Abstract
The Mediterranean Germplasm Gene Bank (MGG) of the CNR-IBBR in Bari (Italy) is the oldest gene bank of the Mediterranean area. Thanks to Vavilov, this area is considered an important gene centre. The first safeguarding activities of the MGG began in 1969 and continue today following traditional and innovative approaches. The strategy followed by the MGG for safeguarding plant genetic resources of Mediterranean origin and of agricultural interest is described in detail together with the activities and methods used. Some examples of rare agrobiodiversity discovered in the area are reported and described. The MGG seed collection (as ex situ conservation) contains about 59,000 accessions from 34 families, 208 genera and 872 species. Over 13,000 samples have been directly collected over time by exploration teams, while others have been acquired from 314 donor institutions through a seed exchange. MGG studies in the Mediterranean region show a severe genetic erosion of about 75%. The approach adopted by the CNR-IBBR research group to combat this phenomenon can be broken down into two main areas. Firstly, new collecting missions could secure still available valuable material as old landraces cultivated in the fields and gardens of less anthropized areas; the considerable experience and knowledge acquired over the span of five decades, accumulated through this endeavour, undoubtedly plays a pivotal role. Moreover, the integration of conservation methods, ex situ and on farm, for cultivated material, and predominantly in situ for wild species, is necessary for the sustainable development and use of Mediterranean plant genetics resources. In pursuit of this objective, the international standing of the MGG and its extensive network of collaborations represent a foundational element.
1. Introduction
Geographically speaking, the Mediterranean basin includes the land surrounding a 2.51 million km2 body of water: the southern belt of Europe, Anatolia and the coastal region of the Middle East, and the northern borders of the African continent. It is no coincidence, therefore, that the Latin word ‘mediterraneus’ literally means ‘in the middle of the land’, since it is an inland sea that currently boarders 23 different nations in which some 450 million people live. The Mediterranean basin also has its own precise definition in a biogeographical sense, as the presence of the sea is fundamental in defining the climate of all coastal areas and part of the inland areas of the countries bordering the Mediterranean Sea.
The Mediterranean climate is characterised by long dry summers (3–5 months) and mild winters, with temperatures never falling below 0 °C, and more or less abundant rainfall (400–900 mm per year), concentrated in the cool season. The combination of dry summers and rainy winters is a typical feature of the Mediterranean climate: in fact, in almost all climates (with the exception of maritime climates, where rainfall is constant, and desert climates, where it hardly ever rains), most of the rain falls during the hot season. Such climatic conditions favour the life of evergreen shrubs and trees.
The Mediterranean is home to remarkable biodiversity, including 22,500 endemic species of vascular plants, and is considered a biodiversity hotspot [1]. The whole area, where agriculture appeared about 12,000 years ago, is considered the centre of origin of staple crops (wheat, lentils, peas, vetches, broad beans, chickpeas), as well as some aromatic (lavender and myrtle) and medicinal (sage and thyme) plants, and where fruits such as olives, vines and figs have been cultivated for millennia [2].
What has made the agrobiodiversity of this area so special is the human migrations that have taken place over the centuries [3], routes that have almost always had the Mediterranean basin as a point of departure or destination, with the consequent transfer of cultural practices and thus cultivation to and from other areas of the planet.
It is estimated that the rate of endemism of the plants present exceeds 40%. The great diversity and high rate of endemism is due to the great geomorphological and habitat complexity, explained by the presence of many mountains, islands and rocky environments, as well as underground biotopes. Because of these conditions, several studies have shown that the Mediterranean region contains numerous “hotspots” of great importance for the biodiversity of the entire continent.
The Mediterranean is the centre of diversity for olive and carob trees, a large number of cultivated vegetables, spices, oil and many ancient species of fodder plants [4,5]. Zeven and Zhukovsky [6] reported that of a total of 167 cultivated plant families distributed in 12 world centres of diversity, 53 (31%) had at least one genus and one species with centres of primary or secondary origin in the Mediterranean. Today, some ancient Mediterranean crops are no longer cultivated due to the introduction of other crops or improved cultivars.
It is evident that the initial biodiversity, particularly agrobiodiversity, is currently facing significant challenges and has been adversely impacted by various factors. The modernisation of agriculture has led to the adoption of increasingly uniform crops, a topic which will be explored in further depth in this study. The repercussions of climate change have also been recently observed, thereby posing a genuine threat to all PGRs that will be unable to adapt to emerging climatic conditions (e.g., elevated temperatures, drier summers).
The famous Vavilov [7] conducted research campaigns in Italy and wrote a brief description of wheat varieties, particularly in Sicily [8]. In 1950, R. Maly, a former collaborator of H. Stubbe, carried out the first comprehensive collection of cultivated plants in Italy [9]. Stubbe, founder of the Institut für Kulturpflanzenforschung (now IPK) in Gatersleben (Germany) and inspired by Vavilov’s ideas, further developed the Vavilovian approach to collecting expeditions and initiated and supported this mission. New impetus came with the establishment of the Germplasm Laboratory (later the Germplasm Institute—IG, now the Institute of Biosciences and Bio-Resources—IBBR) in Bari in 1969 [10].
Over the years, Italy has become the centre of international activities. Under the influence of the FAO, the International Biological Programme (IBP) was established, which culminated in various collection activities. The extensive research programme for the conservation and utilisation of plant genetic resources in Italy has shown that small islands are often the last refuges for plant varieties and may even be richer in plant genetic resources than other continental areas [11].
The loss of much of the variability at the species level (extinction) is relatively slow, and most of the species mentioned by earlier authors as being in cultivation have still been found in recent missions. However, at the intraspecific level (genetic erosion), enormous losses have been observed, with significant examples. In general, this process can be illustrated by the decreasing number of cultivated botanical varieties or landraces and the increasing uniformity of modern agriculture. The influence of the latter in accentuating the process of genetic erosion is evident when the phenomenon is more pronounced in lowland areas than in mountainous areas. Furthermore, while genetic erosion is very high in arable crops (a huge loss can be observed, for example, in cereals and legumes), garden plants show a slower and milder loss of intraspecific variability over time.
In order to combat this phenomenon and to ensure the safeguarding of plant genetic resources of Mediterranean origin and agricultural interest, the Mediterranean Germplasm Gene Bank (MGG) has been hosted by the IBBR in Bari since 1969. Over the years, the MGG has been endowed with almost 60,000 accessions of genetic material at risk of erosion/extinction through collecting missions. The high rate of genetic erosion (approximately 75%) in the Mediterranean region has led the MGG to intensify its efforts in two areas: firstly, it is necessary to intensify the collection of the material that is still available through new missions; secondly, it is necessary to integrate the different conservation methods (ex situ, on farm and in situ) in the best possible way in order to make the use of Mediterranean plant genetic resources truly sustainable.
