Development and Characterization of Sustainable Antimicrobial Food Packaging Films with Incorporated Silver Nanoparticles Synthesized from Olive Oil Mill By-Products
Abstract
1. Introduction
Literature Review
2. Materials and Methods
2.1. Silver Nanoparticle (AgNP) Synthesis
2.2. Film Preparation and AgNP Incorporation
2.3. Silver Nanoparticles and Film Characterization
2.4. Antimicrobial Experiments
2.4.1. Bacterial Strains and Preparation of Their Saline Cellular Suspensions
2.4.2. Film Inoculation with the Bacteria and Refrigerated Storage
2.4.3. Detachment of Adhered Bacteria from the Films and Counting of Surviving Cells
2.5. Statistics
3. Results and Discussion
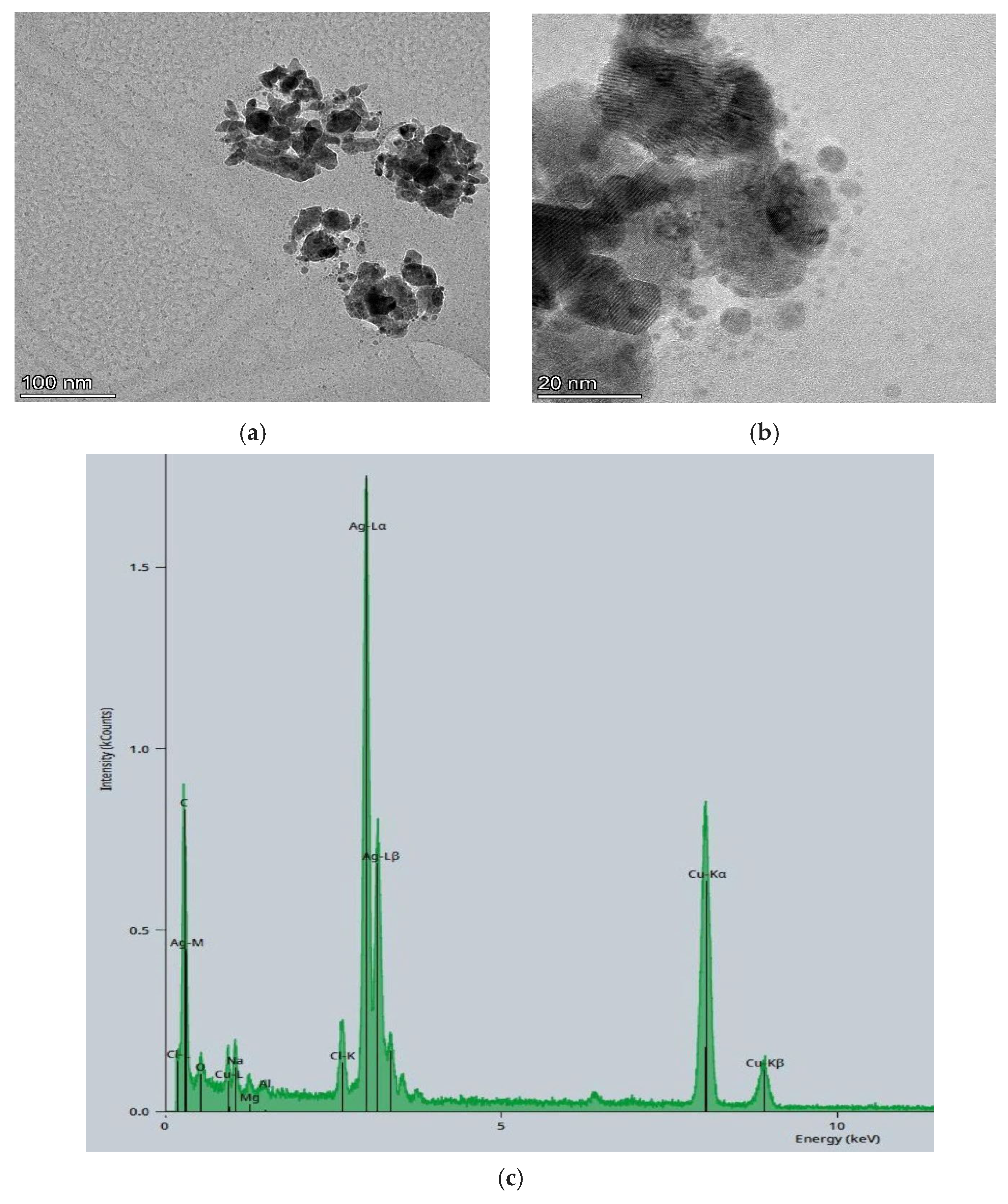
3.1. TEM-EDS Characterization of Silver Nanoparticle Derived from Olive Stone (AgNPs-OSE)
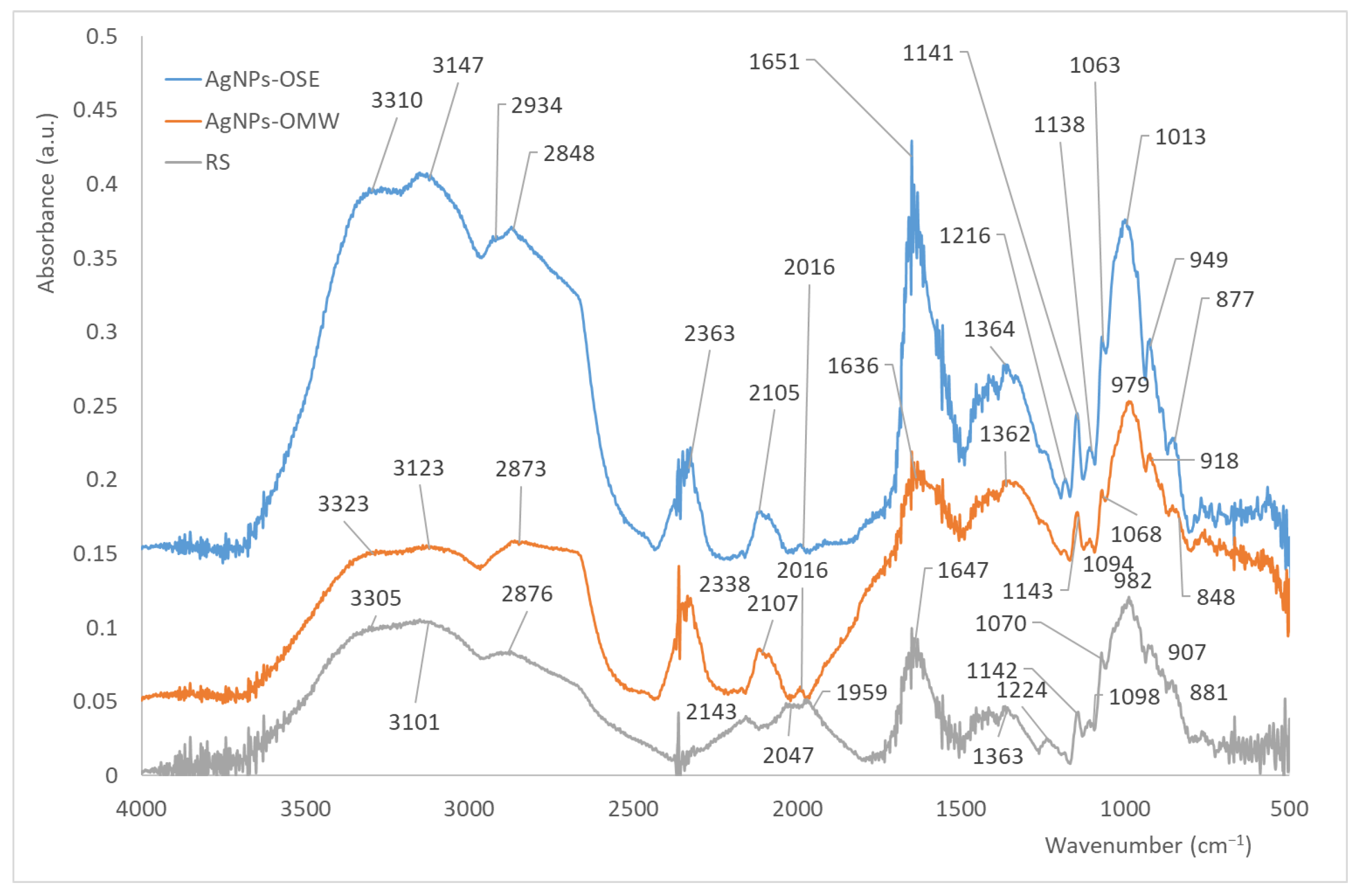
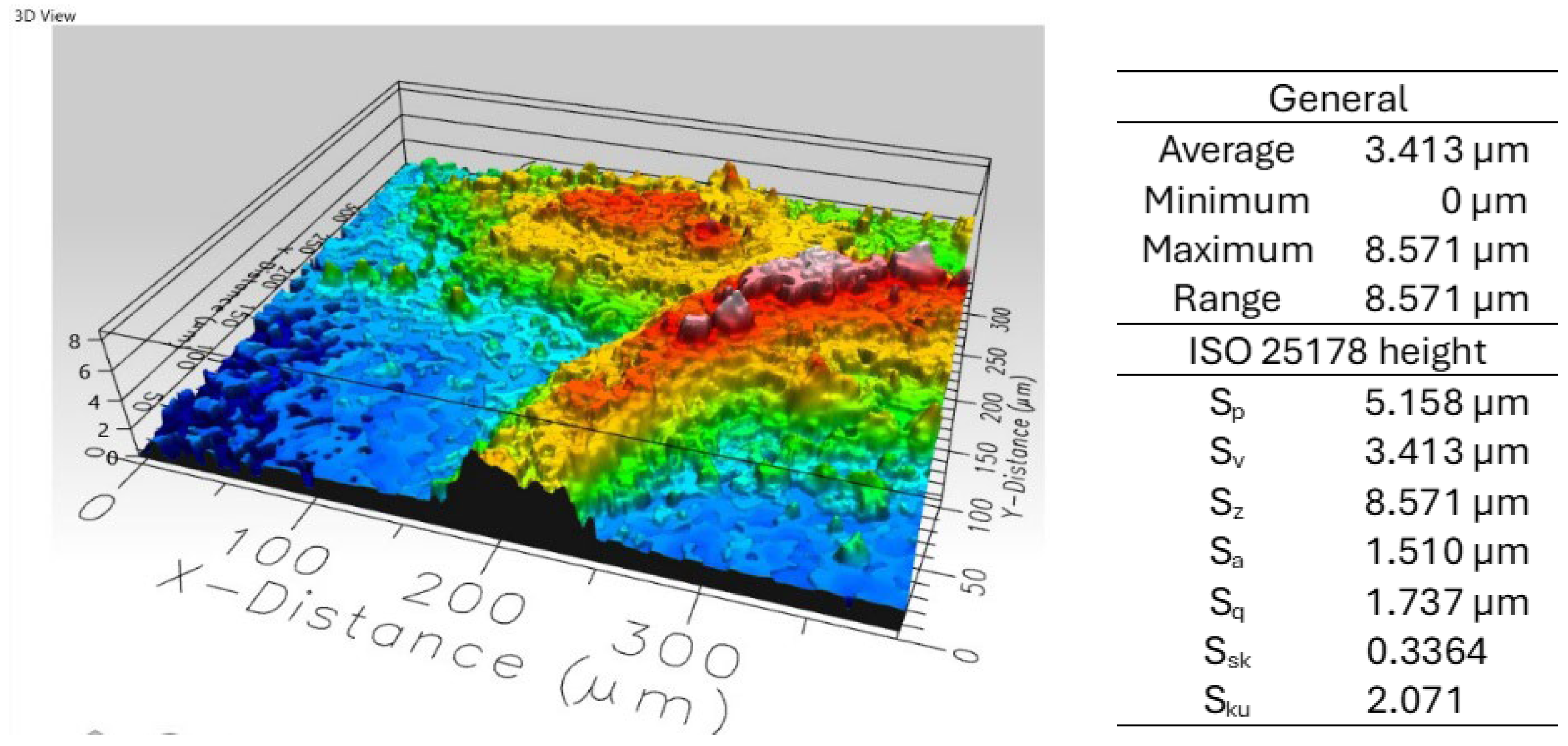
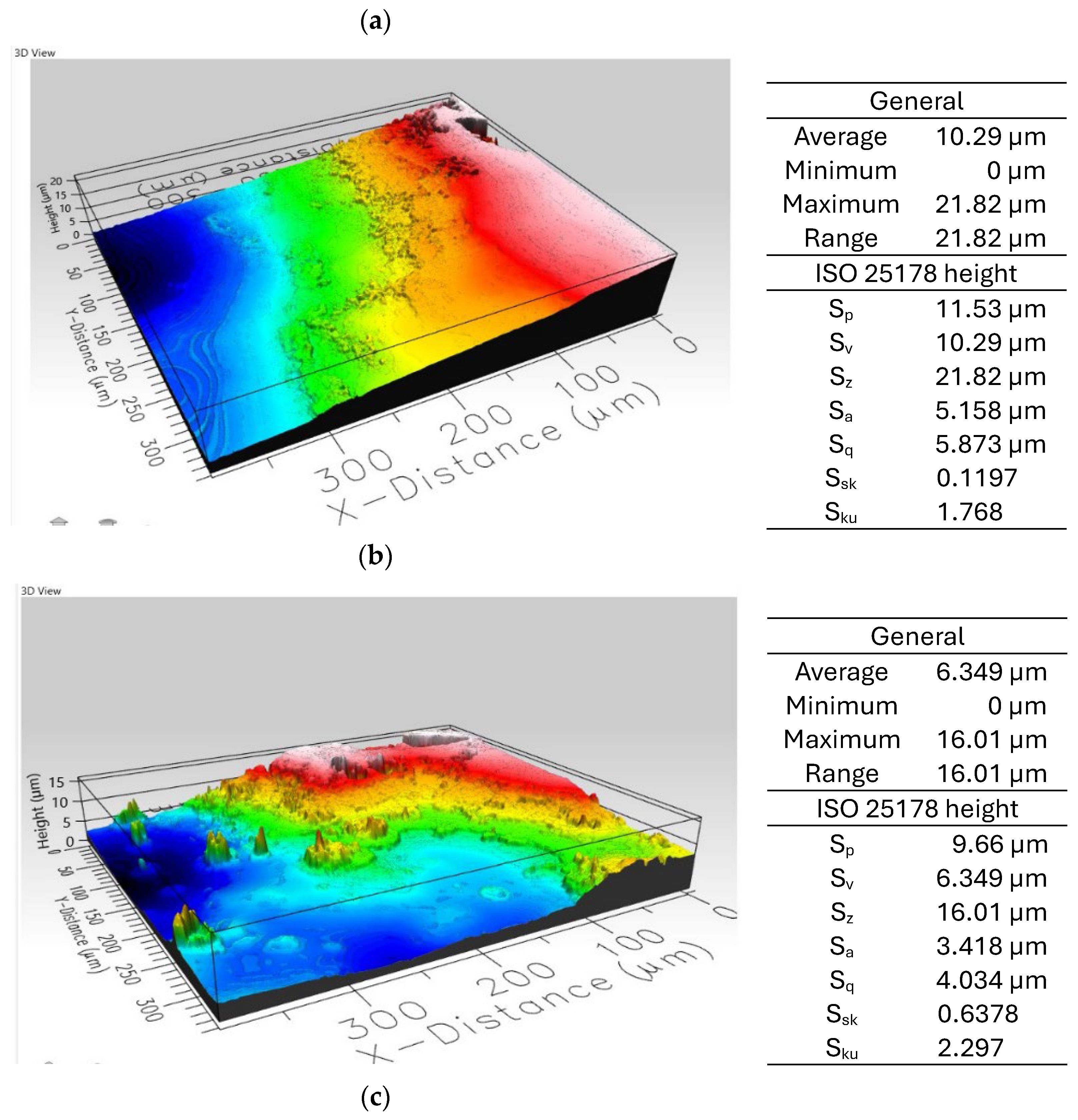
3.2. Film Characterization
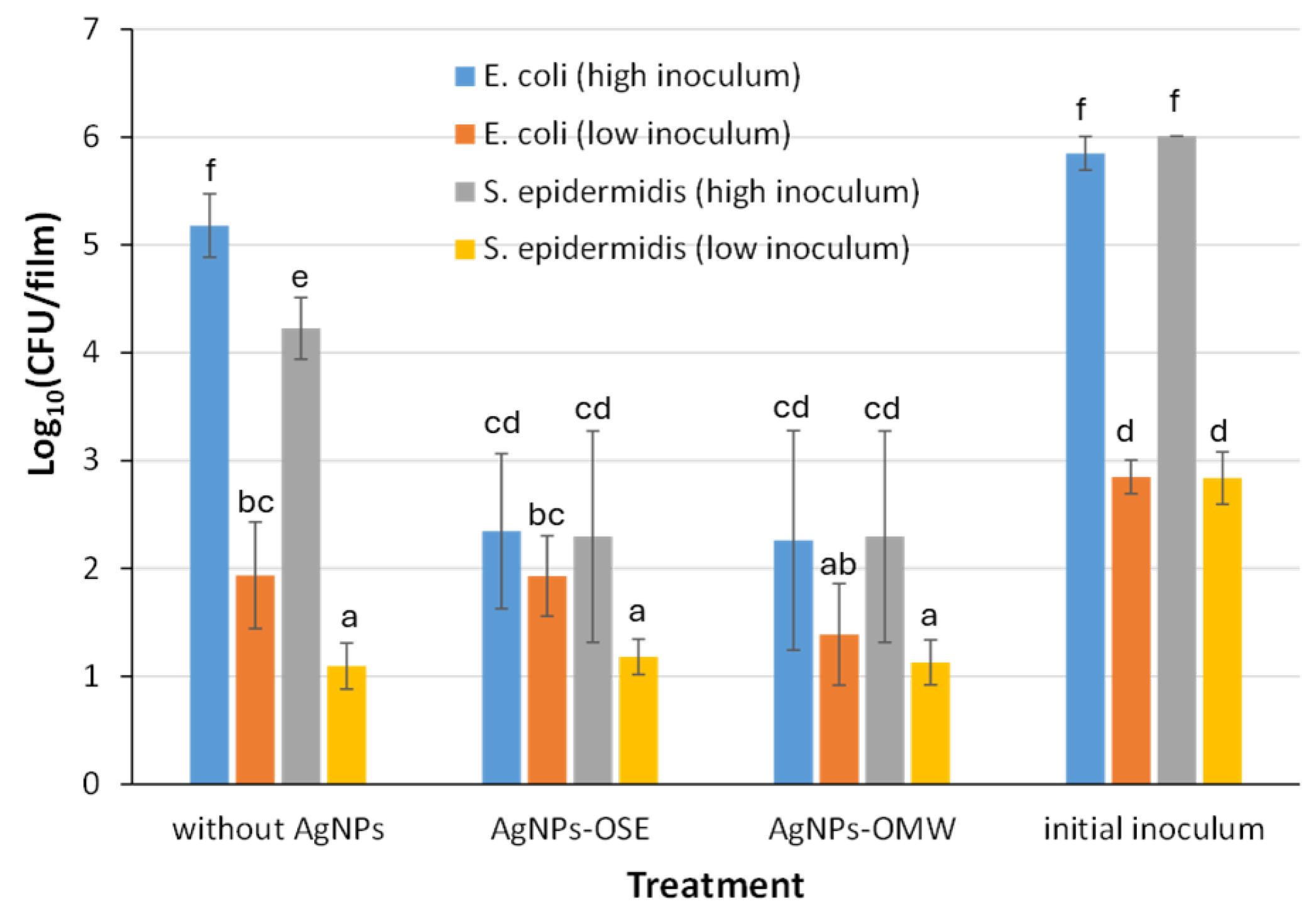
3.3. Films’ Antimicrobial Potential
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macena, M.G.; Carvalho, R.; Cruz-Lopes, L.P.; Guiné, R.P.F. Perceptions and knowledge regarding quality and safety of plastic materials used for food packaging. Open Agric. 2022, 7, 132–146. [Google Scholar] [CrossRef]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. Environmental Impact of Food Packaging Materials: A Review of Contemporary Development from Conventional Plastics to Polylactic Acid Based Materials. Materials 2020, 13, 4994. [Google Scholar] [CrossRef]
- Licciardello, F. Unexpected possible consequences of plastic packaging reuse. Curr. Opin. Food Sci. 2024, 56, 101131. [Google Scholar] [CrossRef]
- Roy, S.; Chawla, R.; Santhosh, R.; Thakur, R.; Sarkar, P.; Zhang, W. Agar-based edible films and food packaging application: A comprehensive review. Trends Food Sci. Technol. 2023, 141, 104198. [Google Scholar] [CrossRef]
- Jang, E.J.; Padhan, B.; Patel, M.; Pandey, J.K.; Xu, B.; Patel, R. Antibacterial and Biodegradable Food Packaging Film from Bacterial Cellulose. Food Control 2023, 153, 109902. [Google Scholar] [CrossRef]
- Rydz, J.; Musiol, M.; Zawidlak-Wegrzyńska, B.; Sikorska, W. Present and Future of Biodegradable Polymers for Food Packaging Applications. Biopolymers for Food Design. In Handbook of Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press Inc.: Cambridge, MA, USA, 2018; pp. 431–467. [Google Scholar]
