The Effect of Hormonal Priming on Morphological Characteristics and Antioxidant Enzyme Activities in Silage Maize Under Salt Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Measurement of Growth and Physiological Parameters
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bozdemir, M.; Bayramoğlu, Z.; Ağızan, K.; Ağızan, S. Prudential expectation analysis in maize production. Turk. J. Agric. -Food Sci. Technol. 2019, 7, 390–400. [Google Scholar]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Wei, X.; Wang, Q.; Liu, W.; Wang, D.; Yao, Y.; Hu, H.; Chen, X.; Huang, S.; Hou, J. Karrikin improves osmotic and salt stress tolerance via the regulation of the redox homeostasis in the oil plant Sapium sebiferum. Front. Plant Sci. 2020, 11, 216. [Google Scholar] [CrossRef]
- Athar, H.-u.-R.; Khan, A.; Ashraf, M. Inducing salt tolerance in wheat by exogenously applied ascorbic acid through different modes. J. Plant Nutr. 2009, 32, 1799–1817. [Google Scholar] [CrossRef]
- Shalaby, O.A. Using Bacillus megaterium as a bio-fertilizer alleviates salt stress, improves phosphorus nutrition, and increases cauliflower yield. J. Plant Nutr. 2024, 47, 926–939. [Google Scholar]
- Murillo-Amador, B.; López-Aguilar, R.; Kaya, C.; Larrinaga-Mayoral, J.; Flores-Hernández, A. Comparative effects of NaCl and polyethylene glycol on germination, emergence and seedling growth of cowpea. J. Agron. Crop Sci. 2002, 188, 235–247. [Google Scholar]
- Grewal, H.S. Water uptake, water use efficiency, plant growth and ionic balance of wheat, barley, canola and chickpea plants on a sodic vertosol with variable subsoil NaCl salinity. Agric. Water Manag. 2010, 97, 148–156. [Google Scholar] [CrossRef]
- Mohammadi, G. The influence of NaCl priming on seed germination and seedling growth of canola (Brassica napus L.) under salinity conditions. Am.-Eurasian J. Agric. Environ. Sci. 2009, 5, 696–700. [Google Scholar]
- Chen, T.-W.; Gomez Pineda, I.M.; Brand, A.M.; Stützel, H. Determining ion toxicity in cucumber under salinity stress. Agronomy 2020, 10, 677. [Google Scholar] [CrossRef]
- Cherifi, K.; Boufous, E.H.; Boubaker, H.; Msanda, F. Comparative salt tolerance study of some Acacia species at seed germination stage. arXiv 2016, arXiv:1610.06033. [Google Scholar]
- Bina, F.; Bostani, A. Effect of Salinity (NaCl) stress on germination and early seedling growth of three medicinal plant species. Adv. Life Sci. 2017, 4, 77–83. [Google Scholar]
- Farooq, M.; Hussain, M.; Wakeel, A.; Siddique, K.H. Salt stress in maize: Effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef]
- Khaliq, A.; Iqbal, M.A.; Zafar, M.; Gulzar, A. Appraising economic dimension of maize production under coherent fertilization in Azad Kashmir, Pakistan. Custos Agronegocio 2019, 15, 243–253. [Google Scholar]
- Masum Billah, M.B.; Latif, M.; Neelima Hossain, N.H.; Uddin, M. Evaluation and selection of salt tolerant hybrid maize under hydroponics culture. Res. Crops 2017, 18, 481–489. [Google Scholar]
- Feng, G.; Zhang, Z.; Wan, C.; Lu, P.; Bakour, A. Effects of saline water irrigation on soil salinity and yield of summer maize (Zea mays L.) in subsurface drainage system. Agric. Water Manag. 2017, 193, 205–213. [Google Scholar] [CrossRef]
