Extraction and Analytical Techniques for Pharmaceuticals and Personal Care Products in Sediments: A Critical Review Towards Environmental Sustainability
Abstract
1. Introduction
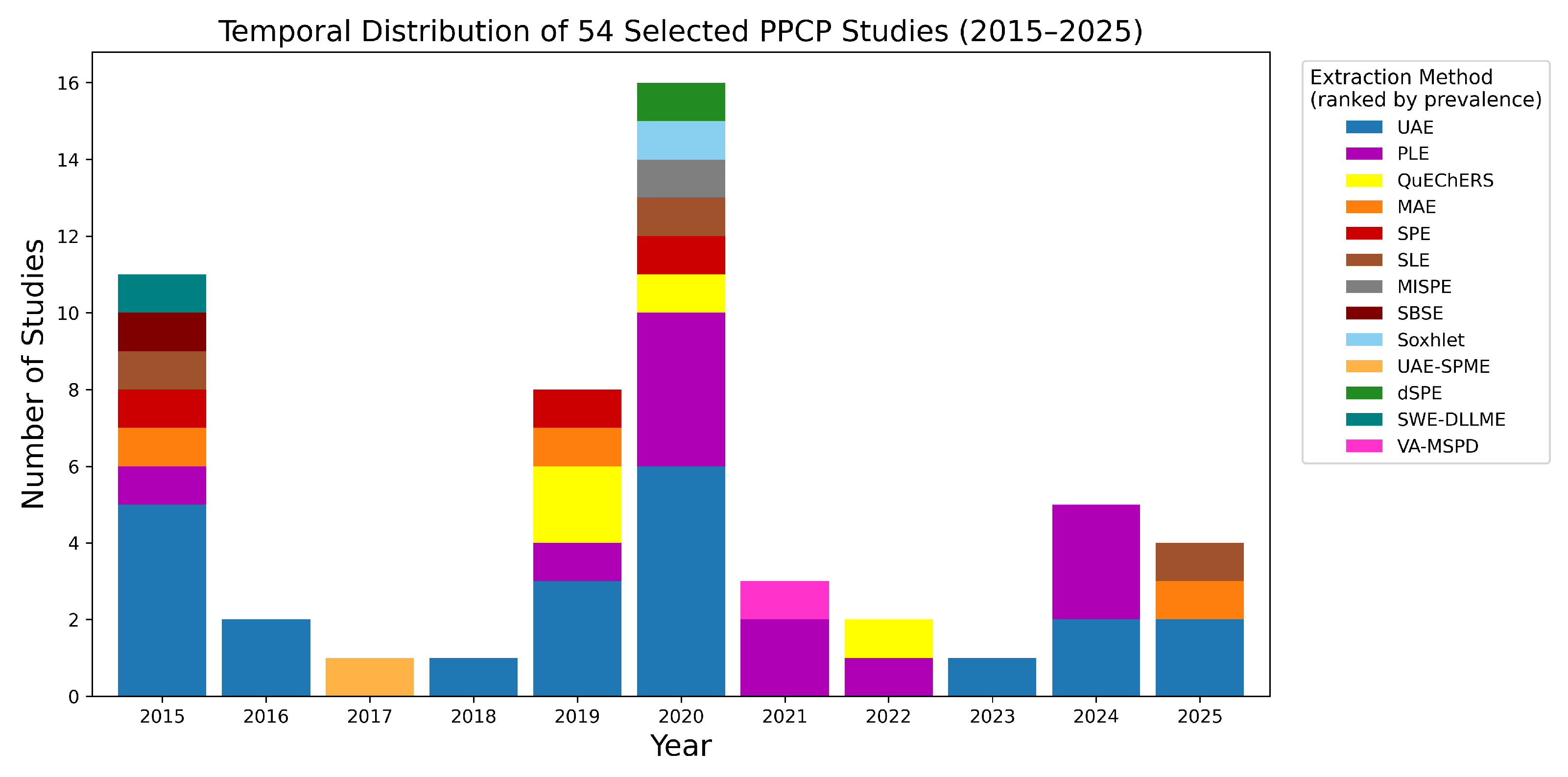
2. Searching Methodology
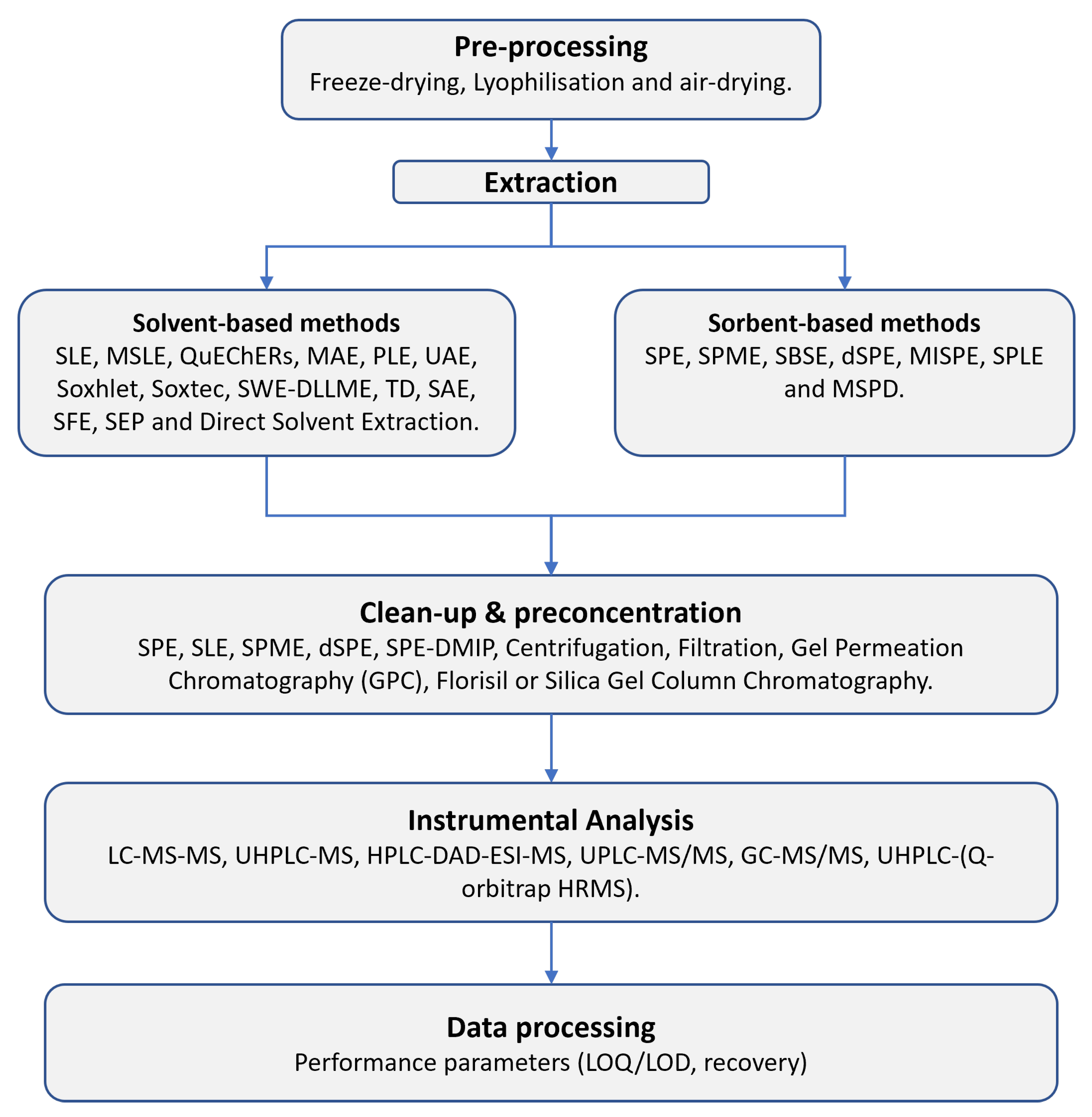
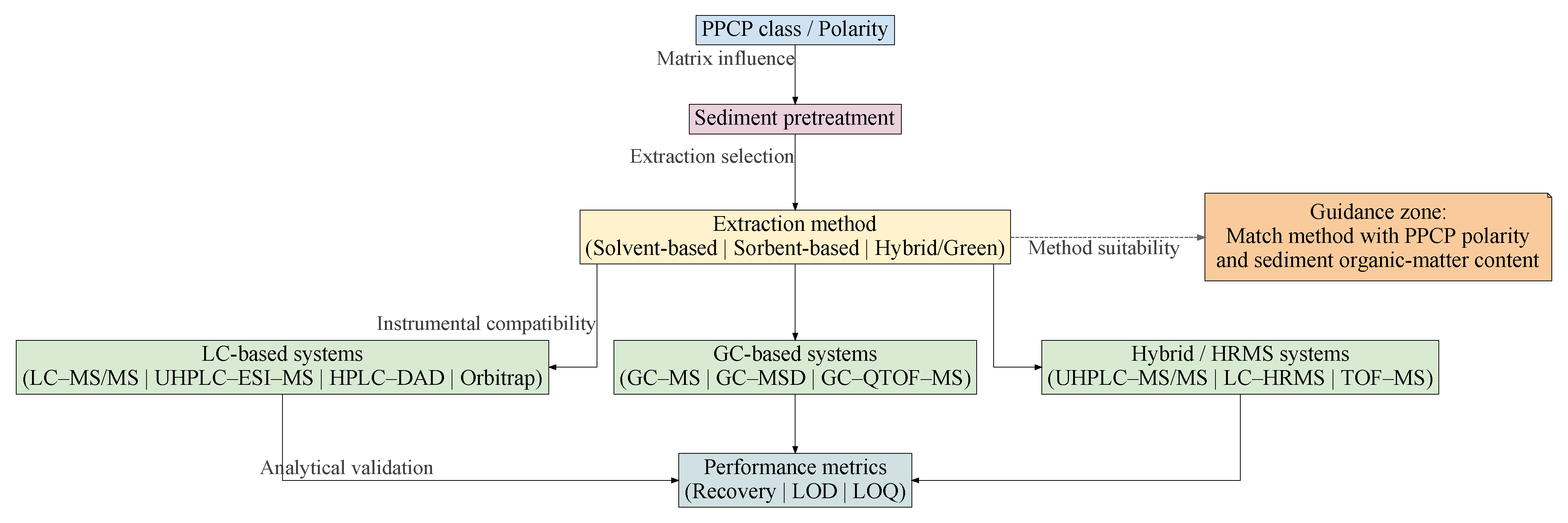
3. Generic Workflow Pipeline of Emerging Pollutant Determination
3.1. Pre-Processing
3.2. Extraction
3.2.1. Distinction Owing to Optimization Parameters
- i.
