Sustainable Management of Bottom Ash and Municipal Sewage Sludge as a Source of Micronutrients for Biomass Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Analysis Methods
2.3. Calculations
- (a)
- Dry matter yield (Y, Mg·ha−1 DM), calculated as a sum for each year of the study period, 2013–2018.
- (b)
- Concentrations of micronutrients Mn, Fe, Mo, Co and Al in the plant biomass, calculated as a mean for each year of the study period (X, mg·kg−1 DM).
- (c)
- Mass ratio for Fe/Mn in the plant biomass, calculated from the respective mean concentration of the micronutrients over the entire study period.
- (d)
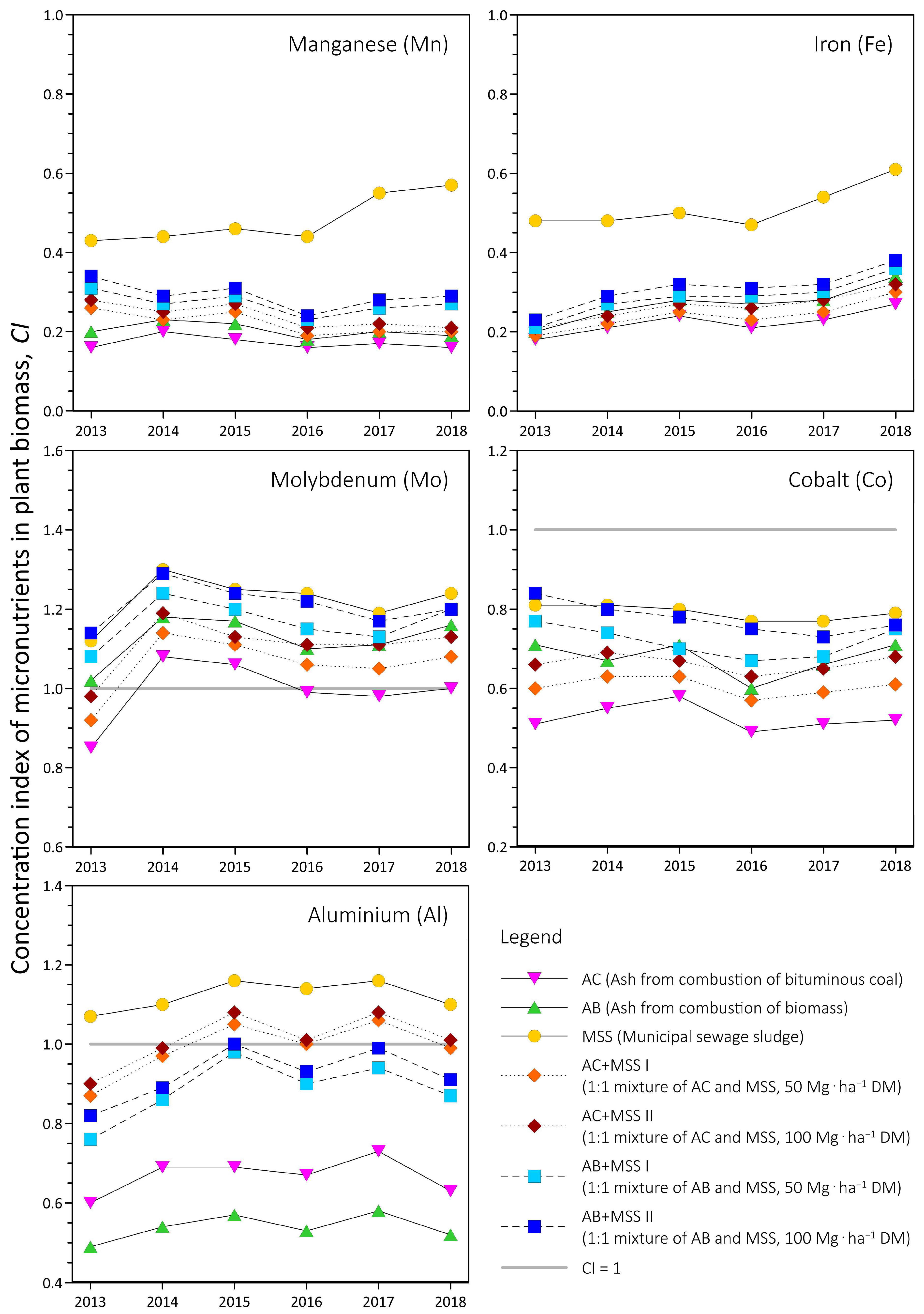
- Concentration index (CI) of micronutrients in the plant biomass, calculated as the ratio of the concentration of a given metal in the waste-applied plots to the metal concentration in the plants grown in the control for each year of the study period.
- (e)
- Uptake of each micronutrient (U = Y × X, g·ha−1), calculated annually and summed up over the entire study period.
- (f)
- Simplified balance (B, g·ha−1 DM) of micronutrients over the entire study period, calculated from the difference between the amounts of elements introduced with the waste (input, I, g·ha−1 DM) and the micronutrients taken up (U) with the plant yield, B = I − U. The recovery rate of micronutrients (R, %) was the percentage of the micronutrients’ uptake in relation to the amount introduced into the soil with the waste. The R was calculated as follows: R = (U/I) × 100.
2.4. Statistical Analysis
3. Results
3.1. Plant Yield
3.2. Concentration in Plant Biomass and Uptake by Plants of Micronutrients
3.3. Simplified Balance and Recovery of Micronutrients by Plants
4. Discussion
4.1. Micronutrient Concentration in Plant Biomass
4.2. Concentration Index
4.3. Micronutrient Uptake
4.4. Simplified Balance and Recovery of Micronutrients by Plants
5. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Treatment | Year | |||||
|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
| Ct | 4.10 ± 0.12 | 6.85 ± 0.74 | 6.98 ± 0.52 | 6.54 ± 0.39 | 7.05 ± 0.21 | 6.62 ± 0.26 |
| vw | jklmno | hijklm | nopq | fghijk | mnop | |
| AC | 3.88 ± 0.11 | 5.92 ± 0.44 | 6.14 ± 0.23 | 6.07 ± 0.25 | 6.32 ± 0.38 | 6.14 ± 0.19 |
| w | s | rs | s | pqrs | qrs | |
| AB | 4.16 ± 0.23 | 6.61 ± 0.34 | 6.64 ± 0.23 | 6.57 ± 0.12 | 6.84 ± 0.19 | 6.52 ± 0.17 |
| vw | mnop | lmnop | nop | klmno | opqr | |
| MSS | 4.32 ± 0.14 | 7.38 ± 0.21 | 7.26 ± 0.53 | 6.85 ± 0.12 | 7.75 ± 0.33 | 7.09 ± 0.19 |
| uv | defgh | efghi | jklmno | bcd | fghijk | |
| AC+MSS I | 4.57 ± 0.07 | 7.27 ± 0.29 | 7.59 ± 0.29 | 6.55 ± 0.22 | 7.67 ± 0.21 | 7.40 ± 0.19 |
| tu | efghi | cde | nop | bcd | defg | |
| AC+MSS II | 4.58 ± 0.09 | 7.24 ± 0.27 | 7.45 ± 0.20 | 7.03 ± 0.31 | 7.76 ± 0.13 | 6.89 ± 0.16 |
| tu | efghij | cdef | ghijkl | bcd | ijklmno | |
| AB+MSS I | 4.85 ± 0.08 | 8.01 ± 0.38 | 8.02 ± 0.10 | 7.41 ± 0.33 | 8.29 ± 0.15 | 7.39 ± 0.24 |
| t | ab | ab | defg | a | defg | |
| AB+MSS II | 4.82 ± 0.06 | 7.75 ± 0.20 | 7.84 ± 0.19 | 6.85 ± 0.25 | 7.76 ± 0.53 | 6.93 ± 0.37 |
| t | bcd | bc | jklmno | bcd | ijklmn | |
| Treatment | Year | |||||
|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
| Ct | 219.93 × 1.05 ±1 | 197.12 × 1.10 ±1 | 166.37 × 1.08 ±1 | 181.29 × 1.11 ±1 | 152.80 × 1.06 ±1 | 129.57 × 1.12 ±1 |
| a | b | d | c | e | f | |
| AC | 35.19 × 1.10 ±1 | 39.90 × 1.12 ±1 | 29.29 × 1.19 ±1 | 28.55 × 1.16 ±1 | 26.74 × 1.18 ±1 | 21.03 × 1.09 ±1 |
| tuv | rs | wxy | xyz | zD | F | |
| AB | 44.97 × 1.08 ±1 | 44.65 × 1.09 ±1 | 35.85 × 1.09 ±1 | 31.03 × 1.12 ±1 | 29.85 × 1.14 ±1 | 24.02 × 1.10 ±1 |
| pq | pq | tuv | w | wx | E | |
| MSS | 94.57 × 1.06 ±1 | 87.16 × 1.09 ±1 | 77.14 × 1.09 ±1 | 78.27 × 1.06 ±1 | 83.31 × 1.08 ±1 | 75.86 × 1.11 ±1 |
| g | h | j | ij | hi | j | |
| AC+MSS I | 56.33 × 1.10 ±1 | 45.32 × 1.17 ±1 | 42.42 × 1.10 ±1 | 34.23 × 1.07 ±1 | 31.16 × 1.16 ±1 | 25.34 × 1.10 ±1 |
| lm | pq | qr | uv | w | DE | |
| AC+MSS II | 60.89 × 1.08 ±1 | 49.52 × 1.14 ±1 | 45.02 × 1.07 ±1 | 36.36 × 1.09 ±1 | 33.54 × 1.11 ±1 | 27.75 × 1.08 ±1 |
| l | o | pq | tu | v | yz | |
| AB+MSS I | 68.96 × 1.07 ±1 | 53.91 × 1.11 ±1 | 47.48 × 1.09 ±1 | 40.57 × 1.11 ±1 | 40.30 × 1.09 ±1 | 35.56 × 1.15 ±1 |
| k | mn | op | r | rs | tuv | |
| AB+MSS II | 74.08 × 1.05 ±1 | 57.27 × 1.08 ±1 | 50.84 × 1.08 ±1 | 42.77 × 1.09 ±1 | 42.55 × 1.07 ±1 | 37.75 × 1.11 ±1 |
| jk | lm | no | qr | qr | st | |
| Treatment | Year | |||||
|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
| Ct | 241.52 × 1.06 ±1 | 208.10 × 1.09 ±1 | 176.40 × 1.08 ±1 | 192.86 × 1.10 ±1 | 173.21 × 1.08 ±1 | 146.58 × 1.10 ±1 |
| a | b | d | c | d | e | |
| AC | 42.89 × 1.07 ±1 | 43.66 × 1.05 ±1 | 41.75 × 1.07 ±1 | 40.34 × 1.04 ±1 | 40.42 × 1.10 ±1 | 40.08 × 1.07 ±1 |
