Abstract
Industrial solid waste fly ash has been widely applied in various fields as a resource for waste repurposing. The use of fly ash can significantly reduce production costs and at the same time reduce environmental pollution to achieve sustainability. This study explores the feasibility of using fly ash as a raw material to formulate high-temperature ceramic glazes, examining the composition, surface phases, and texture patterns of the resultant glazes. This study systematically assesses the impact of formulation modifications on glazing qualities by XRF, XRD, and SEM testing methods. The results show that 1. in high-temperature glazes, the element that determines the degree of transparency in the surface phase is the Ti content; 2. Zinc and Ferrum are important factors that can fine-tune the color shade and crystal mention; and 3. controlling the fly ash content in the glaze can change its color and texture. The novelty of this paper lies in utilizing fly ash to create high-performance, high-value-added ceramic products that feature unique aesthetics and artistic effects. In the future, we can investigate the influence of fly ash on glaze coloration, and the formation of different texture effects, as well as achieve specific color mixing.
1. Introduction
Today, our society is facing huge environmental challenges, including global warming, rising sea levels, and acidification of the oceans, which are exacerbating the dangers to the society and the environment’s biosystems [1]. Population growth, globalization, and industrialization have led to a rapid increase in CO2 emissions in the air. Greenhouse gases and aerosols have a significant impact on global warming, with fossil fuel combustion and biofuels being the main sources of carbon dioxide and man-made aerosols [2,3].
Reducing the burning of primary fossil fuels and promoting the use of renewable and sustainable energy are important steps in the fight against climate warming. For the sake of sustainable development, the United Nations Sustainable Development Goals (SDGs), carbon neutral and peak carbon (dual carbon) targets, the Materials 2030 Roadmap issued by the European Union, and the 14th Five-Year Plan for the Development of the Raw Materials Industry have all issued relevant policies [1,4,5]. Green manufacturing is a modernized manufacturing mode with low consumption, low emission, high efficiency, and high benefit. It is a green sustainable development concept throughout the entire production cycle of products [6]. In this background, the field of materials science has also begun to pursue green manufacturing and reuse.
China consumes a large amount of coal-powered energy, and thermal power generation produces millions of tons of fly ash every year. Stockpiling most of the fly ash results in pollution of the surrounding soil and water resources, as well as damage to the ecological environment [7]. Recycling fly ash, an industrial waste material, realizes waste resource utilization and reduces the waste landfill, lowers CO2 emissions, and mitigates the greenhouse effect.
Ceramic glaze is a glassy layer attached to the surface of ceramics with extremely high density. Its composition mainly consists of substances such as Al2O3, CaO, and silicates [8]. Fly ash contains SiO2, Al2O3, Fe2O3, CaO, etc. [9]; these substances closely match the composition required for preparing ceramic glazes, and their fine particles make them suitable for addition to the glaze process.
Exploring the application of fly ash in ceramic glazes is of enormous importance. This importance arises not only from the ecological significance of fly ash but also from its role as a cost-effective raw material option for the ceramic industry. The ceramic production industry is characterized by high output and a significant demand for coal-fired power generation. The use of fly ash in ceramics alleviates the accumulation of waste materials and facilitates resource recycling [10]. It helps promote green manufacturing and sustainable development. However, most research on fly ash has focused on building materials, road engineering, and soil improvement [8]. Engineering applications account for more than 90% of fly ash utilization and are the main area of application [11]. These mainstream disposal methods can utilize large amounts of fly ash, but the added value of the products is low.
Based on this, developing new ceramic glazes from solid waste fly ash can bring low-cost, high-value-added benefits. Meanwhile, developing new ceramic glazes can achieve the goals of sustainable development and environmental protection. This research attempts to select industrial waste fly ash as a raw material for ceramic glazes. The project aims to achieve sustainable development of materials based on resource recycling. To achieve this goal, we need to leverage the following characteristics of fly ash: 1. it has excellent refractoriness and is suitable for high-temperature firing; 2. its chemical composition is very compatible with the composition of mixed ceramic glazes; 3. the particle texture on the surface of fly ash is similar to the glaze effect, which is more in line with the aesthetic view of ceramic art; 4. the surface texture of ceramic glaze can enhance its artistic value and become a new type of decorative material, and it can also produce innovative features in high value-added products; and 5. replacing kaolin with fly ash as a ceramic glaze material can significantly reduce raw material production costs.
Besides waste minimization, using fly ash also reduces the cost of extracting natural minerals, providing greater benefits at a lower cost. Therefore, developing sustainable new high-temperature glazes is an innovative task. It aims to address the research gap in the use of fly ash, an industrial waste product, as a substitute for natural raw materials (such as kaolin) in ceramic glaze formulations. It promotes the development of high value-added, eco-friendly ceramic materials through green manufacturing and sustainable development principles.