- Zafar, S.; Shah, A.A.; Fatima, A.M.; Ahmad, K.S.; Ashraf, M.A.; Rasheed, R.; Mehmood, A.; Iftikhar, M.; Elansary, O.H.; Shankarappa, S. Seed priming by trehalose improves tolerance of maize seedlings by improving the growth and physiological parameters under Cadmium Toxicity. Pak. Sci. 2024. [CrossRef]
- Salam, A.; Khan, A.R.; Liu, L.; Yang, S.; Azhar, W.; Ulhassan, Z.; Zeeshan, M.; Wu, J.; Fan, X.; Gan, Y. Seed priming with zinc oxide nanoparticles downplayed ultrastructural damage and improved photosynthetic apparatus in maize under cobalt stress. J. Hazard. Mater. 2022, 423, 127021. [Google Scholar] [CrossRef]
- Paparella, S.; Araújo, S.d.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Damalas, C.A.; Koutroubas, S.D.; Fotiadis, S. Hydro-priming effects on seed germination and field performance of faba bean in spring sowing. Agriculture 2019, 9, 201. [Google Scholar] [CrossRef]
- Richter, R.; Behringer, C.; Zourelidou, M.; Schwechheimer, C. Convergence of auxin and gibberellin signaling on the regulation of the GATA transcription factors GNC and GNL in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2013, 110, 13192–13197. [Google Scholar]
- Schwechheimer, C. Gibberellin signaling in plants–the extended version. Front. Plant Sci. 2012, 2, 107. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Fahad, S.; Adnan, M.; Ali, M.; Rana, M.S.; Kamran, M.; Ali, Q.; Hashem, I.A.; Bhantana, P.; Ali, M. Foliar application of gibberellic acid endorsed phytoextraction of copper and alleviates oxidative stress in jute (Corchorus capsularis L.) plant grown in highly copper-contaminated soil of China. Environ. Sci. Pollut. Res. 2020, 27, 37121–37133. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.; Rha, E.S. Gibberellic acid (GA3) enhance seed water uptake, germination and early seedling growth in sugar beet under salt stress. Pak. J. Biol. Sci. 2007, 10, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, S.; Karagöz, F.P.; Dursun, A. Giberellik asit ön uygulamasına tabi tutulmuş hüsnüyusuf (Dianthus barbatus L.) tohumlarının tuz stresinde çimlenmesi. Atatürk Üniversitesi Ziraat Fakültesi Derg. 2017, 48, 1–7. [Google Scholar]
- Souri, M.K.; Tohidloo, G. Effectiveness of different methods of salicylic acid application on growth characteristics of tomato seedlings under salinity. Chem. Biol. Technol. Agric. 2019, 6, 26. [Google Scholar] [CrossRef]
- Apon, T.A.; Ahmed, S.F.; Bony, Z.F.; Chowdhury, M.R.; Asha, J.F.; Biswas, A. Sett priming with salicylic acid improves salinity tolerance of sugarcane (Saccharum officinarum L.) during early stages of crop development. Heliyon 2023, 9, e16030. [Google Scholar] [CrossRef]
- Zhang, R.; Chang, J.; Yue, Z.; Zhou, Y.; Liang, X.; Guo, W. Salicylic acid priming promotes sorghum germination under drought stress: Evidence from comparative metabolomics analysis. Appl. Ecol. Environ. Res. 2023, 21, 3643–3658. [Google Scholar] [CrossRef]