| wx | vwx | xy | y | y | y | |
| AB | 49.22 × 1.05 ±1 | 52.89 × 1.03 ±1 | 49.41 × 1.10 ±1 | 51.90 × 1.04 ±1 | 48.36 × 1.09 ±1 | 49.50 × 1.08 ±1 |
| qrs | mno | qrs | nop | rst | qrs | |
| MSS | 116.46 × 1.07 ±1 | 100.02 × 1.11 ±1 | 88.93 × 1.11 ±1 | 89.54 × 1.06 ±1 | 93.96 × 1.05 ±1 | 88.80 × 1.10 ±1 |
| f | g | i | i | h | i | |
| AC+MSS I | 45.28 × 1.04 ±1 | 46.64 × 1.03 ±1 | 44.74 × 1.06 ±1 | 44.19 × 1.05 ±1 | 43.38 × 1.10 ±1 | 44.28 × 1.06 ±1 |
| uv | tu | uvw | vw | vwx | vw | |
| AC+MSS II | 50.12 × 1.03 ±1 | 50.46 × 1.05 ±1 | 47.84 × 1.04 ±1 | 48.47 × 1.05 ±1 | 47.67 × 1.08 ±1 | 46.62 × 1.05 ±1 |
| pqrs | pqr | st | rst | st | tu | |
| AB+MSS I | 51.54 × 1.05 ±1 | 55.78 × 1.03 ±1 | 51.44 × 1.09 ±1 | 54.25 × 1.03 ±1 | 51.70 × 1.06 ±1 | 52.54 × 1.05 ±1 |
| opq | l | opq | lmn | opq | nop | |
| AB+MSS II | 56.26 × 1.10 ±1 | 60.48 × 1.04 ±1 | 55.84 × 1.04 ±1 | 58.49 × 1.06 ±1 | 55.27 × 1.06 ±1 | 55.54 × 1.06 ±1 |
| kl | j | kl | jk | lm | l | |
| Treatment | Year | Mean | |||||
|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | ||
| Ct | 0.30 ± 0.03 | 0.30 ± 0.03 | 0.33 ± 0.03 | 0.31 ± 0.03 | 0.32 ± 0.03 | 0.31 ± 0.04 | 0.31 ± 0.03 |
| e | |||||||
| AC | 0.25 ± 0.06 | 0.32 ± 0.02 | 0.34 ± 0.02 | 0.30 ± 0.02 | 0.31 ± 0.02 | 0.31 ± 0.04 | 0.31 ± 0.04 |
| e | |||||||
| AB | 0.30 ± 0.06 | 0.35 ± 0.03 | 0.38 ± 0.03 | 0.34 ± 0.02 | 0.35 ± 0.02 | 0.36 ± 0.03 | 0.35 ± 0.04 |
| c | |||||||
| MSS | 0.33 ± 0.05 | 0.39 ± 0.02 | 0.41 ± 0.03 | 0.38 ± 0.03 | 0.38 ± 0.02 | 0.39 ± 0.03 | 0.38 ± 0.04 |
| a | |||||||
| AC+MSS I | 0.27 ± 0.06 | 0.34 ± 0.01 | 0.36 ± 0.03 | 0.33 ± 0.02 | 0.34 ± 0.02 | 0.33 ± 0.02 | 0.33 ± 0.04 |
| d | |||||||
| AC+MSS II | 0.29 ± 0.05 | 0.36 ± 0.01 | 0.37 ± 0.03 | 0.34 ± 0.02 | 0.35 ± 0.02 | 0.35 ± 0.03 | 0.35 ± 0.04 |
| c | |||||||
| AB+MSS I | 0.32 ± 0.06 | 0.37 ± 0.02 | 0.39 ± 0.03 | 0.35 ± 0.02 | 0.36 ± 0.02 | 0.36 ± 0.02 | 0.36 ± 0.04 |
| b | |||||||
| AB+MSS II | 0.34 ± 0.05 | 0.39 ± 0.02 | 0.40 ± 0.02 | 0.37 ± 0.02 | 0.37 ± 0.01 | 0.37 ± 0.02 | 0.38 ± 0.03 |
| a | |||||||
| Mean | 0.30 ± 0.06 | 0.35 ± 0.03 | 0.37 ± 0.04 | 0.34 ± 0.03 | 0.35 ± 0.03 | 0.35 ± 0.04 | |
| G | E | D | F | E | E | ||
| Treatment | Year | Mean | |||||
|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | ||
| Ct | 0.27 ± 0.03 | 0.27 ± 0.02 | 0.29 ± 0.03 | 0.27 ± 0.02 | 0.28 ± 0.03 | 0.27 ± 0.04 | 0.28 ± 0.03 |
| a | |||||||
| AC | 0.14 ± 0.02 | 0.15 ± 0.02 | 0.17 ± 0.03 | 0.13 ± 0.02 | 0.14 ± 0.02 | 0.14 ± 0.04 | 0.14 ± 0.03 |
| f | |||||||
| AB | 0.19 ± 0.02 | 0.18 ± 0.02 | 0.21 ± 0.03 | 0.16 ± 0.02 | 0.18 ± 0.02 | 0.19 ± 0.03 | 0.18 ± 0.03 |
| d | |||||||
| MSS | 0.22 ± 0.02 | 0.21 ± 0.02 | 0.23 ± 0.03 | 0.21 ± 0.03 | 0.21 ± 0.02 | 0.21 ± 0.03 | 0.22 ± 0.03 |
| b | |||||||
| AC+MSS I | 0.16 ± 0.02 | 0.17 ± 0.01 | 0.18 ± 0.03 | 0.16 ± 0.02 | 0.17 ± 0.02 | 0.16 ± 0.02 | 0.17 ± 0.02 |
| e | |||||||
| AC+MSS II | 0.18 ± 0.02 | 0.18 ± 0.02 | 0.19 ± 0.03 | 0.17 ± 0.02 | 0.18 ± 0.02 | 0.18 ± 0.03 | 0.18 ± 0.02 |
| d | |||||||
| AB+MSS I | 0.20 ± 0.02 | 0.20 ± 0.01 | 0.21 ± 0.03 | 0.18 ± 0.02 | 0.19 ± 0.02 | 0.19 ± 0.04 | 0.20 ± 0.02 |
| c | |||||||
| AB+MSS II | 0.22 ± 0.01 | 0.21 ± 0.01 | 0.23 ± 0.03 | 0.20 ± 0.02 | 0.20 ± 0.01 | 0.20 ± 0.02 | 0.21 ± 0.02 |
| b | |||||||
| Mean | 0.20 ± 0.04 | 0.20 ± 0.04 | 0.21 ± 0.05 | 0.19 ± 0.05 | 0.20 ± 0.04 | 0.19 ± 0.05 | |
| E | E | D | E | E | E | ||
| Treatment | Year | |||||
|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
| Ct | 27.42 ± 2.99 | 23.41 ± 2.25 | 22.33 ± 2.52 | 24.02 ± 1.82 | 22.26 ± 1.83 | 24.56 ± 1.61 |
| b | ijk | klmn | ghij | klmn | fgh | |
| AC | 16.22 ± 1.58 | 16.09 ± 1.17 | 15.12 ± 1.71 | 16.01 ± 1.71 | 16.42 ± 1.13 | 15.50 ± 1.73 |
| rs | rs | s | rs | r | rs | |
| AB | 13.27 ± 1.23 | 12.57 ± 1.23 | 12.40 ± 1.10 | 12.61 ± 2.01 | 13.06 ± 1.65 | 12.77 ± 1.56 |
| t | t | t | t | t | t | |
| MSS | 29.19 ± 2.15 | 25.58 ± 1.77 | 25.77 ± 1.87 | 27.38 ± 2.00 | 26.05 ± 1.61 | 27.01 ± 1.77 |
| a | def | de | b | cd | bc | |
| AC+MSS I | 23.48 ± 1.83 | 22.52 ± 1.90 | 23.21 ± 1.45 | 23.82 ± 1.41 | 23.85 ± 1.21 | 24.17 ± 2.16 |
| hijk | klm | ijkl | ghij | ghij | ghij | |
| AC+MSS II | 24.20 ± 1.12 | 23.03 ± 1.94 | 23.94 ± 1.31 | 24.23 ± 1.59 | 24.81 ± 1.34 | 24.77 ± 1.83 |
| ghij | jkl | ghij | ghi | efg | efg | |
| AB+MSS I | 20.63 ± 1.37 | 19.85 ± 1.47 | 21.63 ± 1.41 | 21.05 ± 1.35 | 21.21 ± 1.13 | 21.31 ± 1.45 |
| pq | q | mnop | op | nop | nop | |
| AB+MSS II | 22.21 ± 1.08 | 20.61 ± 1.42 | 22.11 ± 1.18 | 22.15 ± 1.22 | 22.20 ± 1.13 | 22.31 ± 1.77 |
| klmno | pq | lmno | lmno | lmno | klmn | |
| Treatment | Year | |||||
|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
| AC | 0.16 × 1.14 ±1 | 0.20 × 1.19 ±1 | 0.18 × 1.17 ±1 | 0.16 × 1.15 ±1 | 0.17 × 1.21 ±1 | 0.16 × 1.13 ±1 |
| q | lmno | pq | q | pq | q | |
