Abstract
Traditionally, antibiotics have been used to treat human and animal diseases caused by pathogenic bacteria. The aquaculture industry, which is massively expanding currently, also makes use of several antibiotic classes, resulting in potential antibiotic residues in the surrounding aquatic environment, as well as the cultured products raising bacterial resistance. The aim of this study was the optimization, validation, and application of a solid-phase extraction (SPE) method in combination with liquid chromatography (LC)-LTQ/Orbitrap mass spectrometry in order to determine the most commonly used antibiotics in waters sampled from fish farms, both saltwater and freshwater, located in Greece. Under optimum conditions, the method was validated, achieving recoveries in the range of 57.7% (for sulfamethoxazole in river water) to 95.8% (for florfenicol in river water). The method quantification limits were within the range of 0.25 and 10 ng·L−1 in all cases, with relative standard deviations (RSDs) < 15.9%. The application of the proposed methodology revealed the presence of oxytetracycline and trimethoprim traces. Finally, an assessment of the environmental risk posed by the detected antibiotics was performed, calculating either the risk quotient (RQ) for three trophic levels (8.013 × 10−6 < RQ < 0.496) or the mixture RQ (0.005 < RQ < 0.682), proving that in all cases, the risk was medium to low.
1. Introduction
Aquaculture is expanding globally, because of the simultaneous rising of the demand for seafood and the decline in catchable wild fisheries [1,2,3]. Among pharmaceuticals, antibiotics have demonstrated good results over the years, concerning the prevention or treatment of bacterial infections in humans and animals, including aqua species [4]. Although the major input of these compounds is decisive, the massive expansion of aquaculture and the excessive use of antibiotics have posed serious threats to aquatic ecosystems through direct release and to human health via dietary habits.
It has been many years since commercial antibiotics were first developed and they have proliferated enormously, both in variety and number, up to the present day [5,6]. Their excessive use in medicine, veterinary practice, and agriculture contributes to the emergence, selection, and spread of antibiotic-resistant bacteria and genes [4,7]. Many of the antibiotics utilized in aquaculture are also applied in human medicine [8]. As fish may be a source of bacterial infections for humans via consumption, it is crucial to control the emergence of antimicrobial resistance (AMR) and mitigate possible environmental and human health concerns [9]. Therefore, many antibiotics have lost their efficacy and have become partly unreliable or threatening to both human and animal health [10,11].
Greece is a major producer of fish products in Europe, with the majority originating from near-shore and offshore marine aquaculture facilities. It produced 123.620 tons of marine finfish in 2016, primarily gilthead seabream and European seabass, while in 2019, total aquaculture production reached 149.975 tons [12,13].
Among antibiotics approved for use in aquaculture, oxytetracycline (tetracycline), sarafloxacin (fluoroquinolone), florfenicol (amphenicol), erythromycin (macrolide), sulfadimethoxine, sulfamethoxazole (sulfonamides), trimethoprim (diaminopyrimidine), and oxolinic acid (quinolone) are the most frequently used [14,15]. However, each country has set its own regulations regarding the use and the concentrations of antibiotics in aquaculture and food. The Aquatic Animal Health Code (AAHC) was developed by the World Organization for Animal Health (WOAH), in order for the AAHC to establish the guidelines and maximum residue limits (MRLs) for the responsible use of antimicrobial agents in aquatic animals. Therefore, a list of veterinary antibiotics has been published, aiming for the best balance between animal health needs and public health considerations [16,17,18].
Overall, the entry of antibiotics into the environment often leads to the pollution of terrestrial and groundwater ecosystems, finally entering the food chain and ultimately reaching humans. The rapid growth of aquaculture has resulted in an increasing burden on the aquatic ecosystem. Although antibiotics can be partly digested after ingestion, up to 80% of antibiotics are eliminated in the urine or feces without fully decomposing [7,19,20]. Moreover, the overuse and illegal application of antibiotics has resulted in their pseudo-persistent behavior in aquatic environments. Even though many environmental processes, such as photolysis (direct or indirect), could be considered as an effective way to decrease the antibiotics’ concentration in the environment, their continuous addition or discharge in water bodies has led to a consistent detected concentration of these contaminants, especially near fish farming facilities [21,22]. Therefore, a need to control and determine the presence of residues of antibiotic compounds in water bodies possibly impacted by nearby aquaculture facilities has arisen.
Analysis of the presence of such antibiotics is challenging as they often occur at trace levels, which necessitates the use of reliable and sensitive analytical techniques, so as to identify and quantify them in aquatic environments. Despite the existence of different and novel analytical techniques, solid-phase extraction (SPE) remains the most popular, easy to handle, and adaptable extraction method with regard to water samples [23,24,25,26]. Furthermore, when it is combined with up-to-date, accurate-mass and high-resolution instrumentation such as the hybrid LTQ/Orbitrap MS, the selectivity and sensitivity achieved for antibiotic analysis is very reliable. Such methods are of crucial importance in the screening of antibiotic residues—which is still limited in Europe—given the threat these residues pose to aquatic ecosystems.
This study is aimed at optimizing, validating, and applying an analytical, SPE-based methodology for the simultaneous determination of the most commonly used antibiotic compounds in fish farm facilities of both fresh and saltwater. Six antibiotic compounds were selected which are representative members of different chemical groups. The main parameters that influence the extraction technique were optimized for the appropriate conditions to be configured. Separation, identification, and quantification of the selected antibiotic compounds with a high-resolution and accurate mass LC-LTQ/Orbitrap MS system was also investigated. Finally, this study sought to assess an updated and complete environmental risk posed by the detected antibiotics at three trophic levels using the risk quotient (RQ) calculation method.
2. Materials and Methods
2.1. Standards and Materials
Analytical-grade standards (purity greater than 94%) of the selected antibiotics, oxytetracycline (OTC)—oxolinic acid (OXO), erythromycin (ERY), trimethroprim (TMP), and sulfamethoxazole (SMX)—were obtained from Sigma-Aldrich (Taufkirchen, Germany) besides florfenicol (FFC) which was purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany). In Table S1, the physicochemical properties and therapeutic use of each selected analyte are depicted.
All solvents used were of high purity (LC-MS grade), including methanol and water purchased from Fisher Scientific (Leicester, UK) and dichloromethane from Acros Organics (Geel, Belgium). Additionally, hydrochloric acid, sulfuric acid, formic acid (Merck, Darmstadt, Germany), disodium hydrate ethylenediamine tetraacetic acid (EDTA.Na2.2H2O) (Merck, Darmstadt, Germany), as well as ammonium formate (Fluka Analytical Taufkirchen, German) were used during the experimental procedure. Individual stock solutions were prepared at 1000 or 2000 mg L−1 in methanol and stored in amber glassware at −20 °C. Stock solution of oxolinic acid was prepared in methanol by adding 100 μL NaOH 1 M, due to its small solubility in methanol [27]. Erythromycin’s stock solution was prepared in its anhydro erythromycin form (ERY-H2O) since erythromycin is easily degraded in the aquatic environment and finally detected as the above-mentioned degradation product, according to the existing literature: initially, a standard solution of erythromycin of 2000 mg L−1 was prepared and then it was diluted to a new standard solution of erythromycin of 500 mg L−1. The newly prepared solution was acidified using sulfuric acid 0.25 M to obtain a pH value of 3 and left under stirring for 4 h [28,29,30,31] The standard stock solution of oxytetracycline was stored for one month at −20 °C and then it was re-prepared due to the fact that oxytetracycline decomposes easily [32]. All working solutions were stored in amber glassware at 4 °C.
Extraction of the water samples was carried out by solid-phase extraction (SPE) using Oasis HLB extraction cartridges (divinylbenzene/N-vinylpyrrolidone co-polymer, 200 mg, 6 mL) supplied from Waters Corporation (Milford, CT, USA).
2.2. Sampling and Sample Preparation
Water samples (sea and river) were collected and used for the method optimization and validation. Subsequently, a series of seawater samples were collected (Figure S1) in two saltwater fish farms in Greece, located in the Ionian Sea, NW Greece, from two sampling points: one in each fish farm center (S1c, S2c), one sampling point in each fish farm’s exit (S1e, S2e) to the outer sea and one reference point (Sr) around 700 m away from the farms, all at 2 m from surface. In addition, fresh water was sampled (Figure S1) along one of the rivers in NW Greece (Louros river) severely impacted by local aquaculture facilities. The river sampling stations included points starting from the river spring (R1), serving as reference points R2 and R3, two points along river and before fish farms, point R4 inside one fish farm, and the sampling point R5 located at the river estuary. The reference points were used as controls and to carry out the method optimization and validation. Purpose of the selected sampling campaign, apart from exploring the antibiotic load inside and farther from the surrounding fish farms’ aquatic environment, was also to investigate the application of the proposed method in waters of different characteristics such as sea and river waters. Samples were collected in amber glass bottles of 2.5 L and were transferred to the laboratory, filtered through GF/F glass fiber filters (0.7 μm pore size, Whatman International Ltd., Maidstone, UK), and stored at −4 °C until analysis.
