Variability in the Carbon Management Index and Enzymatic Activity Under Distinct Altitudes in the Alpine Wetlands of Lesotho
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
2.2. The Alpine Wetland Features
2.3. Climate
2.4. Design of the Study
2.5. Wetland Selection Criteria
2.6. Selection of Sampling Points
2.7. Soil Sampling and Standard Analytical Procedures
2.8. Soil Enzyme Activities
2.9. Soil Organic Carbon Pools and Carbon Management Index
3. Statistical Analysis
4. Results and Discussion
4.1. Impact of Altitudinal Variations on the Soil Bulk Density, Particle Size Distribution and Texture
4.2. Impact of Altitudinal Variations on Soil Physico-Chemical Attributes
4.3. Impact of Altitudinal Variants on Soil Enzyme Activity
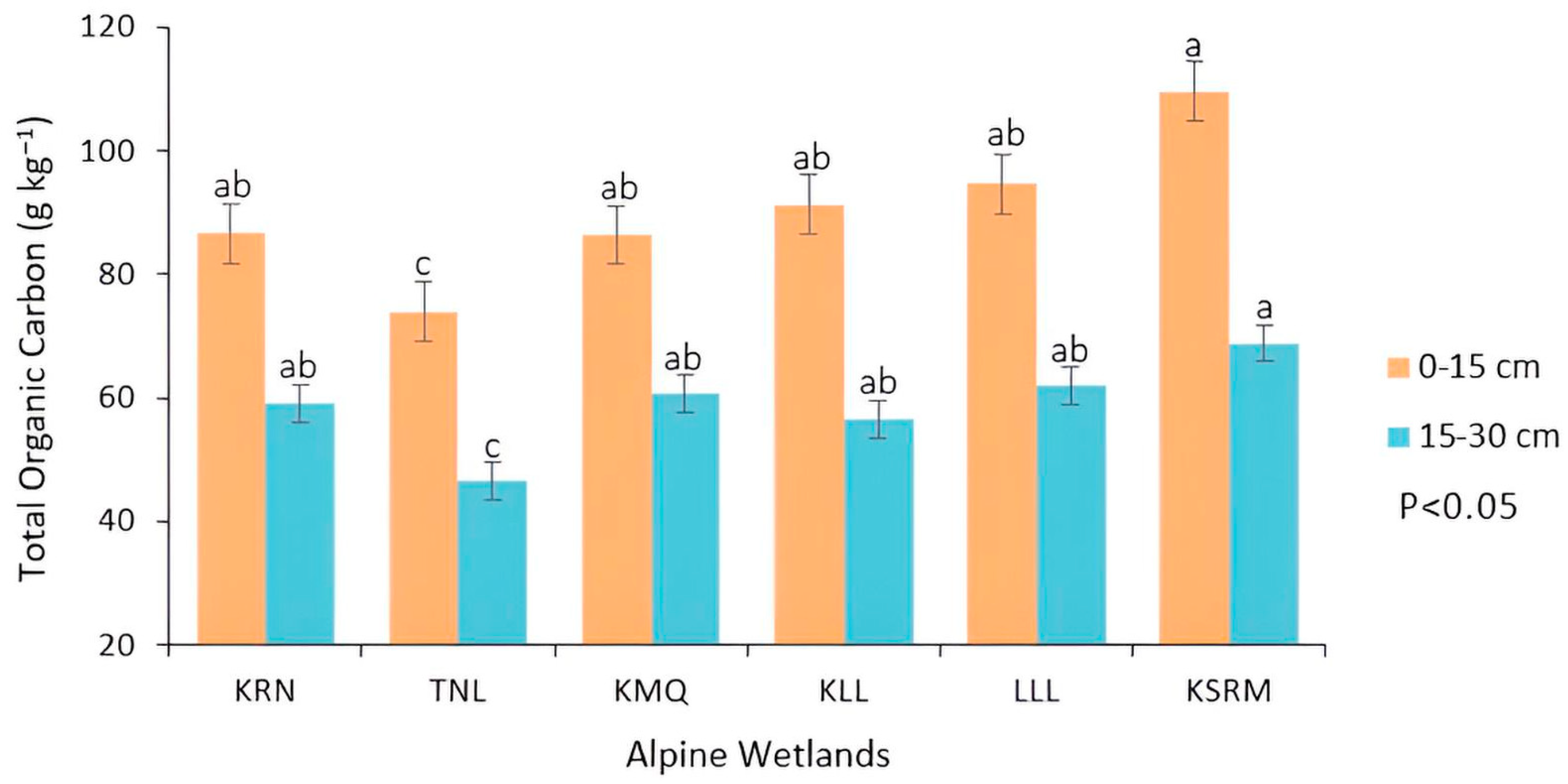