2. Literature Review
Currently, the composition of fly ash typically consists of SiO2, Al2O3, Fe2O3, and CaO. These components can be used as geopolymer additives, concrete additives, and in materials such as raw cement and brick walls. It is currently primarily used in the construction and building industries [12,13]. Fly ash is one of the primary waste products of industrial combustion processes. Fly ash’s potential as a volcanic ash and supplementary cementitious material (SCM) largely drives its value in building materials [14]. Fly ash waste generated from industrial combustion can be used as an SCM to replace cement, thereby promoting the resource utilization of waste [15].
Fly ash research mainly focuses on four aspects: construction materials, mine filling, soil modification, and wastewater treatment, mostly focusing on the engineering-type utilization of fly ash [11,16]. The use of fly ash in construction materials not only improves the durability and strength of concrete but also reduces carbon dioxide emissions in cement production [17]. In terms of concrete utilization, Golewski states that a combination of fly ash and nanoscale additives (NS) can significantly improve the fracture toughness of concrete and enhance the load-bearing capacity [18]. In wastewater treatment, fly ash is capable of adsorbing solid waste due to its inherent porous structure and richness in clay minerals such as silicates. Therefore, the negative charge on its surface can also adsorb harmful ions in water through electrostatic forces [19]. In battery manufacturing, materials developed using fly ash can improve the specific capacity and cycle stability of batteries [17].
It has been proven that fly ash can be used in ceramics and artistic glazes [20]. Fly ash has been proven to be usable in microcrystalline glass ceramics, and the iron oxide, goethite, mullite, and spinel contained in it can all be used as ceramic colorants [21]. It gives ceramics made with fly ash a unique color. The metal elements it contains reduce the amount of coloring agents needed when making ceramic glazes. Fly ash is fine-grained, which makes it easy to use in ceramics. It is worth noting that it can be added to ceramic glazes with almost no pretreatment, which saves on grinding costs [22,23]. Currently, environmental engineering, materials science, and low-temperature materials development are the main fields that use fly ash. In other fields, such as artistic ceramics, the utilization rate of high-temperature functional materials is relatively low.
The oxides in fly ash are very similar to the raw materials used in ceramic production. Traditional ceramic glazes typically contain kaolin, feldspar, quartz, and other natural mineral materials [24]. Since these traditional ceramic glaze materials are natural minerals, the preparation of glazes requires a large amount of mining. The preparation of natural powders generates a significant amount of dust, and it also inevitably produces waste and wastewater, which contributes to energy consumption [22]. Fly ash, however, is a solid waste product from coal-fired power generation, abundant in supply, low in cost, and with virtually zero cost since it is a waste material [23]. Therefore, replacing part of the natural mineral raw materials with fly ash is considered an environmentally friendly and cost-effective option.
There has been some research on the use of fly ash as a raw material in ceramic materials. The inherent qualities of fly ash make it suitable for use in the manufacture of ceramic tiles from waste materials [25]. Haiying’s research found that fly ash has low plasticity and needs to be combined with other highly plastic materials to make ceramic tiles. The research developed the optimal ratio for the manufacture of fly ash ceramic tiles, providing a reference for ceramic tile ratios [26]. Fly ash has been proven to be usable in microcrystalline glass ceramics. Its durability makes it promising for use in industrial ceramic glazes [27]. Research by Fernande et al. shows that by adjusting the fly ash formula and production process, fly ash can be used to make block-shaped nucleated glass ceramics and porous glass ceramic composites [28]. Luo used fly ash to synthesize fibrous tobermorite crystals, which significantly improved the mechanical properties of ceramic materials. Meanwhile, it also led to a reduction in sintering temperature, resulting in significant economic and environmental benefits [29].
These materials usually require low gloss and functionality rather than aesthetics and transparency. Most studies focus on the glaze effect while examining the formulation system. However, research on glaze structure, mechanical properties, and chemical stability is not comprehensive.
In 2004, Karasu discovered that fly ash could be directly added to stoneware glaze and used for coloring [30]. In 2009, Sewri used fly ash in ceramic glaze to explore color changes during the firing process. The results indicated that iron oxide and unburned carbon in fly ash are the primary factors influencing its color [20]. Based on this, fly ash has significant value in ceramic glazes. However, the principles governing the content of fly ash, its interaction with other components, and the formation of glaze properties have not yet been fully explored.
The research explored the feasibility of using fly ash to develop highly transparent, low-consumption, design-oriented colored glazes. The raw material ratio for preparing high-temperature colored glazes for ceramics was determined using fly ash, and attempts were made to replace the kaolin used in traditional glazes with fly ash. The composition, surface phase, and texture were then analyzed. The research used XRF, XRD, and SEM testing methods to systematically evaluate the impact of formula adjustments on glaze performance.