Targeted analytes were extracted using an off line solid-phase extraction (SPE) system (12-port vacuum extraction manifold, Visiprep-DL, Supelco) connected to a vacuum pump by using Oasis HLB cartridges. Initially, an appropriate volume of Na2EDTA solution was added, to achieve a concentration of 0.1% in 250 mL of water sample. Addition of Na2EDTA as a chelator has been shown to prevent antibiotics from forming complexes with metallic ions, resulting in increased recoveries [33]. Sample pretreatment included its acidification at pH 3.5 with 1 M HCl. Then, the Oasis HLB extraction columns were placed in the extraction apparatus and were pre-conditioned with the successive percolation of 6 mL methanol and 6 mL of LC-MS water at a flow rate of ≈1 mL min−1. Immediately after activation and before the adsorbent dries, the pretreated acidified water sample (250 mL) was added for extraction at a flow rate of ≈2 mL min−1. Without leaving the sorbent to dry, 6 mL of LC-MS water was added as a wash step in order to remove interfering substances and cartridge remained under vacuum for 30 min to achieve complete removal of the moisture. The elution of the antibiotics was performed with 2 × 5 mL methanol at a flow rate of ≈2 mL min−1. The eluents were evaporated to dryness under a gentle stream of nitrogen and then reconstituted in 0.5 mL of methanol: water (10:90, v/v) with 0.3% formic acid. The final extract was transferred to an autosampler vial and subjected to LC-LTQ/Orbitrap MS analysis. The validation of the analytical method was performed using fortified samples with an appropriate volume of the target analytes to achieve the concentration range of 0.25–250 ng L−1 based on the latest SANTE guidelines [34]. The application of SPE method to sea and freshwater samples impacted by aquacultures occurred after its optimization and validation. Prior to analysis, the physicochemical characteristics of the water samples were measured, as illustrated in Table S2, by applying standard methods. The temperature, salinity, conductivity, and total dissolved solids (TDS) were measured by a WTW LF 3215 conductivity meter with TetraCon 325 Probe (WTW, Weilheim, Germany), and the pH was directly measured using a Consort C932 analyzer (Constort NT, Turnhout, Belgium) with a HI-1230 pH electrode (Hanna Instruments, Woonsocket, RI, USA).
2.3. LC-LTQ/Orbitrap MS Analysis
Chromatographic separation was accomplished by an Accela LC system (Thermo Fisher Scientific, Inc. GmbH, Bremen, Germany) which consisted of an Accela AS autosampler model 2.1.1 and an Accela quaternary gradient LC-pump. The LC system was coupled with an LTQ-FT Orbitrap XL 2.5.5 SP1 mass spectrometer (Thermo Fisher Scientific, Inc. GmbH, Bremen, Germany). The linear ion trap (LTQ) part of the hybrid MS system was equipped with an Ion Max Electrospray Ionization (ESI) probe, operating in positive and negative ionization mode while the instrument control and data processing were carried out by Xcalibur 2.1 software (Thermo Electron, San Jose, CA, USA).
The target antibiotics were separated on a reversed-phase Hypersil GOLD PFP analytical column (50 mm × 2.1 mm i.d., 1.9 μm) from Thermo Fisher Scientific (Thermo Fisher Scientific, Inc. GmbH, Bremen, Germany). Oxytetracycline, oxolinic acid, trimethoprim, sulfamethoxazole and ERY-H2O were identified as protonated molecular ions [M+H] + in positive ionization mode (PI), while florfenicol formed the deprotonated molecular ion [M−H]− in negative ionization mode (NI). Chromatographic analysis in both cases, PI and NI, was performed using the relevant gradient elution programs (Table S3) with initial mobile phases consisting of (A) water and (B) methanol. In the PI case, 0.1% formic acid was added in both (A) and (B) while for NI 0.1%, formic acid and 5 mM ammonium formate were added accordingly. The flow rate was kept constant at 300 μL min−1 in both modes; the oven temperature was set at 27 °C and the injection volume was set to 10 μL. Elution programs were tested in order to find the best compromise over minimum run time, enhanced sensitivity, and well-shaped compound peaks. All analytes were successfully separated in no more than 9 min runs in total. Antibiotic compounds were identified on the basis of their retention times, their expected molecular ions and their main fragment ions, too.
The instrument main parameters were optimized at the instrument tuning sections, apart from some important ones (injection solvent and volume, scan mode, automatic gain control (AGC) value) that were optimized manually. Single ion monitoring (SIM) in positive (PI) and negative (NI) ionization mode was applied with mass resolving power of 60,000 FWHM, and extracted ion chromatograms were used for identification and quantification purposes. Moreover, a data-dependent acquisition (SIM MS/dd-MS2) using collision-induced dissociation (CID, 35% normalized collision energy, NCE) was applied for the analytes’ confirmation through their main produced fragments (Table S4). Operational parameters of LTQ-Orbitrap HRMS instrument are reported in supporting information section (Table S5). The mass tolerance window was set to 5 ppm.
2.4. Environmental Risk Characterization
The environmental risk was assessed by the risk quotient (RQ) method, according to the revised EMEA guidelines [35]. It is an important tool to characterize the potential ecological risk posed by many contaminants such as antibiotics in aquatic ecosystems. RQ is defined as the ratio of the measured environmental concentration (MEC) to the predicted no-effect concentration (PNEC), for species from at least three trophic levels (see Equation (1)). A PNEC is obtained by dividing the lowest no-observed-effect concentration (NOEC) for the most sensitive species with an appropriate safety factor (Equation (2)). Thus, an ERA requires acute and chronic ecotoxicity data for standard test organisms such as algae, Daphnia (invertebrates), and fish [36].
In accordance with the EMEA guidelines and the technical guidance document on risk assessment by the EU [37,38], the PNEC values for each detected compound were derived from the lowest available values of acute LC50 or EC50 divided by an assessment factor (AF) of 1000. In the case of chronic toxicity, NOEC-value was used, divided by an assessment factor of 100, 50 or 10 with regard to the availability of chronic NOECs for one, two, or three trophic levels, respectively. In this study, information was collected from the literature on the acute or chronic toxicity of the target antibiotics to fish, invertebrates, and algae [39] (Tables S6 and S7). The MEC in the surface waters was analyzed and the PNECs derived were used to calculate the RQ of each antibiotic detected following the “worst-case” scenario; thus, the maximum concentraon value of each antibiotic for river and sea water samples, respectively, was applied. Levels of concern taken into consideration for RQ were defined as high ecological risk for RQ ≥ 1, medium risk for 0.1 < RQ < 1, and low or negligible risk for 0.01 < RQ < 0.1 [40,41].
In fact, multiple antibiotics in the environment may interact simultaneously with aquatic organisms. The water quality that has been investigated substance by substance may lead to underestimation in aquatic risk assessment; thus, the evaluation of the toxic effects of micropollutant mixtures becomes necessary [42]. Therefore, an approach based on summing up the MEC/PNEC ratios for each antibiotic was applied in order to assess the risk at each sampling point where more than one antibiotic was detected, for acute and chronic toxicity. A final mixture risk quotient corresponding to the antibiotic mixture per each sampling point, termed RQMEC⁄PNEC, was calculated according to Equation (3):
A second approach to the estimation of the mixture RQ was also applied; that is, summing the toxic units (STU) of the trophic level with the highest predicted sensitivity to the mixture (maximum STU among trophic levels used) multiplied with the corresponding AF and termed RQSTU (Equation (4)):
The ratio between RQMEC/PNEC and RQSTU is generally found to be in the range of 1−3 [43,44], and the second approach is recommended for use as the next step for cases in which RQMEC⁄PNEC exceeds one [45].
3. Results and Discussion
3.1. LC Separation-LTQ/Orbitrap MS Determination
The target antibiotics were satisfactorily eluted with a methanolic gradient elution program on a pentafluoro phenyl (PFP) analytical column that, alternatively to C18 columns, produces the separation of halogenated compounds and non-halogenated polar compounds. It is noteworthy that selection of the injection solvent is crucial in terms of well-shaped peaks and separation efficiency of such difficult-to-resolve mixtures as the relatively polar-selected antibiotics. Due to the fact that the use of methanol as the injection solvent resulted in peak fronting especially for the most polar analytes, a mixture of water and methanol was tested. It was observed that as the water content in the methanol increased, the chromatographic separation and peak shape optimized. Thus, methanol/water (10:90, v/v) was selected as the optimum injection solvent. Moreover, the addition of formic acid in the injection solvent was found to lead to enhanced peak intensity and morphology with the percent content of 0.3% formic acid being adopted as the optimum (Figure 1).
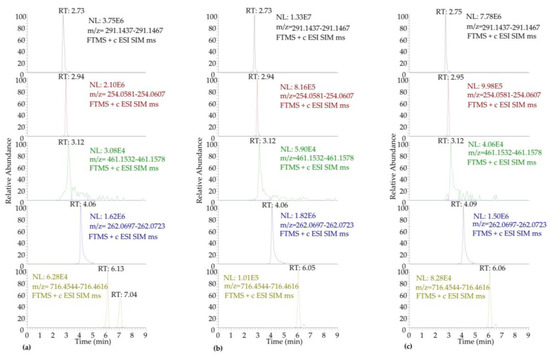
Figure 1.