4.4. Impact of Altitudinal Variations in Soil Organic Carbon (SOC) Pools and Total Organic Carbon (TOC)
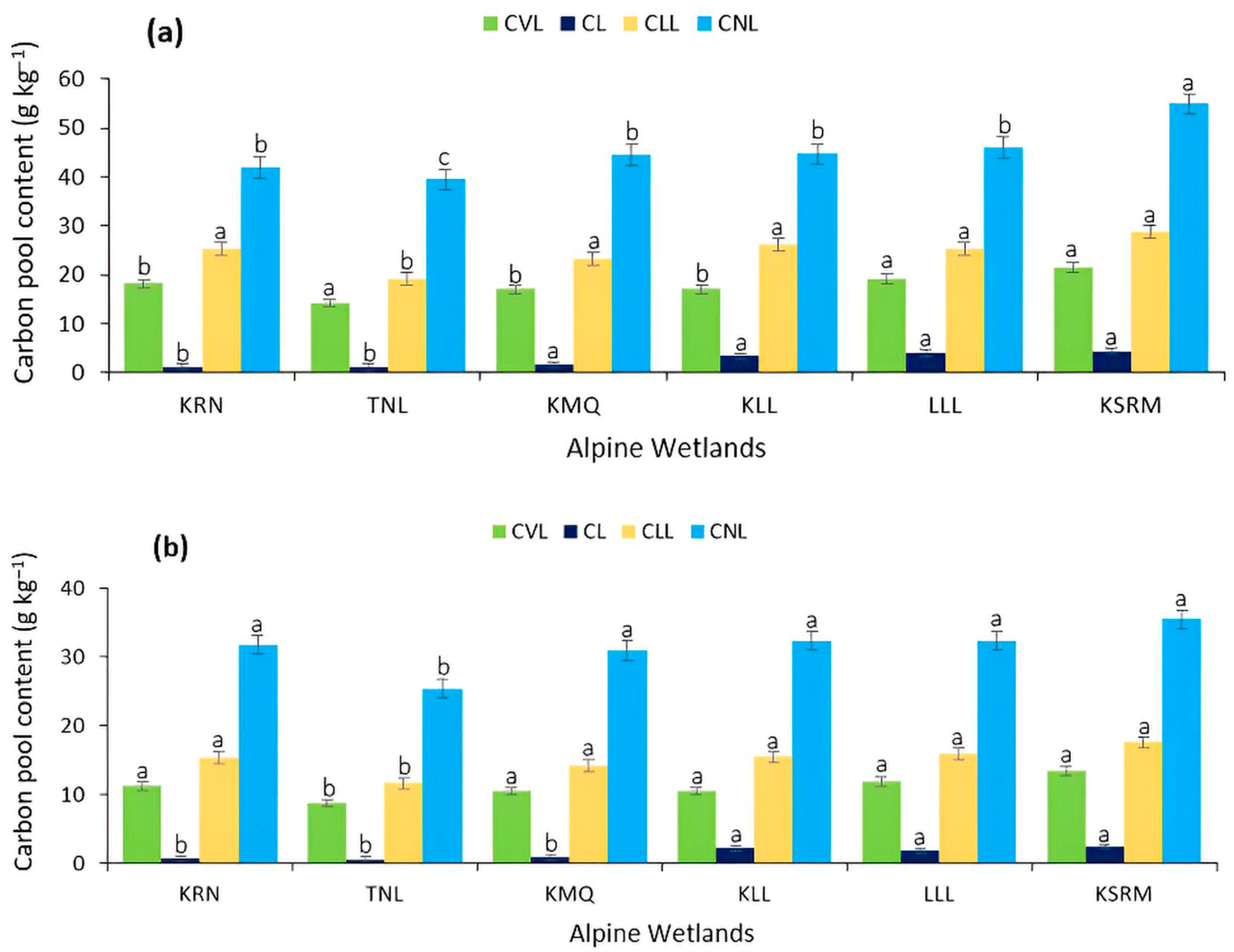
4.5. Active and Passive Pools of Carbon as Influenced by Altitudinal Variations in Alpine Wetlands
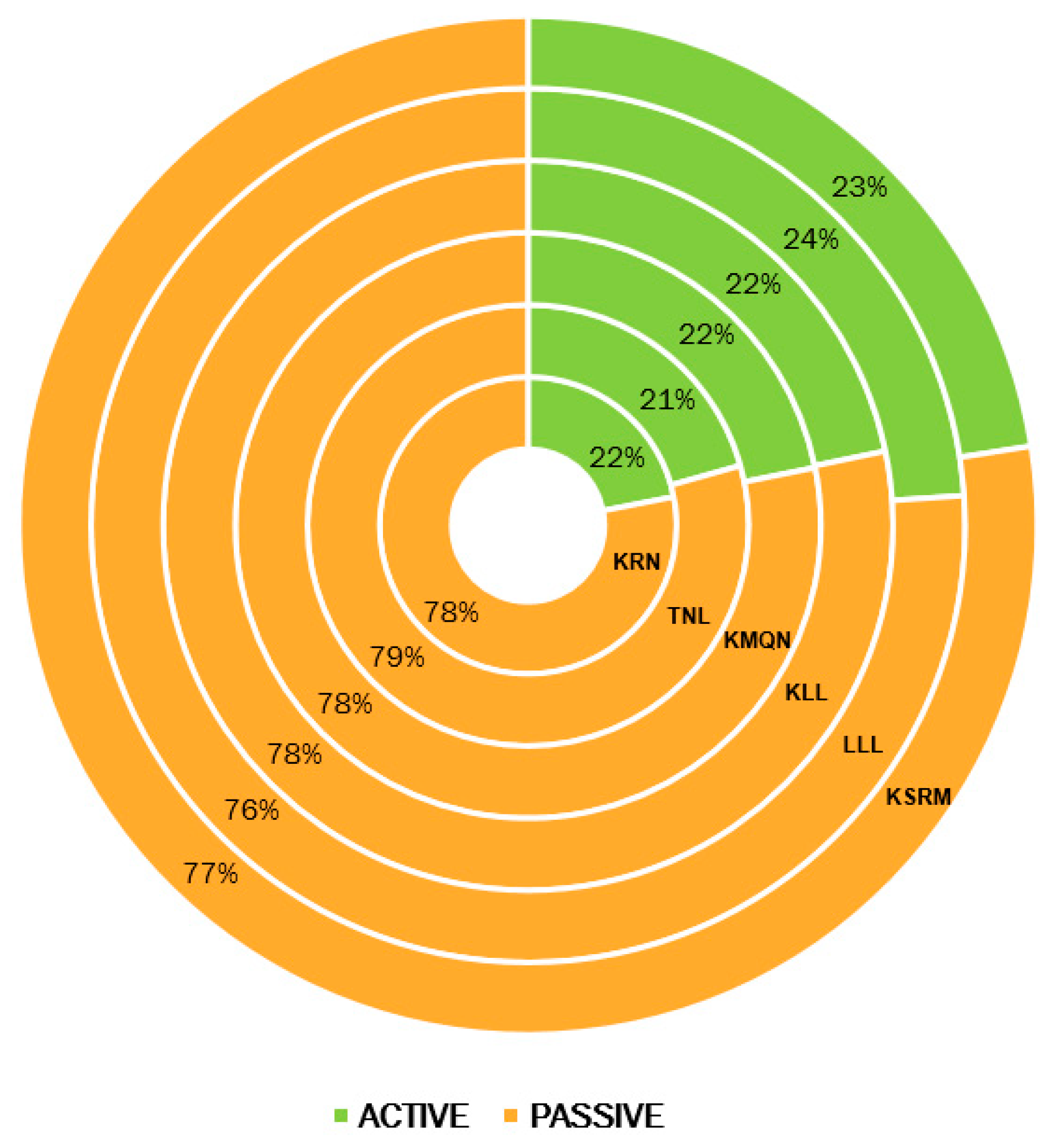
4.6. Percent Contribution of Active and Passive Pools of Carbon to Total Organic Carbon as Influenced by Altitudinal Variations in Alpine Wetlands
4.7. Impact of Altitudinal Variations on the Lability Index, Carbon Pool Index and Carbon Management Index
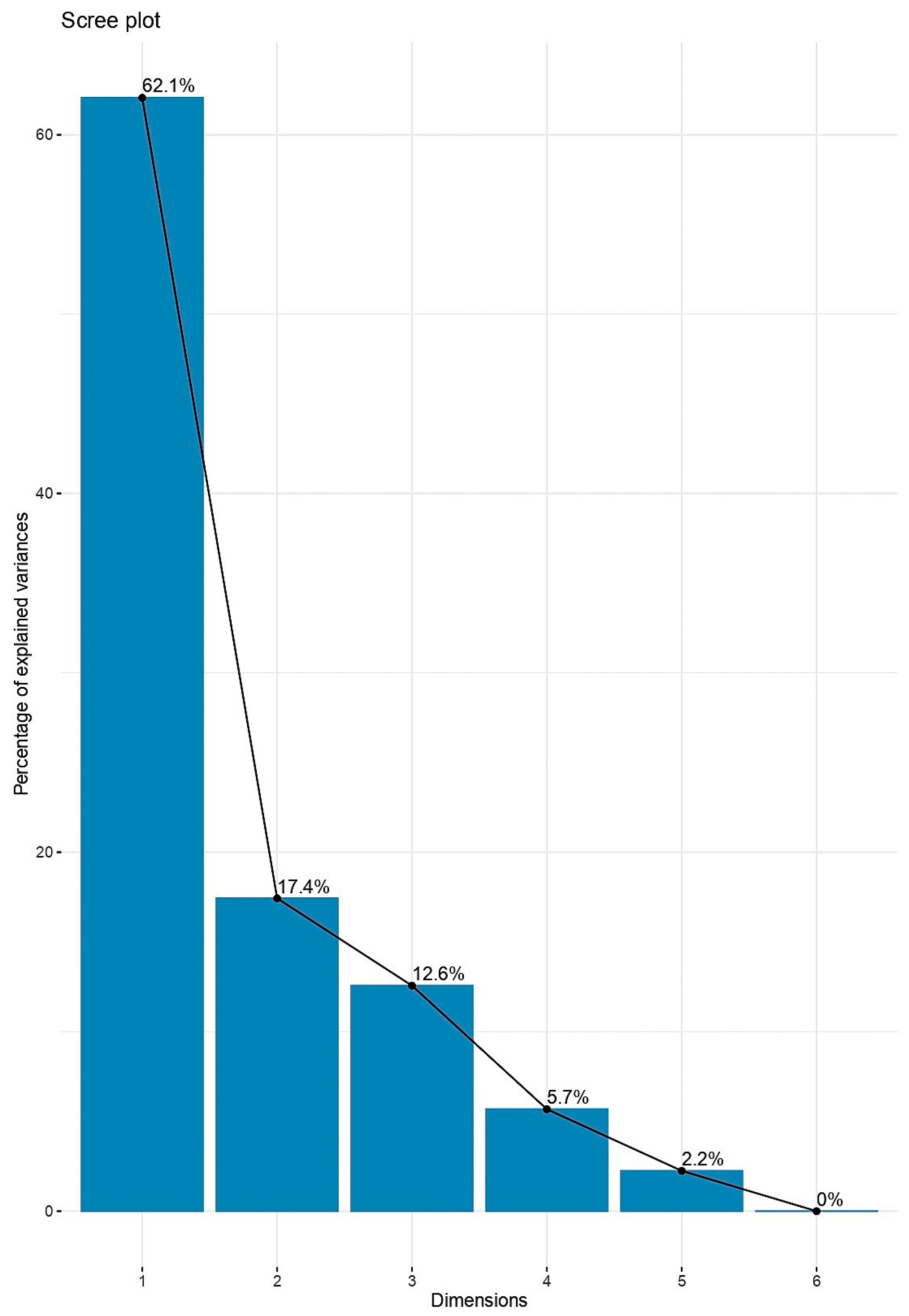
4.8. Principal Component Analysis (PCA) and Variable Selection
5. Conclusions
6. Future Line of Work
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.Y.; Wei, P.J.; Wu, T.H.; Wu, Q.B.; Luo, F.D. Effect of permafrost degradation on carbon sequestration of alpine ecosystems. Sci. Total Environ. 2023, 899, 165642. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, J.; Li, L.; Zhang, L.; Lu, H.; Jiang, X.; Chen, X.; Han, Z.; Dang-zhi, C.; Wang, H.; et al. Evaluation of alpine wetland ecological degradation based on alpine wetland degradation index: A case study in the first meander of the yellow river. Ecol. Indic. 2024, 158, 111414. [Google Scholar] [CrossRef]
- Reza Maddahi. 2015. Carbon Capture and Storage Under the Paris Agreement: A Legal Appraisal of Compatibility and Opportunities. Available online: https://unfccc.int/process/the-paris-agreement/long-term-strategies (accessed on 19 December 2020).
- UNCCD. The Multiple Impacts of Conflict on Land Degradation. 2019. Available online: https://knowledge.unccd.int/publications/multiple0impacts-conflict-land-degradation (accessed on 1 July 2025).
