Toward Sustainability: Electrochemical and Spectroscopic Analysis of Microbial Fuel Cells Using Carrot Pulp
Abstract
1. Introduction
2. Materials and Methods
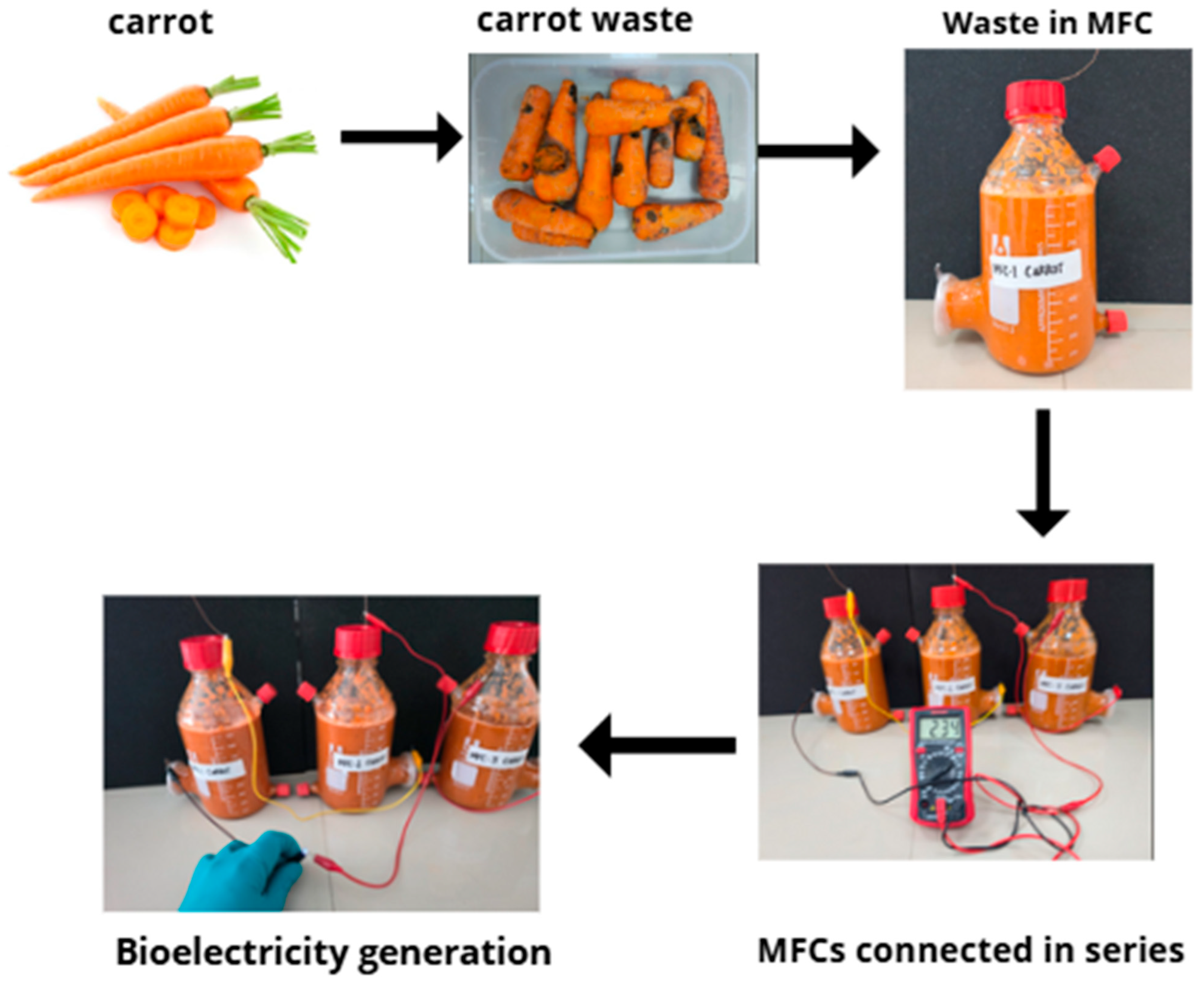
2.1. Design and Manufacture of the MFCs
2.2. Substrate Preparation
2.3. Parameter Monitoring and Microorganism Isolation
2.4. Isolation of Microbial Strains from the Anode
2.5. Molecular Identification of Electrogenic Strains
3. Results and Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kebede, A.A.; Kalogiannis, T.; Van Mierlo, J.; Berecibar, M. A comprehensive review of stationary energy storage devices for large scale renewable energy sources grid integration. Renew. Sustain. Energy Rev. 2022, 159, 112213. [Google Scholar] [CrossRef]
- Ang, T.Z.; Salem, M.; Kamarol, M.; Das, H.S.; Nazari, M.A.; Prabaharan, N. A comprehensive study of renewable energy sources: Classifications, challenges and suggestions. Energy Strategy Rev. 2022, 43, 100939. [Google Scholar] [CrossRef]
- Abdalla, A.N.; Nazir, M.S.; Tao, H.; Cao, S.; Ji, R.; Jiang, M.; Yao, L. Integration of energy storage system and renewable energy sources based on artificial intelligence: An overview. J. Energy Storage 2021, 40, 102811. [Google Scholar] [CrossRef]
- Chen, X.H.; Tee, K.; Elnahass, M.; Ahmed, R. Assessing the environmental impacts of renewable energy sources: A case study on air pollution and carbon emissions in China. J. Environ. Manag. 2023, 345, 118525. [Google Scholar] [CrossRef]
- Saleem, M. Possibility of utilizing agriculture biomass as a renewable and sustainable future energy source. Heliyon 2022, 8, e08905. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, X.; Huang, H.; Zhang, D.; Ghadimi, N. Optimal economic scheduling of microgrids considering renewable energy sources based on energy hub model using demand response and improved water wave optimization algorithm. J. Energy Storage 2022, 55, 105311. [Google Scholar] [CrossRef]