There are several geographical areas that can be explored and the mechanisms for integrating various conservation methods can be refined. Future work around MGG will focus on filling these existing gaps.
2. Relevant Sections and Discussion
2.1. The Mediterranean Germplasm Gene Bank
2.1.1. History
At the “FAO/IBP Technical Conference” held in Rome in 1967, the establishment of three germplasm banks was proposed: one in Lund, Sweden, for northern Europe; one in Braunschweig Folkenrode, Germany, for central Europe; and one in Bari, Italy, representing the Mediterranean region. In 1969, on the proposal of Prof. G.T. Scarascia Mugnozza, the Committee for Agricultural Sciences of the National Research Council (CNR) approved the establishment of the Germplasm Laboratory in Bari. In collaboration with the Faculty of Agriculture of the University of Bari, the Germplasm Laboratory became operational in 1970 [10].
Following the establishment of the institution, a period of considerable enthusiasm ensued, with the commencement of seed and germplasm collection activities at the MGG occurring almost immediately in 1971. This enthusiasm motivated the first collectors, who completed numerous collection expeditions despite considerable technical difficulties. Indeed, lacking access to cellular devices or GPS, they collected all the sourcing data on paper and explored (often with improvised means of transport) foreign territories relying on the directions of locals or on geographical maps that were not always detailed. Since then, more than 120 expeditions have been carried out in southern and eastern Africa, the Middle East and Persian region, and throughout the Mediterranean basin. These expeditions have resulted in the collection of more than 13,000 seed samples, which are currently stored and maintained in the germplasm bank.
This extensive exploration programme for the conservation and use of plant genetic resources (PGRs) has been made possible by tools such as checklists and inventories [12], which are essential for compiling data on PGRs (both cultivated and wild) and for analysing the results of ethnobotanical and eco-geographical studies.
Initially, conservation activities focused on wheat but were later extended to other crops. Currently, as we will see below, accessions belonging to 34 different botanical families are conserved in the MGG collection.
2.1.2. Mission
The main objective of the MGG is to safeguard herbaceous genetic resources of interest to Italian and Mediterranean agriculture.
Particular attention is paid to specific segments of PGRs such as eco-fisheries (including “typical products”), agro-ecotypes threatened by genetic erosion/extinction, Crop Wild Relatives (CWRs) and plants potentially useful for the extraction of bioactive or technological compounds.
From 1970 to the present day, the MGG has undertaken numerous commitments, ranging from the collection, conservation, distribution, characterisation and evaluation of PGRs to the analysis of their genetic structure, selection and pre-breeding and the recognition of useful genes. It is imperative to acknowledge the significance of each of these individual activities in ensuring the effective protection of PGRs. In an ideal work plan, these activities are to be executed in a consecutive manner. It is evident that while the collection of a PGR is indeed an effective method of combating the phenomenon of erosion, the conservation and distribution of the PGR is essential for the execution of characterisation studies, and consequently, its evaluation. It follows that the key to the success of the entire safeguard effort is the possession of detailed knowledge of the genetic basis of a PGR and its practical applications.
2.1.3. Database and Stored Materials
Currently, the collection contains about 60,000 accessions belonging to 34 families, 208 genera and 872 species (Table 1). In addition to the 13,000 specimens directly collected over time by the CNR-IBBR research teams, the remaining material has been acquired from 314 donor institutions through a seed exchange. The conserved material, consisting exclusively of orthodox seeds, comes from 138 countries of origin and is divided into different functional groups: food crops (about 70 percent), fodder crops (about 5 percent), industrial crops (about 3 percent), medicinal plants (about 5 percent), spices and herbs (about 7 percent) and other uses (10 percent).

Table 1.
A summary of the main statistics related to the MGG.
The gene bank conserves several categories of PGR, such as landraces (the largest group), wild and weedy relatives, model plants, garden breeds [13], species potentially useful for the extraction of bioactive compounds and/or of interest to industry, and species of economic importance for Italy and the Mediterranean region.
The gene bank contains two collections: a basic collection stored at −20 °C in glass jars, and an active collection stored in chambers at 0 °C and 35% relative humidity. The latter collection is stored in vacuum-sealed triple-layer aluminium bags.
More than fifteen years ago, the Mediterranean Germplasm Database (MGD) was established and it is still available online at the following URL: https://www.ibbr.cnr.it/mgd/, accessed on 27 October 2025. It is the primary database for the agro-food plant germplasm collection conserved at the CNR-IBBR in Bari, Italy. The database contains passport data for up to 59,000 accessions (over 99% of the total), together with collection site maps for over 12,000 records and around 1,000 images of accessions, all of which are freely available.
2.1.4. Projects
As discussed in Section 1, the ongoing genetic erosion leading to the loss of biodiversity in major crops is a current issue that requires effective responses and decisive action. In this context, gene banks are emerging and operating as repositories of a significant yet largely unknown diversity for many crop species, with even greater diversity likely to be found in farmland and wild habitats. However, detailed information on ex situ accessions is fragmentary, while that on in situ resources is almost non-existent. Consequently, a significant proportion of the material yet to be discovered and analysed may contain key genetic resources with the potential to contribute to agricultural resilience, crop adaptation to global change, and the long-term sustainability of food production.
For this reason, the exchange of genetic material and information between gene banks is essential to conserve PGR and facilitate their better use in research and breeding activities. In recent times, the MGG has embarked on numerous collaborative endeavours and initiatives, with the objective of disseminating information pertaining to material preserved within a network of European and international PGR gene banks and collections. The most salient scientific collaborations currently underway at the MGG are delineated below.
The European Research Catalogue for Plant Genetic Resources (EURISCO) is a comprehensive database that collects and provides information on agrobiological and genetic diversity from over 400 institutes, encompassing approximately 2 million accessions of cultivated species and their wild relatives. The Secretariat of the European Cooperative Programme for Plant Genetic Resources (ECPGR) oversees the management of EURISCO, leveraging a network of national inventories from 43 member countries. The management of the EURISCO on line platform by the IPK in Gatersleben, Germany, commenced in 2014, with the stated aim of providing a comprehensive information repository for the scientific community and plant breeders. MGG has adopted the MCPD (Multi-Crop Passport Data) data model issued by ECPGR, ensuring full interoperability with EURISCO. To date, MGG has entered more than 7000 accessions from its collection into the EURISCO database, which can be viewed and consulted on the online platform of the international gene bank GENESYS-PGR (https://www.genesys-pgr.org/, accessed on 27 October 2025).
MGG is a participant in the RGV-FAO programme, which is based on the International Treaty on Plant Genetic Resources for Food and Agriculture (ITPGRFA). Ratification of the treaty by the Italian government occurred in 2004, and the ratification law established the RGV-FAO long-term programme (currently in its 7th cycle) for the implementation of the ITPGRFA in Italy, in accordance with Article 5 of the Treaty.
