Abstract
Biostimulants play a crucial role in producing high-quality products with increased yields while also positively impacting sustainable agriculture by reducing reliance on chemical fertilizers and promoting soil fertility. In this context, an experiment was developed to evaluate the influence of several commercial foliar fertilizers and biostimulants (Cropmax, FullGreen, Nutrigizer, and Rerum) on the yield and quality parameters of lettuce cultivated in a greenhouse system. The tested products have different formulations, with all containing macronutrients and microelements. Cropmax and Rerum also include amino acids, while Nutrigizer 60 2E contains humic acids. The experiment was arranged in a randomized block design and comprised five treatments, with a control and four biostimulants, Cropmax, FullGreen, Nutrigizer, and Rerum, each replicated five times. Foliar treatments were applied four times to the butterhead lettuce variety ‘Analena’, suitable for spring to autumn production. All foliar treatments resulted in an increase in leaf biomass compared to that for the control variant. Notably, foliar fertilization with Rerum increased the yield by 2.19 times compared to that in the control. Quality indices—dry matter, ascorbic acid, and sugar contents—also improved after treatments. The foliar treatments also significantly enhanced key quality indices, with the Rerum variant showing the most pronounced increases in dry matter (24.11%), ascorbic acid (69.75%), and sugar content (26.38%) compared to those for the control. These results demonstrate that foliar application of biostimulants, particularly Rerum, is an effective sustainable technology strategy for significantly enhancing both the yield and nutritional quality of greenhouse-grown lettuce.
Keywords:
ascorbic acid; biostimulants; dry matter; foliar fertilization; lettuce; leaf biomass; quality; sugars; sustainable; yield 1. Introduction
The importance of the lettuce (Lactuca sativa L., Asteraceae family) crop is due to its versatility, rapid growth cycle, adaptability to various climates, and nutritional value. Lettuce can be grown in various systems, such as greenhouses, fields, and even in hydroponic environments, which highlights its adaptability to different farming practices [1]. Moreover, in line with technological advancements and the development of automated systems, greenhouses controlled by AI algorithms have been developed for growing lettuce [2].
From a nutritional perspective, lettuce is rich in minerals (phosphorus, potassium, magnesium, iron, zinc), vitamins (folate, C, E), phytochemicals (phenolic compounds, carotenoids, chlorophyll), and fiber [3,4,5,6].
Due to its high content of dietary fiber, antioxidants, and bioactive phytochemicals, lettuce is considered highly beneficial for health, offering cardioprotective, anti-cancer, anti-diabetic, immunomodulatory, hepatoprotective, and neuroprotective effects [4]. It is successfully included in healthy diets due to its low calorie and fat content [7].
Considering the agronomic significance of lettuce cultivation, considerable attention has been directed toward optimizing fertilization strategies to ensure an adequate nutrient supply, which enhances both yield and product quality. Optimum fertilization of lettuce positively influences growth, ensuring strong root and foliage development and consequently high yields [8].
Although chemical fertilization can enhance yield and quality in lettuce cultivation, excessive or unbalanced application poses significant environmental and nutritional risks. The overuse of nitrogen fertilizers can result in the accumulation of nitrates in the leaves, which raises health concerns for consumers, particularly when concentrations exceed recommended safety limits [9]. Therefore, rational and balanced fertilization is crucial to ensure both environmental sustainability and the quality and safety of lettuce.
Recently, the use of biostimulants has gained increasing attention due to their positive effects on crop yields and their potential as sustainable fertilization solutions in controlled production systems, a topic explored in research studies [10,11]. Biostimulants are substances or products, either natural or synthetic, that enhance plant growth, improve stress resistance, and boost nutrient efficiency when applied to plants or soil [12]. Biostimulants are agricultural inputs that help reduce reliance on chemical fertilizers by enhancing nutrient uptake, improving crop quality and yield, and influencing plant metabolic processes [13]. In addition, biostimulants provide long-term economic and environmental benefits, particularly in greenhouse systems, despite the initial costs and necessary management adjustments [14,15,16].
A wide range of biostimulants (silicon, seaweed extracts, protein hydrolysates, humic and fulvic acids) has been tested on various crops, and in most cases, their beneficial effects on plant growth, nutrient uptake, and the production of high-quality crops have been demonstrated [17,18,19].
In the case of lettuce cultivation, biostimulants enhance production by improving physiological and biochemical processes, including metabolism, enzymatic activity, and antioxidant defenses. They also boost photosynthetic efficiency and nutrient uptake, leading to increased yield and improved nutritional quality. Through these mechanisms, biostimulants contribute to more sustainable and resilient greenhouse lettuce cultivation [20]. For example, some studies [21] evidence for lettuce crop that the use of biostimulants via foliar application efficiently delivers nutrients directly to the leaves, rapidly correcting nutrient imbalances through quick absorption. Furthermore, the application of commercial plant biostimulants based on protein hydrolysates to red and green romaine lettuce grown under controlled conditions enhanced physiological processes linked to photosynthesis and biomass accumulation, including increases in leaf number, leaf area, leaf weight, and root weight [22].
The application of various biostimulants to lettuce grown under hydroponic conditions improved stomatal conductance, chlorophyll content, nutrient uptake, and overall crop yield [23].
Moreover, evaluation of biostimulants based on humic and fulvic acids, seaweed extracts, silicon, and vegetable proteins in terms of mitigating the effects of deficit irrigation in lettuce has shown that they have variable but generally positive effects [18]. In addition, biostimulants influenced macronutrient content and improved the Na+:Ca2+ and Na+:Mg2+ ratios in different lettuce cultivars [24].
Nevertheless, the widespread adoption of biostimulants is hindered by several factors, including a lack of standardized regulations, an incomplete understanding of their mechanisms of action, limited acceptance among farmers, and relatively high costs [25].
This study aims to evaluate the effect of several commercial biostimulants on the yield and quality of greenhouse-grown ‘Analena’ hybrid lettuce. The selected biostimulants (Cropmax, FullGreen, Nutrigizer, and Rerum) are commercially available products, widely used in horticultural practice, yet there is limited data on their impact on lettuce cultivation, particularly concerning yield and quality. By focusing on these products, we aim to address the gap in the literature, offering valuable insights into their practical application and effectiveness in real-world agricultural systems.
This study examines quality indices for lettuce crop, such as dry matter content, ascorbic acid concentration, and sugar levels. Ascorbic acid serves as an important indicator of the nutritional quality of vegetables, being a valuable antioxidant that the human body cannot synthesize. Dry matter content reflects both the overall nutritional density and post-harvest quality of the product, while sugar levels are closely linked to taste and consumer acceptance.
This research aims to evaluate the potential of these biostimulants to enhance both the productivity and nutritional quality of lettuce in a greenhouse system, exploring their role as sustainable agricultural inputs.
To the best of our knowledge, this is the first study to provide both a comparative evaluation of the effects of Cropmax, FullGreen, Nutrigizer, and Rerum on the yield and a comprehensive profile of quality indices (dry matter, ascorbic acid, sugars) in the ‘Analena’ hybrid lettuce under greenhouse conditions.
Based on previous studies showing their potential, this study hypothesizes that applying commercial biostimulants will improve the yield and key quality traits of ‘Analena’ lettuce, providing sustainable alternatives to conventional fertilization.
2. Material and Methods
2.1. Soil Analyses
Prior to planting, soil analyses were carried out. Soil samples were collected from a 0–20 cm depth. The agrochemical parameters determined for the soil, methods, and instrumentation are presented in Table 1.

Table 1.
Soil agrochemical parameters, analytical methods, and instrumentation.
Soil reaction was achieved through the potentiometric method using a water suspension (1:5, m/V). Humus content (H) was determined based on the organic carbon content using the Walkley–Black method modified by Gogoașă.
Hydrolytic acidity (HAC) and the sum of total exchangeable bases (TEB) were determined using the volumetric Kappen method. These parameters were used to calculate (1) the cation exchange capacity (CEC) and (2) base saturation (BS), using the formulas (1) CEC, mE 100 g−1 = TEB + HAC and (2) BS, % = (TEB/CEC) ∙ 100.
Humus content (H) and base saturation (BS) were used to calculate the nitrogen index (NI) using the formula NI = (H∙BS)/100.
Phosphorus and potassium, in mobile form, were extracted using an ammonium acetate–lactate solution with pH of 3.75 at an extraction ratio of 1:20 (m/V) (using the Egnèr-Riehm-Domingo method). Phosphorus was determined through spectrophotometric means using the molybdenum blue method (λ = 720 nm), while potassium was determined through atomic emission spectrometry.
Carbonates were determined using the volumetric method (the Scheibler method).
Appropriate calibrations of the devices employed in the analyses were performed before determination to ensure the quality of the analytical procedures and the correctness of the obtained data.
The results obtained after the agrochemical analyses are presented in Table 2.

Table 2.
Soil agrochemical characterization at the experimental site.
Lettuce cultivation is demanding in terms of the soil, with medium-textured soils rich in humus and with a pH between 6.00 and 7.00 preferred [26].
2.2. Plant Material
The experiment used the ‘Analena’ hybrid lettuce, chosen for its excellent cold tolerance and suitability for cultivation in spring and autumn, both in greenhouses/tunnels and open fields. Additionally, ‘Analena’ is resistant to downy mildew caused by Bremia lactucae, a major threat to lettuce worldwide [27].
The lettuce crop was established in a greenhouse with a metal frame and an arched shape and covered with professional polyethylene film with a thickness of 150–200 microns, resistant to UV radiation and condensation. Due to the construction type, light enters naturally, and ventilation is achieved through the ends of the greenhouse and along the sides, thereby regulating airflow and humidity. The greenhouse temperature, including at the substrate level, was maintained at 18 °C.
Seeds were sown into 70-cell alveolar trays filled with a Kekkila DSM 3W growth substrate and a Sphagnum peat mixture (particle size: 0–6 mm, pH: 5.9, NPK: 15-5-24) supplemented with micronutrients (Figure 1).

Figure 1.
(a) Emergence and formation of the first leaves; (b) development of the leaf rosette; (c) transplanting; (d) vegetative growth stage; (e,f) head formation (optimal harvest period).
Irrigation was applied according to the moisture level of the substrate until seedling emergence. Following emergence, irrigation was reduced and carefully managed through corrective watering to ensure uniform seedling development across all trays.
Seven days after transplanting (which occurred on 13 March 2024), preventive treatments with acaricide and insecticide to control aphids were applied. The commercial products Floramite 240 SC, Bereta (ecological), Closer, and Voliam Targo were used according to the manufacturers’ recommendations for vegetable crops. These products help maintain the health and quality of the lettuce crop by controlling pest infestations, ensuring optimal growth conditions.
When the seedlings developed 3–4 true leaves, the alveolar trays were soaked in a rooting stimulator solution based on plant-derived amino acids to increase the root number per plant.
2.3. Inputs Used in the Experiment
In the developed experiment, ecological fertilizers and biostimulants were used, whose characteristics are presented in Table 3.

Table 3.
Characterization of inputs used in the experiment.
Cropmax is a 100% organic growth biostimulant, suitable for all types of crops. Its effectiveness results from a synergistic blend of micronutrients, amino acids, and vitamins. It promotes vigorous root development, enhances foliar growth, and supports a 10–30% reduction in the need for NPK fertilizers.
FullGreen is an organic input specifically designed for lettuce crops. It enhances root development and promotes foliage growth. The product contains high levels of fulvic and humic acids, as well as essential micronutrients. In accordance with Regulation (EU) 2018/848 [28], it can be used in organic farming.
Nutrigizer 60 2E is a mineral fertilizer enriched with chelated micronutrients and humic acids, suitable for many crops throughout the entire growing cycle.
