Abstract
Pontechium maculatum (Boraginaceae) is a species of high conservation concern in the Romanian flora. It is assigned the national IUCN category “vulnerable”, legally protected according to the national Biological Diversity Act, and listed in Annex II of the Habitats Directive (Council Directive 92/43/EEC). P. maculatum, formerly known as Echium russicum, is a biennial herbaceous plant, rare in many parts of its range and even critically endangered in some countries. In Romania, populations of this species are found in several areas of Transylvania, Moldova, Dobrogea, and Oltenia, mostly within protected areas, with the number of individuals reaching over 1500, and the populations being stable with a favorable conservation status. In the present study, ten populations of P. maculatum from the “Iron Gates” Natural Park in SW Romania were analyzed. For each density, the composition of the plant community was assessed, as well as some morphological and physiological parameters of the plants. The results indicated the phytocoenotic variability of the species, with a different dominant abundance in the floristic composition of several plant communities. Statistical analysis of quantitative traits revealed variability depending on density (at lower densities, plants have a higher number of leaves and photosynthetic rate). Also, the photosynthetic rate was mainly temperature-dependent, rising with this factor. This research found that in some areas, there are insufficient numbers of individuals due to invasive species. The identified variability of Pontechium maculatum in the “Iron Gates” Natural Park is demonstrated by the interplay of ecological factors, human influence, and conservation efforts. Our findings suggest that effective conservation strategies should focus on managing invasive species and enhancing habitat conditions to support P. maculatum populations.
1. Introduction
Pontechium maculatum (L.) Böhle & Hilgeris (Boraginaceae family) is a Pontic–Pannonian species native to Central and Southern Europe and found further east in the Caucasus and Anatolia regions [1]. It was previously classified as a species within the genus Echium L. under the names of Echium russicum J. F. Gmelin and other synonyms, including E. maculatum L., E. acutifolium Lehm., E. clavatum Willd. ex Lehm., and E. linearifolium K. Koch. The existence of a bicapitate stigma, along with molecular evidence from plastid trnL (UAA) intron, trnL (UAA)–trnF (GAA) spacer, and nuclear (ITS1) DNA sequences, reinforced the classification of Pontechium Böhle & Hilger as a separate genus, with the species being assigned a unique position in the new genus as P. maculatum [2]. This species is known by various common names: Russian bugloss, viper’s bugloss, red-flowered viper’s grass, or snake’s head in Romania and Cretan viper’s bugloss in Georgia and Azerbaijan. Plants are herbaceous and biennial, with erect stems of up to 70 cm. Its crimson blooms are arranged in spike-like inflorescences measuring 25–30 cm, consisting of short bracteate cymes. It is distinguished from other Echium species in Romania by its corolla color, which frequently varies from red to violet hues, while in E. italicum, the corolla is white or reddish-white, and in E. vulgare, the corolla is blue [3].
The Russian bugloss is a popular decorative and therapeutic plant, noted for its antibacterial, wound healing, and antioxidant properties [1,4,5]. The seeds possess nutraceutical potential because of their elevated levels of omega-3 and omega-6 fatty acids [6]. Owing to its flower arrangement, the species holds significant ornamental value and is sold on the European and American markets [1].
P. maculatum is considered rare and endangered in numerous parts of its natural habitat. In many countries, it appears in the national red lists and, in some, even in the Red Books, classified under different categories of threat. It has received the IUCN category of CR (critically endangered) in the Czech Republic [7] and in Poland [1,8], EN (endangered) in Slovakia [9], and VU (vulnerable) in Bulgaria [10]. Additionally, it is mentioned in Annexes II and IV of Habitats Directive 92/43/EU (as modified by Directive 2013/17/EU) [11], which addresses European species that need particular conservation areas and rigorous protection. The conservation status of the species is unfavorable–bad in the Alpine region (Slovakia), in the continental region (Czechia and Poland), and in Pannonian (Slovakia).
P. maculatum exhibits notable variability across its populations in Romania. Ecological factors, propagation techniques, and conservation initiatives influence this variability [12]. The species is widely distributed across the Transylvanian and Moldavian plateaus, as well as Dobrogea, but is restricted in Bucovina and the Romanian Plain [13]. As per the Natura 2000—Standard Data Forms, it can be found in 45 Sites of Community Importance, with a majority of locations having an unfavorable conservation status [3]. It is a rare species, with a favorable conservation status in terms of population and habitats [14]. It has been frequently referenced in floristic and phytosociological research from Romania, beginning with the foundational studies of [15,16] up to contemporary investigations [17,18,19,20,21]. Nonetheless, additional research is required to gain a deeper insight into the biology of this species, already rare in many regions, which may experience significant population declines and potential local extinctions under present climate conditions.
Ecologically, it is a xero-mesophyte, sub-thermophile species, found in meadows, thickets [3], orchards, hayfields, shrubs [22], and along forest edges from the steppe region [23] to the mountains [24].
Due to beautiful and colorful inflorescences, P. maculatum is an invaluable horticultural plant and is capable of generating a substantial quantity of nectar, thus serving as a significant crop for beekeeping, as noted in [1]. The same authors also mention that the species has valuable compounds (rosmarinic acid and shikonin) that possess therapeutic benefits. It is part of Habitats Directive 92/43/EU, which addresses European species requiring strict protection and designated conservation areas. Examination in its natural habitats is crucial since regions with minimal investment in environmental protection and conservation may see species facing higher extinction risk, while climate change could influence the distribution and abundance of plants, increasing their vulnerability to extinction [25].
Invasive species can compete with native plants for resources, leading to decreased populations and changing the balance of an ecosystem.
In Romania, P. maculatum is present in the plant community composition of Natura 2000 Habitats: 62C0* Ponto Sarmatic steppes; 6240* Sub-Pannonic steppic grasslands; 6210* semi-natural dry grasslands and scrub land facies on calcareous substrates (Festuco-Brometalia); and 6250* Pannonic loess steppic grasslands, along with 40A0 subcontinental peri-Pannonic scrub (code specie 4067) [26].
The aim of this study was to examine how density influences the morphology, the phenology, and certain physiological traits of P. maculatum found in the Iron Gates Natural Park. This is situated in southwestern Romania, which is among the country’s protected natural areas with the highest biological diversity [27]. Alongside morpho-metric analysis, an assessment of photosynthesis under natural conditions and a comprehensive floristic and phytocoenotic investigation were performed, facilitating the identification of the plant communities where the examined densities of Russian bugloss are located.
The research carried out by us on significant populations located in a protected zone, specifically within a natural park in Romania, has uncovered new information regarding the phytocoenology and morpho-physiology of this species, which is detailed here.