- FAO. Soil Erosion Key Driver of Wetland Degradation in Lesotho; FAO: Rome, Italy, 2022. [Google Scholar]
- Ogola, H.J.O.; Ijoma, G.N.; Edokpayi, J.N. Exploring the dichotomy: Shotgun metagenomics reveals diversity of beneficial and pathogenic protist community in arid wetlands of northeastern South Africa. Sci. Total Environ. 2024, 946, 174306. [Google Scholar] [CrossRef]
- Bentley, L.K.; Robertson, M.P.; Barker, N.P. Range contraction to a higher elevation: The likely future of the montane vegetation in South Africa and Lesotho. Biodivers. Conserv. 2019, 28, 131–153. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ryan, M.E.; Hamlet, A.F.; Palen, W.J.; Lawler, J.J.; Halabisky, M. Projecting the hydrologic impacts of climate change on montane wetlands. PLoS ONE 2015, 10, e0136385. [Google Scholar] [CrossRef] [PubMed]
- Mofutsanyana, S.S.; Collins, N.B.; Adelabu, S.A.; Chatanga, P.; Sieben, E.J. Changes in plant functional composition of wetland vegetation along an aridity gradient on the Highveld plateau of South Africa. Appl. Veg. Sci. 2020, 23, 622–634. [Google Scholar] [CrossRef]
- Nthebere, K.; Tata, R.; Bhimireddy, P.; Chandran, L.P.; Gudapati, J.; Admala, M.; Sinha, N.K.; Srikanth, T.B.; Prasad, K. Impact of Conservation Agriculture on Soil Quality and Cotton–Maize System Yield in Semi-Arid India. Sustainability 2025, 17, 978. [Google Scholar] [CrossRef]
- Kumar, S.; David-Raj, A.; Kalambukattu, J.G.; Chatterjee, U. Climate change impact on land degradation and soil erosion in hilly and mountainous landscape: Sustainability issues and adaptation strategies. In Ecological Footprints of Climate Change: Adaptive Approaches and Sustainability; Springer International Publishing: Cham, Switzerland, 2023; pp. 119–155. [Google Scholar]
- Padbhushan, R.; Kumar, U.; Sharma, S.; Rana, D.S.; Kumar, R.; Kohli, A.; Kumari, P.; Parmar, B.; Kaviraj, M.; Sinha, A.K. Impact of land-use changes on soil properties and carbon pools in India: A meta-analysis. Front. Environ. Sci. 2022, 9, 794866. [Google Scholar] [CrossRef]
- Sainepo, B.M.; Gachene, C.K.; Karuma, A. Assessment of soil organic carbon fractions and carbon management index under different land use types in Olesharo Catchment, Narok County, Kenya. Carbon Balance Manag. 2018, 13, 4. [Google Scholar] [CrossRef]
- Hill, B.H.; Elonen, C.M.; Herlihy, A.T.; Jicha, T.M.; Serenbetz, G. Microbial ecoenzyme stoichiometry, nutrient limitation, and organic matter decomposition in wetlands of the conterminous United States. Wetl. Ecol. Manag. 2018, 26, 425–439. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Fu, X.; Liu, X.; Shen, J.; Wang, Y.; Wu, J. Three-dimensional spatial variability in soil microorganisms of nitrification and denitrification at a row-transect scale in a tea field. Soil Biol. Biochem. 2016, 103, 452–463. [Google Scholar] [CrossRef]
- Martín-Sanz, A.; Rueda, S.; García-Carneros, A.B.; González-Fernández, S.; Miranda-Fuentes, P.; Castuera-Santacruz, S.; Molinero-Ruiz, L. Genetics, host range, and molecular and pathogenic characterization of Verticillium dahliae from sunflower reveal two differentiated groups in Europe. Front. Plant Sci. 2018, 9, 288. [Google Scholar] [CrossRef]
- Cui, Q.; Yang, H.; Wang, G.; Ma, J.; Feng, L.; Liu, J. Response of soil carbon fractions and enzyme activities to mowing management on in a coastal wetland of the yellow river delta. Front. Mar. Sci. 2022, 9, 993181. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, Y.; Li, G.; Han, Y.; Li, X.; Chen, Y. Influences of environmental variables and their interactions on Chinese farmland soil organic carbon density and its dynamics. Land 2022, 11, 208. [Google Scholar] [CrossRef]
- Munoz, M.A.; Faz, A.; Mermut, A.R. Soil carbon reservoirs at high-altitude ecosystems in the Andean plateau. In Climate Change Impacts on High-Altitude Ecosystems; Springer International Publishing: Cham, Switzerland, 2015; pp. 135–153. [Google Scholar]
- Schmitz, G.; Rooyani, F. Lesotho Geology, Geomorphology, Soils; National University of Lesotho: Roma, Lesotho, 1987; p. 204. [Google Scholar]