- Panagopoulos, A. Water-energy nexus: Desalination technologies and renewable energy sources. Environ. Sci. Pollut. Res. 2021, 28, 21009–21022. [Google Scholar] [CrossRef]
- Awogbemi, O.; Von Kallon, D.V. Pretreatment techniques for agricultural waste. Case Stud. Chem. Environ. Eng. 2022, 6, 100229. [Google Scholar] [CrossRef]
- Waqas, M.; Hashim, S.; Humphries, U.W.; Ahmad, S.; Noor, R.; Shoaib, M.; Naseem, A.; Hlaing, P.T.; Lin, H.A. Composting processes for agricultural waste management: A comprehensive review. Processes 2023, 11, 731. [Google Scholar] [CrossRef]
- Lee, S.H.; Lum, W.C.; Boon, J.G.; Kristak, L.; Antov, P.; Pędzik, M.; Rogoziński, T.; Taghiyari, H.R.; Lubis, M.A.R.; Fatriasari, W.; et al. Particleboard from agricultural biomass and recycled wood waste: A review. J. Mater. Res. Technol. 2022, 20, 4630–4658. [Google Scholar] [CrossRef]
- Riseh, R.S.; Vazvani, M.G.; Hassanisaadi, M.; Thakur, V.K. Agricultural wastes: A practical and potential source for the isolation and preparation of cellulose and application in agriculture and different industries. Ind. Crops Prod. 2024, 208, 117904. [Google Scholar] [CrossRef]
- Onuh, P.; Ejiga, J.O.; Abah, E.O.; Onuh, J.O.; Idogho, C.; Omale, J. Challenges and opportunities in Nigeria’s renewable energy policy and legislation. World J. Adv. Res. Rev. 2024, 23, 2354–2372. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, S.; Alkhanjaf, A.A.M.; Arora, N.K.; Saxena, B.; Umar, A.; Ibrahim, A.A.; Akhtar, M.S.; Mahajan, A.; Negi, S.; et al. Microbial fuel cells for azo dye degradation: A perspective review. J. Ind. Eng. Chem. 2025, 142, 45–67. [Google Scholar] [CrossRef]
- Jalili, P.; Ala, A.; Nazari, P.; Jalili, B.; Ganji, D.D. A comprehensive review of microbial fuel cells considering materials, methods, structures, and microorganisms. Heliyon 2024, 10, e25439. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, J.; Fu, X.; Zhao, G. Finite-time Pade-based adaptive FNN controller implementation for microbial fuel cell with delay and multi-disturbance. Int. J. Hydrog. Energy 2025, 98, 1034–1043. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, H.; Zhang, W.; Lu, Y. Bioanode boosts efficacy of chlorobenzenes-powered microbial fuel cell: Performance, kinetics, and mechanism. Bioresour. Technol. 2024, 405, 130936. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Villacorta, W.; Rojas-Flores, S.; Benites, S.M.; Nazario-Naveda, R.; Romero, C.V.; Gallozzo-Cardenas, M.; Delfín-Narciso, D.; Díaz, F.; Murga-Torres, E. Preliminary study of bioelectricity generation using lettuce waste as substrate by microbial fuel cells. Sustainability 2023, 15, 10339. [Google Scholar] [CrossRef]
- Zafar, H.; Peleato, N.; Roberts, D. A review of the role of pre-treatment on the treatment of food waste using microbial fuel cells. Environ. Technol. Rev. 2022, 11, 72–90. [Google Scholar] [CrossRef]
- Kebaili, H.; Kameche, M.; Innocent, C.; Ziane, F.; Sabeur, S.; Sahraoui, T.; Ouis, M.; Zerrouki, A.; Charef, M.A. Treatment of fruit waste leachate using microbial fuel cell: Preservation of agricultural environment. Acta Ecol. Sin. 2021, 41, 97–105. [Google Scholar] [CrossRef]
- de Mendonça, K.S.; Corrêa, J.L.G.; de Jesus Junqueira, J.R.; de Carvalho, E.E.N.; Silveira, P.G.; Uemura, J.H.S. Peruvian carrot chips obtained by microwave and microwave-vacuum drying. LWT 2023, 187, 115346. [Google Scholar] [CrossRef]
- Boonaert, E.; Depoorter, C.; Marx, A.; Maertens, M. Carrots rather than sticks: Governance of voluntary sustainability standards and farmer welfare in Peru. Sustain. Dev. 2024, 32, 6471–6492. [Google Scholar] [CrossRef]
- Silva, V.D.S.; Arias, L.V.A.; Velasco, J.I.; Fakhouri, F.M.; de Oliveira, R.A. Fat Reduction in Peruvian Carrot (Arracacia xanthorrhiza) Snacks: Effectiveness of Edible Coatings and Optimization of Frying Conditions. Foods 2025, 14, 1895. [Google Scholar] [CrossRef]