2. Materials and Methods
2.1. Area of Study
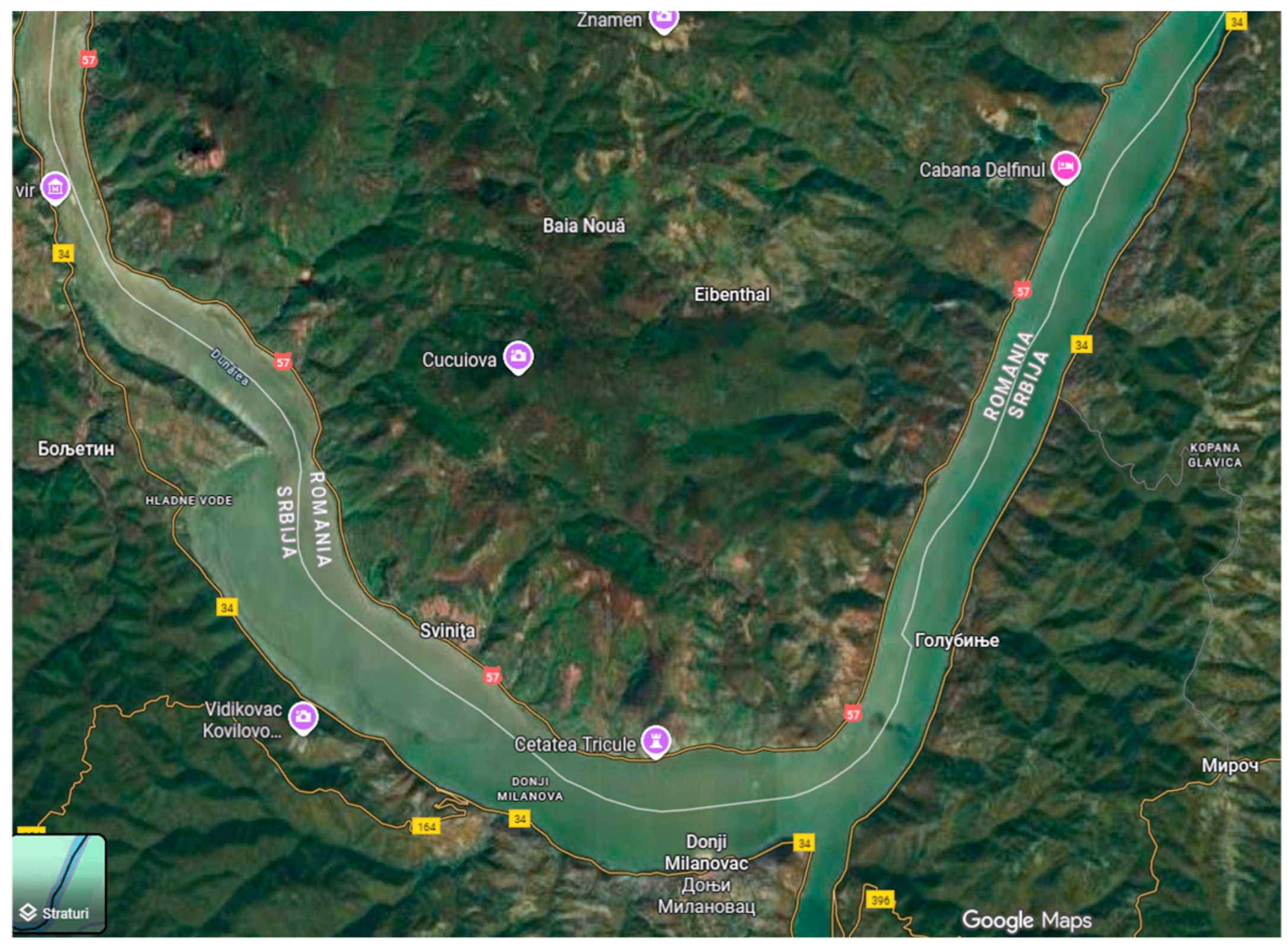
Ten populations (based on the occurrence frequency of P. maculatum/m2) were analyzed in the “Iron Gates” Natural Park, located in the geographical area called the Danube Gorge (Figure 1). These were discovered in two locations that have comparable eco-pedo-climatic conditions, Baia Nouă (44°33′16″ N 22°07′44″ E) and Eibenthal (44°32′42″ N 22°9′51″ E), which are both part of the Dubova Commune, Mehedinți County, Banat region. The elevation of the examined regions ranges from 100 to 400 m. The climate is temperate-continental, featuring an average yearly temperature between 8 °C and 11 °C, and precipitation is moderate, with a relatively balanced distribution throughout the year. The pedological parameter values for Baia Nouă and Eibenthal include these general aspects: the soil type is brown luvic or luvisol, characterized by a profile with an Am-Aca-Cca horizon exhibiting moderate acidity and high fertility, reflecting the temperate forest climate of the Iron Gates National Park. The pH levels of these soils can vary between 4.5 and 6.5. The populations were studied in situ during the summers of 2020–2023.
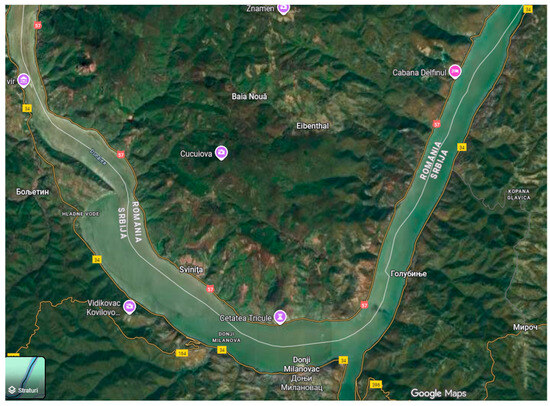
Figure 1.
Map of the study area.
2.2. Floristic and Phytosociological Analysis
Taxa identification relied on the Romanian flora [28] and Flora Europaea [29]. The method of phytosociological research developed by Braun-Blanquet (1932) [30] from the Central European School was utilized for the phytocoenotic research in the study area. The plant communities were recognized and differentiated based on the characteristic, edifying, dominant, and differential species. To identify plant communities and for coenotaxonomic classification, the synthesis works of [31,32,33], along with [34,35,36,37], were used. Data were gathered from ten regions of the two specified locations, where five experimental plots measuring 100 m2 were selected for each plant community type containing the species under investigation. The constancy of the species (K) was observed using data from at least five relevées, with each relevée representing vegetation plots from different locations, following standard phytosociological protocols.
2.3. Morphological and Physiological Determinations
In the investigation of phenotypic diversity, the following traits were measured in the wild for each plant identified in a m2: plant height, number of leaves, and inflorescence length. The evaluation of these growth factors facilitated the estimation of the species’ potential for expansion in the studied regions. A measuring device (meter) was utilized to determine plant height and inflorescence length.
The photosynthetic rate was determined in five plants from each density, utilizing five intact leaves. The recording of this physiological parameter occurred over the course of the day (every two hours, starting with 8:00, 10:00, 12:00, 14:00, 16:00, 18:00 and 20:00), alongside readings of atmospheric temperature. The physiological parameter data were measured at an average value of 250 μmol/m2/s PPFD, with PPFD representing photosynthetic photon flux density, which indicates instantaneous light intensity. The assessments took place every June from 2020 to 2023, utilizing a portable device to determine the photosynthetic rate: Model LCi 300, by ADC BioScientific Ltd., Hoddesdon, UK.
2.4. Statistical Analysis
The statistical analyses of the data were conducted utilizing SPSS Statistics s v. 16 (IBM SPSS Statistics) and Statgraphics Centurion XVI (Statgraphics Tech-601 technologies, The Plains, VA, USA). One-way ANOVA was employed to assess the differences across the analyzed densities, and LSD multiple comparisons tests were conducted at the p ≤ 0.05 significance level. A Pearson correlation was determined for the examined traits. The method of principal component analysis described by Harman (1976) [38] was utilized to extract the components. The variability percentage accounted for by each component was established [38,39]. Principal component analysis was conducted using a bi-plot graphical representation. A hierarchical dendrogram utilizing the Bray–Curtis similarity index was used to conduct the multivariate analysis. Synthetic ecological indices were also computed (abundance, dominance, constancy, and ecological significance index) [40].
3. Results
3.1. Phytosociological Assessment of Pontechium maculatum in Plant Communities
The presence of particular eco-pedo-climatic conditions in the region where the species was found was associated with substantial phytocoenotic variability in the floristic composition of various plant communities, each exhibiting different dominant abundances. Thus, based on the geobotanical research conducted in the examined area, the populations of Russian bugloss that were analyzed are classified into four plant communities: Brachypodio pinnati-Festucetum rupicolae Ghişa 1962, Medicagini-Festucetum valesiacae Wagner 1941, Danthonio Chrysopogonetum grylli Boscaiu 1970 and 1972, and Festucetum valesiaco-rupicolae Csuros et Kovacs 1962 (Figure 2).