- Static mode: A fixed volume of extractant is used. This mode normally requires simpler instrumentation and lower solvent consumption [44].
- ii.
- Dynamic mode: The extractant follows a continuous flow through the sample [45], allowing improved mass transfer, faster solvent movement through the matrix, and reduced compound degradation, especially in challenging extraction situations.
- iii.
- Hybrid mode: Combines the merits of static and dynamic modes [46], aiming to achieve high efficiency while maintaining practical extraction times.
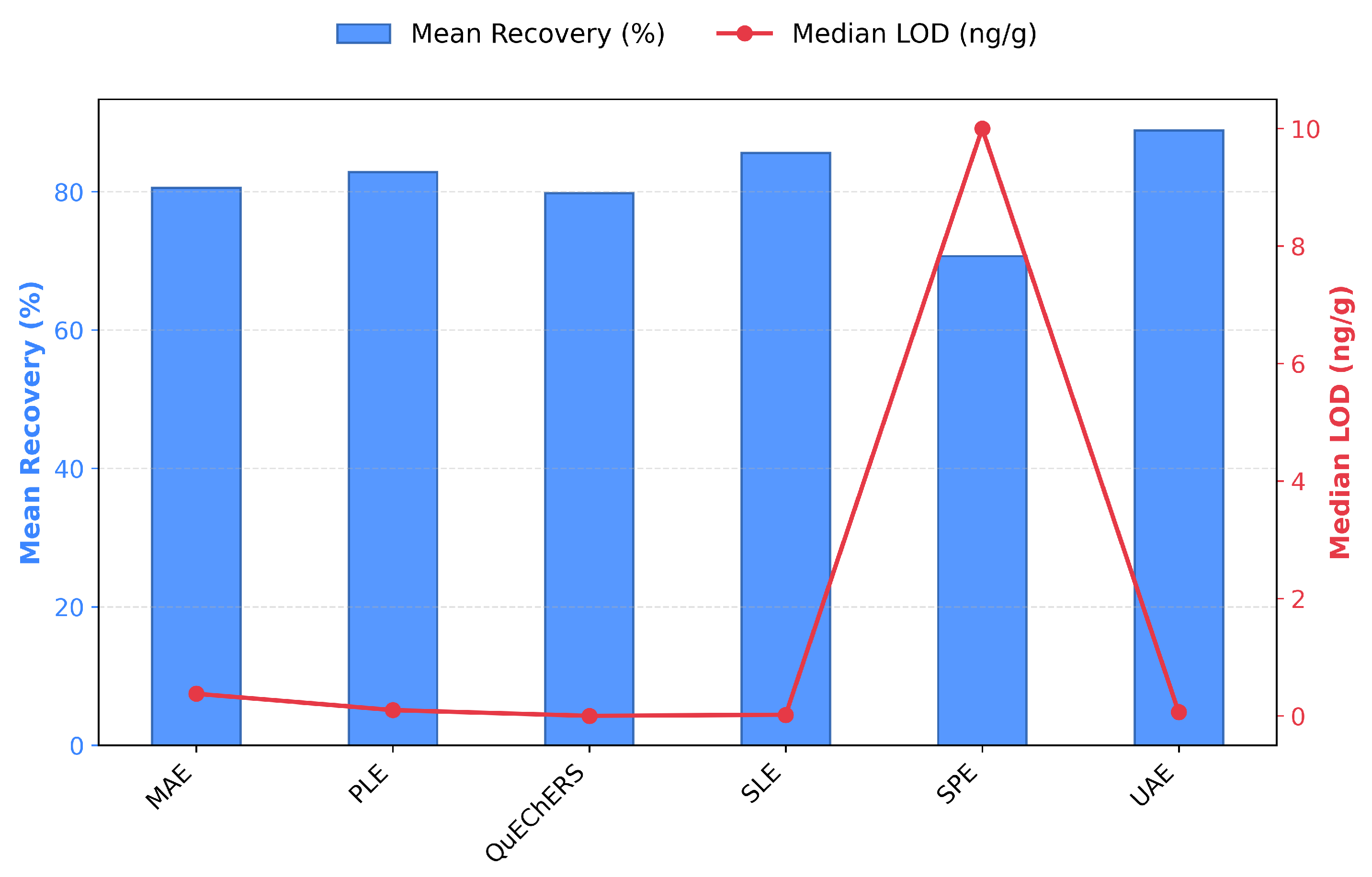
3.2.2. Key Modern Extraction Techniques for Solid Environmental Matrices
3.3. Clean-Up & Preconcentration
3.4. Instrumental Analysis
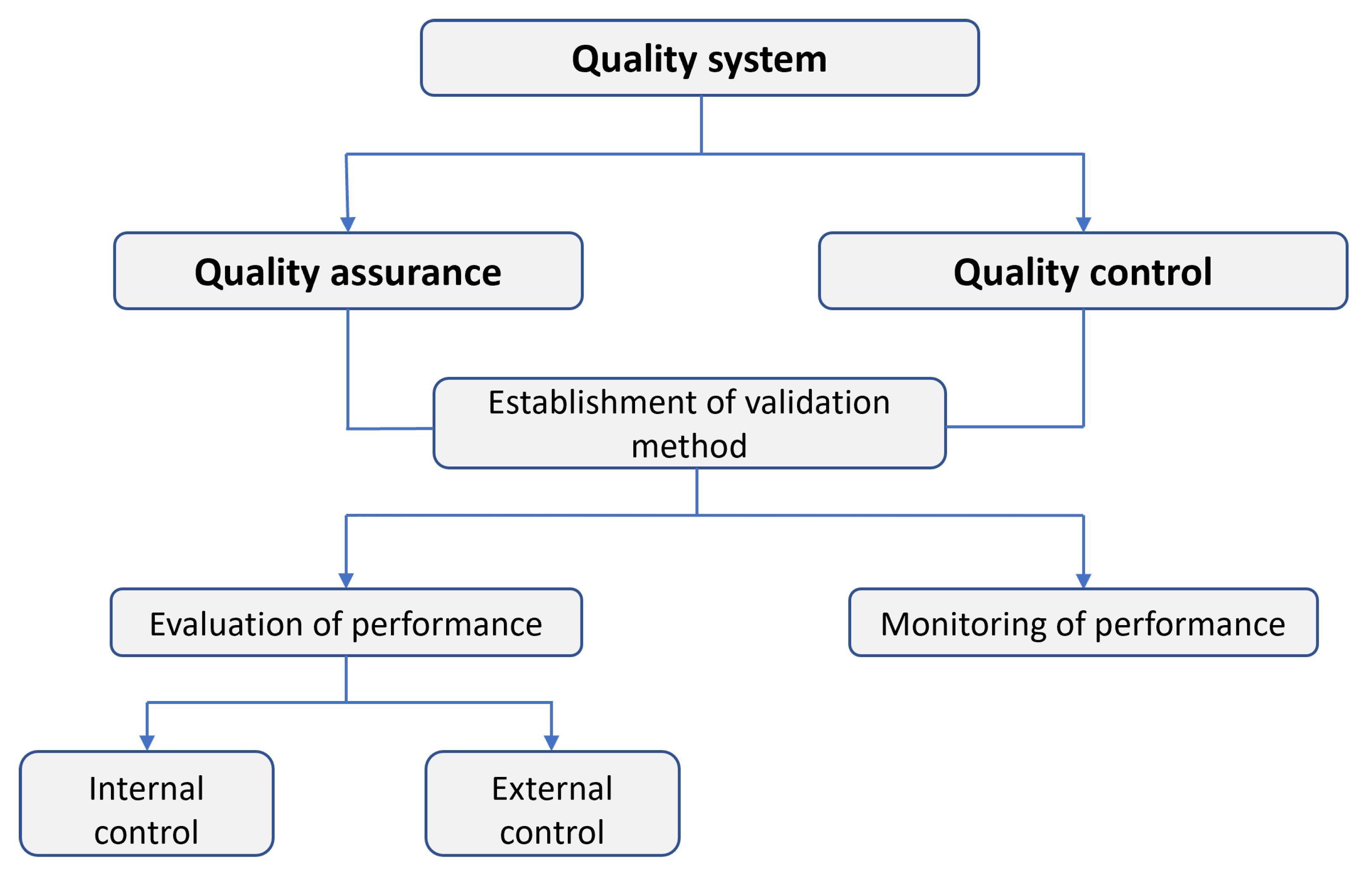
3.5. Quality Assurance and Quality Control (QA/QC)
- i
- Linearity: Define the calibration range and demonstrate a linear response if possible. If calibrants do not exhibit a linear response, appropriate data transformation should be considered.
- ii
- Limit of detection (LOD): Determine the lowest analyte concentration distinguishable from zero with 95% confidence.
- iii
- Precision: Evaluate within-day and between-day coefficients of variation at three concentration levels.
- iv
- Accuracy: Where possible, analyze certified reference materials or perform inter-laboratory comparisons.
