Research on Co-Combustion of High-Calorific Biomass Obtained Using Gasification and Lignite for Sustainable Utilisation of Resources
Abstract
1. Introduction
2. Materials and Methods
2.1. Fuels
- No. 1—Borodino lignite coal;
- No. 2—Carbonisate obtained from larch wood;
- No. 3—Carbonisate obtained from pinewood;
- No. 4—Carbonisate obtained from mixed fuel based on lignite No. 1 and common pinewood;
- No. 5—A mixture based on fuels No. 1 and 2;
- No. 6—A mixture based on fuels No. 1 and 3.
2.2. Main Characteristics of the Fuels Under Study
- The fuels were ground using a Retsch DM200 (Germany) to a grain size of about 1000 µm;
- To obtain a grain size of 140–250 μm (this fuel size is used in solid-fuel flaring combustion), a Retsch AS200 analytical sieving machine (Germany) equipped with sieves with appropriate cells (ISO 3310-1:2016) [35] was used;
- The moisture content (MC) of the samples was determined on an MA-150 (Germany) according to (ISO 18134-1:2022) [36];
- The inorganic residue content (Ad) in a dry state was measured using a Snol 7.2/1300 (Lithuania) muffle furnace (ISO 18122:2022) [37];
- The volatile matter (VCdaf) in a dry ash-free state was also determined using the Snol 7.2/1300 (Lithuania) furnace (ISO 18123:2023) [38];
- The low heat of combustion in working conditions (LHV) was determined on a C6000 (Germany) apparatus (ISO 18125:2017) [39];
- The carbon (Cdaf), hydrogen (Hdaf) and nitrogen (Ndaf) contents in a dry ash-free state were determined (ISO 16948:2015) [40];
- The sulphur content (Sdaf) in a dry ash-free state was determined (ISO 16994:2016) [41];
- Oxygen (Odaf) was determined through subtraction (ISO/TS 20048-1:2020) [42].
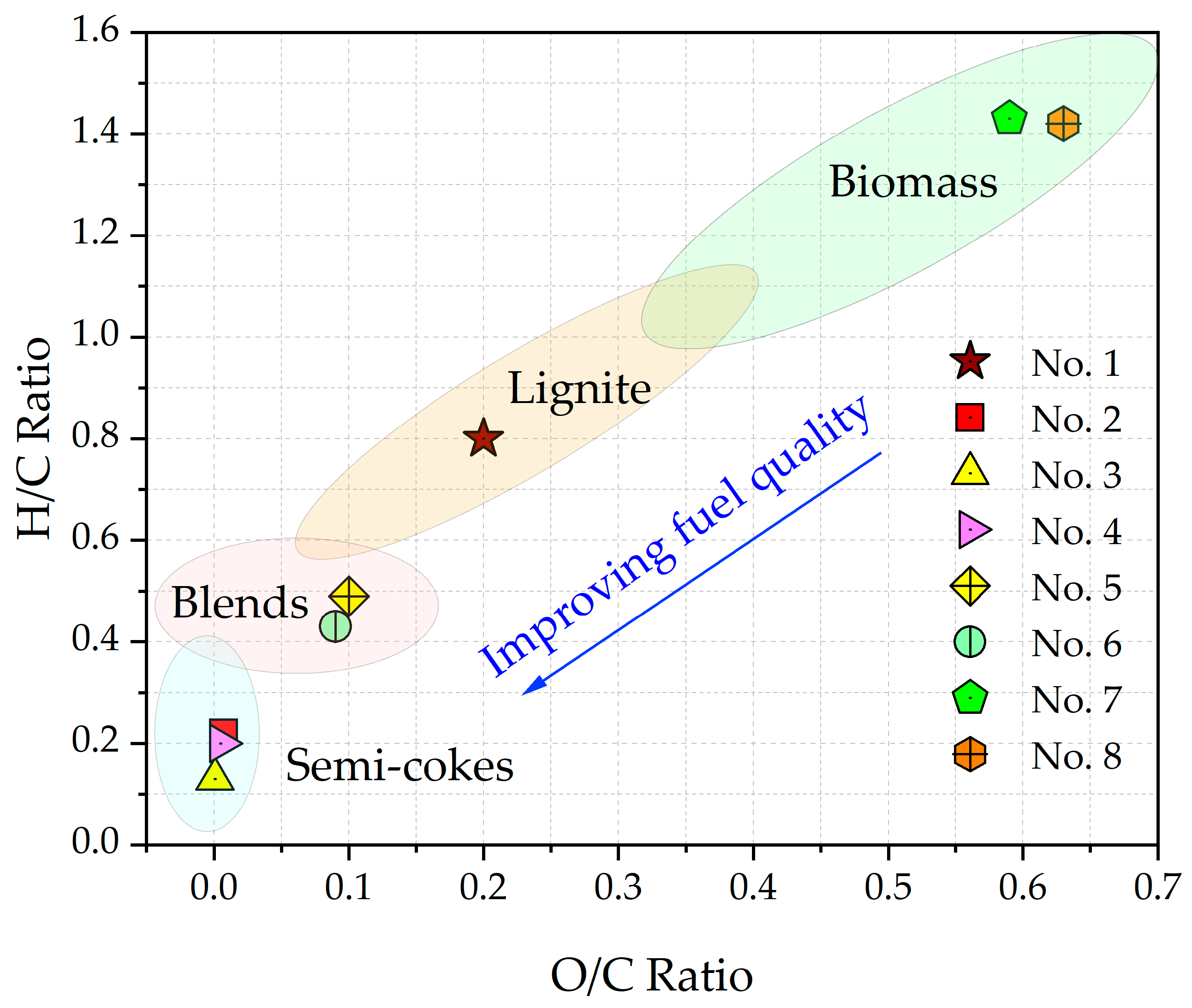
2.3. A Van Krevelen Diagram of the Fuels Under Study
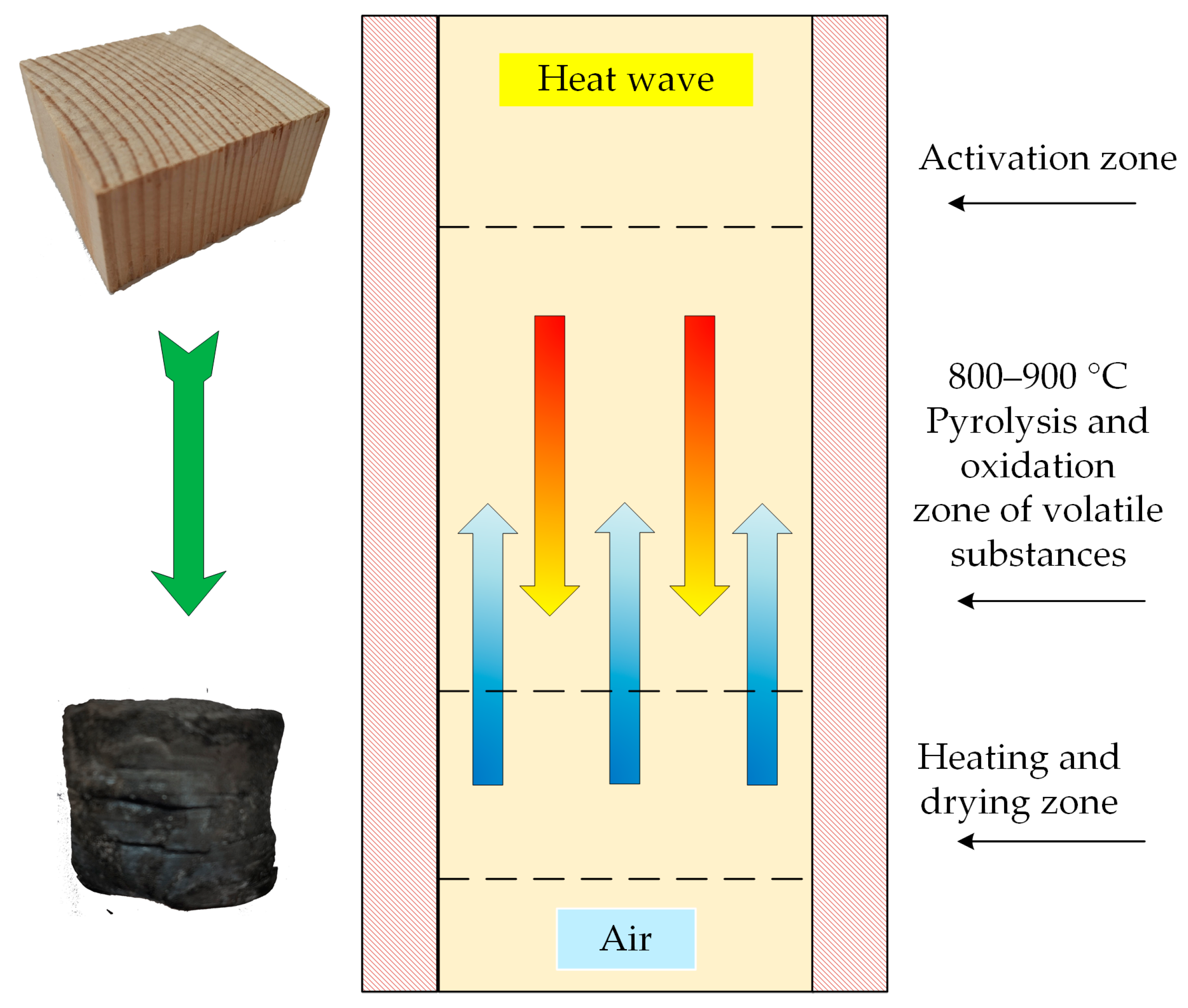
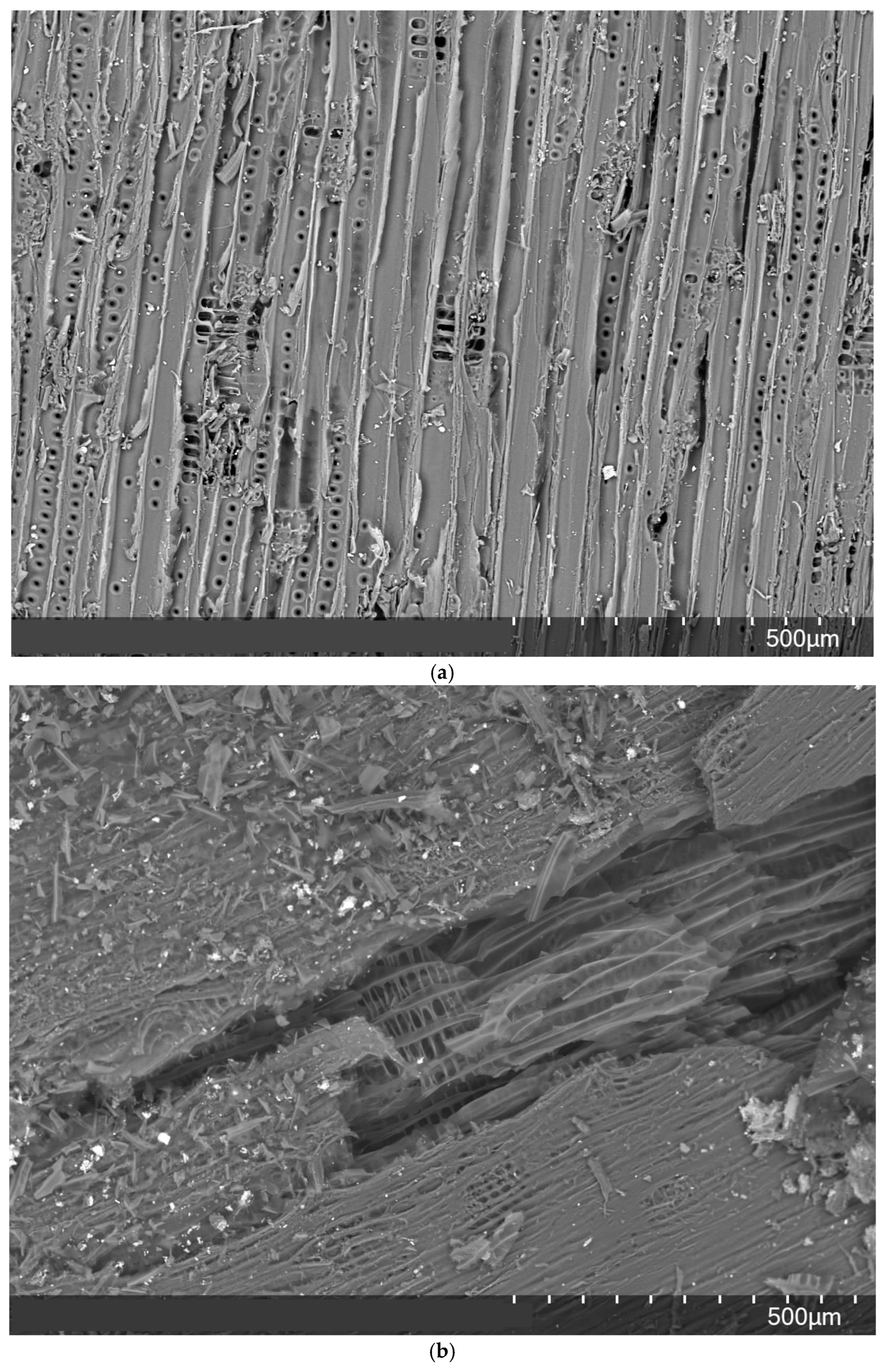
2.4. The Surface Morphology of the Wood Particles Before and After Gasification
2.5. Methodology of Thermal Analysis (TGA)
2.5.1. Determination of the Basic Parameters of Fuel Combustion and Determination of the Synergistic Effects During Heating of the Mixtures
2.5.2. Determination of the Synergistic Effects During Heating of the Mixtures
3. Results and Discussion
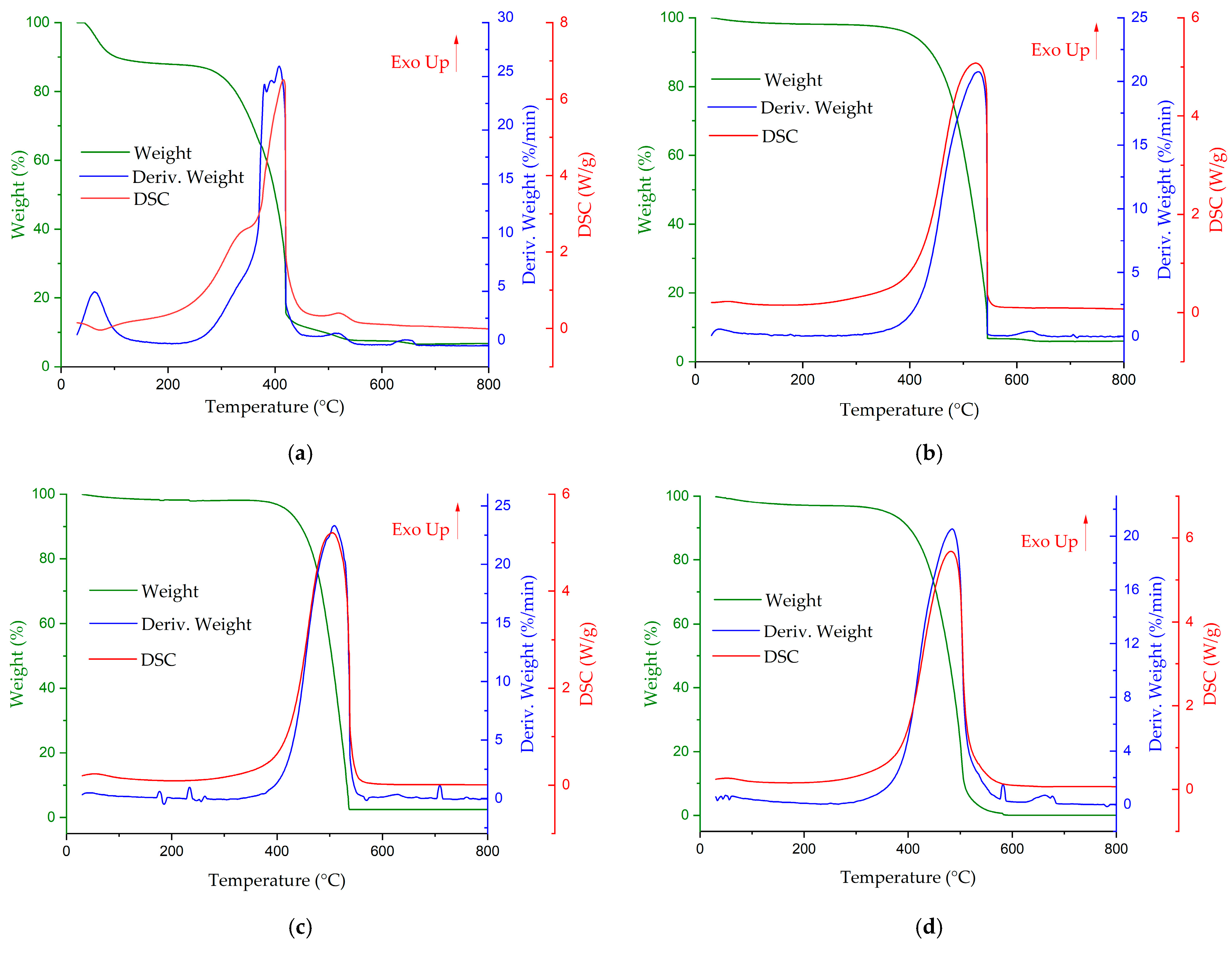
3.1. The Combustion of Individual Fuels