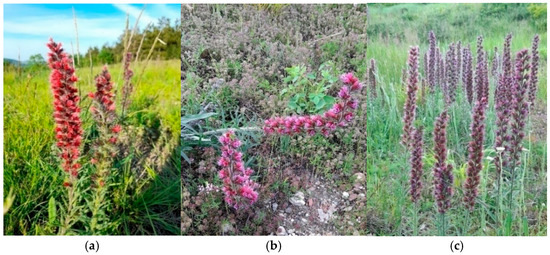
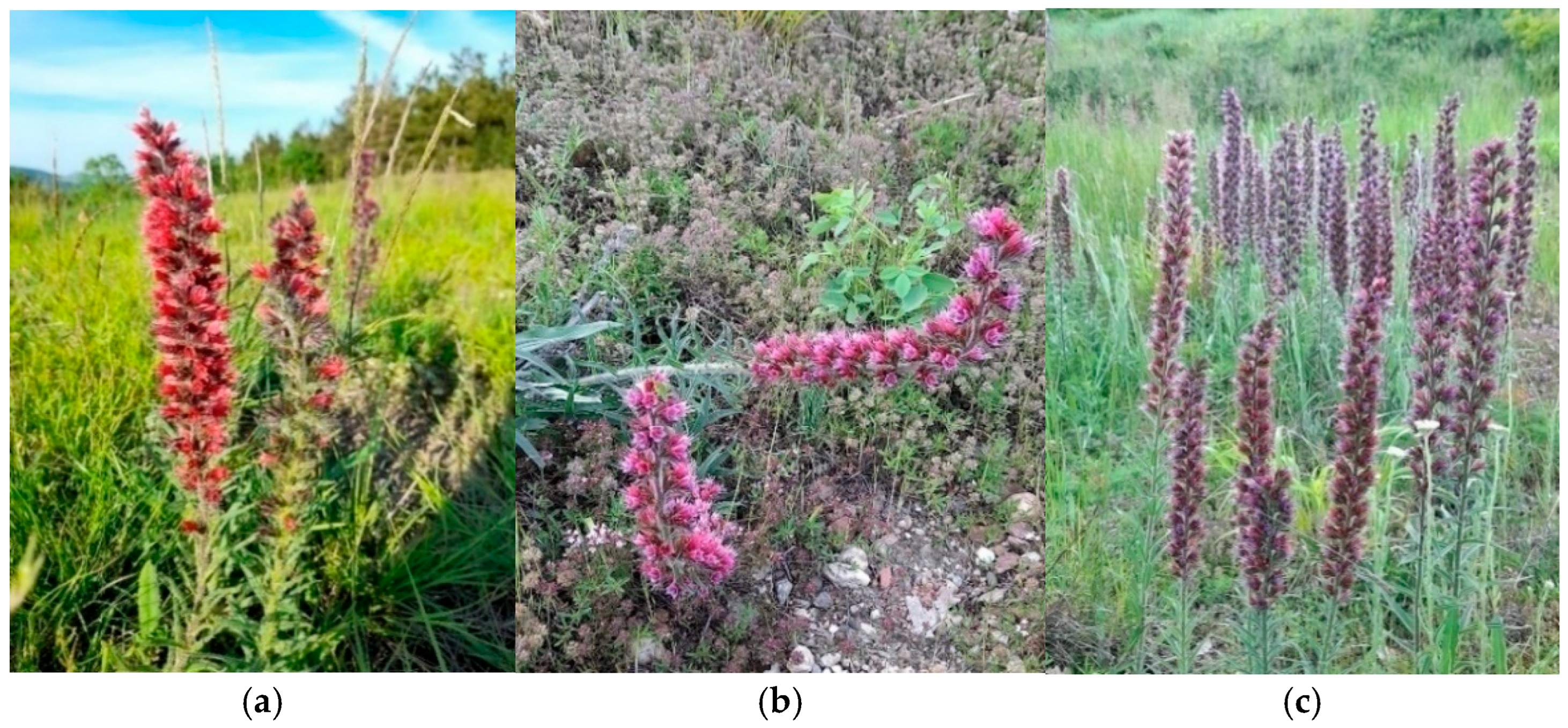
Figure 2.
P. maculatum in the floristic composition of (a) Medicagini-Festucetum valesiacae; (b) Festucetum valesiaco-rupicolae; and (c) Danthonio Chrysopogonetum grylli plant communities.
The Brachypodio pinnati-Festucetum rupicolae plant community (Table 1) characterizes meadows with a heterogeneous structure, covering limited areas in the Tisovița Valley, near the Eibenthal locality, and is adjacent to vegetation typical of the thermophilic and sub-thermophilic regions of the Danube Valley in southwestern Romania. This meadow type is marked by significant human influence, featuring various invasive woody and herbaceous species like Ailanthus altissima, Elaeagnus angustifolia, Morus nigra, Erigeron annuus, Pteridium aquilinum, and Conyza canadensis, which exhibit high abundance and dominance. However, other rare, protected species are present in the floristic composition, including Dianthus giganteus subsp. banaticus, Veronica prostrata, Cleistogenes serotina, Orchis morio, O. militaris, O. coriophora, Centaurea atropurpurea, Dianthus armeria, and others. In this community, the populations of P. maculatum ranged from 4 to 25 individuals. The typical xero-mesophilic and sub-thermophilic ecological conditions that promote healthy morphological growth led to most of these individuals being well developed and exhibiting proper fruiting. To enhance the population of individuals, it is recommended that the unwanted anthropophilic species be removed from the phytocoenoses and meadows be mowed, ensuring that the habitat conservation status and the P. maculatum species remain favorable.

Table 1.
The floristic composition of the investigated plant communities in which Pontechium maculatum was identified.
The Medicagini-Festucetum valesiacae (Table 1) plant community typically occurs in degraded soil areas. The phytocoenoses investigated in this plant community were located on level terrain and sunny slopes and on calcareous soil exhibiting xerophytic conditions, near the Eibenthal locality. From a physiognomic perspective, the analyzed phytocoenoses are characterized by the occurrence of species from the Festucion valesiacae alliance and the Festuco-Brometea class. The structure and floristic physiognomy of these phytocoenoses are quite heterogeneous. Alongside the species Festuca valesiaca, Medicago lupulina, and M. falcata, many species are indicative of the classes Molinio-Arrhenatheretea, Stellarietea mediae, and Artemisietea vulgaris. In the floristic composition of certain relevées, the most developed populations of P. maculatum from the area were identified, consisting of 10 to 53 individuals. The analyzed phytocoenoses exhibited a lower number of ruderal and segetal species, along with invasive species, resulting in a notably less anthropophilic character for the community, thus favoring the conservation status of both the habitat and the species. To maintain the positive conservation status of P. maculatum, the utilization of these meadows must closely align with pastoral standards and suitable management practices.
The Danthonio Chrysopogonetum grylli (Table 1) community covers extensive regions within the analyzed perimeter. Similar to those mentioned earlier, the floristic composition of the phytocoenoses is diverse, including numerous species typical of steppe meadows, particularly dominated by perennial plants from the Festucion valesiacae alliance, as well as from the Festuco-Brometea class. These meso-xerophilic and xerophilic communities thrive on sunny and semi-sunny slopes, utilizing rocky substrates and clayey or sandy sedimentary formations. These meadows are of mixed origin: partly natural and partly anthropogenic, but P. maculatum populations are robust, ranging from 10 to 44 individuals. Among these relevées, invasive species were also recognized, including Erigeron annuus and Conyza canadensis, which exhibited relatively low abundance–dominance. Ecological conditions were favorable, with the plant community situated at the edge of a forest of black pine (Pinus nigra ssp. banatica), allowing P. maculatum individuals to attain optimal growth.
The Festucetum valesiaco-rupicolae community includes phytocoenoses that typically thrive on clayey–marly soils, slightly halophilic. These phytocoenoses were examined in the central part of the Iron Gates Natural Park, around Baia Nouă, close to the old mining quarry, and in Tisovița Valley, Eibenthal. The phytocoenotic structure is defined by the significant abundance–dominance of species belonging to the Festucion valesiacae alliance and the Festuco-Brometea class (Table 1). In the phytocoenoses of this association, the species often observed include Briza media, Holcus lanatus, Dactylis glomerata, Cynosurus cristatus, Anthoxanthum odoratum, Poa pratensis, Trifolium pratense, and Agrostis capillaris. P. maculatum specimens were recognized in the floristic composition of various relevées of this plant community, with their count ranging from 4 to 18, based on the conservation status of the phytocoenoses in this meadow type.
In Tisovița Valley, which passes through the town of Eibenthal, the studied phytocoenoses show a comparatively good conservation status. In the Baia Nouă region, the examined phytocoenoses exhibit a poor conservation status, which adversely affects P. maculatum, with their population reaching only two and showing reduced vigor (the vegetative organs are less developed morphologically, with shorter plants and leaves). Similar to other regions within the surveyed area, certain phytocoenoses that form these meadows where P. maculatum thrives are invaded by bushes of Prunus spinose (from the Medicagini-Festucetum valesiacae Wagner 1941 plant community), Rubus fruticosus (from the Medicagini-Festucetum valesiacae Wagner 1941 and Danthonio Chrysopogonetum grylli Boscaiu (1970, 1972) plant communities), and Rosa canina (from the Medicagini-Festucetum valesiacae Wagner 1941, Danthonio Chrysopogonetum grylli Boscaiu (1970, 1972), and Festucetum valesiaco-rupicolae Csuros et Kovacs 1962 plant communities), while in others, it is dominated by the invasive species Pteridium aquilinum. These shrub species adversely affect P. maculatum populations by overshadowing the plants, harming the grassland habitat where they thrive, and altering its species composition. Non-native species identified include Xanthium strumarium, Ambrosia artemisiifolia, and Erigeron annuus, among others. The Festucetum valesiaco-rupicolae Csuros and Kovacs (1962) plant community corresponds to habitat 6240*, which is part of the Natura 2000 habitat network. The significant pressures that adversely affect P. maculatum include the following: I01—non-native invasive species; I02—unwanted species (Pteridium aquilinium, Prunus spinosa, Rubus fruticosus, and Rosa canina).
This grove is included in the Natura 2000 habitat—6210* semi-natural dry grasslands and scrubland on calcareous substrates (Festuco Brometea) (* priority for important orchid sites); in the classification system of Palearctic habitats, it falls under the type—CLAS. PAL.: 34.31 up to 34.34 and 6240* sub-pannonic steppic grasslands; in the classification system of Palearctic habitats, it is categorized as type—CLAS. PAL.:34.315 [26].
3.2. Morphological and Physiological Analysis
It was found that the individuals particularly thrive in the meadows that are unmowed or ungrazed due to minimal disturbances (Figure 3).