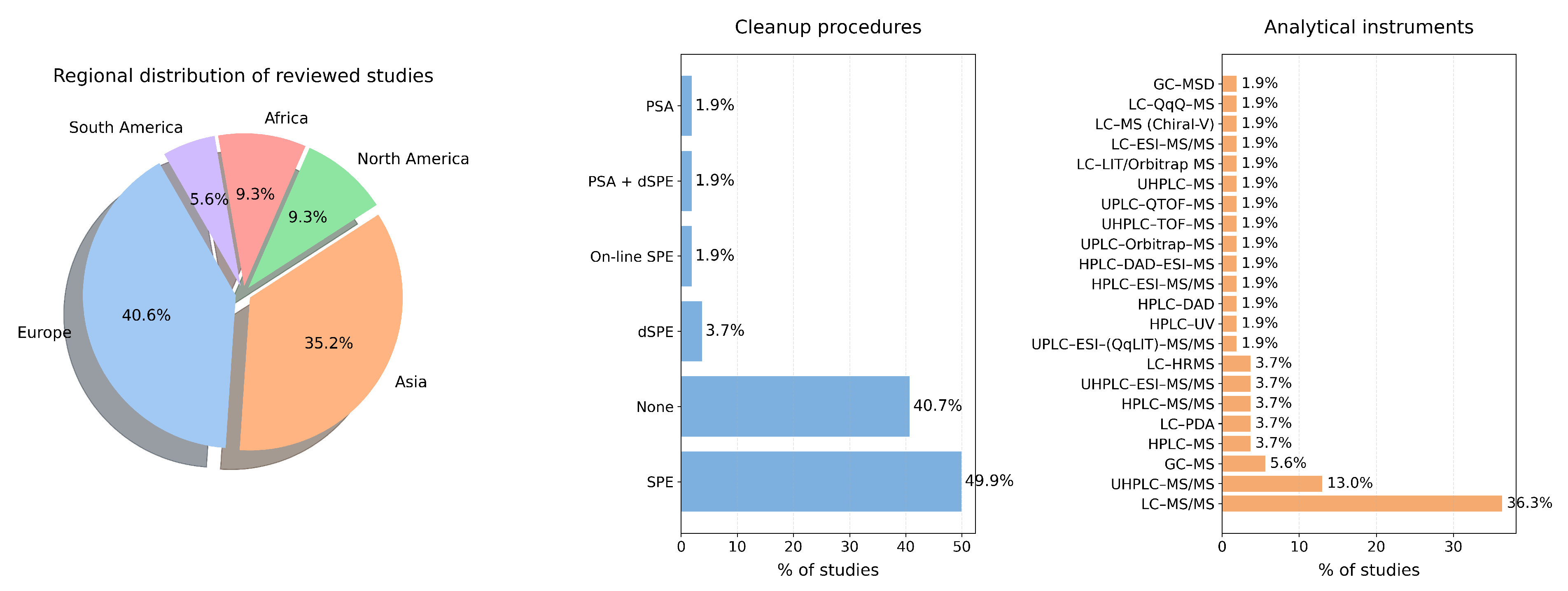
4. Taxonomy of PPCPs in Sediments
4.1. Family of Approaches in Emerging PPCPs
- i
- Heating method: While PLE uses externally applied heat in a pressurized environment, MAE relies on microwave irradiation to generate heat in the sample.
- ii
- Temperature and pressure: Accurate control of both temperature and pressure are feasible in PLE, offering more flexibility in optimizing extraction conditions. In MAE, the temperature is typically controlled by the microwave power and exposure time.
- iii
- Solvent: In MAE, solvents are typically added to the sample to enhance extraction efficiency. Instead, the use of pressurized solvents that are circulated through the sample matrix, facilitate the extraction of target compounds in PLE.
- iv
- Automation/scalability: PLE systems are typically automated and can process multiple samples concurrently, offering higher throughput. MAE configurations can also be automated but are often used for smaller-scale extractions.
| Analytes | Samples/Pre-Treatment | Extraction Method | Conditions (Extraction Process) | Determination | Recovery/LOD | Year | Ref. |
|---|---|---|---|---|---|---|---|
| 13 antibiotics: AZI, ERY, CLA | River sediments/Air-dried | PLE | in MeOH | LC-MS/MS | 80–100%, except for ERY-EE () | 2021 | [31] |
| 19 antibiotics (ABs) | River sediments/freeze-dried | PLE, Clean-up by SPE | (1:1, v/v) | LC-MS/MS | 45–125% | 2022 | [94] |
| 20 Illicit drugs | River sediments/freeze-dried | PLE, clean up by SPE | (9:1, v/v), 1250 psi at 50 °C for 5 min | LC-MS/MS | 90–135%, LOD ng/g (d.w.), except cannabinoid ng/g (d.w.) | 2021 | [32] |
| Anti-bacterial–triclocarban | River sediments/freeze-dried | PLE | (60:40, v/v) | LC–HRMS | , LOD in – ng/g (d.w.) | 2015 | [115] |
| 16 antibiotics, anticonvulsants, NSAIDs, analgesics, stimulants, antibacterial, antifungal agents, insect repellents, hormones | Surface marine sediment/freeze-dried | PLE, clean-up by SPE | (1:1, v/v) | LC–HRMS | 60–, LOD in – ng/g, LOQ in – ng/g | 2024 | [107] |
| 13 pharmaceuticals, 5 personal care products | Lake sediments/freeze-dried | PLE, clean-up by SPE | 80 °C; MeOH and (1:2, v/v) | LC–QqQ–MS | –, LOD in – ng/g, LOQ in – ng/g | 2024 | [122] |
| 36 out of 56 PPCPs (antibiotic, analgesic, antiarrhythmic, antiepileptic, antilipidemic, antitumor, blood vessel dilator, and others) | River sediments | PLE, clean-up by SPE using Oasis HLB cartridges | (1:1, v/v with (v/v) , pH = 11) | LC–MS/MS | – | 2020 | [96] |
| Pharmaceutically active compounds (PhACs): carbamazepine (anticonvulsant and mood stabilizer), ciprofloxacin (antibiotic), sulfamethoxazole (antibiotic) | Lagoon sediments/lyophilized | PLE, clean-up by SPE using Oasis HLB cartridges | MeOH, 60 °C, 3 cycles of 5 min each, pre-heating at 70 °C for 5 min | LC–MS/MS | Carbamazepine: ng/g, ng/g; Ciprofloxacin: ng/g, ng/g | 2024 | [123] |
| Chiral drugs: -blockers, antidepressants, -agonist, antihistamine, stimulants | River sediments/freeze-dried and sieved | PLE, Clean-up by SPE (Oasis HLB) | (1:1, v/v), 100 °C, 1500 psi, 2 cycles | LC-MS/Chiral-V enantioselective column | from (R(-)-chlorpheniramine) to (acebutolol-E1) | 2020 | [116] |
| 31 pharmaceuticals (NSAIDs, sulfonamides, -blockers, psychotropic drugs, hormones) | Marine sediments/freeze-dried | MAE, clean-up by SPE | 400 W, 60 °C, 10 min; (i) 10 mL 10% + sat. (9:1, v/v) + 0.5 mL MeOH; (ii) 10 mL MeOH:DCM (4:1, v/v), acidic pH (non-polar analytes) | LC–MS/MS | <20% to ∼, LOD in –, LOQ in – | 2025 | [124] |
| Veterinary medicine: anthelmintic drugs (ADs), Flubendazole (FLU) and fenbendazole (FEN) | River sediments/air-dried, grounded and sieved | MAE | 30 mL hexane:acetone (1:1, v/v) at 115 °C for 10 min, 400 W | LC-MS/MS | 98.3–103.4% | 2015 | [117] |
| 21 pharmaceuticals | River sediments/freeze-dried | UAE and centrifugation, clean-up by SPE | 2.5 mL 1 M NaCl, 2.5 mL 1 M oxalic acid and 5 mL ethanol | LC-MS/MS | From to , LOD in 1.29–7.20 ng/g | 2023 | [125] |
| 6 pharmaceuticals | River sediments/freeze-dried | UAE, clean-up by SPE | with in MeOH | LC–MS/MS | –, LOD in – ng/g | 2018 | [126] |
| 11 abused drugs (amphetamine, METH, heroin, ketamine, ephedrine, cocaine, codeine, methadone, morphine, benzoylecgonine, methcathinone) | River sediments/freeze-dried and sieved | UAE and centrifugation | 10 mL McIlvain buffer:MeOH (1:1, v/v) | LC-MS/MS | 68–101%, LOD in 0.20–1.50 ng/g | 2019 | [108] |
| 22 out of 28 antibiotics | River sediments/freeze-dried, ground, sieved | UAE & centrifugation | 10 mL ACN:citric acid buffer, pH = 3, (1:1, v/v) | LC-MS/MS | 53–149% | 2020 | [95] |
| 12 antimicrobials | Dam sediments/lyophilization and sieving | UAE, clean-up by on-line SPE | ACN:Citrate Buffer, pH = 3 | LC-MS/MS | 89–119%, LOD in 0.40–5.1 g/kg | 2016 | [66] |
| 32 antibiotics | River sediments/freeze-dried | UAE, clean-up by SPE | McIlvaine buffer:ACN (1:1, v/v) and -·O (96:4, v/v) | LC-MS/MS | 40–127%, (except enrofloxacin and marbofloxacin) LOD in 0.01–0.45 ng/g (d.w.) | 2015 | [97] |
| Antibiotic and antiretroviral drug cocktails (ARVDs) | River sediments/dried | UAE | (4:1, v/v) | LC-ESI-MS/MS | 84.3–111.3% | 2020 | [98] |
| 41 drugs of abuse and metabolites | River sediments/lyophilized | UAE, Clean-up by SPE, MeOH & MeOH–DCM elution | :MeOH (1:1, v/v) pH 4.5 for 10 min | LC-MS/MS | ≥, ng/g (d.w.) | 2015 | [23] |
| 25 pharmaceutical compounds (14 antibiotics, 4 anti-epileptic and antidepressant drugs, 3 analgesic and anti-inflammatory agents, 3 beta-blockers, and 1 lipid regulator) | Lake sediments/freeze-dried | UAE, Clean-up by SPE | ACN & pH 3 citric acid buffer, 15 min | LC–MS/MS | 70–111%, LOD in – ng/kg, LOQ in – ng/kg | 2024 | [127] |
| NSAIDs & anti-epileptic drugs | River sediment/air-dried | UAE, clean-up by SPE | 20 mL ACN:MeOH (1:1, v/v) | LC-PDA | 74–112%, LOD in 0.010–0.027 g/kg | 2020 | [109] |
| , , , | River sediments | UAE | 2 cycles acetone & 2 cycles MeOH, for 25 min | LC–PDA | 69–, LOD in – g/kg, LOQ in – g/kg | 2025 | [128] |
| 85 PPCPs | Estuary sediments | QuEChERS, clean-up by PSA, Secondary Clean-up dSPE | 20 mL ACN:- (MQW, purified ) (1:1, v/v) | LC-MS/MS | from (sulfamethoxazole-13C6), to (alprazolam-d5) | 2022 | [129] |
| Blood lipid regulators, analgesics, anti-inflammatory drugs and -blockers | Lagoon sediments/freeze-dried | QuEChERS, clean up by dSPE | 10 mL buffered ACN | LC-MS/MS | 27–120%, LOD in 0.001– | 2019 | [91] |
| 25 multiclass pharmaceuticals | River sediments/lyophilized | QuEChERS, clean-up by dSPE | 10 mL ACN | LC-LIT/Orbitrap MS | 64–101% | 2019 | [119] |
| EDCs | Marine sediment/air-dried, ground and sieved | Soxhlet, clean-up by SPE | acetone (1:1, v/v) for 8 h | LC-MS/MS | 50.39–129.10% | 2020 | [16] |
| 4 (BLs) and 4 polyether ionophore antibiotics (PEs) | River sediments | SPE | 5 mL MeOH | LC-MS/MS | >, except for AMOX | 2015 | [37] |