- Özyazıcı, M.A.; Açıkbaş, S. The effect of seed priming applications on germination parameters of red clover (Trifolium pratense L.). J. Inst. Sci. Technol. 2021, 11, 3232–3242. [Google Scholar]
- Bagautdinova, Z.Z.; Omelyanchuk, N.; Tyapkin, A.V.; Kovrizhnykh, V.V.; Lavrekha, V.V.; Zemlyanskaya, E.V. Salicylic acid in root growth and development. Int. J. Mol. Sci. 2022, 23, 2228. [Google Scholar] [CrossRef]
- Fardus, J.; Matin, M.A.; Hasanuzzaman, M.; Hossain, M.A. Salicylic acid-induced improvement in germination and growth parameters of wheat under salinity stress. JAPS J. Anim. Plant Sci. 2018, 28, 197–207. [Google Scholar]
- Shihab, M.O.; Hamza, J.H. Seed priming of sorghum cultivars by gibberellic and salicylic acids to improve seedling growth under irrigation with saline water. J. Plant Nutr. 2020, 43, 1951–1967. [Google Scholar] [CrossRef]
- Farooq, M.; Aziz, T.; Cheema, Z.; Hussain, M.; Khaliq, A. Activation of antioxidant system by KCl improves the chilling tolerance in hybrid maize. J. Agron. Crop Sci. 2008, 194, 438–448. [Google Scholar] [CrossRef]
- Health Union. ImageJ v1.54; Health Union: Bethesda, MD, USA, 2012. [Google Scholar]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, H.; Schoenau, J.; Vandenberg, A.; Tayfeh Aligodarz, M.; Bueckert, R.A. Indirect estimations of lentil leaf and plant N by SPAD chlorophyll meter. Int. J. Agron. 2015, 2015, 748074. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K.H.; Nayyar, H. Effects of drought, heat and their interaction on the growth, yield and photosynthetic function of lentil (Lens culinaris Medikus) genotypes varying in heat and drought sensitivity. Front. Plant Sci. 2017, 8, 1776. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, H.; Zeng, Y.; Xiang, L.; Lei, Z.; Huang, Q.; Li, T.; Shen, F.; Cheng, Q. A salt tolerance evaluation method for sunflower (Helianthus annuus L.) at the seed germination stage. Sci. Rep. 2020, 10, 10626. [Google Scholar] [CrossRef]
- Akkemik, E.; Taser, P.; Bayindir, A.; Budak, H.; Ciftci, M. Purification and characterization of glutathione S-transferase from turkey liver and inhibition effects of some metal ions on enzyme activity. Environ. Toxicol. Pharmacol. 2012, 34, 888–894. [Google Scholar] [CrossRef]
- Lakhdar, A.; Iannelli, M.A.; Debez, A.; Massacci, A.; Jedidi, N.; Abdelly, C. Effect of municipal solid waste compost and sewage sludge use on wheat (Triticum durum): Growth, heavy metal accumulation, and antioxidant activity. Journal of the Science of Food and Agriculture 2010, 90, 965–971. [Google Scholar] [CrossRef]
- Çoban, F.K.; Albayrak, A.O. Effect of oleuropein on element distributions in liver of diabetic rats. Uşak Üniversitesi Fen. Doğa Bilim. Derg. 2018, 2, 1–9. [Google Scholar]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1990; Volume 186, pp. 407–421. [Google Scholar]
- Açıkgöz, N.; Açıkgöz, N. Tarımsal araştırmaların istatistiki değerlendirilmesinde yapılan bazı hatalar: I. Tek. Faktörlü Denemeler Anadolu 2001, 11, 135–147. [Google Scholar]
- McDonald, M.B. Seed priming. In Seed Technology and Its Biological Basis; Sheffield Academic Press: Sheffield, UK, 2000; pp. 287–325. [Google Scholar]
