Bone Meal as a Sustainable Amendment for Zinc Retention in Polluted Soils: Adsorption Mechanisms, Characterization, and Germination Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Soil Characteristics
2.3. Characteristics of Bone Meal
- ▪ Ingredients: bones (100% beef, ground).
- ▪ Additives: the manufacturer guarantees that this product contains no additives.
2.4. Characterization of Bone Meal
2.5. Description of Models for Adsorption Equilibrium Study
2.5.1. The Langmuir Model
2.5.2. The Freundlich Equation
2.6. Description of Models for Kinetic Study
- (a)
- The pseudo-second-order adsorption kinetic model is mathematically described according to Equation (5) [34]:
- (b) The pseudo-first-order adsorption kinetic model is mathematically described according to Equation (7) [34]:
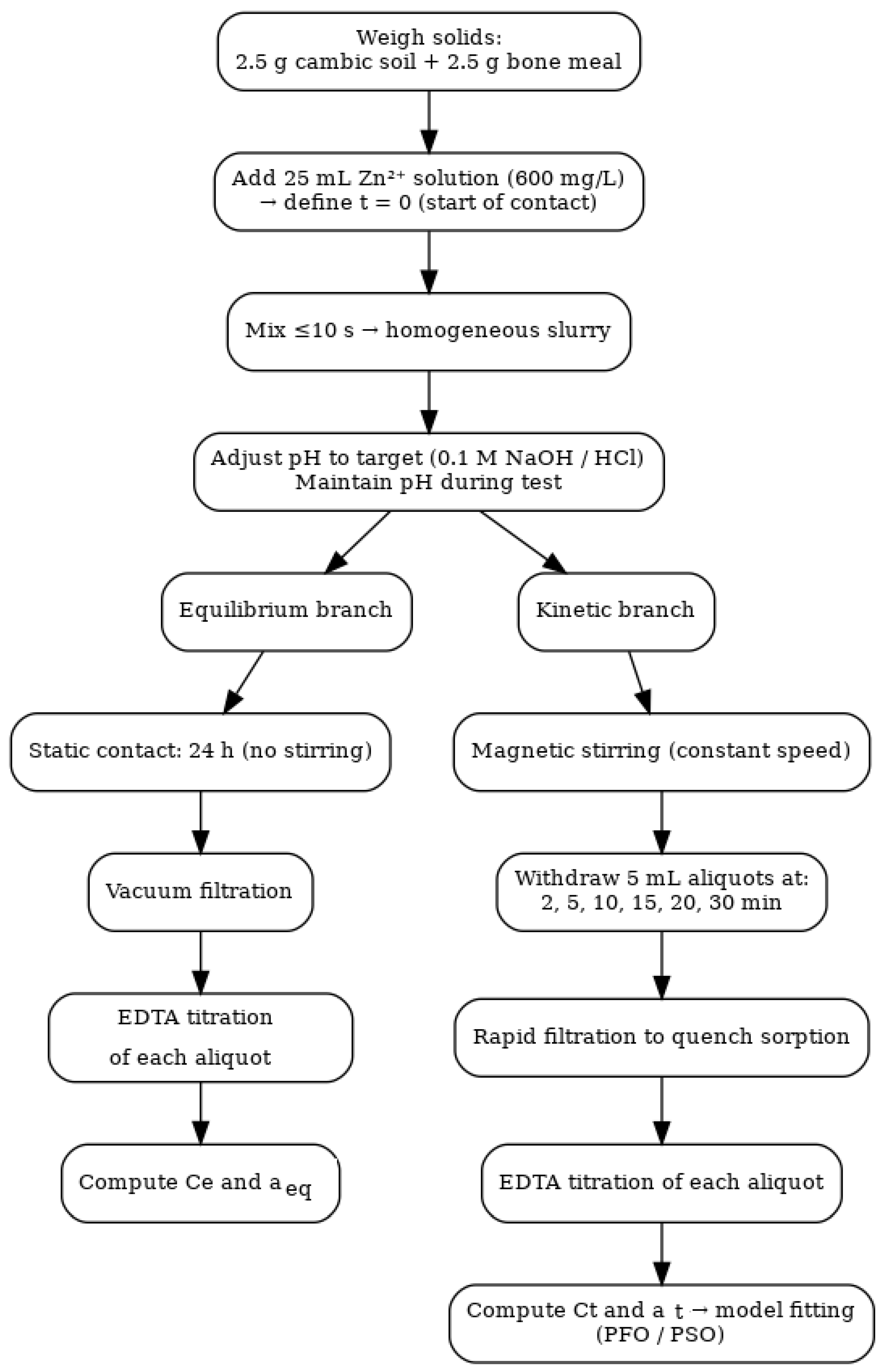
2.7. Methods
2.7.1. Experimental Methods
2.7.2. Calculation of the Germination Rate
2.7.3. Calculation of the Extent of Inhibition
3. Results and Discussion
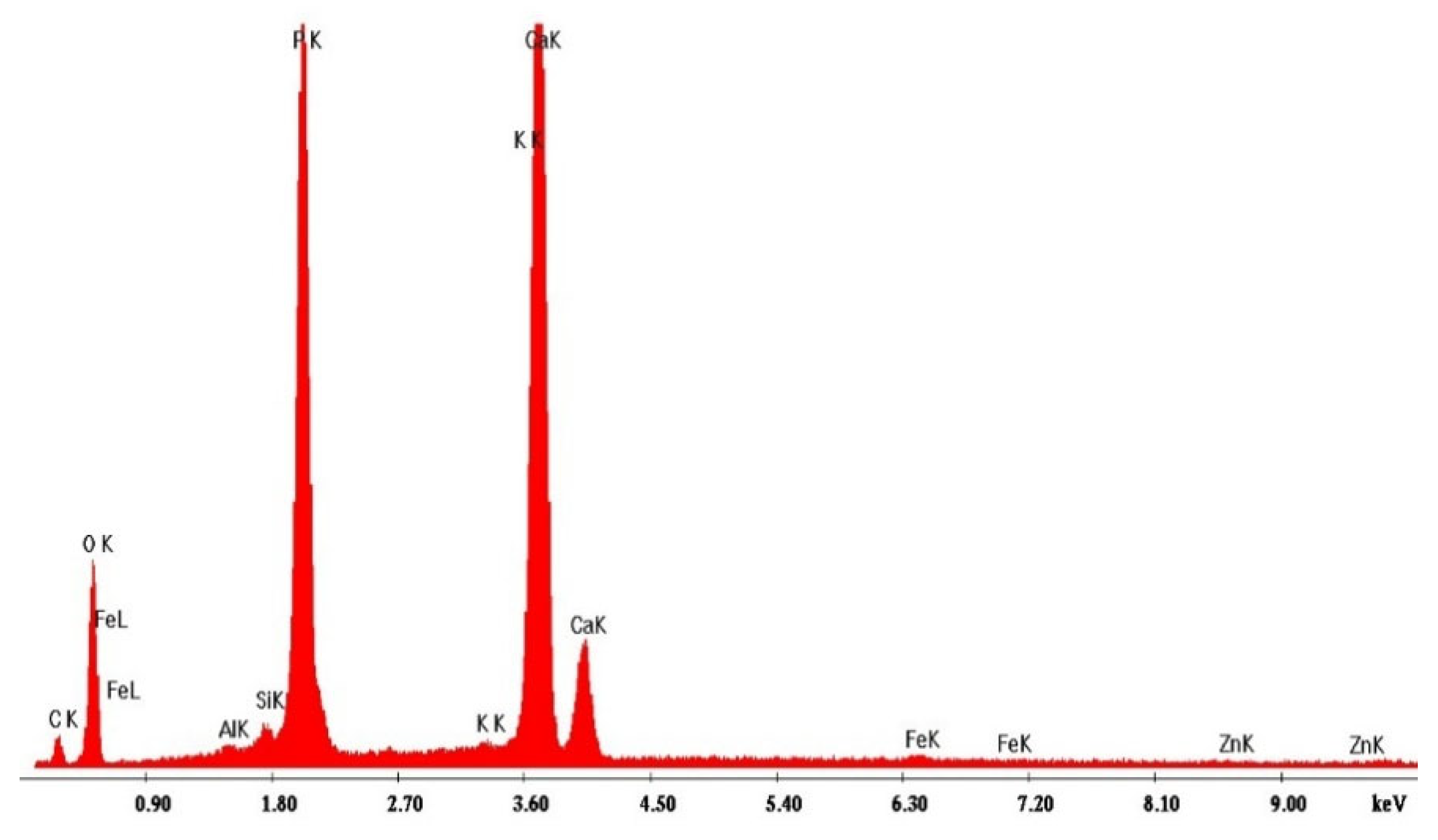
3.1. Characterization of Bone Meal: SEM-EDX
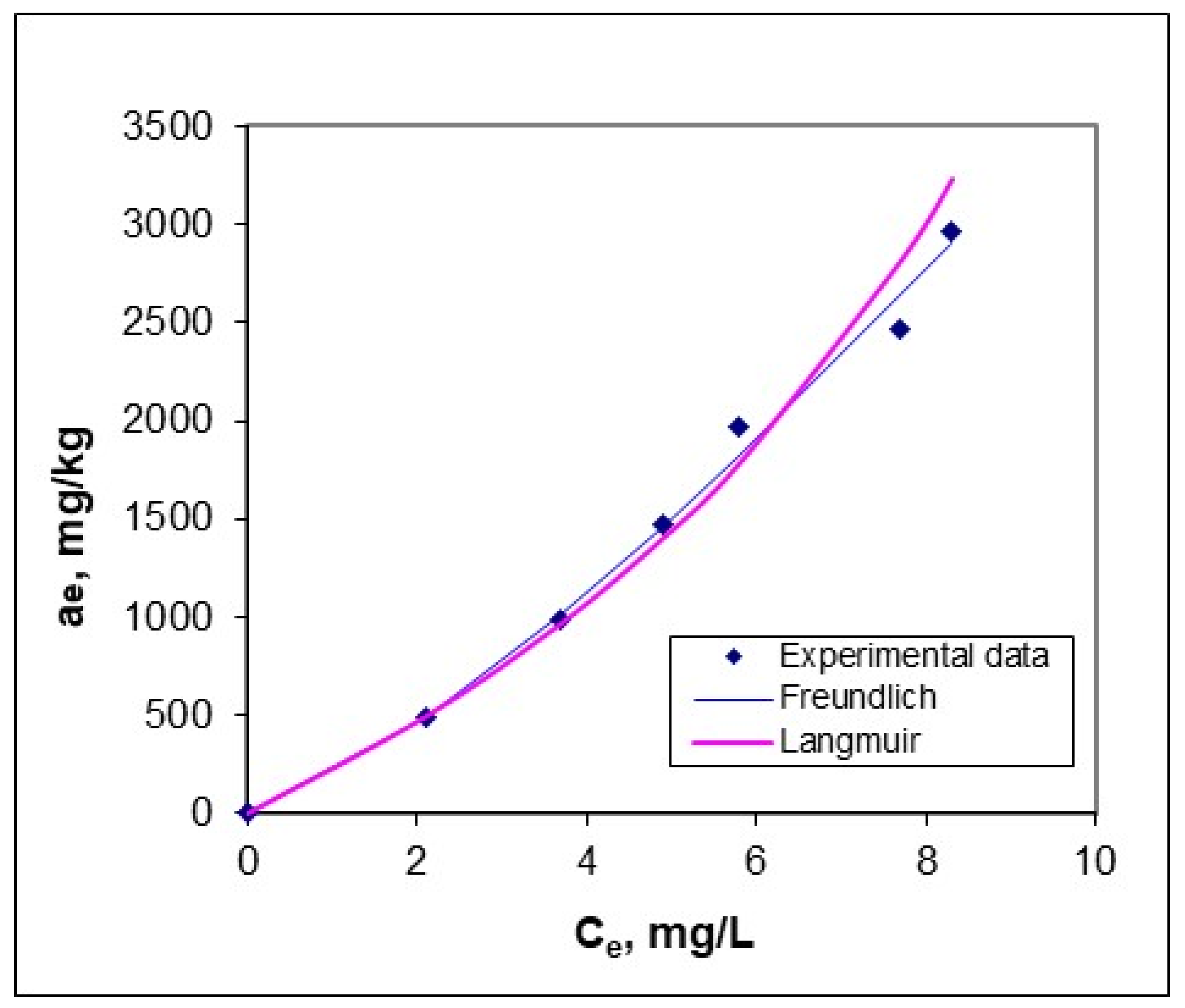
3.2. Adsorption Equilibrium Study
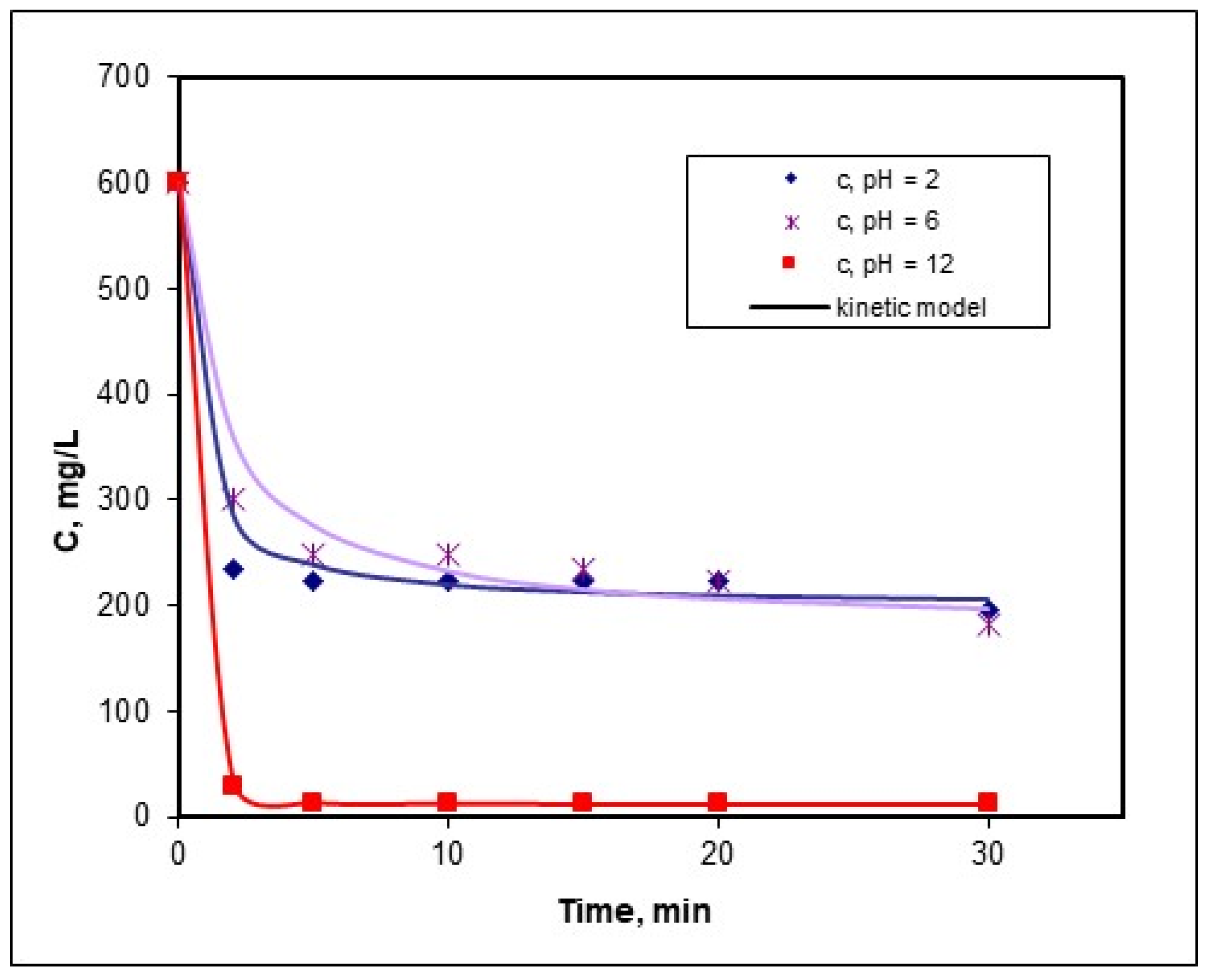
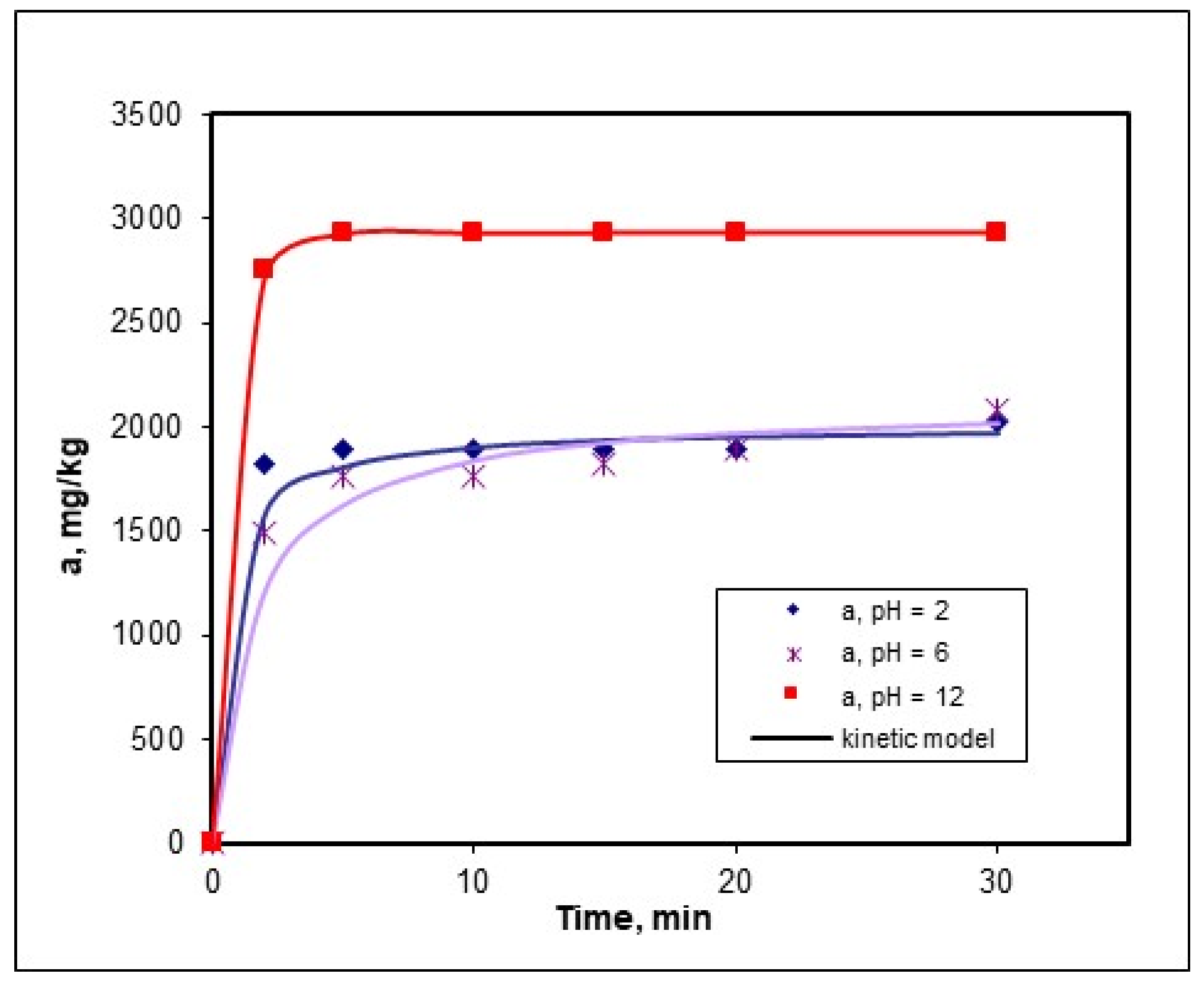
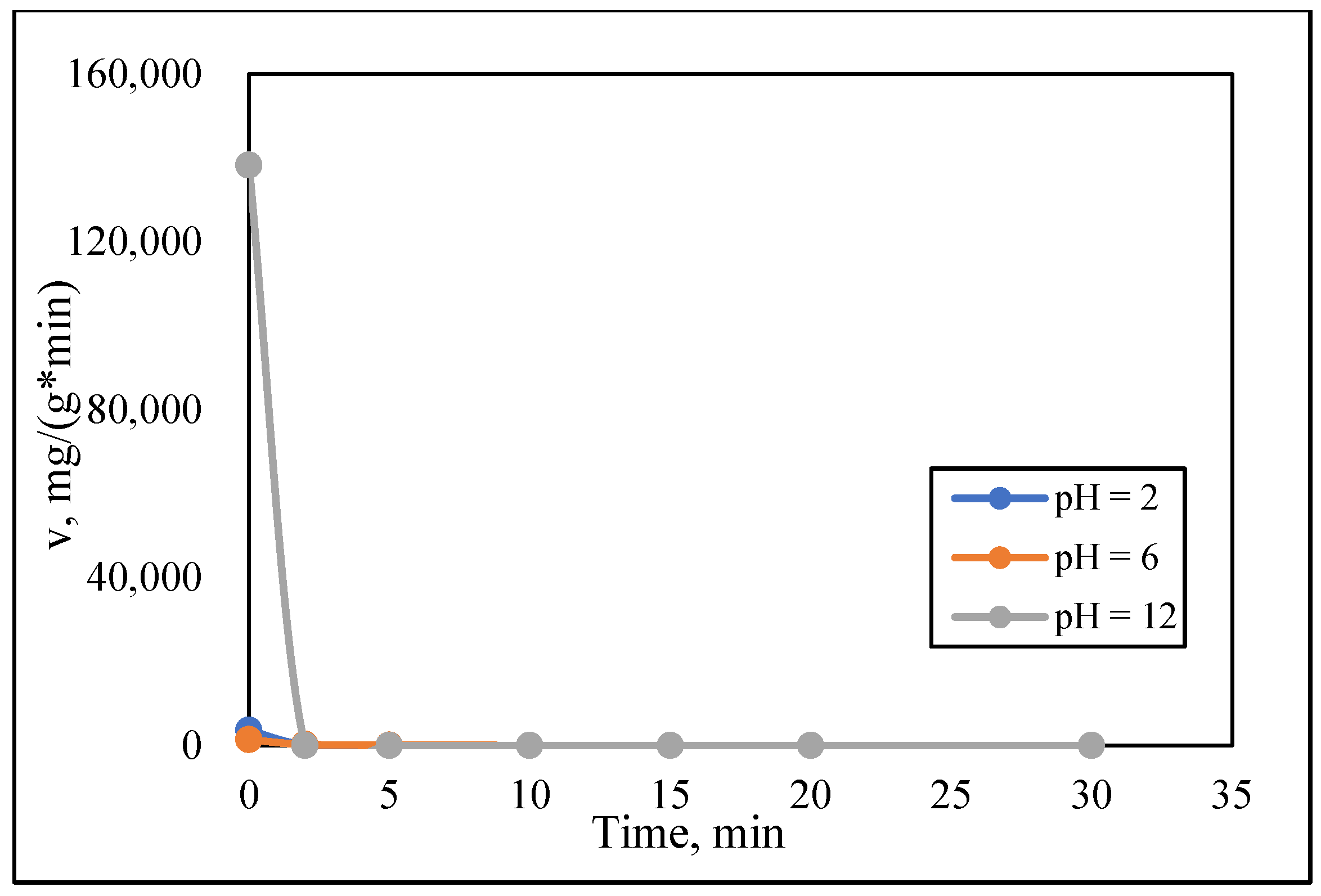
3.3. Kinetic Study
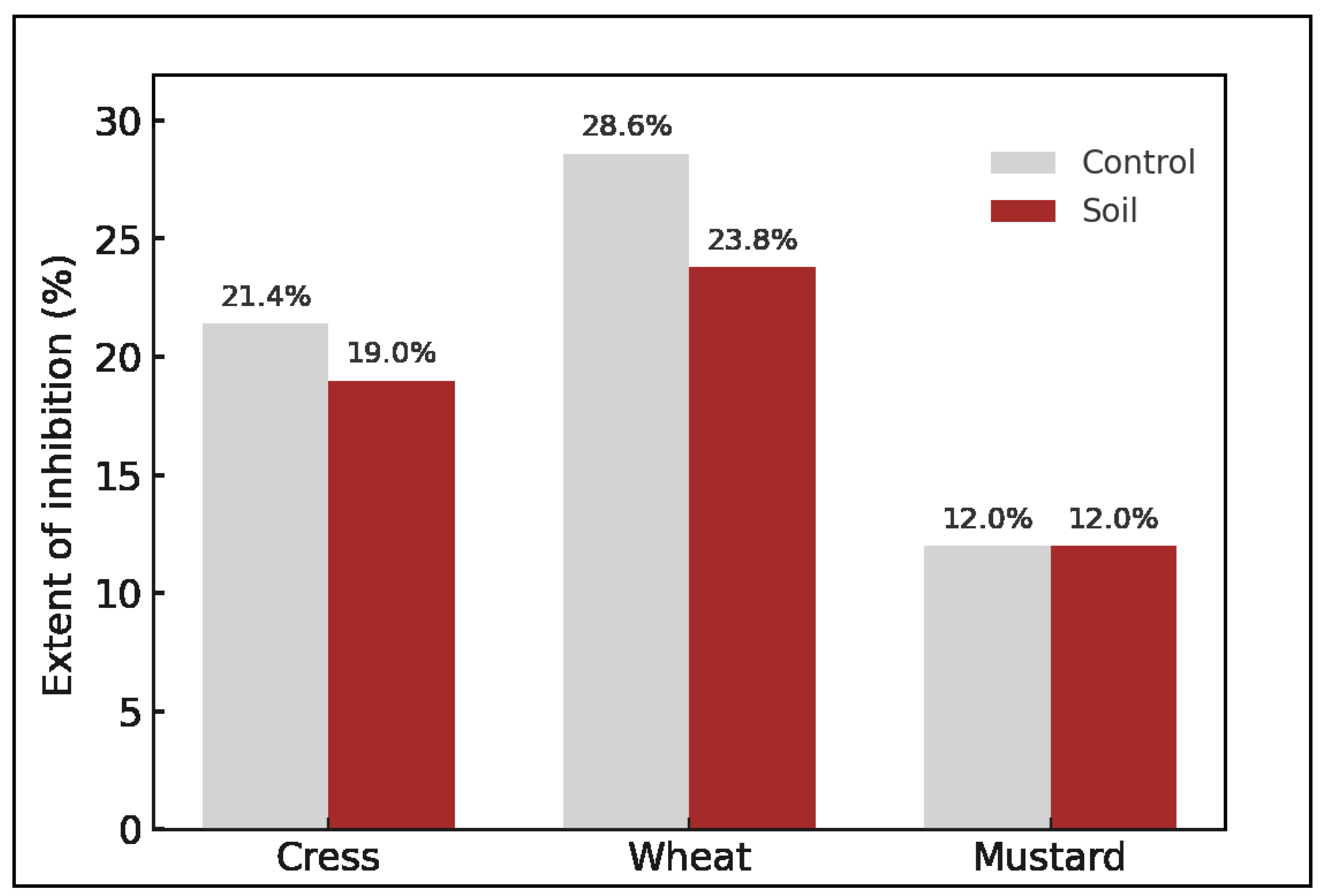