| 40 antibiotics (cephalosporin, fluoroquinolone, lincosamide, macrolide, nitroimidazole, quinolone, sulfonamide and tetracycline groups) | River sediment/freeze-dried and homogenized | dSPE by using 100 mg mix of C18 and PSA (1:2, w/w) and 50 mg | 5 mL MeOH:ACN (1:3, v/v) | LC-MS/MS | 24–162%, 48–151%, 51–159%, and 50–149% for 10, 20, 50 and 100 g/kg spiking levels, respectively | 2020 | [99] |
| 19 anthelmintic drugs (ADs) | River sediments/air-dried | PLE, Clean-up by SPE | MeOH: (1:1, v/v) at 70 °C, 100 bar, 5 min | UHPLC-ESI-MS/MS | 31–90% | 2020 | [111] |
| 34 pharmaceuticals | River sediments/freeze-dried, ground and sieved | UAE and centrifugation, clean-up by SPE with Oasis HLB cartridges | 10 mL citrate buffer (pH 3): ACN (1:1, v/v) | UHPLC-MS/MS | 43–118%, LOD in 0.01–0.6 ng/g (d.w.) | 2019 | [4] |
| 7 Pharmaceuticals, 1 Personal Care Product and 3 Hormones | River sediments/dried | UAE, clean-up by SPE | 5 mL MeOH→ 5 mL MeOH: (1:1, v/v)→ 2 mL acetone | UHPLC-MS/MS | 54.0–94.4% | 2015 | [130] |
| 4 pharmaceuticals: Anti-inflammatory (Ketorolac, Naproxen), Antibiotics (Ofloxacin, Ciprofloxacin), Anti-cancer (Ifosfamide, Cyclophosphamide), -Blockers (Atenolol, Propranolol) | Less complex matrices of sediments | UAE | EtOAc:MeOH (1:1, v/v) | UHPLC-MS/MS | 87–113%, ng/g | 2015 | [100] |
| 111 organic micropollutants (OMPs), anti-epileptic, antineoplastic agents, antihistamine | Lake sediments/air-dried | UAE | (i) (1:1, v/v), formic acid (FA), (ii) ACN, 2-propanol, and (3/3/4, v/v/v, FA) | UHPLC-MS/MS | 81–104% | 2020 | [92] |
| 25 PhACs in 6 categories: 9 antibiotics, 9 hormones, 3 NSAIDs, 2 antipsychotic drugs, 1 hypoglycemic drug, 1 antiviral drug | Marine sediments/freezed | UAE, clean-up by SPE | 20 mL Citric acid buffer (pH 3):ACN (1:1, v/v) | UHPLC-ESI-MS/MS | 61–117% | 2020 | [101] |
| 22 EDCs | River and lake sediments/lyophilized and sieved | UAE, clean-up by SPE with GCB cartridge | 10 mL MeOH:acetone (1:1, v/v) | UHPLC–MS | 75–110% | 2016 | [112] |
| Pharmaceuticals | Marine surface sediments/freeze-dried | UAE, clean-up by SPE using Oasis HLB cartridges | (2:1, v/v) for 15 min at 25 °C | UPLC–QTOF–MS | 75–, LOD in – ng/g | 2025 | [131] |
| 30 PPCPs | River sediments/freeze-dried | UAE, clean-up by SPE | (1:1) | UPLC–Orbitrap/MS | 74–, LOD: – ng/g, LOQ: – ng/g | 2024 | [132] |
| 21 Psychiatric drugs (Carbamazepine, citalopram, fluoxetine, sertraline, trazodone, and venlafaxine) and 6 antibiotics (azithromycin, ciprofloxacin, clarithromycin, moxifloxacin, ofloxacin, and trimethoprim) | River sediments | QuEChERS, clean-up by SPE | 0.5 mL of ACN: ultra-pure water (UPW) (3:7, v/v) | UHPLC-MS/MS | Psychiatric drugs: from (sertraline) to (diazepam). Sulfonamide and fluoroquinolone antibiotics: < and <, respectively. | 2020 | [102] |
| 32 PPCPs | Lagoon and estuary sediments/lyophilized | 2 × SPE: (i) HLB cartridges activated with SDS, (ii) mixed HLB-cation exchange cartridges | 12 mL MeOH: - (1:1, v/v) | UHPLC-MS/MS | of the compounds had recoveries >, ng/g | 2020 | [86] |
| Cardiovascular drugs (atorvastatin, fenofibrate, bezafibrate), anticonvulsant and mood stabilizers (Carbamazepine), benzodiazepine medication (diazepam), opioid medications (codeine, morphine) | River sediments/freeze-dried | SLE, cleaned up by stir-disc SPE | (1:1, v/v) | UHPLC-MS/MS | 50–111%, LOD in 0.02–9.9 ng/g | 2020 | [118] |
| 30 pharmaceuticals | Marine sediments | SLE | ACN + (9:1, v/v) | UHPLC–TOF–MS | –, LOD: – ng/g, LOQ: – ng/g | 2025 | [65] |
| 46 PPs: Antibiotic, anti-inflammatory and cardiovascular | Marine sediments | PLE | (1:1, v/v) | HPLC-MS | > | 2020 | [103] |
| NSAIDs, anticonvulsants, sulfonamides, diuretics, lipid-lowering drugs | Pond sediments/freeze-dried and grounded | MAE | 20 mL of 35/35/30 of MeOH/acetone/miliQ | HPLC-MS | , LOD in 0.69–35.68 ng/g | 2019 | [133] |
| Pharmaceuticals (antibiotics, antipyretics, stimulant, antiepileptic and antipsychotic drugs) | River sediments | UAE and centrifugation, Clean-up by SPE | mL MeOH, 50 mL acetone: Acetic acid (20:1, v/v) and 50 mL EtOAc | HPLC-DAD-ESI-MS | 71.20–118%, LOD in 0.0006–0.7986 ng/g | 2015 | [104] |
| 12 Antibiotics | Lake sediments/lyophilized | UAE & Centrifugation | 30 mL (ACN: EDTA-Mcilvaine buffer, (1:1, v/v) | HPLC–ESI MS/MS | 72.5–113.5%, LOD in 0.11–1.15 g/kg | 2015 | [105] |
| Fluoroquinolone antibiotics (NFX) | Marine sediments/dried | Molecularly imprinted solid-phase extraction (MISPE) | 10 mL DCM, sonicated 20 min, centrifuged 5 min at 4000 rpm | HPLC-DAD | 75.5–91.7%, g/kg | 2020 | [106] |
| Preservatives-parabens (PCPs) | Marine sediments/freeze-dried | off-line SLE/SPE Clean-up with Oasis MCX | (i) 7 mL MeOH for 60 min, (ii) 7 mL of EtOAc | HPLC-MS/MS | 57–105% (blanks), 53–112% (spiked) | 2019 | [134] |
| Pharmaceuticals and personal care products (PPCPs) | Marine sediments/freeze-dried | VA-MSPD | MeOH | HPLC-MS/MS | 60–140%, LOD in 0.13–5.70 ng/g | 2021 | [121] |
| Polar, semi-polar, apolar compounds (NSAIDs & others) | River sediments/dried | stir bar sorptive extraction (SBSE) | Aqueous SBSE (no solvent); desorption with MeOH | HPLC–UV | 81.8–121.3%, LOD in 0.09–0.81 g/L | 2015 | [110] |
| 5 PPs: NSAIDs, antibiotics and antidepressants | River sediments/freeze-dried | SPE | 5 mL MeOH followed by 5 mL deionized water at a rate of 1 mL/min | Ultraperformance TM-ESI-(QqLIT) MS/MS | Ibuprofen , Paracetamol , Diclofenac , Trimethoprim , Citalopram | 2019 | [135] |
| EDCs (such as bisphenol A (BPA) and nonylphenol (NP) | Lake sediments/lyophilized and sieved | UAE | 5 mL Acetone:EtOAc | GC-MS | BPA:, NP: , LOD = (BPA) and (NP) ng/L | 2019 | [113] |
| 8 PPCPs | River sediments/lyophilized | UAE–SPME | 7 mL deionized (pH 3) & MeOH | GC–MS | 56–108%, ng/g | 2017 | [136] |
| 2 PCMs: HHCB and AHTN | River sediments/freeze-dried | UAE & centrifugation | 50 mL of MeOH | GC-MSD | HHCB , AHTN | 2020 | [120] |
| 13 EDCs | River sediments/freeze-dried and sieved | SWE-DLLME | Chlorobenzene (CBz) for DLLME and acetone for SWE at 150 °C | GC–MS | (dienestrol), (4,5-dihydrotestosterone), except for diethyl stilbestrol () and nonylphenols (), LOD in 0.006–0.639 ng/g | 2015 | [114] |
4.2. Discussion on Our Findings
5. Conclusions
Future Research Agenda and Knowledge Gaps
- (i)
- Harmonization and inter-laboratory comparability. The lack of harmonized reference procedures remains a major barrier in environmental-matrix analyses. Establishing validated, consensus protocols for extraction, clean-up, and quantification, along with structured inter-laboratory comparisons and personnel training, will improve data reliability and enable reproducible and comparable results across independent facilities.
- (ii)
- Transformation products (TPs) and real-matrix evaluation. A significant knowledge gap concerns transformation products: Approximately 40–45% of PPCPs lack TP information, and most available data derive from synthetic rather than real sediment matrices [137]. Future studies should prioritize matrix-realistic conditions, harmonized TP identification workflows, and toxicity evaluation of environmentally persistent by-products.