Additionally, the MGG is a partner in the EU-funded Horizon Europe project PRO-GRACE (‘Promoting a Plant Genetic Resource Community for Europe’), launched in 2021 with the aim of developing a new European research infrastructure (GRACE, https://www.grace-ri.eu/, accessed on 27 October 2025) dedicated to the cataloguing, description, conservation and valorisation of European plant genetic resources for food and agriculture. Upon completion, the GRACE Research Infrastructure (RI) will collaborate with global organisations dedicated to PGRs and with other established ESFRI research infrastructures in complementary areas (ELIXIR, European Infrastructure for Multi-scale Plant Phenomics and Simulation “EMPHASIS”, Distributed System of Scientific Collections “DiSSCo”, European Research Infrastructure Consortium “LifeWatch ERIC”, Microbial Resource Research Infrastructure “MIRRI”).
It is also pertinent to mention ITINERIS, a project funded by the EU’s Next Generation programme and the Italian Ministry of Research. The project was initiated with the objective of establishing the Italian hub of scientific–environmental research infrastructures for the observation and study of environmental processes, with a view to providing access to data and services. The project will harmonise and standardise all available data and metadata from the entire national network of biological collections, related to past and ongoing research projects, within an easily accessible platform.
Similarly, at the international level, DiSSCo (Distributed System of Scientific Collections) is a European research infrastructure (RI) that has been included in the ESFRI roadmap since 2018. Its aim is to digitise and digitally unify all natural science collections (including seed banks) in Europe. The potential benefits for MGG of joining the DiSSCo network are significant, particularly in view of the extensive nature of its seed collection. DiSSCo is currently under development and is expected to be fully operational by 2026.
CNR-IBBR is also participating in the PNRR Agritech project, funded by the “Next Generation EU” programme with several research units. One of the most important topics of this joint project, which involves various scientific institutions, is climate change. Information on CNR-IBBR projects aimed at achieving sustainable agricultural production can be found on the MGD website (https://www.ibbr.cnr.it//ibbr/projects, accessed on 27 October 2025).
Finally, the MGG is registered in the Global Register of Scientific Collections [14] which is managed by the Global Biodiversity Information Facility (The GBIF is a major global open-access infrastructure for biodiversity data, funded by governments around the world. The CNR-IBBR has recently been approved as a data publisher in GBIF, and MGG data and metadata are now freely available on the GBIF online platform [15,16,17].
2.2. Exploration and Collecting Activities
As previously referenced, CNR-IBBR research teams have identified more than 13,000 accessions since 1971. The teams of the then newly established Germplasm Laboratory followed the path traced by Vavilov and Maly, who, in 1927 and 1950, respectively, had conducted campaigns to collect genetic material in southern Italy establishing significant PGR collections.
Prior to embarking on a comprehensive exposition of the activities in question, it is essential to clarify the various steps that collectively constitute a PGR finding mission. First, it is imperative to undertake a preliminary evaluation of the accessibility of information concerning the environmental characteristics and distribution of the species targeted by the activity. It is evident that, upon the basis of the knowledge acquired, the region is divided into a limited number of areas. These areas are distinguished in terms of ecology, stationary characters, and land use. The subsequent procedure entails a preliminary excursion to the designated station, with the objective of verifying the identification of the entities to be collected, ascertaining the feasibility of collection, and determining the probable maturity period of the germplasm. This phase is referred to as the ‘exploration phase’.
The exploration phase is followed by the collection phase, although in some cases the two phases may coincide. In order to ensure a statistically accurate collection, the utilisation of a randomised method is strongly recommended. However, in the case of autochthonous entities, it is possible for subpopulations to develop within the natural population. In order to comprehensively explore and document the biodiversity present at a given site, it is essential to identify, collect, and treat these subpopulations as discrete entities.
Collectors typically undertake random or transect-line sampling methodologies. However, the distances and, consequently, the individuals to be sampled depend on the biological morphology of the species; therefore, no single criterion or method is applicable, but a species-specific protocol is required. The quantity of germplasm to be collected is determined by the degree of threat or vulnerability of the species, understood as the risk of extinction. The process of field collection is undertaken with the objective of acquiring a series of specific information and data that facilitate the understanding of the autoecology of a taxon. To ensure the comparability and homogeneity of the data collected from the various banking institutions, field sheets are prepared. The compilation of these field sheets facilitates the collection and processing of data on the stations of the taxa of interest. It is imperative that each specific survey is accompanied by the completion of the relevant sheet.
Table 2 summarises the destinations and times of the 120-plus missions organised and carried out by CNR-IBBR staff. Notably, the geographical scope of these collection campaigns covered southern and eastern Africa, the Middle East, the Persian region and the entire Mediterranean basin.

Table 2.
A summary of the main collecting missions carried out by IBBR-CNR members.
2.2.1. In Italy
Sicily and South Italy
The area under consideration, located in the central region of the Mediterranean diversification centre [1], is characterised by a considerable richness in terms of genetic resources. In this regard, it has been documented that Hammer et al. [18] conducted a study of the geographic areas of origin of 524 taxa found in Southern Italy, identifying that the largest group (202 taxa) was composed of taxa from Southern Italy itself, indicating that their wild ancestors originated in that region. The extant evidence suggests that some of the plants cultivated in Southern Italy may have been introduced to that region when they were already domesticated in other areas. Nevertheless, their interaction with local varieties has exerted a substantial influence on the evolution of the indigenous species. It is evident that the geographical area in question functions as a reservoir of PGR, thereby underscoring its significance.
Since 1980, in collaboration with the Germplasm Bank in Gatersleben (Germany), the CNR-IBBR research team has been systematically exploring southern Italy and collecting a substantial amount of genetic material (more than 1600 samples in the period 1980–1988, mainly garden crops and legumes) [19]. It is imperative to emphasise that the lines delineated by these novel campaigns followed those of Maly’s explorations in 1950, with the objective of comparing the genetic diversity observed in the historical collection with that documented in the material collected between 1980 and 1988 [20,21,22,23,24,25]. The process of genetic erosion has been clearly demonstrated for all crops in southern Italy [19].
However, a distinction must be made between major field crops, in which genetic erosion has occurred most rapidly, and horticultural crops and other minor species, in which this process has occurred with less intensity. This discrepancy can be observed on a global scale and attributed to the fact that the genetic enhancement of horticultural and minor crop species, as well as the subsequent introduction of novel varieties or hybrids, has been comparatively less incentivised than for field crops. Indeed, for the latter, as early as 1988, ten years after the first germplasm retrieval mission, many of the intraspecific taxa were no longer found [19].