- Sher, A.; Sarwar, T.; Nawaz, A.; Ijaz, M.; Sattar, A.; Ahmad, S. Methods of seed priming. In Priming and Pretreatment of Seeds and Seedlings: Implication in Plant Stress Tolerance and Enhancing Productivity in Crop Plants; Springer: Singapore, 2019; pp. 1–10. [Google Scholar]
- Zulfiqar, F. Effect of seed priming on horticultural crops. Sci. Hortic. 2021, 286, 8. [Google Scholar] [CrossRef]
- Mauch-Mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense priming: An adaptive part of induced resistance. Annu. Rev. Plant Biol. 2017, 68, 485–512. [Google Scholar] [CrossRef]
- Shakirova, F.M.; Sakhabutdinova, A.R.; Bezrukova, M.V.; Fatkhutdinova, R.A.; Fatkhutdinova, D.R. Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci. 2003, 164, 317–322. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, A. Influence of gibberellic acid on 14CO2 metabolism, growth, and production of alkaloids in Catharanthus roseus. Photosynthetica 2007, 45, 156–160. [Google Scholar] [CrossRef]
- Senaratna, T.; Touchell, D.; Bunn, E.; Dixon, K. Acetyl salicylic acid (Aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Regul. 2000, 30, 157–161. [Google Scholar] [CrossRef]
- Matsuoka, M. Gibberellin signaling: How do plant cells respond to GA signals? J. Plant Growth Regul. 2003, 22, 123–125. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Ragupathi Gopi, R.G.; Rajaram Panneerselvam, R.P. Alterations in non-enzymatic antioxidant components of Catharanthus roseus exposed to paclobutrazol, gibberellic acid and Pseudomonas fluorescens. Plant Omics 2009, 2, 30–40. [Google Scholar]
- Swain, S.M.; Singh, D.P. Tall tales from sly dwarves: Novel functions of gibberellins in plant development. Trends Plant Sci. 2005, 10, 123–129. [Google Scholar] [CrossRef]
- Al-Khassawneh, N.M.; Karam, N.S.; Shibli, R.A. Growth and flowering of black iris (Iris nigricans Dinsm.) following treatment with plant growth regulators. Sci. Hortic. 2006, 107, 187–193. [Google Scholar] [CrossRef]
- Khan, M.A.; Weber, D.J. Ecophysiology of High Salinity Tolerant Plants; Springer Science & Business Media: Berlin, Germany, 2006; Volume 40. [Google Scholar]
- Khan, M.A.; Gul, B.; Weber, D.J. Action of plant growth regulators and salinity on seed germination of Ceratoides lanata. Can. J. Bot. 2004, 82, 37–42. [Google Scholar] [CrossRef]
- Bejaoui, M. Intéractions entre NaCl et quelques Phytohormones sur la croissance du Soja: Interactions between NaCl and some phytohormones on soybean growth. J. Plant Physiol. 1985, 120, 95–110. [Google Scholar] [CrossRef]
- Szalai, G.; Tari, I.; Janda, T.; Pestenacz, A.; Páldi, E. Effects of cold acclimation and salicylic acid on changes in ACC and MACC contents in maize during chilling. Biol. Plant. 2000, 43, 637–640. [Google Scholar] [CrossRef]
- Horváth, E.; Janda, T.; Szalai, G.; Páldi, E. In vitro salicylic acid inhibition of catalase activity in maize: Differences between the isozymes and a possible role in the induction of chilling tolerance. Plant Sci. 2002, 163, 1129–1135. [Google Scholar] [CrossRef]
- Tari, I. Acclimation of tomato plants to salinity stress after a salicylic acid pre-treatment. Acta Biol. Szeged. 2002, 46, 55–56. [Google Scholar]