Comparative depiction of the SIM-LC-LTQ/Orbitrap MS chromatograms of the antibiotics in PI at concentration 50 μg L−1: (a) injection volume 10 μL, AGC 5 × 105, 0.3% FH; (b) injection volume 10 μL, AGC 5 × 104, 0.3% FH; (c) injection volume 10 μL, AGC 5 × 104, no FH.
All target antibiotics were detected in positive ion mode as [M+H]+ whereas florfenicol was monitored in the negative ion mode as [M−H]−. The molecular ions were identified and quantified in a time-scheduled selected ion monitoring (ts-SIM) acquisition mode. SIM mode was compared with the full scan mode and was found to be more sensitive, achieving lower quantification limits, especially in the case of tetracycline, as depicted in Figure S2. After SIM mode was selected, as many parameters of the acquisition method as possible were optimized in the tuning sections of the instrument by direct infusion of known concentrations of the target antibiotics, in order to acquire maximum sensitivity and selectivity. The common tuning parameters such as tube lens and ion optics voltages, which depend mainly on the molecular structure of the analytes, as well as the capillary voltage, were optimized automatically. For the analytes detected in the positive ion mode, some parameters were found to be important including the injection volume, the content of formic acid in the injection volume, and the automatic gain control (AGC) target value. The tested conditions are depicted in Figure 1, resulting in optimum values of 10 μL for the injection volume, 0.3% formic acid in the injection solvent, and AGC value of 5 × 104. Moreover, a resolution of 60,000 FWHM proved to be ideal in order to simultaneously achieve a good peak shape and width, low mass errors, and adequate data points over the chromatographic peak. It has been reported [46] that fewer peak data points are observed as the analyte’s concentration decreases, without significantly affecting the Orbitrap mass spectrometer’s efficiency to produce high-quality results. Additional confirmation of the antibiotic residues was obtained via their main fragment ions, acquired in data-dependent mode using a collision-induced dissociation (CID 35%) fragmentation process. Table S4 illustrates the main LTQ-Orbitrap mass spectrometric characteristics of the selected antibiotics. In Figure S3, the characteristic ts-SIM-LC-LTQ/Orbitrap HRMS chromatograms are depicted of all analytes of interest in a distilled water matrix-matched substrate at 25 μg L−1 using the optimum conditions, indicating mass errors below 3.0 ppm in all cases.
Under optimal conditions, seven-point solvent calibration curves were constructed to determine the instrument’s analytical characteristics, in the range of 0.1–500 μg L−1 depending on the analyte, using the peak areas. The calibration graphs were constructed using three replicated measurements per point corresponding to peak area. In all cases, experimental points fitted a linear model with relative standard deviation (RSDs) ranging from 0.3 to 4.8%. Excellent linearity in detector response was observed for all target antibiotics (>0.9991), while the instrument limits of quantification (LOQs) ranged from 0.1 μg L−1 for trimethoprim and oxolinic acid to 5 μg L−1 for sulfamethoxazole and anhydro erythromycin form.
3.2. Optimization and Validation of the SPE Method
Antibiotics were extracted from water samples via a modified solid-phase extraction method based on the existing literature [28,41,47,48]. The most crucial parameters were optimized, including the sample pH, the elution solvent, and the extraction cartridge type. The sample pH can significantly control the analyte’s retention and elution from the cartridges. For example, many drugs have acidic or basic properties, and it can be anticipated that their cartridge retention and elution behavior will be affected by the pH of the extraction system [49]. Table S1 illustrates the main physicochemical properties of the selected antibiotics. The wide range of their Kow (lipophilic character) and pKa values is indicative of the need to adjust pH in order for the analyte to correctly interact with the sorbent material of the extraction columns. In addition, an elution solvent system able to obtain antibiotics from the cartridges’ sorbent is crucial. The selection of the type of the cartridge is also dependent on the analyte’s properties, with the reversed-phase being the most commonly used, generating better efficiency overall. These parameters were tested for the compounds monitored in the positive ion mode. Data were obtained after conducting experiments in triplicates. Afterwards, the optimized method was applied for florfenicol analysis (negative ion mode), for which excellent performance was also observed.
Solid-phase extractions using Oasis® HLB (6 cm3, 200 mg) cartridges (divinylbenzene/N-vinylpyrrolidone copolymer) have been known to efficiently extract diverse groups of antibiotics from aqueous matrices [23,50,51,52,53,54,55]. Oasis MCX that is a mixed polymeric-cation exchange column could also efficiently extract acidic, basic, and neutral compounds at low pH values [56]. However, preliminary experiments of these two cartridge types exhibited a significantly lower MCX performance (Rec < 45%) for all analytes and thus, HLB was considered as the most suitable sorbent for the target analytes’ enrichment from aqueous matrices. This was in accordance with the majority of the studies in which HLB columns were selected for the extraction of tetracycline and macrolide antibiotics, since they are silanol-free, avoiding the antibiotics binding [56].
The pH of the sample significantly affects the chemical form of the analytes in the water, their stability, as well as the interaction between the analytes and the sorbent material of the extraction columns. Consequently, for sample preparation it is important to consider the pKa value of each target compound. With regard to the reversed-phase mechanism, analytes should be in their neutral form. Thus, the pH of charged, ionizable compounds should be adjusted to two units above or below the analyte pKa, according to the charged group (basic or acidic compounds) [57]. The pKa (Table S1) of the selected antibiotic compounds range from 1.97 (sulfamethoxazole) to 8.88 (erythromycin)and this is due to the fact that these compounds belong to different groups (tetracyclines, sulfonamides, etc.) which contain acidic and/or basic groups and, therefore, for their ionization, the pH must be adjusted. It is common practice to adjust pH values between 2.0 and 4.0 in the case of multi-residue analyses, whereas few studies exist which do not incorporate pH adjustment. However, in studies involving several water types with different pH values, pH adjustment is preferable when obtaining universal extraction conditions for all samples [56]. Thus, in this study, three different pH values were investigated: 3.5, 5.5, and 7.0 as the best compromise over the selected antibiotics’ properties (pKa values), the type of water samples, the and columns’ sorbent material. The recovery of trimethoprim remained relatively unaffected by the tested pH values, while oxytetracycline presented better recoveries for pH = 5.5 and pH = 3.5. ERY-H2O exhibited improved recovery at acidic pH and especially at pH = 3.5 when compared to neutral pH 7.0, in accordance with the relevant literature [58] (Figure 2). A pH value of 3.5 was selected for antibiotics extraction as the best compromise which is also documented by several authors [59,60].
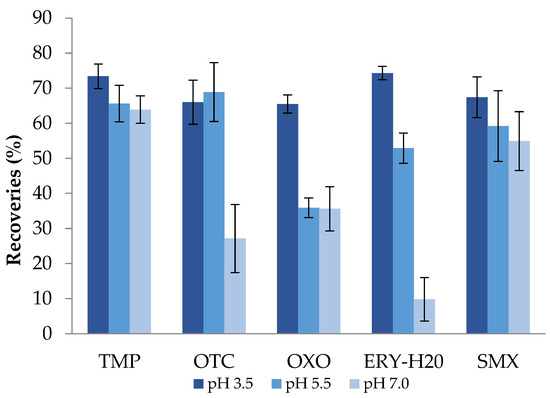
Figure 2.
Recoveries (%) after SPE of the selected antibiotics in different pH conditions (HLB cartridges; methanol as the elution solvent).
Following this, the suitable elution solvent system was explored. Generally, the choice of solvents significantly affects both the retention of the analyte in the adsorbent material and its subsequent elution. Methanol is known to generate strong hydrogen bonds and it has a high permeability for many analytes; thus, it can induce highly efficient elution for the majority of analytes, including antibiotics. The selected antibiotic compounds are relatively polar compounds (Table S1); therefore, the relevant eluents must be selected. Tetracyclines and SAs, for instance, are compounds with great polarity and water solubility, so that they are miscible in bases, acids, and polar organic solvents (especially alcohols) while macrolides (MLs) are mild acids, lipophilic, and poorly water-soluble [61,62]. Quinolone antibiotics have a high polarity and generally amphoteric characteristics and display poor water solubility at pH 6–8 [61]. In general, both acetonitrile and methanol are the common eluents for antibiotics’ recovery from HLB-sorbent material. However, methanol results in better chromatographic peaks and, compared to acetonitrile, evaporates faster under a stream of nitrogen. Due to the multi-class analysis required in this study, methanol was tested alone and acidified along with the inclusion of another polarity grade but still easily evaporating solvent—dichloromethane. The three different elution systems were (1) methanol, (2) methanol acidified with 0.1% formic acid, and (3) methanol followed by methanol-dichloromethane (50:50 v/v). Based on the obtained results, it was observed that methanol and acidified methanol exhibited slight difference; thus, methanol was selected as the most appropriate elution solvent (Figure 3).