- Mendonça, K.S.D.; Corrêa, J.L.G.; Junqueira, J.R.D.J.; Souza, A.U.D. Two-stage power level to improve microwave vacuum drying of restructured Peruvian carrot chips. Ciência E Agrotecnologia 2023, 47, e010523. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Jin, D.; Wu, P. Anammox-coupled microbial fuel cell (Anammox-MFC) for wastewater treatment: A critical review. J. Water Process Eng. 2025, 71, 107285. [Google Scholar] [CrossRef]
- Shadman, P.; Shakeri, A.; Zinadini, S. Incorporating GO-CS-2-aminothiazole-SO3H nanoparticles into sulfonated PES for improved MFC performance in power generation. Renew. Energy 2025, 244, 122580. [Google Scholar] [CrossRef]
- Mulyono, T.; Misto Cahyono, B.E.; Fahmidia, N.H. The impact of adding vegetable waste on the functioning of microbial fuel cell. AIP Conf. Proc. 2022, 2663, 020008. [Google Scholar] [CrossRef]
- Ahmad, A. Conventional vegetable waste: A potential source for the high performance of benthic microbial fuel cells. Biomass Convers. Biorefinery 2024, 14, 24641–24653. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Ma, H.; Sun, W.; Xu, K.; Li, H. Dynamic modeling and active-vibration-suppression for bolted-composite-laminates: An MFC-integrated framework. Int. J. Mech. Sci. 2025, 302, 110580. [Google Scholar] [CrossRef]
- Bagchi, S.; Behera, M. Evaluation of the effect of anolyte recirculation and anolyte pH on the performance of a microbial fuel cell employing ceramic separator. Process Biochem. 2021, 102, 207–212. [Google Scholar] [CrossRef]
- Javed, M.M.; Nisar, M.A.; Ahmad, M.U. Effect of NaCl and pH on bioelectricity production from vegetable waste extract supplemented with cane molasses in dual chamber microbial fuel cell. Pak. J. Zool 2021, 54, 247–254. [Google Scholar] [CrossRef]
- Curiel Alegre, S. Advances in Bioremediation Techniques for the Degradation of Hydrocarbons in Soils. Ph.D. Thesis, University of Burgos, Burgos, Spain, 2024. [Google Scholar]