- (iii)
- Miniaturized and solvent-free extraction technologies. Advances in green analytical chemistry should focus on developing miniaturized, low-solvent, or solvent-free extraction systems, such as deep-eutectic-solvent- or MSPD-based approaches. Emphasis should be placed on solvent reusability, energy efficiency, and quantitative greenness assessment to align PPCP analysis with sustainability principles.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vargas-Berrones, K.; Bernal-Jácome, L.; Díaz de León-Martínez, L.; Flores-Ramírez, R. Emerging pollutants (EPs) in Latin América: A critical review of under-studied EPs, case of study -Nonylphenol-. Sci. Total Environ. 2020, 726, 138493. [Google Scholar] [CrossRef]
- Bunke, D.; Moritz, S.; Brack, W.; Herráez, D.L.; Posthuma, L.; Nuss, M. Developments in society and implications for emerging pollutants in the aquatic environment. Environ. Sci. Eur. 2019, 31, 32. [Google Scholar] [CrossRef]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Xie, H.; Hao, H.; Xu, N.; Liang, X.; Gao, D.; Xu, Y.; Gao, Y.; Tao, H.; Wong, M. Pharmaceuticals and personal care products in water, sediments, aquatic organisms, and fish feeds in the Pearl River Delta: Occurrence, distribution, potential sources, and health risk assessment. Sci. Total Environ. 2019, 659, 230–239. [Google Scholar] [CrossRef]
- Teodosiu, C.; Gilca, A.F.; Barjoveanu, G.; Fiore, S. Emerging pollutants removal through advanced drinking water treatment: A review on processes and environmental performances assessment. J. Clean. Prod. 2018, 197, 1210–1221. [Google Scholar] [CrossRef]
- Vaz, S., Jr. Analytical Chemistry Applied to Emerging Pollutants; Springer: Berlin, Germany, 2018. [Google Scholar] [CrossRef]
- Wang, H.; Xi, H.; Xu, L.; Jin, M.; Zhao, W.; Liu, H. Ecotoxicological effects, environmental fate and risks of pharmaceutical and personal care products in the water environment: A review. Sci. Total Environ. 2021, 788, 147819. [Google Scholar] [CrossRef]
- Kasonga, T.K.; Coetzee, M.A.; Kamika, I.; Ngole-Jeme, V.M.; Benteke Momba, M.N. Endocrine-disruptive chemicals as contaminants of emerging concern in wastewater and surface water: A review. J. Environ. Manag. 2021, 277, 111485. [Google Scholar] [CrossRef] [PubMed]
- Pironti, C.; Ricciardi, M.; Proto, A.; Bianco, P.M.; Montano, L.; Motta, O. Endocrine-Disrupting Compounds: An Overview on Their Occurrence in the Aquatic Environment and Human Exposure. Water 2021, 13, 1347. [Google Scholar] [CrossRef]
- la Farré, M.; Pérez, S.; Kantiani, L.; Barceló, D. Fate and toxicity of emerging pollutants, their metabolites and transformation products in the aquatic environment. TrAC Trends Anal. Chem. 2008, 27, 991–1007. [Google Scholar] [CrossRef]
- Vasilachi, I.C.; Asiminicesei, D.M.; Fertu, D.I.; Gavrilescu, M. Occurrence and Fate of Emerging Pollutants in Water Environment and Options for Their Removal. Water 2021, 13, 181. [Google Scholar] [CrossRef]
- García-Córcoles, M.T.; Rodríguez-Gómez, R.; de Alarcón-Gómez, B.; Çipa, M.; Martín-Pozo, L.; Kauffmann, J.M.; Zafra-Gómez, A. Chromatographic Methods for the Determination of Emerging Contaminants in Natural Water and Wastewater Samples: A Review. Crit. Rev. Anal. Chem. 2019, 49, 160–186. [Google Scholar] [CrossRef]
- Bainbridge, Z.; Lewis, S.; Bartley, R.; Fabricius, K.; Collier, C.; Waterhouse, J.; Garzon-Garcia, A.; Robson, B.; Burton, J.; Wenger, A.; et al. Fine sediment and particulate organic matter: A review and case study on ridge-to-reef transport, transformations, fates, and impacts on marine ecosystems. Mar. Pollut. Bull. 2018, 135, 1205–1220. [Google Scholar] [CrossRef]
- Datta, A.R.; Kang, Q.; Chen, B.; Ye, X. Chapter Four—Fate and Transport Modelling of Emerging Pollutants from Watersheds to Oceans: A Review. In Emerging Pollutants and Their Effects on Marine Ecosystems; Advances in Marine Biology; Chen, B., Zhang, B.H., Zhu, Z.J., Lee, K., Eds.; Academic Press: Oxford, UK, 2018; Volume 81, pp. 97–128. [Google Scholar] [CrossRef]
- Law, J.C.F.; Huang, Y.; Chow, C.H.; Lam, T.K.; Leung, K.S.Y. Comparative physicochemical properties and toxicity of organic UV filters and their photocatalytic transformation products. Environ. Pollut. 2021, 286, 117551. [Google Scholar] [CrossRef]
- Ismail, N.A.H.; Wee, S.Y.; Haron, D.E.M.; Kamarulzaman, N.H.; Aris, A.Z. Occurrence of endocrine disrupting compounds in mariculture sediment of Pulau Kukup, Johor, Malaysia. Mar. Pollut. Bull. 2020, 150, 110735. [Google Scholar] [CrossRef]
- aus der Beek, T.; Weber, F.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the Environment—Global Occurrences and Perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Katsikaros, A.G.; Chrysikopoulos, C.V. Occurrence and Distribution of Pharmaceuticals and Personal Care Products (PPCPs) Detected in Lakes Around the World—A Review. Environ. Adv. 2021, 6, 100131. [Google Scholar] [CrossRef]
- Kayode-Afolayan, S.D.; Ahuekwe, E.F.; Nwinyi, O.C. Impacts of Pharmaceutical Effluents on Aquatic Ecosystems. Sci. Afr. 2022, 17, e01288. [Google Scholar] [CrossRef]
- El-Fattah, H.A.; Maged, A.; Kamel, R.; Kharbish, S. Recent Technologies for the Elimination of Pharmaceutical Compounds from Aqueous Solutions: A Review. Front. Sci. Res. Technol. 2023, 5, 52–72. [Google Scholar] [CrossRef]
- Boahen, E.; Owusu, L.; Adjei-Anim, S.O. A Comprehensive Review of Emerging Environmental Contaminants of Global Concern. Discov. Environ. 2025, 3, 144. [Google Scholar] [CrossRef]
- Peng, X.; Xiong, S.; Ou, W.; Wang, Z.; Tan, J.; Jin, J.; Tang, C.; Liu, J.; Fan, Y. Persistence, temporal and spatial profiles of ultraviolet absorbents and phenolic personal care products in riverine and estuarine sediment of the Pearl River catchment, China. J. Hazard. Mater. 2017, 323, 139–146. [Google Scholar] [CrossRef]
- Álvarez-Ruiz, R.; Andrés-Costa, M.J.; Andreu, V.; Picó, Y. Simultaneous determination of traditional and emerging illicit drugs in sediments, sludges and particulate matter. J. Chromatogr. A 2015, 1405, 103–115. [Google Scholar] [CrossRef]
- Simpson, S.; Batley, G. (Eds.) Sediment Quality Assessment: A Practical Guide, 2nd ed.; CSIRO Publishing: Clayton, Australia, 2016; p. 346. [Google Scholar] [CrossRef]
- Albaseer, S.S.; Nageswara Rao, R.; Swamy, Y.; Mukkanti, K. An overview of sample preparation and extraction of synthetic pyrethroids from water, sediment and soil. J. Chromatogr. A 2010, 1217, 5537–5554. [Google Scholar] [CrossRef]
- Otte, J.M.; Blackwell, N.; Soos, V.; Rughöft, S.; Maisch, M.; Kappler, A.; Kleindienst, S.; Schmidt, C. Sterilization impacts on marine sediment—Are we able to inactivate microorganisms in environmental samples? FEMS Microbiol. Ecol. 2018, 94, fiy189. [Google Scholar] [CrossRef]
- Cabrol, L.; Quéméneur, M.; Misson, B. Inhibitory effects of sodium azide on microbial growth in experimental resuspension of marine sediment. J. Microbiol. Methods 2017, 133, 62–65. [Google Scholar] [CrossRef]
- Ouendi, F.; Zentar, R. Investigating the Influence of Particle Size Ranges on the Physical, Mineralogical, and Environmental Properties of Raw Marine Sediment. Constr. Build. Mater. 2023, 409, 133987. [Google Scholar] [CrossRef]
- Zentar, R.; Ouendi, F.; Wang, H. Effects of Sample Preparation Methods on Measured Characteristics of Marine and Fluvial Sediment. Int. J. Sediment Res. 2024, 39, 15–27. [Google Scholar] [CrossRef]
- Wu, Z.; Yin, Y.; Pan, W.; Tang, J.; Cao, X.; Zhang, W. New Perspective into the Impact of Drying Pretreatment on Phosphorus Performance in Sediments. Curr. Pollut. Rep. 2024, 10, 362–373. [Google Scholar] [CrossRef]
- Senta, I.; Terzic, S.; Ahel, M. Analysis and occurrence of macrolide residues in stream sediments and underlying alluvial aquifer downstream from a pharmaceutical plant. Environ. Pollut. 2021, 273, 116433. [Google Scholar] [CrossRef]
- López-García, E.; Mastroianni, N.; Ponsá-Borau, N.; Barceló, D.; Postigo, C.; López de Alda, M. Drugs of abuse and their metabolites in river sediments: Analysis, occurrence in four Spanish river basins and environmental risk assessment. J. Hazard. Mater. 2021, 401, 123312. [Google Scholar] [CrossRef]
- Xie, J.; Sun, Y.; Cheng, Y.; Chen, Y.; Chen, L.; Xie, C.; Dai, S.; Luo, X.; Zhang, L.; Mai, B. Halogenated flame retardants in surface sediments from fourteen estuaries, South China. Mar. Pollut. Bull. 2021, 164, 112099. [Google Scholar] [CrossRef]
- Hoang, A.Q.; Aono, D.; Watanabe, I.; Kuwae, M.; Kunisue, T.; Takahashi, S. Contamination levels and temporal trends of legacy and current-use brominated flame retardants in a dated sediment core from Beppu Bay, southwestern Japan. Chemosphere 2021, 266, 129180. [Google Scholar] [CrossRef]
- Qi, Y.; He, Z.; Yuan, J.; Ma, X.; Du, J.; Yao, Z.; Wang, W. Comprehensive evaluation of organophosphate ester contamination in surface water and sediment of the Bohai Sea, China. Mar. Pollut. Bull. 2021, 163, 112013. [Google Scholar] [CrossRef] [PubMed]
- Alkan, N.; Alkan, A.; Castro-Jiménez, J.; Royer, F.; Papillon, L.; Ourgaud, M.; Sempéré, R. Environmental occurrence of phthalate and organophosphate esters in sediments across the Gulf of Lion (NW Mediterranean Sea). Sci. Total Environ. 2021, 760, 143412. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Yang, S.; Carlson, K.H. Occurrence of β-lactam and polyether ionophore antibiotics in surface water, urban wastewater, and sediment. Geosyst. Eng. 2015, 18, 140–150. [Google Scholar] [CrossRef]
- Hyötyläinen, T. Critical evaluation of sample pretreatment techniques. Anal. Bioanal. Chem. 2009, 394, 743–758. [Google Scholar] [CrossRef]
- Pintado-Herrera, M.G.; González-Mazo, E.; Lara-Martín, P.A. Environmentally friendly analysis of emerging contaminants by pressurized hot water extraction–stir bar sorptive extraction–derivatization and gas chromatography–mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Biel-Maeso, M.; Corada-Fernández, C.; Lara-Martín, P.A. Determining the distribution of pharmaceutically active compounds (PhACs) in soils and sediments by pressurized hot water extraction (PHWE). Chemosphere 2017, 185, 1001–1010. [Google Scholar] [CrossRef]