| AB | 0.20 × 1.13 ±1 | 0.23 × 1.17 ±1 | 0.22 × 1.11 ±1 | 0.18 × 1.12 ±1 | 0.20 × 1.17 ±1 | 0.19 × 1.16 ±1 |
| lmno | jk | kl | pq | no | op | |
| MSS | 0.43 × 1.08 ±1 | 0.44 × 1.11 ±1 | 0.46 × 1.09 ±1 | 0.44 × 1.13 ±1 | 0.55 × 1.13 ±1 | 0.57 × 1.14 ±1 |
| b | b | b | b | a | a | |
| AC+MSS I | 0.26 × 1.09 ±1 | 0.23 × 1.17 ±1 | 0.25 × 1.08 ±1 | 0.19 × 1.18 ±1 | 0.20 × 1.20 ±1 | 0.20 × 1.19 ±1 |
| ghi | jk | hi | nop | lmno | mno | |
| AC+MSS II | 0.28 × 1.11 ±1 | 0.25 × 1.17 ±1 | 0.27 × 1.07 ±1 | 0.21 × 1.12 ±1 | 0.22 × 1.16 ±1 | 0.21 × 1.16 ±1 |
| efgh | hi | efgh | lmn | kl | klm | |
| AB+MSS I | 0.31 × 1.09 ±1 | 0.27 × 1.11 ±1 | 0.29 × 1.10 ±1 | 0.23 × 1.10 ±1 | 0.26 × 1.13 ±1 | 0.27 × 1.20 ±1 |
| cd | efgh | def | jk | fghi | efgh | |
| AB+MSS II | 0.34 × 1.08 ±1 | 0.29 × 1.10 ±1 | 0.31 × 1.06 ±1 | 0.24 × 1.11 ±1 | 0.28 × 1.11 ±1 | 0.29 × 1.15 ±1 |
| c | de | cd | ij | efg | de | |
| Treatment | Year | |||||
|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
| AC | 0.18 × 1.11 ±1 | 0.21 × 1.07 ±1 | 0.24 × 1.05 ±1 | 0.21 × 1.11 ±1 | 0.23 × 1.12 ±1 | 0.27 × 1.14 ±1 |
| w | tu | pqr | tu | qr | jklm | |
| AB | 0.20 × 1.10 ±1 | 0.25 × 1.09 ±1 | 0.28 × 1.07 ±1 | 0.27 × 1.11 ±1 | 0.28 × 1.14 ±1 | 0.34 × 1.14 ±1 |
| uv | mnop | ijk | jkl | ijk | ef | |
| MSS | 0.48 × 1.09 ±1 | 0.48 × 1.14 ±1 | 0.50 × 1.12 ±1 | 0.47 × 1.13 ±1 | 0.54 × 1.12 ±1 | 0.61 × 1.17 ±1 |
| c | c | bc | c | b | a | |
| AC+MSS I | 0.19 × 1.09 ±1 | 0.22 × 1.09 ±1 | 0.25 × 1.07 ±1 | 0.23 × 1.15 ±1 | 0.25 × 1.15 ±1 | 0.30 × 1.16 ±1 |
| vw | rst | mnop | qr | nopq | gh | |
| AC+MSS II | 0.21 × 1.05 ±1 | 0.24 × 1.09 ±1 | 0.27 × 1.06 ±1 | 0.26 × 1.14 ±1 | 0.28 × 1.15 ±1 | 0.32 × 1.14 ±1 |
| tu | opq | jklm | lmno | jkl | fg | |
| AB+MSS I | 0.21 × 1.10 ±1 | 0.27 × 1.10 ±1 | 0.29 × 1.06 ±1 | 0.29 × 1.12 ±1 | 0.30 × 1.13 ±1 | 0.36 × 1.12 ±1 |
| stu | klmn | hij | hijk | ghi | de | |
| AB+MSS II | 0.23 × 1.09 ±1 | 0.29 × 1.10 ±1 | 0.32 × 1.07 ±1 | 0.31 × 1.12 ±1 | 0.32 × 1.12 ±1 | 0.38 × 1.11 ±1 |
| qrs | hij | fg | gh | fg | d | |
| Treatment | Year | Mean | |||||
|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | ||
| AC | 0.85 ± 0.22 | 1.08 ± 0.13 | 1.06 ± 0.12 | 0.99 ± 0.13 | 0.98 ± 0.07 | 1.00 ± 0.13 | 1.00 ± 0.15 |
| e | |||||||
| AB | 1.02 ± 0.25 | 1.18 ± 0.14 | 1.17 ± 0.14 | 1.10 ± 0.13 | 1.11 ± 0.12 | 1.16 ± 0.12 | 1.13 ± 0.16 |
| c | |||||||
| MSS | 1.12 ± 0.24 | 1.30 ± 0.13 | 1.25 ± 0.14 | 1.24 ± 0.16 | 1.19 ± 0.16 | 1.24 ± 0.13 | 1.23 ± 0.16 |
| a | |||||||
| AC+MSS I | 0.92 ± 0.22 | 1.14 ± 0.10 | 1.11 ± 0.15 | 1.06 ± 0.12 | 1.05 ± 0.10 | 1.08 ± 0.11 | 1.07 ± 0.15 |
| d | |||||||
| AC+MSS II | 0.98 ± 0.22 | 1.19 ± 0.11 | 1.13 ± 0.16 | 1.11 ± 0.13 | 1.11 ± 0.11 | 1.13 ± 0.11 | 1.12 ± 0.15 |
| c | |||||||
| AB+MSS I | 1.08 ± 0.25 | 1.24 ± 0.12 | 1.20 ± 0.17 | 1.15 ± 0.12 | 1.13 ± 0.09 | 1.20 ± 0.15 | 1.17 ± 0.16 |
| b | |||||||
| AB+MSS II | 1.14 ± 0.23 | 1.29 ± 0.13 | 1.24 ± 0.15 | 1.22 ± 0.14 | 1.17 ± 0.10 | 1.20 ± 0.15 | 1.21 ± 0.15 |
| a | |||||||
| Mean | 1.01 ± 0.25 | 1.20 ± 0.14 | 1.17 ± 0.16 | 1.12 ± 0.15 | 1.11 ± 0.13 | 1.15 ± 0.15 | |
| E | D | DE | FG | G | EF | ||
| Treatment | Year | Mean | |||||
|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | ||
| AC | 0.51 ± 0.07 | 0.55 ± 0.09 | 0.58 ± 0.12 | 0.49 ± 0.08 | 0.51 ± 0.07 | 0.52 ± 0.13 | 0.53 ± 0.10 |
| e | |||||||
| AB | 0.71 ± 0.08 | 0.67 ± 0.11 | 0.71 ± 0.15 | 0.60 ± 0.08 | 0.66 ± 0.11 | 0.71 ± 0.14 | 0.67 ± 0.12 |
| c | |||||||
| MSS | 0.81 ± 0.11 | 0.81 ± 0.10 | 0.80 ± 0.14 | 0.77 ± 0.12 | 0.77 ± 0.14 | 0.79 ± 0.13 | 0.79 ± 0.12 |
| a | |||||||
| AC+MSS I | 0.60 ± 0.08 | 0.63 ± 0.07 | 0.63 ± 0.14 | 0.57 ± 0.07 | 0.59 ± 0.08 | 0.61 ± 0.10 | 0.61 ± 0.10 |
| d | |||||||
| AC+MSS II | 0.66 ± 0.07 | 0.69 ± 0.09 | 0.67 ± 0.15 | 0.63 ± 0.08 | 0.65 ± 0.09 | 0.68 ± 0.12 | 0.66 ± 0.10 |
| c | |||||||
| AB+MSS I | 0.77 ± 0.10 | 0.74 ± 0.07 | 0.70 ± 0.17 | 0.67 ± 0.09 | 0.68 ± 0.09 | 0.75 ± 0.15 | 0.72 ± 0.11 |
| b | |||||||
| AB+MSS II | 0.84 ± 0.08 | 0.80 ± 0.09 | 0.78 ± 0.15 | 0.75 ± 0.08 | 0.73 ± 0.08 | 0.76 ± 0.13 | 0.77 ± 0.11 |
| a | |||||||
| Mean | 0.70 ± 0.14 | 0.70 ± 0.12 | 0.69 ± 0.15 | 0.64 ± 0.13 | 0.65 ± 0.12 | 0.69 ± 0.15 | |
| D | D | D | E | E | D | ||
| Treatment | Year | Mean | |||||
|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | ||
| AC | 0.60 ± 0.09 | 0.69 ± 0.07 | 0.69 ± 0.13 | 0.67 ± 0.10 | 0.73 ± 0.11 | 0.63 ± 0.09 | 0.67 ± 0.11 |
| e | |||||||
| AB | 0.49 ± 0.09 | 0.54 ± 0.06 | 0.57 ± 0.10 | 0.53 ± 0.09 | 0.58 ± 0.10 | 0.52 ± 0.08 | 0.54 ± 0.09 |
| f | |||||||
| MSS | 1.07 ± 0.11 | 1.10 ±0.10 | 1.16 ± 0.12 | 1.14 ± 0.10 | 1.16 ± 0.08 | 1.10 ± 0.08 | 1.13 ± 0.10 |
| a | |||||||
| AC+MSS I | 0.87 ± 0.12 | 0.97 ± 0.07 | 1.05 ± 0.12 | 1.00 ± 0.07 | 1.06 ± 0.09 | 0.99 ± 0.10 | 0.99 ± 0.11 |
| b | |||||||
