Improving Shrimp Preservation Quality Through Edible Coatings Based on Starch Modified with Aqueous Plant Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Rooibos Extract
2.3. Preparation of Garlic Extract
2.4. Starch Dispersions and Films Obtaining
2.5. Methods
2.5.1. Fourier Transform Infrared Spectroscopy (ATR/FTIR)
2.5.2. Scanning Electron Microscope (SEM)
2.5.3. Differential Scanning Calorimetry (DSC)
2.5.4. Contact Angle (CA)
2.5.5. Water Vapor Transmission Rate (WVTR)
- WVTR—water vapor transmission rate (mg cm−2 h−1),
- W1—mass of water remaining in the permeation cell after the first hour (mg),
- W2—mass of water remaining after the second hour (mg),
- t—duration of the measurement interval (1 h),
- A—surface of evaporation (19.625 cm2).
2.5.6. Mechanical Properties
2.5.7. Sample Preparation for Microbiological Analysis
2.5.8. Microbiological Testing
- Nc—the bacterial count in the uncoated control sample,
- Nt—the bacterial count in the coated sample.
2.5.9. Statistical Analysis
3. Results and Discussion
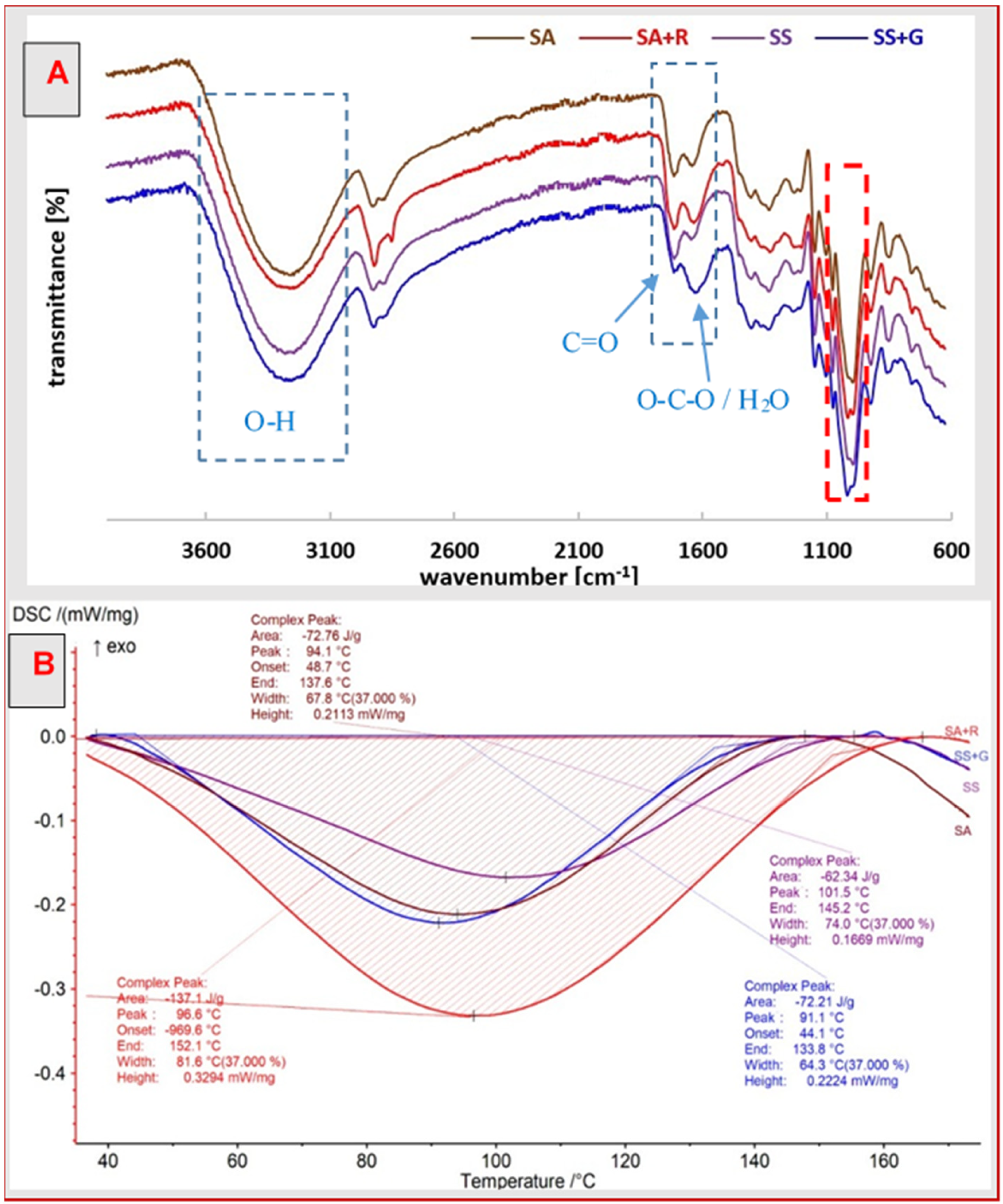
3.1. Chemical Structure
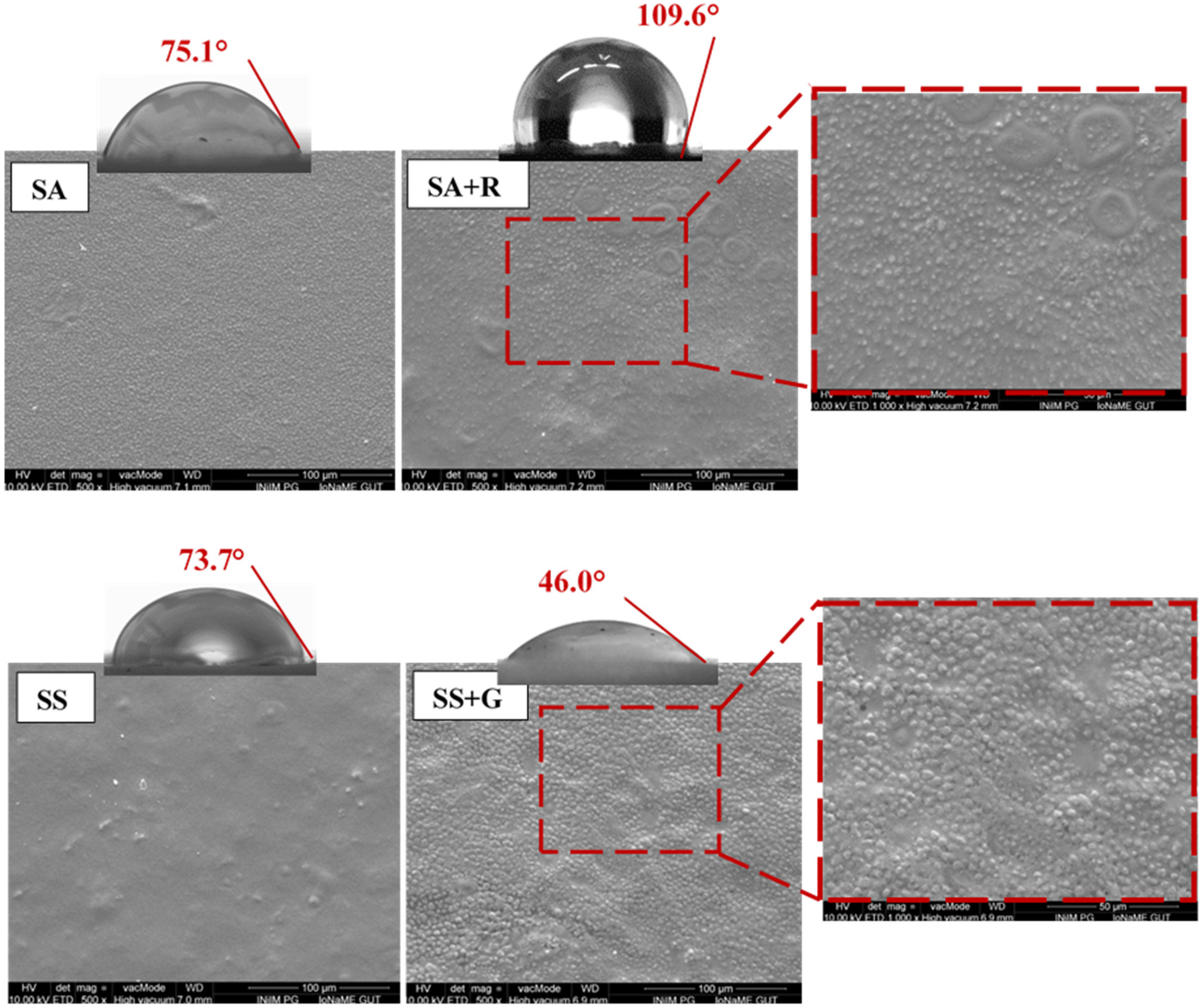
3.2. Surface Structure and Hydrophilicity
3.3. Mechanical Properties
3.4. Thermal Properties
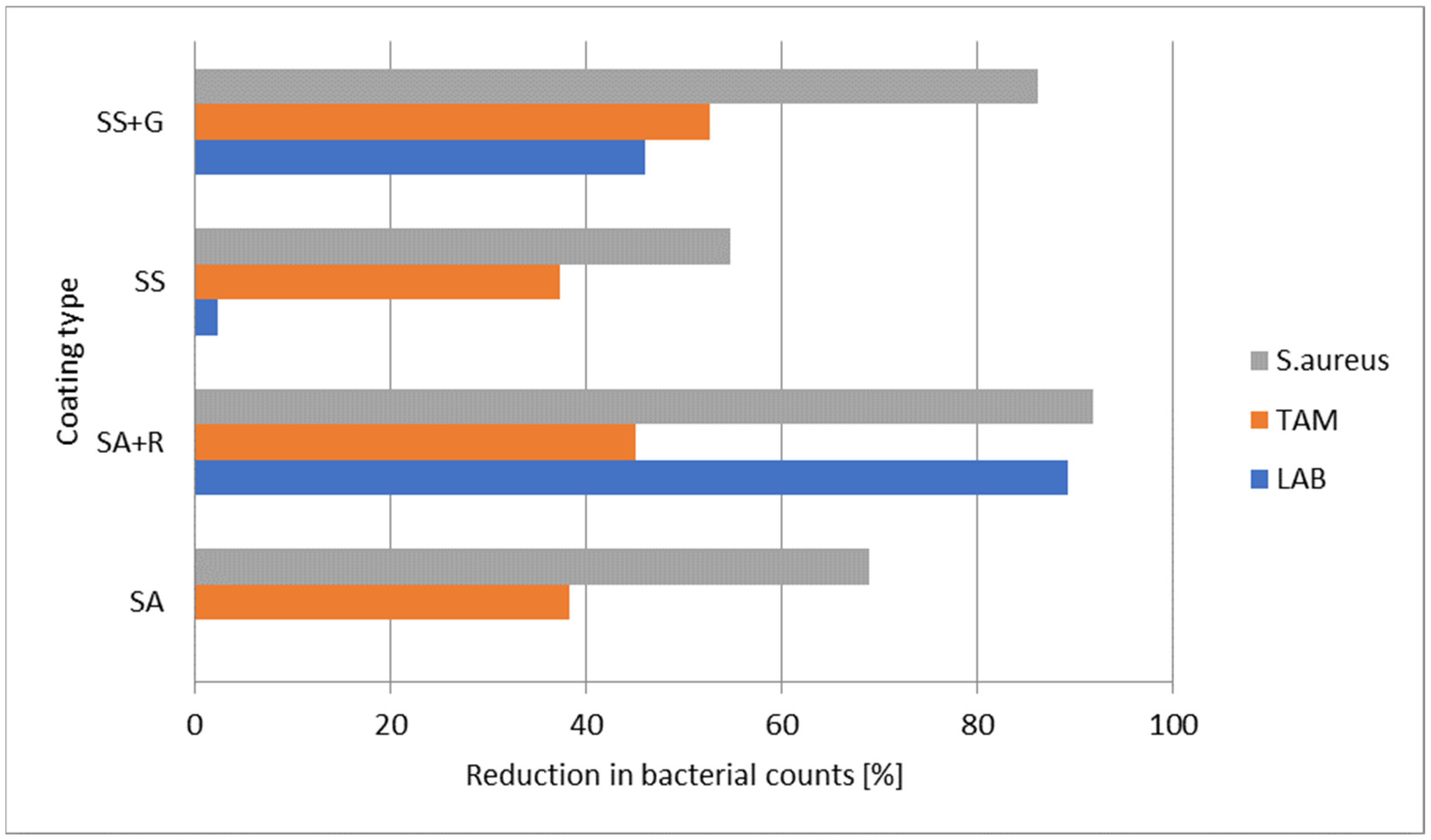
3.5. Microbiological Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATR-FTIR | Fourier transform infrared spectroscopy recorded with an attenuated total reflection mode |
| DSC | Differential Scanning Calorimetry |
| SEM | Scanning Electron Microscope |
| CA | Contact Angle |
| WVTR | Water Vapor Transmission Rate |
| SA | Starch Arrowroot |
| SS | Starch Sago |
| R | Rooibos |
| G | Garlic |
| CS | Cooked (blanched) Shrimp |
| RS | Raw (defrosted) Shrimp |
| U | Untreated Shrimp |
| TAM | Total Aerobic Mesophilic |
| LAB | Lactic Acid Bacteria |
References
- Ćurčić, S.; Milunović, S.; Savović, I.; Đurić, M. Logistics Information Support for Environmental Management for Organizations in the Food Chain. Int. J. Qual. Res. 2008, 2, 165–170. [Google Scholar]
- Yu, Q.; Liu, J.; Yang, J.; Lou, Y.; Li, Y.; Zhang, M. Postharvest Preservation Technologies for Marine-Capture Shrimp: A Review. Food Bioprocess Technol. 2023, 16, 2343–2358. [Google Scholar] [CrossRef]