- Han, G.; Rui, G.; Yu, Z.; Chen, G. Progress on biodegradable films for antibacterial food packaging. E3S Web Conf. 2019, 145, 01036. [Google Scholar] [CrossRef]
- Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.C.; Kaya, D.A.; Andronescu, E. Biodegradable Antimicrobial Food Packaging. Foods 2020, 9, 1438. [Google Scholar] [CrossRef] [PubMed]
- Arruda, T.R.; Machado, G.d.O.; Marques, C.S.; de Souza, A.L.; Pelissari, F.M.; de Oliveira, T.V.; Silva, R.R.A. An Overview of Starch-Based Materials for Sustainable Food Packaging: Recent Advances, Limitations, and Perspectives. Macromol 2025, 5, 19. [Google Scholar] [CrossRef]
- Karnwal, A.; Rauf, A.; Jassim, A.Y.; Selvaraj, M.; Al-Tawaha, A.R.M.S.; Kashyap, P.; Kumar, D.; Malik, T. Advanced starch-based films for food packaging: Innovations in sustainability and functional properties. Food Chem. X 2025, 29, 102662. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, P. A dielectric relaxation study of starch-water and starch-glycerol films. Ionics 2012, 18, 413–423. [Google Scholar] [CrossRef]
- Wu, Y.; Geng, F.; Chang, P.R.; Yu, J.; Ma, X. Effect of agar on the microstructure and performance of potato starch film. Carbohydr. Polym. 2009, 76, 299–304. [Google Scholar] [CrossRef]
- Phan The, D.; Debeaufort, F.; Voilley, A.; Luu, D. Biopolymer interactions affect the functional properties of edible films based on agar, cassava starch and arabinoxylan blends. J. Food Eng. 2009, 90, 548–558. [Google Scholar] [CrossRef]
- Wongphan, P.; Harnkarnsujarit, N. Characterization of starch, agar and maltodextrin blends for controlled dissolution of edible films. Int. J. Biol. Macromol. 2020, 156, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, M.J.; Fromm, K.M.; Akbar Ashkarran, A.; Jimenez de Aberasturi, D.; Larramendi, I.R.D.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Starch/agar—Based functional films integrated with enoki mushroom- mediated silver nanoparticles for active packaging applications. Food Biosci. 2022, 49, 101867. [Google Scholar] [CrossRef]
- Ortega, F.; Giannuzzi, L.; Arce, V.B.; García, M.A. Active composite starch films containing green synthesized silver nanoparticles. Food Hydrocoll. 2017, 70, 152–162. [Google Scholar] [CrossRef]