- Coal had a lower ignition temperature compared to that of biomass carbonisate;
- The carbonisate from larch and pine had a burnout temperature approximately equal to that of coal;
- The values of the combustion index and peak mass change intensity (the combustion intensity) of pine carbonisate were only slightly lower than these values in coal, provided that the volatile content of carbonisate was 14 times lower;
- It was determined that the values of the combustion index of all of the carbonisates were higher than one, which indicated their good combustibility, even under the conditions of a low content of gaseous substances in their composition, and the peak value of the combustion intensity was 20%/min, which also confirmed their high-combustibility properties.
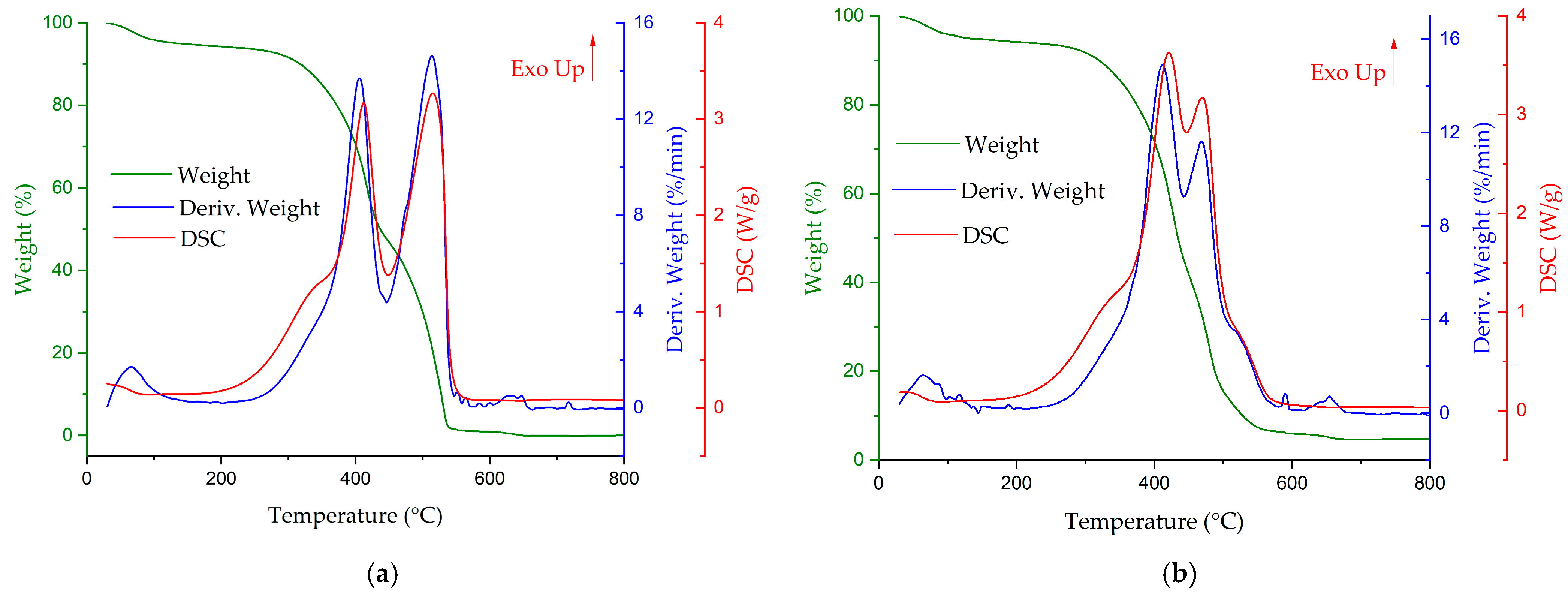
3.2. Combustion of the Fuel Mixtures
- The ignition temperature of mixtures No. 5 and 6 decreased in comparison with that of carbonisates No. 2–4.
- There was a decrease in the flammability of mixtures No. 5 and 6 compared to that of the individual components in these mixtures due to a decrease in the peak combustion intensity (%/min).