- Aggarwal, S.; Kathuria, D.; Singh, N. Edible Films and Coatings, Its Chemical Crosslinking, Starch-Protein Interaction and Application in Food System: A Systematic Review. Int. J. Biol. Macromol. 2025, 306, 141726. [Google Scholar] [CrossRef]
- Djordjevic, M.Z.; Puskaric, H. Management of Process Safety in Food Chain. Int. J. Qual. Res. 2013, 7, 141–152. [Google Scholar]
- Valdés, A.; Ramos, M.; Beltrán, A.; Jiménez, A.; Garrigós, M. State of the Art of Antimicrobial Edible Coatings for Food Packaging Applications. Coatings 2017, 7, 56. [Google Scholar] [CrossRef]
- Kurek, M.; Pišonić, P.; Ščetar, M.; Janči, T.; Čanak, I.; Vidaček, S.F.; Benbettaieb, N.; Debeaufort, F.; Galič, K. Edible Coatings for Fish Preservation: Literature Data on Storage Temperature, Product Requirements, Antioxidant Activity, and Coating Performance—A Review. Antioxidants 2024, 13, 1417. [Google Scholar] [CrossRef] [PubMed]
- Liyanapathiranage, A.; Dassanayake, R.; Gamage, A.; Karri, R.; Manamperi, A.; Evon, P.; Merah, O. Recent Developments in Edible Films and Coatings for Fruits and Vegetables. Coatings 2023, 13, 1177. [Google Scholar] [CrossRef]
- Song, D.-H.; Hoa, V.; Kim, H.; Khang, S.; Cho, S.-H.; Ham, J.-S.; Seol, K.-H. Edible Films on Meat and Meat Products. Coatings 2021, 11, 1344. [Google Scholar] [CrossRef]
- Xie, F.; Mingzhu, M.L.; Feng, X.; He, Z.; Chen, Q.; Zhou, J. Tannic acid one-step induced quaternized chitin-based edible and easy-cleaning coatings with multifunctional preservation for perishable products. Food Hydrocoll. 2025, 159, 110636. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, C.; Liu, S.; Gao, J.; Cui, S.W.; Xia, W. Coating White Shrimp (Litopenaeus vannamei) with Edible Fully Deacetylated Chitosan Incorporated with Clove Essential Oil and Kojic Acid Improves Preservation during Cold Storage. Int. J. Biol. Macromol. 2020, 162, 1276–1282. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, L.; Li, B. Chitosan-Based Edible Coatings for Food Preservation: A Review. Carbohydr. Polym. 2014, 103, 345–356. [Google Scholar] [CrossRef]
- Wang, J.; Qin, M.; Wang, W.; Xia, Y.; Wu, G.; Deng, H.; Lin, Q. Konjac Glucomannan Modification for Sustainable Functional Materials. Food Hydrocoll. 2025, 165, 111340. [Google Scholar] [CrossRef]