| AC+MSS II | 0.90 ± 0.13 | 0.99 ± 0.09 | 1.08 ± 0.12 | 1.01 ± 0.05 | 1.08 ± 0.13 | 1.01 ± 0.09 | 1.02 ± 0.11 |
| b | |||||||
| AB+MSS I | 0.76 ± 0.11 | 0.86 ± 0.10 | 0.98 ± 0.14 | 0.90 ± 0.10 | 0.94 ± 0.13 | 0.87 ± 0.11 | 0.89 ± 0.12 |
| d | |||||||
| AB+MSS II | 0.82 ± 0.08 | 0.89 ± 0.11 | 1.00 ± 0.11 | 0.93 ± 0.07 | 0.99 ± 0.11 | 0.91 ± 0.07 | 0.92 ± 0.11 |
| c | |||||||
| Mean | 0.79 ± 0.21 | 0.86 ± 0.20 | 0.93 ± 0.24 | 0.88 ± 0.21 | 0.94 ± 0.21 | 0.86 ± 0.21 | |
| F | E | D | E | D | E | ||
| Treatment | Year | |||||
|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
| Ct | 893.98 × 1.02 ±1 | 1359.22 × 1.13 ±1 | 1186.73 × 1.09 ±1 | 1197.06 × 1.09 ±1 | 1070.57 × 1.04 ±1 | 875.74 × 1.12 ±1 |
| d | a | b | b | c | d | |
| AC | 138.70 × 1.08 ±1 | 236.48 × 1.08 ±1 | 186.30 × 1.08 ±1 | 179.21 × 1.05 ±1 | 175.98 × 1.10 ±1 | 129.77 × 1.11 ±1 |
| B | uv | yz | yz | z | B | |
| AB | 189.24 × 1.09 ±1 | 297.68 × 1.07 ±1 | 240.20 × 1.06 ±1 | 209.17 × 1.04 ±1 | 210.57 × 1.04 ±1 | 157.51 × 1.03 ±1 |
| yz | op | tuv | wx | w | A | |
| MSS | 405.39 × 1.05 ±1 | 648.92 × 1.05 ±1 | 568.53 × 1.09 ±1 | 540.61 × 1.03 ±1 | 658.67 × 1.06 ±1 | 529.75 × 1.03 ±1 |
| ij | e | f | f | e | f | |
| AC+MSS I | 253.60 × 1.04 ±1 | 342.20 × 1.06 ±1 | 331.18 × 1.03 ±1 | 224.27 × 1.04 ±1 | 249.01 × 1.01 ±1 | 187.78 × 1.05 ±1 |
| rstu | lm | mn | vw | stu | yz | |
| AC+MSS II | 278.96 × 1.03 ±1 | 372.46 × 1.05 ±1 | 341.87 × 1.02 ±1 | 259.93 × 1.06 ±1 | 268.44 × 1.02 ±1 | 192.77 × 1.02 ±1 |
| pq | jkl | m | qrst | qrs | xy | |
| AB+MSS I | 332.91 × 1.08 ±1 | 441.44 × 1.08 ±1 | 389.10 × 1.04 ±1 | 308.36 × 1.06 ±1 | 338.60 × 1.03 ±1 | 271.56 × 1.06 ±1 |
| mn | gh | ijk | no | m | qr | |
| AB+MSS II | 359.56 × 1.05 ±1 | 451.53 × 1.02 ±1 | 407.69 × 1.04 ±1 | 298.99 × 1.05 ±1 | 331.40 × 1.09 ±1 | 267.90 × 1.07 ±1 |
| klm | g | hi | op | mn | qrs | |
| Treatment | Year | |||||
|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
| Ct | 977.49 × 1.01 ±1 | 1432.05 × 1.13 ±1 | 1256.41 × 1.09 ±1 | 1270.81 × 1.09 ±1 | 1212.30 × 1.05 ±1 | 977.53 × 1.12 ±1 |
| c | a | b | b | b | c | |
| AC | 168.23 × 1.07 ±1 | 259.22 × 1.07 ±1 | 260.12 × 1.06 ±1 | 245.19 × 1.04 ±1 | 254.35 × 1.10 ±1 | 245.49 × 1.03 ±1 |
| v | rs | rs | st | rs | st | |
| AB | 206.49 × 1.07 ±1 | 349.24 × 1.05 ±1 | 335.99 × 1.04 ±1 | 341.89 × 1.02 ±1 | 333.13 × 1.04 ±1 | 321.16 × 1.01 ±1 |
| u | mno | nop | mnop | op | p | |
| MSS | 500.30 × 1.07 ±1 | 760.01 × 1.06 ±1 | 657.34 × 1.09 ±1 | 614.82 × 1.01 ±1 | 734.47 × 1.05 ±1 | 618.29 × 1.04 ±1 |
| f | d | e | e | d | e | |
| AC+MSS I | 209.22 × 1.02 ±1 | 339.42 × 1.05 ±1 | 345.17 × 1.04 ±1 | 287.60 × 1.05 ±1 | 334.44 × 1.05 ±1 | 327.96 × 1.05 ±1 |
| u | mnop | mnop | q | op | op | |
| AC+MSS II | 228.13 × 1.04 ±1 | 364.81 × 1.03 ±1 | 360.36 × 1.02 ±1 | 339.80 × 1.02 ±1 | 375.90 × 1.03 ±1 | 321.08 × 1.01 ±1 |
| t | klm | lmn | mnop | jkl | p | |
| AB+MSS I | 253.90 × 1.03 ±1 | 445.55 × 1.04 ±1 | 422.98 × 1.03 ±1 | 401.40 × 1.05 ±1 | 430.30 × 1.02 ±1 | 389.37 × 1.03 ±1 |
| rs | gh | hi | ij | hi | jk | |
| AB+MSS II | 266.73 × 1.04 ±1 | 471.89 × 1.03 ±1 | 441.00 × 1.03 ±1 | 402.70 × 1.03 ±1 | 433.74 × 1.06 ±1 | 384.11 × 1.07 ±1 |
| r | fg | gh | ij | h | jkl | |
| Treatment | Year | |||||
|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
| Ct | 1.21 ± 0.10 | 2.09 ± 0.28 | 2.31 ± 0.26 | 1.98 ± 0.08 | 2.25 ± 0.11 | 2.07 ± 0.17 |
| v | no | jklm | opq | klmn | nop | |
| AC | 1.01 ± 0.06 | 1.90 ± 0.18 | 2.12 ± 0.11 | 1.84 ± 0.11 | 1.98 ± 0.13 | 1.90 ± 0.10 |
| w | pq | no | qr | opq | pq | |
| AB | 1.29 ± 0.06 | 2.33 ± 0.20 | 2.49 ± 0.10 | 2.21 ± 0.09 | 2.43 ± 0.12 | 2.33 ± 0.14 |
| uv | jkl | hij | lmn | ij | jkl | |
| MSS | 1.46 ± 0.06 | 2.86 ± 0.11 | 2.95 ± 0.18 | 2.59 ± 0.12 | 2.96 ± 0.21 | 2.74 ± 0.09 |
| tu | cde | abc | fghi | abc | def | |
| AC+MSS I | 1.30 ± 0.02 | 2.47 ± 0.11 | 2.71 ± 0.17 | 2.14 ± 0.08 | 2.59 ± 0.11 | 2.46 ± 0.10 |
| uv | hij | efg | mno | fghi | hij | |
| AC+MSS II | 1.38 ± 0.03 | 2.58 ± 0.11 | 2.72 ± 0.13 | 2.42 ± 0.10 | 2.74 ± 0.14 | 2.41 ± 0.07 |
| uv | fghi | efg | ijk | def | ijk | |
| AB+MSS I | 1.61 ± 0.06 | 2.96 ± 0.16 | 3.07 ± 0.12 | 2.62 ± 0.11 | 3.00 ± 0.10 | 2.74 ± 0.18 |
| st | abc | ab | fgh | abc | defg | |
| AB+MSS II | 1.70 ± 0.05 | 2.97 ± 0.10 | 3.10 ± 0.02 | 2.56 ± 0.14 | 2.91 ± 0.18 | 2.58 ± 0.12 |
| rs | abc | a | ghi | bcd | fghi | |
| Treatment | Year | |||||
|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
| Ct | 1.09 ± 0.08 | 1.74 ± 0.14 | 2.16 ± 0.08 | 1.77 ± 0.07 | 1.98 ± 0.09 | 1.82 ± 0.13 |
| rst | cde | a | cd | b | c | |
| AC | 0.52 ± 0.04 | 0.87 ± 0.11 | 1.02 ± 0.10 | 0.81 ± 0.06 | 0.91 ± 0.07 | 0.85 ± 0.12 |
| z | vwx | tu | wxy | uvw | vwx | |
| AB | 0.78 ± 0.03 | 1.17 ± 0.13 | 1.31 ± 0.08 | 1.07 ± 0.08 | 1.27 ± 0.10 | 1.23 ± 0.13 |
| xy | qrs | lmnop | st | nopq | pq | |