3.4. Effects of Zinc and Bone Meal on Plant Germination and Growth in Treated Soil
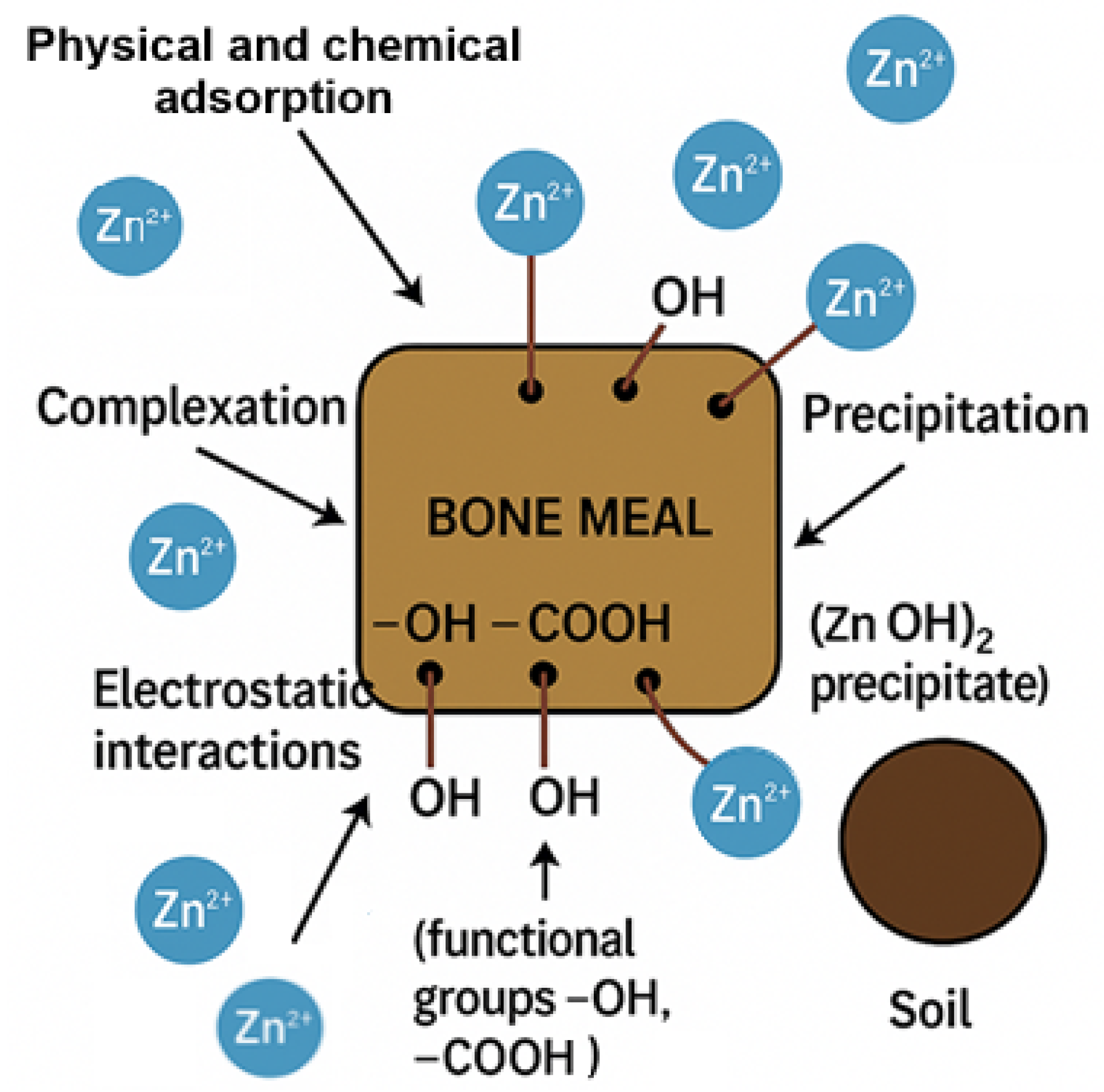
3.5. Mechanism of Zinc Retention in Soil–Bone Meal Mixture
- (a)
- Alkaline Precipitation
- (b) Surface Complexation with Functional Groups
- (c) Electrostatic interactions
- (d) Physical and Chemical Adsorption
4. Conclusions
Limitations and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Angon, P.B.; Islam, S.; KC, S.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, Effects and Present Perspectives of Heavy Metals Contamination: Soil, Plants and Human Food Chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef]
- Natasha, N.; Shahid, M.; Bibi, I.; Iqbal, J.; Khalid, S.; Murtaza, B.; Bakhat, H.F.; Farooq, A.B.U.; Amjad, M.; Hammad, H.M.; et al. Zinc in Soil-Plant-Human System: A Data-Analysis Review. Sci. Total Environ. 2022, 808, 152859. [Google Scholar] [CrossRef]
- Azeem, M.; Shaheen, S.M.; Ali, A.; Jeyasundar, P.G.S.A.; Latif, A.; Abdelrahman, H.; Li, R.; Almazroui, M.; Niazi, N.K.; Sarmah, A.K.; et al. Removal of Potentially Toxic Elements from Contaminated Soil and Water Using Bone Char Compared to Plant- and Bone-Derived Biochars: A Review. J. Hazard. Mater. 2022, 427, 128131. [Google Scholar] [CrossRef]