Rerum is an amino-acid-based agricultural biostimulant, suitable for a wide range of crops and recommended for foliar application.
2.4. Experimental Design
A monofactorial experiment was performed with 5 experimental variants, coded as V1–V5 (V1—control variant; V2—Cropmax variant; V3—FullGreen variant; V4—Nutrigizer variant; V5—Rerum variant), and 5 replications, coded as R1–R5, arranged into randomized blocks (Figure 2).
Figure 2.
The layout of treatments and replications within the experiment (V1—control variant; V2—Cropmax variant; V3—FullGreen variant; V4—Nutrigizer variant; V5—Rerum variant; R1–R5—replications).
The lettuce crop was established in strips, with a 30 cm distance between rows, 60 cm between strips, and 25–30 cm between plants within rows. Each experimental variant covered 6 m, with 24 plants per variant. Considering there were 5 repetitions, this resulted in a total of 720 lettuce plants.
2.5. Fertilization Protocol
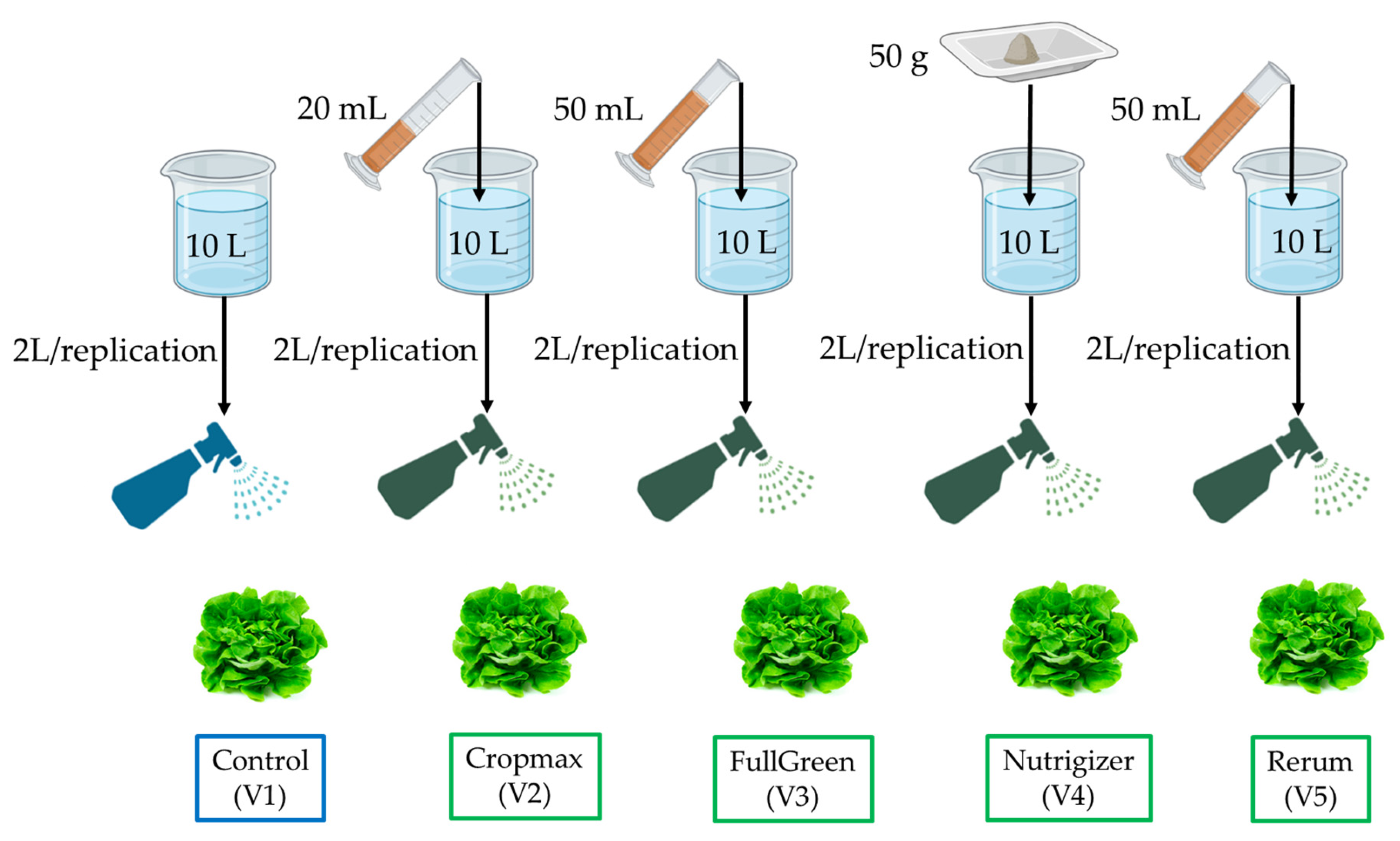
Foliar fertilization treatments for all biostimulants were carried out as follows, 20 March 2024 (F1); 29 March 2024 (F2); 13 April 2024 (F3); and 20 April 2024 (F4), using the doses of the commercial products shown in Figure 3. The products were applied foliarly, and the concentrations used were as follows: 2 mL L−1 (Cropmax), 5 mL L−1 (FullGreen and Rerum), and 5 g L−1 (Nutrigizer). Each variant was treated with a 2.0 L foliar application of the solution. The control variant did not receive any foliar treatments with biostimulants, only preventive treatments for pest infestations.
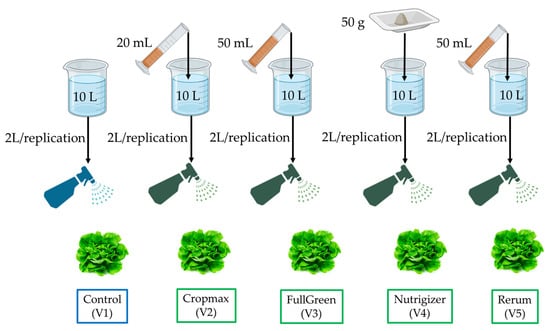
Figure 3.
Preparation and application scheme of foliar treatments in lettuce experiment (V1—control variant; V2—Cropmax variant; V3—FullGreen variant; V4—Nutrigizer variant; V5—Rerum variant; R1–R5—replications).
For the foliar treatments, the same volume of solution, the same method, and the same type of equipment were used (a manual sprayer with a constant pressure, equipped with a fan nozzle for uniform distribution of the solution on the leaf surface). Spraying was carried out from a distance of approximately 30–40 cm from the leaves, moving uniformly and slowly across the entire plant’s surface to avoid concentrating the solution in one spot. To ensure that each plant received an equivalent amount of solution, multiple uniform passes were made.
2.6. Plant Analyses
The total dry matter content (%) was determined through the gravimetric method, which involves measuring the weight loss of the plant material after heating it to 105 °C.
The ascorbic acid content was determined volumetrically, through the iodometric method. This method is based on the oxidation of L-ascorbic acid into dehydroascorbic acid in a 2% hydrochloric acid (HCl) medium, followed by the blue coloration of 2,6-dichlorophenolindophenol in the solution and its reduction into the red form at a pH of 4.2.
The sugar content was estimated using the Fehling’s method, a volumetric procedure based on the reduction of copper (II) ions into copper(I) oxide by reducing sugars [29].
2.7. Statistical Processing of the Data
Data were analyzed using a one-way ANOVA, and means were separated using Duncan’s multiple-range test at a significance level of p ≤ 0.05. The statistical package IBM SPSS (Version 26) was used for analysis.
3. Results and Discussion
3.1. Impact of Foliar Treatments on the Average Leaf Mass After Fertilization Treatments
All applied foliar treatments resulted in an increase in lettuce leaf biomass compared to that for the control variant (Table S1). Beneficial effects of biostimulants on an increase in plant biomass have been demonstrated and reported [17,30,31].
The results obtained on the lettuce crop (Table S1) are consistent with those from previous studies [30] where biostimulant applications significantly improved vegetative growth. For instance, treatment with Green Leaves, a biostimulant containing Macrocystis algae extract, amino acids, corn steep liquor, calcium, and glycine betaine, resulted in up to a 26% increase in lettuce plant growth compared to that for untreated controls.
3.1.1. The Influence of Foliar Treatments on the Average Leaf Mass After the First Fertilization (F1)
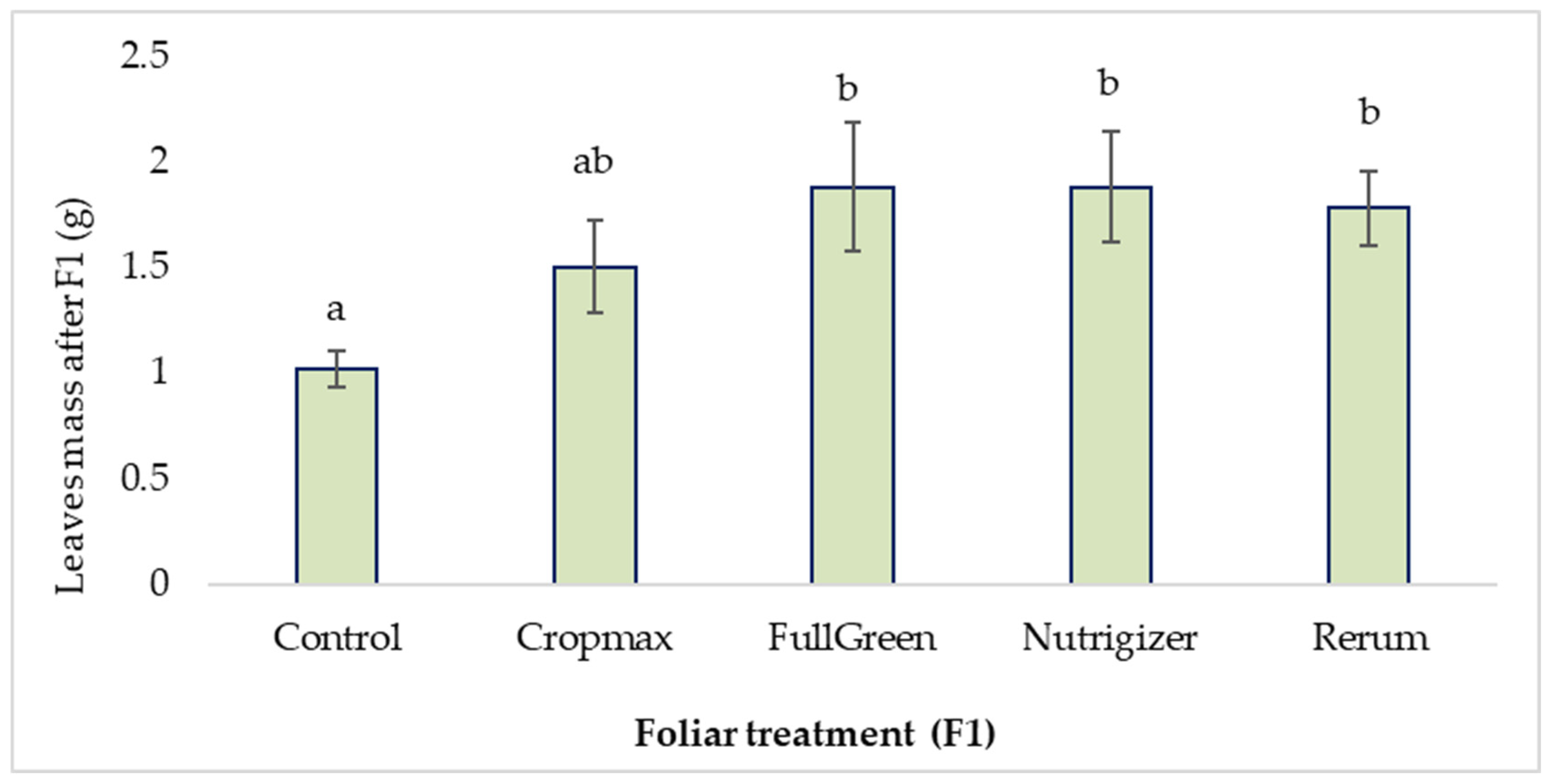
The variance analysis using Duncan’s test, with the significance level set at 0.05, indicates significant differences between the control and the application of Rerum (0.76 g), as well as FullGreen and Nutrigizer (0.86 g), in terms of the average leaf mass after the first fertilization (F1).
