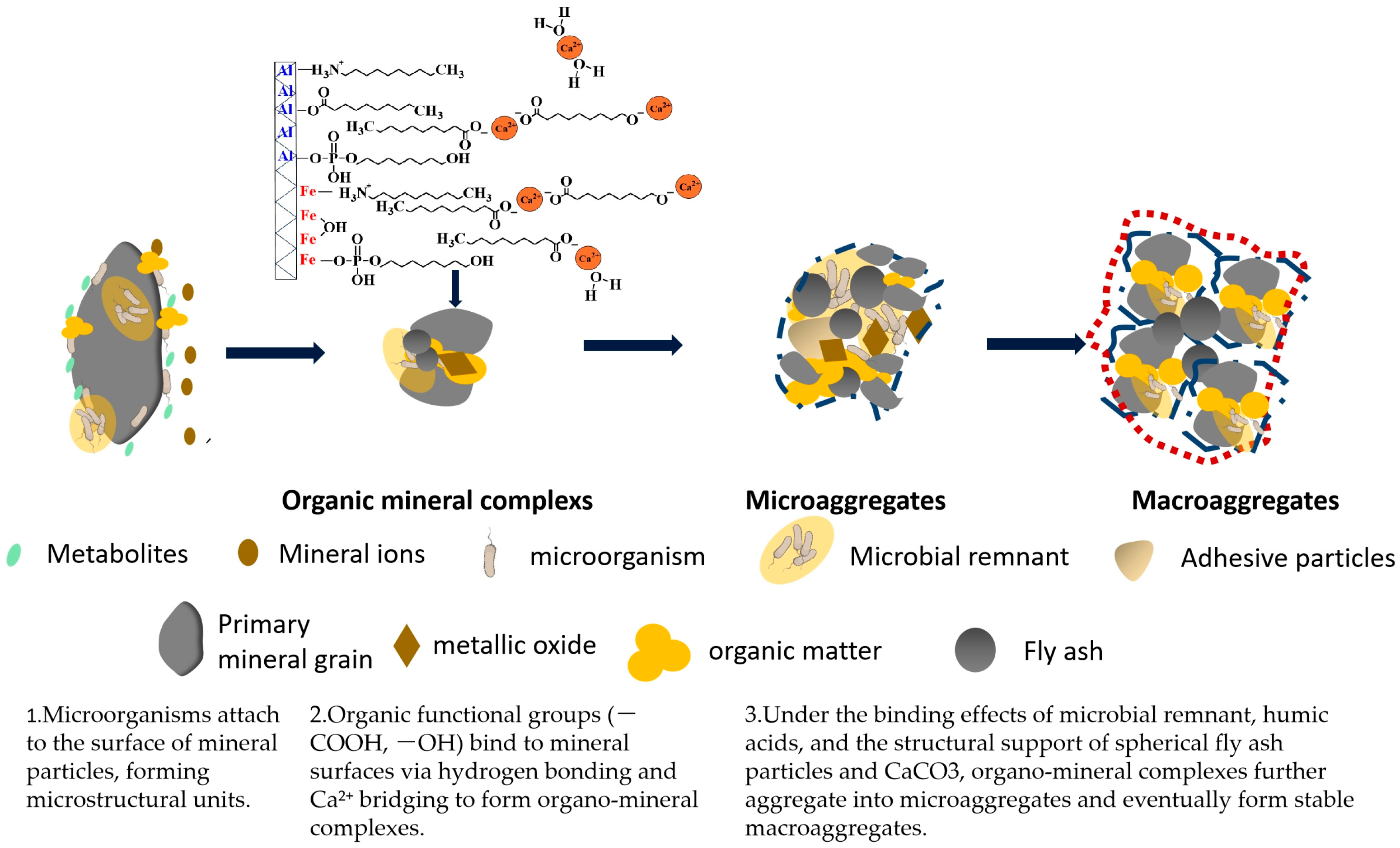
4.4. Mechanism Diagram of the Formation of Artificial Soil Aggregate
- (1)
- Microorganisms attach to mineral surfaces to form microstructural units while promoting the weathering of gangue minerals and releasing mineral ions [43].