- Kim, J.-H.; Hong, W.-S.; Oh, S.-W. Effect of Layer-by-Layer Antimicrobial Edible Coating of Alginate and Chitosan with Grapefruit Seed Extract for Shelf-Life Extension of Shrimp (Litopenaeus vannamei) Stored at 4 °C. Int. J. Biol. Macromol. 2018, 120, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Lee, S.-Y.; Oh, S.-W. Enhancing Safety and Quality of Shrimp by Nanoparticles of Sodium Alginate-Based Edible Coating Containing Grapefruit Seed Extract. Int. J. Biol. Macromol. 2021, 189, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Marta, H.; Chandra, S.; Cahyana, Y.; Sukri, N.; Pangawikan, A.D.; Yuliana, T.; Arifin, H.R. Arrowroot (Maranta arundinaceae L.) Starch-Based Edible Coating Formulation and Its Application to Shelf-Life Extension of Tomato (Solanum lycopersicum L.). Carbohydr. Polym. Technol. Appl. 2025, 9, 100674. [Google Scholar] [CrossRef]
- Devi, L.S.; Jaiswal, A.K.; Jaiswal, S. Lipid Incorporated Biopolymer-Based Edible Films and Coatings in Food Packaging: A Review. Curr. Res. Food Sci. 2024, 8, 100720. [Google Scholar] [CrossRef]
- Balti, R.; Mansour, M.B.; Zayoud, N.; Le Balc’h, R.; Brodu, N.; Arhaliass, A.; Massé, A. Active Exopolysaccharides-Based Edible Coatings Enriched with Red Seaweed (Gracilaria gracilis) Extract to Improve Shrimp Preservation during Refrigerated Storage. Food Biosci. 2020, 34, 100522. [Google Scholar] [CrossRef]
- Khazaei, N.; Esmaiili, M.; Emam-Djomeh, Z. Effect of Active Edible Coatings Made by Basil Seed Gum and Thymol on Oil Uptake and Oxidation in Shrimp during Deep-Fat Frying. Carbohydr. Polym. 2016, 137, 249–254. [Google Scholar] [CrossRef]
- Rossi-Márquez, G.; Dávalos-Saucedo, C.; Di Pierro, P. Edible Films and Coatings Applied in the Food Industry. Coatings 2023, 13, 670. [Google Scholar] [CrossRef]
- Agarwal, A.; Mirza, S.; Nimbalkar, T. Report: Sustainable Manufacturing Market—Global Forecast to 2029; MarketsandMarkets™: Delray Beach, FL, USA; London, UK, 2024. [Google Scholar]
- Abidin, M.Z.; Kourmentza, K.; Niranjan, K. Chitin Oligosaccharide N,N′-Diacetylchitobiose (GlcNAc2) as Antimicrobial Coating against Listeria monocytogenes on Ready-to-Eat Shrimp. Sustainability 2023, 15, 10099. [Google Scholar] [CrossRef]
- Afifi, M.R.; Ariaii, P.; Soltani, M.S.; Jafarian, S. The Effect of Nanocomposite Coating (Pullulan–Nano Clay) Activated with Nanoliposomes Containing the Watercress Essential Oil on the Quality of Pacific White Shrimp during Refrigerated Storage. J. Food Meas. Charact. 2023, 17, 2651–2662. [Google Scholar] [CrossRef]