- The high ignition temperature of the carbonisate is due to the low content of volatile substances, which in turn can cause ignition in a lower temperature range due to the intense increase in the temperature of the carbon residue caused by the intense heat transfer from the burning gaseous substances to the carbon residue. The addition of coal with a high content of volatile substances shifts the ignition process to lower temperatures due to an increase in the volatile content of the mixture compared to the carbonisate (Table 1).
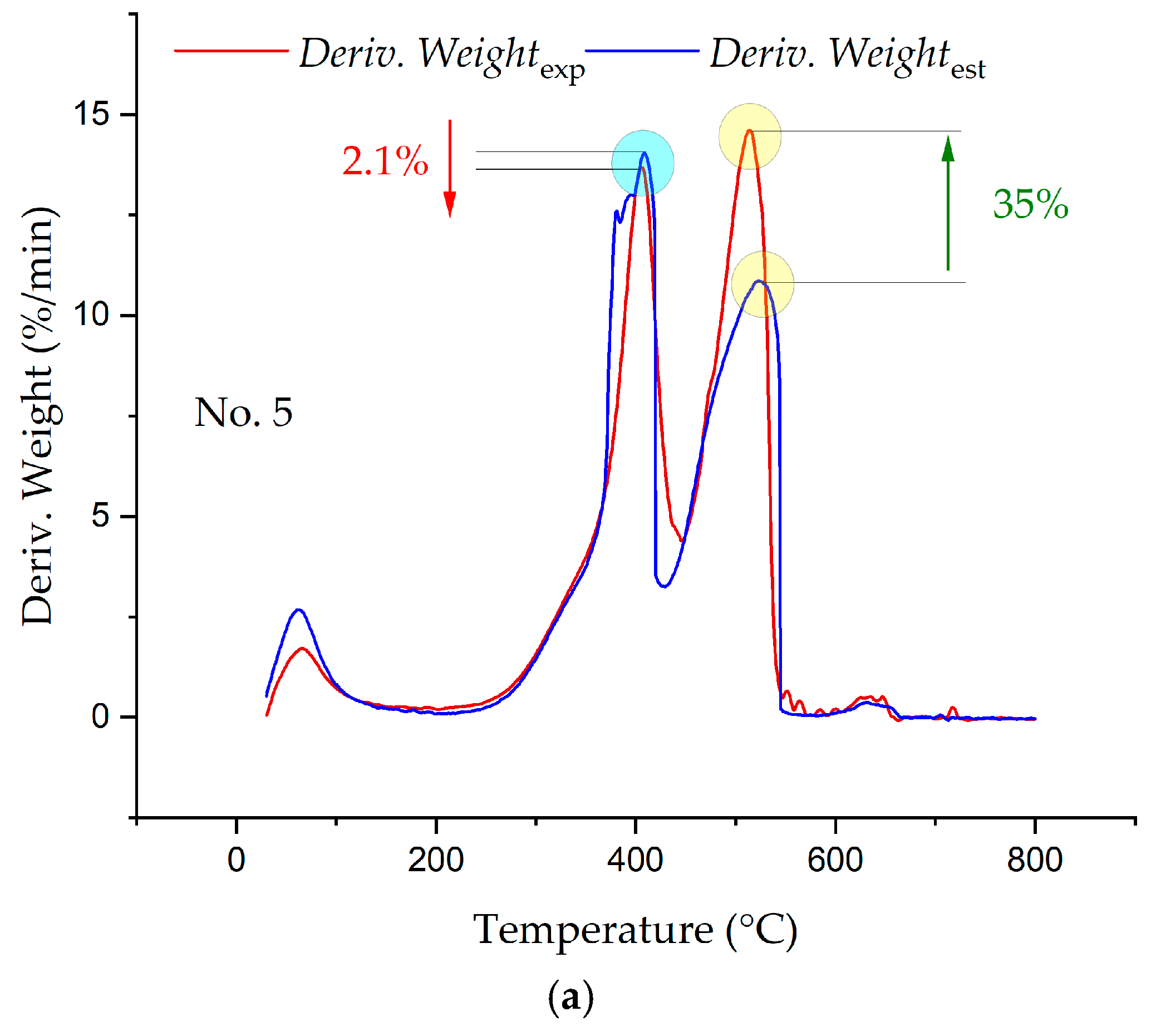
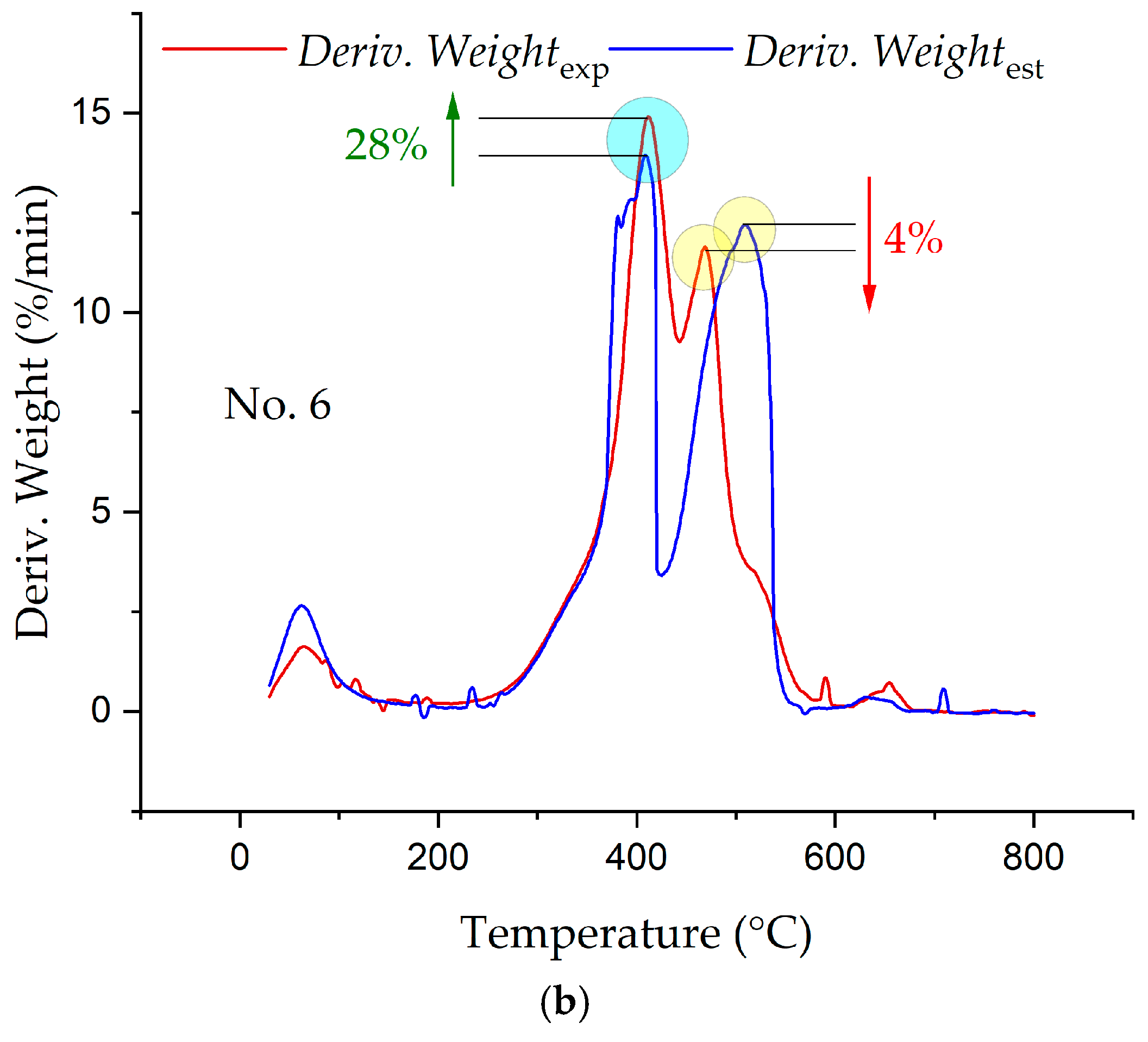
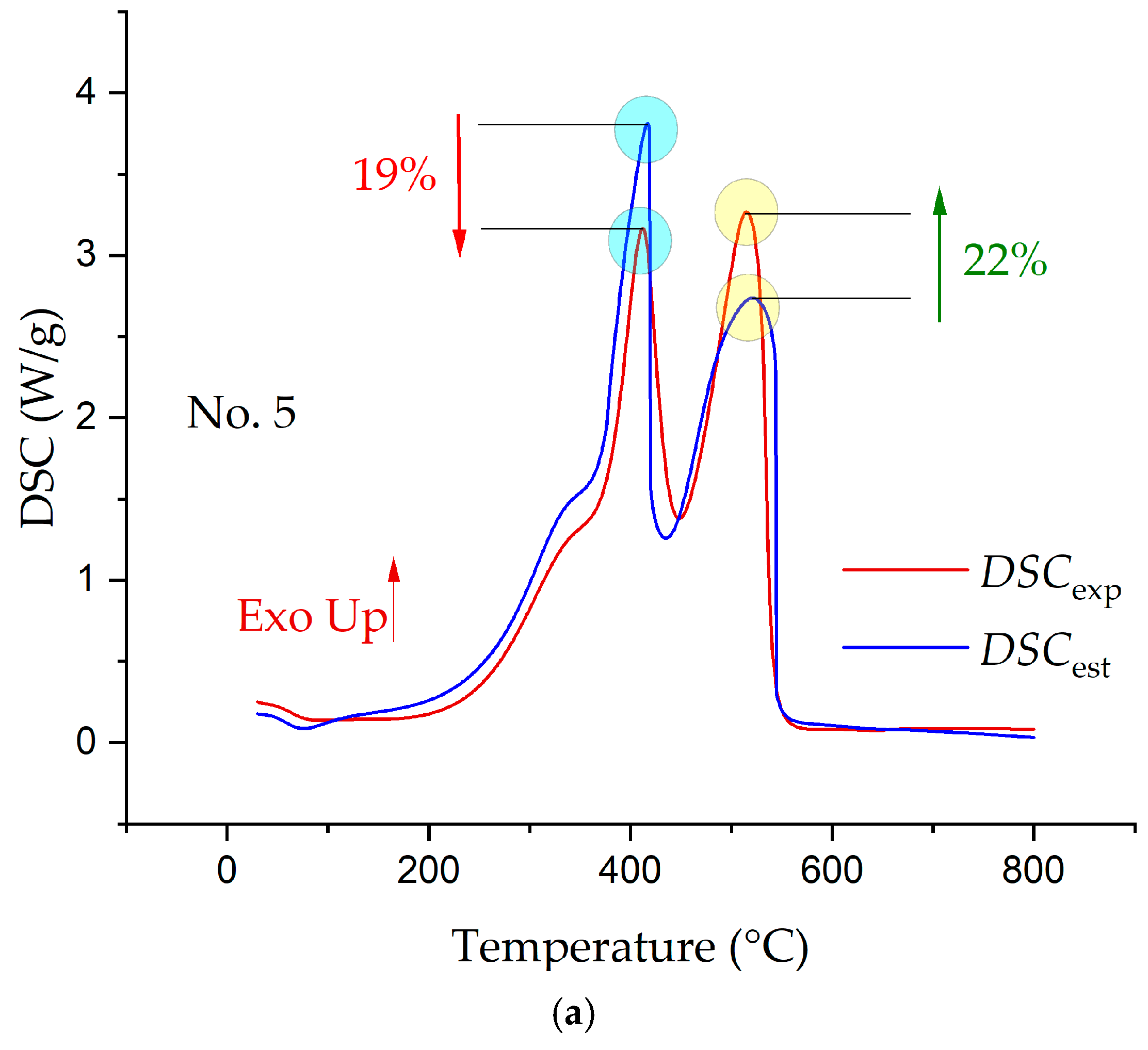
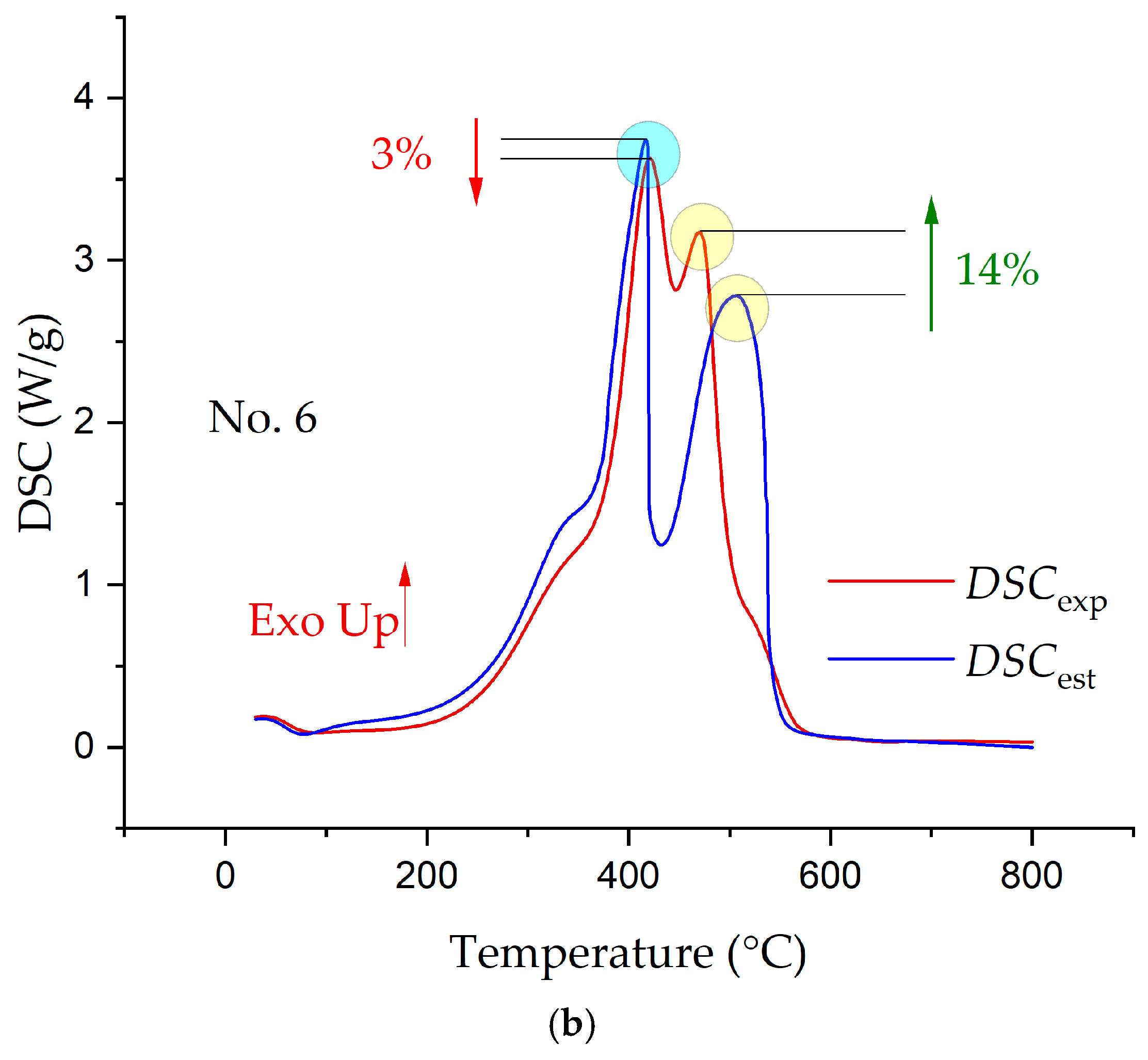
3.3. Synergetic Effects in the Combustion of the Fuel Mixtures
4. Conclusions
- Carbonisate produced from coal and pinewood has the following advantages: Its ignition temperature is lower by 7% compared to that of carbonisate produced from wood; the combustion index is higher than that of carbonisate produced from larch wood by 16%; and the lower calorific value is higher than that of coal by 86%.