- Roy, S.; Shankar, S.; Rhim, J.-W. Melanin-mediated synthesis of silver nanoparticles and its use for the preparation of carrageenan-based antibacterial films. Food Hydrocoll. 2019, 88, 237–246. [Google Scholar] [CrossRef]
- Mahuwala, A.A.; Hemant, V.; Meharwade, S.D.; Deb, A.; Chakravorty, A.; Grace, A.N.; Raghavan, V. Synthesis and characterisation of starch/agar nanocomposite films for food packaging application. IET Nanobiotechnol. 2020, 9, 809–814. [Google Scholar] [CrossRef]
- Sadeghi, B.; Jamali, M.; Kia, S.; Amininia, A.; Ghafari, S. Synthesis and characterization of silver nanoparticles for antibacterial activity. Int. J. Nano Dimens. 2010, 1, 119–124. [Google Scholar]
- Zulfiqar, Z.; Khan, R.R.M.; Summer, M.; Saeed, Z.; Pervaiz, M.; Rasheed, S.; Shehzad, B.; Kabir, F.; Ishaq, S. Plant-mediated green synthesis of silver nanoparticles: Synthesis, characterization, biological applications, and toxicological considerations: A review. Biocatal. Agric. Biotechnol. 2024, 57, 103121. [Google Scholar] [CrossRef]
- Wasiq, M.; Ahmed, F.; Saeed, F.; Raza, A.; Abdullah, H.M.; Saleem, S.; Shoukat, L.; Nuzhyma, N.; Afzaal, M. From waste to wellness: Green synthesis of nanoparticles from vegetable waste. Sustain. Environ. 2025, 11, 2491870. [Google Scholar] [CrossRef]
- Mathew, S.S.; Sunny, N.E.; Shanmugam, V. Green synthesis of anatase titanium dioxide nanoparticles using Cuminum cyminum seed extract; effect on Mung bean (Vigna radiata) seed germination. Inorg. Chem. Commun. 2021, 126, 108485. [Google Scholar] [CrossRef]
- Hamimed, S.; Chamekh, A.; Slimi, H.; Chatti, A. How olive mill wastewater could turn into valuable bionanoparticles in improving germination and soil bacteria. Ind. Crops Prod. 2022, 188, 115682. [Google Scholar] [CrossRef]
- Xu, J.; Yıldıztekin, M.; Han, D.; Keskin, C.; Baran, A.; Baran, M.F.; Eftekhari, A.; Aytuğ Ava, C.; İrtegün Kandemir, S.; Bariş Cebe, D.; et al. Biosynthesis, chara-cterization, and investigation of antimicrobial and cytotoxic activities of silver nanoparticles using Solanum tuberosum peel aqueous extract. Heliyon 2023, 9, e19061. [Google Scholar] [CrossRef]
- Daniel, S.; Gladis, L.R. Investigation of Antimicrobial Potential of Silver Nanoparticles Synthesised from Vegetable Peel Extracts. Nanochem. Res. 2024, 9, 242–247. [Google Scholar]