- Wang, L.; Sun, H.; Zhang, G.; Sun, S.; Fu, X. Application of ionic liquids for the extraction and passive sampling of endocrine-disrupting chemicals from sediments. J. Soils Sediments 2013, 13, 450–459. [Google Scholar] [CrossRef]
- Mortada, W.I. Recent developments and applications of cloud point extraction: A critical review. Microchem. J. 2020, 157, 105055. [Google Scholar] [CrossRef]
- Pereira, F.P. Miniaturization in Sample Preparation; De Gruyter Open Poland: Warsaw, Poland, 2014. [Google Scholar] [CrossRef]
- Ramos, L.; Vreuls, J.; Brinkman, U. Miniaturised pressurised liquid extraction of polycyclic aromatic hydrocarbons from soil and sediment with subsequent large-volume injection–gas chromatography. J. Chromatogr. A 2000, 891, 275–286. [Google Scholar] [CrossRef]
- Sun, M.; Al-hamimi, S.; Sandahl, M.; Turner, C. Dynamic extraction coupled on-line to liquid chromatography with a parallel sampling interface—A proof of concept for monitoring extraction kinetics. Anal. Bioanal. Chem. 2019, 411, 3675–3683. [Google Scholar] [CrossRef]
- Luque-Garciá, J.; Luque de Castro, M. Comparison of the static, dynamic and static-dynamic pressurised liquid extraction modes for the removal of nitrated polycyclic aromatic hydrocarbons from soil with on-line filtration-preconcentration. J. Chromatogr. A 2003, 1010, 129–140. [Google Scholar] [CrossRef]
- Teo, C.C.; Tan, S.N.; Yong, J.W.H.; Hew, C.S.; Ong, E.S. Pressurized hot water extraction (PHWE). J. Chromatogr. A 2010, 1217, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Morales-Muñoz, S.; Luque-García, J.L.; Luque de Castro, M.D. Pressurized Hot Water Extraction with On-Line Fluorescence Monitoring: A Comparison of the Static, Dynamic, and Static-Dynamic Modes for the Removal of Polycyclic Aromatic Hydrocarbons from Environmental Solid Samples. Anal. Chem. 2002, 74, 4213–4219. [Google Scholar] [CrossRef] [PubMed]
- Naeeni, M.H.; Yamini, Y.; Rezaee, M. Combination of supercritical fluid extraction with dispersive liquid–liquid microextraction for extraction of organophosphorus pesticides from soil and marine sediment samples. J. Supercrit. Fluids 2011, 57, 219–226. [Google Scholar] [CrossRef]
- Mandal, S.C.; Mandal, V.; Das, A.K. Chapter 6—Classification of Extraction Methods. In Essentials of Botanical Extraction; Mandal, S.C., Mandal, V., Das, A.K., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 83–136. [Google Scholar] [CrossRef]
- Pintado-Herrera, M.G.; González-Mazo, E.; Lara-Martín, P.A. In-cell clean-up pressurized liquid extraction and gas chromatography–tandem mass spectrometry determination of hydrophobic persistent and emerging organic pollutants in coastal sediments. J. Chromatogr. A 2016, 1429, 107–118. [Google Scholar] [CrossRef]
- Santana-Viera, S.; Tuček, J.; Torres-Padrón, M.E.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J.; Halko, R. Cytostatic compounds in sludge and sediment: Extraction and determination by a combination of microwave-assisted extraction and UHPLC–MS/MS. Anal. Bioanal. Chem. 2020, 412, 3639–3651. [Google Scholar] [CrossRef]
- Baur, S.; Reemtsma, T.; Stärk, H.J.; Wagner, S. Surfactant assisted extraction of incidental nanoparticles from road runoff sediment and their characterization by single particle-ICP-MS. Chemosphere 2020, 246, 125765. [Google Scholar] [CrossRef] [PubMed]
- Picó, Y. 3.28—Recent Advances in Sample Preparation for Pesticide Analysis. In Comprehensive Sampling and Sample Preparation; Pawliszyn, J., Ed.; Academic Press: Oxford, UK, 2012; pp. 569–590. [Google Scholar] [CrossRef]
- Tena, M.T. Extraction | Pressurized Liquid Extraction. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Oxford, UK, 2019; pp. 78–83. [Google Scholar] [CrossRef]
- ElKhori, S.; Paré, J.J.; Bélanger, J.M.; Pérez, E. The microwave-assisted process (MAPTM1): Extraction and determination of fat from cocoa powder and cocoa nibs. J. Food Eng. 2007, 79, 1110–1114. [Google Scholar] [CrossRef]
- Chan, C.H.; Yusoff, R.; Ngoh, G.C.; Kung, F.W.L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef]
- Weggler, B.A.; Gruber, B.; Teehan, P.; Jaramillo, R.; Dorman, F.L. Chapter 5—Inlets and sampling. In Basic Multidimensional Gas Chromatography; Separation Science and Technology; Snow, N.H., Ed.; Academic Press: Oxford, UK, 2020; Volume 12, pp. 141–203. [Google Scholar] [CrossRef]
- Blicharski, T.; Oniszczuk, A. Extraction Methods for the Isolation of Isoflavonoids from Plant Material. Open Chem. 2017, 15, 34–45. [Google Scholar] [CrossRef]
- Khadhraoui, B.; Ummat, V.; Tiwari, B.; Fabiano-Tixier, A.; Chemat, F. Review of ultrasound combinations with hybrid and innovative techniques for extraction and processing of food and natural products. Ultrason. Sonochem. 2021, 76, 105625. [Google Scholar] [CrossRef]
- Rodríguez-González, N.; González-Castro, M.J.; Beceiro-González, E.; Muniategui-Lorenzo, S. Development of a matrix solid phase dispersion methodology for the determination of triazine herbicides in marine sediments. Microchem. J. 2017, 133, 137–143. [Google Scholar] [CrossRef]
- Zhao, P.; Zhao, J.; Lei, S.; Guo, X.; Zhao, L. Simultaneous enantiomeric analysis of eight pesticides in soils and river sediments by chiral liquid chromatography-tandem mass spectrometry. Chemosphere 2018, 204, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Leite, M.; Freitas, A.; Cenáculo, D.; Bessa, F.; Pardal, M.A.; Ramos, F.; Leston, S. Pharmaceuticals in coastal sediments: Validation of an ultra-high-performance liquid chromatography coupled with time-of-flight mass spectrometry (UHPLC-TOF-MS) methodology. Environ. Pollut. 2025, 380, 126571. [Google Scholar] [CrossRef]
- Monteiro, S.H.; Francisco, J.G.; Andrade, G.C.R.M.; Botelho, R.G.; Figueiredo, L.A.; Tornisielo, V.L. Study of spatial and temporal distribution of antimicrobial in water and sediments from caging fish farms by on-line SPE-LC-MS/MS. J. Environ. Sci. Health Part B 2016, 51, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Al-Khazrajy, O.S.A.; Boxall, A.B.A. Determination of pharmaceuticals in freshwater sediments using ultrasonic-assisted extraction with SPE clean-up and HPLC-DAD or LC-ESI-MS/MS detection. Anal. Methods 2017, 9, 4190–4200. [Google Scholar] [CrossRef]
- Abdulra’uf, L.B.; Sirhan, A.Y.; Huat Tan, G. Recent developments and applications of liquid phase microextraction in fruits and vegetables analysis. J. Sep. Sci. 2012, 35, 3540–3553. [Google Scholar] [CrossRef] [PubMed]
- Abou-Elwafa Abdallah, M.; Drage, D.; Harrad, S. A one-step extraction/clean-up method for determination of PCBs, PBDEs and HBCDs in environmental solid matrices. Environ. Sci. Process. Impacts 2013, 15, 2279–2287. [Google Scholar] [CrossRef]
- Yao, W.; Ge, J.; Hu, Q.; Ma, J.; Yuan, D.; Fu, X.; Qi, Y.; Volmer, D.A. An advanced LC–MS/MS protocol for simultaneous detection of pharmaceuticals and personal care products in the environment. Rapid Commun. Mass Spectrom. 2023, 37, e9397. [Google Scholar] [CrossRef]
- Urge, A.Y.; Pampanin, D.M.; Martino, M.E.; Knudsen, D.L.; Brede, C. Salting-Out Assisted Liquid-Liquid Extraction for UPLC-MS/MS Determination of Thyroxine and Steroid Hormones in Human Serum and Fish Plasma. Separations 2023, 10, 240. [Google Scholar] [CrossRef]
- Pedersen-Bjergaard, S.; Rasmussen, K.E.; Grønhaug Halvorsen, T. Liquid–liquid extraction procedures for sample enrichment in capillary zone electrophoresis. J. Chromatogr. A 2000, 902, 91–105. [Google Scholar] [CrossRef]
- Zhou, W.; Wieczorek, M.N.; Javanmardi, H.; Pawliszyn, J. Direct solid-phase microextraction-mass spectrometry facilitates rapid analysis and green analytical chemistry. TrAC Trends Anal. Chem. 2023, 166, 117167. [Google Scholar] [CrossRef]
- Islas, G.; Ibarra, I.S.; Hernandez, P.; Miranda, J.M.; Cepeda, A. Dispersive Solid Phase Extraction for the Analysis of Veterinary Drugs Applied to Food Samples: A Review. Int. J. Anal. Chem. 2017, 2017, 8215271. [Google Scholar] [CrossRef] [PubMed]
- Munjanja, B.K.; Nomngongo, P.N.; Mketo, N. Organochlorine pesticides in vegetable oils: An overview of occurrence, toxicity, and chromatographic determination in the past twenty-two years (2000–2022). Crit. Rev. Food Sci. Nutr. 2023, 64, 10204–10220. [Google Scholar] [CrossRef] [PubMed]
- Brennan, A.A.; You, J.; Lydy, M.J. Comparison of Cleanup Methods for Fipronil and Its Degradation Products in Sediment Extracts. Talanta 2009, 78, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Massei, R.; Byers, H.; Beckers, L.M.; Prothmann, J.; Brack, W.; Schulze, T.; Krauss, M. A Sediment Extraction and Cleanup Method for Wide-Scope Multitarget Screening by Liquid Chromatography–High-Resolution Mass Spectrometry. Anal. Bioanal. Chem. 2018, 410, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Muijs, B.; Jonker, M.T.O. Evaluation of Clean-Up Agents for Total Petroleum Hydrocarbon Analysis in Biota and Sediments. J. Chromatogr. A 2009, 1216, 5182–5189. [Google Scholar] [CrossRef]
- James, A.T.; Martin, A.J.P.; Smith, G.H. Gas-liquid partition chromatography: The separation and micro-estimation of ammonia and the methylamines. Biochem. J. 1952, 52, 238–242. [Google Scholar] [CrossRef]
- Lee, T.D. Introduction to Modern Liquid Chromatography, Third Edition. J. Am. Soc. Mass Spectrom. 2011, 22, 196. [Google Scholar] [CrossRef]
- Fialkov, A.B.; Lehotay, S.J.; Amirav, A. Less than one minute low-pressure gas chromatography—Mass spectrometry. J. Chromatogr. A 2020, 1612, 460691. [Google Scholar] [CrossRef] [PubMed]
- Lehotay, S.J.; de Zeeuw, J.; Sapozhnikova, Y.; Michlig, N.; Rousova Hepner, J.; Konschnik, J.D. There Is No Time to Waste: Low-Pressure Gas Chromatography–Mass Spectrometry Is a Proven Solution for Fast, Sensitive, and Robust GC–MS Analysis. LCGC N. Am. 2020, 38, 457–466. Available online: https://www.chromatographyonline.com/view/there-is-no-time-to-waste-low-pressure-gas-chromatography-mass-spectrometry-is-a-proven-solution (accessed on 31 October 2025).