- (2)
- The metabolites produced by microbial activity, including HA, other OM, and mineral particles, form an organic–mineral complex. At this stage, the CG and FA particles primarily serve as carriers in the formation of the complex. In particular, the gypsum phase formed by microbial treatment of CG and the calcium carbonate present in FA act as “cation bridges”, connecting the minerals and OM through the production of Ca2+ [39]. Additionally, the abundant iron and aluminum oxides in coal gangue and fly ash may carry a substantial positive charge in the weakly acidic microenvironment induced by water erosion or microbial metabolism. These oxides can serve as binding agents between clay particles and organic molecules, jointly promoting the formation of microaggregates [44].
- (3)
- The organic–mineral complexes aggregate to form microaggregates, which subsequently coalesce into macroaggregates. FA particles are primarily used as bridging carriers during the formation of both microaggregate and macroaggregate structures.
5. Conclusions and Future Outlook
- (1)
- Microorganisms can effectively activate OM in CG and promote the conversion of coal and other OM to HA, thereby improving the soil’s WHC, reducing bulk density, and promoting the formation of a stable macroaggregate structure. The LMA screened from the CG demonstrated significant advantages over the CMA in enhancing artificial soil properties.
- (2)
- Both FA and microbial agents showed similar trends in enhancing the quality of CGAS. Under the synergistic effect of microorganisms and FA, compared with the untreated gangue, the HA content increased by 2.06 times, the content of water-stable macroaggregates increased to 11.46%, and the bulk density decreased by 39.71% to 1.26 g/cm3. Additionally, the OM content and WHC of the soil were effectively improved.
- (3)
- The formation of soil macroaggregates in the new CGAS system involves four primary stages: the development of microstructural units, formation of organic–mineral complexes, aggregation into microaggregates, and growth into macroaggregates. The resulting soil exhibits good properties and a stable aggregate structure formed through the combined action of microorganisms, organic binders (such as HA), and inorganic binders (such as iron and aluminum oxides and calcium ions). The FA particles serve as carriers and bridging agents during the formation of the CGAS structure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CG | Coal gangue |
| CGAS | Coal gangue-based artificial soil. |
| LMA | Local microbial agent |
| CMA | Commercial microbial agent |
| FA | Fly ash |
| FTIR | Fourier transform Infrared spectroscopy |
| OM | Organic matter |
| HA | Humic acid |
| WHC | Water-holding capacity |
| MWD | Mean weight diameter |
| GMD | Geometric mean diameter |
References
- Xu, M.Y.; Mao, Y.C.; Yan, Z.L.; Zhang, M.Q.; Xiao, D. Coal and Gangue Classification Based on Laser-Induced Breakdown Spectroscopy and Deep Learning. ACS Omega 2023, 8, 47646–47657. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.Q.; Peng, J.F.; Xu, D.J.; Tian, J.J.; Liu, T.H.; Jiang, B.B.; Zhang, F.C. Leaching Characteristics and Pollution Risk Assessment of Potentially Harmful Elements from Coal Gangue Exposed to Weathering for Different Periods of Time. Environ. Sci. Pollut. Res. 2023, 30, 63200–63214. [Google Scholar] [CrossRef]
- Gao, H.; Zhao, G.H.; Guo, L.H.; Zhou, L.Q.; Yuan, K.K. Utilization of Coal Gangue as Coarse Aggregates in Structural Concrete. Constr. Build. Mater. 2021, 268, 121212. [Google Scholar] [CrossRef]
- He, Z.X.; Zhao, X.; Ye, M.C.; Zuo, W.; Nie, X.X.; Zhao, J.J. Study on the Effect of Basalt Fiber Content and Length on Mechanical Properties and Durability of Coal Gangue Concrete. Sustainability 2024, 16, 9310. [Google Scholar] [CrossRef]
- Xu, Y.H.; Wu, H.J.; Dong, Z.F.; Wang, Q.Q.; Chen, X.Y. Life Cycle Energy Use Efficiency and Greenhouse Gas Emissions of Circulating Fluidized Bed Coal-Fired Plant with Coal Gangue and Coal Co-Combustion. Environ. Dev. Sustain. 2024, 26, 0049–0071. [Google Scholar] [CrossRef]