- Rezaei, F.; Hosseinzadeh, S.; Basiri, S.; Golmakani, M.-T.; Gholamhosseini, A.; Shekarforoush, S.S. The Effects of Shirazi thyme (Zataria multiflora) Oil Nanoemulsion on the Quality of Shrimp (Litopenaeus vannamei) during Refrigerated Storage. J. Food Sci. Technol. 2023, 60, 710–719. [Google Scholar] [CrossRef]
- Hübsch, Z.; Van Vuuren, S.F.; Van Zyl, R.L. Can Rooibos (Aspalathus linearis) Tea Have an Effect on Conventional Antimicrobial Therapies? S. Afr. J. Bot. 2014, 93, 148–156. [Google Scholar] [CrossRef]
- Vhangani, L.N.; Favre, L.C.; Rolandelli, G.; Van Wyk, J.; del Pilar Buera, M. Optimising the Polyphenolic Content and Antioxidant Activity of Green Rooibos (Aspalathus linearis) Using Beta-Cyclodextrin Assisted Extraction. Molecules 2022, 27, 3556. [Google Scholar] [CrossRef]
- Santos, J.S.; Deolindo, C.T.P.; Esmerino, L.A.; Genovese, M.I.; Fujita, A.; Marques, M.B.; Rosso, N.D.; Daguer, H. Effects of Time and Extraction Temperature on Phenolic Composition and Functional Properties of Red Rooibos (Aspalathus linearis). Food Res. Int. 2016, 89, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Gutiérrez, R.; Lago-Lestón, A.; Vargas-Albores, F.; Cicala, F.; Martínez-Porchas, M. Exploring the Garlic (Allium sativum) Properties for Fish Aquaculture. Fish Physiol. Biochem. 2021, 47, 1179–1198. [Google Scholar] [CrossRef]
- Magryś, A.; Olender, A.; Tchórzewska, D. Antibacterial Properties of Allium sativum L. against the Most Emerging Multidrug-Resistant Bacteria and Its Synergy with Antibiotics. Arch. Microbiol. 2021, 203, 2257–2268. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, M.; Kunimura, K.; Suzuki, J.-I.; Kodera, Y. Antimicrobial Properties of Hydrophobic Compounds in Garlic: Allicin, Vinyldithiin, Ajoene and Diallyl Polysulfides (Review). Exp. Ther. Med. 2020, 19, 1550–1553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xiao, D.; Zhu, C.; Younis, K.; Yousuf, O. Shelf-Life Extension and Quality Changes of Fresh-Cut Apple via Sago and Soy-Oil-Based Edible Coatings. Coatings 2024, 14, 1202. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A.; Ilyas, R.A.; Zainudin, E.S. Thermal, Flammability, and Antimicrobial Properties of Arrowroot (Maranta arundinacea) Fiber Reinforced Arrowroot Starch Biopolymer Composites for Food Packaging Applications. Int. J. Biol. Macromol. 2022, 213, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Paramitasari, D.; Musa, M.; Putra, O.N.; Suparman, S.; Pramana, Y.S.; Elisa, S.; Hidayat, T.; Tjahjono, A.E.; Meidiawati, D.P.; Pudjianto, K.; et al. Hydroxypropylation for Functional Enhancement of Sago Starch: The Effects of Low Propylene Oxide Concentration Using Response Surface Methodology. J. Agric. Food Res. 2024, 15, 100933. [Google Scholar] [CrossRef]
- Surendran, N.; Raju, M.V.; Chandrasekaran, M.K.; Ahalliya, R.M.; Palanisamy, C.P.; Chandrasekaran, G.; Kanniappan, G.V. Physicochemical Characterization of Starch from Maranta arundinacea L. (Arrowroot) Rhizomes and Its Inhibition of COX-2: In Vivo Validation. Bioact. Carbohydr. Diet. Fibre 2025, 33, 100465. [Google Scholar] [CrossRef]
- Duay, B.S.C.; De Leon, M.S.; Santos, A.C. Proximate Analysis of Maranta arundinacea L. Flour. Int. J. Multidiscip. Res. Dev. 2023, 2. [Google Scholar] [CrossRef]
- Taharuddin, N.H.; Jumaidin, R.; Ilyas, R.A.; Kamaruddin, Z.H.; Mansor, M.R.; Md Yusof, F.A.; Knight, V.F.; Norrrahim, M.N.F. Effect of Agar on the Mechanical, Thermal, and Moisture Absorption Properties of Thermoplastic Sago Starch Composites. Materials 2022, 15, 8954. [Google Scholar] [CrossRef]
- Nunes, N.B.; Reis, J.O.; Castro, V.S.; Machado, M.A.M.; Cunha-Neto, A.; Figueiredo, E.E. Optimizing the Antimicrobial Activity of Sodium Hypochlorite (NaClO) over Exposure Time for the Control of Salmonella spp. In Vitro. Antibiotics 2024, 13, 68. [Google Scholar] [CrossRef]
- Putri, T.R.; Adhitasari, A.; Paramita, V.; Yulianto, M.E.; Ariyanto, H.D. Effect of Different Starch on the Characteristics of Edible Film as Functional Packaging in Fresh Meat or Meat Products: A Review. Mater. Today Proc. 2023, 87, 192–199. [Google Scholar] [CrossRef]
- Morawska, M.; Kukułowicz, A.; Brzeska, J. Green Chemistry in Medical Applications: Preliminary Assessment of Kuzu Starch Films with Plant-Based Antiseptics. Sustainability 2023, 15, 16541. [Google Scholar] [CrossRef]
- PN-EN ISO 4833-1:2013/A1:2016; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. Polish Committee for Standardization (PKN): Warsaw, Poland, 2016.