- Tongwanichniyom, S.; Phewrat, N.; Rangsarikorn, N.; Leasen, S.; Luangkamin, S.; Chumnanvej, N. Green synthesis of silver nanoparticles using mature-pseudostem extracts of Alpinia nigra and their bioactivities. Green Process. Synth. 2024, 13, 20230226. [Google Scholar] [CrossRef]
- Elshahat Abdallah, S.; Elmessery, W.M.; Elfallawi, F.E.; Shoueir, K.R. Utilizing agricultural biowaste for food safety: Integrating naturally synthesized silver nanoparticles as antibacterial coating. Inorg. Chem. Commun. 2024, 163, 112337. [Google Scholar] [CrossRef]
- Rigopoulos, N.; Gkaliouri, C.M.; Ioannou, Z.; Sakavitsi, V.; Gournis, D. Green synthesis of silver nanoparticles using olive mill wastewater and olive stones extract and testing their antimicrobial activities against Escherichia coli and Staphylococcus epidermidis. NanoExpress 2024, 5, 015026. [Google Scholar] [CrossRef]
- Rao, M.M.V.; Mohammad, N.; Banerjee, S.; Khanna, P.K. Synthesis and food packaging application of silver nanoparticles: A review. Hybrid Adv. 2024, 6, 100230. [Google Scholar] [CrossRef]
- Basumatary, K.; Daimary, P.; Das, S.K.; Thapa, M.; Singh, M.; Mukherjee, A.; Κumar, S. Lagerstroemia speciosa fruit-mediated synthesis of silver nanoparticles and its application as filler in agar-based nanocomposite films for antimicrobial food packaging. Food Packag. Shelf Life 2018, 17, 99–106. [Google Scholar] [CrossRef]
- Liu, J.; Ma, Z.; Liu, Y.; Zheng, X.; Pei, Y.; Tang, K. Soluble soybean polysaccharide films containing in-situ generated silver nanoparticles for antibacterial food packaging applications. Food Packag. Shelf Life 2022, 31, 100800. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, W.; Choi, J.W.; Bumbudsanpharoke, N.; Ko, S. A Facile Green Fabrication and Characterization of Cellulose-Silver Nanoparticle Composite Sheets for an Antimicrobial Food Packaging. Front. Nutr. 2021, 8, 778310. [Google Scholar] [CrossRef] [PubMed]
- Pandian, H.; Senthilkumar, K.; Venkata Ratnam, M.; Naveenkumar, M.; Samraj, S. Azadirachta indica leaf extract mediated silver nanoparticles impregnated nano composite film (AgNP/MCC/starch/whey protein) for food packaging applications. Environ. Res. 2023, 216, 114641. [Google Scholar] [CrossRef] [PubMed]
- Zhong, K.; Yang, B.; Liu, C.; Xiong, Y.; Wen, Q.; Li, W.; Ren, J. Progress in the application of polysaccharide/nanomaterial composites in food safety. Food Chem. 2025, 495, 146317. [Google Scholar] [CrossRef]