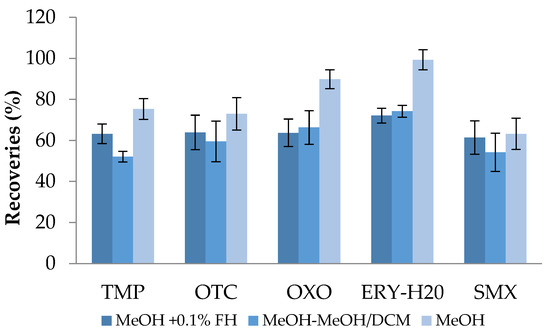
Figure 3.
Antibiotics’ recoveries (%) after distilled water extraction using SPE testing of different elution solvent systems (HLB cartridges; pH 3.5).
The optimized SPE method was then validated in terms of trueness, linearity range, limits of detection (LODs), and quantification (LOQs) along with precision as relative standard deviation (RSD %) and matrix effect (ME %). Method validation was performed in water samples of different origins (distilled, river, and sea water) (Table 1). For that purpose, aqueous samples were fortified with a mixture of the target antibiotics at the appropriate concentration levels, were subjected to the optimized SPE methodology as described in Section 2.2, and then injected into the liquid chromatography-LTQ/Orbitrap mass spectrometry system.

Table 1.
Main analytical method characteristics after SPE validation at 50 ngL−1.
Linearity range was evaluated by spiking each water sample with a mixture of the antibiotics, to obtain concentrations ranging from 0.25 to 250 ng L−1. The response of the detector was found to be linear in the range of LOQ to 250 ng L−1 depending on the analyte, with coefficients of determination (R2) above 0.997 in all cases.
The method’s trueness was based on the calculation of the recoveries in fortified distilled, river, and sea water samples. The recoveries of antibiotics were calculated for the concentration level of 50 ng L−1 and were analyzed in triplicates (n = 3). The level selection was based on the concentration levels at which the selected compounds are generally found in the environment [23,63,64,65,66,67,68,69,70,71]. As shown in Table 1, mean recoveries in distilled water ranged from 61.6% (sulfamethoxazole) to 89.5% (florfenicol), in river water from 57.7% (sulfamethoxazole) to 95.8% (florfenicol) and in seawater from 74.1% (sulfamethoxazole) to 94.6% (trimethoprim). As it can be deduced, recoveries were enhanced in seawater when compared to other substrates, due to the fact that the ionic strength is one of the factors that positively affect the antibiotics’ extraction. The extraction capacity of the selected compounds was increased due to the salting-out effect observed in the case of seawater extraction [72]. Five replicates (n = 5) of each kind of fortified water of 50 ng L−1, were analyzed in the same day to calculate the repeatability of the method (RSDr), and in five consecutive days to obtain the reproducibility of the method (RSDR). The method’s repeatability ranged from 1.3% to 7.1% for distilled water, from 1.9% to 6.6% for river water, and from 1.1% to 8.3% for seawater, while reproducibility was in the range of 6.5% (sea water) and 15.9% (river water) (Table 1).
The suggested SPE method’s LOQs, determined as the signal to noise ratio equal to 10, were found to range from 0.25 ng L−1 (Trimethoprim) to 10.0 ng L−1 (Oxytetracycline and ERY-H2O) in distilled water, in river water they ranged from 0.30 ng L−1 (Trimethoprim) to 10.0 ng L−1 (Oxytetracycline and Erythromycin-H2O), and in seawater ranged from 1.00 ng L−1 (Oxolinic acid) to 10.0 ng L−1 (Oxytetracycline and Erythromycin-H2O) (Table 1). Finally, the method’s LODs, determined as a signal to noise ratio of 3, were found to be in the range of 0.07 ng L−1 to 3.00 ng L−1 for the three matrices. Generally, the method offered comparable or improved performance features compared to previous SPE-LC–MS/MS methodologies for the determination of antibiotics in several types of surface waters [73,74,75,76].
The matrix effect (ME) is generally defined as the combined effect of all the components of which a sample may be composed, in the analytical signal, except for the substances to be determined. It is a substantial concern in LC/MS studies, especially in electrospray mass spectrometry due to the fact that ESI is very liable to other components presence in the sample which may lead to inaccurate results [41]. To estimate the matrix effect, it is necessary to compare the calibration curve slopes derived from the standard solutions and matrix-matched standards (substrate-simulated solutions). Experiments were conducted in triplicates. Slight matrix effect (ME) values (Figure 4) were observed ranging between −20% and 20% in many cases. In river water, the effect of the matrix negatively contributed to the signal response for all compounds except florfenicol for which the effect was not significant. For seawater, all target antibiotics exhibited an insignificant matrix effect, while medium signal suppression was observed for trimethoprim and, on the contrary, signal enhancement was obvious in the case of florfenicol. Finally, when the distilled water was tested for a matrix effect, a slight to strong increase in the signal was observed in the case of sulfamethoxazole.
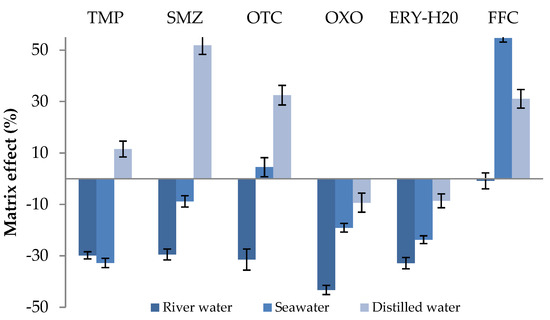
Figure 4.
Matrix effect (%ME) for the studied antibiotics.
3.3. Occurrence in Surface Water Samples Impacted by Aquacultures
The optimized and validated analytical methodology for the detection and identification of the six targeted antibiotic compounds was further applied in real surface water samples impacted by aquacultures. The selected sampling points are described in Section 2.2 in detail. Results are depicted in Figure S4.
Concerning the river water samples, none of the target antibiotics were detected in the sampling points, R1, R2, and R3 as expected. However, in sampling point R4 (inside the fish farm), oxytetracycline was found at a concentration level of 38.8 ng L−1 followed by trimethoprim that was also detected but at a lower concentration (2.5 ng L−1). Oxytetracycline was also detected at the river estuary (sampling point R5), though at a much lower concentration than sampling point R4 (15.5 ng L−1). Sulfamethoxazole, oxolinic acid, erythromycin, and florfenicol were not detected in any river samples.
As far as the seawater samples are concerned, oxytetracycline and trimethoprim were again the predominant compounds detected (Figure S4). Oxytetracycline was detected in all sampling points except the reference one, at concentrations ranging from 12.6 ng L−1 (S1e) to 84.4 ng L−1 (S1c). Trimethoprim was also detected but in lower concentrations, in the center of the first facility (S1c) (3.02 ng L−1) and at its exit, sampling point S1e (16.3 ng L−1), while its concentration in the center of the second fish farm (S2c) was below the method quantification limit. Concerning the other antibiotics, sulfamethoxazole in the facility center (S1c) and florfenicol at its exit (S1e) were also found at trace levels. Oxolinic acid and erythromycin were not detected in any of the samples. A positive trimethoprim detection in the exit of the first saltwater fish farm is depicted in Figure 5.
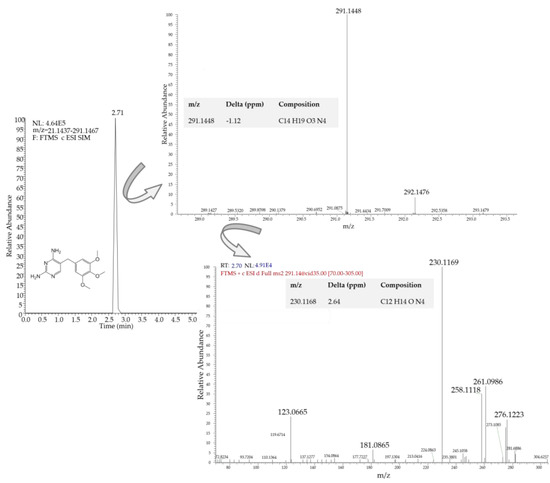
Figure 5.
LC-LTQ/Orbitrap MS2 extracted ion chromatogram (EIC) of seawater (S1e) containing trimethoprim at 16.3 ng L−1.
In summary, oxytetracycline and trimethoprim were the most frequently detected antibiotics in both fresh and seawater samples impacted by nearby aquaculture facilities. This is due to the fact that these two substances are among the most common antibiotic compounds used in fish farms of both salt and fresh water. In seawater fish farms, enhanced concentration levels of oxytetracycline were found in the center of the farms, while as the distance from the center increased, the concentration decreased, which is easily attributed to the sea currents and further dilution of the concentration. Oxytetracycline was also detected in river water fish farm samples and, as has been observed, the concentration decreased approaching the estuary. It is worth noting that at the time of sampling, the river was at a sufficient volume, which may explain the non-detection of compounds at points outside the unit.