- Cruz González, X.A. Análisis Genotípico, Fenotípico y Funcional de Bacterias Aisladas de Nódulos de Cicer arietimum L. para la Evaluación de su Potencial Como Biofertilizantes Agrícolas en Cultivos de Garbanzo y Trigo. Ph.D. Thesis, University of Salamanca, Salamanca, Spain, 2018. [Google Scholar]
- Munfarida, I.; Auvaria, S.W. The Role of EM4 (Effective Microorganisms) in Solid Waste-Powered Microbial Fuel Cells: Investigating Voltage Output and Electrical Conductivity For Bioelectricity Generation. Int. J. Environ. Sustain. Soc. Sci. 2024, 5, 1270–1279. [Google Scholar] [CrossRef]
- Moqsud, M.A.; Yoshitake, J.; Bushra, Q.S.; Hyodo, M.; Omine, K.; Strik, D. Compost in plant microbial fuel cell for bioelectricity generation. Waste Manag. 2015, 36, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Naveenkumar, M.; Senthilkumar, K. Microbial fuel cell for harvesting bio-energy from tannery effluent using metal mixed biochar electrodes. Biomass Bioenergy 2021, 149, 106082. [Google Scholar] [CrossRef]
- Raychaudhuri, A.; Behera, M. Enhancement of bioelectricity generation by integrating acidogenic compartment into a dual-chambered microbial fuel cell during rice mill wastewater treatment. Process Biochem. 2021, 105, 19–26. [Google Scholar] [CrossRef]
- Utomo, H.D.; Yu, L.S.; Yi, D.C.Z.; Jun, O.J. Recycling solid waste and bioenergy generation in MFC dual-chamber model. Energy Procedia 2017, 143, 424–429. [Google Scholar] [CrossRef]
- Streletskiy, O.A.; Zavidovskiy, I.A.; Nuriahmetov, I.F.; Nishchak, O.Y.; Pavlikov, A.V.; Savchenko, N.F. Resistive gas sensors based on porous Sp-containing films obtained by dehydrohalogenation of PVDC and PVDC-PVC copolymer. C 2023, 9, 82. [Google Scholar] [CrossRef]
- Rady, A.M.; Sugiharto, S.; Adedeji, A.A. Evaluation of carrot quality using visiblenear infrared spectroscopy and multivariate analysis. J. Food Res. 2018, 7, 80–93. [Google Scholar] [CrossRef]
- Joda, B.A.; Abed Al-Kadhim, Z.M.; Ahmed, H.J.; Al-Khalaf, A.K. A convenient green method to synthesize β-carotene from edible carrot and nanoparticle formation. Karbala Int. J. Mod. Sci. 2022, 8, 20–27. [Google Scholar] [CrossRef]