- Akila, V.; Badwaik, L.S. Recent advancement in improvement of properties of polysaccharides and proteins-based packaging film with added nanoparticles: A review. Int. J. Biol. Macromol. 2022, 203, 515–525. [Google Scholar]
- Dejene, B.K. Eco-friendly synthesis of metallic nanoparticles from agri-food waste extracts: Applications in food packaging and healthcare–A critical review. Mater. Today Chem. 2025, 45, 102619. [Google Scholar] [CrossRef]
- Kyziol-Komosinska, J.; Dzieniszewska, A.; Czupioł, J. Behavior of silver species in soil: Ag nanoparticles versus ionic Ag. Molecules 2024, 29, 5531. [Google Scholar] [CrossRef]
- Benoit, R.; Wilkinson, K.J.; Sauvé, S. Partitioning of silver and chemical speciation of free Ag in soils amended with nanoparticles. Chem. Cent. J. 2013, 7, 75. [Google Scholar] [CrossRef]
- Saleeb, N.; Gooneratne, R.; Cavanagh, J.; Bunt, C.; Mofasser Hossain, A.K.M.; Gaw, S.; Robinson, B. The mobility of silver nanoparticles and silver ions in the soil-plant system. J. Environ. Qual. 2019, 48, 1835–1841. [Google Scholar] [CrossRef]
- Pradas del Real, A.E.; Castillo-Michel, H.; Kaegi, R.; Sinnet, B.; Magnin, V.; Findling, N.; Villanova, J.; Carrière, M.; Santaella, C.; Fernańdez-Martıńez, A.; et al. Fate of Ag-NPs in Sewage Sludge after Application on Agricultural Soils. Environ. Sci. Technol. 2016, 50, 1759–1768. [Google Scholar] [CrossRef]
- Maymuna, A.; Annisa, W.D.; Nandiyanto, A.B.D.; Ragadhita, R. Economic Perspective in the Production of Silver Nanoparticles on the Bacterial Cellulose Membrane as Antibacterial Material. Int. J. Energetica 2019, 4, 17–22. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [PubMed]
- Silver Nanoparticles Market. Available online: https://www.alliedmarketresearch.com/silver-nanoparticles-marketA06923#:~:text=The%20global%20silver%20nanoparticles%20market,11.7%25%20from%202025%20to%202034 (accessed on 25 September 2025).
- Silver Nitrate Price. Available online: https://www.metal.com/Silver/202203220001 (accessed on 25 September 2025).
- Silver Nitrate Price. Available online: https://www.alibaba.com/product-detail/Factory-Price-Silver-Nitrate-PowderAgno3_1600290497590.html?spm=a2700.7724857.0.0.1cb77187PiLhA9 (accessed on 25 September 2025).
- Global Cassava Starch Price. Available online: https://dir.tridge.com/prices/cassava-starch (accessed on 25 September 2025).