Central and Northern Italy
The encouraging results obtained in the southern Italian region have led to the extension of the study to the central and northern parts of the country, commencing in 1987 [26] and concluding in 1996. In general, the new catalogue exhibits a substantially reduced proportion of PGR compared to the previous catalogue from South Italy (486 accessions compared to 1622, with 200 out of 486 being cereals and grasses). Furthermore, a general erosion for landraces in South Italy could be estimated at 75% [6] thanks to the comparison with material collected in 1950 [9]. However, data of this kind have not been available for the central and northern parts of Italy. Nevertheless, the transition from cropping systems to animal production throughout the Alpine region, coupled with the progressive development of tourism, suggests that the area in question has undergone significant genetic erosion, with a reported loss of more than 90% of its original genetic diversity.
Small and Minor Islands
The role of islands in biodiversity discussions is of particular significance. This constitutes the rationale behind CNR-IBBR and IPK’s initiation of a collection programme across a multitude of Italian islands in 1994 (as in Figure 1), after the findings of preceding studies. The impact of isolation on the biodiversity of landraces was found to be a surprising revelation [27]. A comparison with the largest Italian islands (i.e., Sicily and Sardinia) revealed that, on average, small islands have a higher number of landraces per square kilometre (2 vs. 0.5). This trend is more pronounced when comparing the same parameter with that of Central-North Italian mainland (2 vs. 0.2) and with the case of South Italy (2 vs. 0.8). However, a sensible genetic erosion was also observed in ‘rich in landraces’ islands, where an erosion rate of up to 12.2% was calculated [28].
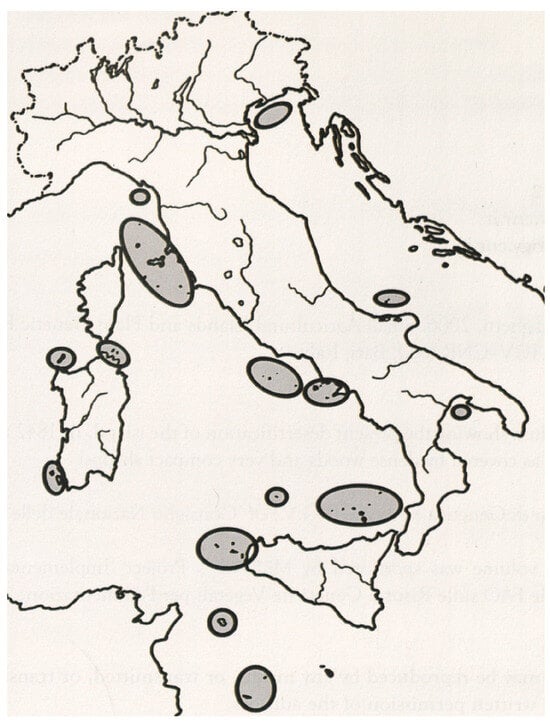
Figure 1.
A map of all the Italian minor islands (highlighted) explored by the CNR-IBBR and IPK since 1994.
Linguistic Islands
It is important to note the significant experience of researching, collecting and preserving PGR related to language islands in Italy, which commenced in 1996 and focused in particular on towns of Albanian origin [29]. In this case, the linguistic factor was used as a guide: if in a town of Albanian origin a certain landrace was called by an Albanian name, it was highly probable that, over the centuries, the Albanians had transported it from there and that it was already part of their history; it was on these materials that the research focused. Between 1980 and 2008, 210 accessions from these areas were collected: mainly vegetables and, to a lesser extent, cereals [29]. The work was concluded with a comparison of the Albanian agrobiodiversity with that of the Italian regions of Albanian origin, using the same materials.
2.2.2. Abroad
The collection campaign in Ethiopia in 1973 signifies the inaugural initiative undertaken by the CNR-IBBR group on an international scale. In conjunction with the subsequent survey conducted the following year within the same nation, a total of 921 accessions were collected, predominantly classified under the genera Triticum and Hordeum (which collectively constituted 67% of the total), accompanied by a notable presence of Pisum and Vicia [30]. It is imperative to emphasise that, in one of the world’s primary centres of crop evolution and origin, genetic erosion resulted, over the years, in the extinction of certain accessions that had been meticulously preserved ex situ at the MGG in Bari, shortly following the initial collecting campaigns.
As previously stated, these were followed by missions to further regions of high interest in terms of genetic diversity: southern and eastern Africa, the Middle East, the Persian region and the entire Mediterranean basin, including major islands (e.g., Corsica, Crete) and minor islands (e.g., Ionian Islands, Cheradi and the Maltese archipelago).
A particularly noteworthy experience is represented by the four emergency missions to Albania funded by the Food and Agriculture Organization of the United Nations (FAO) and conducted in collaboration with researchers from Germany and Poland [6]. The main project’s objective was to collect all regional materials of particular interest in the aftermath of the dissolution of the communist regime (1990), which had previously isolated the nation from the rest of the world for a period of 44 years. The relaxation of border restrictions, as anticipated, precipitated a systematic withdrawal of all local PGR. The collection yielded a total of 354 samples, representing 75 distinct species. In order of importance, the largest category was represented by vegetables, followed by pulses and cereals. This distribution can be attributed to the geographical characteristics of the Albanian territory, which is predominantly mountainous and thus well-suited to livestock farming and small gardens, rather than to field crops.
2.3. Case Studies of Rare Landraces Found
As was stated in the preceding section, in the course of exploration missions, whenever rare landraces were encountered, they were subjected to detailed analysis, and the results of these studies were published separately. These include, for example, landraces that have the capacity to thrive in particular ecological niches, landraces that have only recently been domesticated, and landraces whose area of origin extends over vast distances. Such discoveries, in addition to being significant due to their rarity, offer a significant opportunity to study and investigate the genetic variability inherent in the resources examined, as well as their unique characteristics of environmental adaptation.
In the following paragraphs, a comprehensive analysis of several case studies will be conducted.
2.3.1. ‘Mugnoli’
During a collection mission conducted in Italy in 2004, a rare landrace of ‘mugnoli’ (a sub-taxon form of Brassica oleracea var. italica) was found [31]. However, it is important to note that this brassicaceous plant is traditionally cultivated in Salento, a region located in Apulia, in southern Italy, but does not occur in the rest of the region and the rest of Italy [32]. From a morphological perspective, it bears similarities to mature broccoli and can be regarded as a parallel variant of ‘broccolo rapa’ or ‘cima di rapa’, both of which are well-known and cultivated in the rest of Italy [31], even if, as illustrated in Figure 2, there are some observable discrepancies. However, subsequent genetic research has revealed that ‘mugnoli’ are an ancestor in the evolutionary line that subsequently led to broccoli [31].

Figure 2.
The inflorescences of ‘‘mugnoli’’ and their large flower bracts: one of the main diagnostic traits for differentiation between this crop and ‘broccoli’.
The cultivation of ‘mugnoli’ is primarily undertaken at the domestic level, with a limited proportion being allocated for local market distribution. The rationale behind this restricted distribution can be attributed to the fact that analogous crops, such as broccoli, have been demonstrated to yield significantly higher returns. Consequently, the cultivation of ‘mugnoli’ is a practice that has been perpetuated over time exclusively by those who particularly appreciate their flavour.