- PN-ISO 15214:2002; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Mesophilic Lactic acid Bacteria—Colony-Count Technique at 30 °C. Polish Committee for Standardization (PKN): Warsaw, Poland, 2002.
- PN-EN ISO 6888-2:2001/A1:2004; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species)—Part 2: Method Using Rabbit Plasma Fibrinogen Agar. Polish Committee for Standardization (PKN): Warsaw, Poland, 2004.
- PN-ISO 16649-2:2004; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of β-Glucuronidase-Positive Escherichia coli—Part 2: Colony-count technique at 44 °C Using 5-bromo-4-chloro-3-indolyl β-D-glucuronide. Polish Committee for Standardization (PKN): Warsaw, Poland, 2004.
- Abdillah, A.A.; Lee, R.-C.; Charles, A.L. Improving Physicomechanical Properties of Arrowroot Starch Films Incorporated with Kappa-Carrageenan: Sweet Cherry Coating Application. Int. J. Biol. Macromol. 2024, 277, 133938. [Google Scholar] [CrossRef] [PubMed]
- Hamed, I.; Jakobsen, A.N.; Lerfall, J. Sustainable Edible Packaging Systems Based on Active Compounds from Food Processing Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 198–226. [Google Scholar] [CrossRef]
- Sondari, D.; Restu, W.K.; Septevani, A.A.; Suryaningrum, R.; Burhani, D.; Widyaningrum, B.A.; Putri, R. Effect of Catalyst and Cross-Linker Concentrations on the Functional and Chemical Properties of Sago Starch. Starch-Stärke 2022, 74, 2000266. [Google Scholar] [CrossRef]
- Shivaraju, V.K.; Appukuttan, S.V.; Kumar, S. The Influence of Bound Water on the FTIR Characteristics of Starch and Starch Nanocrystals Obtained from Selected Natural Sources. Starch-Stärke 2019, 71, 1700026. [Google Scholar] [CrossRef]
- Yin, Y.; Zhuang, Y.; Sun, L.; Gu, Y.; Zhang, G.; Fan, X.; Ding, Y. How does high hydrostatic pressure treatment improve the esterification of quinoa (Chenopodium quinoa Willd.) starch? Food Chem. 2025, 463, 141166. [Google Scholar] [CrossRef]
- Travalini, A.P.; Lamsal, B.; Magalhães, W.L.E.; Demiate, I.M. Cassava Starch Films Reinforced with Lignocellulose Nanofibers from Cassava Bagasse. Int. J. Biol. Macromol. 2019, 139, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Xu, R.; McClements, D.J.; Liu, T.; Li, Q.; Su, G.; Zhao, M.; Zhao, Q. Effect of Preheating-Induced Structural Changes of Mung Bean Starch and Protein on the Phase Behavior, Physicochemical Properties, and Digestibility of Composite Hydrogels. Food Hydrocoll. 2025, 166, 111346. [Google Scholar] [CrossRef]
- Sun, C.; Du, K.; He, Z.; Zhu, Z.; Hu, Y.; Wang, C.; Mei, L.; Xie, Q.; Chen, Y.; Liu, Y.; et al. Liquid Nitrogen Ball-Milled Mechanochemical Modification of Starches with Typically Selected A, B and C Crystal Types on Multiscale Structure and Physicochemical Properties. Food Chem. 2025, 463, 141148. [Google Scholar] [CrossRef] [PubMed]
- Wan Zullkiplee, W.S.H.; Khairuddin, N.; Ramaiya, S.D.; Sarbini, S.R.; Ngaini, Z. Characterization and pH Response of Passiflora suberosa Extract as a Novel Biosensor Intended for Smart Food Packaging Film. J. Food Process Eng. 2025, 48, e70077. [Google Scholar] [CrossRef]
- Hornung, P.S.; Ávila, S.; Apea-Bah, F.B.; Liu, J.; Teixeira, G.L.; Ribani, R.H.; Beta, T. Sustainable Use of Ilex paraguariensis Waste in Improving Biodegradable Corn Starch Films’ Mechanical, Thermal and Bioactive Properties. J. Polym. Environ. 2020, 28, 1696–1709. [Google Scholar] [CrossRef]