- Global Agar Price. Available online: https://dir.tridge.com/prices/agar (accessed on 25 September 2025).
- Sodium Hydroxide Price Index. Available online: https://businessanalytiq.com/procurementanalytics/index/sodium-hydroxide-price-index/ (accessed on 25 September 2025).
- Glycerol Price Index. Available online: https://businessanalytiq.com/procurementanalytics/index/glycerol-price-index/ (accessed on 25 September 2025).
- Puritan Springs. Available online: https://shop.puritansprings.com/55-gallon-drum-industrial-distilled-water/ (accessed on 25 September 2025).
- Cano, A.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Development and characterization of active films based on starch-PVA, containing silver nanoparticles. Food Packag. Shelf Life 2016, 10, 16–24. [Google Scholar] [CrossRef]
- Krásniewska, K.; Galus, S.; Gniewosz, M. Biopolymers-Based Materials Containing Silver Nanoparticles as Active Packaging for Food Applications—A Review. Int. J. Mol. Sci. 2020, 21, 698. [Google Scholar] [CrossRef]
- Moulahoum, H.; Ghorbanizamani, F. Navigating the development of silver nanoparticles-based food analysis through the power of artificial intelligence. Food Chem. 2024, 445, 138800. [Google Scholar] [CrossRef]
- Paidari, S.; Tahergorabi, R.; Anari, E.S.; Nafchi, A.M.; Zamindar, N.; Goli, M. Migration of Various Nanoparticles into Food Samples: A Review. Foods 2021, 10, 2114. [Google Scholar] [CrossRef]
- Rybalchenko, N.P.; Demchenko, V.L.; Hnatiuk, T.T.; Vasyliuk, O.M.; Iurzhenko, T.V.; Rybalchenko, T.V.; Sytnyk, I.O.; Shtepa, D.V.; Marynin, A.I. Antimicrobial effect of biopolymer packaging materials with silver nanoparticles for food storage. Microbiol. J. 2024, 86, 30–41. [Google Scholar] [CrossRef]
- Suvarna, V.; Nair, A.; Mallya, R.; Khan, T.; Omri, A. Antimicrobial Nanomaterials for Food Packaging. Antibiotics 2022, 11, 729. [Google Scholar] [CrossRef]
- Trotta, F.; Da Silva, S.; Massironi, A.; Mirpoor, S.F.; Lignou, S.; Ghawi, S.K.; Charalampopoulos, D. Silver bionanocomposites as active food packaging: Recent advances & future trends tackling the food waste crisis. Polymers 2023, 15, 4243. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-water Quality, Recommendations, 3rd ed. 2004, Volume 1. Available online: https://www.who.int/publications/i/item/9789241547611 (accessed on 6 October 2025).