In contrast to previous low-temperature or brick-based applications, the novelty of this study lies in the application of fly ash to high-performance, high-value-added ceramic products. Consider decorative and ornamental ceramics as an example. This work has theoretical and practical significance. Using fly ash as a ceramic glaze material exchanges the low cost of raw materials for high efficiency. It effectively promotes the reuse of industrial waste. The metal elements inherent in fly ash also reduce the need for colorants, resulting in resource conservation. The granular texture on the surface of fly ash imparts a rustic and steady feel. There are also innovations in the artistic aspects of the glaze.
3. Experimental Part
3.1. Sample Preparation
Ceramic preparation: This study revives traditional ceramic art production techniques and demonstrates the role of fly ash in glaze. Fly ash completely replaces the kaolin required in traditional glazes. The body is selected from high-white clay produced by Jingdezhen Firefly Glaze Clay Company in China. It belongs to the commonly used clay material kaolin. The unglazed body was formed into 3 cm square samples using ceramic pressing technology, as shown in Figure 1. An electric kiln was used as the tool for firing the experimental samples, as it offers convenient temperature and atmosphere control, uniform heat distribution, and ease of controlling variables [31]. The samples were fired at 850 °C for 5 h to produce green bodies. This step mimics the rapid firing process of traditional ceramic products. It transforms the clay into a pottery state, enhancing hardness and thereby improving the yield rate. During the glaze firing process, we use a three-stage firing method. The first stage is from room temperature to 350 °C. Slow heating allows the clay body to dry. The second stage takes 5.1 h to heat from 350 °C to 1270 °C. The third stage holds the temperature at 1270 °C for half an hour.

Figure 1.
Data collection process for identifying social innovation in relation to forestry.
Glaze preparation: Fly ash, lithium feldspar, feldspar, and quartz were selected as the basic ingredients for the transparent glaze. By adjusting the proportions of different colorants, five glaze samples with distinct color tendencies were formulated. After firing, they were named tcsy-1, tcsy-2, tcsy-3, tcsy-4, and tcsy-5 according to the different colorant ratios, where FA refers to fly ash. The specific contents are shown in Table 1.

Table 1.
Content of 5 samples.
Pass the glaze slurry through a 200-mesh ultra-fine sieve to remove larger impurities. Then, using a cross-hatch technique, apply the prepared glaze evenly to the green body in three coats. After allowing it to dry naturally, all samples are fired to 1270 °C using a traditional medium-temperature artistic glaze firing curve. After firing for 8 h, the temperature is allowed to cool naturally. The final test samples are obtained. As shown in Figure 1, the post-firing section reveals that the overall color of the samples tends toward yellowish brown.
3.2. Characterization Methods
The chemical composition of the five different formulations of color glazes was tested for chemical performance by XRF (Malvern Panalytical-Zetium, Malvern, Worcestershire, UK).
The glaze crystal structure was analyzed using an X-ray diffractometer (XRD: Malvern Panalytical X’pert3 Powder, Malvern, Worcestershire, UK) to analyze the samples. The micromorphology of the glaze samples was observed using a scanning electron microscope (SEM: Hitachi S-4800, Tokyo, Japan) operated at a voltage of 20 kV with a scale of 30 μm and 100 μm.
4. Analysis of Results
4.1. Chemical Composition Analysis Results
Five samples had different glassy transparent textures and color tendencies, which are crucial elements in the application of ceramic glazes. The chemical characterization of the glazes was analyzed using XRF detection with variations in glaze morphology. The specific chemical characterization data are presented in Table 2.

Table 2.
XRF assay chemical composition (wt%) of the 5 samples.
As we all know, the content of SiO2 is a crucial factor in determining how the glaze exhibits a glassy texture, and it also serves as an important index for assessing the glaze’s “feel.” Its level can affect the glass phase of the glaze, its viscosity, and its fluidity during firing [32]. Al2O3 will determine the degree of glass phase stabilization of the glaze. P2O5, on the other hand, will promote glaze precipitation and fine-tune the viscosity [33,34].
From Table 2, the sum of the five samples is around 79.3–88.1 wt% in SiO2 + Al2O3 + P2O5, which indicates that they all belong to the high-silica–aluminum glass phase. The SiO2 values of all five samples are around 62.7–69.5 wt%, which is not a big difference. Among them, tcsy-1 belongs to the high-silica–medium-aluminum matrix (63.16, 15.86), where both high silica and BaO promote the synthesis of BaSi2O5 [35]. This results in a foggy, glassy texture. tcsy-5 is a double-high Si and Al basis (69.57, 18.22) with a high glassy texture. tcsy-3 and tcsy-4 embody the highest K2O (3.72) and Cl (0.138), respectively, and would have a high crystalline texture.