- The combustion index of carbonisate made of pinewood is lower than that of coal by only 2.5%, that of carbonisate made of larch wood by 36%, that of carbonisate made of coal and that of coal by 17%.
- The ignition temperature of coal is lower than that of carbonisate by more than 100 °C, so the addition of 50% of highly reactive coal to carbonisate reduces the ignition temperature by 25%, and the LHV of the mixture is higher than the LHV of coal by 50%.
- The analysis of the interaction of the fuels between themselves in the process of their joint combustion showed the presence of synergetic interactions, which led both to a decrease in the intensity of combustion and heat release and to increases.
- During the combustion of the carbon residue carbonisate obtained from a mixture of coal and pinewood (No. 4), a shift in the peak values of the combustion intensity and the heat release intensity to lower temperatures was observed.
- The results of this study may be useful for retrofitting coal-fired boilers to run on a mixture containing carbonisate and lignite. If carbonisate is produced from biomass, the resulting gas could be used as an energy fuel by burning it in a coal-fired boiler.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Ad | ash (%) |
| Cdaf, Hdaf, Ndaf, Odaf, Sdaf | fraction of carbon, hydrogen, nitrogen, oxygen and sulphur converted in a dry ash-free state (%) |
| Deriv. Weightmax | value of peak mass change rate (%/min) |
| DSCmax | peak heat release value (W/g) |
| LHV | low heating value (MJ/kg) |
| MC | moisture content (%) |
| S | combustion index (min–2 °C−3) |
| Tb | burnout temperature (°C) |
| TDeriv. Weight | Deriv. Weightmax temperature (°C) |
| TDSC | DSCmax temperature (°C) |
| Ti | ignition temperature (°C) |
| VCdaf | gaseous content in an ash-free dry state (%) |
| peak | maximum |
References
- Hou, H.; Lu, W.; Liu, B.; Hassanein, Z.; Mahmood, H.; Khalid, S. Exploring the Role of Fossil Fuels and Renewable Energy in Determining Environmental Sustainability: Evidence from OECD Countries. Sustainability 2023, 15, 2048. [Google Scholar] [CrossRef]
- Paramati, S.R.; Shahzad, U.; Doğan, B. The role of environmental technology for energy demand and energy efficiency: Evidence from OECD countries. Renew. Sustain. Energy Rev. 2022, 153, 111735. [Google Scholar] [CrossRef]
- Lai, D.; Luo, C.; Wang, Z.; Shi, Z.; Luo, T.; Zhang, L.; Li, X.; Wu, F.; Zhang, Z. Process simulation and techno-economic analysis of CO2 capture by coupling calcium looping with concentrated solar power in coal-fired power plant. Sep. Purif. Technol. 2025, 358, 130228. [Google Scholar] [CrossRef]
- Zha, X.; Zhang, Z.; Yang, L.; Zhao, Z.; Wu, F.; Li, X.; Luo, C.; Zhang, L. Experimental study on the catalytic effect of AAEMs on NO reduction during coal combustion in O2/CO2 atmosphere. Carbon Capture Sci. Technol. 2024, 10, 100159. [Google Scholar] [CrossRef]
- Naidoo, S. Commentary on the contribution of working group III to the sixth assessment report of the intergovernmental panel on climate change. S. Afr. J. Sci. 2022, 118, 9–10. [Google Scholar] [CrossRef]
- Zhao, H.-X.; Li, J.-C.; Wang, Y.; Guo, Y.-R.; Li, S.; Pan, Q.-J. An environment-friendly technique for direct air capture of carbon dioxide via a designed cellulose and calcium system. Sep. Purif. Tech. 2023, 307, 122774. [Google Scholar] [CrossRef]
- Ling, J.L.J.; Yang, W.; Park, H.S.; Lee, H.E.; Lee, S.H. A comparative review on advanced biomass oxygen fuel combustion technologies for carbon capture and storage. Energy 2023, 284, 128566. [Google Scholar] [CrossRef]
- Demirbas, A. Combustion of biomass. Energy Sources Recovery Util. Environ. Eff. 2007, 29, 549–561. [Google Scholar] [CrossRef]
- Jenkins, B.M.; Baxter, L.L.; Miles, T.R., Jr.; Miles, T.R. Combustion properties of biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Zhai, J.; Burke, I.T.; Stewart, D.I. Beneficial management of biomass combustion ashes. Renew. Sustain. Energy Rev. 2021, 151, 111555. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Ling, P.; An, X.; Han, H.; Chen, Y.; Jiang, L.; Wang, Y.; Su, S.; Hu, S.; et al. A study on the release characteristics and formation mechanism of SO2 during co-combustion of sewage sludge and coal slime. Fuel 2023, 333, 126511. [Google Scholar] [CrossRef]
- Sami, M.; Annamalai, K.; Wooldridge, M. Co-firing of coal and biomass fuel blends. Prog. Energy Combust. Sci. 2001, 27, 171–214. [Google Scholar] [CrossRef]
- Sahu, S.G.; Chakraborty, N.; Sarkar, P. Coal-biomass co-combustion: An overview. Renew. Sustain. Energy Rev. 2014, 39, 575–586. [Google Scholar] [CrossRef]
- Verma, M.; Loha, C.; Sinha, A.N.; Chatterjee, P.K. Drying of biomass for utilising in co-firing with coal and its impact on environment—A review. Renew. Sustain. Energy Rev. 2017, 71, 732–741. [Google Scholar] [CrossRef]
- Yang, W.; Pudasainee, D.; Gupta, R.; Li, W.; Wang, B.; Sun, L. An overview of inorganic particulate matter emission from coal/biomass/MSW combustion: Sampling and measurement, formation, distribution, inorganic composition and influencing factors. Fuel Process. Technol. 2021, 213, 106657. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, Y.; Vassilev, S.V.; He, C.; Vassileva, C.G.; Wei, Y. Migration behavior of chlorine and sulfur during gasification and combustion of biomass and coal. Biomass Bioenergy 2024, 182, 107080. [Google Scholar] [CrossRef]