Mean levels of OTC, TMP, and FFC detected in sites near trout farms of the Nera river (Italy) were 73.9, 38.5, and 34.8 ng L−1, respectively [19]. According to Pereira et al. [77] levels of OTC were also detected in freshwater aquaculture in the Caima river (Portugal), at the range of 3 to 11.9 ng L−1. Literature concerning the concentration levels of the selected antibiotics in seawater aquafarms in Europe is limited, with the majority of studies dealing with the detection of residues in sediments or tissues of cultivated species [78,79]. It is noteworthy that even if the range of the targeted antibiotics differs between countries, their residues—trimethoprim, and sulfamethoxazole—retain their place as some of the most detected antibiotics [80].
3.4. Environmental Risk Assessment
For each compound identified in river and sea samples “based on the worst-case scenario”, the maximum concentration values were used to assess the potential risk posed by the antibiotics on the three trophic levels. The RQs were calculated for acute and chronic exposure. Results for the RQ values for fish, invertebrates and algae are depicted in Figure 6 and Figure 7, and in Table S6. Data concerning acute and chronic toxicity for the target antibiotics are shown in Tables S7 and S8.
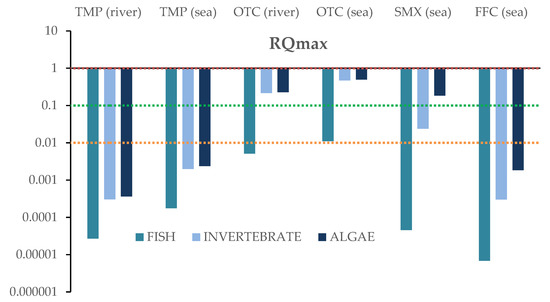
Figure 6.
Risk quotients (RQs) based on maximum concentrations in salt and river water impacted by aquaculture facilities, for three taxonomic classes: algae, invertebrates, and fish, and acute toxicity levels.
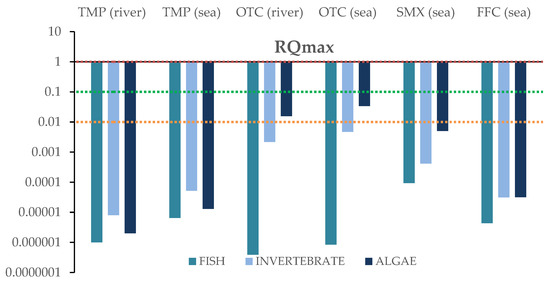
Figure 7.
Risk quotients (RQs) based on maximum concentrations in salt and river water impacted by aquaculture facilities, for three taxonomic classes: algae, invertebrates, and fish, and chronic toxicity levels.
As far as acute toxicity is concerned, none of the detected antibiotics exhibited RQs < 1; thus, none of them posed a high risk to any of the three trophic levels tested. However, oxytetracycline seemed to be capable of posing medium risk on invertebrates and algae in both fresh (RQ = 0.216 and 0.229, respectively) and salt waters (RQ = 0.469 and 0.496, respectively), demonstrating RQs between 0.1 and 1. It should be noted that its maximum concentrations were observed inside the river as well as at the sea fish farms. Moreover, a moderate risk was probably posed to algae by sulfamethoxazole’s presence in the sea water (RQ = 0.185). With the exception of sulfamethoxazole, that exhibited low risk to invertebrates, in all other cases, no risk was posed, especially on fish.
Regarding to chronic toxicity, trimethoprim, sulfamethoxazole, and florfenicol posed no risk to the three trophic levels of aquatic life in both fresh and salt waters. On the other hand, oxytetracycline showed a low risk only to algae for river and sea water.
Overall, the selected antibiotics’ associated risk assessment highlighted the negligible danger of these compounds in the receiving aquatic ecosystems, with the exception of tetracycline for which, however, no high risk was detected in all cases. This is in accordance with other studies, indicating that antibiotics pose a medium or low risk to the aquatic environment [81,82,83,84].
The above estimation of RQs was made for each compound separately but it must be taken into consideration that, in the aquatic environment, antibiotics are frequently present in mixtures which may lead to toxicity risks that did not result from single compounds [84,85].
Table 2 shows the calculated RQMEC/PNEC, RQSTU, and their ratio, for acute and chronic toxicity in each sampling point where more than one antibiotic was present. Thus, the four sampling points consisted of one along the river (R4, inside fish farm) and three in the sea (S1c and S1e inside and at the exit of fish farm 1, and S2c inside fish farm 2).

Table 2.
Calculated RQMEC/PNEC, RQSTU, and their ratio, based on acute and chronic toxicity for the sampling points where more than one antibiotic was detected.
According to the results, the calculated values of risk quotients, with both approaches, for the antibiotic mixtures were less than 1 for all relevant sampling points, indicating the absence of a high risk for the aquatic environment, even when antibiotics are present in mixtures. In the case of acute toxicity, risk reached the medium level inside all fish farms (one river and two sea) while for chronic exposure assessment, low risk was posed by the antibiotic’s mixture in all cases. The ratio of RQMEC/PNEC and RQSTU based on acute and chronic toxicity reached values equal to 1 in almost all sampling sites. Similar results were found in other studies concerning the risk assessment of these antibiotic compounds in surface water [86,87,88,89,90].
The application of the concept of mixture risk toxicity decreases deviations in the evaluation of toxicity and may more accurately predict the potential effects of antibiotics on aquatic organisms [91].
4. Conclusions
A targeted analytical methodology, based on SPE followed by high resolution LC-LTQ/Orbitrap MS analysis, has been optimized and validated for the determination of the most commonly used antibiotic compounds in fresh and salt water fish farms. Its excellent analytical characteristics (accuracy, precision, linearity, and limits of quantification) proved the method’s suitability for the application in sea and river water samples impacted by nearby aquacultures. Waters were collected from the relevant sampling points in Greece. Generally, oxytetracycline, along with trimethoprim, was detected at concentrations below 84.4 ng L−1, whereas other antibiotics were not found or were below quantification limits in all sampling locations.
The need for their continuous monitoring has become obvious due to their wide and intensive use resulting in their dispersion in sea and river waters, especially those hosting fish farm facilities. Furthermore, a more comprehensive study could follow, including more location points along impacted rivers or the related seas. The expansion of this study to more aquaculture systems in Greece could offer an integrated assessment of the ecological health of one of the biggest Mediterranean Sea aquatic system contributors. However, the induced risk for the aquatic environment was proven to be medium or low at three trophic levels in the worst case scenario, and so is the crucial mixture toxicity estimation, since the antibiotics’ presence in mixtures may induce different toxic effects. To conclude, systematic control of the pollutant load related to the aquaculture waters and surrounding aquatic environment (including fish and other aquatic organisms) could be a future addition to this study and always in a prominent place among the research community.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su15129199/s1, Figure S1: sampling location; Figure S2: comparative depiction of the SIM—LC-LTQ/Orbitrap MS chromatograms of the selected antibiotics in positive ionization at concentration 50 μg L−1 with (a) full scan acquisition mode and (b) time-scheduled SIM acquisition mode (all other parameters were the same); Figure S3: SIM-LC-LTQ/Orbitrap MS chromatogram of the selected antibiotics matrix-matched solution at concentration 25 μg L−1. Peaks are: (a) florfenicol (FFC), (b) trimethoprim (TMP), (c) sulfamethoxazole (SMX), (d) oxytetracycline (OTC), (e) oxolinic acid (OXO), (f) erythromycin-H2O (ERY-H2O); Figure S4: The selected antibiotics’ concentration levels expressed as percent (%) occurrence detected in each sampling point (R1-5 and S1-2 exit and center) of surface waters impacted by aquaculture facilities; Table S1: list of the surveyed antibiotics with their physicochemical properties and therapeutic use; Table S2: physicochemical properties values of water samples depending on their origin; Table S3: Gradient elution programs for positive (PI) and negative ionization (NI); Table S4: Detection parameters for SIM MS/dd-MS2 analysis of the selected antibiotics; Table S5: operational parameters of LTQ-Orbitrap HRMS instrument in positive and negative ionization, respectively; Table S6. Acute and chronic RQ values obtained after the potential risk assessment for each identified antibiotic in river and sea samples “based on the worst-case scenario” for the three trophic levels.; Table S7: acute toxicity data (EC50, mg/L) of antibiotics on fish, invertebrates and algae (lower values indicated in bold font); Table S8: chronic toxicity data (NOEC, mg/L) of antibiotics on fish, invertebrates and algae (lower values indicated in bold font).
Author Contributions
Conceptualization, V.B. and T.A.; Data curation, V.B., V.T. and C.E.; Formal analysis, V.T. and C.E.; Funding acquisition, T.A.; Investigation, V.B. and V.T.; Methodology, V.B. and T.A.; Project administration, V.B.; Resources, T.A.; Supervision, T.A.; Validation, V.T.; Visualization, V.B. and C.E.; Writing—original draft, V.B. and C.E.; Writing—review and editing, V.B. and T.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project, “Development of research infrastructure for the design, production, development of quality characteristics and safety of agrofoods and functional foods (RI-Agrofoods)” (MIS 5047235) which is implemented under the Action “Reinforcement of the Research and Innovation Infrastructure”, funded by the Operational Program “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014-2020) and co-financed by Greece and the European Union (European Regional Development Fund).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article and the supporting information file.