The color of oxides corresponds to that of glazes. The chemical values of variable oxides are an important basis for determining color. In Table 2, the chemical elements that can affect glaze color are TiO2, Fe2O3, ZnO, BaO, and Cr2O3, which appear only in tcsy-5. Among them, the elements Ti and Fe control the development of glaze colors toward yellow, dark, and red [36]. Zinc oxide and barium oxide act as promoters, facilitating crystal precipitation by lowering the melting point. This results in a soft cream color. Cr2O3 is a strong colorant that produces a deep green hue in the glaze. In tcsy-1, tcsy-3, and tcsy-4, TiO2 is equal to or exceeds 7 wt%. The TiO2 content enhances the opacity of the glaze surface. tcsy-1 has the highest TiO2 value, reaching 9.72, and meanwhile contains a large amount of BaO. It provides the glaze a matte beige color. tcsy-2 contains a large amount of iron oxide and zinc oxide. The higher the iron content, the darker the glaze color. The presence of zinc oxide increases fluidity during firing and often produces ZnFe2O4 spinel [37]. It has a matte dark brown texture. tcsy-3 has a more balanced content of titanium, iron, and zinc, and its glaze is yellowish brown. In comparison, tcsy-4 has twice the TiO2 and Fe2O3 content of the other samples.
Additionally, high concentrations of zinc oxide cause the glaze surface to appear rough and feature yellowish-brown streaks. Table 2 shows that tcsy-2, in contrast to tcsy-4 and tcsy-5, has a higher zinc oxide content. Under high-temperature reduction conditions, it can promote crystal precipitation together with Ti. The TiO2 value of tcsy-5 is only 0.50, and the Si-Al value is the highest, so it has a pure glass texture. The sample had only a small amount of Cr2O3 (0.029), possibly due to volatile adsorption in the firing kiln. Combined with the fact that the sample color did not turn green, it had no effect on this research and can therefore be ignored.
4.2. XRD Crystal Phase Analysis Results
Figure 2 shows the XRD spectra of the five sample glazes. The spectrum in tcsy-1 shows that a large amount of TiO2 crystallization is found at 27.8° (PDF Number 73-2224) and 54.3° (PDF Number 73-2224) and is the sample with the highest crystal talk of the five samples. It shows that TiO2 in this sample is mainly rutile. Rutile precipitates in the glaze and form scattering centers, which reduce gloss by disrupting the uniformity of the glass phase [38,39]. Therefore, the evidence corroborates that the glaze in tcsy-1 is the reason for the apparent matte texture, in line with the chemical characterization results of XRF.
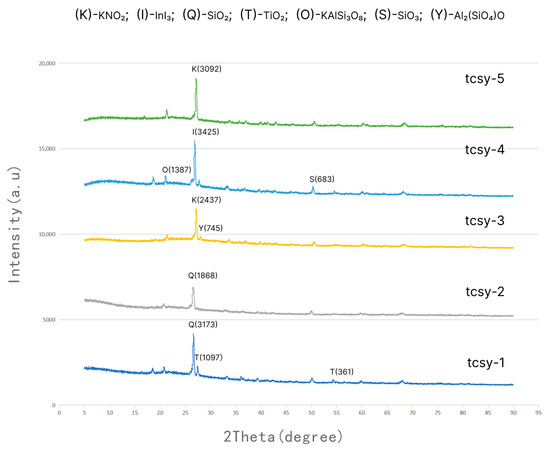
Figure 2.
XRD spectra of 5 sample glazes.
The tcsy-3 spectrum shows a distinct diffraction peak at 28.1° (PDF No. 89-0886), along with a small number of secondary peaks at 28.9° (PDF No. 84-2369). The sample is considered a raw material because some of the kaolin or hydromica powders in the glaze were incompletely fired. Such residual crystals result in a pitting texture in the glaze, creating a yellowish-brown glaze fleck texture.
The characteristic peaks in the graph of tcsy-4 are 21.1° (PDF No. 76-0823) for orthoclase and 50.3° (PDF No. 78-1253) for SiO3, which are distinguished from the high silica texture of tcsy-1 and tcsy-2. The high KAlSi3O8 values and low SiO3 are due to the fact that there may still be quartzite and feldspar residues in the glaze, which increase the refractive index of the glaze. In addition, the low SiO3 leads to a low vitrification of the glaze, resulting in a frosted texture glaze feature. Unlike the others, the figure for tcsy-5 has no obvious spikes, only a glass halo, and the crystallinity is also essentially 0. From this, it can be inferred that tcsy-5 is a highly vitrified (mirrored) glaze feature.