| MSS | 0.91 ± 0.05 | 1.59 ± 0.10 | 1.66 ± 0.10 | 1.42 ± 0.12 | 1.64 ± 0.16 | 1.53 ± 0.07 |
| uvwx | ghi | defg | jkl | defgh | hij | |
| AC+MSS I | 0.70 ± 0.03 | 1.21 ± 0.06 | 1.37 ± 0.13 | 1.03 ± 0.05 | 1.28 ± 0.09 | 1.20 ± 0.08 |
| y | pqr | klmn | tu | mnopq | pqr | |
| AC+MSS II | 0.78 ± 0.02 | 1.32 ± 0.07 | 1.40 ± 0.09 | 1.22 ± 0.06 | 1.42 ± 0.13 | 1.24 ± 0.07 |
| xy | lmnop | jklm | pq | jkl | opq | |
| AB+MSS I | 0.98 ± 0.05 | 1.56 ± 0.10 | 1.65 ± 0.11 | 1.36 ± 0.07 | 1.59 ± 0.07 | 1.48 ± 0.15 |
| tuv | ghi | defg | klmno | fghi | ijk | |
| AB+MSS II | 1.06 ± 0.04 | 1.63 ± 0.07 | 1.72 ± 0.02 | 1.39 ± 0.10 | 1.59 ± 0.10 | 1.40 ± 0.06 |
| st | efgh | cdef | klmn | ghi | jklm | |
| Treatment | Year | |||||
|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | |
| Ct | 112.30 ± 9.41 | 153.67 ± 13.42 | 159.03 ± 9.46 | 155.43 ± 10.73 | 161.55 ± 16.08 | 161.08 ± 2.88 |
| p | n | jklmn | mn | jklmn | jklmn | |
| AC | 63.43 ± 4.52 | 94.53 ± 5.89 | 92.05 ± 4.64 | 97.73 ± 8.81 | 103.38 ± 8.87 | 97.05 ± 4.70 |
| x | stuv | tuvw | rstu | pqrs | rstu | |
| AB | 55.63 ± 5.82 | 82.98 ± 7.89 | 81.38 ± 6.74 | 84.18 ± 9.17 | 88.10 ± 5.76 | 85.40 ± 8.00 |
| x | w | w | vw | uvw | vw | |
| MSS | 124.55 ± 3.10 | 190.98 ± 11.83 | 189.03 ± 15.46 | 185.15 ± 8.40 | 202.40 ± 8.88 | 188.80 ± 3.66 |
| o | bc | bcd | bcde | a | bcd | |
| AC+MSS I | 107.83 ± 4.14 | 166.20 ± 7.62 | 177.98 ± 10.43 | 154.45 ± 5.23 | 183.18 ± 8.77 | 178.23 ± 14.28 |
| pqr | ijklm | defgh | n | bcdef | defgh | |
| AC+MSS II | 111.28 ± 4.40 | 169.53 ± 5.56 | 180.53 ± 4.32 | 168.93 ± 7.73 | 192.75 ± 2.87 | 170.08 ± 4.26 |
| pq | ghijk | cdefg | hijkl | ab | ghij | |
| AB+MSS I | 100.23 ± 3.68 | 158.38 ± 8.79 | 175.10 ± 8.76 | 156.50 ± 5.81 | 175.60 ± 4.64 | 157.75 ± 8.95 |
| qrst | klmn | efghi | mn | efghi | lmn | |
| AB+MSS II | 107.43 ± 4.03 | 159.18 ± 2.20 | 175.25 ± 4.93 | 150.30 ± 6.69 | 173.20 ± 11.43 | 155.25 ± 10.58 |
| pqr | jklmn | efghi | n | fghi | mn | |
References
- COM 614. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions Closing the Loop—An EU Action Plan for the Circular Economy; Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions: Brussels, Belgium, 2015. [Google Scholar]
- Luo, J.; Zhao, C.; Huang, W.; Wang, F.; Fang, F.; Su, L.; Wang, D.; Wu, Y. A holistic valorization of treasured waste activated sludge for directional high-valued products recovery: Routes, key technologies and challenges. Environ. Res. 2024, 262, 119904. [Google Scholar] [CrossRef]
- Han, W.; Jin, P.K.; Chen, D.W.; Liu, X.K.; Jin, H.; Wang, R.; Liu, Y.J. Resource reclamation of municipal sewage sludge based on local conditions: A case study in Xi’an, China. J. Clean. Prod. 2021, 316, 128189. [Google Scholar] [CrossRef]
- Kirchmann, H.; Börjesson, G.; Kätterer, T.; Cohen, Y. From agricultural use of sewage sludge to nutrient extraction: A soil science outlook. Ambio 2017, 46, 143–154. [Google Scholar] [CrossRef]
- Chojnacka, K.; Mikula, K.; Skrzypczak, D.; Izydorczyk, G.; Gorazda, K.; Kulczycka, J.; Kominko, H.; Moustakas, K.; Witek-Krowiak, A. Practical aspects of biowastes conversion to fertilizers. Biomass Convers. Biorefinery 2024, 14, 1515–1533. [Google Scholar] [CrossRef]
- Hidalgo, D.; Corona, F.; Martín-Marroquín, J. Nutrient recycling: From waste to crop. Biomass Convers. Biorefinery 2021, 11, 207–217. [Google Scholar] [CrossRef]
- Antonkiewicz, J.; Baran, A.; Kopta, T.; Lošák, T.; Ryant, P. Chapter 11—From biowaste to fertiliser. In The Circular Bioeconomy, Institutional and Production Perspectives, 1st ed.; Pink, M., Józefowska, A., Eds.; Routledge: London, UK, 2025; pp. 322–353. [Google Scholar] [CrossRef]
- Antonkiewicz, J.; Popławska, A.; Kołodziej, B.; Ciarkowska, K.; Gambuś, F.; Bryk, M.; Babula, J. Application of ash and municipal sewage sludge as macronutrient sources in sustainable plant biomass production. J. Environ. Manag. 2020, 264, 110450. [Google Scholar] [CrossRef]
- Buneviciene, K.; Drapanauskaite, D.; Mazeika, R.; Tilvikiene, V.; Baltrusaitis, J. Granulated biofuel ash as a sustainable source of plant nutrients. Waste Manag. Res. 2021, 39, 806–817. [Google Scholar] [CrossRef]
- Wierzbowska, J.; Sienkiewicz, S.; Żarczyński, P.; Krzebietke, S. Environmental Application of Ash from Incinerated Biomass. Agronomy 2020, 10, 482. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Eloffy, M.G.; Priya, A.K.; Yogeshwaran, V.; Yang, Z.; Elwakeel, K.Z.; Lopez-Maldonado, E.A. Biosolids management and utilizations: A review. J. Clean. Prod. 2024, 451, 141974. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No. 1069/2009 and (EC) No. 1107/2009 and Repealing Regulation (EC) No. 2003/2003 (Text with EEA Relevance); Official Journal of the European Union L 170: 2019; European Union: Maastricht, The Netherlands, 2019; Volume 1009, pp. 1–114.