- Latawiec, A. Evolution of Multiplexing Technology for High-Throughput LC/MS Analyses. In High-Throughput Mass Spectrometry in Drug Discovery; Wiley Online Books: Hoboken, NJ, USA, 2023; pp. 103–119. [Google Scholar] [CrossRef]
- Black, G.P.; Woodward, E.E.; Sanders, C.J.; Gross, M.S.; Hladik, M.L. Multiresidue extraction of current-use pesticides from complex solid matrices using energized dispersive guided extraction with analysis by gas and liquid chromatography tandem mass spectroscopy. Chemosphere 2023, 327, 138550. [Google Scholar] [CrossRef]
- Lescord, G.L.; Lepage, A.T. Making waves: Report your quality assurance and control (QA/QC) information when publishing analytical data. Water Res. 2025, 278, 123167. [Google Scholar] [CrossRef]
- Sadutto, D.; Álvarez-Ruiz, R.; Picó, Y. Systematic assessment of extraction of pharmaceuticals and personal care products in water and sediment followed by liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2020, 412, 113–127. [Google Scholar] [CrossRef]
- Darwano, H.; Duy, S.V.; Sauvé, S. A New Protocol for the Analysis of Pharmaceuticals, Pesticides, and Hormones in Sediments and Suspended Particulate Matter from Rivers and Municipal Wastewaters. Arch. Environ. Contam. Toxicol. 2014, 66, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Langford, K.H.; Reid, M.; Thomas, K.V. Multi-Residue Screening of Prioritised Human Pharmaceuticals, Illicit Drugs and Bactericides in Sediments and Sludge. J. Environ. Monit. 2011, 13, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- Şana Sungur. Chapter 6—Pharmaceutical and personal care products in the environment: Occurrence and impact on the functioning of the ecosystem. In Emerging Contaminants in the Environment; Sarma, H., Dominguez, D.C., Lee, W.Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 137–157. [Google Scholar] [CrossRef]
- Nazarov, V.; Miroshnichenko, D.; Ivakh, O.; Pyshyev, S.; Korchak, B. State of the Art in Industrial Application of Amino-1,2,4-Triazoles. Mini-Rev. Org. Chem. 2023, 20, 394–402. [Google Scholar] [CrossRef]
- Rashid, A.; Wang, Y.; Li, Y.; Yu, C.P.; Sun, Q. Simultaneous Analysis of Multiclass Contaminants of Emerging Concern in Sediments by Liquid Chromatography with Tandem Quadrupole Mass Spectrometry. Environ. Toxicol. Chem. 2019, 38, 1409–1422. [Google Scholar] [CrossRef] [PubMed]
- Golovko, O.; Rehrl, A.L.; Köhler, S.; Ahrens, L. Organic micropollutants in water and sediment from Lake Mälaren, Sweden. Chemosphere 2020, 258, 127293. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.K.; Markert, N.; Leser, K.; Kämpfer, D.; Crawford, S.E.; Schäffer, A.; Segner, H.; Hollert, H. Assessing endocrine disruption in freshwater fish species from a “hotspot” for estrogenic activity in sediment. Environ. Pollut. 2020, 257, 113636. [Google Scholar] [CrossRef]
- Chabilan, A.; Landwehr, N.; Horn, H.; Borowska, E. Impact of log(Kow) Value on the Extraction of Antibiotics from River Sediments with Pressurized Liquid Extraction. Water 2022, 14, 2534. [Google Scholar] [CrossRef]
- Chen, L.; Li, H.; Liu, Y.; Cui, Y.; Li, Y.; Yang, Z. Distribution, residue level, sources, and phase partition of antibiotics in surface sediments from the inland river: A case study of the Xiangjiang River, south-central China. Environ. Sci. Pollut. Res. 2020, 27, 2273–2286. [Google Scholar] [CrossRef]
- Ngo, T.H.; Van, D.A.; Tran, H.L.; Nakada, N.; Tanaka, H.; Huynh, T.H. Occurrence of pharmaceutical and personal care products in Cau River, Vietnam. Environ. Sci. Pollut. Res. 2021, 28, 12082–12091. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yi, Q.; Hong, J.; Zhang, L.; Lin, K.; Yuan, D. Simultaneous determination of 32 antibiotics and 12 pesticides in sediment using ultrasonic-assisted extraction and high performance liquid chromatography-tandem mass spectrometry. Anal. Methods 2015, 7, 1896–1905. [Google Scholar] [CrossRef]
- Kairigo, P.; Ngumba, E.; Sundberg, L.R.; Gachanja, A.; Tuhkanen, T. Contamination of Surface Water and River Sediments by Antibiotic and Antiretroviral Drug Cocktails in Low and Middle-Income Countries: Occurrence, Risk and Mitigation Strategies. Water 2020, 12, 1376. [Google Scholar] [CrossRef]
- Rashid, A.; Mazhar, S.H.; Zeng, Q.; Kiki, C.; Yu, C.P.; Sun, Q. Simultaneous analysis of multiclass antibiotic residues in complex environmental matrices by liquid chromatography with tandem quadrupole mass spectrometry. J. Chromatogr. B 2020, 1145, 122103. [Google Scholar] [CrossRef]
- López Zavala, M.Á.; Reynoso-Cuevas, L. Simultaneous extraction and determination of four different groups of pharmaceuticals in compost using optimized ultrasonic extraction and ultrahigh pressure liquid chromatography–mass spectrometry. J. Chromatogr. A 2015, 1423, 9–18. [Google Scholar] [CrossRef]
- Peng, Q.; Song, J.; Li, X.; Yuan, H.; Liu, M.; Duan, L.; Zuo, J. Pharmaceutically active compounds (PhACs) in surface sediments of the Jiaozhou Bay, north China. Environ. Pollut. 2020, 266, 115245. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.J.; Paíga, P.; Silva, A.; Llaguno, C.P.; Carvalho, M.; Vázquez, F.M.; Delerue-Matos, C. Antibiotics and antidepressants occurrence in surface waters and sediments collected in the north of Portugal. Chemosphere 2020, 239, 124729. [Google Scholar] [CrossRef]
- Feo, M.L.; Bagnati, R.; Passoni, A.; Riva, F.; Salvagio Manta, D.; Sprovieri, M.; Traina, A.; Zuccato, E.; Castiglioni, S. Pharmaceuticals and other contaminants in waters and sediments from Augusta Bay (southern Italy). Sci. Total Environ. 2020, 739, 139827. [Google Scholar] [CrossRef]
- Matongo, S.; Birungi, G.; Moodley, B.; Ndungu, P. Occurrence of selected pharmaceuticals in water and sediment of Umgeni River, KwaZulu-Natal, South Africa. Environ. Sci. Pollut. Res. 2015, 22, 10298–10308. [Google Scholar] [CrossRef]
- Lei, X.; Lu, J.; Liu, Z.; Tong, Y.; Li, S. Concentration and distribution of antibiotics in water–sediment system of Bosten Lake, Xinjiang. Environ. Sci. Pollut. Res. 2015, 22, 1670–1678. [Google Scholar] [CrossRef]
- Qin, D.; Zhao, M.; Wang, J.; Lian, Z. Selective extraction and detection of norfloxacin from marine sediment and seawater samples using molecularly imprinted silica sorbents coupled with HPLC. Mar. Pollut. Bull. 2020, 150, 110677. [Google Scholar] [CrossRef]
- Rauseo, J.; Spataro, F.; Pescatore, T.; Patrolecco, L. Multiresidue determination and predicted risk assessment of emerging contaminants in sediments from Kongsfjorden, Svalbard. Sci. Total Environ. 2024, 922, 171156. [Google Scholar] [CrossRef]
- Hu, P.; Guo, C.; Zhang, Y.; Lv, J.; Zhang, Y.; Xu, J. Occurrence, distribution and risk assessment of abused drugs and their metabolites in a typical urban river in north China. Front. Environ. Sci. Eng. 2019, 13, 56. [Google Scholar] [CrossRef]
- Hlengwa, N.B.; Mahlambi, P.N. Ultrasonic Followed by Solid Phase Extraction and Liquid Chromatography-Photodiode Array for Determination of Pharmaceutical Compounds in Sediment and Soil. Bull. Environ. Contam. Toxicol. 2020, 104, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; He, M.; Chen, B.; Hu, B. Simultaneous determination of polar and apolar compounds in environmental samples by a polyaniline/hydroxyl multi-walled carbon nanotubes composite-coated stir bar sorptive extraction coupled with high performance liquid chromatography. J. Chromatogr. A 2015, 1394, 36–45. [Google Scholar] [CrossRef]
- Li, Y.; Gan, Z.; Liu, Y.; Chen, S.; Su, S.; Ding, S.; Tran, N.H.; Chen, X.; Long, Z. Determination of 19 anthelmintics in environmental water and sediment using an optimized PLE and SPE method coupled with UHPLC-MS/MS. Sci. Total Environ. 2020, 719, 137516. [Google Scholar] [CrossRef]
- Cavaliere, C.; Capriotti, A.L.; Ferraris, F.; Foglia, P.; Samperi, R.; Ventura, S.; Laganà, A. Multiresidue analysis of endocrine-disrupting compounds and perfluorinated sulfates and carboxylic acids in sediments by ultra-high-performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2016, 1438, 133–142. [Google Scholar] [CrossRef]