- Carbone, M.; Donia, D.T.; Sabbatella, G.; Antiochia, R. Silver nanoparticles in polymeric matrices for fresh food packaging. J. King Saud Univ. Sci. 2016, 28, 273–279. [Google Scholar] [CrossRef]
- Lambre, C.; Baviera, J.M.B.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, Ε.; Mengelers, M.; et al. Safety assessment of the substance silver nanoparticles for use in food contact materials, Enzymes and Processing Aids (CEP), Scientific opinion. EJ EFSA J. 2021, 19, 6790. [Google Scholar]
- Chadha, U.; Bhardwaj, P.; Selvaraj, S.; Arasu, K.; Praveena, S.; Pavan, A.; Khanna, M.; Singh, P.; Singh, S.; Chakravorty, A.; et al. Current trends and future perspectives of nanomaterials in food packaging application. J. Nanomater. 2022, 2022, 2745416. [Google Scholar] [CrossRef]
- Marchiore, N.G.; Manso, I.J.; Kaufmann, K.C.; Lemes, G.F.; de Oliveira Pizolli, A.P.; Droval, A.A.; Bracht, L.; Gonçalves, O.H.; Leimann, F.V. Migration evaluation of silver nanoparticles from antimicrobial edible coating to sausages. LWT-Food Sci. Technol. 2017, 76, 203–208. [Google Scholar] [CrossRef]
- Thapliyal, D.; Karale, M.; Diwan, V.; Kumra, S.; Arya, R.K.; Verros, G.D. Current Status of Sustainable Food Packaging Regulations: Global Perspective. Sustainability 2024, 16, 5554. [Google Scholar] [CrossRef]
- Rigopoulos, N.; Gkaliouri, C.M.; Sakavitsi, V.; Gournis, D. Full Factorial Design Synthesis of Silver Nanoparticles Using Origanum vulgare. Reactions 2023, 4, 505–517. [Google Scholar] [CrossRef]
- ImageJ. Image Processing and Analysis in Java. Available online: https://imagej.net/ij/ (accessed on 28 September 2025).
- Iwuji, C.; Saha, H.; Ghann, W.; Dotson, D.; Bhuiya, M.A.K.; Parvez, M.S.; Jahangir, Z.M.G.S.; Rahman, M.M.; Chowdhury, F.I.; Uddin, J. Synthesis and characterization of silver nanoparticles and their promising antimicrobial effects. Chem. Phys. Impact 2024, 9, 100758. [Google Scholar] [CrossRef]
- Qi, Z.; Xue, X.; Zhou, H.; Yuan, H.; Li, W.; Yang, G.; Xie, P.; Whang, C. The aqueous assembly preparation of OPs-AgNPs with phenols from olive mill wastewater and its mechanism on antimicrobial function study. Food Chem. 2022, 376, 131924. [Google Scholar] [CrossRef]
- Stozhko, N.; Tarasov, A.; Tamoshenko, V.; Bukharinova, M.; Khamzina, E.; Kolotygina, V. Green Silver Nanoparticles: Plant-Extract-Mediated Synthesis, Optical and Electrochemical Properties. Physchem 2024, 4, 402–419. [Google Scholar] [CrossRef]
- Scroccarello, A.; Junior, B.M.H.; Della Pelle, F.; Ciancetta, J.; Ferraro, G.; Fratini, E.; Valbonetti, L.; Copez, C.C.; Compagnone, D. Effect of phenolic compounds-capped AgNPs on growth inhibition of Aspergillus niger. Colloids Surf. B Biointerfaces 2021, 199, 111533. [Google Scholar] [CrossRef]
- Sudha, P.N.; Gomathi, T.; Vinodhini, P.A.; Nasreen, K. Marine Carbohydrates of Wastewater Treatment. In Advances in Food and Nutrition Research; Se-Kwon, K., Ed.; Academic Press Inc.: Cambridge, MA, USA, 2014; Volume 73, pp. 103–143. [Google Scholar] [CrossRef]
- Robyt, J.F. General Principles, General Properties, Occurrence and Preparation of Carbohydrates. In Glycoscience, Chemistry and Chemical Biology, 2nd ed.; Fraser-Reid, B.O., Tatsuta, K., Thiem, J., Eds.; Springer: Heidelberg, Germany, 2008; Volume 1, p. 72. [Google Scholar]
- Abdullah, A.H.D.; Chalimah, S.; Primadona, I.; Hanantyo, M.H.G. Physical and chemical properties of corn, cassava, and potato starchs. IOP Conf. Ser. Earth Environ. Sci. 2018, 160, 012003. [Google Scholar] [CrossRef]
- Karthik, L.; Kumar, G.; Kirthi, A.V.; Rahuman, A.A.; Bhaskara Rao, K.V. Streptomyces sp. LK3 mediated synthesis of silver nanoparticles and its biomedical application. Bioprocess Biosyst. Eng. 2014, 37, 261–267. [Google Scholar] [CrossRef]
- 79 Niraimathi, K.L.; Sudha, V.; Lavanya, R.; Brindha, P. Biosynthesis of silver nanoparticles using Alternanthera sessilis (Linn.) extract and their antimicrobial, antioxidant activities. Colloids Surf. B Biointerfaces 2013, 102, 288–291. [Google Scholar] [CrossRef]
- 80 Prakash, P.; Gnanaprakasam, P.; Emmanuel, R.; Arokiyaraj, S.; Saravanan, M. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Colloids Surf. B Biointerfaces 2013, 108, 255–259. [Google Scholar] [CrossRef]
- Hemavathy, R.V.; Kattupalli, R.; Sridevi, V.; Talib Hamzah, H.; Prasad, K.S.N.V.; Yaashikaa, P.R. Green synthesis of Pelargonium radens derived silver nanoparticles: Characterization studies and anticancer activity. Mater. Lett. 2024, 372, 137075. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; Iqbal, M.Z.; Rana, M.M.; Hossain, N.; Shahin, M.; Islam, M.A.; Rahman, M.M. Green synthesis of novel green ceramic-based nanoparticles prepared by sol-gel technique for diverse industrial application. Results Surf. Interfaces 2024, 14, 100178. [Google Scholar] [CrossRef]