- Liu, L.; Memon, M.Z.; Xie, Y.; Gao, S.; Guo, Y.; Dong, J.; Gao, Y.; Li, A.; Ji, G. Recent advances of research in coal and biomass co-firing for electricity and heat generation. Circ. Econ. 2023, 2, 100063. [Google Scholar] [CrossRef]
- Ramos, A.; Monteiro, E.; Rouboa, A. Biomass pre-treatment techniques for the production of biofuels using thermal conversion methods—A review. Energy Convers. Manag. 2022, 270, 116271. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Hammad, A.; El-Sherif, D.M.; Abouzid, M.; Gaballah, M.S.; Elwakeel, K.Z. Thermochemical conversion strategies of biomass to biofuels, techno-economic and bibliometric analysis: A conceptual review. J. Environ. Chem. Eng. 2021, 9, 106503. [Google Scholar] [CrossRef]
- Zhuikov, A.V.; Mokhirev, A.P.; Tarasov, I.V.; Nazirov, R.A.; Zyryanov, M.A. Combustion Characteristics of Larch Sawmill Wastes and Their Partial Gasification Products. Coke Chem. 2022, 65, 412–417. [Google Scholar] [CrossRef]
- Rauch, R.; Hrbek, J.; Hofbauer, H. Biomass gasification for synthesis gas production and applications of the syngas. WIREs Energy Environ. 2014, 3, 343–362. [Google Scholar] [CrossRef]
- Song, W.; Song, G.; Qi, X.; Lu, Q. Transformation characteristics of sodium in Zhundong coal under circulating fluidized bed gasification. Fuel 2016, 182, 660–667. [Google Scholar] [CrossRef]
- Lackner, M.; Fei, Q.; Guo, S.; Yang, N.; Guan, X.; Hu, P. Biomass Gasification as a Scalable, Green Route to Combined Heat and Power (CHP) and Synthesis Gas for Materials: A Review. Fuels 2024, 5, 625–649. [Google Scholar] [CrossRef]
- Sampaolesi, S.; Briand, L.E.; Saparrat, M.C.N.; Toledo, M.V. Potentials of Biomass Waste Valorization: Case of South America. Sustainability 2023, 15, 8343. [Google Scholar] [CrossRef]
- Konstantinavičienė, J.; Vitunskienė, V. Definition and Classification of Potential of Forest Wood Biomass in Terms of Sustainable Development: A Review. Sustainability 2023, 15, 9311. [Google Scholar] [CrossRef]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Li, X.T.; Grace, J.R.; Lim, C.J.; Watkinson, A.P.; Chen, H.P.; Kim, J.R. Biomass gasification in a circulating fluidized bed. Biomass Bioenergy 2004, 26, 171–193. [Google Scholar] [CrossRef]
- Adeleke, A.A.; Ikubanni, P.P.; Emmanuel, S.S.; Fajobi, M.O.; Nwachukwu, P.; Adesibikan, A.A.; Odusote, J.K.; Adeyemi, E.O.; Abioye, O.M.; Okolie, J.A. A comprehensive review on the similarity and disparity of torrefied biomass and coal properties. Renew. Sustain. Energy Rev. 2024, 199, 114502. [Google Scholar] [CrossRef]
- Roy, R.; Hewetson, B.; Schooff, B.; Bandi, S.; LaTour, P.; Iverson, B.D.; Fry, A. Steam explosion treated biomass as a renewable fuel source: A review from collection to combustion. Fuel 2024, 378, 132883. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, M.; Jiang, E.; Wang, D.; Zhang, K.; Ren, Y.; Jiang, Y. Pyrolysis of Torrefied Biomass. Trends Biotechnol. 2018, 36, 1287–1298. [Google Scholar] [CrossRef]
- Liu, H.-P.; Liang, W.-X.; Qin, H.; Wang, Q. Thermal behavior of co-combustion of oil shale semi-coke with torrefied cornstalk. Appl. Therm. Eng. 2016, 109, 413–422. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, W.; Liang, S.; Pan, X. Study on the co-combustion behavior of semi-coke and typical biomass: Combustion, NO emission and ash characteristics analysis. Fuel 2024, 358, 130068. [Google Scholar] [CrossRef]
- Wang, P.; Wang, C.; Wang, C.; Du, Y.; Che, D. Experimental investigation on co-combustion characteristics of semi-coke and coal: Insight into synergy and blending method. Process Saf. Environ. Prot. 2023, 175, 290–302. [Google Scholar] [CrossRef]
- Ni, Z.; Song, Z.; Bi, H.; Jiang, C.; Sun, H.; Qiu, Z.; He, L.; Lin, Q. The effect of cellulose on the combustion characteristics of coal slime: TG-FTIR, principal component analysis, and 2D-COS. Fuel 2023, 333, 126310. [Google Scholar] [CrossRef]
- ISO 3310-1:2016; Test sieves—Technical Requirements and Testing—Part 1: Test Sieves of Metal Wire Cloth. International Organization for Standardization: Geneva, Switzerland.
- ISO 18134-1:2022; Solid biofuels—Determination of Moisture Content—Part 1: Reference Method. International Organization for Standardization: Geneva, Switzerland.
- ISO 18122:2022; Solid Biofuels—Determination of Ash Content. International Organization for Standardization: Geneva, Switzerland.
- ISO 18123:2023; Solid Biofuels—Determination of Volatile Matter. International Organization for Standardization: Geneva, Switzerland.
- ISO 18125:2017; Solid Biofuels—Determination of Calorific Value. International Organization for Standardization: Geneva, Switzerland.
- ISO 16948:2015; Solid Biofuels—Determination of Total Content of Carbon, Hydrogen and Nitrogen. International Organization for Standardization: Geneva, Switzerland.
- ISO 16994:2016; Solid Biofuels—Determination of Total Content of Sulfur and Chlorine. International Organization for Standardization: Geneva, Switzerland.
- ISO/TS 20048-1:2020; Solid Biofuels—Determination of Off-Gassing and Oxygen Depletion Characteristics—Part 1: Laboratory Method for the Determination of Off-Gassing and Oxygen Depletion Using Closed Containers. International Organization for Standardization: Geneva, Switzerland.