4.3. SEM Inspection and Analysis
The microscopic results of the five samples were examined using the SEM approach. Figure 3(1a–5a) are the surfaces observed at 100 μm for samples tcsy-1 to 5. Figure 3(1b–5b) are surfaces at 30 μm of tcsy-1 to 5, as shown in Figure 3. The samples were analyzed at 20.0 kV for low magnification of the base’s overall distribution arrangement and high magnification of the crystal shape and number.
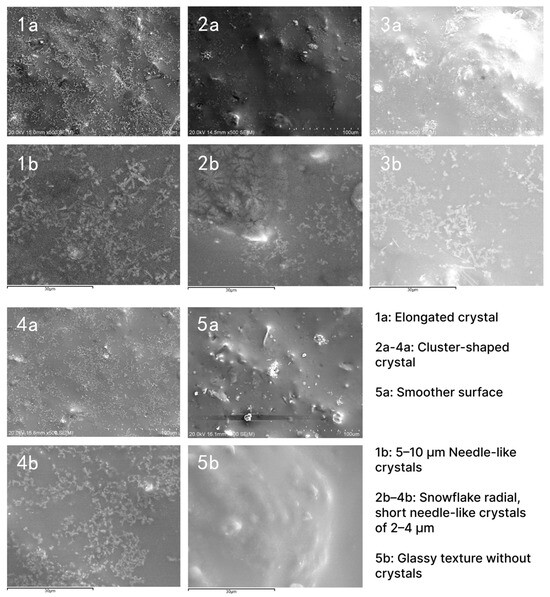
Figure 3.
SEM images of surface enamel of 5 samples.
Macrostructural examination at 100 μm from Figure 3(1a–5a) showed a microstructural transition from the 0 compact elongated crystal form of Figure 3(1a) to the cluster-shaped crystal structure of Figure 3(2a–4a). Finally, Figure 3(5a) has a smoother surface with no needle-like crystals formed. Combined with Figure 4 to see the distribution of the elements, tcsy-1–5 show a decreasing relationship between the density of Ti elements, from sparse to very little. It confirms the high and low number of crystallized crystals in the samples.
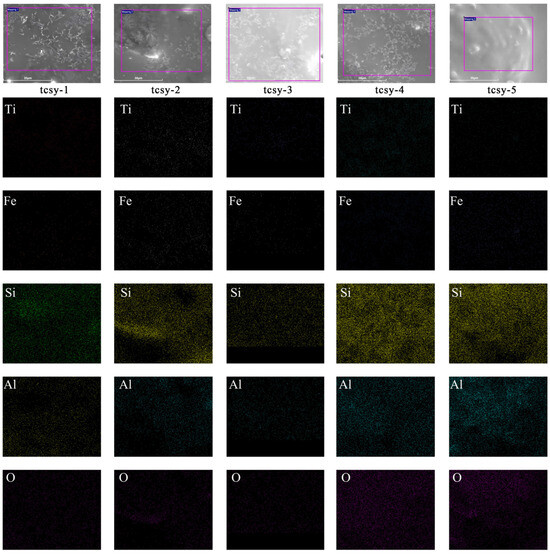
Figure 4.
A localized SEM image of the glaze surface and a chemical element map.
Observed in microscopic detail at 30 μm, Figure 3(1b) exhibits abundant and tightly packed 5–10 μm needle-like crystals. This coincides with the previously detected strong peaks of the highest Al2TiO5, which form a misty glaze with multiple scattering states [40]. Figure 3(2b–4b) show snowflake radial, short needle-like crystals of 2–4 μm, respectively. It indicates that the individual samples show different degrees of crystallization, which, in combination with the coloring oxides, will result in matte and soft glaze characteristics. Combined with chemical element Figure 4, tcsy-2 and tcsy-4 have higher ZnO, and Fe2O densities. The zinc oxide and iron contents are 0.19 wt% and 4.3 wt%, and 0.16 wt% and 4.20 wt%, respectively. This combination may result in the formation of ZnFe2O4 crystals, which is consistent with the SEM characterization appearing as short needle crystals. In contrast, Figure 3(5b) realizes a glassy texture without crystals as well as a bubbly character. Additionally, the high SiO2 and Al2O3 elemental distribution shown in Figure 4 contributes to this observation. It appears to be a highly vitrified glaze, which is also consistent with previous studies.
5. Conclusions
This study confirms the feasibility of industrial waste fly ash as a raw material in ceramic glazes. The texture and color of the glaze vary depending on the different formulation proportions used. Such different textures and colors are in accordance with the appearance design requirements of ceramic products.