In the case of the treatments with Cropmax, FullGreen, Nutrigizer, and Rerum, no significant differences were observed among them; however, the highest increases compared to the Control were recorded with FullGreen and Nutrigizer, showing the greatest difference of 84.31% after the first fertilization (Table S1, Figure 4).
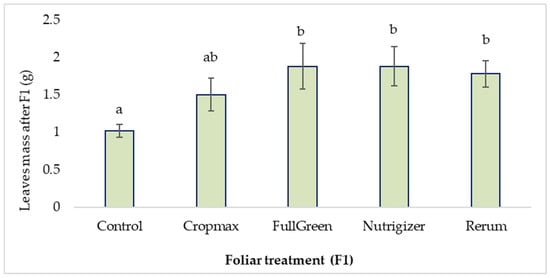
Figure 4.
Effects of foliar treatment (F1) on leaf mass. Each value is a mean of 5 replicates with the standard error mean (mean ± SEM). Means with different letters (a, b) are significantly different at the 0.05 probability level according to Duncan’s multiple-range test.
3.1.2. The Influence of Foliar Treatments on the Average Leaf Mass After the Second Fertilization (F2)
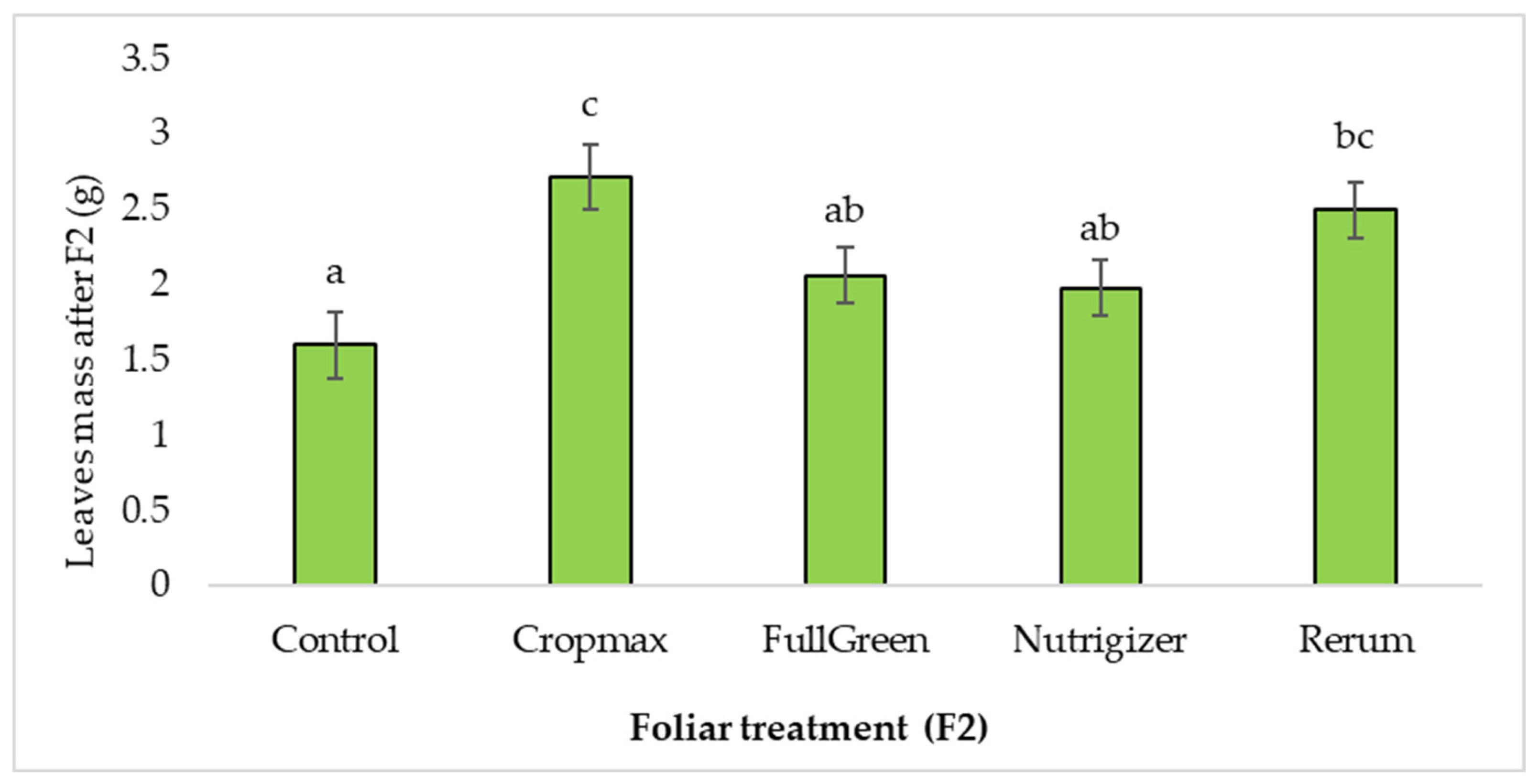
After the second fertilization (F2), the plants treated with Cropmax and Rerum developed a significantly larger leaf mass than that for those treated with FullGreen or Nutrigizer (Table S1). Duncan’s test indicated significant differences in mean leaf mass for the Cropmax (1.12 g) and Rerum (0.90 g) treatments and non-significant differences for FullGreen (0.46 g) and Nutrigizer (0.38 g) compared to the control. The greatest difference from the control (70%) was recorded in the variant fertilized with Cropmax (Table S1 and Figure 5).
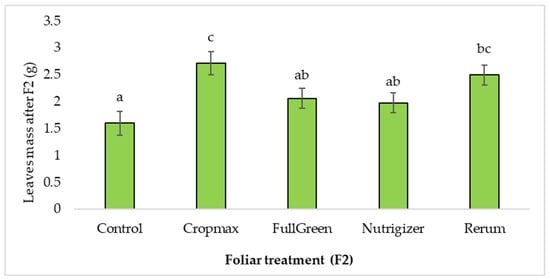
Figure 5.
Effects of foliar treatment (F2) on leaf mass. Each value is a mean of 5 replicates with standard error mean (Mean ± SEM). Means with different letters (a, b, c) are significantly different at the 0.05 probability level according to Duncan’s multiple-range test.
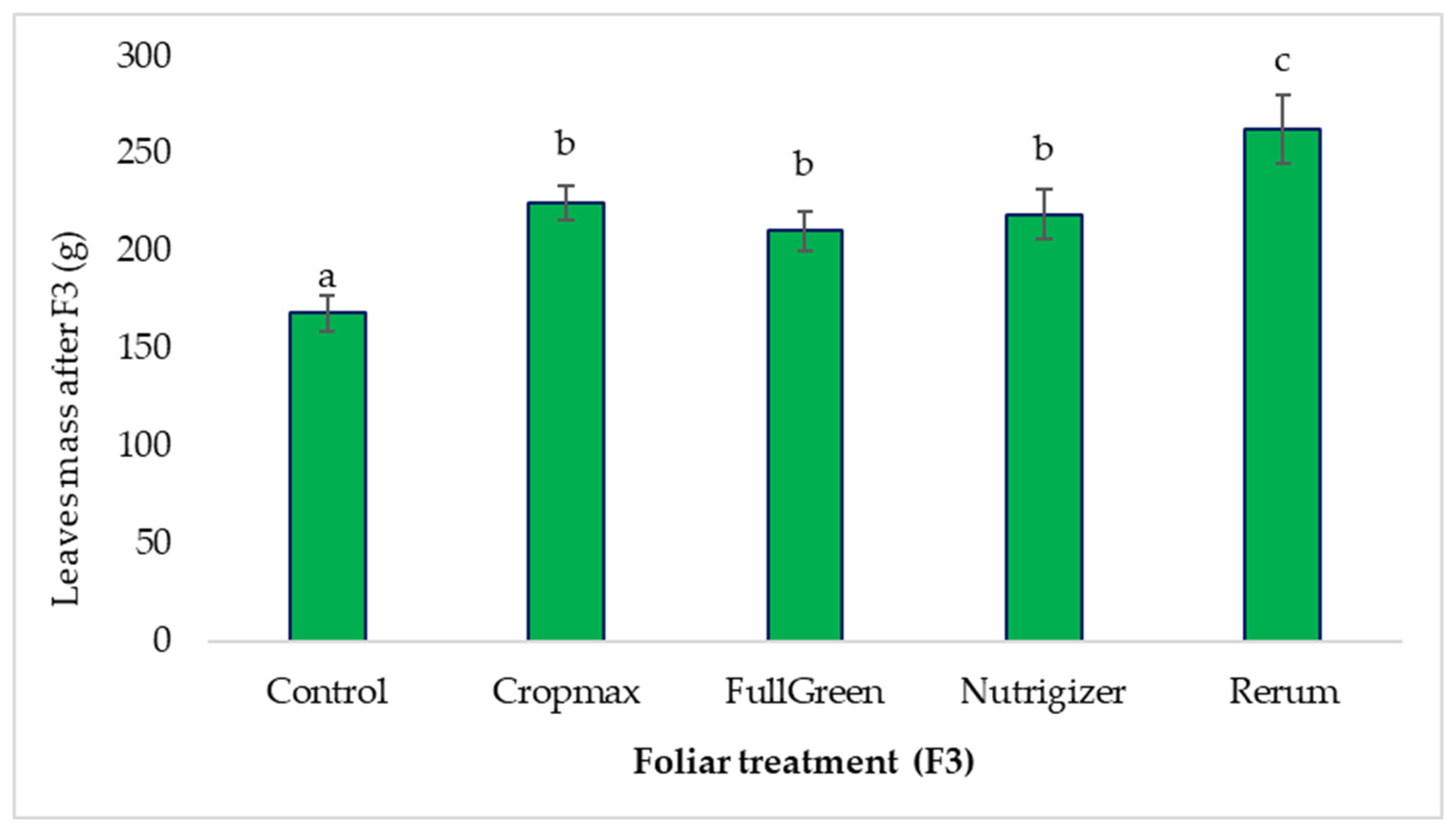
3.1.3. The Influence of Foliar Treatments on the Average Leaf Mass After the Third Fertilization (F3)
After the third fertilization (F3), a 56.12% increase in the average leaf mass was observed in the Rerum-treated variant compared to that in the control, surpassing the Cropmax-treated variant, which had previously shown the highest increase in average leaf mass after the second fertilization (Table S1).
Following the third fertilization (F3), the average leaf mass for the FullGreen, Nutrigizer, and Cropmax treatments was significantly greater than that for the control, by 42.4 g, 50.8 g, and 56.6 g, respectively (Table S1).
No significant differences were found among the FullGreen, Nutrigizer, and Cropmax variants; however, there are significant differences between this group and the Rerum treatment. The Rerum-treated variant showed the most substantial significant increase compared to the control (94.4 g) after the third fertilization (Table S1 and Figure 6).