Acknowledgments
The authors would like to thank the Unit of Environmental, Organic and Biochemical high-resolution analysis–Orbitrap-LC–MS of the University of Ioannina for providing access to the facilities. In addition, we acknowledge the support of this work the by the project, “Development of research infrastructure for the design, production, development of quality characteristics and safety of agrofoods and functional foods (RI-Agrofoods)” (MIS 5047235) which is implemented under the Action “Reinforcement of the Research and Innovation Infrastructure”, funded by the Operational Program ”Competitiveness, Entrepreneurship and Innovation” (NSRF 2014-2020) and co-financed by Greece and the European Union (European Regional Development Fund).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, J.; Huang, L.; Wang, Q.; Zeng, H.; Xu, J.; Chen, Z. Antibiotics in aquaculture ponds from Guilin, South of China: Occurrence, distribution, and health risk assessment. Environ. Res. 2022, 204, 112084. [Google Scholar] [CrossRef] [PubMed]
- Charuaud, L.; Jarde, E.; Jaffrezic, A.; Thomas, M.-F.; Le Bot, B. Veterinary pharmaceutical residues from natural water to tap water: Sales, occurrence and fate. J. Hazard. Mater. 2018, 361, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Flores-Kossack, C.; Montero, R.; Köllner, B.; Maisey, K. Chilean aquaculture and the new challenges: Pathogens, immune response, vaccination and fish diversification. Fish Shellfish. Immunol. 2019, 98, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef] [PubMed]
- Danner, M.C.; Robertson, A.; Behrends, V.; Reiss, J. Antibiotic pollution in surface fresh waters: Occurrence and effects. Sci. Total Environ. 2019, 664, 793–804. [Google Scholar] [CrossRef]
- Beza, P. Simultaneous Determination and Degradation Study of the Antibiotics Oxytetracycline, Oxolinic Acid, Trimethoprim and Sulfadiazine in Surface Waters. Ph.D. Thesis, University of Ioannina, Ioannina, Greek, 2010. [Google Scholar]
- Carrizo, J.C.; Griboff, J.; Bonansea, R.I.; Nimptsch, J.; Valdés, M.E.; Wunderlin, D.A.; Amé, M.V. Different antibiotic profiles in wild and farmed Chilean salmonids. Which is the main source for antibiotic in fish? Sci. Total Environ. 2021, 800, 149516. [Google Scholar] [CrossRef]
- Kolar, B.; Arnuš, L.; Jeretin, B.; Gutmaher, A.; Drobne, D.; Durjava, M.K. The toxic effect of oxytetracycline and trimethoprim in the aquatic environment. Chemosphere 2014, 115, 75–80. [Google Scholar] [CrossRef]
- Leonard, A.F.; Morris, D.; Schmitt, H.; Gaze, W.H. Natural recreational waters and the risk that exposure to antibiotic resistant bacteria poses to human health. Curr. Opin. Microbiol. 2021, 65, 40–46. [Google Scholar] [CrossRef]
- Done, H.Y.; Halden, R.U. Reconnaissance of 47 antibiotics and associated microbial risks in seafood sold in the United States. J. Hazard. Mater. 2015, 282, 10–17. [Google Scholar] [CrossRef]
- González-Gaya, B.; García-Bueno, N.; Buelow, E.; Marin, A.; Rico, A. Effects of aquaculture waste feeds and antibiotics on marine benthic ecosystems in the Mediterranean Sea. Sci. Total Environ. 2021, 806, 151190. [Google Scholar] [CrossRef]
- Statistical Office of the European Union, Eurostat, Production from Aquaculture Excluding Hatcheries and Nurseries (From 2008 Onwards, Online Database). Available online: https://ec.europa.eu/eurostat/databrowser/view/FISH_AQ2A__custom_1938198/default/table?lang=en (accessed on 8 July 2022).
- Federation of Greek Maricultures. Aquaculture in Greece. Annual Report. 2022. Available online: https://fishfromgreece.com/wp-content/flipbook/nov22/ (accessed on 8 July 2022).
- Guidi, L.R.; Santos, F.A.; Ribeiro, A.C.S.R.; Fernandes, C.; da Silva, L.H.M.; Gloria, M.B.A. Quinolones and tetracyclines in aquaculture fish by a simple and rapid LC-MS/MS method. Food Chem. 2018, 245, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- ECDC. Summary of the Latest Data on Antibiotic Consumption in the European Union; European Centre for Disease Prevention and Control: Solna, Sweden, 2014. [Google Scholar]
- FAO; WOAH. Maximum Residue Limits (MRLs) and Risk Management Recommendations (RMRs) for Residues of Veterinary Drugs in Foods; FAO: Rome, Italy, 2018. [Google Scholar]
- Manage, P.M. Heavy use of antibiotics in aquaculture: Emerging human and animal health problems—A review. Sri Lanka J. Aquat. Sci. 2018, 23, 13–27. [Google Scholar] [CrossRef]
- Official Journal of the European Union. COMMISSION REGULATION (EU) No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin; European Commission: Brussels, Belgium, 2010. [Google Scholar]
- Sargenti, M.; Bartolacci, S.; Luciani, A.; Di Biagio, K.; Baldini, M.; Galarini, R.; Giusepponi, D.; Capuccella, M. Investigation of the Correlation between the Use of Antibiotics in Aquaculture Systems and Their Detection in Aquatic Environments: A Case Study of the Nera River Aquafarms in Italy. Sustainability 2020, 12, 5176. [Google Scholar] [CrossRef]
- Muziasari, W.I.; Managaki, S.; Pärnänen, K.; Karkman, A.; Lyra, C.; Tamminen, M.; Suzuki, S.; Virta, M. Sulphonamide and Trimethoprim Resistance Genes Persist in Sediments at Baltic Sea Aquaculture Farms but Are Not Detected in the Surrounding Environment. PLoS ONE 2014, 9, e92702. [Google Scholar] [CrossRef] [PubMed]
- dos Louros, V.L.; Silva, C.P.; Nadais, H.; Otero, M.; Esteves, V.I.; Lima, D. Oxolinic acid in aquaculture waters: Can natural attenuation through photodegradation decrease its concentration? Sci. Total Environ. 2020, 749, 141661. [Google Scholar] [CrossRef]
- Mangla, D.; Sharma, A.; Ikram, S. Critical review on adsorptive removal of antibiotics: Present situation, challenges and future perspective. J. Hazard. Mater. 2022, 425, 127946. [Google Scholar] [CrossRef]
- Martinaiou, P.; Manoli, P.; Boti, V.; Hela, D.; Makou, E.; Albanis, T.; Konstantinou, I. Quality Control of Emerging Contaminants in Marine Aquaculture Systems by Spot Sampling-Optimized Solid Phase Extraction and Passive Sampling. Sustainability 2022, 14, 3452. [Google Scholar] [CrossRef]
- Fagnani, E.; Montemurro, N.; Pérez, S. Multilayered solid phase extraction and ultra performance liquid chromatographic method for suspect screening of halogenated pharmaceuticals and photo-transformation products in freshwater-comparison between data-dependent and data-independent acquisition mass spectrometry. J. Chromatogr. A 2021, 1663, 462760. [Google Scholar] [CrossRef]
- Korashy, M.A.R.; Gawad, S.A.A.; Hassan, N.Y.; AbdelKawy, M. Solid Phase Extraction and Simultaneous Chromatographic Quantification of some Non-steroidal Anti-inflammatory Drug Residues; an Application in Pharmaceutical Industrial Wastewater Effluent. Braz. J. Pharm. Sci. 2022, 58, 1–14. [Google Scholar] [CrossRef]
- Fontanals, N.; Zohar, J.; Borrull, F.; Ronka, S.; Marcé, R. Development of a maleic acid-based material to selectively solid-phase extract basic compounds from environmental samples. J. Chromatogr. A 2021, 1647, 462165. [Google Scholar] [CrossRef]
- Ibãnez, M.; Sancho, J.V.; Hernandez, F. Screening of antibiotics in surface and wastewater samples by ultra-high-pressure liquid chromatography coupled to hybrid quadrupole time-of-flight mass spectrometry. J. Chromatogr. 2009, 1216, 2529–2539. Available online: https://www.academia.edu/13790021/Screening_of_antibiotics_in_surface_and_wastewater_samples_by_ultra_high_pressure_liquid_chromatography_coupled_to_hybrid_quadrupole_time_of_flight_mass_spectrometry (accessed on 22 March 2022). [CrossRef] [PubMed]
- Dasenaki, M.E.; Thomaidis, N.S. Multianalyte method for the determination of pharmaceuticals in wastewater samples using solid-phase extraction and liquid chromatography–tandem mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 4229–4245. [Google Scholar] [CrossRef]
- Kalaboka, M.; Chrimatopoulos, C.; Jiménez-Holgado, C.; Boti, V.; Sakkas, V.; Albanis, T. Exploring the Efficiency of UHPLC-Orbitrap MS for the Determination of 20 Pharmaceuticals and Acesulfame K in Hospital and Urban Wastewaters with the Aid of FPSE. Separations 2020, 7, 46. [Google Scholar] [CrossRef]
- McArdell, C.S.; Molnar, E.; Suter, M.J.-F.; Giger, W. Occurrence and Fate of Macrolide Antibiotics in Wastewater Treatment Plants and in the Glatt Valley Watershed, Switzerland. Environ. Sci. Technol. 2003, 37, 5479–5486. [Google Scholar] [CrossRef]
- Abuin, S.; Codony, R.; Compañó, R.; Granados, M.; Prat, M.D. Analysis of macrolide antibiotics in river water by solid-phase extraction and liquid chromatography–mass spectrometry. J. Chromatogr. A 2006, 1114, 73–81. [Google Scholar] [CrossRef]
- European Commission. Commission Decision of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results (Text with EEA Relevance) (Notified under Document Number C(2002) 3044); European Commission: Brussels, Belgium, 2002. [Google Scholar]
- Papageorgiou, M.; Kosma, C.; Lambropoulou, D. Seasonal occurrence, removal, mass loading and environmental risk assessment of 55 pharmaceuticals and personal care products in a municipal wastewater treatment plant in Central Greece. Sci. Total Environ. 2016, 543, 547–569. [Google Scholar] [CrossRef] [PubMed]
- SANTE 11312/2021. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed, SANTE 11312/2021. European Union (EU). 2021. Available online: https://www.eurl-pesticides.eu/docs/public/tmplt_article.asp?CntID=727 (accessed on 22 November 2022).