We made five kinds of high-temperature glazes using solid waste fly ash as the main raw material and analyzed the laws between their composition, surface phase, and texture. The findings indicate the following:
- In high-temperature glazes, the titanium content dictates the level of transparency based on its face phase. When TiO2 is equal to or above 7 wt%, there are pronounced and moderate peaks of rutile and Al2TiO5, which represent the essential threshold for achieving glaze transparency texture [39,41]. The increase in TiO2 is associated with a rise in both the quantity of rutile and the number of crystals.
- Zinc and Ferrum are important factors that can fine-tune the color shades and crystal characteristics. Iron oxide and zinc oxide showed peaks in the tcsy-2 and tcsy-4 samples, accounting for approximately 12% of the cluster. This analysis also suggests that the appearance of dark red and reddish brown glazes does not occur until Fe and Zn reach this ratio [42].
This indicates that using fly ash in ceramic glazes at concentrations under 30% is viable. From the perspective of manufacturers, including fly ash in ceramic glaze production decreases kaolin expenses while addressing solid waste from industrial operations. It facilitates the transition of the ceramics industry to technological innovation.
This research approach successfully controls the texture and color of the glaze, demonstrating its feasibility. This research method for producing sustainable glazes is feasible in terms of controlling the texture and color of glazes and achieves resource recycling through waste reuse. In industrial glaze production, controlling the iron oxide content between 0.19 and 4.20 yields varying depths of reddish-brown hues.
This method is suitable for producing a wide range of ceramic items, including decorative daily necessities and decorations, while achieving environmentally friendly and unique aesthetic effects. It provides a highly efficient conversion path for the circular economy.
Limitation and Future Study
However, the research still has certain limitations. First is the variability in raw material composition. Fly ash from different batches and origins exhibits slight variations in composition. Differences between batches may influence the results to some extent. This study did not systematically evaluate the impact of raw material variability on the final glaze performance. Second, while the research confirmed the influence of iron, zinc, and titanium elements on ceramic glaze surfaces, further testing of the glaze’s safety properties is required to advance its practical application in daily-use ceramics. This investigation includes assessing durability, pressure resistance, and food safety to comprehensively evaluate the feasibility of this technology for everyday porcelain.
Author Contributions
Conceptualization, Y.D.; Methodology, K.W.; Validation, M.C.; Writing—original draft, Y.D. and M.C.; Visualization, T.W.; Supervision, L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Hebei Natural Science Foundation grant number E2024105053.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nations, U. Transforming our World: The 2030 Agenda for Sustainable Development. 2015. Available online: https://www.un.org/zh/documents/treaty/A-RES-70-1 (accessed on 30 June 2025).
- Hansen, J.E.; Pushker, K.; Makiko, S.; George, T.; Joseph, K.; Bauer, S.E.; Reto, R.; Eunbi, J.; Qinjian, J.; Eric, R.; et al. Global Warming Has Accelerated: Are the United Nations and the Public Well-Informed? Environ. Sci. Policy Sustain. Dev. 2025, 67, 6–44. [Google Scholar] [CrossRef]
- Bilgili, M.; Tumse, S.; Nar, S. Comprehensive Overview on the Present State and Evolution of Global Warming, Climate Change, Greenhouse Gasses and Renewable Energy. Arab. J. Sci. Eng. 2024, 49, 14503–14531. [Google Scholar] [CrossRef]
- Communist Party of China (or Chinese Communist Party). ‘Opinion of the Central Committee of the Communist Party of China and the State Council on Comprehensively and Accurately Implementing the New Development Philosophy and Doing a Good Job in Carbon Peaking and Carbon Neutrality Work’. 2021. Available online: https://www.gov.cn/gongbao/content/2021/content_5649728.htm (accessed on 30 June 2025).
- Communist Party of China (or Chinese Communist Party). ‘The 14th Five-Year Plan for the Development of the Raw Materials Industry Issued—Enhancing the Raw Materials Industry’s Capacity to Ensure Supply’. 2022. Available online: https://www.gov.cn/zhengce/2022-01/03/content_5666170.htm (accessed on 30 June 2025).
- Communist Party of China (or Chinese Communist Party). Green Manufacturing Series National Standards Released. 2023. Available online: https://www.miit.gov.cn/jgsj/jns/lszz/art/2023/art_70a2a2c3b0574e5da6fe77fa971ac9a1.html (accessed on 30 June 2025).