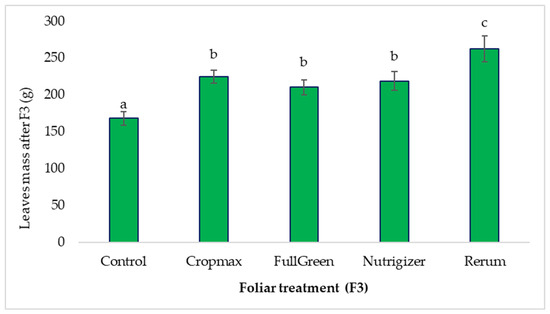
Figure 6.
Effects of foliar treatment (F3) on leaf mass. Each value is a mean of 5 replicates with standard error mean (Mean ± SEM). Means with different letters (a, b, c) are significantly different at the 0.05 probability level according to Duncan’s multiple-range test.
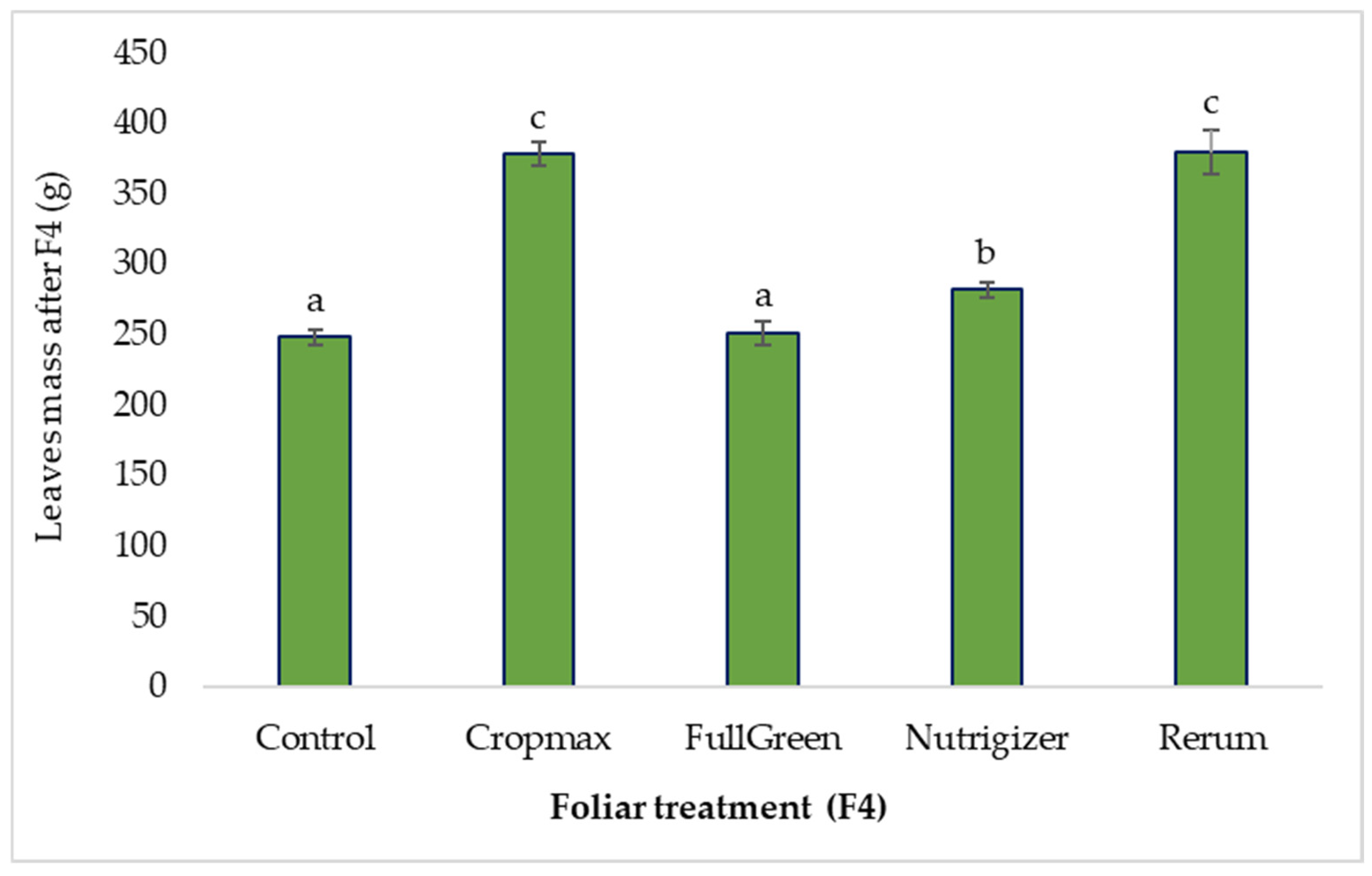
3.1.4. The Influence of Foliar Treatments on the Average Leaf Mass After the Fourth Fertilization (F4)
After the fourth fertilization (at the head formation stage), a similar increase in average leaf mass was observed in the Cropmax and Rerum treatments—52.25% and 52.73%, respectively—compared to the control. The use of the biostimulant containing humic and fulvic acids (FullGreen) did not influence the average leaf mass, with an increase of only 1.20% compared to the control.
The impact of biostimulant treatments based on humic and fulvic acids on lettuce growth has been documented in the literature [18,32], demonstrating their potential to increase yield through enhanced leaf development.
As shown in Table S1, the analysis of variance indicates significant differences in average leaf mass compared to the control for the Cropmax (52.25 g), Rerum (52.73 g), and Nutrigizer (33.4 g) treatments after the fourth fertilization. Fertilization with FullGreen did not result in a statistically significant increase in average leaf mass compared to the control at the F4 stage (Table S1 and Figure 7).
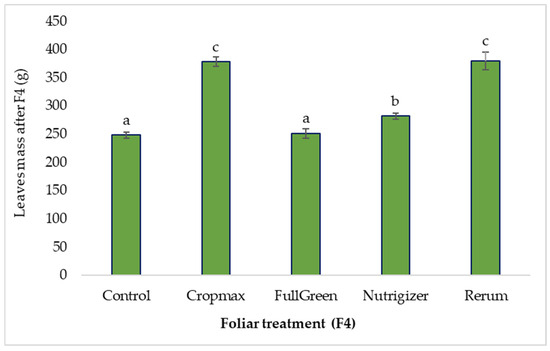
Figure 7.
Effects of foliar treatment (F4) on leaf mass. Each value is a mean of 5 replicates with standard error mean (Mean ± SEM). Means with different letters (a, b, c) are significantly different at the 0.05 probability level according to Duncan’s multiple-range test.
3.2. Results on Lettuce Yield Under the Influence of Applied Foliar Treatments
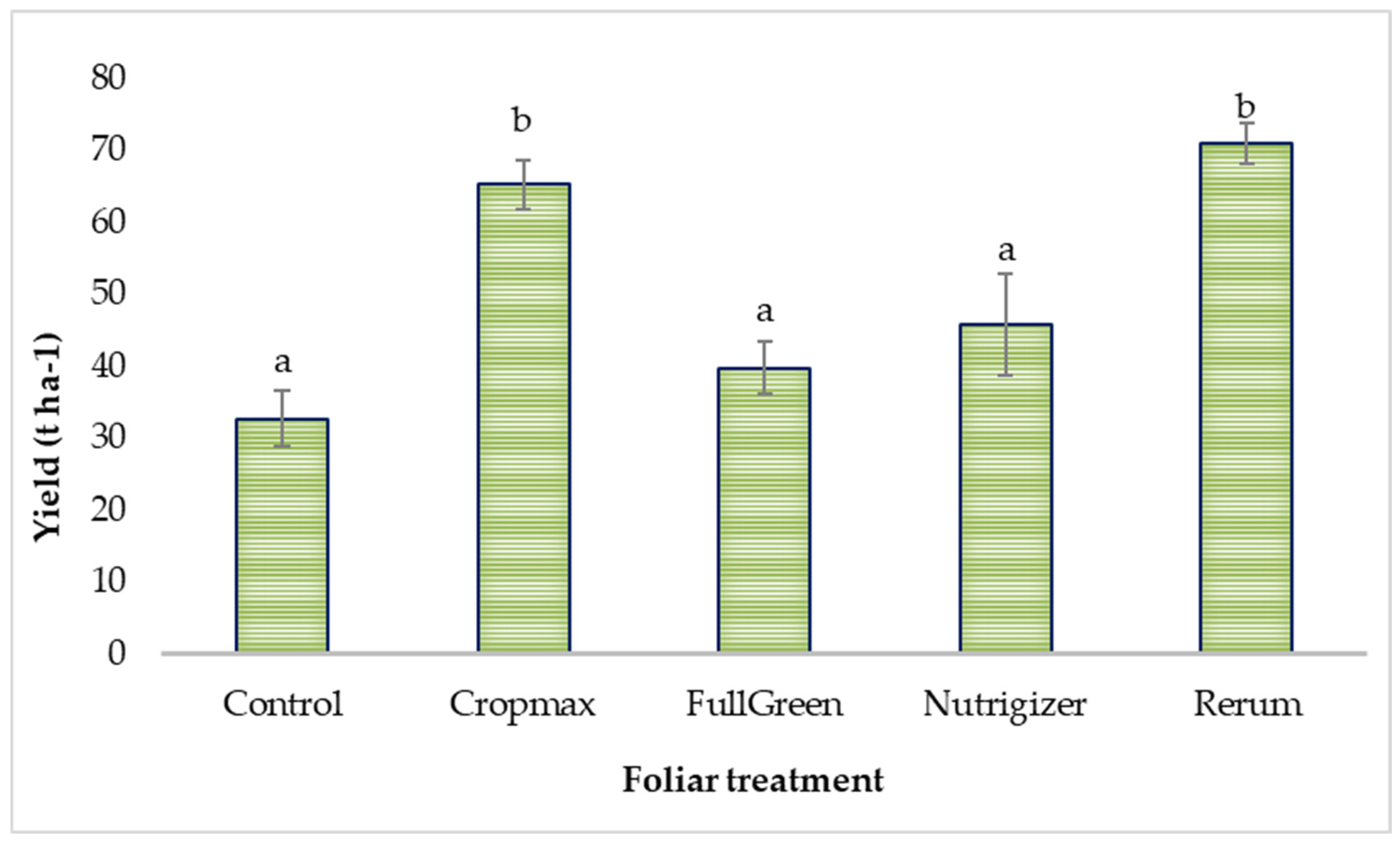
The foliar treatments applied influenced lettuce yield, as can be seen in Table S2. It was found that the Rerum-treated variant recorded a 117% increase in yield compared to that for the control, while the Cropmax-treated variant showed a 99.51% increase in yield over that for the control.
Duncan’s test applied to lettuce yield (t ha−1), based on the type of foliar fertilizer used, indicated significant differences of 32.56 t ha−1 and 38.28 t ha−1 compared to the control for the Cropmax and Rerum treatments, respectively.
Lettuce yield was not significantly affected by fertilization with the FullGreen and Nutrigizer treatments (Table S2 and Figure 8). According to another study [33], yields between 25 and 32.7 t ha−1 were obtained for different lettuce hybrids cultivated under conditions comparable to those for the control treatment in the current experiment.
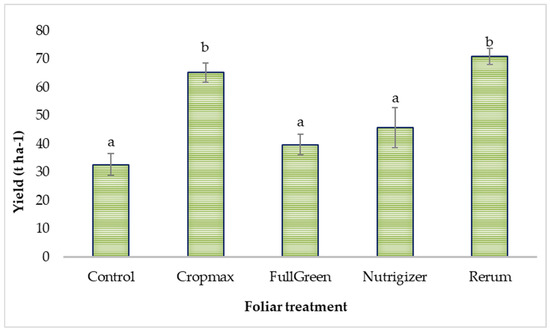
Figure 8.