- Baranitharan, E.; Khan, M.R.; Prasad, D.M.R.; Teo, W.F.A.; Tan, G.Y.A.; Jose, R. Effect of biofilm formation on the performance of microbial fuel cell for the treatment of palm oil mill effluent. Bioprocess Biosyst. Eng. 2015, 38, 15–24. [Google Scholar] [CrossRef]
- You, J.; Chen, H.; Xu, L.; Zhao, J.; Ye, J.; Zhang, S.; Chen, C.; Cheng, Z. Anodic-potential-tuned bioanode for efficient gaseous toluene removal in an MFC. Electrochim. Acta 2021, 375, 137992. [Google Scholar] [CrossRef]
- Banerjee, A.; Calay, R.K.; Mustafa, M. Review on material and design of anode for microbial fuel cell. Energies 2022, 15, 2283. [Google Scholar] [CrossRef]
- Yanuka-Golub, K.; Dubinsky, V.; Korenblum, E.; Reshef, L.; Ofek-Lalzar, M.; Rishpon, J.; Gophna, U. Anode surface bioaugmentation enhances deterministic biofilm assembly in microbial fuel cells. MBio 2021, 12, 10–1128. [Google Scholar] [CrossRef]
- Hemdan, B.A.; El-Taweel, G.E.; Naha, S.; Goswami, P. Bacterial community structure of electrogenic biofilm developed on modified graphite anode in microbial fuel cell. Sci. Rep. 2023, 13, 1255. [Google Scholar] [CrossRef] [PubMed]
- González-Nava, C.; Canul-Chan, M.; Campos, J.; Caballero-Pérez, J.; Houbron, E.; Godínez, L.A.; Rodríguez-Valadez, F.J. Analysis of the potential of the electroactive biofilm growth in a microbial fuel cell type H. Rev. Int. De Contam. Ambient. 2024, 40, 560–591. [Google Scholar] [CrossRef]
- Rincón-Catalán, N.I.; Cruz-Salomón, A.; Sebastian, P.; Pérez-Fabiel, S.; Hernández-Cruz, M.D.C.; Sánchez-Albores, R.M.; Hernández-Méndez, J.M.E.; Domínguez-Espinosa, M.E.; Esquinca-Avilés, H.A.; Ríos-Valdovinos, E.I.; et al. Banana waste-to-energy valorization by microbial fuel cell coupled with anaerobic digestion. Processes 2022, 10, 1552. [Google Scholar] [CrossRef]