- Aftab, T.; Hakeem, K.R. (Eds.) Sustainable Plant Nutrition: Molecular Interventions and Advancements for Crop Improvement; Elsevier: Amsterdam, The Netherlands; Academic Press: London, UK, 2022. [Google Scholar] [CrossRef]
- Cao, X.; Chen, C.; Zhang, D.; Shu, B.; Xiao, J.; Xia, R. Influence of nutrient deficiency on root architecture and root hair morphology of trifoliate orange (Poncirus trifoliata L. Raf.) seedlings under sand culture. Sci. Hortic. 2013, 162, 100–105. [Google Scholar] [CrossRef]
- Schmidt, S.B.; Jensen, P.E.; Husted, S. Manganese deficiency in plants: The impact on Photosystem II. Trends Plant Sci. 2016, 21, 622–632. [Google Scholar] [CrossRef]
- Moosavi, A.A.; Ronaghi, A. Growth And Iron-Manganese Relationships In Dry Bean As Affected By Foliar And Soil Applications Of Iron And Manganese In A Calcareous Soil. J. Plant Nutr. 2010, 33, 1353–1365. [Google Scholar] [CrossRef]
- Soltangheisi, A.; Rahman, Z.A.; Ishak, C.F.; Musa, H.M.; Zakikhani, H. Interaction effects of zinc and manganese on growth, uptake response and chlorophyll content of sweet corn (Zea mays var. saccharata). Asian J. Plant Sci. 2014, 13, 26–33. [Google Scholar] [CrossRef]
- Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Ning, X.; Lin, M.; Huang, G.; Mao, J.; Gao, Z.; Wang, X. Research progress on iron absorption, transport, and molecular regulation strategy in plants. Front. Plant Sci. 2023, 14, 1190768. [Google Scholar] [CrossRef] [PubMed]
- Albin, M.; Oskarsson, A. Chapter 23—Molybdenum. In Handbook on the Toxicology of Metals, 5th ed.; Nordberg, G.F., Costa, M., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: London, UK, 2022; pp. 601–614. [Google Scholar] [CrossRef]
- Kaiser, B.N.; Gridley, K.L.; Ngaire Brady, J.; Phillips, T.; Tyerman, S.D. The Role of Molybdenum in Agricultural Plant Production. Ann. Bot. 2005, 96, 745–754. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; Bagy, M.K.; El-enany, A.W.E.S.; Bashandy, S.R. Activation of Rhizobium tibeticum with Flavonoids Enhances Nodulation, Nitrogen Fixation, and Growth of Fenugreek (Trigonella foenum-graecum L.) Grown in Cobalt-Polluted Soil. Arch. Environ. Contam. Toxicol. 2014, 66, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wei, X.; Ling, J.; Chen, J. Cobalt: An Essential Micronutrient for Plant Growth? Front. Plant Sci. 2021, 12, 768523. [Google Scholar] [CrossRef]
- Karuppanapandian, T.; Kim, W. Cobalt-induced oxidative stress causes growth inhibition associated with enhanced lipid peroxidation and activates antioxidant responses in Indian mustard (Brassica juncea L.) leaves. Acta Physiol. Plant. 2013, 35, 2429–2443. [Google Scholar] [CrossRef]
- Li, H.-F.; Gray, C.; Mico, C.; Zhao, F.-J.; McGrath, S.P. Phytotoxicity and bioavailability of cobalt to plants in a range of soils. Chemosphere 2009, 75, 979–986. [Google Scholar] [CrossRef]
- Chatzistathis, T. Chapter 7—Physiological Importance of Manganese, Cobalt and Nickel and the Improvement of Their Uptake and Utilization by Plants. In Plant Micronutrient Use Efficiency; Hossain, M.A., Kamiya, T., Burritt, D.J., Phan Tran, L.-S., Fujiwara, T., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: London, UK, 2018; pp. 123–135. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a Friend or Foe of Higher Plants in Acid Soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef]
- Kocjan, A.; Kwaśniewska, J.; Szurman-Zubrzycka, M. Understanding plant tolerance to aluminum: Exploring mechanisms and perspectives. Plant Soil 2025, 507, 195–219. [Google Scholar] [CrossRef]
- Siecińska, J.; Nosalewicz, A. Aluminium Toxicity to Plants as Influenced by the Properties of the Root Growth Environment Affected by Other Co-Stressors: A Review. In Reviews of Environmental Contamination and Toxicology; de Voogt, P., Gunther, F.A., Eds.; Springer: Cham, Switzerland, 2017; Volume 243, pp. 1–26. [Google Scholar] [CrossRef]
- Xu, J.-Q.; Yu, R.-L.; Dong, X.-Y.; Hu, G.-R.; Shang, X.-S.; Wang, Q.; Li, H.-W. Effects of municipal sewage sludge stabilized by fly ash on the growth of Manilagrass and transfer of heavy metals. J. Hazard. Mater. 2012, 217–218, 58–66. [Google Scholar] [CrossRef]
- Sivapatham, P.; Potts, M.C.; Delise, J.A.; Sajwan, K.S.; Alva, A.K.; Jayaraman, K.; Chakraborty, P. Evaluation of wastewater treatment by-products as soil amendment: Growth of sorghum-sudan grass and trace elements concentrations. J. Environ. Sci. Health Part A 2012, 47, 1678–1686. [Google Scholar] [CrossRef]
- Esperschuetz, J.; Anderson, C.; Bulman, S.; Lense, O.; Horswell, J.; Dickinson, N.; Hofmann, R.; Robinson, B.H. Production of Biomass Crops Using Biowastes on Low-Fertility Soil: 1. Influence of Biowastes on Plant and Soil Quality. J. Environ. Qual. 2016, 45, 1960–1969. [Google Scholar] [CrossRef] [PubMed]
- Eid, E.M.; El-Bebany, A.F.; Alrumman, S.A.; Hesham, A.E.; Taher, M.A.; Fawy, K.F. Effects of different sewage sludge applications on heavy metal accumulation, growth and yield of spinach (Spinacia oleracea L.). Int. J. Phytoremediation 2017, 19, 340–347. [Google Scholar] [CrossRef]
- Eid, E.M.; Alrumman, S.A.; El-Bebany, A.F.; Hesham, A.; Taher, M.A.; Fawy, K.F. The effects of different sewage sludge amendment rates on the heavy metal bioaccumulation, growth and biomass of cucumbers (Cucumis sativus L.). Environ. Sci. Pollut. Res. 2017, 24, 16371–16382. [Google Scholar] [CrossRef] [PubMed]
- Eid, E.M.; Alrumman, S.A.; El-Bebany, A.F.; Fawy, K.F.; Taher, M.A.; Hesham, A.; El-Shaboury, G.A.; Ahmed, M.T. Evaluation of the potential of sewage sludge as a valuable fertilizer for wheat (Triticum aestivum L.) crops. Environ. Sci. Pollut. Res. 2019, 26, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Eid, E.M.; El-Bebany, A.F.; Taher, M.A.; Alrumman, S.A.; Hussain, A.A.; Galal, T.M.; Shaltout, K.H.; Sewelam, N.A.; Ahmed, M.T.; El-Shaboury, G.A. Influences of sewage sludge amended soil on heavy metal accumulation, growth and yield of rocket plant (Eruca sativa). Appl. Ecol. Environ. Res. 2020, 18, 3027–3040. [Google Scholar] [CrossRef]
- Eid, E.M.; Shaltout, K.H.; Alamri, S.A.M.; Alrumman, S.A.; Hussain, A.A.; Sewelam, N.; Ragab, G.A. Sewage sludge enhances tomato growth and improves fruit-yield quality by restoring soil fertility. Plant Soil Environ. 2021, 67, 514–523. [Google Scholar] [CrossRef]