- European Medicines Agency (EMEA). Committee for Medicinal Products for Human Use (CHMP) Guideline on Safety and Efficacy Follow-Up and Risk Management of Advanced Therapy Medicinal Products (EMEA/149995/2008 Rev.1); Science Medicines Health: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Hommen, U.; Baveco, J.M.H.; Galic, N.; van den Brink, P.J. Potential application of ecological models in the European environmental risk assessment of chemicals I: Review of protection goals in EU directives and regulations. Integr. Environ. Assess Manag. 2010, 6, 325–337. [Google Scholar] [CrossRef]
- European Commission. Technical Guidance Document in Support of Commission Directive 93/67/EEC on Risk Assessment for New Notified Substances and Commission Regulation (EC) No 1488/94 on Risk Assessment for Existing Substances, Part II; European Commission: Brussels, Belgium, 2003. [Google Scholar]
- De Bruijn, J.; Hansen, B.; Johansson, S.; Luotamo, M.; Munn, S.; Musset, C.; Olsen, S.; Olsson, H.; Paya-Perez, A.; Pedersen, F.; et al. Technical Guidance Document on risk Assessment. Part 1. Part 2; European Commission: Brussels, Belgium, 2002. [Google Scholar]
- U.S. EPA. Report on the 2011 U.S. Environmental Protection Agency (EPA) Decontamination Research and Development Conference; U.S. EPA: Washington, DC, USA, 2011. [Google Scholar]
- Hernando, M.; Mezcua, M.; Fernandezalba, A.; Barceló, D. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 2006, 69, 334–342. [Google Scholar] [CrossRef]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Investigation of PPCPs in wastewater treatment plants in Greece: Occurrence, removal and environmental risk assessment. Sci. Total Environ. 2014, 466–467, 421–438. [Google Scholar] [CrossRef]
- Shao, Y.; Chen, Z.; Hollert, H.; Zhou, S.; Deutschmann, B.; Seiler, T.-B. Toxicity of 10 organic micropollutants and their mixture: Implications for aquatic risk assessment. Sci. Total Environ. 2019, 666, 1273–1282. [Google Scholar] [CrossRef]
- Backhaus, T.; Karlsson, M. Screening level mixture risk assessment of pharmaceuticals in STP effluents. Water Res. 2014, 49, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, T.; Faust, M. Predictive Environmental Risk Assessment of Chemical Mixtures: A Conceptual Framework. Environ. Sci. Technol. 2012, 46, 2564–2573. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Fu, X.; Liang, Y.; Qin, L.; Mo, L. Risk assessment of an organochlorine pesticide mixture in the surface waters of Qingshitan Reservoir in Southwest China. RSC Adv. 2018, 8, 17797–17805. [Google Scholar] [CrossRef] [PubMed]
- Cahill, M.G.; Dineen, B.A.; Stack, M.A.; James, K.J. A critical evaluation of liquid chromatography with hybrid linear ion trap—Orbitrap mass spectrometry for the determination of acidic contaminants in wastewater effluents. J. Chromatogr. A 2012, 1270, 88–95. [Google Scholar] [CrossRef]
- Gros, M.; Rodríguez-Mozaz, S.; Barceló, D. Fast and comprehensive multi-residue analysis of a broad range of human and veterinary pharmaceuticals and some of their metabolites in surface and treated waters by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry. J. Chromatogr. A 2012, 1248, 104–121. [Google Scholar] [CrossRef]
- Gros, M.; Petrović, M.; Barceló, D. Development of a multi-residue analytical methodology based on liquid chromatography–tandem mass spectrometry (LC–MS/MS) for screening and trace level determination of pharmaceuticals in surface and wastewaters. Talanta 2006, 70, 678–690. [Google Scholar] [CrossRef]
- Nigel, J.K. Simpson and Marcel Dekker, Solid-Phase Extraction—Principles, Techniques, and Applications; Marcel Dekker: New York, NY, USA, 2000. [Google Scholar]
- Goessens, T.; Huysman, S.; De Troyer, N.; Deknock, A.; Goethals, P.; Lens, L.; Vanhaecke, L.; Croubels, S. Multi-class analysis of 46 antimicrobial drug residues in pond water using UHPLC-Orbitrap-HRMS and application to freshwater ponds in Flanders, Belgium. Talanta 2020, 220, 121326. [Google Scholar] [CrossRef]
- Kosma, C.I.; Kapsi, M.G.; Konstas, P.-S.G.; Trantopoulos, E.P.; Boti, V.I.; Konstantinou, I.K.; Albanis, T.A. Assessment of multiclass pharmaceutical active compounds (PhACs) in hospital WWTP influent and effluent samples by UHPLC-Orbitrap MS: Temporal variation, removals and environmental risk assessment. Environ. Res. 2020, 191, 110152. [Google Scholar] [CrossRef]
- Do, T.C.M.V.; Nguyen, D.Q.; Nguyen, T.D.; Le, P.H. Development and Validation of a LC-MS/MS Method for Determination of Multi-Class Antibiotic Residues in Aquaculture and River Waters, and Photocatalytic Degradation of Antibiotics by TiO2 Nanomaterials. Catalysts 2020, 10, 356. [Google Scholar] [CrossRef]
- Xue, M.; Wu, H.; Liu, S.; Huang, X.; Jin, Q.; Ren, R. Simultaneous determination of 44 pharmaceutically active compounds in water samples using solid-phase extraction coupled with ultra-performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2020, 412, 203–222. [Google Scholar] [CrossRef]
- Shen, F.; Xu, Y.-J.; Wang, Y.; Chen, J.; Wang, S. Rapid and ultra-trace levels analysis of 33 antibiotics in water by on-line solid-phase extraction with ultra-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2022, 1677, 463304. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.; Spisso, B.; Ferreira, R.; Pereira, M.; Grutes, J.; De Andrade, B.; D’avila, L. Development and Validation of Liquid Chromatography-Tandem Mass Spectrometry Methods for Determination of Beta-Lactams, Macrolides, Fluoroquinolones, Sulfonamides and Tetracyclines in Surface and Drinking Water from Rio de Janeiro, Brazil. J. Braz. Chem. Soc. 2017, 29, 801–813. [Google Scholar] [CrossRef]
- Seifrtová, M.; Nováková, L.; Lino, C.; Pena, A.; Solich, P. An overview of analytical methodologies for the determination of antibiotics in environmental waters. Anal. Chim. Acta 2009, 649, 158–179. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; El-Nouby, M.A.M.; Kimani, P.K.; Lim, L.W.; Rabea, E.I. A review of the modern principles and applications of solid-phase extraction techniques in chromatographic analysis. Anal. Sci. 2022, 38, 1457–1487. [Google Scholar] [CrossRef]
- Li, B.; Zhang, T.; Xu, Z.; Fang, H.H.P. Rapid analysis of 21 antibiotics of multiple classes in municipal wastewater using ultra performance liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2009, 645, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Bouyou, P.A.L.; Weisser, J.J.; Strobel, B.W. Determination of sulfadiazine in phosphate- and DOC-rich agricultural drainage water using solid-phase extraction followed by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 5019–5030. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-J.; Ying, G.-G.; Liu, S.; Zhao, J.-L.; Chen, F.; Zhang, R.-Q.; Peng, F.-Q.; Zhang, Q.-Q. Simultaneous determination of human and veterinary antibiotics in various environmental matrices by rapid resolution liquid chromatography–electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2012, 1244, 123–138. [Google Scholar] [CrossRef]
- Jafari Ozumchelouei, E.; Hamidian, A.H.; Zhang, Y.; Yang, M. Physicochemical properties of antibiotics: A review with an emphasis on detection in the aquatic environment. Water Environ. Res. 2020, 92, 177–188. [Google Scholar] [CrossRef]