- Brzeska, J.; Jasik, G.; Sikorska, W.; Mendrek, B.; Karczewski, K.; Kowalczuk, M.; Rutkowska, M. Susceptibility to Degradation in Soil of Branched Polyesterurethane Blends with Polylactide and Starch. Polymers 2022, 14, 2086. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Fakhouri, F.M.; de Oliveira, R.A. Extraction and Characterization of Arrowroot (Maranta arundinacea L.) Starch and Its Application in Edible Films. Carbohydr. Polym. 2018, 186, 64–72. [Google Scholar] [CrossRef]
- Savita, K.; Kaur, J.; Kaur, N.; Arya, A.; Siwach, K.; Dhiman, R.; Sharma, P.K.; Sharma, A. Impact of Functionalised Silicon Carbide NPs on the Mechanical and Dielectric Performance of Sago Starch-Based Nanocomposites. Mater. Chem. Phys. 2025, 332, 130264. [Google Scholar] [CrossRef]
- Tananuwong, K.; Reid, D.S. DSC and NMR Relaxation Studies of Starch–Water Interactions during Gelatinization. Carbohydr. Polym. 2004, 58, 345–358. [Google Scholar] [CrossRef]
- Mano, J.F.; Koniarova, D.; Reis, R.L. Thermal Properties of Thermoplastic Starch/Synthetic Polymer Blends with Potential Biomedical Applicability. J. Mater. Sci. Mater. Med. 2003, 14, 127–135. [Google Scholar] [CrossRef]
- Iaccheri, E.; Siracusa, V.; Ragni, L.; De Aguiar Saldanha Pinheiro, A.C.; Romani, S.; Rocculi, P.; Rosa, M.D.; do Amaral Sobral, P.J. Studying Physical State of Films Based on Cassava Starch and/or Chitosan by Dielectric and Thermal Properties and Effects of Pitanga Leaf Hydroethanolic Extract. J. Food Eng. 2023, 339, 111280. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Meneghetti, B.B.; Tagliari, I.H.B.S.; Soares, C.T.; Bevilaqua, G.; Fakhouri, F.M.; de Oliveira, R.A. Multipurpose Arrowroot Starch Films with Anthocyanin-Rich Grape Pomace Extract: Color Migration for Food Simulants and Monitoring the Freshness of Fish Meat. Int. J. Biol. Macromol. 2024, 265, 130934. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.A.V.T.; Garcia, C.F.; Gomes, F.A.A. Pharmaceutical and Biomedical Applications of Native and Modified Starch: A Review. Starch-Stärke 2020, 72, 1900270. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible Cassava Starch Films Carrying Rosemary Antioxidant Extracts for Potential Use as Active Food Packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Endurocide. A Quick Guide to Log Reductions. Available online: https://endurocide.com/a-quick-guide-to-log-reductions/ (accessed on 13 November 2025).
- Guarechahi, M.; Moosavi, H.; Forghani, M. Effect of Surface Roughness and Materials Composition. J. Biomed. Nanotechnol. 2012, 3, 541. [Google Scholar] [CrossRef]
- Bohinc, K.; Dražić, G.; Fink, R.; Oder, M.; Jevšnik, M.; Nipič, D.; Godič-Torkar, K.; Raspor, P. Available Surface Dictates Microbial Adhesion Capacity. Int. J. Adhes. Adhes. 2014, 50, 265–272. [Google Scholar] [CrossRef]
- Syamaladevi, R.M.; Tang, J.; Zhong, Q.P. Water Diffusion from a Bacterial Cell in Low-Moisture Foods. J. Food Sci. 2016, 81, 2129–2134. [Google Scholar] [CrossRef]


| Sample | Water (mL) | Extract Kind/Volume (mL) | Starch Kind/Mass (g) | Glycerol (g) | Citric Acid (g) |
|---|---|---|---|---|---|
| SA | 92.5 | - | Arrowroot/5 | 2 | 0.5 |
| SA+R | - | Rooibos/92.5 | Arrowroot/5 | 2 | 0.5 |
| SS | 92.5 | - | Sago/5 | 2 | 0.5 |
| SS+G | 67.5 | Garlic/25 | Sago/5 | 2 | 0.5 |
| Sample | Contact Angle (°) ± SD | Water Vapor Transmission Rate (mg cm−2 h−1) | ||