- Yang, H.Y.; Guo, H.L.; Sun, H.J.; Peng, T.J. Surface modification study of CaO-Al2O3-SiO2-Fe2O3 base system high temperature phase reconstruction. Front. Mater. 2023, 9, 1109363. [Google Scholar] [CrossRef]
- Xu, X.; Sang, S.; Li, Y.; Xu, Y.; Wang, Q. Study on the Formation of High-Temperature Glaze Layers on the Surface of Corundum Castable Refractories and Their Resistance to Alkali Erosion. Refract. Mater. 2016, 50, 329–334. [Google Scholar] [CrossRef]
- Yao, Z.; Ji, X.; Sarker, P.K.; Tang, J.; Ge, L.; Xia, M.; Xi, Y. A comprehensive review on the applications of coal fly ash. Earth-Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef]
- Zimmer, A.; Bergmann, C.P. Fly ash of mineral coal as ceramic tiles raw material. Waste Manag. 2007, 27, 59–68. [Google Scholar] [CrossRef]
- Hao, L.; Qin, S.; Pang, W.; Li, S.; Lyu, D.; Zheng, X.; Hou, J.; Men, C. Research Progress on the Migration Patterns of Harmful Elements and Environmental Risk Assessment During the Storage and Utilisation of Fly Ash. Silic. Bull. 2025, 44, 151–168. [Google Scholar] [CrossRef]
- Alterary, S.S.; Marei, N.H. Fly ash properties, characterization, and applications: A review. J. King Saud Univ.-Sci. 2021, 33, 101536. [Google Scholar] [CrossRef]
- Vilakazi, A.Q.; Ndlovu, S.; Chipise, L.; Shemi, A. The Recycling of Coal Fly Ash: A Review on Sustainable Developments and Economic Considerations. Sustainability 2022, 14, 1958. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Y.; Li, Z.; Yang, M.; Zheng, Y. Study on the Adsorption Performance of a New Modified Fly Ash Material on Heavy Metal-Contaminated Wastewater. Contemp. Chem. Ind. 2024, 53, 1931–1934+1943. [Google Scholar] [CrossRef]
- Amran, M.; Fediuk, R.; Murali, G.; Avudaiappan, S.; Ozbakkaloglu, T.; Vatin, N.; Karelina, M.; Klyuev, S.; Gholampour, A. Fly Ash-Based Eco-Efficient Concretes: A Comprehensive Review of the Short-Term Properties. Materials 2021, 14, 4264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, C.; Cheng, G.; Von Lau, E. Towards carbon neutrality: A comprehensive study on the utilization and resource recovery of coal-based solid wastes. Int. J. Coal Sci. Technol. 2025, 12, 34. [Google Scholar] [CrossRef]
- Sharma, V.; Dash, S.; Gupta, P. Comprehensive Review of Fly Ash: Environmental Impact and Applications. Environ. Qual. Manag. 2024, 34, e22338. [Google Scholar] [CrossRef]
- Golewski, G.L. Enhancement fracture behavior of sustainable cementitious composites using synergy between fly ash (FA) and nanosilica (NS) in the assessment based on digital image processing procedure. Theor. Appl. Fract. Mech. 2024, 131, 104442. [Google Scholar] [CrossRef]
- Dong, S.; Qiao, J.; Kang, C.; Sun, M.; Yang, S.; Zhao, Y.; Yang, Y.; Sun, W.; Duan, C. A review of coal solid waste-based catalytic materials in wastewater treatment: Preparation and application. Sep. Purif. Technol. 2025, 367, 132800. [Google Scholar] [CrossRef]
- Sewri; Park, S.; Chung, Y.J. A Study on the Ceramic Body and Art Glaze by Using Coal Ash. J. Korean Ceram. Soc. 2009, 46, 548–553. [Google Scholar] [CrossRef]
- Grabias-Blicharz, E.; Franus, W. A critical review on mechanochemical processing of fly ash and fly ash-derived materials. Sci. Total Environ. 2023, 860, 160529. [Google Scholar] [CrossRef]
- Communist Party of China (or Chinese Communist Party). HJ2304-2018; Feasible Technology Guidelines for Pollution Prevention and Control in the Ceramic Industry. China Environment Publishing Group: Beijing, China, 2018.