- Alkurdi, S.S.A.; Herath, I.; Bundschuh, J.; Al-Juboori, R.A.; Vithanage, M.; Mohan, D. Biochar versus Bone Char for a Sustainable Inorganic Arsenic Mitigation in Water: What Needs to Be Done in Future Research? Environ. Int. 2019, 127, 52–69. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Z.; Wang, Y.; Mu, G.; Wu, X.; Liu, Y.; Luo, W.; Wen, X. Remediation of Soil Contaminated by Heavy Metals Using Biochar: Strategies and Future Prospects. Pol. J. Environ. Stud. 2023, 32, 27–40. [Google Scholar] [CrossRef]
- Majewska, M.; Hanaka, A. Biochar in the Bioremediation of Metal-Contaminated Soils. Agronomy 2025, 15, 273. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Sandilya, S.P.; Sarma, B.; Pandey, A.K.; Dutta, J.; Mahanta, K.; Lesueur, D.; Nath, B.C.; Borah, D.; Borgohain, D.J. Biochar as Soil Amendment in Climate-Smart Agriculture: Opportunities, Future Prospects, and Challenges. J. Soil Sci. Plant Nutr. 2024, 24, 135–158. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Xu, H.; Li, F.; Wang, Y. Combined effects of biochar and zeolite on the immobilization of heavy metals in contaminated soils: A meta-analysis. Environ. Pollut. 2025, 336, 123456. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Tsadilas, C.D.; Rinklebe, J. A critical review of biochar-based materials for the immobilization of potentially toxic elements in contaminated soils. Environ. Int. 2023, 170, 107606. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, X.; Li, Z.; Wu, W.; Li, J. Meta-analysis of modified biochar for cadmium immobilization in contaminated soils. Front. Environ. Sci. 2024, 12, 1413047. [Google Scholar] [CrossRef]
- Abdelhafez, A.A.; Li, J.; Abbas, M.H.H. Feasibility of using hydroxyapatite for remediation of heavy metal contaminated soils: A review. Toxics 2020, 8, 102. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Hanafiah, M.A.K.M. Removal of Heavy Metal Ions from Wastewater by Chemically Modified Plant Wastes as Adsorbents: A Review. Bioresour. Technol. 2008, 99, 3935–3948. [Google Scholar] [CrossRef]
- Natasha, N.; Shahid, M.; Khalid, S.; Bibi, I.; Naeem, M.A.; Niazi, N.K.; Tack, F.M.; Ippolito, J.A.; Rinklebe, J. Influence of Biochar on Trace Element Uptake, Toxicity and Detoxification in Plants and Associated Health Risks: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2803–2843. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A Review of Biochars’ Potential Role in the Remediation, Revegetation and Restoration of Contaminated Soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil Amendments for Immobilization of Potentially Toxic Elements in Contaminated Soils: A Critical Review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W. Biochar Reduces the Bioavailability and Phytotoxicity of Heavy Metals. Plant Soil 2011, 348, 439–451. [Google Scholar] [CrossRef]