- Chinnusamy, V.; Jagendorf, A.; Zhu, J.K. Understanding and improving salt tolerance in plants. Crop Sci. 2005, 45, 437–448. [Google Scholar] [CrossRef]
- Hughes, B.; Davenport, D.; Dohle, L. Standard Soil Test Methods and Guidelines for Interpretation of Soil Results; Government of South Australia: Adelaide, Australia, 1996. [Google Scholar]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant responses to salt stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Bouallegue, A.; Horchani, F.; Souissi, F.; Tebini, M.; Jalali, K.; Ahmed, H.B.; Abbes, Z.; Mhadhbi, H. Enhancement of plant growth in lentil (Lens culinaris) under salinity stress by exogenous application or seed priming with salicylic acid and hydrogen peroxide. PLoS ONE 2025, 20, e0326093. [Google Scholar] [CrossRef]
- Zare, M.; Oladi, A.; Zadeh, S. Investigation of GA3 and kinetin effects on seed germination and seedling growth of wheat under salinity stress. J. Agric. Sci. 2007, 12, 855–865. [Google Scholar]
- Iqbal, H.; Khalid, M.; Tahir, A.; Ahmed, A.; Rasul, E. Gibberellin alleviation of NaCl salinity in chickpea (Cicer arietinum L.). Pak. J. Biol. Sci. 2001, 4, 378–380. [Google Scholar] [CrossRef]
- Ebrahimian, E.; Bybordi, A. Effect of salinity, salicylic acid, silicium and ascorbic acid on lipid peroxidation, antioxidant enzyme activity and fatty acid content of sunflower. Afr. J. Agric. Res. 2012, 7, 3685–3694. [Google Scholar] [CrossRef]
- Stanton, E. The Ability of Salicylic Acid to Reduced the Damaging Effects of Salt Water Stress on Phaseolus vulgaris; California State University: Northridge, CA, USA, 2004. [Google Scholar]
- Tari, I.; Csiszar, J.; Horvath, E.; Poor, P.; Takács, Z.; Szepesi, A. The alleviation of the adverse effects of salt stress in the tomato plant by salicylic acid shows a time-and organ-specific antioxidant response. Acta Biol. Cracoviensia Ser. Bot. 2015, 57, 21–30. [Google Scholar] [CrossRef]
- Shah, S. Effects of salt stress on mustard as affected by gibberellic acid application. Gen. Appl. Plant Physiol. 2007, 33, 97–106. [Google Scholar]
- Shaddad, M.; Abd El-Samad, H.; Mostafa, D. Role of gibberellic acid (GA3) in improving salt stress tolerance of two wheat cultivars. Int. J. Plant Physiol. Biochem. 2013, 5, 50–57. [Google Scholar]
- Ali, A.Y.A.; Ibrahim, M.E.H.; Zhou, G.; Nimir, N.E.A.; Elsiddig, A.M.I.; Jiao, X.; Zhu, G.; Salih, E.G.I.; Suliman, M.S.E.S.; Elradi, S.B.M. Gibberellic acid and nitrogen efficiently protect early seedlings growth stage from salt stress damage in Sorghum. Sci. Rep. 2021, 11, 6672. [Google Scholar] [CrossRef] [PubMed]
- Sedláková, V.; Zeljković, S.Ć.; Štefelová, N.; Smýkal, P.; Hanáček, P. Phenylpropanoid content of chickpea seed coats in relation to seed dormancy. Plants 2023, 12, 2687. [Google Scholar] [CrossRef]
- Ramezani, E.; Sepanlou, M.G.; Badi, H.A.N. The effect of salinity on the growth, morphology and physiology of Echium amoenum Fisch. & Mey. Afr. J. Biotechnol. 2011, 10, 8765–8773. [Google Scholar]