- Xie, S.R.; Pan, H.; Gu, W.Z.; Zhu, L.; Yue, D.; Chen, D.D.; Song, T.Q.; Jiang, Z.S. Technology and Engineering Test of Filling Goaf with Coal Gangue Slurry. Sci. Rep. 2023, 13, 20536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.T.; Xu, Y.X.; Liao, J.B.; Liu, S.K.; Liu, Z.; Gao, W.H.; Yi, L.W. Study on the Particle Strength and Crushing Patterns of Coal Gangue Coarse-Grained Subgrade Fillers. Sustainability 2024, 16, 5155. [Google Scholar] [CrossRef]
- Zheng, Y.S.; Zhou, J.X.; Ma, Z.J.; Weng, X.Y.; Cheng, L.; Tang, G.R. Preparation of a High-Silicon ZSM-5 Molecular Sieve Using Only Coal Gangue as the Silicon and Aluminum Sources. Materials 2023, 16, 4338. [Google Scholar] [CrossRef]
- Wu, C.L.; Wang, W.L.; Wang, X.J. Research Status and Innovative Utilization Strategy of Coal Gangue Resource in Building Material Field. Energy Environ. Prot. 2023, 37, 167–177. [Google Scholar]
- Peng, S.P.; Bi, Y.L. Strategic Consideration and Core Technology About Environmental Ecological Restoration in Coal Mine Areas in the Yellow River Basin of China. J. China Coal Soc. 2020, 45, 4. [Google Scholar]
- Li, S.Q.; Li, X.Q.; Qiang, X.L.; Zhao, Y.; Li, H.Y.; Sun, Z.J.; Li, Q.; He, J.; Han, L.; Zhao, N.X. Improving Saline–Alkaline Soil and Ryegrass Growth with Coal Gangue Treatments. Plants 2024, 13, 3419. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Wang, D.M.; Bai, Y.J.; Zhang, Z.Z. Optimizing the Formulation of Coal Gangue Planting Substrate Using Wastes: The Sustainability of Coal Mine Ecological Restoration. Ecol. Eng. 2020, 143, 105669. [Google Scholar] [CrossRef]
- Luo, C.; Li, S.H.; Ren, P.G.; Yan, F.; Wang, L.; Guo, B.; Zhao, Y.M.; Yang, Y.; Sun, J.; Ga, P.C.; et al. Enhancing the Carbon Content of Coal Gangue for Composting Through Sludge Amendment: A Feasibility Study. Environ. Pollut. 2024, 348, 123439. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; He, X.M.; Hu, H.M.; Zhang, Q.W. Cogrinding with Alkaline Metal Salts to Enhance the Reactivity of Silicate Mineral to Serve as Silicon Fertilizer. Chem. Phys. Lett. 2020, 747, 137347. [Google Scholar] [CrossRef]
- Shao, S.; Ma, B.Z.; Wang, C.Y.; Chen, Y.Q. Extraction of Valuable Components from Coal Gangue through Thermal Activation and Hno3 Leaching. J. Ind. Eng. Chem. 2022, 113, 564–574. [Google Scholar] [CrossRef]
- Bi, Y.L.; Xiao, L.; Liu, R.R. Response of Arbuscular Mycorrhizal Fungi and Phosphorus Solubilizing Bacteria to Remediation Abandoned Solid Waste of Coal Mine. Int. J. Coal Sci. Technol. 2019, 6, 603–610. [Google Scholar] [CrossRef]
- Lv, Y.; Li, J.; Ye, H.P.; Du, D.Y.; Sun, P.; Ma, M.Y.; Zhang, T.C. Bioleaching of Silicon in Electrolytic Manganese Residue (Emr) by Paenibacillus mucilaginosus: Impact of Silicate Mineral Structures. Chemosphere 2020, 256, 127043. [Google Scholar] [CrossRef]
- Zhu, X.B.; Gong, W.H.; Li, W.; Bai, X.Y.; Zhang, C.X. Reclamation of Waste Coal Gangue Activated by Stenotrophomonas maltophilia for Mine Soil Improvement: Solubilizing Behavior of Bacteria on Nutrient Elements. J. Environ. Manag. 2022, 320, 115865. [Google Scholar] [CrossRef]
- Regelink, I.C.; Cathelijne, R.S.; Svetla, R.; Weng, L.P.; Georg, J.L.; Pavel, K.; Nikolaos, P.N.; Kercheva, M.; Banwart, S.; Comans, R.N.J. Linkages Between Aggregate Formation, Porosity and Soil Chemical Properties. Geoderma 2015, 247, 24–37. [Google Scholar] [CrossRef]
- Rillig, M.C.; Ludo, A.H.M.; Anika, L. Soil Aggregates as Massively Concurrent Evolutionary Incubators. ISME J. 2017, 11, 1943–1948. [Google Scholar] [CrossRef]
- Garland, G.; Bünemann, E.K.; Oberson, A.; Frossard, E.; Snapp, S.; Chikowo, R.; Six, J. Phosphorus Cycling Within Soil Aggregate Fractions of a Highly Weathered Tropical Soil: A Conceptual Model. Soil Biol. Biochem. 2018, 116, 91–98. [Google Scholar] [CrossRef]
- Cheng, Z.; Gaohang, C.; Zheng, Y.; Haohang, G.; Zening, G.; Daili, Z.; Chen, X. Improvement of the Salinized Soil Properties of Fly Ash by Freeze-Thaw Cycles: An Impact Test Study. Sustainability 2021, 13, 2908. [Google Scholar] [CrossRef]
- NY/T 1121.6-2006; Soil Testing—Part 6: Determination of Organic Matter. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2006.