It is a commonly held belief that the ingestion of ‘mugnoli’ has a stimulatory effect on lactation in both women and bovines. The flavour profile of ‘mugnoli’ is characterised by a subtle sweetness and an aromatic quality that surpasses that of broccoli. It is evident that a significant number of traditional recipes provide compelling evidence of the profound cultural significance of this landrace at the local level.
In order to increase the production of ‘mugnoli’, it is imperative to stimulate the interest of the preserved food industry. Empirical evidence has demonstrated the efficacy of mugnoli for frozen preservation, and thus this should be a priority in future research.
The CNR-IBBR germplasm bank in Bari is home to ten accessions of ‘mugnoli’. A slight morphological variation has been observed between the stored accessions. A study on the genetic variability of this material is currently underway at the CNR-IBBR. Preliminary findings suggest that the genetic variability of ‘mugnoli’ is minimal. It is probable that ‘mugnoli’ populations are descended from a single original form. Furthermore, local farmers have been meticulous in their efforts to eradicate foreign types that have arisen from unintentional spontaneous crossbreeding with other Brassica taxa.
2.3.2. Salicornia spp.
The genus Salicornia L. comprises halophytic herbs characterised by articulated succulent stems and an extreme degree of phenotypic plasticity.
It is important to note the findings reported by Urbano et al. [32] on the cultivation of Salicornia patula Duval-Jouve in the northernmost part of the Apulia region (Figure 3). This species, inhabiting the driest regions along the periphery of coastal lagoons, on mudflats or sands that become inundated during the winter months, has a long history of gathering from the wild as a source of sustenance. To date, there has been no report on the domestication of S. patula in the rest of Italy. In the Gargano area of Apulia, this species was domesticated forty years ago as a minor cultivation, limited to private gardens, and is also present in the wild: at present, there is still no genetic difference between the two PGR. The increasing demand for this vegetable, both for fresh consumption and processing in packinghouses, has recently prompted some farmers to cultivate it for marketing purposes. S. patula has the potential to become a lucrative new cash crop for marsh marginal lands; however, the success of this endeavour is contingent upon the selection of superior genotypes and the implementation of appropriate agro-techniques.

Figure 3.
Edible branches of S. patula.
Salicornia spp. have a plethora of applications and potential, ranging from their ancient use as an additive in the production of soap and glass [33,34] to their recent environmental protection uses as a plant biofilter for marine aquaculture effluent [35] or as a viable source of biofuel for aviation [36].
The collection of wild plants is a very ancient and widespread practice in the Apulia region. Salicornia spp. have been recognised as a source of food and feed, and as a medicinal resource, for a considerable duration. The ancient culinary tradition has been passed down through successive generations, and in the contemporary era, Salicornia europea L. is recognised as a traditional product of Apulia by the Ministry of Agriculture and Forestry.
The propagation of this species is achieved through the dissemination of seeds. The implementation of both direct seeding and transplanting methods of planting has been observed [32]. Presently, the commercial production necessitates genetic enhancement to obtain varieties that manifest superior and reproducible quality characteristics, in conjunction with a stable quantitative yield. Concurrently, concerted efforts are imperative to enhance the agro-technical approach for this species.
2.3.3. Lathyrus cicera
Lathyrus cicera L. is regarded as one of the most ancient cultivated plants worldwide, with origins in the Mediterranean centre of diversity. It is hypothesised that, in conjunction with its close relative, L. sativus, it was the first crop to be domesticated in Europe (Iberian Peninsula and southern France) (Figure 4). In the contemporary era, L. cicera finds itself in a similar situation to numerous legume, cereal and other once extensively cultivated species, which have become largely neglected and underutilised. The cultivation of food and feed is characterised by its extreme sporadic nature, with a paucity of breeding strategies in place. Furthermore, the species has been observed to frequently escape from local agricultural practices into neighbouring wild flora. Hammer et al. [37] conducted an exploratory study of a small area in south-west Italy (the Camerotan hamlet of Lentiscosa), where L. cicera is still well known and celebrated with the local name of ‘maracuoccio’. The promotion of regional folk customs is as important as current ex situ collecting across the world for its reintroduction. In this regard, it is imperative to emphasise the noteworthy intraspecific diversity exhibited by L. cicera, which serves as a substantial foundation for its enhancement and reintroduction.

Figure 4.
The seeds of the landraces of (left) Lathyrus cicera and (right) Lathyrus sativus.
The available genetic resources of this ancient legume in some of the largest ex situ collections are not easily quantifiable, leaving us with a rough estimate of between 4000 and 6000 accessions worldwide in all gene banks [38,39]. These numbers may be interpreted as a cautionary indication that the existing ex situ genetic resources of L. cicera are somewhat fragile and require adequate conservation, successful preservation, appropriate characterisation and evaluation with developed core collections [40,41,42].
In addition to the aforementioned examples, the CNR-IBBR working group has identified and examined a considerable number of rare landraces over the years, including Solanum aethiopicum L., ‘farro’, and numerous others (e.g., ‘Lenticchia di Altamura’, Scolymus hispanicus L., Citrus medica L. ‘Diamante’).
2.4. Safeguarding Strategies
In general, biodiversity conservation strategies (ex situ, in situ, on farm) are not uniform in their operational characteristics, despite their complementary nature; the overarching objective is to ensure the preservation of PGR from genetic erosion and extinction.
2.4.1. Ex Situ Conservation
The term ‘ex situ conservation’ refers to the conservation of genetic resources outside their natural environment [43]. The primary objective of ex situ conservation of populations is to contribute to the protection of threatened taxa and their genetic diversity. This approach to conservation is imperative in circumstances where a species or population has become extinct in the wild or is at imminent risk of extinction, as well as for genetic resources that hold significant cultural, economic, or scientific value [44].
Ex situ conservation of biological diversity is achieved through the utilisation of gene banks, which can be categorised into four distinct classifications: (i) seed banks; (ii) in vivo collections; (iii) in vitro banks; and (iv) cryopreservation banks (DNA, pollen).
The primary approach to ex situ conservation is the utilisation of seed banks, which facilitates the effective preservation of both intrapopulation and interpopulation diversity. As demonstrated in Section 2.1, the activity of the MGG predominantly falls within this specific approach.
In vivo collections comprise plants cultivated in controlled environments, including botanical gardens, greenhouses, arboreta, alpine gardens, nurseries field gene bank collections and analogous facilities. The conservation of wild species is primarily overseen by botanical gardens, which have historically amassed a vast collection of wild plants from various global regions [45]. Initially, this collection was undertaken for the purpose of research and documentation, but subsequently, a shift occurred towards the conservation of these specimens. In vivo collections also play a significant role in the protection of species or cultivars of economic interest, especially those whose seeds cannot be preserved (e.g., forest trees, fruit trees, garlic) [46]. However, this method requires large spaces, and with the increasing demand for cultivated land, there has been a progressive reduction in the available areas. To date, CNR-IBBR still maintains a field collection of globe artichoke.