Effects of foliar treatments on lettuce yield. Each value is a mean of 5 replicates with standard error mean (mean ± SEM). Means with different letters (a, b) are significantly different at the 0.05 probability level according to Duncan’s multiple-range test.
Evaluation of the lettuce yield (Table S2) indicated that foliar application of the product Rerum led to the highest yield (71 t ha−1), followed by that for the variant treated with Cropmax (65.28 t ha−1). These results can be attributed to the composition of the products, particularly the presence of amino acids, which play a key role in enhancing plant growth, photosynthesis optimization, and productivity [34]. In addition, Rerum incorporates an auxin biostimulator, conferring a distinct advantage over other products, as evidenced by the resulting data.
The effectiveness of biostimulants based on fulvic acid, amino acids, and vermicompost in terms of lettuce yield was reported in a study [35] in which it resulted in a 17.4–18.2% increase over the control. The use of a plant-derived biostimulant based on amino acids resulted in a 21% increase in lettuce yield compared to that for the untreated control [36].
3.3. Results on the Variability in Quality Indices Under the Influence of Applied Foliar Treatments
The evaluation of quality indices for lettuce (dry matter, ascorbic acid, and sugars) led to the results presented in Table S3. As can be observed, the application of foliar fertilization with the biostimulants under study resulted in increased values for these quality parameters compared to those for the control variant.
3.3.1. Variability in Dry Matter Content
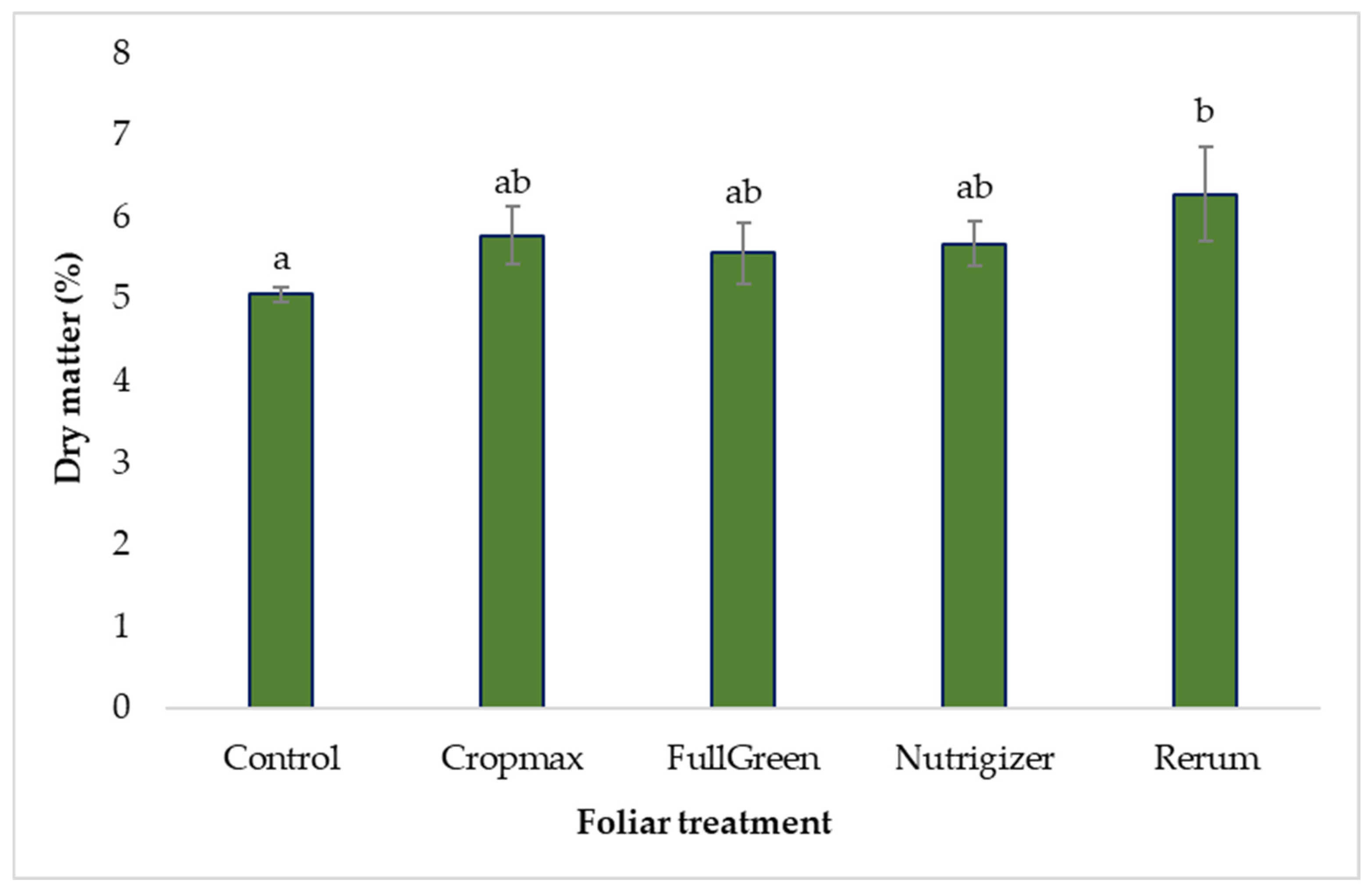
The dry matter accumulation content varied as follows, control < FullGreen < Nutrigizer < Cropmax < Rerum, with an increase of 24.11% observed in the case of the Rerum variant compared to the control.
According to other studies, the dry matter content for different lettuce hybrids cultivated in different regimes ranges between 5.30% and 6.22% [33], 4.27% and 4.92% [37], and 4.36 and 6.41% [38].
As shown in Table S3, the analysis of variance indicates statistically significant differences in the case of Rerum fertilization, with an increase of 1.22% in dry matter accumulation compared to that for the control, depending on the type of foliar fertilizer applied. For the other experimental treatments, the application of foliar fertilizers did not result in statistically significant differences compared to the control (Table S3 and Figure 9).
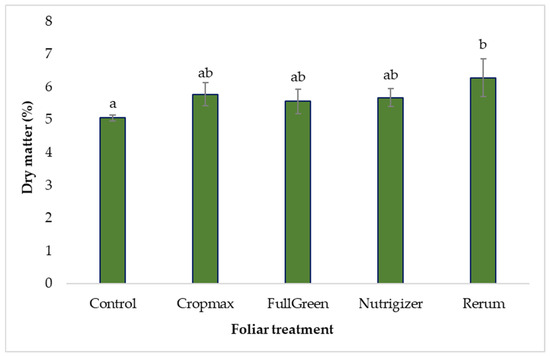
Figure 9.
Effects of foliar treatments on lettuce dry matter content. Each value is a mean of 5 replicates with standard error mean (mean ± SEM). Means with different letters (a, b) are significantly different at the 0.05 probability level according to Duncan’s multiple-range test.
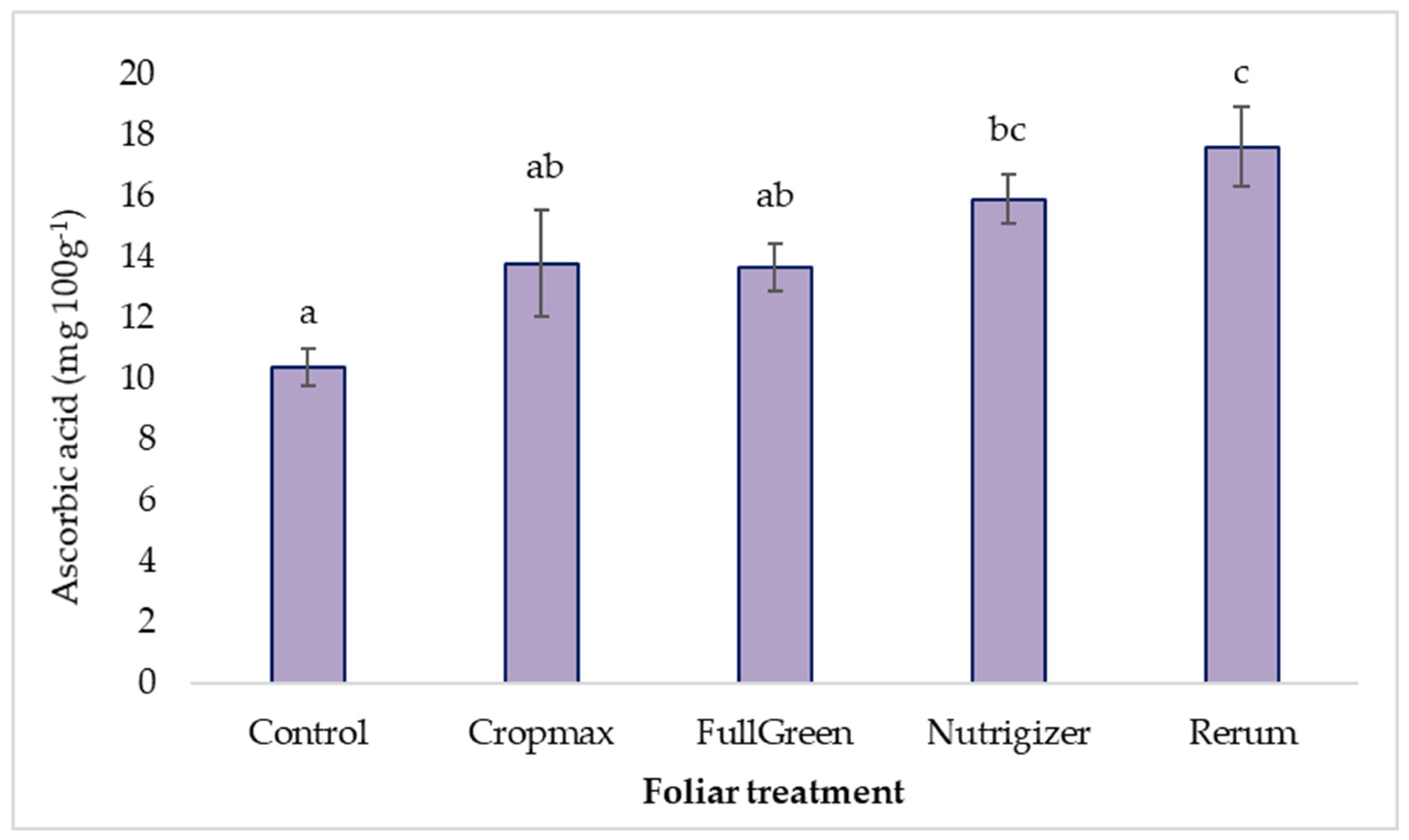
3.3.2. Variability in Ascorbic Acid Content
The ascorbic acid content in lettuce is generally lower than that in other leafy greens, broccoli, cauliflower, or pepper [4]. Nevertheless, its main advantage is that it is mostly consumed raw, and because of this, even though it has a lower content, it is fully utilized by the body.
In the experiment, the highest concentration of ascorbic acid was observed in the lettuce plants fertilized with Rerum (17.62 mg 100 g−1), representing an increase of 69.74% relative to the control variant. Additionally, the treatment with Nutrigizer demonstrated a notable increase of 53.17% compared to the control.
Statistical analysis (Duncan’s test) indicates significant differences of 5.52 mg 100 g−1 for the variant fertilized with Nutrigizer and 7.24 mg 100 g−1 for the variant fertilized with Rerum. For the variants fertilized with Cropmax and FullGreen, the analysis of variance revealed non-significant differences of 3.40 mg 100 g−1 and 3.28 mg 100 g−1, respectively, compared to the control, regarding the ascorbic acid content (Table S3 and Figure 10).