- Segundo, R.-F.; Benites, S.M.; De La Cruz-Noriega, M.; Vives-Garnique, J.; Otiniano, N.M.; Rojas-Villacorta, W.; Gallozzo-Cardenas, M.; Delfín-Narciso, D.; Díaz, F. Impact of dragon fruit waste in microbial fuel cells to generate friendly electric energy. Sustainability 2023, 15, 7316. [Google Scholar] [CrossRef]
- Du, H.; Li, F. Size effects of potato waste on its treatment by microbial fuel cell. Environ. Technol. 2016, 37, 1305–1313. [Google Scholar] [CrossRef]
- Petroudy, S.R.D.; Syverud, K.; Chinga-Carrasco, G.; Ghasemain, A.; Resalati, H. Effects of bagasse microfibrillated cellulose and cationic polyacrylamide on key properties of bagasse paper. Carbohydr. Polym. 2014, 99, 311–318. [Google Scholar] [CrossRef]
- Segundo, R.-F.; Magaly, D.L.C.-N.; Benites, S.M.; Daniel, D.-N.; Angelats-Silva, L.; Díaz, F.; Luis, C.-C.; Fernanda, S.-P. Increase in electrical parameters using sucrose in tomato waste. Fermentation 2022, 8, 335. [Google Scholar] [CrossRef]
- Flores, S.J.R.; Benites, S.M.; Rosa, A.L.R.A.L.; Zoilita, A.L.Z.A.L.; Luis, A.S.L. The Using Lime (Citrus × aurantiifolia), Orange (Citrus × sinensis), and Tangerine (Citrus reticulata) Waste as a Substrate for Generating Bioelectricity: Using lime (Citrus × aurantiifolia), orange (Citrus × sinensis), and tangerine (Citrus reticulata) waste as a substrate for generating bioelectricity. Environ. Res. Eng. Manag. 2022, 76, 24–34. [Google Scholar]

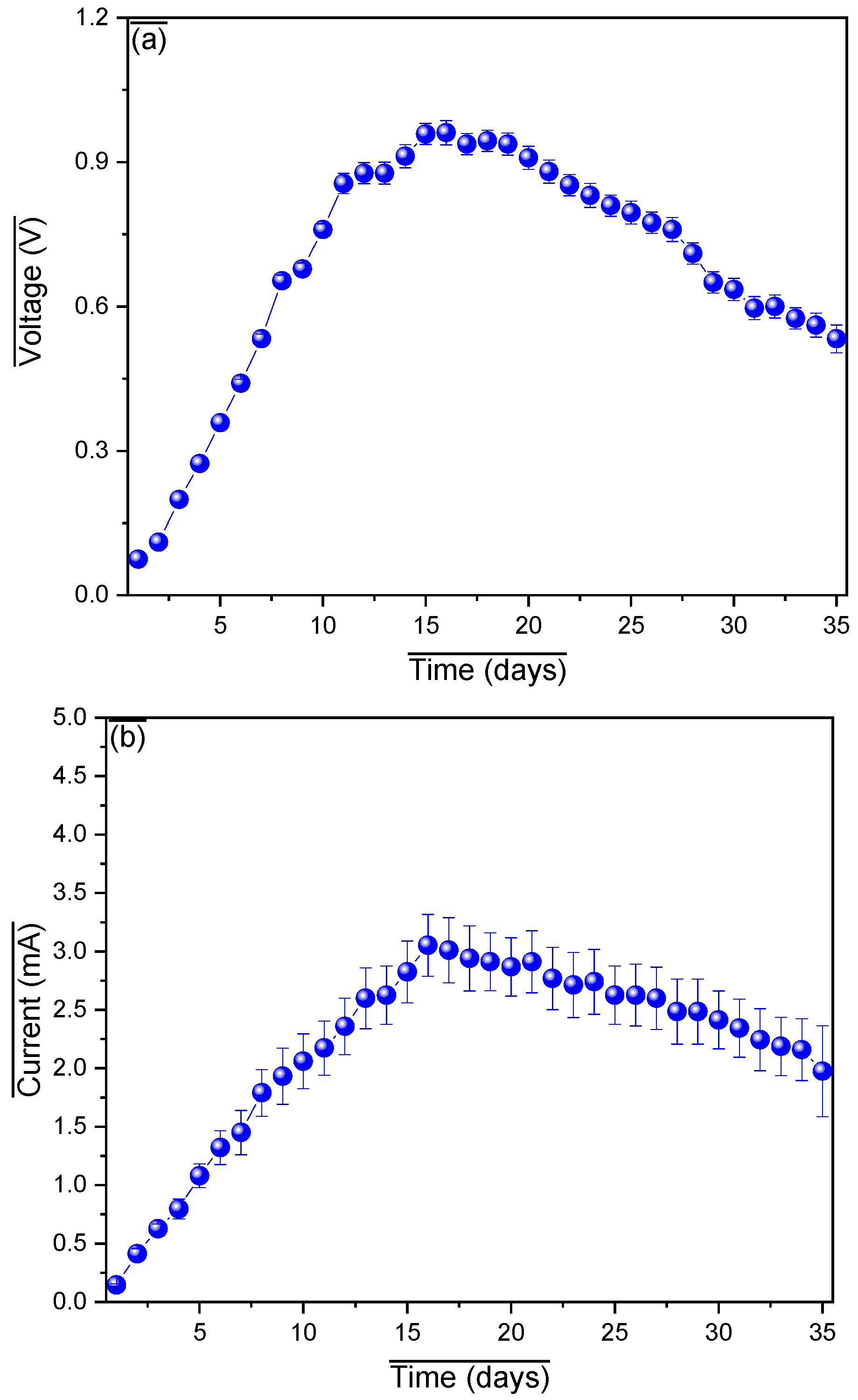
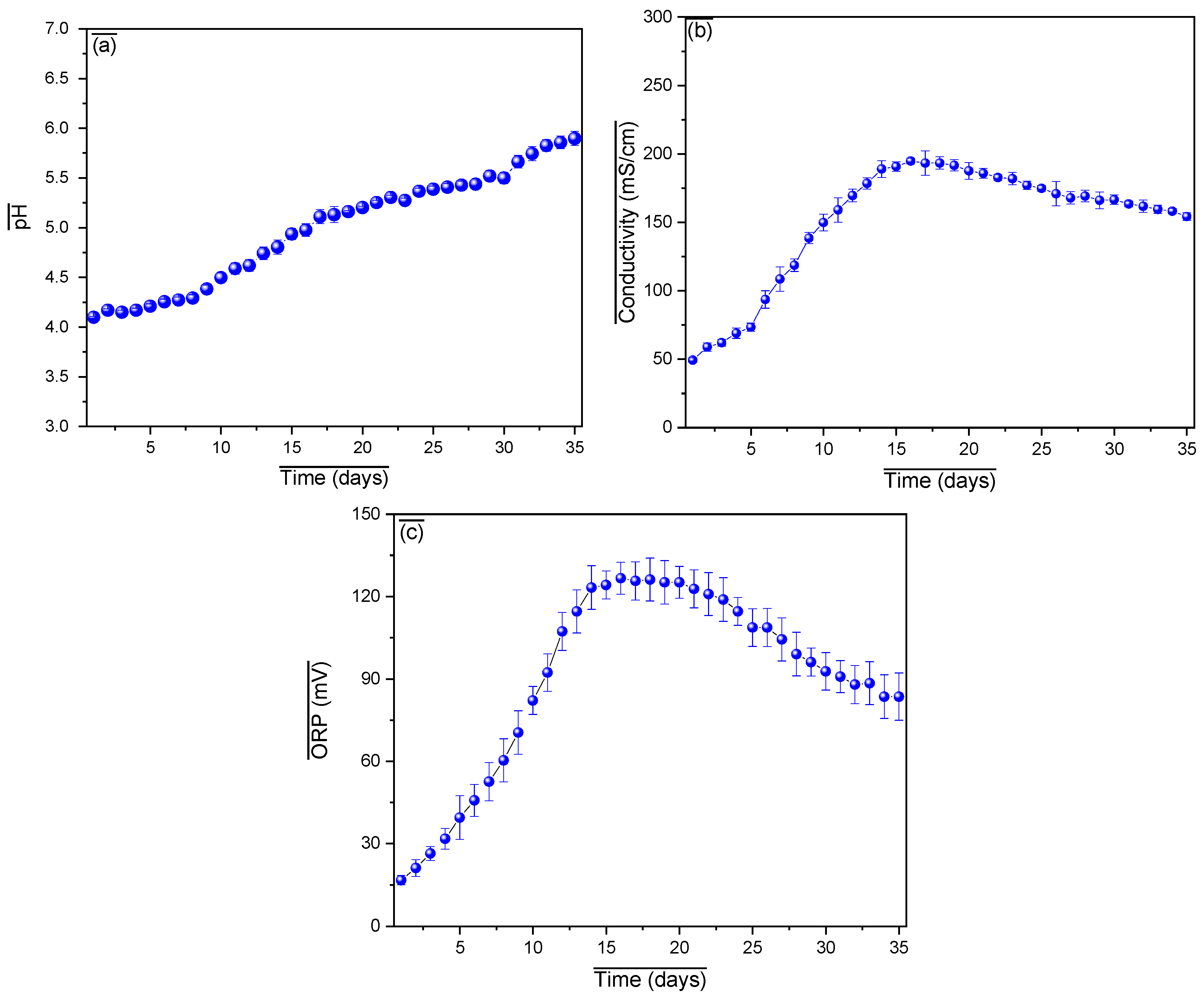
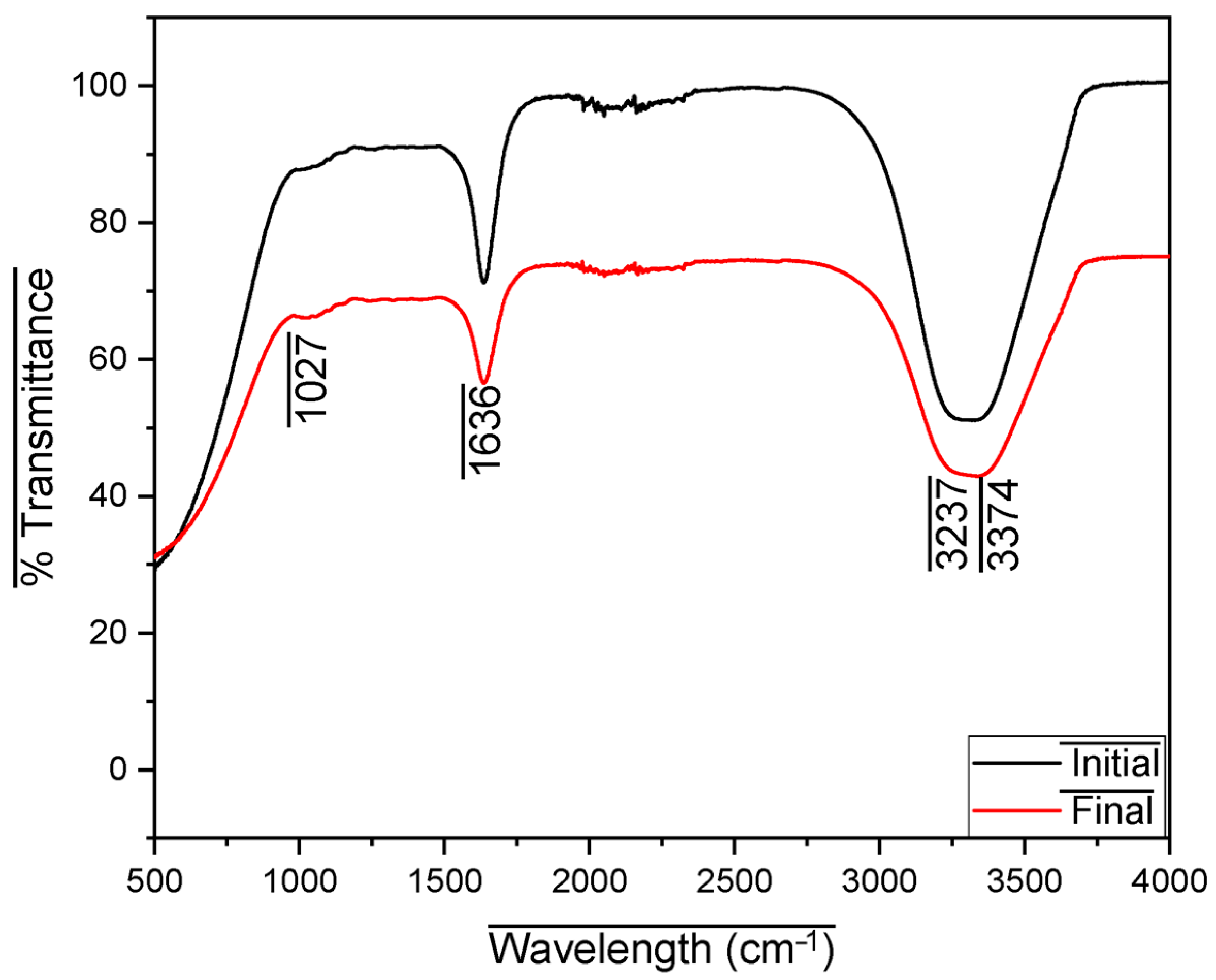
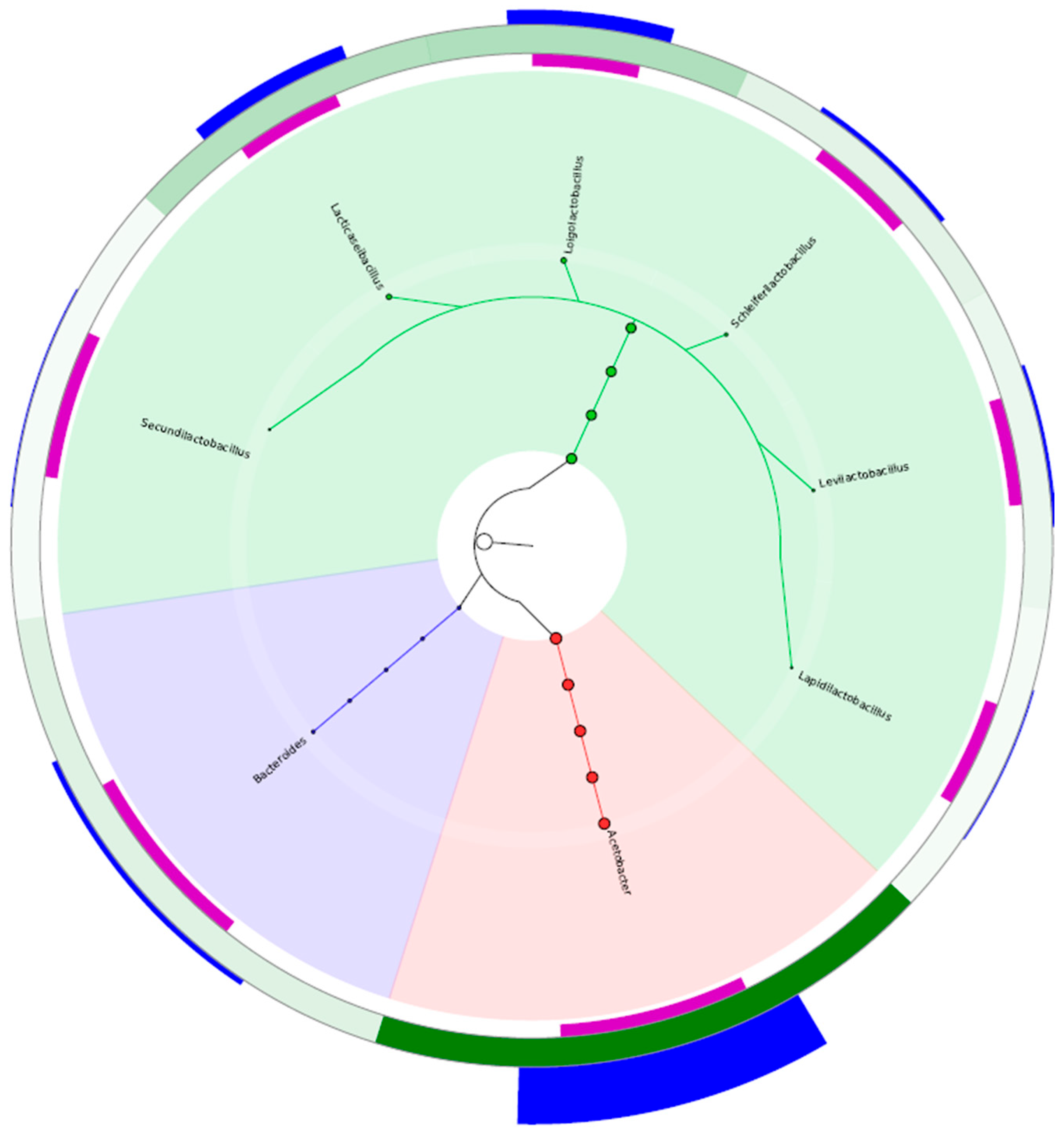
| Substrate | Pretreatment | Organic Composition | Power Density (mW/m2) | Operational Stability | Remarks |
|---|---|---|---|---|---|
| Carrot Pulp [This investigation]. | None | Simple sugars + insoluble fiber | Moderate | Stable | Fiber may limit biodegradability; good performance without pretreatment |
| Lettuce [17] | None | Water + cellulose | Low | Variable | Low energy density; requires load optimization |
| Banana peel [47] | None | Starch + fiber | Moderate | Stable | Balanced biodegradability and energy availability |