Figure 3.
Vigorous specimens of P. maculatum from Tisovița Valley.
The statistical analysis of the quantitative characteristics was performed according to the density of P. maculatum plants. For plant height, the highest average values were those calculated at the highest densities. The same situation was also found for the inflorescence length characteristic. Concerning the number of leaves, the highest average values were observed at the lowest densities (Table 2). Concerning the ratio between inflorescence length and the total length of the plant, a decrease was observed as density increased. The decrease in the values of this ratio indicates that at high densities, shading takes place, leading to a decrease in the number of flowers.

Table 2.
Variations in the mean values of morphological characteristics according to the density of the plants.
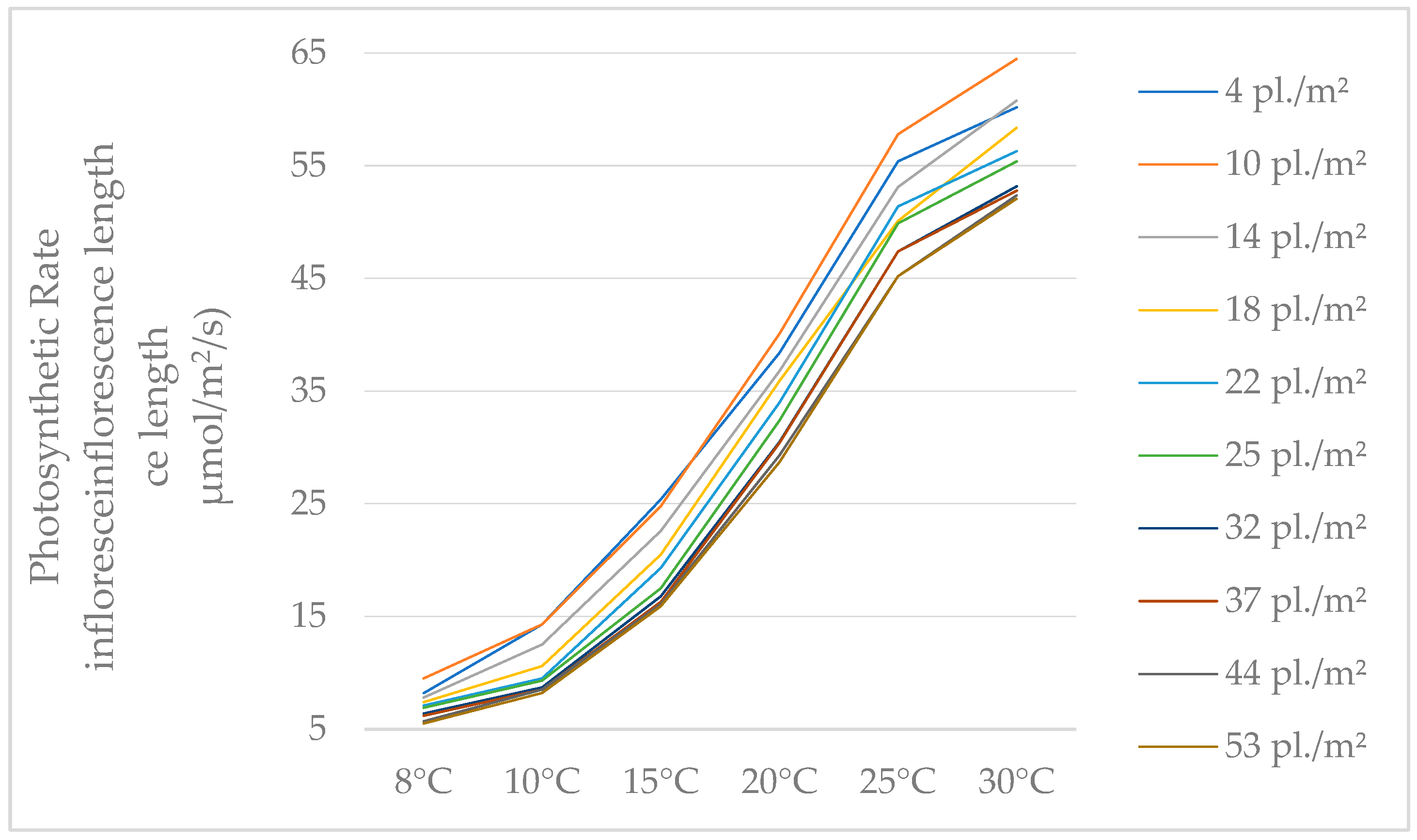
Our study of photosynthesis shows that, typically independent of the temperature during the measurements, the highest values were observed in plants with low densities. Additionally, it can be observed that the values recorded for density of 37 pl./m2, 44 pl./m2, and 53 pl./m2 are small differences, regardless of the temperature (Table 3).

Table 3.
Analysis of photosynthesis variation depending on temperature and P. maculatum density.
Concerning the changes in photosynthesis values, this is influenced by temperature, independent of density, and the rate of photosynthesis rises with temperature (Figure 4).
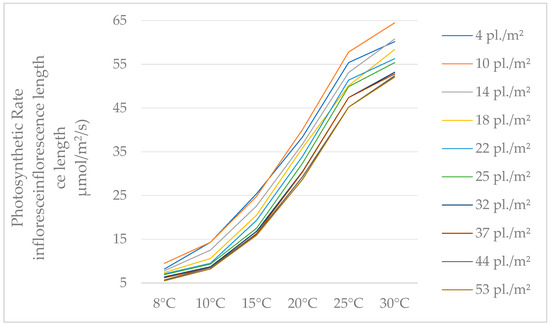
Figure 4.
The values of photosynthesis rates, depending on temperature.
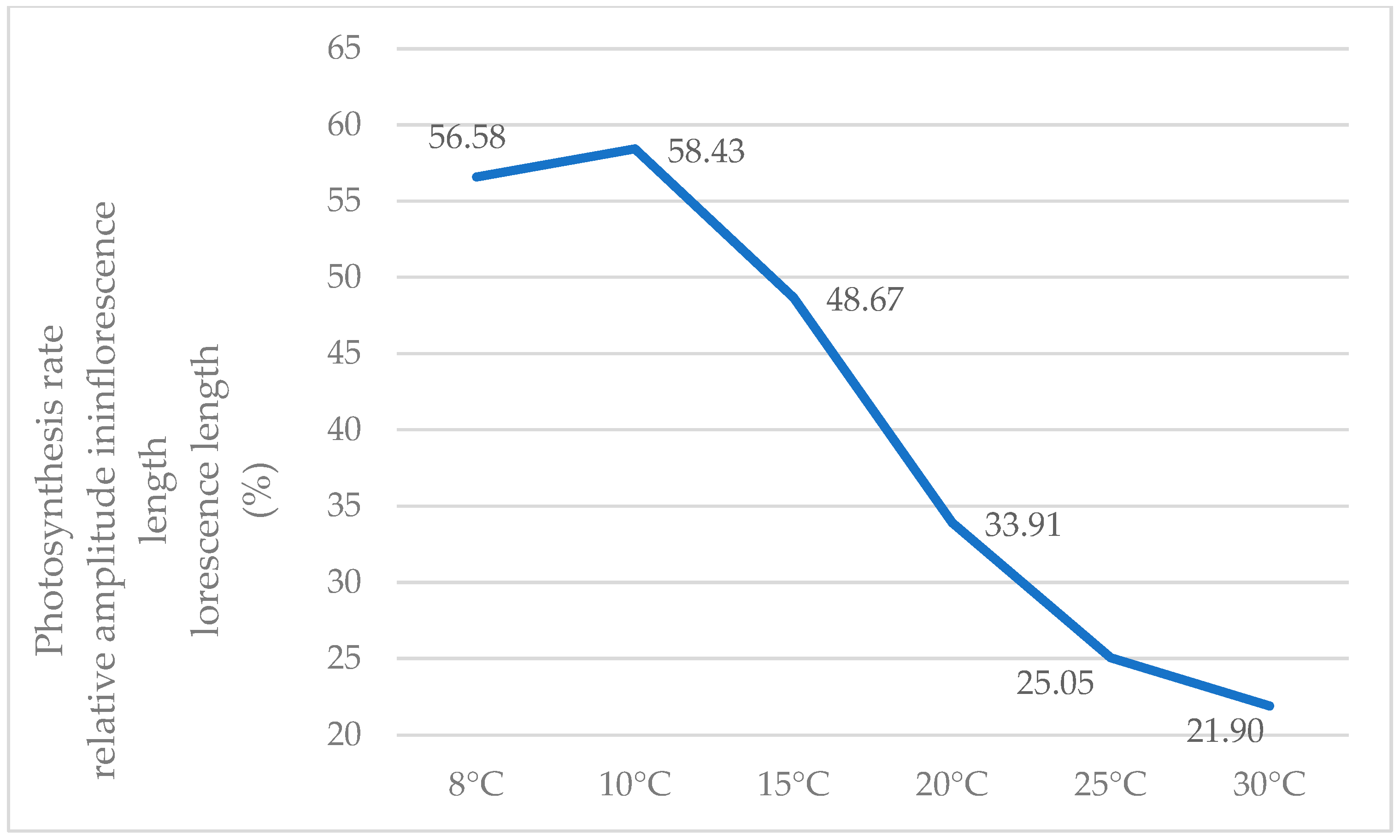
The highest percentage differences, influenced by temperature, occur at low temperatures (8 °C, 10 °C, and 15 °C), and as the temperature rises, the relative amplitude diminishes. Therefore, at 5 °C and 10 °C, relative amplitudes exceeding 55% were calculated, while at 25 °C and 30 °C, this index decreases to 25 and 21%, respectively. In other words, at low temperatures, plants with low densities exhibit photosynthetic activity that is over 50% higher than that of high densities, whereas this ratio generally diminishes with increasing temperatures (Figure 5).