- Zhuikov, A.; Pyanykh, T.; Grishina, I.; Chicherin, S.; Zhuikova, Y. Thermogravimetric Analysis of Combustion of Semi-Coke Obtained from Coniferous Wood and Mixtures on Their Basis. Fire 2024, 7, 385. [Google Scholar] [CrossRef]
- Riaz, S.; Oluwoye, I.; Al-Abdeli, Y.M. Torrefaction of densified biomass using flue gases in a fixed bed combustor. Appl. Therm. Eng. 2023, 233, 121157. [Google Scholar] [CrossRef]
- Zhu, G.; Wen, C.; Liu, T.; Xu, M.; Ling, P.; Wen, W.; Li, R. Combustion and co-combustion of biochar: Combustion performance and pollutant emissions. Appl. Energy 2024, 376, 124292. [Google Scholar] [CrossRef]
- Kumar Mondal, A.; Hinkley, C.; Kondaveeti, S.; Vo, P.H.N.; Ralph, P.; Kuzhiumparambil, U. Influence of pyrolysis time on removal of heavy metals using biochar derived from macroalgal biomass (Oedogonium sp.). Bioresour. Technol. 2024, 414, 131562. [Google Scholar] [CrossRef]
- Li, G.; Wang, Z.; Zuo, L.; Zhang, T.; Xiao, W.; Yang, T.; Tursunov, O.; Zhao, N.; Zhou, Y. Regulating phenol tar in pyrolysis of lignocellulosic biomass: Product characteristics and conversion mechanisms. Bioresour. Technol. 2024, 409, 131259. [Google Scholar] [CrossRef]
- Wang, C.; Wang, F.; Yang, Q.; Liang, R. Thermogravimetric studies of the behavior of wheat straw with added coal during combustion. Biomass Bioenergy 2009, 33, 50–56. [Google Scholar] [CrossRef]
- Raza, M.; Abu-Jdayil, B.; Al-Marzouqi, A.H.; Inayat, A. Inayat Kinetic and thermodynamic analyses of date palm surface fibers pyrolysis using Coats-Redfern method. Renew. Energy 2022, 183, 67–77. [Google Scholar] [CrossRef]
- Zhuikov, A.V.; Glushkov, D.O.; Kuznetsov, P.N.; Grishina, I.I.; Samoilo, A.S. Ignition of two-component and three-component fuel mixtures based on brown coal and char under slow heating conditions. J. Therm. Anal. Calorim. 2022, 147, 11965–11976. [Google Scholar] [CrossRef]
- Isaac, K.; Bada, S.O. The co-combustion performance and reaction kinetics of refuse derived fuels with South African high ash coal. Heliyon 2020, 6, e03309. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Quek, A.; Hoekman, S.K.; Srinivasan, M.P.; Balasubramanian, R. Thermogravimetric investigation of hydrochar-lignite co-combustion. Bioresour. Technol. 2012, 123, 646–652. [Google Scholar] [CrossRef]
- Oladejo, J.M.; Adegbite, S.; Pang, C.H.; Liu, H.; Parvez, A.M.; Wu, T. A novel index for the study of synergistic effects during the co-processing of coal and biomass. Appl. Energy 2017, 188, 215–225. [Google Scholar] [CrossRef]
- Moon, C.; Sung, Y.; Ahn, S.; Kim, T.; Choi, G.; Kim, D. Effect of blending ratio on combustion performance in blends of biomass and coals of different ranks. Exp. Therm. Fluid Sci. 2013, 47, 232–240. [Google Scholar] [CrossRef]
- Zhao, R.; Qin, J.; Chen, T.; Wu, J. TG-FTIR study on co-combustion of bituminous coal semicoke and lignite. J. Therm. Anal. Calorim. 2022, 147, 1849–1858. [Google Scholar] [CrossRef]
- Yuan, Y.; Zuo, H.; Wang, J.; Gao, Y.; Xue, Q.; Wang, J. Co-combustion behavior, kinetic and ash melting characteristics analysis of clean coal and biomass pellet. Fuel 2022, 324, 124727. [Google Scholar] [CrossRef]
- Armakan, S.; Civan, M.; Yurdakul, S. Determining co-combustion characteristics, kinetics and synergy behaviors of raw and torrefied forms of two distinct types of biomass and their blends with lignite. J. Therm. Anal. Calorim. 2022, 147, 12855–12869. [Google Scholar] [CrossRef]
| Fuels | MC | Ad | VCdaf | Cdaf | Hdaf | Ndaf | Sdaf | Odaf | LHV |
|---|---|---|---|---|---|---|---|---|---|
| % | MJ/kg | ||||||||
| No. 1 | 11.6 | 9.2 | 47.3 | 74.8 | 5.1 | 1.0 | 0.3 | 21.1 | 16.1 |
| No. 2 | 1.7 | 4.1 | 4.7 | 97.1 | 1.8 | 0.2 | 0.1 | 0.9 | 32.6 |
| No. 3 | 4.7 | 3.7 | 3.4 | 98.3 | 1.1 | 0.4 | 0.1 | 0.1 | 31.6 |
| No. 4 | 1.8 | 10.8 | 7.5 | 96.1 | 1.6 | 1.1 | 0.5 | 0.6 | 30.1 |
| No. 5 | 6.7 | 6.7 | 26.0 | 86.0 | 3.5 | 0.6 | 0.2 | 11.0 | 24.4 |
| No. 6 | 8.2 | 6.5 | 25.4 | 86.6 | 3.1 | 0.8 | 0.2 | 10.6 | 23.9 |
| Parameters | Fuels | |||||
|---|---|---|---|---|---|---|
| No. 1 | No. 2 | No. 3 | No. 4 | No. 5 | No. 6 | |
| Ti, °C | 355 | 468 | 464 | 435 | 375 | 359 |
| Deriv. Weightmax, %/min | 26.0 | 20.8 | 23.3 | 20.5 | 14.6 | 14.9 |
| TDeriv. Weight, °C | 408 | 528 | 509 | 485 | 514 | 412 |
| DSCmax, W/g | 6.5 | 5.1 | 5.2 | 5.7 | 3.3 | 3.6 |
| TDSC, °C | 416 | 523 | 505 | 482 | 515 | 421 |
| Tb, °C | 539 | 545 | 552 | 574 | 556 | 570 |
| S, min–2 °C−3 | 1.67 | 1.23 | 1.63 | 1.43 | 0.69 | 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuikov, A.; Pyanykh, T.; Grishina, I.; Chicherin, S.; Zhuikova, Y. Research on Co-Combustion of High-Calorific Biomass Obtained Using Gasification and Lignite for Sustainable Utilisation of Resources. Sustainability 2025, 17, 2845. https://doi.org/10.3390/su17072845
Zhuikov A, Pyanykh T, Grishina I, Chicherin S, Zhuikova Y. Research on Co-Combustion of High-Calorific Biomass Obtained Using Gasification and Lignite for Sustainable Utilisation of Resources. Sustainability. 2025; 17(7):2845. https://doi.org/10.3390/su17072845
Chicago/Turabian StyleZhuikov, Andrey, Tatyana Pyanykh, Irina Grishina, Stanislav Chicherin, and Yana Zhuikova. 2025. "Research on Co-Combustion of High-Calorific Biomass Obtained Using Gasification and Lignite for Sustainable Utilisation of Resources" Sustainability 17, no. 7: 2845. https://doi.org/10.3390/su17072845
APA StyleZhuikov, A., Pyanykh, T., Grishina, I., Chicherin, S., & Zhuikova, Y. (2025). Research on Co-Combustion of High-Calorific Biomass Obtained Using Gasification and Lignite for Sustainable Utilisation of Resources. Sustainability, 17(7), 2845. https://doi.org/10.3390/su17072845