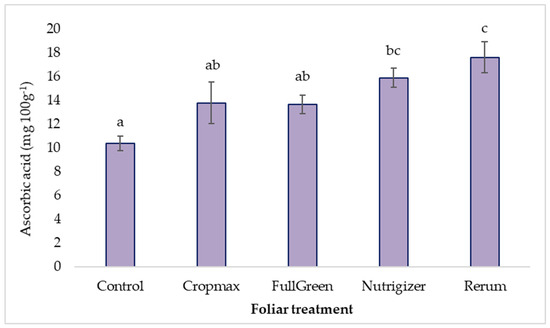
Figure 10.
Effects of foliar treatments on ascorbic acid content. Each value is a mean of 5 replicates with standard error mean (mean ± SEM). Means with different letters (a, b, c) are significantly different at the 0.05 probability level according to Duncan’s multiple-range test.
The ascorbic acid contents of all experimental variants exceed those reported in the literature, a phenomenon most likely associated with the ‘Analena’ hybrid used in the experiment. In general, according to data from the literature, ascorbic acid content is correlated with the type of lettuce. It has been found that leaf lettuce contains higher levels of ascorbic acid compared to head lettuce.
The reported ascorbic acid contents range from 5.25 to 9.60 mg 100 g−1 for leaf lettuce and from 3.85 to 4.99 mg 100 g−1 for head lettuce. In addition, colored leaf varieties have the lowest contents [39]. Similar research [33] has also indicated ascorbic acid contents of 6.68–7.27 mg 100 g−1 for leaf lettuce, compared to 5.77–6.29 mg 100 g−1 for head lettuce.
Furthermore, the ascorbic acid content reported [30] for leaf lettuce grown under different fertilization regimes, including biofertilizer treatments, ranged between 7.00 and 13.20 mg 100 g−1.
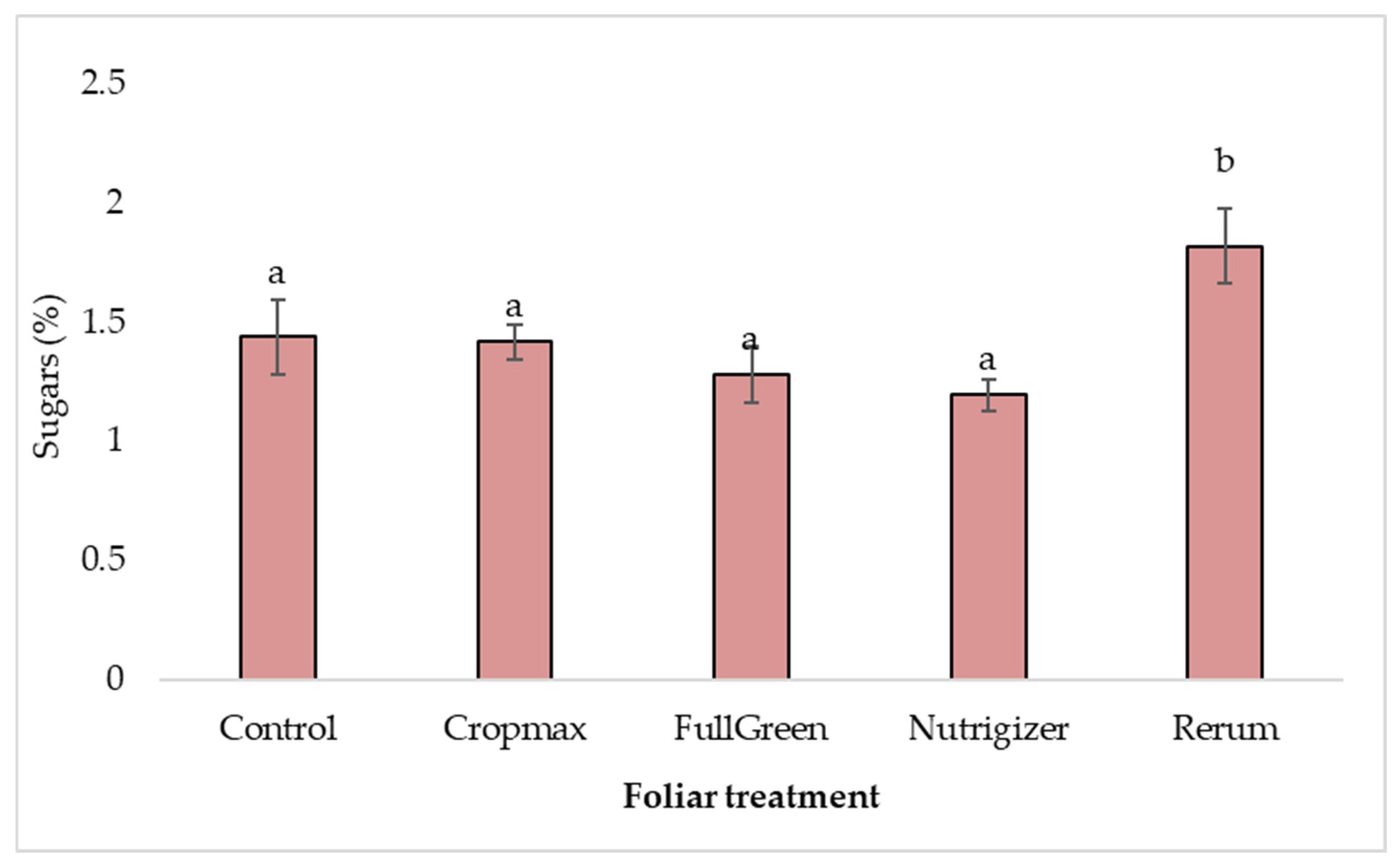
3.3.3. Variability in Sugar Content
Regarding sugar levels, laboratory analyses indicated a content of 1.82% for the Rerum variant. The ‘Analena’ lettuce variety naturally has a high sugar content, as evidenced by the control variant’s value of 1.44%, demonstrating that even without fertilization, it can provide a significant amount of sugars, which contribute to its distinctive taste properties.
For the Rerum fertilizer, the increase in sugar content (0.38%) was statistically significant compared to the control (Table S3).
Regarding the influence of foliar fertilization on the sugar content in the variants treated with Cropmax, FullGreen, and Nutrigizer, the analysis of variance revealed non-significant differences (Table S3 and Figure 11).
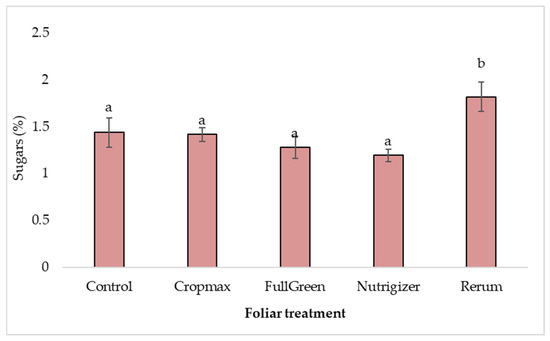
Figure 11.
Effects of foliar treatments on sugar content. Each value is a mean of 5 replicates with standard error mean (mean ± SEM). Means with different letters (a, b) are significantly different at the 0.05 probability level according to Duncan’s multiple-range test.
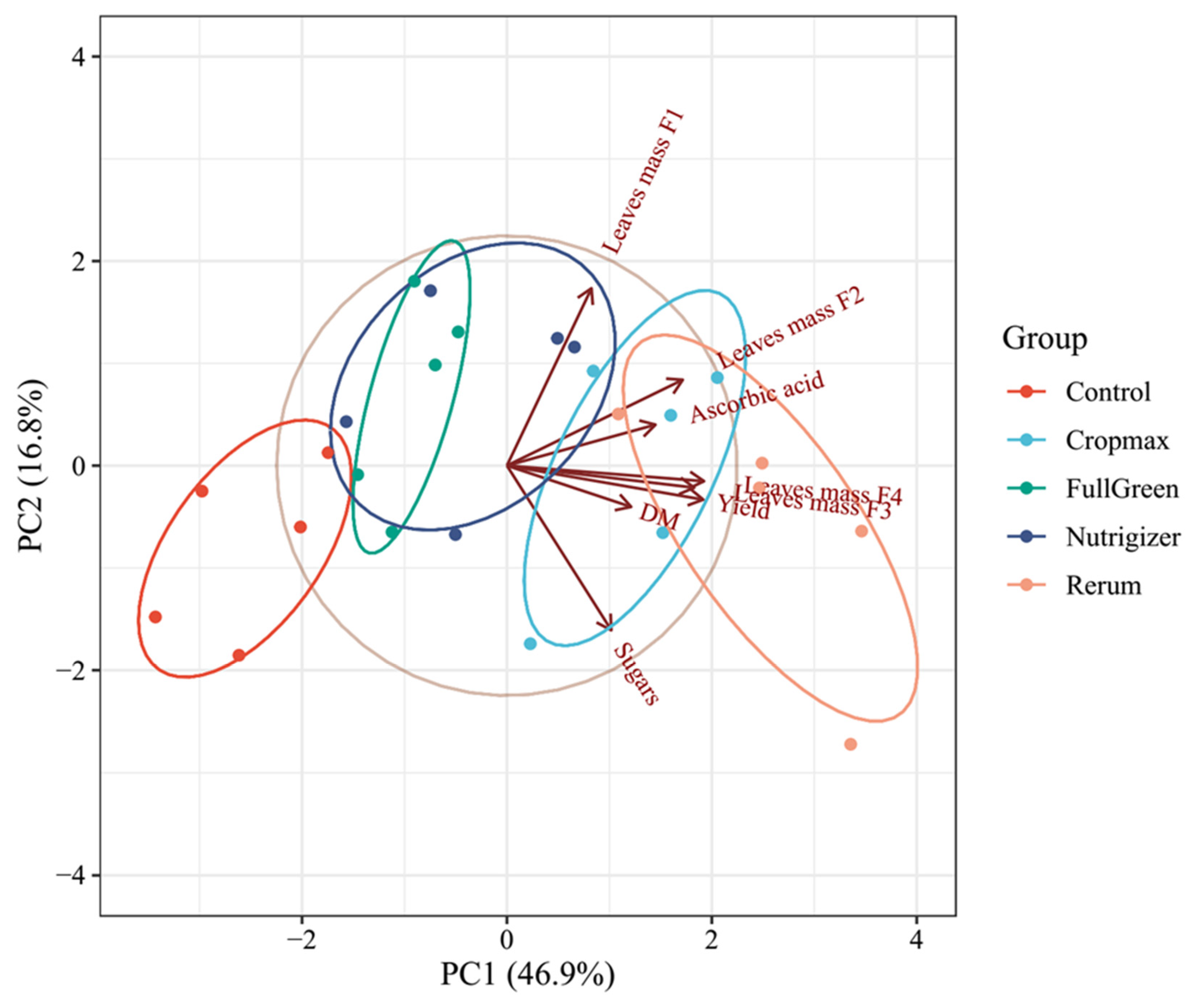
3.4. Analysis of Data Variability Through Principal Component Analysis (PCA)
A data matrix with five rows (number of samples, i.e., control, Cropmax, FullGreen, Nutrigizer, and Rerum) and eight columns (number of variables, i.e., leaf mass F1, leaf mass F2, leaf mass F3, leaf mass F4, yield, dry matter (DM), ascorbic acid, and sugar content) was used in the principal component analysis (PCA).