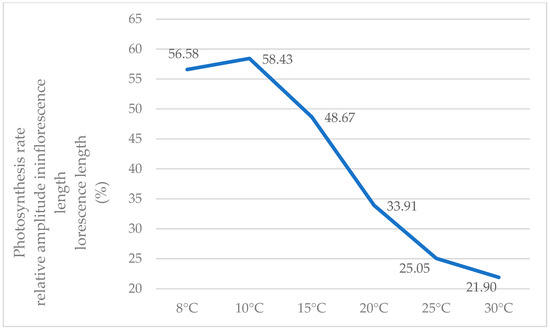
Figure 5.
Variations in the relative amplitude for the recorded photosynthesis values depending on temperature (%).
3.3. Multivariate Analysis of the Results
Concerning the correlation analysis between the examined traits, the coefficient values differ with plant density (Table 4). Thus, in the case of the correlation between the average plant height and the number of leaves/plants, the highest values were calculated for low densities, with the value of the correlation coefficient decreasing with increasing density. At higher densities, the correlation between the two traits decreases. At low density, the correlation is weak. The same situation can be seen between plant height and photosynthesis. The photosynthetic rate has smaller values at higher densities, comparative with the one from low densities. Concerning the number of leaves and inflorescence length, the correlation values increase at high densities compared with those of low densities. In relation to photosynthesis, the correlation coefficients between the rate of photosynthesis and the number of leaves are higher at high densities compared to those at low densities.

Table 4.
The Pearson correlation coefficients between the studied characteristics according to density.
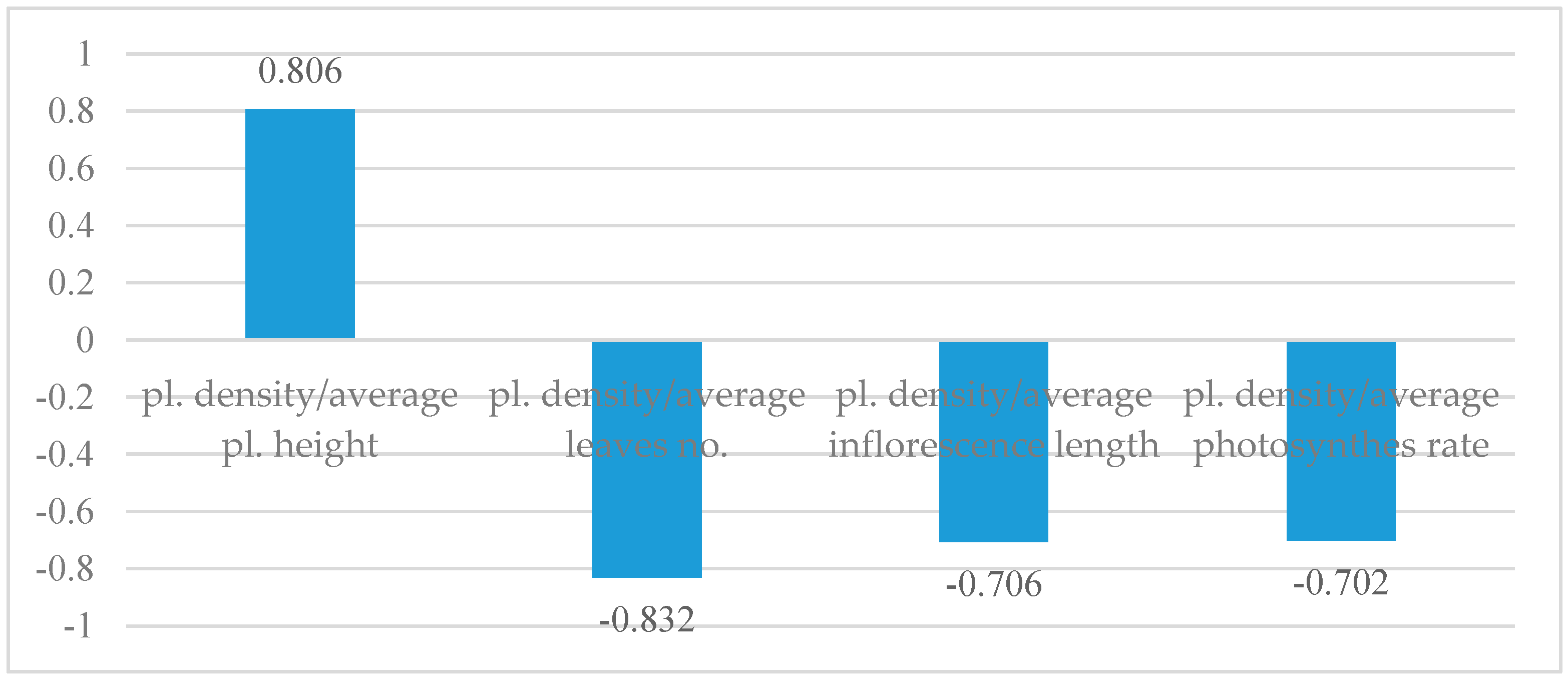
A stronger correlation between plant density and plant height was identified. Therefore, as the plant density increases, the tendency of plants to grow taller also rises. The correlation value between the number of leaves and plant density is high, yet negative (Figure 6). Thus, plants at high densities produce fewer leaves. A similar situation can be observed in the correlation between plant density and inflorescence length, as well as the photosynthesis rate, with the values remaining low because of mutual shading.
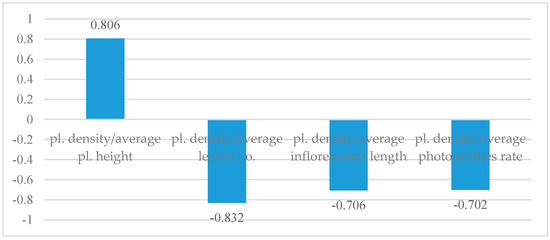
Figure 6.
Correlation between plant density and other studied characteristics.