- Kleynhans, C.J. A qualitative procedure for the assessment of the habitat integrity status of the Luvuvhu River (Limpopo system, South Africa). J. Aquat. Ecosyst. Health 1996, 5, 41–54. [Google Scholar] [CrossRef]
- Macfarlane, D.; Kotze, D.; Ellery, W.; Walters, D.; Koopman, V.; Goodman, P.; Goge, M. WET-Health: A Technique for Rapidly Assessing Wetland Health; Water Research Commission: Pretoria, South Africa, 2009; pp. 235–247. [Google Scholar]
- Malebajoa, M. Climate Change Impacts on Crop Yields and Adaptive Measures for Agricultural Sector in the Lowlands of Lesotho. Master’s Thesis, Department of Physical Geography and Ecosystem Analysis, Lund University, Lund, Sweden, 2010. Available online: https://lup.lub.lu.se/student-papers/search/publication/2438787 (accessed on 1 July 2025).
- Paulsen, S.G.; Larsen, D.P.; Kaufmann, P.R.; Whittier, T.R.; Baker, J.R. EMAP-Surface Waters Monitoring and Research Strategy (No. PB-91-168518/XAB.; EPA-600/3-91/022); Environmental Protection Agency, Environmental Research Lab: Corvallis, OR, USA, 1991; p. 91. [Google Scholar]
- Gee, W.G.; Or, D. Particle-Size Analysis. In Methods of Soil Analysis; Dane, J., Topp, G.C., Eds.; Book Series: 5. Part 4; Soil Science Society of America: Madison, WI, USA, 2002; pp. 255–293. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis. In An Advanced Course, 2nd ed.; University of Wisconsin: Madison, WI, USA, 1973. [Google Scholar]
- Blake, G.R.; Hartge, K.H. Bulk Density. In Methods of Soil Analysis: Part 1 Physical and Mineralogical Methods; American Society of Agronomy, Inc.: Madison, WI, USA, 1986; Volume 5, pp. 363–375. [Google Scholar]
- Walkley, A.; Black, C.A. Estimation of organic carbon by chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Wu, X.; Yao, Z.; Brüggemann, N.; Shen, Z.Y.; Wolf, B.; Dannenmann, M.; Butterbach-Bahl, K. Effects of soil moisture and temperature on CO2 and CH4 soil–atmosphere exchange of various land use/cover types in a semiarid grassland in Inner Mongolia, China. Soil Biol. Biochem. 2010, 42, 773–787. [Google Scholar] [CrossRef]
- Casida, L.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Green, V.S.; Stott, D.E.; Diack, M. Assay for fluorescein diacetate hydrolytic activity: Optimization for soil samples. Soil Biol. Biochem. 2006, 38, 693–701. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Bueis, T.; Turrión, M.B.; Bravo, F.; Pando, V.; Muscolo, A. Factors determining enzyme activities in soils under Pinus halepensis and Pinus sylvestris plantations in Spain: A basis for establishing sustainable forest management strategies. Ann. For. Sci. 2018, 75, 34. [Google Scholar] [CrossRef]
- Daunoras, J.; Kačergius, A.; Gudiukaitė, R. Role of soil microbiota enzymes in soil health and activity changes depending on climate change and the type of soil ecosystem. Biology 2024, 13, 85. [Google Scholar] [CrossRef]
- Chan, K.Y.; Bowman, A.; Oates, A. Oxidizible organic carbon fractions and soil quality changes in an oxic paleustalf under different pasture leys. Soil Sci. 2001, 166, 61–67. [Google Scholar] [CrossRef]
- Jha, P.; Biswas, A.K.; Lakaria, B.L.; Saha, R.; Singh, M.; Rao, A.S. Predicting total organic carbon content of soils from Walkley and Black analysis. Commun. Soil Sci. Plant Anal. 2014, 45, 713–725. [Google Scholar] [CrossRef]
- Blair, G.J.; Lefroy, R.D.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural system. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Panse, V.G.; Sukhatme, P.V. Statistical Methods for Agricultural Works; ICAR: New Delhi, India, 1978. [Google Scholar]
- Mohanty, M. SQI CAL Software—A Tool for Soil Health Assessment; Division of Soil Physics, Indian Institute of Soil Science: Bhopal, India, 2020. [Google Scholar]
- Eyayu, M.; Heluf, G.; Tekalign, M.; Mohammed, A. Effects of land-use change on selected soil properties in the Tera Gedam Catchment and adjacent agroecosystems, north–west Ethiopia. Ethiop. J. Nat. Resour. 2009, 11, 35–62. [Google Scholar]
- Reichert, J.M.; Gubiani, P.I.; dos Santos, D.R.; Reinert, D.J.; Aita, C.; Giacomini, S.J. Soil properties characterization for land-use planning and soil management in watersheds under family farming. Int. Soil Water Conserv. Res. 2022, 10, 119–128. [Google Scholar] [CrossRef]