In vitro conservation is a frequently employed approach for species that demonstrate resistance [47]. However, it is important to note that this method has significant disadvantages, including short-term conservation and high labour requirements. Notwithstanding, this technique remains the sole means of conservation for species that have lost their capacity for sexual reproduction, signifying that their propagation is exclusively feasible through vegetative means, as exemplified by bananas [48].
The most recent method for preserving genetic material involves the extraction of DNA from plants [49]. Furthermore, the utilisation of genetic engineering techniques facilitates the extraction of beneficial genes for subsequent use [50]. It is evident that an additional botanical component that can be preserved is the pollen grain, from which the male gamete will derive [51]. It is evident that this process exclusively permits the preservation of the paternal haploid set. This solution is distinguished by its minimal spatial requirements, which consequently result in lower storage costs.
2.4.2. In Situ Conservation
The term ‘conservation in situ’ is employed to denote the preservation of natural habitats and ecosystems in their original locations.
In situ conservation, defined as the protection and preservation of biological diversity in a specific natural environment, aims to preserve species and ecosystems in their natural environment, ensuring the continuity of the ecological relationships that characterise them [52]. The conservation in question can be defined as “dynamic”, as populations evolve in response to selective pressures exerted by the biotic community and pedo-climatic factors [53]. This process is conducive to the adaptation of species or populations of local ecotypes to the abiotic and biotic factors of the surrounding environment, through a mechanism of co-evolution with the environment as a whole.
The fundamental prerequisite for ecosystem management is the recognition that it is a dynamic and open system, consisting of a complex network of interactions between living organisms. In order to fulfil their protective function effectively, management plans must take this assumption into account. Therefore, before formulating realistic conservation measures, it is essential to understand the number of populations, their genetic variability, their reproductive system and their seed dispersal mechanisms, since plants which reproduce sexually tend to exhibit greater variability than those which self-pollinate or reproduce asexually [54].
Intraspecific genetic variability, that is to say, the diversity in the genetic heritage of populations of the same species, provides useful information for understanding the evolution of species, the relationships between different populations and their ability to adapt to environmental changes [55]. The loss of significant portions of a species’ distribution area is reflected in a corresponding reduction in its genetic variability, with negative consequences for its ability to adapt to environmental changes [56].
However, a further differentiation in biodiversity conservation strategies is possible. It is possible to distinguish between “in situ” conservation, which is suitable for wild materials within their reference ecosystems, and “on farm” conservation, which involves the introduction of the genetic resources being conserved into farms located in the same territories of origin and, therefore, within agro-ecosystems [57].
In this context, as part of projects launched in synergy with the Apulia Region, the CNR-IBBR has proposed protocols entrusted to custodian farmers, who are responsible for conserving small portions of durum and soft wheat found and stored within the MGG on farms. The protocols in question have been developed in accordance with the guidelines set out in the Italian National Plan for Agricultural Biodiversity. The protocols delineate a comprehensive set of guidelines that encompass all facets of agricultural management of genetic resources. These protocols are species-specific and are also contingent on the agro-climatic conditions of the cultivation site and the cultivation techniques employed (e.g., taking into account soil selection, sowing timing and density, irrigation and fertilisation management, pest and weed control, seed harvesting and storage practices).
2.5. Genetic Erosion in the Mediterranean Area
Brush [58] popularised and analysed the term “genetic erosion”, which he defined as “the global process whereby landraces, which have adapted over 10,000 years to different natural and anthropogenic conditions, are gradually being replaced by modern varieties characterised by uniformity and high production performance”. Utilising wheat as a point of reference, it is evident that genetic erosion advanced at a rapid rate in Italy between the 1920s and 1950s (13.2% p.a.) [28]. Subsequently, in accordance with statistical laws, genetic erosion remained at less striking levels (0.48-4% p.a. until the 1980s), yet a rapid decline in biodiversity was observed on some small islands. For instance, genetic erosion affecting wheat stood at 12.2% per annum between 1986 and 2000 in Favignana [28]. In geographically constrained contexts, such elevated rates invariably result in the reduction in wheat cultivation.
As indicated in Section 2.2.2, a further case study is provided by the experience documented in Albania in 1941, where some plant genetic resources were collected for the first time by Stubbe. This was followed by a subsequent collection in 1993 [6]. The collecting of identical resources (mainly cereals) from the same sites and employing the same methodologies permitted a precise comparison of the state of genetic erosion. Specifically, an average genetic erosion of 72.4% was recorded over the 52-year period between collections: to illustrate this point, consider the 1941 collection of 15 samples of Triticum spp., in which only a single sample was identified in the 1993 collection [6].
3. Conclusions
The MGG has excellent functionality and supplies seed samples worldwide. The gene bank is highly regarded both nationally and internationally. Some Italian regions have entrusted their native crop genetic resources to its conservation chambers.
It is evident that the IBBR-CNR working group cannot claim to have amassed all the necessary data; therefore, further collection missions are imperative in regions that have remained unexplored. As demonstrated, the accelerating rate of genetic erosion on a global scale signifies that efforts to preserve biodiversity must be made with utmost urgency.
In parallel with this, there is an expectation that the MGG will become involved in an increasing number of international cooperation programmes and establish networks with new or recently established gene banks to which it can transfer experience and know-how, as well as seeds.
In addition to traditional activities related to plant genetic resource conservation and management, other lines of research have been developed at the CNR-IBBR in genetics, genomics, proteomics and biotechnology. Consolidated approaches in biochemistry, molecular biology, and bioinformatics are used, as well as innovative approaches in genotyping, phenotyping, and “omics” technologies (genomics, transcriptomics, proteomics) to study species evolution and food quality and safety. The MGG seed collection is a valuable resource of material, and, at the same time, the results of these research activities provide new and valuable data for the characterisation and evaluation of the material conserved in gene banks.
Some units are still in the early stages, such as the DNA bank, while others are missing, such as in vitro culture and cryopreservation of vegetatively propagated plant species, a pollen bank and a plant symbionts bank.
Within the scope of the CNR-IBBR gene bank, the concept of conservation is closely linked to utilisation. An excellent conservation programme is not only judged by the quality of methods and materials maintained, but also by its utilisation. The utilisation of plant germplasm includes both direct and indirect use. The main use of the gene bank collection is for breeding purposes. However, as seen, the collections can also be used for reintroduction programmes and basic and applied research.
In conclusion, as a fully functional gene bank, the MGG will help address the global loss of agrobiodiversity, which is overlooked compared to that in wild systems.