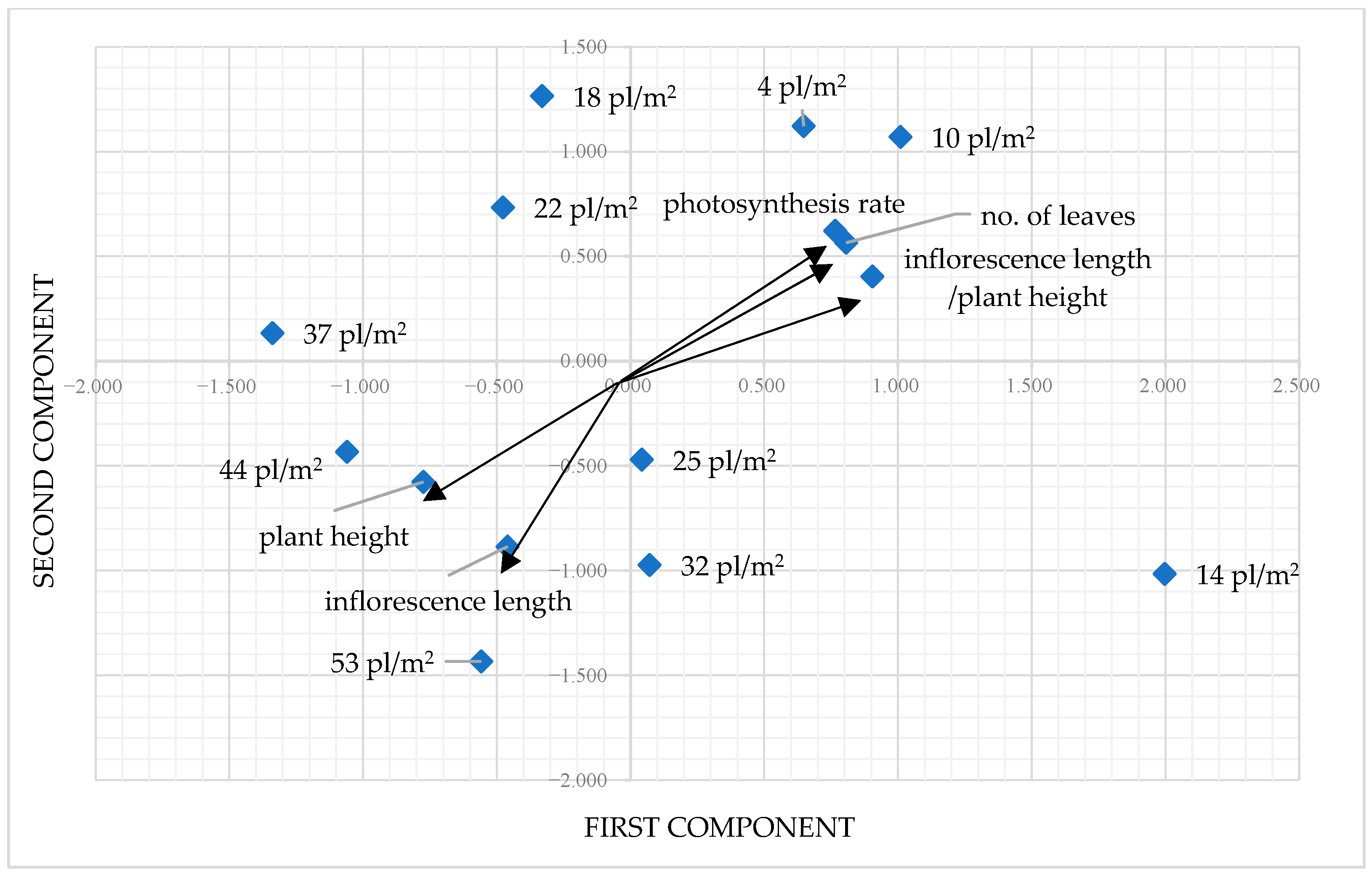
Concerning the principal components analysis, the initial two components account for 96.966% of the total variance, with the first component at 92.371% and the second component at 4.595% (Table 5).

Table 5.
Total variance explained by the five components.
The parameters associated with the first component included the number of leaves per plant, the inflorescence height-to-total plant height ratio, and the photosynthesis rate, whereas the factor that achieved a high value for the second component was the photosynthesis rate. The initial component is characterized by plants’ capacity to produce both sizable leaves and extended inflorescence concurrently, while the second component is the plant’s capacity to generate many leaves that efficiently perform photosynthesis.
The PCA facilitated the distinction of four groups located along the axes of the two components (Figure 7). The variants featuring both positive components were the ones from low density (4 and 10 pl./m2), while the variants with both negative components came from high density (44 and 53 pl./m2). It is evident that one component relates to a plant’s ability to effectively produce inflorescence and an increased number of leaves that contribute to a high rate of photosynthesis, while a second component pertains to the plant’s ability to adjust to greater densities by decreasing height and inflorescence length.
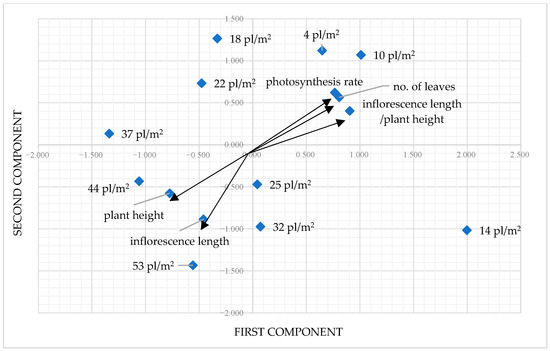
Figure 7.
Principal component analysis of P. maculatum population densities and their morpho-physiological characteristics.
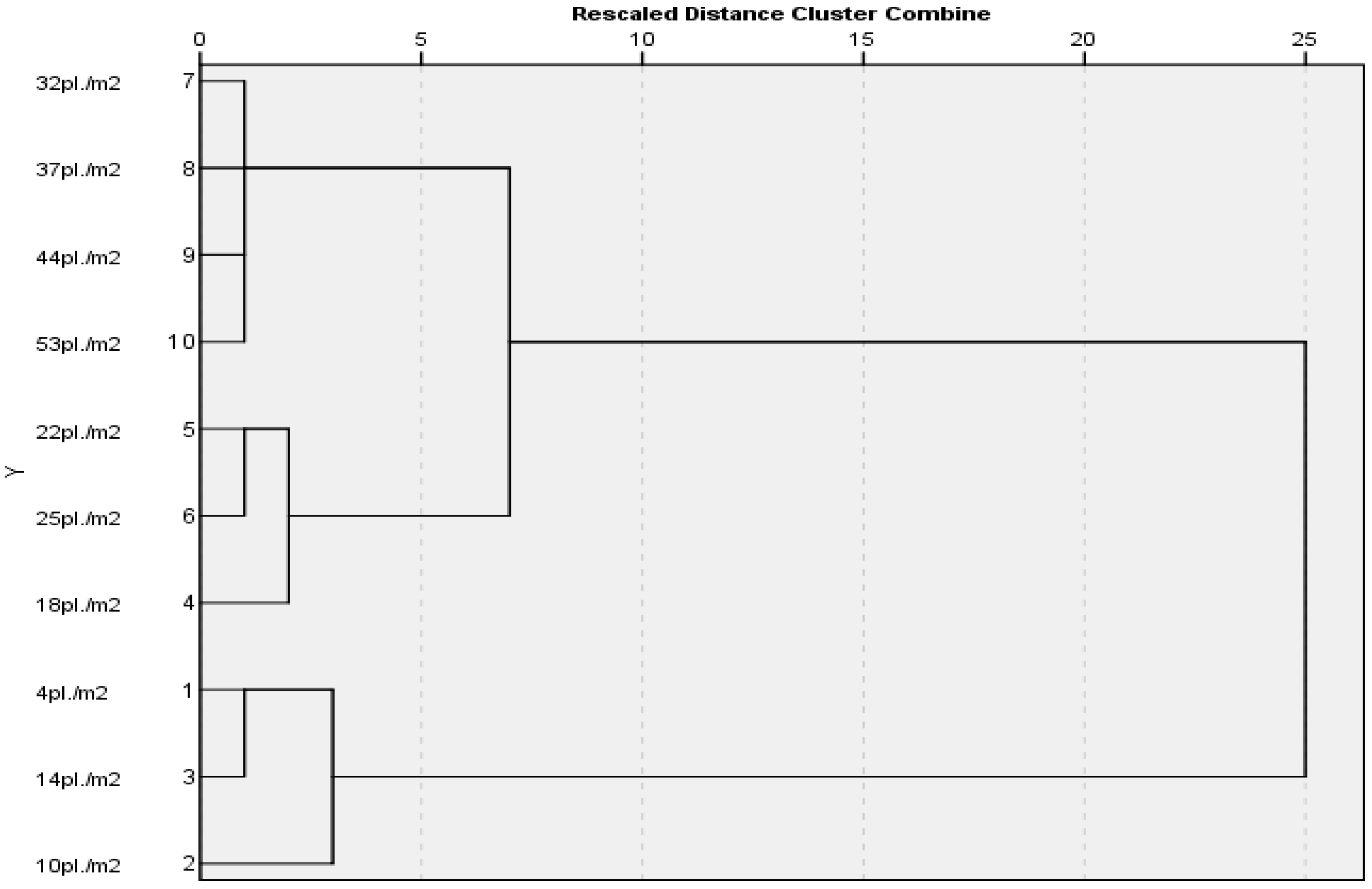
After conducting PCA, four clusters were recognized. The initial cluster consists of plants at densities of 4, 10, and 14 pl./m2. This group includes plants with low densities and elevated photosynthetic rate values. The second cluster includes plants with densities of 18, 22, and 25 pl./m2, characterized by medium densities and moderate photosynthetic rate values. The third cluster includes plants at the greatest densities experiencing shading, resulting in a low rate of photosynthesis.
The maximum distance was determined between the densities of 4 and 53 pl./m2. A greater similarity was found between clusters 2 and 3, while cluster 1 showed the least connection (Figure 8).
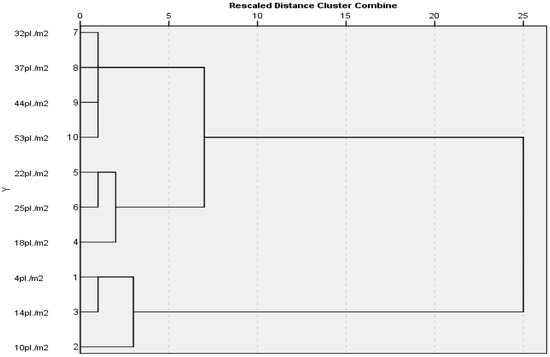
Figure 8.