- Joshi, R.; Joshi, R.; Amanah, H.Z.; Faqeerzada, M.A.; Jayapal, P.K.; Kim, G.; Baek, I.; Park, E.S.; Masithoh, R.E.; Cho, B.K. Quantitative analysis of glycerol concentration in red wine using Fourier transform infrared spectroscopy and chemometrics analysis. Korean J. Agric. Sci. 2021, 48, 299–310. [Google Scholar] [CrossRef]
- Alardhi, S.M.; Salih, H.G.; Ali, N.S.; Khalbas, A.H.; Salih, I.K.; Saady, N.M.C.; Zendehboudi, S.; Albayati, T.M.; Harharah, H.N. Olive stone as an eco-friendly bio-adsorbent for elimination of methylene blue dye from industrial wastewater. Sci. Rep. 2023, 13, 21063. [Google Scholar] [CrossRef]
- Ponsanti, K.; Tangnorawich, B.; Ngernyuang, N.; Pechyen, C. A flower shape-green synthesis and characterization of silver nanoparticles (AgNPs) with different starch as a reducing agent. J. Mater. Res. Technol. 2020, 9, 11003–11012. [Google Scholar] [CrossRef]
- Hanry, E.L.; Redzwan, N.F.M.; Badeges, N.F.A.K.; Surugau, N. Characterization of biofilms developed from alginate extracted from Padina sp. incorporated with calcium chloride (CaCL2). J. Phys. Conf. Ser. 2022, 2314, 012022. [Google Scholar] [CrossRef]
- Agarwal, S.; Hoque, M.; Bandara, N.; Pal, K.; Sarkar, P. Synthesis and characterization of tamarind kernel powder-based antimicrobial edible films loaded with geraniol. Food Packag. Shelf Life 2020, 26, 100562. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, B.; Zhao, S.; Qiao, D.; Xie, F. Plasticized starch/agar composite films: Processing, morphology, structure, mechanical properties and surface hydrophilicity. Coatings 2021, 11, 311. [Google Scholar] [CrossRef]
- Tiwari, H.; Samal, K.; Geed, S.R.; Bera, S.; Das, C.; Mohanty, K. Green synthesis of silver nanoparticles for ultrafiltration membrane surface modification and antimicrobial activity. Sustain. Chem. Clim. Action 2023, 3, 100031. [Google Scholar] [CrossRef]
- Sheng, Y.-J.; Jiang, S.; Tsao, H.-K. Effects of geometrical characteristics of surface roughness on droplet wetting. J. Chem. Phys. 2007, 127, 234704. [Google Scholar] [CrossRef]
- Abbott, J.P.R.; Zhu, H. 3D optical surface profiler for quantifying leaf surface roughness. Surf. Topogr. Metrol. Prop. 2019, 7, 045016. [Google Scholar] [CrossRef]
- Ahmad, S.A.; Das, S.S.; Khatoon, A.; Ansari, M.T.; Afzal, M.; Hasnain, M.S.; Nayak, A.K. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Georgakopoulos-Soares, I.; Papazoglou, E.L.; Karmiris-Obratański, P.; Karkalos, N.E.; Markopoulos, A.P. Surface antibacterial properties enhanced through engineered textures and surface roughness: A review. Colloids Surf. B Biointerfaces 2023, 231, 113584. [Google Scholar] [CrossRef]
- Niu, H.; Gu, J.; Zhang, Y. Bacterial persisters: Molecular mechanisms and therapeutic development. Signal Transduct. Target. Ther. 2024, 9, 174. [Google Scholar] [CrossRef]
- Serrano, S.; Grujović, M.Z.; Marković, K.G.; Barreto-Crespo, M.T.; Semedo-Lemsaddek, T. From dormancy to eradication: Strategies for controlling bacterial persisters in food settings. Foods 2025, 14, 1075. [Google Scholar] [CrossRef]
- Mahmoudi-Qashqay, S.; Zamani-Meymian, M.R.; Sadati, S.J. Improving antibacterial ability of Ti-Cu thin films with co-sputtering method. Sci. Rep. 2023, 13, 16593. [Google Scholar] [CrossRef]



| Samples | L | a | b |
|---|---|---|---|
| AgNPs-OSE | 27.83 ± 0.64 | 11.13 ± 0.64, | 28.03 ± 1.21 |
| AgNPs-OMW | 32.10 ± 0.45 | 7.77 ± 0.34 | 22.27 ± 2.02 |
| RS | 87.46 ± 0.17 | 2.39 ± 1.25 | −9.84 ± 2.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkaliouri, C.M.; Rigopoulos, N.; Ioannou, Z.; Giaouris, E.; Giannakopoulos, K.P.; Ellinas, K. Development and Characterization of Sustainable Antimicrobial Food Packaging Films with Incorporated Silver Nanoparticles Synthesized from Olive Oil Mill By-Products. Sustainability 2025, 17, 8916. https://doi.org/10.3390/su17198916
Gkaliouri CM, Rigopoulos N, Ioannou Z, Giaouris E, Giannakopoulos KP, Ellinas K. Development and Characterization of Sustainable Antimicrobial Food Packaging Films with Incorporated Silver Nanoparticles Synthesized from Olive Oil Mill By-Products. Sustainability. 2025; 17(19):8916. https://doi.org/10.3390/su17198916
Chicago/Turabian StyleGkaliouri, Christina M., Nikolas Rigopoulos, Zacharias Ioannou, Efstathios Giaouris, Konstantinos P. Giannakopoulos, and Kosmas Ellinas. 2025. "Development and Characterization of Sustainable Antimicrobial Food Packaging Films with Incorporated Silver Nanoparticles Synthesized from Olive Oil Mill By-Products" Sustainability 17, no. 19: 8916. https://doi.org/10.3390/su17198916
APA StyleGkaliouri, C. M., Rigopoulos, N., Ioannou, Z., Giaouris, E., Giannakopoulos, K. P., & Ellinas, K. (2025). Development and Characterization of Sustainable Antimicrobial Food Packaging Films with Incorporated Silver Nanoparticles Synthesized from Olive Oil Mill By-Products. Sustainability, 17(19), 8916. https://doi.org/10.3390/su17198916