|---|---|---|---|---|
| 0 min. | 1 min. | 3 min. | ||
| SA | 75.1 ± 4.4 a | 51.1 ± 1.9 a | 36.5 ± 3.1 a | 18.3 |
| SA+R | 109.6 ± 4.3 b | 102.4 ± 6.9 b | 93.8 ± 7.5 b | 25.7 |
| SS | 73.7 ± 12.5 a | 61.3 ± 11.1 a | 46.9 ± 12.4 a | 26.6 |
| SS+G | 46.0 ± 0.6 c | 29.5 ± 0.4 c | 23.3 ± 1.2 a | 40.1 |
| Sample | Thickness (mm) | Tensile Strength ± SD (MPa) | Elongation at Break ± SD (%) |
|---|---|---|---|
| SA | 0.20 ± 0.02 a | 4.0 ± 0.9 a | 45.0 ± 6.9 a |
| SA+R | 0.24 ± 0.03 b | 1.5 ± 0.2 b | 67.4 ± 16.0 b |
| SS | 0.19 ± 0.03 a | 2.0 ± 0.4 c | 56.0 ± 9.5 a |
| SS+G | 0.32 ± 0.04 c | 0.5 ± 0.0 b | 79.8 ± 15.2 b |
| Sample | Tonset (°C) | Tend (°C) | Tpeak (°C) | ΔH (J/g) |
|---|---|---|---|---|
| SA | 48.7 | 137.6 | 94.1 | 72.8 |
| SA+R | 38.5 | 152.1 | 96.6 | 137.1 |
| SS | 38.1 | 145.2 | 101.5 | 62.3 |
| SS+G | 44.1 | 133.8 | 91.1 | 72.2 |
| Factor | Comparison | LAB (M ± SD) | TAM (M ± SD) | S. aureus (M ± SD) | t | p-Value | Significant Difference |
|---|---|---|---|---|---|---|---|
| Shrimp type | Raw (RS)/Cooked (CS) | 3.39 ± 0.47/1.98 ± 0.35 | 6.97 ± 1.18/6.63 ± 1.15 | 2.64 ± 0.37/2.37 ± 0.41 | 13.49/1.15/2.77 | <0.001/0.255/0.007 | LAB, S. aureus |
| Coating type | U/SA | 2.89 ± 0.80/2.65 ± 0.73 | 6.72 ± 1.23/6.82 ± 1.17 | 2.67 ± 0.43/2.60 ± 0.30 | 0.82/−0.21/0.45 | 0.417/0.832/0.656 | ns |
| U/SA+R | 2.89 ± 0.80/2.63 ± 0.78 | 6.72 ± 1.23/6.70 ± 1.21 | 2.67 ± 0.43/2.19 ± 0.28 | 0.85/0.04/3.31 | 0.403/0.967/0.003 | S. aureus | |
| U/SS | 2.89 ± 0.80/2.64 ± 0.87 | 6.72 ± 1.23/6.96 ± 1.19 | 2.67 ± 0.43/2.59 ± 0.34 | 0.79/−0.52/0.49 | 0.439/0.607/0.624 | ns | |
| U/SS+G | 2.89 ± 0.80/2.55 ± 0.99 | 6.72 ± 1.23/6.84 ± 1.20 | 2.67 ± 0.43/2.41 ± 0.50 | 1.00/−0.26/1.47 | 0.328/0.793/0.154 | ns | |
| Storage time (days) | 0/2 | 2.78 ± 1.05/2.63 ± 0.89 | 5.80 ± 0.68/6.15 ± 0.92 | 2.29 ± 0.19/2.39 ± 0.26 | 0.30/−0.70/−0.68 | 0.767/0.489/0.506 | ns |
| 0/4 | 2.78 ± 1.05/2.56 ± 0.84 | 5.80 ± 0.68/6.60 ± 1.14 | 2.29 ± 0.19/2.69 ± 0.43 | 0.46/−1.33/−1.75 | 0.652/0.197/0.094 | ns | |
| 0/7 | 2.78 ± 1.05/2.85 ± 0.71 | 5.80 ± 0.68/7.86 ± 0.66 | 2.29 ± 0.19/2.48 ± 0.49 | −0.15/−5.62/−0.76 | 0.878/<0.001/0.458 | TAM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morawska, M.; Brzeska, J.; Kukułowicz, A.; Karczewski, J.; Prześniak-Welenc, M. Improving Shrimp Preservation Quality Through Edible Coatings Based on Starch Modified with Aqueous Plant Extracts. Sustainability 2025, 17, 10592. https://doi.org/10.3390/su172310592
Morawska M, Brzeska J, Kukułowicz A, Karczewski J, Prześniak-Welenc M. Improving Shrimp Preservation Quality Through Edible Coatings Based on Starch Modified with Aqueous Plant Extracts. Sustainability. 2025; 17(23):10592. https://doi.org/10.3390/su172310592
Chicago/Turabian StyleMorawska, Magda, Joanna Brzeska, Anita Kukułowicz, Jakub Karczewski, and Marta Prześniak-Welenc. 2025. "Improving Shrimp Preservation Quality Through Edible Coatings Based on Starch Modified with Aqueous Plant Extracts" Sustainability 17, no. 23: 10592. https://doi.org/10.3390/su172310592
APA StyleMorawska, M., Brzeska, J., Kukułowicz, A., Karczewski, J., & Prześniak-Welenc, M. (2025). Improving Shrimp Preservation Quality Through Edible Coatings Based on Starch Modified with Aqueous Plant Extracts. Sustainability, 17(23), 10592. https://doi.org/10.3390/su172310592