The principal component analysis clearly demonstrated that biostimulant application substantially affected the physiological and biochemical performance of lettuce plants (Figure 12). The first two components (PC1 = 46.9% and PC2 = 16.8%) together explained 63.7% of the total variability, providing a reliable representation of the overall dataset. The strong separation among treatments along PC1 indicates that the biostimulants induced distinct metabolic and growth responses compared with those in the untreated control.
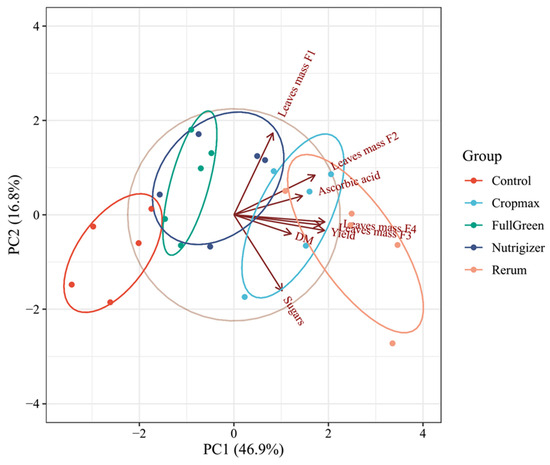
Figure 12.
Principal component analysis (PCA) illustrating the effects of biostimulant treatments on lettuce growth and quality traits. Projections of variables (leaf mass F1–F4, yield, dry matter (DM), ascorbic acid, and sugar content) and samples (control, Cropmax, FullGreen, Nutrigizer and, Rerum) onto the factor plane PC1–PC2.
Treatments with Rerum and Cropmax were positioned on the positive side of PC1 and were associated with higher values for leaf mass, yield, dry matter, and ascorbic acid, suggesting an enhancement in both growth and nutritional quality parameters. In contrast, the control group clustered into the opposite quadrant, characterized by lower values for these variables, confirming the positive influence of biostimulant application. FullGreen and Nutrigizer formed partially overlapping clusters, reflecting intermediate effects, which may indicate moderate stimulation of growth and biochemical activity relative to that in the control.
The close association of the ascorbic acid, yield, and leaf biomass vectors suggests that the increase in antioxidant capacity was accompanied by improved vegetative growth, possibly as a result of enhanced nutrient assimilation and metabolic efficiency promoted by the biostimulants. Biostimulant formulations can modulate plant metabolism, leading to improved productivity and crop quality. Overall, the PCA supports the conclusion that the tested biostimulants, particularly Rerum and Cropmax, exerted the most pronounced positive effects on lettuce growth performance and quality-related biochemical attributes.
3.5. Comparative Analysis of Biostimulants’ Effects on Lettuce
A comparative analysis of the effects of the biostimulants used in the experiment on lettuce growth, yield, and quality parameters (dry matter, ascorbic acid content, and sugar levels) is presented in Table 4.

Table 4.
Comparative effects of biostimulants on lettuce growth, yield, and quality parameters.
Cropmax application resulted in high leaf biomass, yield, dry matter, and sugar levels, with a moderate effect on ascorbic acid. This response is attributed to the presence of amino acids in the biostimulant which are readily absorbed through the leaves and enhance protein and chlorophyll synthesis, promoting vegetative growth and high leaf biomass [20,34,40]. Additionally, amino acids indirectly influence sugar synthesis in plant tissues, contributing to the elevated sugar content observed [41,42].
A similar study [30] evidenced that biostimulants based on amino acids enhance the photosynthetic rate in lettuce, as well as the accumulation of soluble sugars and proteins.
Lettuce treated with FullGreen showed the lowest values among the applied biostimulants for leaf biomass, yield, and quality parameters. This weak response can be explained by the limited composition of the biostimulant, which contains mainly macroelements and only a few microelements, lacking the amino acids or growth-promoting compounds necessary to strongly enhance plant metabolism and growth.
The application of Nutrigizer 60 2E resulted in high levels of ascorbic acid, while its effects on leaf biomass, yield, and dry matter were moderate. Although humic acids alone do not significantly influence lettuce yield [43], biostimulants containing humic acids can enhance ascorbic acid content [44] and improve overall plant growth by stimulating nutrient absorption [45]. A recent study [46] demonstrated that humic acids derived from leonardite enhance the antioxidant capacity in lettuce by increasing ascorbic acid levels.
Lettuce treated with Rerum exhibited the highest values for leaf biomass (particularly after the F4 treatment), yield, and quality parameters (dry matter, ascorbic acid, and sugar content). These results can be attributed to the balanced composition of amino acids, hormones, and essential nutrients, which enhance overall plant metabolism. Additionally, auxin-like compounds in Rerum promote root development [47], improving nutrient uptake and contributing to the observed increased yields [48].
To conclude, the comparative analysis of the biostimulants used on the lettuce crop evidenced that
(a) Rerum was the best overall performer due to its balanced composition of amino acids, hormones, and nutrients;
(b) Cropmax strongly enhanced growth and yield with a moderate increase in antioxidants (ascorbic acid);
(c) Nutrigizer 60 2E primarily improved the antioxidant potential, possibly due to the humic acids in its composition;
(d) FullGreen showed the weakest overall response, likely due to its limited composition.
4. Conclusions and Future Perspectives
This study highlights the positive impact of biostimulants applied via foliar fertilization on both yield and quality parameters (dry matter, ascorbic acid, sugars) in the ‘Analena’ hybrid lettuce. Among the tested products, Rerum (V5) demonstrated the most significant benefits, with the highest yield increase (117%) and superior quality indices, including a 1.22% higher dry matter content and a 69.75% increase in ascorbic acid as compared with these values for the control. Additionally, the sugar content increased by 26.38% in the Rerum-treated plants. Overall, Rerum surpassed the other biostimulants (Cropmax, FullGreen, and Nutrigizer) in both yield and nutritional quality, proving to be the most effective treatment. The high efficiency of the Rerum product can be attributed to its composition, which, in addition to microelements, includes auxin biostimulator and amino acids that play a crucial role in promoting plant growth, enhancing nutrient uptake, and improving the nutrient profile.
These findings demonstrate the potential benefits of biostimulants for enhancing crop productivity and nutritional quality in the ‘Analena’ hybrid lettuce. They also suggest that such biostimulants could contribute to more sustainable agricultural practices.
In addition to the benefits for crop yield and quality, the use of biostimulants significantly contributes to reducing the carbon footprint by improving fertilizer efficiency, minimizing the need for synthetic fertilizers, and decreasing nutrient runoff. From an economic perspective, biostimulants provide cost savings by reducing input needs and boosting crop yields, positioning them as a sustainable and profitable choice for contemporary agricultural practices.
Future research will explore the effects of biostimulants on other lettuce hybrids and vegetable species, broadening our understanding of their broader applicability.
Given the rapid advancement of technology and the growing demand for high-quality, high-yield production, future perspectives will focus on assessing the effectiveness of biostimulants under stress conditions like salinity and drought, evaluating their economic feasibility, and exploring their integration with AI-controlled greenhouse systems. These directions will further enhance our knowledge of biostimulants’ potential to optimize agricultural practices in diverse and challenging conditions.
Overall, plant biostimulants offer a promising advancement toward more sustainable and resilient farming systems, balancing productivity with effective environmental management.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/su17219736/s1. Table S1: The influence of foliar treatments on leaf mass; Table S2: Results concerning lettuce yield after foliar treatments; Table S3: Results concerning lettuce quality indices after foliar treatments.
Author Contributions
Conceptualization: R.M.M. and G.V.S.; data curation: M.C.G.; formal analysis: G.V.S.; investigation: G.V.S. and M.C.G.; methodology: R.M.M.; software: R.M.M.; validation: G.V.S.; writing—original draft preparation: G.V.S. and M.C.G.; writing—review and editing: R.M.M.; visualization: G.V.S.; supervision: R.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Torres, J.D.; Jaeger, S.; Puerta, P.; Tarrega, A. How do Spanish consumers perceive different lettuce cultivation systems? Insights from explicit and implicit methods. Appl. Food Res. 2025, 5, 100709. [Google Scholar] [CrossRef]
- Petropoulou, A.S.; van Marrewijk, B.; de Zwart, F.; Elings, A.; Bijlaard, M.; van Daalen, T.; Jansen, G.; Hemming, S. Lettuce Production in Intelligent Greenhouses—3D Imaging and Computer Vision for Plant Spacing Decisions. Sensors 2023, 23, 2929. [Google Scholar] [CrossRef] [PubMed]
- Baslam, M.; Garmendia, I.; Goicoechea, N. Enhanced Accumulation of Vitamins, Nutraceuticals and Minerals in Lettuces Associated with Arbuscular Mycorrhizal Fungi (AMF): A Question of Interest for Both Vegetables and Humans. Agriculture 2013, 3, 188–209. [Google Scholar] [CrossRef]
- Shi, M.; Gu, J.; Wu, H.; Rauf, A.; Emran, T.B.; Khan, Z.; Mitra, S.; Aljohani, A.S.M.; Alhumaydhi, F.A.; Al-Awthan, Y.S.; et al. Phytochemicals, Nutrition, Metabolism, Bioavailability, and Health Benefits in Lettuce—A Comprehensive Review. Antioxidants 2022, 11, 1158. [Google Scholar] [CrossRef]
- Volpe, M.L.; Vargas, V.C.S.; Morón, A.; González, R.E. Bioactive Compounds, Antioxidant Activity and Growth Behavior in Lettuce Cultivars Grown under Field and Greenhouse Conditions. Proceedings 2021, 70, 52. [Google Scholar] [CrossRef]
- Cho, E.; Gurdon, C.; Zhao, R.; Peng, H.; Poulev, A.; Raskin, I.; Simko, I. Phytochemical and Agronomic Characterization of High-Flavonoid Lettuce Lines Grown under Field Conditions. Plants 2023, 12, 3467. [Google Scholar] [CrossRef]
- Tokarz, B.; Gajewski, Z.; Makowski, W.; Mazur, S.; Kiełkowska, A.; Kunicki, E.; Jeremiasz, O.; Szendera, W.; Wesołowski, W.; Tokarz, K.M. The Effect of Head Lettuce (Lactuca sativa var. capitata L.) Cultivation Under Glass with a Light Spectrum-Modifying Luminophore on Crop Traits. Agronomy 2025, 15, 2090. [Google Scholar] [CrossRef]