- ISO 12739:2006; Zinc Sulfide Concentrates—Determination of Zinc—Ion-Exchange/EDTA Titrimetric Method. International Organization for Standardization: Geneva, Switzerland, 2006.
- SR EN ISO 11885:2009; Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). ASRO—Romanian Institute for Standardization: Bucharest, Romania, 2009.
- STAS 7184/10-79; Soluri. Determinarea Compoziţiei Granulometrice [Soils. Determination of Granulometric Composition]. ASRO—Romanian Institute for Standardization: Bucharest, Romania, 1979.
- ISO 10390:2021; Soil, Treated Biowaste and Sludge—Determination of pH. International Organization for Standardization (ISO): Geneva, Switzerland, 2021.
- STAS 7184/21-82; Soluri. Determinarea Conținutului de Humus [Soils. Determination of Humus Content]. ASRO—Romanian Institute for Standardization: Bucharest, Romania, 1982.
- STAS 7184/16-80; Soluri. Determinarea Carbonaţilor Alcalino-Pământoşi [Soils. Determination of Alkaline Earth Carbonates]. ASRO—Romanian Institute for Standardization: Bucharest, Romania, 1980.
- SR ISO 11261:2000; Soil quality—Determination of Total Nitrogen—Modified Kjeldahl Method. ASRO—Romanian Institute for Standardization: Bucharest, Romania, 2000.