- Chen, J.; Sun, R.; Pan, C.-G.; Sun, Y.; Mai, B.-X.; Li, Q.X. Antibiotics and Food Safety in Aquaculture. J. Agric. Food Chem. 2020, 68, 11908–11919. [Google Scholar] [CrossRef]
- Kim, S.; Carlson, K. Quantification of human and veterinary antibiotics in water and sediment using SPE/LC/MS/MS. Anal. Bioanal. Chem. 2007, 387, 1301–1315. [Google Scholar] [CrossRef]
- Nozaki, K.; Tanoue, R.; Kunisue, T.; Tue, N.M.; Fujii, S.; Sudo, N.; Isobe, T.; Nakayama, K.; Sudaryanto, A.; Subramanian, A.; et al. Pharmaceuticals and personal care products (PPCPs) in surface water and fish from three Asian countries: Species-specific bioaccumulation and potential ecological risks. Sci. Total Environ. 2023, 866, 161258. [Google Scholar] [CrossRef] [PubMed]
- Fisch, K.; Waniek, J.J.; Schulz-Bull, D.E. Occurrence of pharmaceuticals and UV-filters in riverine run-offs and waters of the German Baltic Sea. Mar. Pollut. Bull. 2017, 124, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Nödler, K.; Voutsa, D.; Licha, T. Polar organic micropollutants in the coastal environment of different marine systems. Mar. Pollut. Bull. 2014, 85, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed]
- Paíga, P.; Santos, L.H.; Ramos, S.; Jorge, S.; Silva, J.G.; Delerue-Matos, C. Presence of pharmaceuticals in the Lis river (Portugal): Sources, fate and seasonal variation. Sci. Total Environ. 2016, 573, 164–177. [Google Scholar] [CrossRef]
- Johnson, A.C.; Keller, V.; Dumont, E.; Sumpter, J.P. Assessing the concentrations and risks of toxicity from the antibiotics ciprofloxacin, sulfamethoxazole, trimethoprim and erythromycin in European rivers. Sci. Total Environ. 2015, 511, 747–755. [Google Scholar] [CrossRef]
- Bu, Q.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Pharmaceuticals and personal care products in the aquatic environment in China: A review. J. Hazard. Mater. 2013, 262, 189–211. [Google Scholar] [CrossRef]
- Tamtam, F.; Mercier, F.; Le Bot, B.; Eurin, J.; Dinh, Q.T.; Clément, M.; Chevreuil, M. Occurrence and fate of antibiotics in the Seine River in various hydrological conditions. Sci. Total Environ. 2008, 393, 84–95. [Google Scholar] [CrossRef]
- Boti, V. Analytical Techniques for the Determination of Selected Compounds with Endocrinal Disruptive Activity (EDCs) in Natural Water Samples, Sediments and Food-Application of Chemometric Methods. Ph.D. Thesis, University of Ioannina, Ioannina, Greek, 2009. [Google Scholar]
- Klančar, A.; Trontelj, J.; Roškar, R. Development of a Multi-Residue Method for Monitoring 44 Pharmaceuticals in Slovene Surface Water by SPE-LC-MS/MS. Water Air Soil Pollut. 2018, 229, 192. [Google Scholar] [CrossRef]
- Kokoszka, K.; Wilk, J.; Felis, E.; Bajkacz, S. Application of UHPLC-MS/MS method to study occurrence and fate of sulfonamide antibiotics and their transformation products in surface water in highly urbanized areas. Chemosphere 2021, 283, 131189. [Google Scholar] [CrossRef]
- Yi, X.; Lin, C.; Ong, E.J.L.; Wang, M.; Zhou, Z. Occurrence and distribution of trace levels of antibiotics in surface waters and soils driven by non-point source pollution and anthropogenic pressure. Chemosphere 2018, 216, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Tang, S.; Bao, Y.; Daniels, K.D.; How, Z.T.; El-Din, M.G.; Wang, J.; Tang, L. Fully-automated SPE coupled to UHPLC-MS/MS method for multiresidue analysis of 26 trace antibiotics in environmental waters: SPE optimization and method validation. Environ. Sci. Pollut. Res. 2021, 29, 16973–16987. [Google Scholar] [CrossRef]
- Pereira, A.M.P.T.; Silva, L.J.G.; Meisel, L.M.; Pena, A. Fluoroquinolones and Tetracycline Antibiotics in a Portuguese Aquaculture System and Aquatic Surroundings: Occurrence and Environmental Impact. J. Toxicol. Environ. Heal. Part A 2015, 78, 959–975. [Google Scholar] [CrossRef] [PubMed]
- González-Gaya, B.; Cherta, L.; Nozal, L.; Rico, A. An optimized sample treatment method for the determination of antibiotics in seawater, marine sediments and biological samples using LC-TOF/MS. Sci. Total Environ. 2018, 643, 994–1004. [Google Scholar] [CrossRef]
- Kalantzi, I.; Rico, A.; Mylona, K.; Pergantis, S.A.; Tsapakis, M. Fish farming, metals and antibiotics in the eastern Mediterranean Sea: Is there a threat to sediment wildlife? Sci. Total Environ. 2020, 764, 142843. [Google Scholar] [CrossRef]
- Zhang, R.; Kang, Y.; Zhang, R.; Han, M.; Zeng, W.; Wang, Y.; Yu, K.; Yang, Y. Occurrence, source, and the fate of antibiotics in mariculture ponds near the Maowei Sea, South China: Storm caused the increase of antibiotics usage. Sci. Total Environ. 2020, 752, 141882. [Google Scholar] [CrossRef]
- Straub, J.O. An Environmental Risk Assessment for Human-Use Trimethoprim in European Surface Waters. Antibiotics 2013, 2, 115–162. [Google Scholar] [CrossRef]
- Liu, X.; Lu, S.; Guo, W.; Xi, B.; Wang, W. Antibiotics in the aquatic environments: A review of lakes, China. Sci. Total Environ. 2018, 627, 1195–1208. [Google Scholar] [CrossRef]
- Biel-Maeso, M.; Baena-Nogueras, R.M.; Corada-Fernández, C.; Lara-Martín, P.A. Occurrence, distribution and environmental risk of pharmaceutically active compounds (PhACs) in coastal and ocean waters from the Gulf of Cadiz (SW Spain). Sci. Total Environ. 2018, 612, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhao, H.; Liu, S.; Xie, H.; Wang, Y.; Chen, J. Antibiotics in the coastal water of the South Yellow Sea in China: Occurrence, distribution and ecological risks. Sci. Total Environ. 2017, 595, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Kosma, C. Analysis, Transportation and Photolytic Degradation of Pharmaceuticals in Environment. Ph.D. Thesis, University of Ioannina, Ioannina, Greek, 2013. [Google Scholar]
- De Liguoro, M.; Di Leva, V.; Bona, M.D.; Merlanti, R.; Caporale, G.; Radaelli, G. Sublethal effects of trimethoprim on four freshwater organisms. Ecotoxicol. Environ. Saf. 2012, 82, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Ilechukwu, I.; Okonkwo, C.J.; Olusina, T.A.; Mpock, J.A.; Ilechukwu, C. Occurrence and risk assessment of selected pharmaceuticals in water and sediments of Usuma Dam, Abuja, Nigeria. Int. J. Environ. Anal. Chem. 2021, 101, 1–13. [Google Scholar] [CrossRef]
- Liu, N.; Jin, X.; Feng, C.; Wang, Z.; Wu, F.; Johnson, A.C.; Xiao, H.; Hollert, H.; Giesy, J.P. Ecological risk assessment of fifty pharmaceuticals and personal care products (PPCPs) in Chinese surface waters: A proposed multiple-level system. Environ. Int. 2020, 136, 105454. [Google Scholar] [CrossRef] [PubMed]
- Riva, F.; Zuccato, E.; Davoli, E.; Fattore, E.; Castiglioni, S. Risk assessment of a mixture of emerging contaminants in surface water in a highly urbanized area in Italy. J. Hazard. Mater. 2019, 361, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, M.; Li, Y.; Rehman, M.S.U.; Zubair, M.; Mustafa, G.; Nazar, M.F.; Yu, C.-P.; Sun, Q. Occurrence, spatial variation and risk assessment of pharmaceuticals and personal care products in urban wastewater, canal surface water, and their sediments: A case study of Lahore, Pakistan. Sci. Total Environ. 2019, 688, 653–663. [Google Scholar] [CrossRef]
- Backhaus, T. Environmental Risk Assessment of Pharmaceutical Mixtures: Demands, Gaps, and Possible Bridges. AAPS J. 2016, 18, 804–813. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).