- Hong, J.; Xu, F.; Chen, G.; Huang, X.; Wang, S.; Du, L.; Ding, G. Evaluation of the Effects of Nitrogen, Phosphorus, and Potassium Applications on the Growth, Yield, and Quality of Lettuce (Lactuca sativa L.). Agronomy 2022, 12, 2477. [Google Scholar] [CrossRef]
- Luetic, S.; Knezovic, Z.; Jurcic, K.; Majic, Z.; Tripkovic, K.; Sutlovic, D. Leafy Vegetable Nitrite and Nitrate Content: Potential Health Effects. Foods 2023, 12, 1655. [Google Scholar] [CrossRef]
- Acin-Albiac, M.; García-Jiménez, B.; Marín Garrido, C.; Borda Casas, E.; Velasco-Alvarez, J.; Serra, N.S.; Acedo, A. Lettuce Soil Microbiome Modulated by an L-α-Amino Acid-Based Biostimulant. Agriculture 2023, 13, 344. [Google Scholar] [CrossRef]
- Mesmar, A.K.; Albedwawi, S.T.; Alsalami, A.K.; Alshemeili, A.R.; Abu-Elsaoud, A.M.; El-Tarabily, K.A.; Al Raish, S.M. The Effect of Recycled Spent Coffee Grounds Fertilizer, Vermicompost, and Chemical Fertilizers on the Growth and Soil Quality of Red Radish (Raphanus sativus) in the United Arab Emirates: A Sustainability Perspective. Foods 2024, 13, 1997. [Google Scholar] [CrossRef] [PubMed]
- Sible, C.N.; Seebauer, J.R.; Below, F.E. Plant Biostimulants: A Categorical Review, Their Implications for Row Crop Production, and Relation to Soil Health Indicators. Agronomy 2021, 11, 1297. [Google Scholar] [CrossRef]
- Li, J.; Van Gerrewey, T.; Geelen, D. A Meta-Analysis of Biostimulant Yield Effectiveness in Field Trials. Front. Plant Sci. 2022, 13, 836702. [Google Scholar] [CrossRef] [PubMed]
- Szparaga, A.; Kuboń, M.; Kocira, S.; Czerwińska, E.; Pawłowska, A.; Hara, P.; Kobus, Z.; Kwaśniewski, D. Towards Sustainable Agriculture—Agronomic and Economic Effects of Biostimulant Use in Common Bean Cultivation. Sustainability 2019, 11, 4575. [Google Scholar] [CrossRef]
- Rajabi Hamedani, S.; Rouphael, Y.; Colla, G.; Colantoni, A.; Cardarelli, M. Biostimulants as a Tool for Improving Environmental Sustainability of Greenhouse Vegetable Crops. Sustainability 2020, 12, 5101. [Google Scholar] [CrossRef]
- Ginter, A.; Zarzecka, K.; Gugała, M. Effect of Herbicide and Biostimulants on Production and Economic Results of Edible Potato. Agronomy 2022, 12, 1409. [Google Scholar] [CrossRef]
- Cristofano, F.; El-Nakhel, C.; Rouphael, Y. Biostimulant Substances for Sustainable Agriculture: Origin, Operating Mechanisms and Effects on Cucurbits, Leafy Greens, and Nightshade Vegetables Species. Biomolecules 2021, 11, 1103. [Google Scholar] [CrossRef]
- Chaski, C.; Petropoulos, S.A. The Alleviation Effects of Biostimulants Application on Lettuce Plants Grown under Deficit Irrigation. Horticulturae 2022, 8, 1089. [Google Scholar] [CrossRef]
- Mezeyová, I.; Kollárová, I.; Golian, M.; Árvay, J.; Mezey, J.; Šlosár, M.; Galovičová, L.; Rosa, R.; Bakalár, M.; Horečná, T. The Effect of Humic-Based Biostimulants on the Yield and Quality Parameters of Chili Peppers. Horticulturae 2024, 10, 998. [Google Scholar] [CrossRef]
- Oliveira, T.M.L.d.; Pires, J.S.B.; Oliveira, V.d.S.; Jeveaux Machado, A.J.C.; Fernandes, A.A.; Arantes, L.d.O.; Dousseau-Arantes, S. Potential of the Use of Biostimulants in Lettuce Production. Plants 2025, 14, 2416. [Google Scholar] [CrossRef]
- Fragalà, F.; Salvagno, E.; La Bella, E.; Saccone, R.; Padoan, E.; Montoneri, E.; Miccichè, J.; Ferrarello, D.; Baglieri, A.; Puglisi, I. Enhancing Lettuce Yield through Innovative Foliar Spray of Biopolymers Derived from Municipal Biowastes. Plants 2024, 13, 1664. [Google Scholar] [CrossRef]
- Zahra, A.M.; Sinaga, A.N.K.; Nugroho, B.D.A.; Masithoh, R.E. Effect of Plant Biostimulants on Red and Green Romaine Lettuce (Lactuca sativa) Growth in Indoor Farming. IOP Conf. Ser. Earth Environ. Sci. 2024, 1297, 012008. [Google Scholar] [CrossRef]
- Ikiz, B.; Dasgan, H.Y.; Balik, S.; Kusvuran, S.; Gruda, N. The use of biostimulants as a key to sustainable hydroponic lettuce farming under saline water stress. BMC Plant Biol. 2024, 24, 808. [Google Scholar] [CrossRef]
- Yaseen, A.A.; Takacs-Hajos, M. The Effect of Plant Biostimulants on the Macronutrient Content and Ion Ration of Several Lettuce (Lactuca sativa l.) Cultivars grown in a Plastic House. S. Afr. J. Bot. 2022, 147, 223–230. [Google Scholar] [CrossRef]
- Mannino, G. A New Era of Sustainability: Plant Biostimulants. Int. J. Mol. Sci. 2023, 24, 16329. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.; Shackley, S.; Sohi, S.; Suy, T.B.; Haefele, S. The Impact of Biochar Application on Soil Properties and Plant Growth of Pot Grown Lettuce (Lactuca sativa) and Cabbage (Brassica chinensis). Agronomy 2013, 3, 404–418. [Google Scholar] [CrossRef]
- Ban, S.; Tian, M.; Hu, D.; Xu, M.; Yuan, T.; Zheng, X.; Li, L.; Wei, S. Evaluation and Early Detection of Downy Mildew of Lettuce Using Hyperspectral Imagery. Agriculture 2025, 15, 444. [Google Scholar] [CrossRef]
- Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on Organic Production and Labelling of Organic Products and Repealing Council Regulation (EC) No 834/2007. Available online: https://eur-lex.europa.eu/eli/reg/2018/848/oj/eng (accessed on 13 May 2025).
- Godlewska, K.; Pacyga, P.; Najda, A.; Michalak, I. Investigation of Chemical Constituents and Antioxidant Activity of Biologically Active Plant-Derived Natural Products. Molecules 2023, 28, 5572. [Google Scholar] [CrossRef]
- Atero-Calvo, S.; Izquierdo-Ramos, M.J.; García-Huertas, C.; Rodríguez-Alcántara, M.; Navarro-Morillo, I.; Navarro-León, E. An Evaluation of the Effectivity of the Green Leaves Biostimulant on Lettuce Growth, Nutritional Quality, and Mineral Element Efficiencies under Optimal Growth Conditions. Plants 2024, 13, 917. [Google Scholar] [CrossRef]
- Marin, M.d.P.; Robledo-Olivo, A.; Camposeco, N.; Charles-Rodriguez, A.V.; González-Morales, S.; Cabrera-De La Fuente, M.; Juarez-Maldonado, A. The application of a whey protein hydrolysates enhance the productivity and quality of lettuce under nft system. AgroLife Sci. J. 2024, 13, 168–175. [Google Scholar]
- Hernandez, O.; Calderin, A.; Huelva, R.; Martinez-Balmori, D.; Guridi, F.; Aguiar, N.; Olivares, F.; Canellas, L.P. Humic substances from vermicompost enhance urban lettuce production. Agron. Sustain. Dev. 2015, 35, 225–232. [Google Scholar] [CrossRef]
- Stef, A.V.; Apahidean, S.; Carbunar, M.; Bei, M.; Apahidean, A.I.; Domocos, D.; Laczi, E. Culture starting time can influence the production of some salad cultivars grown in the field. J. Hortic. For. Biotehnol. 2018, 22, 99–104. [Google Scholar]
- Khan, S.; Yu, H.; Li, Q.; Gao, Y.; Sallam, B.N.; Wang, H.; Liu, P.; Jiang, W. Exogenous Application of Amino Acids Improves the Growth and Yield of Lettuce by Enhancing Photosynthetic Assimilation and Nutrient Availability. Agronomy 2019, 9, 266. [Google Scholar] [CrossRef]
- Keskin, B.; Akhoundnejad, Y.; Dasgan, H.Y.; Gruda, N.S. Fulvic Acid, Amino Acids, and Vermicompost Enhanced Yield and Improved Nutrient Profile of Soilless Iceberg Lettuce. Plants 2025, 14, 609. [Google Scholar] [CrossRef] [PubMed]
- Ottaiano, L.; Di Mola, I.; Cozzolino, E.; El-Nakhel, C.; Rouphael, Y.; Mori, M. Biostimulant Application under Different Nitrogen Fertilization Levels: Assessment of Yield, Leaf Quality, and Nitrogen Metabolism of Tunnel-Grown Lettuce. Agronomy 2021, 11, 1613. [Google Scholar] [CrossRef]
- Dasgan, H.Y.; Yilmaz, D.; Zikaria, K.; Ikiz, B.; Gruda, N.S. Enhancing the Yield, Quality and Antioxidant Content of Lettuce through Innovative and Eco-Friendly Biofertilizer Practices in Hydroponics. Horticulturae 2023, 9, 1274. [Google Scholar] [CrossRef]
- Sularz, O.; Smoleń, S.; Koronowicz, A.; Kowalska, I.; Leszczyńska, T. Chemical Composition of Lettuce (Lactuca sativa L.) Biofortified with Iodine by KIO3, 5-Iodo-, and 3.5-Diiodosalicylic Acid in a Hydroponic Cultivation. Agronomy 2020, 10, 1022. [Google Scholar] [CrossRef]
- Acamovic-Djokovic, G.; Pavlovic, R.; Mladenovic, J.; Djuric, M. Vitamin C content of different types of lettuce varieties. Acta Agric. Serb. 2011, 32, 83–89. [Google Scholar]
- Deveikytė, J.; Blinstrubienė, A.; Burbulis, N. Amino Acids as Biostimulants: Effects on Growth, Chlorophyll Content, and Antioxidant Activity in Ocimum basilicum L. Agriculture 2025, 15, 1496. [Google Scholar] [CrossRef]
- Al-Karaki, G.; Othman, Y. Effect of foliar application of amino acid biostimulants on growth, macronutrient, total phenol contents and antioxidant activity of soiless grown lettuce cultivars. S. Afr. J. Bot. 2023, 154, 225–231. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [PubMed]
- Cimrin, M.; Yilmaz, I. Humic acid applications to lettuce do not improve yiled but do improve phosphorus availability. Acta Agric. Scand. Sect. B Soil Plant Sci. 2005, 55, 58–63. [Google Scholar] [CrossRef]
- Soare, R.; Dinu, M.; Babeanu, C.; Botu, M. The influence of foliar fertilization with humic acids–based products on the quality of tomato fruits. Sci. Pap. Ser. B Hortic. 2024, LXVIII, 511–515. [Google Scholar]
- Nabi, F.; Sarfaraz, A.; Kama, R.; Kanwal, R.; Li, H. Structure-Based Function of Humic Acid in Abiotic Stress Alleviation in Plants: A Review. Plants 2025, 14, 1916. [Google Scholar] [CrossRef]
- Atero-Calvo, S.; Magro, F.; Masetti, G.; Navarro-León, E.; Rios, J.J.; Ruiz, J.M. Assaying the Use of a Leonardite-Suspension Concentrate-Based Product as a Potential Biostimulant to Enhance Growth, NPK Use Efficiency, and Antioxidant Capacity in Lactuca sativa L. Agronomy 2024, 14, 64. [Google Scholar] [CrossRef]
- Xiang, Y.; Peng, J.; Shao, Y.; Son, J.E.; Tagawa, K.; Yamada, S.; Yamada, M.; Baiyin, B.; Yang, Q. Auxin Responds to Flowing Nutrient Solution to Accelerate the Root Growth of Lettuce in Hydroponic Culture. Int. J. Mol. Sci. 2025, 26, 7742. [Google Scholar] [CrossRef]
- Zain, N.M.; Zainal, M.D.; Abu Bakar, T.H.S.T.; Zakaria, S.; Mukhtar, N.K.; Naher, L. Efficacy of auxin foliar application on the growth and yield of green Romaine (Lactuca sativa L. var. Jericho) grown under nutrient film technique (NFT) hydroponic system. IOP Conf. Ser. Earth Environ. Sci. 2022, 1102, 012012. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).