- STAS 7184/19-82; Soluri. Determinarea Fosforului Extractibil în Acetat-Lactat de Amoniu [Soils. Determination of Phosphorus Extractable in Ammonium Acetate-Lactate]. ASRO—Romanian Institute for Standardization: Bucharest, Romania, 1982.
- STAS 7184/14-79; Soluri. Determinarea Fosforului Total [Soils. Determination of Total Phosphorus]. ASRO—Romanian Institute for Standardization: Bucharest, Romania, 1979.
- STAS 7184/18-80; Soluri. Determinarea Conţinutului de Potasiu Accesibil şi Potenţial Accesibil Pentru Plante [Soils. Determination of Plant-Available and Potentially Available Potassium Content]. ASRO—Romanian Institute for Standardization: Bucharest, Romania, 1980.
- SR ISO 11047:1999; Soil Quality—Determination of Cadmium, Chromium, Cobalt, Copper, Lead, Manganese, Nickel and Zinc in Soil Extracts with Aqua Regia. Methods by Flame Atomic Absorption Spectrometry and Electrothermal Atomization. ASRO—Romanian Institute for Standardization: Bucharest, Romania, 1999.
- ISO 11260:2018; Soil Quality—Determination of Effective Cation Exchange Capacity and Base Saturation Level Using Barium Chloride Solution. International Organization for Standardization (ISO): Geneva, Switzerland, 2018.
- STAS 7184/6-87; Soluri. Determinarea Coeficientului de Higroscopicitate [Soils. Determination of Hygroscopicity Coefficient]. ASRO – Romanian Institute for Standardization: Bucharest, Romania, 1987.