- Chauhan, A.; AbuAmarah, B.A.; Kumar, A.; Verma, J.; Ghramh, H.A.; Khan, K.A.; Ansari, M.J. Influence of gibberellic acid and different salt concentrations on germination percentage and physiological parameters of oat cultivars. Saudi J. Biol. Sci. 2019, 26, 1298–1304. [Google Scholar] [CrossRef]
- Dheeba, B.; Sampathkumar, P.; Kannan, K. Fertilizers and mixed crop cultivation of chromium tolerant and sensitive plants under chromium toxicity. J. Toxicol. 2015, 2015, 367217. [Google Scholar] [CrossRef]
- El-Tayeb, M. Response of barley grains to the interactive e. ect of salinity and salicylic acid. Plant Growth Regul. 2005, 45, 215–224. [Google Scholar] [CrossRef]
- Gutiérrez-Coronado, M.A.; Trejo-López, C.; Larqué-Saavedra, A. Effects of salicylic acid on the growth of roots and shoots in soybean. Plant Physiol. Biochem. 1998, 36, 563–565. [Google Scholar] [CrossRef]
- Khodary, S. Effect of salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt stressed maize plants. Int. J. Agric. Biol. 2004, 6, 5–8. [Google Scholar]
- Egamberdieva, D.; Wirth, S.J.; Shurigin, V.V.; Hashem, A.; Abd_Allah, E.F. Endophytic bacteria improve plant growth, symbiotic performance of chickpea (Cicer arietinum L.) and induce suppression of root rot caused by Fusarium solani under salt stress. Front. Microbiol. 2017, 8, 1887. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.; Açıkbaş, S. Determination of germination and seedling characteristics of common grasspea (Lathyrus sativus L.) genotypes under salt stress. Ege Üniv. Ziraat Fak. Derg. 2024, 61, 425–436. [Google Scholar] [CrossRef]
- Alyemeni, M.N.; Hayat, Q.; Wijaya, L.; Hayat, S. Effect of salicylic acid on the growth, photosynthetic efficiency and enzyme activities of leguminous plant under cadmium stress. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 440–445. [Google Scholar] [CrossRef]
- Zadehbagheri, M. Salicylic acid priming in corn (Zea mays L. var. Sc. 704) reinforces NaCl tolerance at germination and the seedling growth stage. Int. J. Biosci. 2014, 4, 187–197. [Google Scholar]
- Espanany, A.; Fallah, S. Seed germination of dill (Anethum graveolens L.) in response to salicylic acid and halopriming under cadmium stress. Iran. J. Plant Physiol. 2016, 6, 1702–1713. [Google Scholar]
- Zhou, H.; Shi, H.; Yang, Y.; Feng, X.; Chen, X.; Xiao, F.; Lin, H.; Guo, Y. Insights into plant salt stress signaling and tolerance. J. Genet. Genom. 2024, 51, 16–34. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Sankar, B.; Sridharan, R.; Panneerselvam, R. Soil salinity alters growth, chlorophyll content, and secondary metabolite accumulation in Catharanthus roseus. Turk. J. Biol. 2008, 32, 79–83. [Google Scholar]
- Meloni, D.A.; Oliva, M.A.; Martinez, C.A.; Cambraia, J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot. 2003, 49, 69–76. [Google Scholar] [CrossRef]
- Adalatzadeh-Aghdam, S.; Toorchi, M.; Zarei, M. Fennel (Foeniculum vulgare Mill) plants responses to salicylic acid foliar application as chemical priming agent under salt stress. Biol. Bull. 2021, 48, S45–S53. [Google Scholar] [CrossRef]