- Håkansson, I.; Lipiec, J. A review of the usefulness of relative bulk density values in studies of soil structure and compaction. Soil Tillage Res. 2000, 53, 71–85. [Google Scholar] [CrossRef]
- Teron, G.; Bordoloi, R.; Paul, A.; Singha, L.B.; Tripathi, O.P. Effect of Altitude on Soil Physico-Chemical Properties and Microbial Biomass Carbon in the Eaglenest Wildlife Sanctuary of Arunachal Pradesh. Geol. Ecol. Landsc. 2024, 1–19. [Google Scholar] [CrossRef]
- Charan, G.; Bharti, V.K.; Jadhav, S.E.; Kumar, S.; Acharya, S.; Kumar, P.; Srivastava, R.B. Altitudinal variations in soil physico-chemical properties at cold desert high altitude. J. Soil Sci. Plant Nutr. 2013, 13, 267–277. [Google Scholar] [CrossRef]
- Kamal, A.; Mian, I.A.; Akbar, W.A.; Rahim, H.U.; Irfan, M.; Ali, S.; Alrefael, A.F.; Zaman, W. Effects of Soil Depth and Altitude on Soil Texture and Soil Quality Index. Appl. Ecol. Environ. Res. 2023, 21, 4135–4142. [Google Scholar] [CrossRef]
- Ramesh, T.; Bolan, N.S.; Kirkham, M.B.; Wijesekara, H.; Kanchikerimath, M.; Rao, C.S.; Sandeep, S.; Rinklebe, J.; Ok, Y.S.; Choudhury, B.U.; et al. Soil organic carbon dynamics: Impact of land use changes and management practices: A review. Adv. Agron. 2019, 156, 1–107. [Google Scholar]
- Wang, J.; Xiao, S.; Hayat, K.; Liao, X.; Chen, J.; Zhang, L.; Xie, Y. Investigating the Effects of Elevation on Microbial Communities and Soil Properties at Fanjing Mountain, China. Forests 2024, 15, 1980. [Google Scholar] [CrossRef]
- Nozari, S.; Borůvka, L. The effects of slope and altitude on soil organic carbon and clay content in different land-uses: A case study in the Czech Republic. Soil Water Res. 2023, 18, 204–218. [Google Scholar] [CrossRef]
- Olaleye, A.; Mating, R.; Nkheloane, T.; Samuel, T.K.; Akande, T.Y. Wetland Health in Two Agro-Ecological Zones of Lesotho: Soil Physico-Chemical Properties, Nutrient Dynamics and Vegetation Isotopic N-15; IntechOpen: London, UK, 2022. [Google Scholar]
- Fan, S.; Sun, H.; Yang, J.; Qin, J.; Shen, D.; Chen, Y. Variations in soil enzyme activities and microbial communities along an altitudinal gradient on the eastern Qinghai–Tibetan plateau. Forests 2021, 12, 681. [Google Scholar] [CrossRef]
- Ekenler, M.; Tabatabai, M.A. Effects of liming and tillage systems on microbial biomass and glycosidases in soils. Biol. Fertil. Soils 2003, 39, 51–61. [Google Scholar] [CrossRef]
- Kumar, D.; Bhardwaj, D.R.; Sharma, P.; Bharti; Sankhyan, N.; Al-Ansari, N.; Linh, N.T.T. Population dynamics of Juniperus macropoda Bossier forest ecosystem in relation to soil physico-chemical characteristics in the cold desert of North-Western Himalaya. Forests 2022, 13, 1624. [Google Scholar] [CrossRef]
- Imtimongla, A.M.; Saya, D.; Phuncho, T. Properties of soil in relation to altitude. Just Agric. 2021, 12, 1–13. [Google Scholar]
- Feyissa, A.; Raza, S.T.; Cheng, X. Soil carbon stabilization and potential stabilizing mechanisms along elevational gradients in alpine forest and grassland ecosystems of Southwest China. Catena 2023, 229, 107210. [Google Scholar] [CrossRef]
- Dinesh, G.K.; Sharma, D.K.; Jat, S.L.; Bandyopadhyay, K.; Srinivasa Rao, C.; Venkatramanan, V.; Boomiraj, K. Effect of Conservation Agriculture Practices on Carbon Pools in a Sandy Loam Soil of Indo-Gangetic Plains. Commun. Soil Sci. Plant Anal. 2023, 54, 2845–2862. [Google Scholar] [CrossRef]
- Sahoo, U.K.; Singh, S.L.; Gogoi, A.; Kenye, A.; Sahoo, S.S.; Wu, F. Active and passive soil organic carbon pools as affected by different land use types in Mizoram, Northeast India. Public Libr. Sci. One 2019, 14, 21–32. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J.; Lambers, H.; Wu, J.; Qin, G.; Li, Y.; Wang, F. Intensified rainfall in the wet season alters the microbial contribution to soil carbon storage. Plant Soil 2022, 476, 337–351. [Google Scholar] [CrossRef]
- Von-Lützow, M.; Kögel-Knabner, I.; Ekschmitt, K.; Flessa, H.; Guggenberger, G.; Matzner, E.; Marschner, B. SOM fractionation methods: Relevance to functional pools and to stabilization mechanisms—A review. Soil Biol. Biochem. 2007, 39, 2183–2207. [Google Scholar] [CrossRef]