- GB/T 11957-2001; Soil Quality—Determination of Particle Size Distribution. Standards Press of China: Beijing, China, 2001.
- NY/T 1121.22-2010; Soil Testing—Part 22: Determination of Available Potassium. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2010.
- NY/T 1121.19-2008; Soil Testing—Part 19: Determination of Available Phosphorus. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2008.
- NY/T 1121.4-2006; Soil Testing—Part 4: Determination of Soil pH. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2006.
- Liang, C.; Cheng, G.; Wixon, D.L.; Balser, T.C. An Absorbing Markov Chain Approach to Understanding the Microbial Role in Soil Carbon Stabilization. Biogeochemistry 2011, 106, 303–309. [Google Scholar] [CrossRef]
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative Assessment of Microbial Necromass Contribution to Soil Organic Matter. Glob. Change Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef]
- Ye, J.X.; An, N.; Chen, H.; Ying, Z.Y.; Zhang, S.H.; Zhao, J.K. Performance and Mechanism of Carbon Dioxide Fixation by a Newly Isolated Chemoautotrophic Strain Paracoccus denitrificans PJ-1. Chemosphere 2020, 252, 126473. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Wang, X.H.; Ou, Y.J.; Du, H. Biodegradation of Organic Compounds in the Coal Gangue by Bacillus Sp. Into Humic Acid. Biodegradation 2023, 34, 125–138. [Google Scholar] [CrossRef]
- Li, S.Y.; Tan, J.F.; Wang, Y.; Li, P.P.; Hu, D.S.; Shi, Q.Z.; Yue, Y.J.; Li, F.; Han, Y.L. Extraction Optimization and Quality Evaluation of Humic Acids from Lignite Using the Cell-Free Filtrate of Penicillium ortum MJ51. RSC Adv. 2022, 12, 528–539. [Google Scholar] [CrossRef]
- Liang, C.; Lu, L.; Tao, X.; Kang, G.; Liu, Z.; Li, B. The Pseudomonas Ligninolytic Catalytic Network Reveals the Importance of Auxiliary Enzymes in Lignin Biocatalysts. Proc. Natl. Acad. Sci. USA 2025, 4, e2417343122. [Google Scholar] [CrossRef]
- Nuraly, A.; Digel, I.; Qiao, X.H.; Tastambe, K.; Zhubanova, A. Lignite Biosolubilization by Bacillus sp. RKB 2 and Characterization of its Products. Geomicrobiol. J. 2020, 37, 255–261. [Google Scholar]
- Hazrin, C.; Hazlin, N.; Marjo, C.E.; Theerthankar, D.; Anne, M.R.; Mike, M. Surface Analysis Reveals Biogenic Oxidation of Sub-Bituminous Coal by Pseudomonas fluorescens. Appl. Microbiol. Biotechnol. 2014, 98, 6443–6452. [Google Scholar] [CrossRef]
- Adessi, A.; Carvalho, R.C.D.; Philippis, R.D.; Branquinho, C.; Silva, J.M.D. Microbial Extracellular Polymeric Substances Improve Water Retention in Dryland Biological Soil Crusts. Soil Biol. Biochem. 2018, 116, 67–69. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The Interplay Between Microbial Communities and Soil Properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Tirado-Corbalá, R.; Slater, B.K.; Dick, W.A.; Bigham, J.; Muñoz-Muñoz, M. Gypsum Amendment Effects on Micromorphology and Aggregation in No-Till Mollisols and Alfisols from Western Ohio, USA. Geoderma Reg. 2019, 16, e00217. [Google Scholar] [CrossRef]
- Pihlap, E.; Steffens, M.; Kögel-Knabner, I. Initial Soil Aggregate Formation and Stabilisation in Soils Developed from Calcareous Loess. Geoderma 2021, 385, 114854. [Google Scholar] [CrossRef]