- Antonkiewicz, J.; Kowalewska, A.; Mikołajczak, S.; Kołodziej, B.; Bryk, M.; Spychaj-Fabisiak, E.; Koliopoulos, T.; Babula, J. Phytoextraction of heavy metals after application of bottom ash and municipal sewage sludge considering the risk of environmental pollution. J. Environ. Manag. 2022, 306, 114517. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; International Union of Soil Sciences: Vienna, Austria, 2022. [Google Scholar]
- Beck, H.; Zimmermann, N.; McVicar, T.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Jones, J.B.; Case, V.W. Sampling, handling, and analyzing plant tissue samples. In Soil Testing and Plant Analysis, 3rd ed.; Westerman, R.L., Ed.; SSSA Book Series, Soil Science Society of America; Wiley: Madison, WI, USA, 1990; pp. 389–427. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Kumari, S.; Ahirwal, J.; Maiti, S.K. Reclamation of industrial waste dump using grass-legume mixture: An experimental approach to combat land degradation. Ecol. Eng. 2022, 174, 106443. [Google Scholar] [CrossRef]
- McBride, M.B.; Richards, B.K.; Steenhuis, T.; Spiers, G. Molybdenum Uptake by Forage Crops Grown on Sewage Sludge-Amended Soils in the Field and Greenhouse. J. Environ. Qual. 2000, 29, 848–854. [Google Scholar] [CrossRef]
- Kępka, W.; Antonkiewicz, J.; Gambuś, F.; Witkowicz, R. The effect of municipal sewage sludge on the content, use and mass ratios of some elements in spring barley biomass. Soil Sci. Annu. 2017, 68, 99–105. [Google Scholar] [CrossRef]
- Adamcová, V.; Valica, M.; Gubiš, J.; Gubišová, M.; Ondreičková, K.; Dulanská, S.; Horník, M. Municipal sewage sludge as a source of microelements in sustainable plant production: A laboratory lysimeter study. Nova Biotechnol. Chim. 2022, 20, e1258. [Google Scholar] [CrossRef]
- Kicińska, A.; Kosa-Burda, B.; Kozub, P. Utilization of a sewage sludge for rehabilitating the soils degraded by the metallurgical industry and a possible environmental risk involved. Hum. Ecol. Risk Assess. 2018, 24, 1990–2010. [Google Scholar] [CrossRef]
- Audet, P.; Charest, C. Heavy metal phytoremediation from a meta-analytical perspective. Environ. Pollut. 2007, 147, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Szady, C.; Picarillo, G.; Davis, E.J.; Drapanauskaite, D.; Buneviciene, K.; Baltrusaitis, J.; Navea, J.G. Iron dissolution and speciation from combustion particles under environmentally relevant conditions. Environ. Chem. 2023, 20, 171–182. [Google Scholar] [CrossRef]
- Jakubus, M.; Bakinowska, E.; Tobiašová, E. Valorisation of sewage sludge humic compounds in the aspect of its application in natural environment. Environ. Prot. Eng. 2021, 47, 67–83. [Google Scholar] [CrossRef]
- Kominko, H.; Gorazda, K.; Wzorek, Z. Formulation and evaluation of organo-mineral fertilizers based on sewage sludge optimized for maize and sunflower crops. Waste Manag. 2021, 136, 57–66. [Google Scholar] [CrossRef]
- Sharma, B.; Sarkar, A.; Singh, P.; Singh, R.P. Agricultural utilization of biosolids: A review on potential effects on soil and plant grown. Waste Manag. 2017, 64, 117–132. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash. Part 1. Phase–mineral and chemical composition and classification. Fuel 2013, 105, 40–76. [Google Scholar] [CrossRef]
- You, M.; Xu, M.; Hu, Y.; Xue, S.; Zhao, J. Chemical Speciation, Leaching Behavior, and Environmental Risk Assessment of Trace Elements in the Bottom Ash from Biomass Power Plant. ACS Omega 2024, 9, 18480–18487. [Google Scholar] [CrossRef]
- Pogrzeba, M.; Galimska-Stypa, R.; Krzyżak, J.; Sas-Nowosielska, A. Sewage sludge and fly ash mixture as an alternative for decontaminating lead and zinc ore regions. Environ. Monit. Assess. 2015, 187, 4120. [Google Scholar] [CrossRef]
- Xu, F.; Qin, S.J.; Li, S.Y.; Wang, J.X.; Qi, D.E.; Lu, Q.F.; Xing, J.K. Distribution, occurrence mode, and extraction potential of critical elements in coal ashes of the Chongqing Power Plant. J. Clean. Prod. 2022, 342, 130910. [Google Scholar] [CrossRef]
- Hannl, T.K.; Häggström, G.; Hedayati, A.; Skoglund, N.; Kuba, M.; Öhman, M. Ash transformation during single-pellet gasification of sewage sludge and mixtures with agricultural residues with a focus on phosphorus. Fuel Process. Technol. 2022, 227, 107102. [Google Scholar] [CrossRef]
- National Research Council. Mineral Tolerance of Animals: Second Revised Edition, 2005; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar] [CrossRef]
- Kao, P.T.; Darch, T.; McGrath, S.P.; Kendall, N.R.; Buss, H.L.; Warren, H.; Lee, M.R.F. Chapter Four—Factors influencing elemental micronutrient supply from pasture systems for grazing ruminants. In Advances in Agronomy; Sparks, D.L., Ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: London, UK, 2020; Volume 164, pp. 161–229. [Google Scholar] [CrossRef]
- Ryzhakova, N.K.; Babeshina, L.G.; Kondratyeva, A.G.; Sechnaya, D.Y. Contents of macro-, microelements and long-lived radionuclides in the medicinal plants belonging to the wetland community of Siberian region, Russia. Phytochem. Lett. 2017, 22, 280–286. [Google Scholar] [CrossRef]
- Liu, H.Y.; Wang, R.Z.; Lu, X.T.; Cai, J.P.; Feng, X.; Yang, G.J.; Li, H.; Zhang, Y.G.; Han, X.G.; Jiang, Y. Effects of nitrogen addition on plant-soil micronutrients vary with nitrogen form and mowing management in a meadow steppe. Environ. Pollut. 2021, 289, 117969. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Navasero, S.A. Interaction between iron and manganese in the rice plant. Soil Sci. Plant Nutr. 1966, 12, 29–33. [Google Scholar] [CrossRef]
- Yu, S.L.; Tao, R.Z.; Tan, H.Z.; Zhou, A.; Deng, S.H.; Wang, X.B.; Zhang, Q.F. Migration characteristics and ecological risk assessment of heavy metals in ash from sewage sludge co-combustion in coal-fired power plants. Fuel 2023, 333, 126420. [Google Scholar] [CrossRef]
- Chojnacka, K.; Skrzypczak, D.; Szopa, D.; Izydorczyk, G.; Moustakas, K.; Witek-Krowiak, A. Management of biological sewage sludge: Fertilizer nitrogen recovery as the solution to fertilizer crisis. J. Environ. Manag. 2023, 326, 116602. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; Hu, D.W.; Zhao, F.J. Molybdenum: More than an essential element. J. Exp. Bot. 2022, 73, 1766–1774. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.V.; Pilbeam, D.J. (Eds.) Handbook of Plant Nutrition, 1st ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2006. [Google Scholar] [CrossRef]