- Horchani, F.; Bouallegue, A.; Namsi, A.; Abbes, Z. Exogenous application of ascorbic acid mitigates the adverse effects of salt stress in two contrasting barley cultivars through modulation of physio-biochemical attributes, K+/Na+ homeostasis, osmoregulation and antioxidant defense system. Russ. J. Plant Physiol. 2023, 70, 219. [Google Scholar] [CrossRef]
- Gohari, G.; Alavi, Z.; Esfandiari, E.; Panahirad, S.; Hajihoseinlou, S.; Fotopoulos, V. Interaction between hydrogen peroxide and sodium nitroprusside following chemical priming of Ocimum basilicum L. against salt stress. Physiol. Plant. 2020, 168, 361–373. [Google Scholar] [CrossRef]
- Xu, L.; Chen, H.; Zhang, T.; Deng, Y.; Yan, J.; Wang, L. Salicylic acid improves the salt tolerance capacity of Saponaria officinalis by modulating its photosynthetic rate, osmoprotectants, antioxidant levels, and ion homeostasis. Agronomy 2022, 12, 1443. [Google Scholar] [CrossRef]
- Ma, X.; Zheng, J.; Zhang, X.; Hu, Q.; Qian, R. Salicylic acid alleviates the adverse effects of salt stress on Dianthus superbus (Caryophyllaceae) by activating photosynthesis, protecting morphological structure, and enhancing the antioxidant system. Front. Plant Sci. 2017, 8, 600. [Google Scholar] [CrossRef]
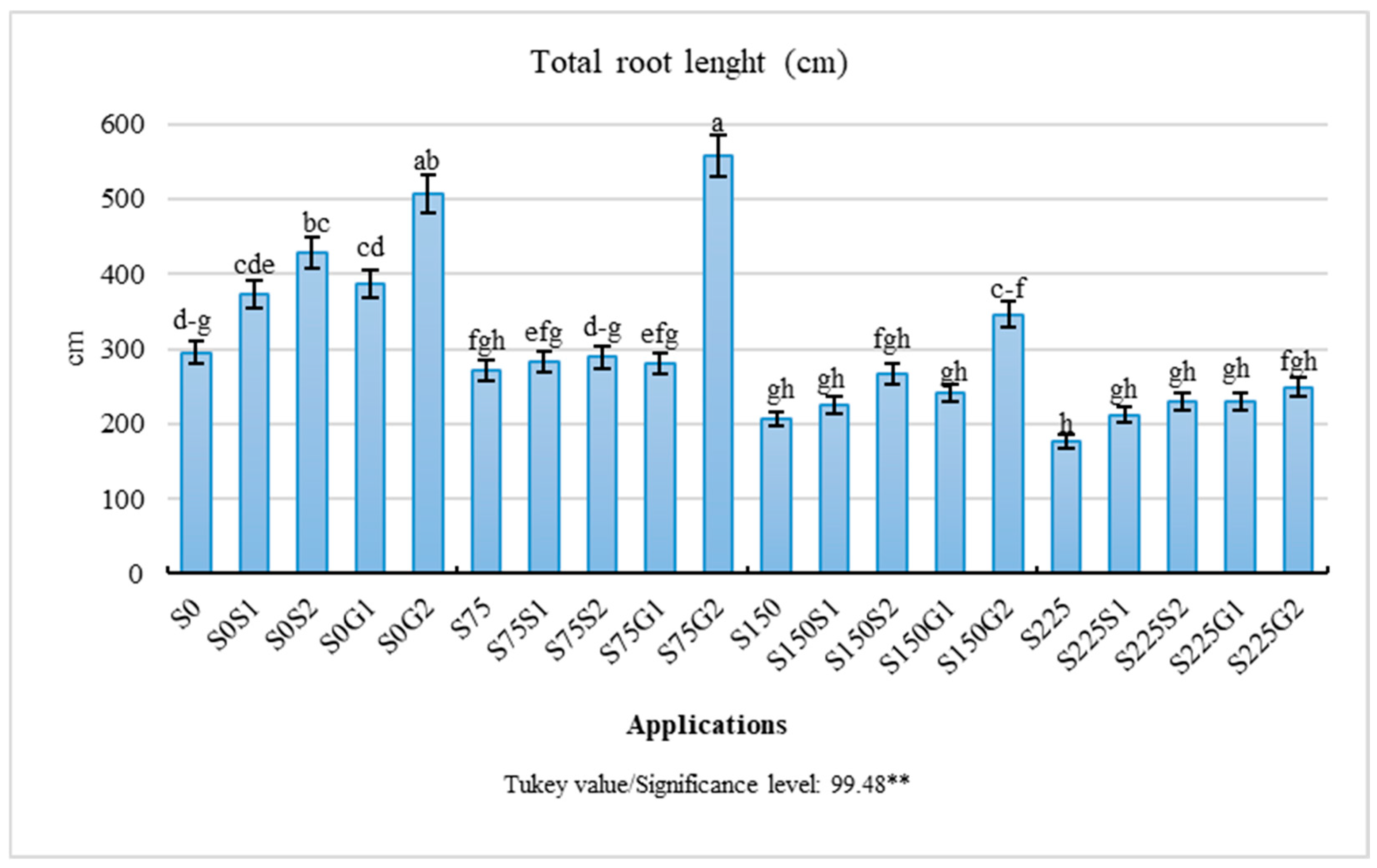
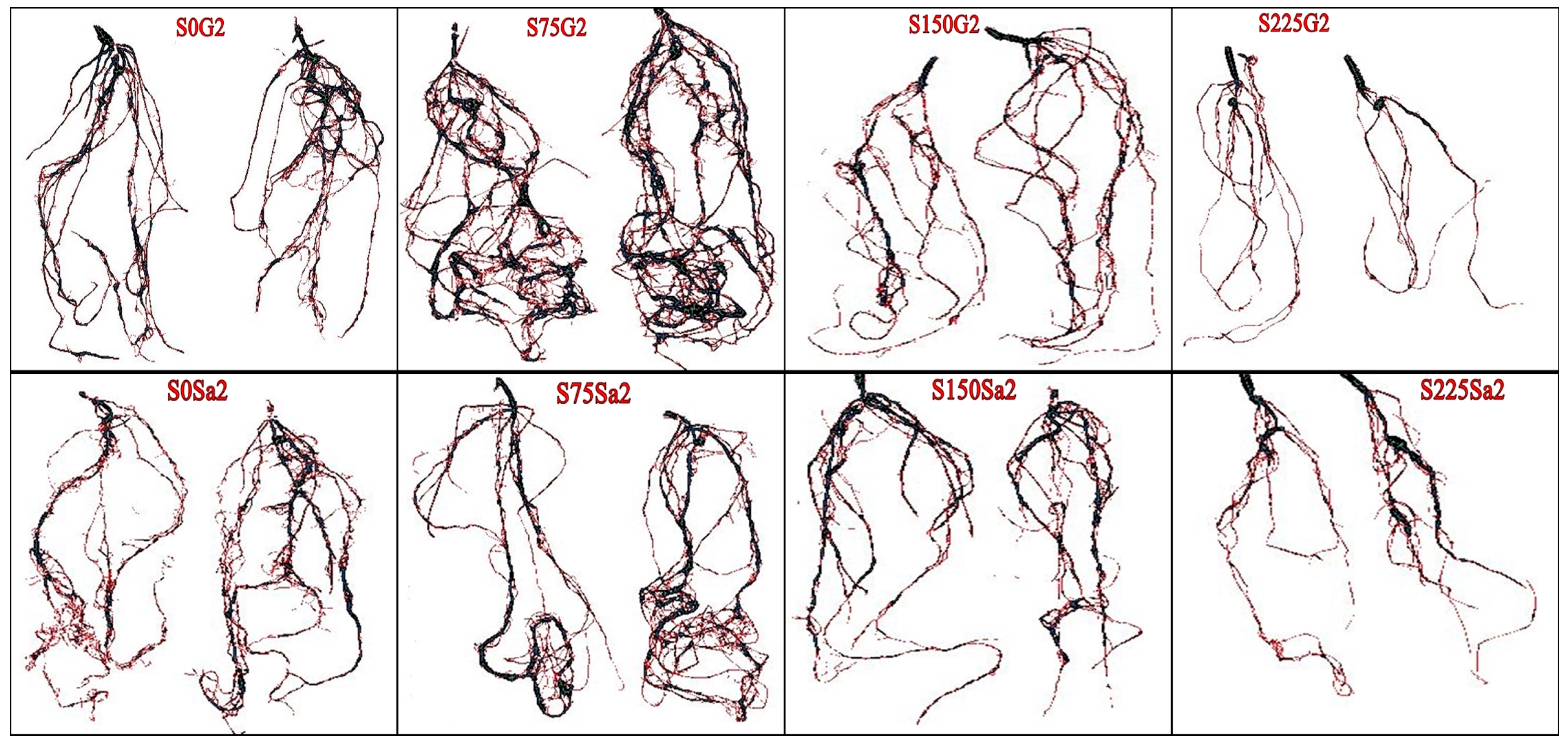
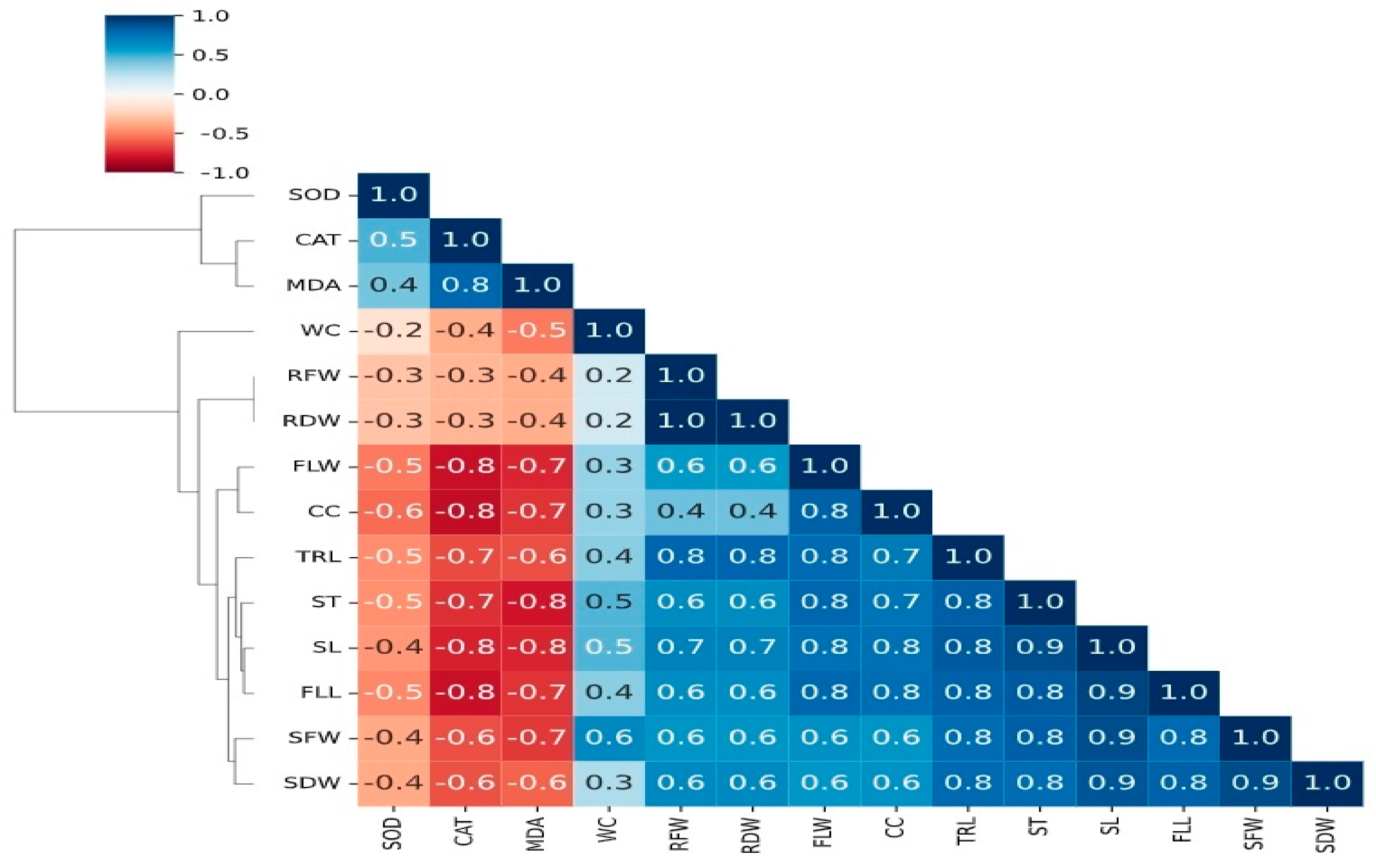
| Treatments | Shoot Length (cm) | Shoot Fresh Weight (g) | Shoot Dry Weight (g) | Root Fresh Weight (g) | Root Dry Weight (g) |
|---|---|---|---|---|---|
| S0 | 87.8 cde | 13.1 cde | 1.34 abc | 2.39 b–f | 0.206 d–j |
| S75 | 82.0 d–g | 10.9 efg | 0.96 b–f | 1.51 d–g | 0.210 d–j |
| S150 | 68.4 f–i | 8.4 e–h | 0.85 b–f | 1.52 d–g | 0.183 f–j |
| S225 | 53.1 i | 5.3 h | 0.57 f | 1.56 d–g | 0.163 hij |
| S0S1 | 100.4 abc | 17.6 bc | 1.38 ab | 2.26 b–f | 0.196 d–j |
| S75S1 | 80.8 d–h | 16.4 bcd | 0.50 ef | 1.08 g | 0.151 ij |
| S150S1 | 67.4 ghi | 8.2 fgh | 0.95 b–f | 1.78 b–g | 0.135 j |
| S225S1 | 69.3 f–i | 7.3 gh | 0.74 def | 1.64 c–g | 0.192 e–j |
| S0S2 | 98.0 bcd | 19.1 b | 1.34 abc | 2.67 abc | 0.311 abc |
| S75S2 | 67.4 ghi | 9.4 e–h | 0.57 f | 1.17 g | 0.193 e–j |
| S150S2 | 69.7 f–i | 10.4 efg | 0.50 f | 1.58 d–g | 0.178 g–j |
| S225S2 | 64.6 hi | 7.9 fgh | 0.77 c–f | 1.98 b–g | 0.222 d–i |
| S0G1 | 93.9 bcd | 16.4 bcd | 1.35 abc | 2.56 a–d | 0.281 bcd |
| S75G1 | 85.4 c–f | 13.2 cde | 0.84 b–f | 1.40 fg | 0.247 c–h |
| S150G1 | 76.1 e–h | 8.8 e–h | 0.80 b–f | 1.48 efg | 0.273 b–e |
| S225G1 | 72.7 e–h | 8.7 e–h | 1.10 b–e | 2.04 b–g | 0.251 c–g |
| S0G2 | 105.3 ab | 24.4 a | 1.76 a | 2.81 ab | 0.380 a |
| S75G2 | 117.4 a | 18.2 b | 1.72 a | 3.57 a | 0.346 ab |
| S150G2 | 87.8 cde | 12.5 def | 1.07 b–f | 2.50 a–e | 0.322 abc |
| S225G2 | 85.1 c–f | 11.1 efg | 1.25 a–d | 2.08 b–g | 0.268 b–f |
| Tukey value/Significance | 17.25 ** | 3.83 ** | 0.60 ** | 1.06 ** | 0.09 ** |
| Treatments | Chlorophyll Content (Spad) | Flag Leaf Length (cm) | Flag Leaf Width (cm) | Stem Thickness (mm) | Water Content |
|---|---|---|---|---|---|
| S0 | 44.3 a | 66.7 a–d | 2.43 a | 5.31 cde | 92.2 |
| S75 | 31.5 efg | 58.3 a–e | 1.79 a–e | 4.96 def | 91.3 |
| S150 | 31.1 efg | 55.0 a–e | 1.63 b–e | 4.52 ef | 91.8 |