Author Contributions
Conceptualization, G.L. and M.Z.; methodology, G.L.; software, G.L. and M.Z.; validation, G.L. and M.Z.; formal analysis, G.L. and M.Z.; investigation, G.L. and M.Z.; resources, G.L. and M.Z.; data curation, G.L. and M.Z.; writing—original draft preparation, G.L. and M.Z.; writing—review and editing, G.L. and M.Z.; visualization, G.L. and M.Z.; supervision, G.L.; project administration, G.L.; funding acquisition, G.L. and M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Hammer, K.; Esquivel Pérez, M.Á.; Long, C. (Eds.) Diversity and Geography of Cultivated Plants, 1st ed.; CRC Press: Boca Raton, FL, USA, 2025. [Google Scholar] [CrossRef]
- Abulafia, D. The Great Sea: A Human History of the Mediterranean; Allen Lane: London, UK, 2011. [Google Scholar]
- Perrino, P. The diversity in Vavilov’s Mediterranean Gene Center. Kulturpflanze 1988, 36, 85–105. [Google Scholar] [CrossRef]
- Hammer, K.; Knüpffer, H.; Xhuveli, L.; Perrino, P. Estimating genetic erosion in landraces—Two case studies. Genet. Resour. Crop Evol. 1996, 43, 329–336. [Google Scholar] [CrossRef]
- Zeven, A.; Zhukovsky, P. Dictionary of Cultivated Plants and Their Centres of Diversity. Excluding Ornamentals, Forest Trees and Lower Plants; PUDOC: Wageningen, The Netherlands, 1975. [Google Scholar]
- Vrugtman, F.; Vavilov, N.I. Origin and geography of cultivated plants. (Translated by Doris Löve). Arch. Nat. Hist. 1994, 21, 142. [Google Scholar] [CrossRef]
- Schreiber, L. Wheats of the islands of the Mediterranean. Bull. Appl. Bot. Leningr. 1934, 5, 41–240. [Google Scholar]
- Maly, R.; Hammer, K.; Lehmann, C.O. Sammlung pflanzlicher genetischer Ressourcen in Süditalien—Ein Reisebericht aus dem Jahre 1950 mit Bemerkungen zum Schicksal der Landsorten “in situ” und in der Genbank. Kulturpflanze 1987, 35, 109–134. [Google Scholar] [CrossRef]
- Scarascia Mugnozza, G.T.; Porceddu, E. Il problema della salvaguardia delle risorse genetiche vegetali in Italia. In Proceedings of the II Simposio Nazionale sulla Conservazione della Natura, Bari, Italy, 26–30 April 1972; pp. 337–345. [Google Scholar]
- Hammer, K.; Heller, J.; Engels, J. Monographs on underutilized and neglected crops. Genet. Resour. Crop Evol. 2001, 48, 3–5. [Google Scholar] [CrossRef]
- Hammer, K.; Knüpffer, H.; Perrino, P. A checklist of the South Italian cultivated plants. Kulturpflanze 1990, 38, 191–310. [Google Scholar] [CrossRef]
- Zeven, A. Landraces: A review of definitions and classifications. Euphytica 1998, 104, 127–139. [Google Scholar] [CrossRef]
- GrSciColl. Available online: https://www.gbif.org/grscicoll (accessed on 27 October 2025).
- Mediterranean Germplams Genebank—Triticum Collection (ITA436-MGG-Triticum). Available online: https://doi.org/10.15468/frm59p (accessed on 27 October 2025).
- Mediterranean Germplams Genebank—Miscellaneous Species (ITA436-MGG-Miscellaneous). Available online: https://doi.org/10.15468/rpej8x (accessed on 27 October 2025).
- Mediterranean Germplasm Genebank—Hordeum Dataset (ITA436-MGG-Hordeum). Available online: https://doi.org/10.15468/bxc8pe (accessed on 27 October 2025).
- Hammer, K.; Knüpffer, H.; Laghetti, G.; Perrino, P. Seeds from the Past. A Catalogue of Crop Germplasm in South Italy and Sicily; Germplasm Institute of CNR: Bari, Italy, 1992; pp. 2+173. [Google Scholar]
- Hammer, K.; Laghetti, G.; Perrino, P. Collection of plant genetic resources in South Italy, 1988. Kulturpflanze 1989, 37, 401–414. [Google Scholar] [CrossRef]
- Perrino, P.; Hammer, K.; Lehmann, C.O. Collection of plant genetic resources in South Italy, 1981. Kulturpflanze 1982, 30, 181–190. [Google Scholar] [CrossRef]
- Perrino, P.; Hammer, K.; Hanelt, P. Collection of land-races of cultivated plants in South Italy 1983. Kulturpflanze 1984, 32, 207–216. [Google Scholar] [CrossRef]
- Perrino, P.; Hammer, K. Collecting in Southern Italy [landraces of cultivated plants]. In Plant Genetic Resources Newsletter; IBPGR: Wageningen, The Netherlands; FAO: Rome, Italy, 1983; p. 54. [Google Scholar]
- Perrino, P.; Hammer, K. Collection of land-races of cultivated plants in South Italy, 1984. Kulturpflanze 1985, 33, 225–236. [Google Scholar] [CrossRef]
- Hammer, K.; Cifarelli, S.; Perrino, P. Collection of land-races of cultivated plants in South Italy, 1985. Kulturpflanze 1986, 34, 261–273. [Google Scholar] [CrossRef]
- Hammer, K.; Perrino, P.; Pignone, D. Collection of plant genetic resources in South Italy, 1986. Kulturpflanze 1987, 35, 389–399. [Google Scholar] [CrossRef]
- Perrino, P.; Laghetti, G.; Hammer, K. Collection of plant genetic resources in Italy, 1987. Kulturpflanze 1988, 36, 377–390. [Google Scholar] [CrossRef]
- Hammer, K.; Laghetti, G. Small Agricultural Islands and Plant Genetic Resources—Le Piccole Isole Rurali Italiane; IGV-CNR: Bari, Italy, 2006; pp. 10+246. ISBN 88-900347-4-2. [Google Scholar]
- Hammer, K.; Laghetti, G. Genetic Erosion—Examples from Italy 1,2. Genet. Resour. Crop Evol. 2005, 52, 629–634. [Google Scholar] [CrossRef]
- Hammer, K.; Laghetti, G.; Pignone, D. (Eds.) Linguistic Islands and Plant Genetic Resources—The Case of the Arbëreshë; ARACNE: Rome, Italy, 2011; ISBN 978-88-548-3958-8. [Google Scholar]
- Porceddu, E.; Perrino, P. Wheat in Ethiopia. Preliminary report of a collecting mission. Plant Genet. Resour. Newsl. 1973, 30, 33–36. [Google Scholar]
- Laghetti, G.; Martignano, F.; Falco, V.; Cifarelli, S.; Gladis, T.; Hammer, K. “Mugnoli”: A neglected race of Brassica oleracea L. from Salento (Italy). Genet. Resour. Crop Evol. 2005, 52, 635–639. [Google Scholar] [CrossRef]