- Hazra, K.K.; Ghosh, P.K.; Venkatesh, M.S.; Nath, C.P.; Kumar, N.; Singh, M.; Nadarajan, N. Improving soil organic carbon pools through inclusion of summer mungbean in cereal–cereal cropping systems in Indo-Gangetic plain. Arch. Agron. Soil Sci. 2018, 64, 1690–1704. [Google Scholar] [CrossRef]
- Parihar, C.M.; Jat, S.L.; Singh, A.K.; Datta, A.; Parihar, M.D.; Varghese, E.; Jat, M.L. Changes in carbon pools and biological activities of a sandy loam soil under medium-term conservation agriculture and diversified cropping systems. Eur. J. Soil Sci. 2018, 69, 902–912. [Google Scholar] [CrossRef]
- Babu, S.; Mohapatra, K.P.; Das, A.; Yadav, G.S.; Tahasildar, M.; Singh, R.; Chandra, P. Designing energy-efficient, economically sustainable and environmentally safe cropping system for the rainfed maize–fallow land of the Eastern Himalayas. Sci. Total Environ. 2020, 722, 137874. [Google Scholar] [CrossRef]
- Vieira, F.C.B.; Bayer, C.; Zanatta, J.A.; Dieckow, J.; Mielniczuk, J.; He, Z.L. Carbon management index based on physical fractionation of soil organic matter in an Acrisol under long-term no-till cropping systems. Soil Tillage Res. 2007, 96, 195–204. [Google Scholar] [CrossRef]
- Parihar, C.M.; Singh, A.K.; Jat, S.L.; Ghosh, A.; Dey, A.; Nayak, H.S.; Jat, M.L. Dependence of temperature sensitivity of soil organic carbon decomposition on nutrient management options under conservation agriculture in a subtropical Inceptisol. Soil Tillage Res. 2019, 190, 50–60. [Google Scholar] [CrossRef]
- Mangral, Z.A.; Islam, S.U.; Tariq, L.; Kaur, S.; Ahmad, R.; Malik, A.H.; Dar, T.U.H. Altitudinal gradient drives significant changes in soil physico-chemical and eco-physiological properties of Rhododendron anthopogon: A case study from Himalaya. Front. For. Glob. Change 2023, 6, 1181299. [Google Scholar] [CrossRef]
- Behera, M.C.; Sahoo, U.K.; Mohanty, T.L. Soil organic carbon pools and carbon management index of the tropical moist deciduous forests in Indian Eastern Ghats. Sci. Rep. 2025, 15, 12640. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Jiang, W.; Zou, S.; Kang, D.; Yan, X. Microbial carbohydrate-active enzymes influence soil carbon by regulating plant-and fungal-derived biomass decomposition in plateau peat wetlands under differing water conditions. Front. Microbiol. 2023, 14, 1266016. [Google Scholar] [CrossRef] [PubMed]
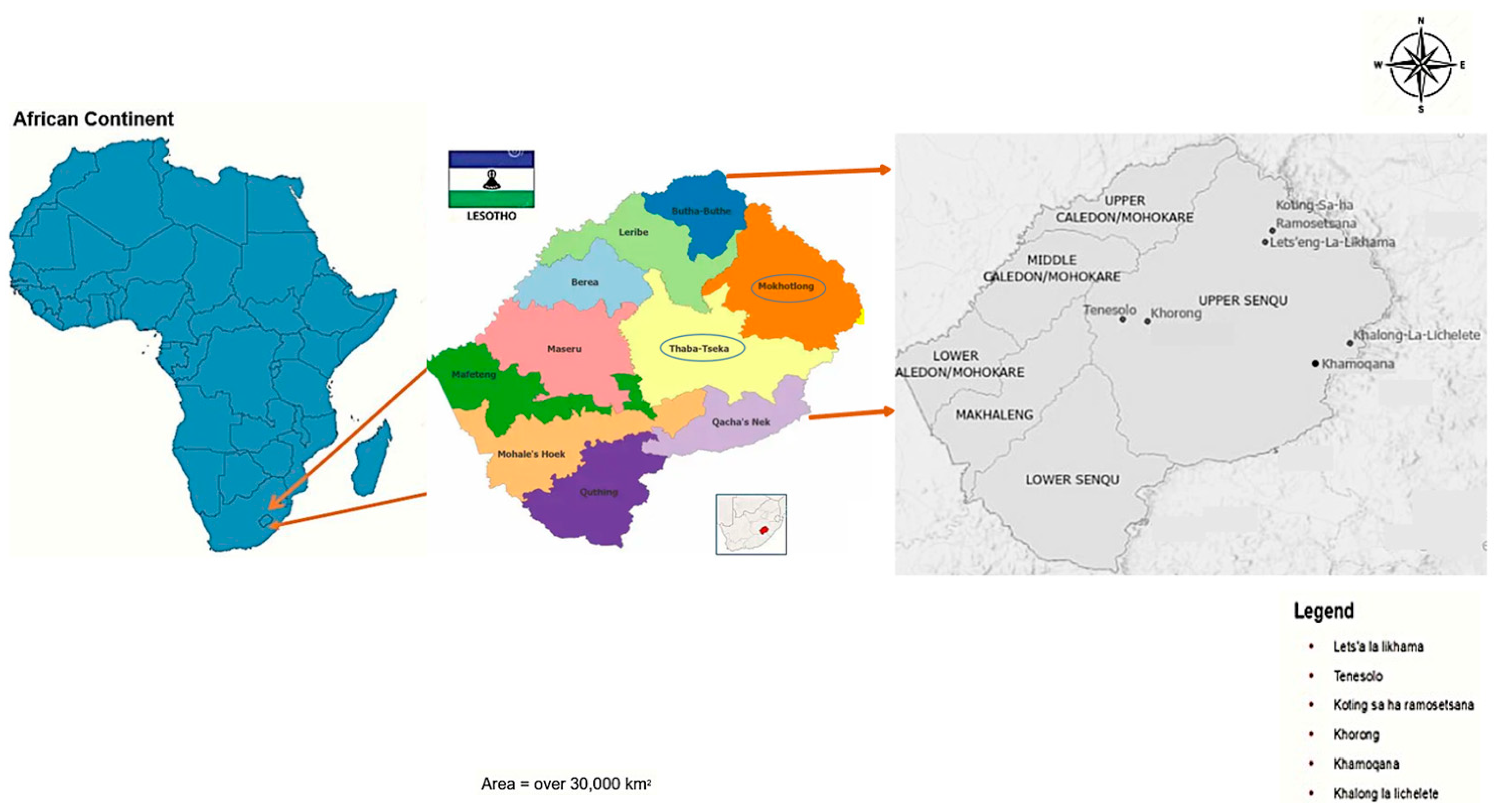
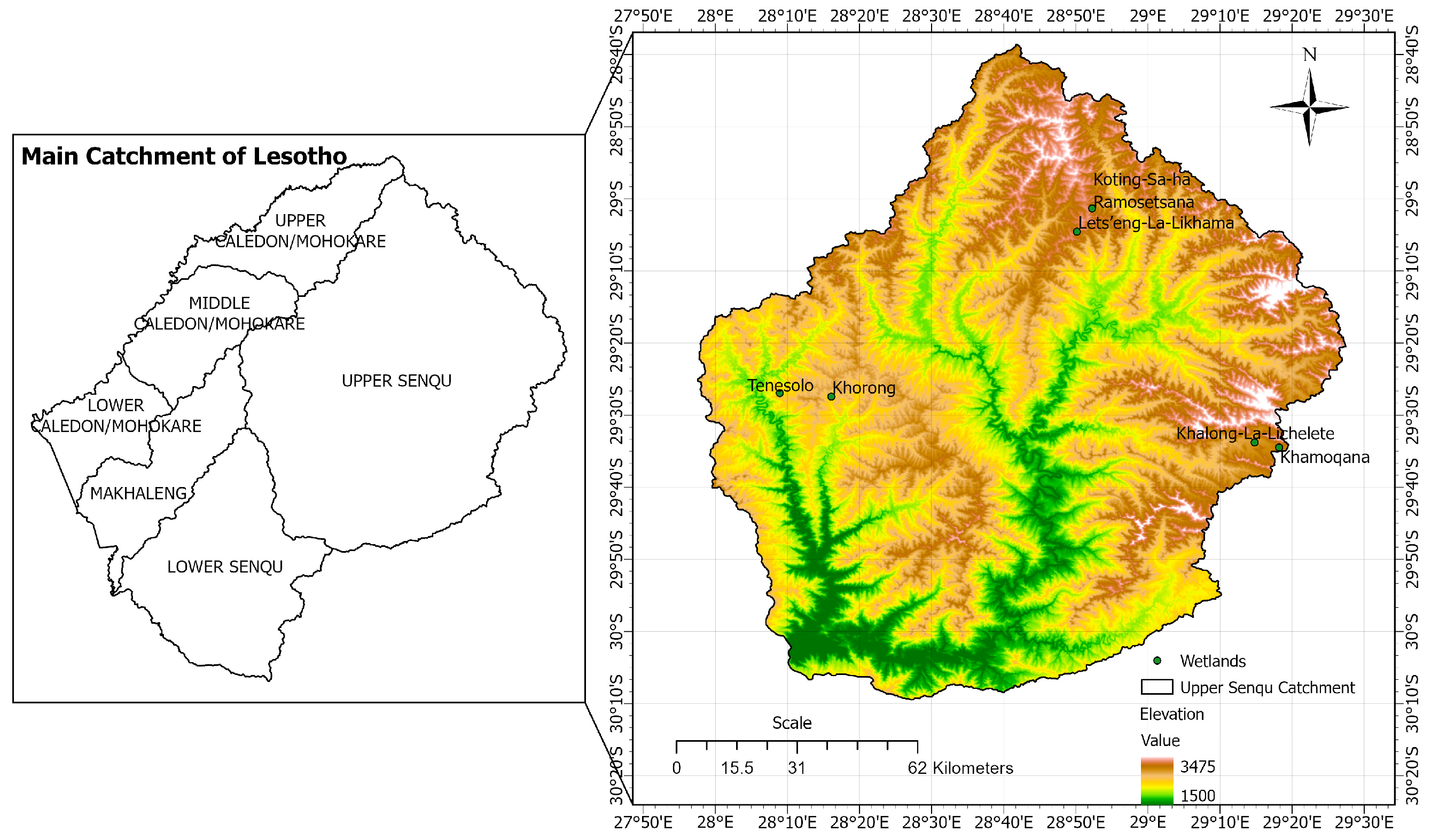

| Alpine Wetlands | Latitude N | Longitude E | Soil Degradation Level Assessed with WET Health According to Kleynhans [21] and Macfarlane et al. [22]. | |
|---|---|---|---|---|
| PES Score (%) | Description | |||
| Khorong | −29.457168 | 28.268082 | 80 | Largely natural with few modifications. A slight change in ecosystem processes is discernible, and a small loss of natural habitats and biota may have taken place. |
| Tenesolo | −29.449256 | 28.149214 | 45 | Extensively altered and alterations in ecological functions accompanied by the disappearance of natural habitats and native species. |
| Khamoqana | −29.457178 | 28.268094 | 30 | Seriously modified, the change in ecosystem processes, great loss of natural habitat and biota but some remaining natural habitat features are still being recognized. |
| Khalong-La-Lichelete | −29.563552 | 29.247207 | 90 | Unmodified natural wetland |
| Lets’eng-La-Likhama | −29.076355 | 28.836095 | 40 | Largely modified with a large change in ecosystem processes and loss of natural habitat, and biota has occurred. |
| Koting-Sa-ha Ramosetsana | −29.022686 | 28.871324 | 85 | Largely natural with few modifications and a slight change in ecosystem processes being discernible and a small loss of natural habitats and biota may have taken place. |
| Treatment(s) | |
|---|---|
| Alpine Wetlands | Altitude (m) asl |
| Khorong | 2500–2550 |
| Tenesolo | 2552–2600 |
| Khamoqana | 2839–2880 |
| Khalong-La-Lichelete | 2891–2995 |
| Lets’eng-La-Likhama | 3040–3080 |
| Koting-Sa-ha Ramosetsana | 3087–3155 |
| S.No | Soil Property | Method | Reference |
|---|---|---|---|
| 2 | Mechanical separates | Hydrometer method | Bouyoucos [25] |
| Sand (%) | |||
| Silt (%) | |||
| Clay (%) | |||
| 3 | Soil reaction (pH) | Soil: water suspension (1:2.5) | Jackson [26] |