- Ding, J.; Cheng, Y.; Hua, Z.; Yuan, C.; Wang, X. The Effect of Dissolved Organic Matter (DOM) on the Release and Distribution of Endocrine-Disrupting Chemicals (Edcs) from Sediment under Hydrodynamic Forces, A Case Study of Bisphenol A (BPA) and Nonylphenol (NP). Int. J. Environ. Res. Public Health 2019, 16, 1724. [Google Scholar] [CrossRef]
- Yuan, K.; Kang, H.; Yue, Z.; Yang, L.; Lin, L.; Wang, X.; Luan, T. Determination of 13 endocrine disrupting chemicals in sediments by gas chromatography–mass spectrometry using subcritical water extraction coupled with dispersed liquid–liquid microextraction and derivatization. Anal. Chim. Acta 2015, 866, 41–47. [Google Scholar] [CrossRef]
- Souchier, M.; Benali-Raclot, D.; Benanou, D.; Boireau, V.; Gomez, E.; Casellas, C.; Chiron, S. Screening triclocarban and its transformation products in river sediment using liquid chromatography and high resolution mass spectrometry. Sci. Total Environ. 2015, 502, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Eaglesham, A.; Scott, A.; Petrie, B. Multi-residue enantioselective analysis of chiral drugs in freshwater sediments. Environ. Chem. Lett. 2020, 18, 2119–2126. [Google Scholar] [CrossRef]
- Wagil, M.; Maszkowska, J.; Białk-Bielińska, A.; Stepnowski, P.; Kumirska, J. A comprehensive approach to the determination of two benzimidazoles in environmental samples. Chemosphere 2015, 119, S35–S41. [Google Scholar] [CrossRef] [PubMed]
- Tomai, P.; Gentili, A.; Fanali, S.; Picó, Y. Multi-residue determination of organic micro-pollutants in river sediment by stir-disc solid phase extraction based on oxidized buckypaper. J. Chromatogr. A 2020, 1621, 461080. [Google Scholar] [CrossRef]
- Nannou, C.I.; Boti, V.I.; Albanis, T.A. A modified QuEChERS approach for the analysis of pharmaceuticals in sediments by LC-Orbitrap HRMS. Anal. Bioanal. Chem. 2019, 411, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bu, Q.; Wu, D.; Yu, G. Polycyclic musks in surface water and sediments from an urban catchment in the megacity Beijing, China. Environ. Pollut. 2020, 263, 114548. [Google Scholar] [CrossRef]
- Soares, K.L.; Sunyer-Caldú, A.; Barbosa, S.C.; Primel, E.G.; Fillmann, G.; Diaz Cruz, M.S. Rapid and cost-effective multiresidue analysis of pharmaceuticals, personal care products, and antifouling booster biocides in marine sediments using matrix solid phase dispersion. Chemosphere 2021, 267, 129085. [Google Scholar] [CrossRef]
- Teysseire, F.; Cabana, H.; Segura, P.A. Method development and comprehensive study of matrix effects for the determination of trace organic contaminants in lake sediments. J. Chromatogr. A 2024, 1738, 465504. [Google Scholar] [CrossRef]
- Becerra-Rueda, O.F.; Rodríguez-Figueroa, G.M.; Marmolejo-Rodríguez, A.J.; Aguíñiga-García, S.; Durán-Álvarez, J.C. Pharmaceutical Residues in Sediments of a Coastal Lagoon in Northwest Mexico—Occurrence and Environmental Risk Assessment. J. Xenobiotics 2024, 14, 1757–1770. [Google Scholar] [CrossRef]
- Rojewska, A.; Godlewska, K.; Paszkiewicz, M.; Siedlewicz, G.; Pazdro, K.; Białk-Bielińska, A. Occurrence and risk assessment of the residues of pharmaceuticals and other micropollutants in the marine sediments–Preliminary study for the Baltic Sea. Mar. Pollut. Bull. 2025, 215, 117875. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Shi, G.; Liu, Y.; Li, Y.; Liu, C.; Hao, Q.; Cao, W.; Li, Q. Pharmaceuticals in multi-media environment from the Jin River to adjacent marine embayment in Southeast China. Environ. Sci. Pollut. Res. 2023, 30, 29909–29920. [Google Scholar] [CrossRef]
- Al-Khazrajy, O.S.; Bergström, E.; Boxall, A.B. Factors affecting the dissipation of pharmaceuticals in freshwater sediments. Environ. Toxicol. Chem. 2018, 37, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Nantaba, F.; Wasswa, J.; Kylin, H.; Bouwman, H.; Palm, W.U.; Kümmerer, K. Spatial trends and ecotoxic risk assessment of selected pharmaceuticals in sediments from Lake Victoria, Uganda, East Africa. Sci. Total Environ. 2024, 906, 167348. [Google Scholar] [CrossRef] [PubMed]
- Thavarayan, L.; Moodley, B. Development of solid phase extraction method for the LC-PDA detection of selected pharmaceuticals and their metabolites in surface water and sediment from the Isipingo River, KZN, South Africa. Sci. Afr. 2025, 28, e02655. [Google Scholar] [CrossRef]
- Foster, G.; Leahigh, A.; Huff, T. Surface Water Processes Influencing Alterations in Pharmaceutical Chemical Composition following Wastewater Discharge into a Freshwater Estuary. Toxics 2022, 10, 702. [Google Scholar] [CrossRef]
- de Sousa, D.N.R.; Grosseli, G.M.; Mozeto, A.A.; Carneiro, R.L.; Fadini, P.S. Ultrasound-assisted extraction method for the simultaneous determination of emerging contaminants in freshwater sediments. J. Sep. Sci. 2015, 38, 3454–3460. [Google Scholar] [CrossRef]
- Wanjeri, V.W.O.; Okuku, E.; Ngila, J.C.; Kariuki, J.N.; Omondi, C.O.; Gachanja, M. Distribution of pharmaceuticals in marine surface sediment and macroalgae (Ulvophyceae) around Mombasa peri-urban creeks and Gazi Bay, Kenya. Environ. Sci. Pollut. Res. 2025, 32, 4103–4123. [Google Scholar] [CrossRef]
- Dawood, A.; Drage, D.S.; Harrad, S.; Abdallah, M.A. Concentrations, partitioning and ecological risk of pharmaceuticals and personal care products in UK freshwater sediment. Environ. Pollut. Manag. 2024, 1, 87–98. [Google Scholar] [CrossRef]
- Liu, F.; Nielsen, A.H.; Vollertsen, J. Sorption and Degradation Potential of Pharmaceuticals in Sediments from a Stormwater Retention Pond. Water 2019, 11, 526. [Google Scholar] [CrossRef]
- Liao, C.; Shi, J.; Wang, X.; Zhu, Q.; Kannan, K. Occurrence and distribution of parabens and bisphenols in sediment from northern Chinese coastal areas. Environ. Pollut. 2019, 253, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Letsinger, S.; Kay, P.; Rodríguez-Mozaz, S.; Villagrassa, M.; Barceló, D.; Rotchell, J.M. Spatial and temporal occurrence of pharmaceuticals in UK estuaries. Sci. Total Environ. 2019, 678, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Díaz, A.; Peña-Alvarez, A. A Simple Method for the Simultaneous Determination of Pharmaceuticals and Personal Care Products in River Sediment by Ultrasound-Assisted Extraction Followed by Solid-Phase Microextraction Coupled with Gas Chromatography–Mass Spectrometry. J. Chromatogr. Sci. 2017, 55, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Bonnot, K.; Benoit, P.; Mamy, L.; Patureau, D. Transformation of PPCPs in the Environment: Review of Knowledge and Classification of Pathways According to Parent Molecule Structures. Crit. Rev. Environ. Sci. Technol. 2022, 53, 47–69. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aouant, A.D.; Hela, D. Extraction and Analytical Techniques for Pharmaceuticals and Personal Care Products in Sediments: A Critical Review Towards Environmental Sustainability. Sustainability 2025, 17, 10025. https://doi.org/10.3390/su172210025
Aouant AD, Hela D. Extraction and Analytical Techniques for Pharmaceuticals and Personal Care Products in Sediments: A Critical Review Towards Environmental Sustainability. Sustainability. 2025; 17(22):10025. https://doi.org/10.3390/su172210025
Chicago/Turabian StyleAouant, Alia D., and Dimitra Hela. 2025. "Extraction and Analytical Techniques for Pharmaceuticals and Personal Care Products in Sediments: A Critical Review Towards Environmental Sustainability" Sustainability 17, no. 22: 10025. https://doi.org/10.3390/su172210025
APA StyleAouant, A. D., & Hela, D. (2025). Extraction and Analytical Techniques for Pharmaceuticals and Personal Care Products in Sediments: A Critical Review Towards Environmental Sustainability. Sustainability, 17(22), 10025. https://doi.org/10.3390/su172210025