- Ferreira, C.; Ribeiro, A.; Ottosen, L. Possible applications for municipal solid waste fly ash. J. Hazard. Mater. 2003, 96, 201–216. [Google Scholar] [CrossRef]
- Chop, H.; Arnold, B.J. Utilization of Coal Wastes for the Production of Ceramic Materials: A Review. Min. Metall. Explor. 2025, 42, 1001–1023. [Google Scholar] [CrossRef]
- Alves, C.L.; Mueller, J.d.O.M.; de Noni, A., Jr.; Heinrich, S. Challenges and opportunities for increase sustainability and energy efficiency in ceramic tile industry. Int. J. Appl. Ceram. Technol. 2025, 22, e15097. [Google Scholar] [CrossRef]
- Haiying, Z.; Youcai, Z.; Jingyu, Q. Utilization of municipal solid waste incineration (MSWI) fly ash in ceramic brick: Product characterization and environmental toxicity. Waste Manag. 2011, 31, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Quesada, E.; Villaquirán-Caicedo, M.A.; Gutiérrez, R.M.d. New glass-ceramic from ternary–quaternary mixtures based on Colombian industrial wastes: Blast furnace slag, cupper slag, fly ash and glass cullet. Boletín Soc. Española Cerámica Vidr. 2022, 61, 284–299. [Google Scholar] [CrossRef]
- Fernandes, H.R.; Gunduz, O.; Tulyaganov, D.U. Synthesis of Bulk-Nucleated Glass-Ceramics and Porous Glass-Ceramic Composites through Utilization of Fly Ashes. Ceramics 2024, 7, 1014–1029. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, S.; Zhao, Z.; Wang, Z.; Zheng, S.; Wang, X. Preparation and characterization of whisker-reinforced ceramics from coal fly ash. Ceram. Int. 2017, 43, 1–11. [Google Scholar] [CrossRef]
- Karasu, B.; Kaya, G.; Aydasgil, A.; Kurama, H. Use of tuncbilek thermal power plant’s fly ash in stoneware glazes as a colouring agent. In Euro Ceramics Viii, Pts 1–3; Mandal, H., Ovecoglu, L., Eds.; Key Engineering Materials; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2004; Volume 264–268, pp. 2501–2504. [Google Scholar]
- Gould, R.E.; Toole, M.G. Preliminary Experiments for the Development of a Continuous Electric Ceramic Kiln for High-Temperature Burning. Trans. Electrochem. Soc. 1936, 70, 111. [Google Scholar] [CrossRef]
- Xu, J.; Ren, F.; Liu, C.; Tang, C.; Han, S.; Chen, Y.; Song, Y.; Lin, J. Effect of Glaze Composition on the Impact Resistance of Glass Lining of Glass-Lined Vessels. J. Phys. Conf. Ser. 2020, 1676, 012016. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Li, X.; Chen, W.; Bai, M.; Li, X.; Wang, Y. Effect of CaF2−, TiO2− and P2O5− on crystallization and properties of CaO-Al2O3-SiO2-ZrO2-based glass-ceramic glaze. Ceram. Int. 2024, 50, 14139–14150. [Google Scholar] [CrossRef]
- Suvaci, E.; Yildiz, B. Roles of CaO, MgO and SiO2 on crystallization and microstructure development in diopside-based glass-ceramic glazes under industrial fast-firing condition. J. Aust. Ceram. Soc. 2017, 53, 75–81. [Google Scholar] [CrossRef]
- Feng, M.; Chen, M.H.; Yu, Z.D.; Chen, Z.X.; Chen, J.H.; Zhu, S.L.; Wang, F.H. Crystallization and wear behavior of SiO2-Al2O3-ZrO2-Ba(Sr, Ca)O glass-ceramics added with Cr2O3 by different methods. Ceram. Int. 2019, 45, 22617–22624. [Google Scholar] [CrossRef]
- Sun, S.J.; Ding, H.; Ao, W.H.; Liu, Y.G.; Chang, L.; Zhang, J.M. Preparation of a CaCO3-TiO2 composite based opaque glaze: Insight into the mechanism of opacification and glaze yellowing inhibition. J. Eur. Ceram. Soc. 2020, 40, 6171–6180. [Google Scholar] [CrossRef]
- Romero, M.; Rincón, J.M.; Acosta, A. Effect of iron oxide content on the crystallisation of a diopside glass-ceramic glaze. J. Eur. Ceram. Soc. 2002, 22, 883–890. [Google Scholar] [CrossRef]
- Teixeira, S.; Bernardin, A.M. Development of TiO2 white glazes for ceramic tiles. Dye Pigment 2009, 80, 292–296. [Google Scholar] [CrossRef]
- Ke, S.J.; Cheng, X.S.; Wang, Q.H.; Wang, Y.M.; Pan, Z.D. Preparation of a photocatalytic TiO2/ZnTiO3 coating on glazed ceramic tiles. Ceram. Int. 2014, 40, 8891–8895. [Google Scholar] [CrossRef]
- Kim, I.J.; Gauckler, L.G. Formation, Decomposition and Thermal Stability of Al2TiO5 Ceramics. J. Ceram. Sci. Technol. 2012, 3, 49–59. [Google Scholar] [CrossRef]
- Zhou, X.J.; Zhang, X.Z.; Zou, C.H.; Chen, R.H.; Cheng, L.L.; Han, B.T.; Liu, H.F. Insight into the Effect of Counterions on the Chromatic Properties of Cr-Doped Rutile TiO2-Based Pigments. Materials 2022, 15, 2049. [Google Scholar] [CrossRef]
- Konvicka, T.; Solc, Z.; Mosner, P.; Kalendová, A. Comparison of various analytic methods in the study of the reaction between zinc oxide and Fe(III) oxide. J. Therm. Anal. Calorim. 1998, 54, 845–853. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).