| 4 | Electrical conductivity (dS m−1) | ||
| 5 | Bulk density (Mg m−3) | Core sampler | Blake and Hartge [27] |
| 6 | Soil organic carbon (g kg−1) | Wet oxidation | Walkley and Black [28] |
| Treatment(s) | BD (Mg m−3) | Sand | Silt | Clay | Textural Class | |
|---|---|---|---|---|---|---|
| Wetlands | Altitude (m) asl | (%) | ||||
| Khorong | 2500–2550 | 1.30 | 64.98 | 23.67 | 11.36 | Sandy loam |
| Tenesolo | 2552–2600 | 1.52 | 60.63 | 28.92 | 10.45 | Sandy loam |
| Khamoqana | 2839–2880 | 1.28 | 52.82 | 34.04 | 13.14 | Loam |
| Khalong-La-Lichelete | 2891–2995 | 1.26 | 64.43 | 21.92 | 13.65 | Sandy loam |
| Lets’eng-La-Likhama | 3040–3080 | 1.27 | 46.38 | 35.72 | 17.90 | Loam |
| Koting-Sa-ha Ramosetsana | 3087–3155 | 1.09 | 39.79 | 35.02 | 25.19 | Loam |
| SEM± | 0.016 | 0.279 | 0.140 | 0.245 | ||
| CD (p < 0.05) | 0.049 | 0.868 | 0.307 | 0.764 | ||
| Treatment(s) | pH | EC (dS m−1) | SOC (g kg−1) | ||
|---|---|---|---|---|---|
| Wetlands | Altitude (m) asl | 0–15 cm | 15–30 cm | ||
| Khorong | 2500–2550 | 5.76 | 0.34 | 84.67 | 53.34 |
| Tenesolo | 2552–2600 | 6.04 | 0.35 | 69.14 | 43.56 |
| Khamoqana | 2839–2880 | 5.98 | 0.35 | 73.24 | 46.14 |
| Khalong-La-Lichelete | 2891–2995 | 5.80 | 0.33 | 80.27 | 50.57 |
| Lets’eng-La-Likhama | 3040–3080 | 5.53 | 0.29 | 94.34 | 59.43 |
| Koting-Sa-ha Ramosetsana | 3087–3155 | 6.01 | 0.32 | 95.80 | 60.35 |
| SEM± | 0.072 | 0.014 | 8.28 | 3.69 | |
| CD (p < 0.05) | 0.223 | NS | 18.24 | 11.48 | |
| Treatment(s) | DHA (μg TPF g−1 Dry Soil Day−1) | β-GaA (nmol p-Nitrophenol g−1 Dry Soil hr−1) | FDA (µg.Fluorescein g−1 Dry Soil 3 hr−1) | |
|---|---|---|---|---|
| Wetlands | Altitude (m) asl | |||
| Khorong | 2500–2550 | 49.63 | 173.22 | 227.72 |
| Tenesolo | 2552–2600 | 35.49 | 153.23 | 188.27 |
| Khamoqana | 2839–2880 | 36.70 | 140.00 | 187.60 |
| Khalong-La-Lichelete | 2891–2995 | 39.82 | 151.44 | 220.64 |
| Lets’eng-La-Likhama | 3040–3080 | 29.03 | 126.49 | 119.01 |
| Koting-Sa-ha Ramosetsana | 3087–3155 | 34.15 | 150.48 | 172.26 |
| SEM± | 2.068 | 7.633 | 16.157 | |
| CD (p < 0.05) | 6.442 | 23.779 | 50.335 | |
| Treatment(s) | LI | CPI | CMI | LI | CPI | CMI | |
|---|---|---|---|---|---|---|---|
| Wetlands | Altitude (m) asl | 0–15 cm | 15–30 cm | ||||
| Khorong | 2500–2550 | 0.98 | 0.75 | 73.13 | 0.96 | 0.65 | 61.32 |
| Tenesolo | 2552–2600 | 0.96 | 0.58 | 57.24 | 0.93 | 0.53 | 47.97 |
| Khamoqana | 2839–2880 | 1.06 | 0.71 | 75.36 | 1.02 | 0.61 | 62.72 |
| Khalong-La-Lichelete | 2891–2995 | 1.06 | 0.65 | 69.01 | 1.04 | 0.56 | 57.79 |
| Lets’eng-La-Likhama | 3040–3080 | 1.00 | 0.84 | 80.37 | 0.94 | 0.72 | 67.07 |
| Koting-Sa-ha Ramosetsana | 3087–3155 | 1.07 | 0.85 | 91.05 | 1.04 | 0.73 | 75.88 |
| SEM± | 0.093 | 0.139 | 4.801 | 0.095 | 0.045 | 4.002 | |
| CD (p < 0.05) | NS | 0.045 | 14.597 | NS | 0.140 | 12.467 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nthebere, K.; Mazvimavi, D.; Marake, M.; Mochala, M.; Raliengoane, T.; Mohseni, B.; Kujinga, K.; Onema, J.M.K. Variability in the Carbon Management Index and Enzymatic Activity Under Distinct Altitudes in the Alpine Wetlands of Lesotho. Sustainability 2025, 17, 8571. https://doi.org/10.3390/su17198571
Nthebere K, Mazvimavi D, Marake M, Mochala M, Raliengoane T, Mohseni B, Kujinga K, Onema JMK. Variability in the Carbon Management Index and Enzymatic Activity Under Distinct Altitudes in the Alpine Wetlands of Lesotho. Sustainability. 2025; 17(19):8571. https://doi.org/10.3390/su17198571
Chicago/Turabian StyleNthebere, Knight, Dominic Mazvimavi, Makoala Marake, Mosiuoa Mochala, Tebesi Raliengoane, Behrooz Mohseni, Krasposy Kujinga, and Jean Marie Kileshye Onema. 2025. "Variability in the Carbon Management Index and Enzymatic Activity Under Distinct Altitudes in the Alpine Wetlands of Lesotho" Sustainability 17, no. 19: 8571. https://doi.org/10.3390/su17198571
APA StyleNthebere, K., Mazvimavi, D., Marake, M., Mochala, M., Raliengoane, T., Mohseni, B., Kujinga, K., & Onema, J. M. K. (2025). Variability in the Carbon Management Index and Enzymatic Activity Under Distinct Altitudes in the Alpine Wetlands of Lesotho. Sustainability, 17(19), 8571. https://doi.org/10.3390/su17198571