Dendrogram constructed on the basis of distances estimated using the rate of photosynthesis recorded at different temperatures.
4. Discussion
P. maculatum is a species characteristic of pasture- and meadow-type phytocoenoses in the forest–steppe zone, with phytocoenotic variability related to the plant associations in which it is found. In the studied areas, this species occurs in different associations, but the population density varies significantly, reflecting not only local environmental conditions but also other causes. In this study, the population density of P. maculatum in the two areas ranges between 4 plants/m2 and 53 plants/m2, with variable heights and numbers of leaves. In addition, the inflorescences are of a medium size, and the number of leaves is reduced. This morphological variability is probably influenced by ecological factors and is determined by an expression of plant adaptation to the environment, which causes the significant differences in the mentioned characters. We can assume that the species has efficient photosynthetic adaptations for moderate environmental conditions and tolerance to temperature and precipitation variations.
Other studies have shown that P. maculatum faces significant challenges due to climate change and human activities. Populations have declined due to agricultural expansion and climate variability [41]. This study reveals that the density of P. maculatum differs significantly, but the variability of characters was not directly correlated with the spatial density of individuals, suggesting complex ecological interactions, similar to those reported for other species [42]. These morphological differences reflect adaptation to the environment in the two areas analyzed. Previous studies on P. maculatum in Romania have highlighted a considerable impact caused by various anthropogenic factors, especially overgrazing, which has led to a significant reduction in the size and number of populations throughout the country [13]. In addition, due to global warming, the average annual temperature in this geographical area has increased, while precipitation levels have decreased, which has led to aggravation of drought conditions [43]. These changes influence the vegetative development and reproductive efficiency of various plant species, including P. maculatum. Fluctuations in temperature and humidity disrupt plant growth cycles and reproductive phases [1]. Frequent assessments of climate impacts on local ecosystems can support effective adaptation of conservation strategies [13]. Extreme heat events can negatively impact plant physiological processes, potentially reducing reproductive success and overall viability [44].
The increased frequency of extreme weather events, such as floods and storms, can disrupt local ecosystems [45], further jeopardizing the stability of P. maculatum populations. Overgrazing and agricultural expansion, together with climate stress, further affect the habitats where P. maculatum thrives. The abandonment of traditional grazing methods also affects the ecological balance essential for its survival [12].
The vegetation in the area of the Iron Gates Natural Park includes forests, shrubs, meadows, and ruderal formations, and their dispersion depends on the oro-pedo-climatic particularities of the substrate. This study highlights the morphological variability of P. maculatum and provides data on its ecology and potential for spread within plant communities, the level of vitality, viability, and the ability of individuals to fructify from each phytocoenosis. This variability is influenced by ecological factors, propagation methods, and conservation efforts. P. maculatum is suffering considerably from climate change due to higher temperatures, decreased rainfall, habitat destruction, and extreme weather events. Managing these challenges is crucial for the species’ conservation.
Also, the identification of invasive species causes a reduction in phytocoenotic diversity and the elimination of valuable local species, including rare or protected ones. Consequently, the long-term maintenance of P. maculatum is influenced by the sustainable management of grassland habitats and the monitoring of invasive species. These conclusions are in line with those reported by [13].
Population viability analysis is used as a method in the study of plant population dynamics and management for their conservation [46]. The assessment of population size is essential to not only guarantee the survival of viable communities in sites where they are already few but also to support large populations in areas where species are well represented and well developed [47,48]. The objective should be to ensure minimum viability or to maintain large populations in their regions of optimal development. Although in some situations it may be argued that a population’s viability requires a larger number of individuals than assessed, the population may still be viable, and the goal should not be to create populations larger than the minimum appropriate. In general, populations with fewer than 50 individuals should be carefully considered to determine whether appropriate management could increase the population size. When necessary, population consolidation can be achieved, with an ideal stocking density based on local conditions.
The variable number of P. maculatum individuals in different plant communities is largely influenced by environmental and anthropogenic factors. The last group can damage ecosystems, and population size can decrease or even disappear [49].
5. Conclusions
P. maculatum benefits from legal protection under the National Law on Biological Diversity and is listed in Annex II of Council Directive 92/43/EEC (Habitats Directive), with the associated IUCN national category VU (vulnerable), and, therefore, requires considerable attention for conservation. Compared to other countries in the Balkan Peninsula, a larger number of populations are found in Romania, the number of individuals being quite significant, the populations being found in both the continental and alpine biogeographic regions, this being due to favorable eco-pedo-climatic and cenotic factors
From a coenotic point of view, this species is of major importance in the creation of phytocoenoses of plant communities in Ponto-Sarmatian and sub-Pannonic steppe meadows and Pannonic or dry meadows on calcareous substrate.
The analyzed P. maculatum densities belonged to the four mentioned plant communities, indicating the phytocenotic variability of this species in close connection with the environmental factors and biological diversity in the studied area. The number of individuals varied between 4 and 53 per population, and most of them were well grown due to the characteristic xero-mesophilic and subthermophilic ecological conditions, which support optimal development. The evolution of the individuals was satisfactory or excellent, depending on the nature and intensity of anthropogenic factors, the variability of the constantly changing seasonal conditions, and the impact of invasive or undesirable species. For the optimal development of the populations, it is recommended that unwanted species with anthropophilic characteristics be eliminated, that these meadows be mowed to ensure a beneficial conservation state for both the habitat and the P. maculatum species, and that the conservation measures be implemented correctly (according to pastoral arrangements and norms).
The variability of the P. maculatum species in the Iron Gates Natural Park is due to a complex interaction between ecological factors, human impact, and conservation measures. Continued research and effective management methods are essential for the conservation of this species and its habitats, which are increasingly threatened by climate change and anthropogenic pressure. Addressing these challenges through dedicated conservation initiatives is crucial for the future of this species.
Author Contributions
Conceptualization, M.N. and P.I.; methodology, M.N.; software, O.F.P.; validation, M.N., P.I. and O.F.P.; formal analysis, M.N.; investigation, M.N.; resources, M.N.; data curation, O.F.P.; writing—original draft preparation, P.I.; writing—review and editing, P.I.; visualization, M.N.; supervision, M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nowak, B.; Sitek, E.; Augustynowicz, J. Sourcing and Propagation of Pontechium maculatum for Horticulture and Species Restoration. Biology 2020, 9, 317. [Google Scholar] [CrossRef]
- Hilger, H.H.; Böhle, U.R. Pontechium: A new genus distinct from Echium and Lobostemon (Boraginaceae). Taxon 2000, 49, 737–746. [Google Scholar] [CrossRef]
- Goriup, P.; Anastasiu, P.; Barbos, M.; Nicolin, A.; Niculescu, M.; Oprea, A. Natura 2000 in Romania Species Fact Sheets; Romanian Ministry of Environment and Sustainable Development: Bucharest, Romania, 2008.
- Dresler, S.; Kubrak, T.; Bogucka-Kocka, A.; Szymczak, G. Determination of Shikonin and Rosmarinic Acid in Echium vulgare L. and Echium russicum J.F. Gmel. by Capillary Electrophoresis. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 698–701. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Daironas, Z.V.; Zilfikarov, I.N. Shikonin and Rosmarinic-Acid Derivatives from Echium russicum Roots. Chem. Nat. Compd. 2017, 53, 953–955. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; García-Maroto, F.; Vilches-Ferrón, M.A.; López-Alonso, D. Gamma-linolenic acid from fourteen Boraginaceae species. Ind. Crop. Prod. 2003, 18, 85–89. [Google Scholar] [CrossRef]
- Holub, J.; Procházka, F. Red List of vascular plants of the Czech Republic—2000. Preslia 2000, 72, 187–230. [Google Scholar]
- Chmielewski, P.; Czarnecka, B.; Kucharczyk, M. Echium russicum J.F.Gmel. In Polish Plant Red Data Book; Pteridophyta and Spermatophyta; Akademia Nauk: Kraków, Poland, 2014; pp. 417–418. [Google Scholar]
- Turis, P.; Kliment, J.; Feráková, V.; Dítě, D.; Eliáš, P.; Hrivnák, R.; Košťál, J.; Šuvada, R.; Mráz, P.; Bernátová, D. Red List of vascular plants of the Carpathian part of Slovakia. Thaiszia J. Bot. 2014, 24, 35–87. [Google Scholar]
- Petrova, A.; Vladimirov, V. Red List of Bulgarian vascular plants. Phytol. Balc. 2009, 15, 63–94. [Google Scholar]
- EU Habitats Directive. Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora—Consolidated Version 01/01/2007. (as Modified by Directive 2013/17/EU). 1992. Available online: https://environment.ec.europa.eu/topics/nature-and-biodiversity/habitats-directive_en (accessed on 27 June 2025).