| Passion fruit Pulp [48] | None | Sugars + organic acids | High | Stable | High microbial activity; efficient bioelectrochemical conversion |
| Potato peel [49] | None | Starch + phenolic compounds | Moderate | Variable | Pretreatment may enhance degradation and performance |
| Sugarcane bagasse [50] | Yes (crushed) | Fiber + residual sugars | High | Stable | High energy availability; good performance with minimal processing |
| Tomato Pulp [51]. | None | Sugars + organic acids | Moderate | Stable | Good microbial response; high water content may dilute organic load |
| Orange peel [52]. | None | Sugars + essential oils | Low–Moderate | Variable | Antimicrobial compounds may inhibit electrogenic activity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Flores, S.J.; Nazario-Naveda, R.; Benites, S.M.; Delfin-Narciso, D.; Gallozzo Cardenas, M. Toward Sustainability: Electrochemical and Spectroscopic Analysis of Microbial Fuel Cells Using Carrot Pulp. Sustainability 2025, 17, 9114. https://doi.org/10.3390/su17209114
Rojas-Flores SJ, Nazario-Naveda R, Benites SM, Delfin-Narciso D, Gallozzo Cardenas M. Toward Sustainability: Electrochemical and Spectroscopic Analysis of Microbial Fuel Cells Using Carrot Pulp. Sustainability. 2025; 17(20):9114. https://doi.org/10.3390/su17209114
Chicago/Turabian StyleRojas-Flores, Segundo Jonathan, Renny Nazario-Naveda, Santiago M. Benites, Daniel Delfin-Narciso, and Moisés Gallozzo Cardenas. 2025. "Toward Sustainability: Electrochemical and Spectroscopic Analysis of Microbial Fuel Cells Using Carrot Pulp" Sustainability 17, no. 20: 9114. https://doi.org/10.3390/su17209114
APA StyleRojas-Flores, S. J., Nazario-Naveda, R., Benites, S. M., Delfin-Narciso, D., & Gallozzo Cardenas, M. (2025). Toward Sustainability: Electrochemical and Spectroscopic Analysis of Microbial Fuel Cells Using Carrot Pulp. Sustainability, 17(20), 9114. https://doi.org/10.3390/su17209114