- Urbano, M.; Tomaselli, V.; Bisignano, V.; Veronico, G.; Hammer, K.; Laghetti, G. Salicornia patula Duval-Jouve: From gathering of wild plants to some attempts of cultivation in Apulia region (southern Italy). Genet. Resour. Crop Evol. 2017, 64, 1465–1472. [Google Scholar] [CrossRef]
- Kurinsky, S. The Glassmakers: An Odyssey of the Jews, the First Three Thousand Years; Hippocrene Books: New York, NY, USA, 1991. [Google Scholar]
- Liebezeit, G. Ethnobotany and phytochemistry of plants dominant in salt marshes of the Lower Saxonian Wadden Sea, southern North Sea. Senckenb. Marit. 2008, 38, 1–30. [Google Scholar] [CrossRef]
- Shpigel, M.; Ben-Ezra, D.; Shauli, L.; Sagi, M.; Ventura, Y.; Samocha, T.; Lee, J.J. Constructed wetland with Salicornia as a biofilter for mariculture effluents. Aquaculture 2013, 412–413, 52–63. [Google Scholar] [CrossRef]
- Hashem, H. Why Research Won’t Give Up on Salicornia. MIT Technology Review, 24 March 2015. [Google Scholar]
- Hammer, K.; Montesano, V.; Direnzo, P.; Laghetti, G. Conservation of crop genetic resources in Italy with a focus on vegetables and a case study of a neglected race of Brassica oleracea. Agriculture 2018, 8, 105. [Google Scholar] [CrossRef]
- Mathur, P.N.; Alercia, A.; Jain, C. Lathyrus Germplasm Collections Directory; Bioversity International: Rome, Italy, 2005. [Google Scholar]
- Vaz Patto, M.C.; Rubiales, D. Lathyrus diversity: Available resources with relevance to crop improvement—L. sativus and L. cicera as case studies. Ann. Bot. 2014, 113, 895–908. [Google Scholar] [CrossRef]
- Burlyaeva, M.O.; Solovyeva, A.E.; Nikishkina, M.A.; Rasulova, M.A.; Zolotov, S.V. Species of the genus Lathyrus, L. from N.I. Vavilov Institute (VIR) Collection—The source of initial material for high-protein forage varieties breeding. Legum Groat Crop 2012, 4, 62–71. [Google Scholar]
- Vishnyakova, M.A.; Aleksandrova, T.G.; Buravtseva, T.V.; Bulyntsev, S.V.; Burlyaeva, M.O.; Egorova, G.P.; Semenova, E.V.; Seferova, I.V.; Yankov, I.I. Strategy and Tactics of Grain Legumes Genetic Resources Mobilization in VIR Collection at the Turn of XX–XXI Centuries. Proc. Appl. Bot. Genet. Breed. 2012, 169, 41–52. [Google Scholar]
- Vishnyakova, M.A.; Aleksandrova, T.G.; Bulyntsev, S.V.; Buravtseva, T.V.; Burlyaeva, M.O.; Egorova, G.P.; Semenova, E.V.; Seferova, I.V.; Yan’kov, I.I. Grain legumes genetic resources of Mediterranean origin in VIR collection: Diversity and use in breeding (review). Agric. Biol. 2016, 51, 31–45. [Google Scholar]
- Engelmann, F.; Engels, J.M.M. Technologies and strategies for ex situ conservation. In Managing Plant Genetic Diversity; CABI Publishing: Wallingford, UK, 2002; pp. 89–103. [Google Scholar]
- Hawkes, J.G.; Maxted, N.; Ford-Lloyd, B.V. The plant genetic resources Conservation movement. In The Ex Situ Conservation of Plant Genetic Resources; Springer: Dordrecht, The Netherlands, 2000. [Google Scholar] [CrossRef]
- Smith, P.; Pence, V. The role of botanic gardens in ex situ conservation. In Plant Conservation Science and Practice: The Role of Botanic Gardens; Cambridge University Press: Cambridge, UK, 2017; pp. 102–133. [Google Scholar]
- Panis, B.; Nagel, M.; Van den houwe, I. Challenges and Prospects for the Conservation of Crop Genetic Resources in Field Genebanks, in In Vitro Collections and/or in Liquid Nitrogen. Plants 2020, 9, 1634. [Google Scholar] [CrossRef]
- Withers, L.A. 4. In-vitro conservation. Biol. J. Linn. Soc. 1991, 43, 31–42. [Google Scholar] [CrossRef]
- Oliveira, R.P.D.; Silva, S.D.O.; Silva, K.M.D.; Silveira, D.G. In vitro conservation of diploid banana accessions. Sci. Agric. 2000, 57, 245–249. [Google Scholar] [CrossRef]
- Hodkinson, T.R.; Waldren, S.; Parnell, J.A.N.; Kelleher, C.T.; Salamin, K.; Salamin, N. DNA banking for plant breeding, biotechnology and biodiversity evaluation. J. Plant Res. 2007, 120, 17–29. [Google Scholar] [CrossRef]
- Ye, X.; Lei, B. The current status and trends of DNA extraction. BioEssays 2023, 45, 2200242. [Google Scholar] [CrossRef]
- Chen, P.H.; Pan, Y.B.; Chen, R.K. High-throughput procedure for single pollen grain collection and polymerase chain reaction in plants. J. Integr. Plant Biol. 2008, 50, 375–383. [Google Scholar] [CrossRef]
- Meilleur, B.A.; Hodgkin, T. In situ conservation of crop wild relatives: Status and trends. Biodivers. Conserv. 2004, 13, 663–684. [Google Scholar] [CrossRef]
- Rotach, P. In situ conservation methods. In Conservation and Management of Forest Genetic Resources in Europe; Arbora: Zvolen, Slovakia, 2005; pp. 535–565. [Google Scholar]
- Lei, S.A. Benefits and costs of vegetative and sexual reproduction in perennial plants: A review of literature. J. Ariz.-Nev. Acad. Sci. 2010, 42, 9–14. [Google Scholar] [CrossRef]
- Ramel, C. Biodiversity and intraspecific genetic variation. Pure Appl. Chem. 1998, 70, 2079–2084. [Google Scholar] [CrossRef]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef]
- Hammer, K.; Gladis, T.; Diederichsen, A. In situ and on-farm management of plant genetic resources. Eur. J. Agron. 2003, 19, 509–517. [Google Scholar] [CrossRef]
- Brush, S.B. Genetic erosion of crop populations in centers of diversity: A revision. In Proceedings of the Technical Meeting on the Methodology of the FAO World Information and Early Warning System on Plant Genetic Resources, Research Institute of Crop Production, Prague, Czech Republic, 21–23 June 1999; pp. 34–44. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).