- ISO 11272:2017; Soil Quality—Determination of Dry Bulk Density. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Guo, X.; Wang, L. Comparison of Linearization Methods for Modeling the Langmuir Adsorption Isotherm. J. Mol. Liq. 2019, 296, 111850. [Google Scholar] [CrossRef]
- Mekonnen, D.T.; Alemayehu, E.; Lennartz, B. Adsorptive Removal of Phosphate from Aqueous Solutions Using Low-Cost Volcanic Rocks: Kinetics and Equilibrium Approaches. Materials 2021, 14, 1312. [Google Scholar] [CrossRef]
- Orbeci, C. Thermodynamic and Kinetic Aspects Concerning Cr(VI) Retention from Aqueous Solutions by Strong Base Anion Exchangers with Pyridine Structures. Bull. Sci. UPB Ser. A Chem. Chem. Eng. 2005, 67, 9–15. [Google Scholar]
- Demirbas, E.; Kobya, M.; Senturk, E.; Ozkan, T. Adsorption Kinetics for the Removal of Chromium(VI) from Aqueous Solutions on the Activated Carbons Prepared from Agricultural Wastes. Water SA 2004, 30, 533–539. [Google Scholar] [CrossRef]
- Ministerul Apelor, Pădurilor și Protecției Mediului. Ordin nr. 756 din 3 noiembrie 1997 pentru aprobarea Reglementării privind evaluarea poluării mediului. Monitorul Oficial al României, Partea I. [Ministry of Waters, Forests and Environmental Protection. (1997, November 3). Order No. 756 of November 3, 1997 for the Approval of the Regulation on Environmental Pollution Assessment. Official Gazette of Romania, Part I]; Ministry of Waters, Forests and Environmental Protection: Bucharest, Romania, 1997. [Google Scholar]
- Thomas, M.; Melichová, Z.; Šuránek, M.; Kuc, J.; Więckol Ryk, A.; Lochyński, P. Removal of Zinc from Concentrated Galvanic Wastewater by Sodium Trithiocarbonate: Process Optimization and Toxicity Assessment. Molecules 2023, 28, 546. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.; Hilhorst, H.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Wani, P.A.; Khan, M.S.; Zaidi, A. Effect of Metal-Tolerant Plant Growth-Promoting Rhizobium on Growth, Symbiosis, Seed Yield and Metal Uptake by Lentil in Soil Amended with Heavy Metals. Chemosphere 2007, 70, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Joseph, S. (Eds.) Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Routledge: London, UK, 2015; pp. 563–594. [Google Scholar] [CrossRef]
- Guo, M.; Song, W.; Tian, J. Biochar-Facilitated Soil Remediation: Mechanisms and Efficacy Variations. Front. Environ. Sci. 2020, 8, 521512. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Z.; Xu, L.; Buyong, F.; Chay, T.C.; Li, Z.; Cai, Y.; Hu, B.; Zhu, Y.; Wang, X. Modified Biochar: Synthesis and Mechanism for Removal of Environmental Heavy Metals. Carbon Res. 2022, 1, 8. [Google Scholar] [CrossRef]
- Idris, M.O.; Yaqoob, A.A.; Ibrahim, M.N.M.; Ahmad, A.; Alshammari, M.B. Introduction of Adsorption Techniques for Heavy Metals Remediation. In Emerging Techniques for Treatment of Toxic Metals from Wastewater; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–18. [Google Scholar] [CrossRef]
- Cheung, C.W.; Porter, J.F.; McKay, G. Sorption Kinetics for the Removal of Copper and Zinc from Effluents Using Bone Char. Sep. Purif. Technol. 2000, 19, 55–64. [Google Scholar] [CrossRef]
- Hart, A.; Porbeni, D.W.; Omonmhenle, S.I.; Peretomode, E. Waste bone char-derived adsorbents: Characteristics, adsorption mechanism and model approach. Environ. Technol. Rev. 2023, 12, 175–204. [Google Scholar] [CrossRef]
- Cai, P.; Zheng, H.; Wang, C.; Ma, H.; Hu, J.; Pu, Y.; Liang, P. Competitive adsorption characteristics of fluoride and phosphate on calcined Mg-Al-CO3 layered double hydroxides. J. Hazard. Mater. 2012, 213–214, 100–108. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Removal of natural organic matter (NOM) and its constituents from water by adsorption—A review. Chemosphere 2017, 166, 497–510. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Awasthi, M.K.; Ali, A.; Zhang, Z.; Lahori, A.H.; Mahar, A. Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios. Sci. Total Environ. 2016, 559, 121–129. [Google Scholar] [CrossRef]
- Kösesakal, T.; Ünal, M. Effects of Zinc Toxicity on Seed Germination and Plant Growth in Tomato (Lycopersicon esculentum Mill.). Fresenius Environ. Bull. 2012, 2, 315–321. [Google Scholar]
- Sethy, S.K.; Ghosh, S. Effect of Heavy Metals on Germination of Seeds. J. Nat. Sci. Biol. Med. 2013, 4, 272–275. [Google Scholar] [CrossRef]
- Rajoria, S.; Vashishtha, M.; Sangal, V.K. Heavy Metal Ions in Wastewater: A Review on Detection and Toxicity. Chem. Eng. Process Tech. 2023, 10, 1082. [Google Scholar]
- Abd Elnabi, M.K.; Elkaliny, N.E.; Elyazied, M.M.; Azab, S.H.; Elkhalifa, S.A.; Elmasry, S.; Mouhamed, M.S.; Shalamesh, E.M.; Alhorieny, N.A.; Abd Elaty, A.E.; et al. Toxicity of Heavy Metals and Recent Advances in Their Removal: A Review. Toxics 2023, 11, 580. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Harris, W. Properties of Dairy-Manure-Derived Biochar Pertinent to Its Potential Use in Remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, B.; Zimmerman, A.R.; Fang, J.; Sun, Y.; Cao, X. Sorption of Heavy Metals on Chitosan-Modified Biochars and Its Biological Effects. Chem. Eng. J. 2013, 231, 512–518. [Google Scholar] [CrossRef]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in Soil Using Amendments—A Review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef]
- Uchimiya, M.; Klasson, K.T.; Wartelle, L.H.; Lima, I.M. Influence of Soil Properties on Heavy Metal Sequestration by Biochar Amendment: 1. Copper Sorption Isotherms and the Release of Cations. Chemosphere 2011, 82, 1431–1437. [Google Scholar] [CrossRef]