- Wershaw, R.L.; Llaguno, E.C.; Leenheer, J.A. Mechanism of Formation of Humus Coatings on Mineral Surfaces 3. Composition of Adsorbed Organic Acids from Compost Leachate on Alumina by Solid-state 13C NMR. Colldid Surface A 1996, 108, 213–223. [Google Scholar] [CrossRef]
- Kleber, M.; Sollin, P.; Sutton, R. A Conceptual Model of Organo-Mineral Interactions in Soils: Self-Assembly of Organic Molecular Fragments into Zonal Structures on Mineral Surfaces. Biogeochemistry 2007, 85, 9–24. [Google Scholar] [CrossRef]
- Zeynali, Y.; Niroumand, H.; Moayed, R.Z. Stabilizing Cohesive Soils with Micro- and Nano- Fly Ash as Eco-Friendly Materials: An Experimental Study. Constr. Build. Mater. 2023, 399, 132490. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J.M. Microbe-Mineral Interactions: The Impact of Surface Attachment on Mineral Weathering and Element Selectivity by Microorganisms. Chem. Geol. 2015, 403, 13–23. [Google Scholar] [CrossRef]
- Zhe, L.; Huang, Z.G.; Liao, D.L.; Huang, W.X.; Huang, J.; Deng, Y.S. Effects of Soil Organic Matter Components and Iron Aluminum Oxides on Aggregate Stability During Vegetation Succession in Granite Red Soil Eroded Areas. J. Mt. Sci. 2022, 19, 2634–2650. [Google Scholar]


| Sample | Chemical Composition (wt%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | SO3 | Fe2O3 | K2O | TiO2 | MgO | CaO | Na2O | P2O5 | |
| CG | 57.03 | 22.30 | 4.14 | 5.13 | 3.74 | 1.90 | 1.25 | 2.89 | 1.23 | 0.12 |
| FA | 46.21 | 34.70 | 1.77 | 5.15 | 0.92 | 2.49 | 0.99 | 6.48 | 0.55 | 0.23 |
| Sample Name | CG (%) | FA (%) | Microbial Agents (%) |
|---|---|---|---|
| CG | 100 | 0 | 0 |
| CG + LMA | 100 | 0 | 0.1 |
| CG + CMA | 100 | 0 | 0.1 |
| CG + FA | 70 | 30 | 0 |
| CG + FA + LMA | 70 | 30 | 0.1 |
| CG + FA + CMA | 70 | 30 | 0.1 |
| Sample Name | WHC (%) |
|---|---|
| CG | 38.47 |
| CG + LMA | 49.44 |
| CG + CMA | 46.50 |
| CG + FA | 49.41 |
| CG + FA + LMA | 54.88 |
| CG + FA + CMA | 51.95 |
| Sample Name | MWD | GMD |
|---|---|---|
| CG | 0.14 | 0.11 |
| CG + LMA | 0.24 | 0.20 |
| CG + CMA | 0.20 | 0.16 |
| CG + FA | 0.18 | 0.13 |
| CG + FA + LMA | 0.36 | 0.24 |
| CG + FA + CMA | 0.31 | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, W.; Hui, H.; Ma, S.; Ji, J.; Jiang, H. Preparation of Coal Gangue-Based Artificial Soil and Investigation of the Mechanism of Aggregate Structure Formation. Sustainability 2025, 17, 3318. https://doi.org/10.3390/su17083318
Gong W, Hui H, Ma S, Ji J, Jiang H. Preparation of Coal Gangue-Based Artificial Soil and Investigation of the Mechanism of Aggregate Structure Formation. Sustainability. 2025; 17(8):3318. https://doi.org/10.3390/su17083318
Chicago/Turabian StyleGong, Weinan, Helong Hui, Shuhua Ma, Jianbing Ji, and Hongtao Jiang. 2025. "Preparation of Coal Gangue-Based Artificial Soil and Investigation of the Mechanism of Aggregate Structure Formation" Sustainability 17, no. 8: 3318. https://doi.org/10.3390/su17083318
APA StyleGong, W., Hui, H., Ma, S., Ji, J., & Jiang, H. (2025). Preparation of Coal Gangue-Based Artificial Soil and Investigation of the Mechanism of Aggregate Structure Formation. Sustainability, 17(8), 3318. https://doi.org/10.3390/su17083318