- Sava, A.R.; Boz, I.; Costică, N. Contribution to the knowledge of vegetative organs structure at Echium russicum J.F. GMELIN. Analele Ştiinţifice Univ. “Al. I. Cuza” Iaşi II Biol. Veg. 2019, 65, 44–54. [Google Scholar]
- Chirilă, S.D.; Vassilev, K. Habitat preference for the populations of the endangered species Pontechium maculatum (Boraginaceae) in Romania. Tuexenia 2024, 44, 131–157. [Google Scholar] [CrossRef]
- Mountford, O.; Gafta, D.; Anastasiu, P.; Bărbos, M.; Nicolin, A.; Niculescu, M.; Oprea, A. Natura 2000 in Romania; Habitat Fact Sheets; Ministry of Environment and Sustainable Development: Bucharest, Romania, 2008; pp. 252, 502.
- Prodan, I. Aspecte din vegetația zonei de vest a R.P.R. Bul. Șt. Acad. Rom. Biol. Șt. Agr. București 1956, 8, 5–45. [Google Scholar]
- Borza, A. Cercetări asupra florei și vegetației din Câmpia Română. Contrib. Bot. Cluj Napoca 1968, 2, 149–183. [Google Scholar]
- Ciocârlan, V. Flora Ilustrată a României, Pteridophyta et Spermatophyta, 2nd ed.; Edit. Ceres: Bucharest, Romania, 2000; Volume 2, 1138p. [Google Scholar]
- Ciocârlan, V. Flora ilustrată a României, Pteridophyta et Cormophyta; Ed. Ceres: Bucharest, Romania, 2009. [Google Scholar]
- Mititelu, D.; Chifu, T. Flora și vegetația județului Botoșani. Stud. Comun. Muz. Ști. Naț. Bacău 1993, 13, 109–126. [Google Scholar]
- Oprea, A. Lista Critică a Plantelor Vasculare din România; Editura Universitatii A.I. Cuza: Iasi, Romania, 2005; 668p. [Google Scholar]
- Oprea, A. Flora și Vegetația Câmpiei Tecuciului; Editura Universitatii A.I. Cuza: Iasi, Romania, 2021; 596p. [Google Scholar]
- Grințescu, I. Echium L. In Flora Republicii Populare Romîne; Săvulescu, T., Ed.; Academia Republicii Populare: Bucharest, Romania, 1960; Volume VII, pp. 230–237. [Google Scholar]
- Popescu, I.E. The reservation of centuries-old hay from Valea lui David Iaşi. In Miroslava Commune. About Places and People; Susai, Ș., Ed.; Masterprint: Iasi, Romania, 2013; pp. 169–186. [Google Scholar]
- Sârbu, I.; Ștefan, N.; Oprea, A. Vascular Plants from Romania—Illustrated Field Determinant; Victor B Victor: Bucharest, Romania, 2013; 1320p. [Google Scholar]
- Available online: https://www.europarl.europa.eu/portal/en (accessed on 9 October 2024).
- Gafta, D.; Mountford, J.O. (Eds.) Manual de Interpretare a Habitatelor Natura 2000 din România; Editura Risoprint: Cluj-Napoca, Romania, 2008. [Google Scholar]
- Rozylowicz, L.; Nita, A.; Manolache, S.; Popescu, V.D.; Hartel, T. Navigating protected areas networks for improving diffusion of conservation practices. J. Environ. Manag. 2019, 230, 413–421. [Google Scholar] [CrossRef]
- Săvulescu, T. (Ed.) Flora R.P Române. R.S România, 1-XIII; Editura Academiei Romane: Bucharest, Romania, 1952–1976. [Google Scholar]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, C.M.; Webb, D.A. (Eds.) Flora Europaea, 2nd ed.; Cambridge University Press: Cambridge, UK, 1964–1980 & 1993; Volume 1–5. [Google Scholar]
- Braun-Blanquet, J. Plantsociology. In The Study of Plant Communities; Fullerand, G.D.; Conrad, H.S., Translators; McGraw-Hill: New York, NY, USA, 1932; 539p. [Google Scholar]
- Coldea, G. Prodrome des associations végétales des Carpates du sud-est (Carpates Roumaines). Doc. Phytosociol. 1991, 13, 317–539. [Google Scholar]
- Sanda, V.; Popescu, A.; Barabaş, N. Cenotaxonomia şi caracterizarea grupărilor vegetale din România. In Studii şi Comunicări Biologie Vegetală; Edit. “I. Borcea”: Bacău, Romania, 1998; 342p. [Google Scholar]
- Sanda, V.; Popescu, A.; Stancu, D.I. Structura Cenotica si Caracterizarea Ecologica a Fitocenozelor din România; Editura Cophis: Pitesti, Romania, 2001. [Google Scholar]
- Oberdorfer, E. Teil IV: Wälder und Gebüsche 2. In Süddeutsche Pflanzen—Gesellschaften; Stark berabeilete Auflage Texband; Gustav Fischer: Jena, Germany, 1992. [Google Scholar]
- Mucina, L.; Grabherr, G.; Ellmauer, T.; Wallnöfer, B. (Eds.) Die Pflanzengesellschaften Österreichs; Band I–III; G. Fischer: Jena, Germany, 1993. [Google Scholar]
- Mucina, L.; Bültmann, H.; Dierßen, K.; Theurillat, J.P.; Raus, T.; Tichý, L. Vegetation of Europe: Hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl. Veg. Sci. 2016, 19, 3–264. [Google Scholar] [CrossRef]
- Rodwell, J.S.; Schaminée, J.H.J.; Mucina, L.; Pignatti, S.; Dring Moss, J.D. The Diversity of European Vegetation—An Overview of Phyto-Sociological Alliances and Their Relationships to EUNIS Habitats; [report no. EC-LNV 2002(054)]; National Reference Centre for Agriculture, Nature and Fisheries: Wageningen, The Netherlands, 2002; p. 109.
- Harman, H.H. Modern Factor Analysis, 3rd ed.; University of Chicago Press: Chicago, IL, USA, 1976; p. 376. [Google Scholar]
- Sharma, S. Applied Multivariate Techniques, 1st ed.; John Wiley and Sons: New York, NY, USA, 1996; 493p. [Google Scholar]
- Stugren, B. Bazele Ecologiei Generale; Ed. Ştiinţă şi Enciclopedică: Bucureşti, România, 1982; 435p. [Google Scholar]
- Tofan, D.E.; Ionita, O.; Ghendov, V. In situ and ex situ conservation of Ponthechium maculatum (L.) Böhle et Hilger (Boraginaceae) in Republic of Moldova. AGROFOR Int. J. 2024, 9, 69–76. [Google Scholar]
- Gheorghe, I.F.; Memedemin, D.; Graza, A.A. Important Species of Plants for Biodiversity Conservation in the North Dobrogean Plateau—Conservation Status. In Proceedings of the Delta Dunării VIII, Tulcea, Romania, 12–17 May 2020; pp. 47–62. Available online: https://biblioteca-digitala.ro/reviste/Delta-Dunarii/Revista-Delta-Dunarii-08-2020_05.pdf (accessed on 9 October 2024).
- Zaldea, G.; Nechita, A.; Damian, D.; Ghiur, A.D.; Cotea, V.V. Climate changes in recent decades, the evolution of the drought phenomenon and their influence on vineyards in north-eastern Romania. Not. Bot. Horti Agrobot. Cluj Napoca 2021, 49, 12448. [Google Scholar] [CrossRef]
- Working Group on Fighting Climate Change: An Integrated Approach. In Executive Summary of the Report Limiting Climate Change and Its Impact: An Integrated Approach for Romania; English Translation; Presidential Administration of Romania, Department of Climate and Sustainability: Bucharest, Romania, 2023.
- Available online: https://climate-adapt.eea.europa.eu/ (accessed on 9 October 2024).
- Chaudhary, V.; Oli, M.K. A critical appraisal of population viability analysis. Conserv. Biol. 2020, 34, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Iriondo, J.M. The survey and modelling of small plant populations as a basis for developing conservation strategies. Bocconea 1996, 5, 151–157. [Google Scholar]
- Gauthier, P.; Pons, V.; Letourneau, A.; Klesczewski, M.; Papuga, G.; Thompson, J.D. Combining population monitoring with habitat vulnerability to assess conservation status in populations of rare and endangered plants. J. Nat. Conserv. 2017, 37, 83–95. [Google Scholar] [CrossRef]
- Kaya, O.F.; Ertekin, A.S. Evaluation of the taxonomy and conservation status of Podonosma sintenisii (Boraginaceae). Botanica 2024, 30, 31–41. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).