| S225 | 30.8 efg | 41.5 e | 1.42 de | 4.15 f | 89.3 |
| S0S1 | 43.2 ab | 76.0 ab | 2.27 abc | 6.62 ab | 92.6 |
| S75S1 | 26.0 g | 55.0 a–e | 1.72 a–e | 5.87 bcd | 93.4 |
| S150S1 | 31.2 efg | 55.2 a–e | 1.66 a–e | 4.95 ef | 92.3 |
| S225S1 | 32.9 d–g | 52.6 b–e | 1.45 de | 4.58 ef | 90.3 |
| S0S2 | 40.1 a–d | 71.6 abc | 2.06 a–d | 6.34 ab | 92.3 |
| S75S2 | 25.4 g | 49.6 cde | 1.69 a–e | 5.97 bc | 92.4 |
| S150S2 | 27.6 fg | 46.2 de | 1.26 e | 4.72 ef | 92.1 |
| S225S2 | 31.0 efg | 49.5 cde | 1.45 de | 4.69 ef | 91.8 |
| S0G1 | 40.1 a–d | 72.3 abc | 2.43 a | 6.30 ab | 92.6 |
| S75G1 | 34.3 c–f | 60.0 a–e | 1.77 a–e | 5.87 bcd | 93.3 |
| S150G1 | 30.5 efg | 53.2 b–e | 1.37 de | 4.80 ef | 92.1 |
| S225G1 | 35.1 b–f | 54.5 a–e | 1.38 de | 4.79 ef | 90.8 |
| S0G2 | 42.0 abc | 78.2 a | 2.28 abc | 6.96 a | 92.6 |
| S75G2 | 41.4 abc | 78.7 a | 2.31 ab | 7.06 a | 92.5 |
| S150G2 | 32.7 d–g | 66.5 a–d | 1.60 b–e | 4.92 ef | 92.5 |
| S225G2 | 37.9 a–e | 63.4 a–e | 1.52 cde | 4.83 ef | 90.3 |
| Tukey value/Significance | 8.13 ** | 24.40 ** | 0.77 ** | 0.91 ** | 4.35 ns |
| Treatments | Superoxide Dismutase (EU/mL) | Catalase (EU/mL) | Malondialdehyde (nmol/mL) |
|---|---|---|---|
| S0 | 166.5 i | 25.4 ij | 0.743 g |
| S75 | 168.6 hi | 38.1 fgh | 0.935 ef |
| S150 | 178.2 abc | 45.3 bcd | 1.283 b |
| S225 | 173.5 ef | 56.6 a | 1.531 a |
| S0S1 | 168.3 hi | 28.4 i | 0.768 g |
| S75S1 | 167.4 hi | 38.3 fgh | 0.763 g |
| S150S1 | 180.3 a | 45.1 cd | 1.075 de |
| S225S1 | 177.2 a–d | 55.8 a | 1.222 bc |
| S0S2 | 168.5 hi | 23.0 j | 0.746 g |
| S75S2 | 177.3 a–d | 36.1 gh | 0.732 g |
| S150S2 | 179.3 ab | 41.8 c–f | 1.017 de |
| S225S2 | 178.5 abc | 50.3 b | 1.123 cd |
| S0G1 | 176.5 b–e | 21.9 j | 0.724 g |
| S75G1 | 174.5 def | 34.8 h | 0.796 fg |
| S150G1 | 172.5 fg | 44.6 cde | 1.299 b |
| S225G1 | 173.4 ef | 46.7 bc | 1.341 b |
| S0G2 | 170.2 gh | 24.7 ij | 0.732 g |
| S75G2 | 169.5 ghi | 39.6 e–h | 0.759 g |
| S150G2 | 172.3 fg | 40.4 d–g | 1.288 b |
| S225G2 | 175.8 cde | 40.7 d–g | 1.329 b |
| Tukey value/Significance | 3.15 ** | 5.09 ** | 0.14 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acikbas, S.; Bulut, A.T. The Effect of Hormonal Priming on Morphological Characteristics and Antioxidant Enzyme Activities in Silage Maize Under Salt Stress. Sustainability 2025, 17, 8917. https://doi.org/10.3390/su17198917
Acikbas S, Bulut AT. The Effect of Hormonal Priming on Morphological Characteristics and Antioxidant Enzyme Activities in Silage Maize Under Salt Stress. Sustainability. 2025; 17(19):8917. https://doi.org/10.3390/su17198917
Chicago/Turabian StyleAcikbas, Semih, and Abidin Tayga Bulut. 2025. "The Effect of Hormonal Priming on Morphological Characteristics and Antioxidant Enzyme Activities in Silage Maize Under Salt Stress" Sustainability 17, no. 19: 8917. https://doi.org/10.3390/su17198917
APA StyleAcikbas, S., & Bulut, A. T. (2025). The Effect of Hormonal Priming on Morphological Characteristics and Antioxidant Enzyme Activities in Silage Maize Under Salt Stress. Sustainability, 17(19), 8917. https://doi.org/10.3390/su17198917