- Ullah, M.S.; Malekian, R.; Randhawa, G.S.; Gill, Y.S.; Singh, S.; Esau, T.J.; Zaman, Q.U.; Afzaal, H.; Du, D.L.; Farooque, A.A. The Potential of Biochar Incorporation into Agricultural Soils to Promote Sustainable Agriculture: Insights from Soil Health, Crop Productivity, Greenhouse Gas Emission Mitigation and Feasibility Perspectives—A Critical Review. Rev. Environ. Sci. Bio/Technol. 2024, 23, 1105–1130. [Google Scholar] [CrossRef]
- Nziguheba, G.; Palm, C.A.; Buresh, R.J.; Smithson, P.C. Soil phosphorus fractions and adsorption as affected by organic and inorganic sources. Plant Soil 1998, 198, 159–168. [Google Scholar] [CrossRef]
- Stevenson, F.J.; Cole, M.A. Cycles of Soil: Carbon, Nitrogen, Phosphorus, Sulfur, Micronutrients, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1999; ISBN 978-0-471-33217-3. [Google Scholar]
- Haynes, R.J.; Naidu, R. Influence of lime, fertilizer and manure applications on soil organic matter content and soil physical conditions: A review. Nutr. Cycl. Agroecosyst. 1998, 51, 123–137. [Google Scholar] [CrossRef]
- Richardson, A.E.; Simpson, R.J. Soil microorganisms mediating phosphorus availability. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef]
- Sharpley, A.N.; Jarvie, H.P.; Buda, A.; May, L.; Spears, B.; Kleinman, P. Phosphorus legacy: Overcoming the effects of past management practices to mitigate future water quality impairment. J. Environ. Qual. 2013, 42, 1308–1326. [Google Scholar] [CrossRef]
- Withers, P.J.A.; Doody, D.G.; Sylvester-Bradley, R. Stewardship to tackle global phosphorus inefficiency: The case of Europe. Ambio 2015, 44, 193–206. [Google Scholar] [CrossRef]
- Sneddon, I.R.; Orueetxebarria, M.; Hodson, M.E.; Schofield, P.F.; Valsami-Jones, E. Use of bone meal amendments to immobilise Pb, Zn and Cd in soil: A leaching column study. Environ. Pollut. 2006, 144, 816–825. [Google Scholar] [CrossRef]










| Granular Fraction Name | Grain Diameter (mm) | Composition (%) |
|---|---|---|
| Coarse sand | 0.2 | 0.35 |
| Fine sand | 0.02–0.2 | 17.21 |
| Dusti | 0.002–0.02 | 39.52 |
| Clay | <0.002 | 42.92 |
| Property | Value | Analysis Method |
|---|---|---|
| pH 1:2.5 | 5.4 | [20] |
| Humus (%) | 2.9 | [21] |
| CaCO3 (%) | 0.00 | [22] |
| Ntotal (%) | 0.18 | [23] |
| PAL (ppm) | 90.00 | [24] |
| Ptotal (%) | 0.090 | [25] |
| KAL (ppm) | 315 | [26] |
| Zn (ppm) | 3.69 | [27] |
| Cu (ppm) | 3.01 | |
| Fe (ppm) | 40.37 | |
| Al (ppm) | 170 | |
| Mn (ppm) | 4.5 | |
| Alkali saturation level (%) | 83.2 | [28] |
| Exchange alkali (me/100 g sol) | 28.3 | [28] |
| Hygroscopicity coefficient (%) | 6.9 | [29] |
| Volumetric weight (g/cm3) | 1.25 | [30] |
| (a) | |
|---|---|
| Component | Content (% w/w) |
| Moisture | 20.9 |
| Ash | 72.8 |
| Protein | 0.6 |
| Fat | 0.3 |
| Fiber | 0.3 |
| Others | 5.1 |
| (b) | |
| Mineral | Content (% w/w) * |
| Calcium (Ca) | 22.6 |
| Phosphorus (P) | 17.7 |
| Magnesium (Mg) | 0.022 |
| Sodium (Na) | 0.030 |
| pH | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| K (mg−1·L) | amax (mg·kg−1) | R2 | K | n–1 | R2 | |
| 2 | 0.0139 | 101.88 | 0.5016 | 1.4559 | 0.8180 | 0.7572 |
| 6 | 0.0389 | 2375.33 | 0.8717 | 115.39 | 0.6966 | 0.9071 |
| 12 | −0.0562 | −3692.79 | 0.9961 | 186.44 | 1.2976 | 0.9944 |
| pH | C (mg·L−1) | Pseudo First Order | Pseudo Second Order | ||||
|---|---|---|---|---|---|---|---|
| k′ (min−1) | R2 | K″ (kg·mg−1·min−1) | v0 (mg·g−1·min−1) | ae (mg·kg−1) | R2 | ||
| 2 | 600 | 0.1801 | 0.7304 | 0.0009 | 3610.18 | 2010.91 | 0.9974 |
| 6 | 600 | 0.1079 | 0.8514 | 0.0003 | 1382.52 | 2116.10 | 0.9931 |
| 12 | 600 | 0.1670 | 0.7590 | 0.0160 | 138,250.19 | 2937.53 | 1 |
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Squares | F Value | p Value |
|---|---|---|---|---|---|
| Between Groups | 9.733 | 2 | 4.867 | 11.231 | 0.002 |
| Within Groups | 5.200 | 12 | 0.433 | ||
| Total | 14.933 | 14 |
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Squares | F Value | p Value |
|---|---|---|---|---|---|
| Between Groups | 4.133 | 2 | 2.067 | 3.263 | 0.074 |
| Within Groups | 7.600 | 12 | 0.633 | ||
| Total | 11.733 | 14 |
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Squares | F Value | p Value |
|---|---|---|---|---|---|
| Between Groups | 1.200 | 2 | 0.600 | 1.125 | 0.357 |
| Within Groups | 6.400 | 12 | 0.533 | ||
| Total | 7.600 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cișmașu, M.; Modrogan, C.; Orbuleț, O.D.; Bosomoiu, M.; Răileanu, M.; Dăncilă, A.M. Bone Meal as a Sustainable Amendment for Zinc Retention in Polluted Soils: Adsorption Mechanisms, Characterization, and Germination Response. Sustainability 2025, 17, 8027. https://doi.org/10.3390/su17178027
Cișmașu M, Modrogan C, Orbuleț OD, Bosomoiu M, Răileanu M, Dăncilă AM. Bone Meal as a Sustainable Amendment for Zinc Retention in Polluted Soils: Adsorption Mechanisms, Characterization, and Germination Response. Sustainability. 2025; 17(17):8027. https://doi.org/10.3390/su17178027
Chicago/Turabian StyleCișmașu (Enache), Mirela, Cristina Modrogan, Oanamari Daniela Orbuleț, Magdalena Bosomoiu, Madălina Răileanu, and Annette Madelene Dăncilă. 2025. "Bone Meal as a Sustainable Amendment for Zinc Retention in Polluted Soils: Adsorption Mechanisms, Characterization, and Germination Response" Sustainability 17, no. 17: 8027. https://doi.org/10.3390/su17178027
APA StyleCișmașu, M., Modrogan, C., Orbuleț, O. D., Bosomoiu, M., Răileanu, M., & Dăncilă, A. M. (2025). Bone Meal as a Sustainable Amendment for Zinc Retention in Polluted Soils: Adsorption Mechanisms, Characterization, and Germination Response. Sustainability, 17(17), 8027. https://doi.org/10.3390/su17178027