- Santos, E.F.; Santini, J.M.K.; Paixao, A.P.; Furlani, E.; Lavres, J.; Campos, M.; dos Reis, A.R. Physiological highlights of manganese toxicity symptoms in soybean plants: Mn toxicity responses. Plant Physiol. Biochem. 2017, 113, 6–19. [Google Scholar] [CrossRef]
- Bloem, E.; Haneklaus, S.; Haensch, R.; Schnug, E. EDTA application on agricultural soils affects microelement uptake of plants. Sci. Total Environ. 2017, 577, 166–173. [Google Scholar] [CrossRef]
- Lin, W.Y.; Ng, W.C.; Wong, B.S.E.; Teo, S.L.M.; Sivananthan, G.; Baeg, G.H.; Ok, Y.S.; Wang, C.H. Evaluation of sewage sludge incineration ash as a potential land reclamation material. J. Hazard. Mater. 2018, 357, 63–72. [Google Scholar] [CrossRef]
- Srivastava, A.N.; Chakma, S. Bioavailability reduction of heavy metals through dual mode anaerobic Co-landfilling of municipal solid waste and industrial organic sludge. Chem. Eng. J. 2022, 439, 135725. [Google Scholar] [CrossRef]
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, Å.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef]
- Ociepa, E.; Mrowiec, M.; Lach, J. Influence of fertilisation with sewage sludge-derived preparation on selected soil properties and prairie cordgrass yield. Environ. Res. 2017, 156, 775–780. [Google Scholar] [CrossRef]
- Grzegórska, A.; Czaplicka, N.; Antonkiewicz, J.; Rybarczyk, P.; Baran, A.; Dobrzynski, K.; Zabrocki, D.; Rogala, A. Remediation of soils on municipal rendering plant territories using Miscanthus × giganteus. Environ. Sci. Pollut. Res. 2023, 30, 22305–22318. [Google Scholar] [CrossRef]
- Ustak, S.; Munoz, J. Cup-plant potential for biogas production compared to reference maize in relation to the balance needs of nutrients and some microelements for their cultivation. J. Environ. Manag. 2018, 228, 260–266. [Google Scholar] [CrossRef]
- Anuwattana, R.; Khummongkol, P. Conventional hydrothermal synthesis of Na-A zeolite from cupola slag and aluminum sludge. J. Hazard. Mater. 2009, 166, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.V.; Tiwari, D.; Mok, Y.S.; Kim, D.J. Recovery of aluminum from water treatment sludge for phosphorus removal by combined calcination and extraction. J. Ind. Eng. Chem. 2021, 103, 195–204. [Google Scholar] [CrossRef]


| Treatment | Waste Doses, Mg∙ha−1 DM | |||
|---|---|---|---|---|
| Symbol | Description | Bituminous Coal Ash | Biomass Ash | Municipal Sewage Sludge |
| Ct | Control | – | – | – |
| AC | Ash from combustion of bituminous coal | 50 | – | – |
| AB | Ash from combustion of biomass | – | 50 | – |
| MSS | Municipal sewage sludge | – | – | 50 |
| AC+MSS I | 1:1 mixture of bituminous coal ash and municipal sewage sludge, 50 Mg·ha−1 DM | 25 | – | 25 |
| AC+MSS II | 1:1 mixture of bituminous coal ash and municipal sewage sludge, 100 Mg·ha−1 DM | 50 | – | 50 |
| AB+MSS I | 1:1 mixture of biomass ash and municipal sewage sludge, 50 Mg·ha−1 DM | – | 25 | 25 |
| AB+MSS II | 1:1 mixture of biomass ash and municipal sewage sludge, 100 Mg·ha−1 DM | – | 50 | 50 |
| Micronutrient | Soil | Ash from Combustion of Bituminous Coal | Ash from Combustion of Biomass | Municipal Sewage Sludge |
|---|---|---|---|---|
| Manganese (Mn) | 139.1 ± 4.1 | 6475 ± 88 | 5187 ± 191 | 259.9 ± 13.3 |
| Iron (Fe) | 12,659 ± 49 | 19,125 ± 127 | 18,565 ± 111 | 1500 ± 49 |
| Molybdenum (Mo) | 0.65 ± 0.06 | 4.31 ± 0.19 | 6.41 ± 0.18 | 5.07 ± 0.24 |
| Cobalt (Co) | 4.28 ± 0.29 | 3.58 ± 0.10 | 7.12 ± 0.04 | 7.21 ± 0.12 |
| Aluminium (Al) | 11,536 ± 326 | 28,116 ± 412 | 24,977 ± 658 | 7718 ± 492 |
| Parameter | Soil | Ash from Combustion of Bituminous Coal | Ash from Combustion of Biomass | Municipal Sewage Sludge |
|---|---|---|---|---|
| Dry matter, DM, % | – | 78.4 ± 1.7 | 75.3 ± 2.5 | 23.3 ± 0.8 |
| pHH2O | 7.02 ± 0.04 | 12.30 ± 0.23 | 10.20 ± 0.38 | 7.12 ± 0.20 |
| pHKCl | 6.52 ± 0.09 | 11.60 ± 0.11 | 9.89 ± 0.16 | 6.98 ± 0.17 |
| Total organic carbon, TOC, g∙kg−1 DM | 10.24 ± 1.83 | 2.31 ± 0.17 | 6.96 ± 0.10 | 269.0 ± 25.2 |
| Treatment | Fe/Mn |
|---|---|
| Ct (Control) | 1.08 ± 0.01 a |
| AC (Ash from combustion of bituminous coal) | 1.37 ± 0.04 d |
| AB (Ash from combustion of biomass) | 1.45 ± 0.02 e |
| MSS (Municipal sewage sludge) | 1.16 ± 0.02 c |
| AC+MSS I (1:1 mixture of AC and MSS, 50 Mg·ha−1 DM) | 1.16 ± 0.02 c |
| AC+MSS II (1:1 mixture of AC and MSS, 100 Mg·ha−1 DM) | 1.16 ± 0.02 c |
| AB+MSS I (1:1 mixture of AB and MSS, 50 Mg·ha−1 DM) | 1.12 ± 0.02 b |
| AB+MSS II (1:1 mixture of AB and MSS, 100 Mg·ha−1 DM) | 1.13 ± 0.01 b |
| Micronutrient | Treatment | Input, I g·ha−1 DM | Uptake, U g·ha−1 DM | Balance, B g·ha−1 DM | Recovery, R % |
|---|---|---|---|---|---|
| Mn | Ct | 0 | 6603 | −6603 | n/a 1 |
| AC | 323,750 | 1049 | 322,701 | 0.32 | |
| AB | 259,377 | 1306 | 258,071 | 0.50 | |
| MSS | 12,995 | 3356 | 9639 | 25.82 | |
| AC+MSS I | 168,373 | 1589 | 166,783 | 0.94 | |
| AC+MSS II | 336,745 | 1715 | 335,030 | 0.51 | |
| AB+MSS I | 136,186 | 2085 | 134,101 | 1.53 | |
| AB+MSS II | 272,372 | 2119 | 270,252 | 0.78 | |
| CV, % | 70.00 | 73.00 | 72.00 | 218.18 | |
| Fe | Ct | 0 | 7111 | −7111 | n/a |
| AC | 956,250 | 1435 | 954,815 | 0.15 | |
| AB | 928,250 | 1889 | 926,361 | 0.20 | |
| MSS | 75,000 | 3890 | 71,110 | 5.19 | |
| AC+MSS I | 515,625 | 1845 | 513,780 | 0.36 | |
| AC+MSS II | 1,031,250 | 1991 | 1,029,259 | 0.19 | |
| AB+MSS I | 501,625 | 2345 | 499,280 | 0.47 | |
| AB+MSS II | 1,003,250 | 2402 | 1,000,848 | 0.24 | |
| CV, % | 67.00 | 65.00 | 67.00 | 191.75 | |
| Mo | Ct | 0 | 11.91 | −11.91 | n/a |
| AC | 216 | 10.75 | 204.75 | 4.99 | |
| AB | 321 | 13.07 | 307.43 | 4.08 | |
| MSS | 254 | 15.55 | 237.95 | 6.13 | |
| AC+MSS I | 235 | 13.66 | 220.84 | 5.82 | |
| AC+MSS II | 469 | 14.24 | 454.76 | 3.04 | |
| AB+MSS I | 287 | 15.98 | 271.02 | 5.57 | |
| AB+MSS II | 574 | 15.82 | 558.18 | 2.76 | |
| CV, % | 59.00 | 13.75 | 61.02 | 29.33 | |
| Co | Ct | 0 | 10.55 | −10.55 | n/a |
| AC | 179 | 4.97 | 174.03 | 2.78 | |
| AB | 356 | 6.83 | 349.17 | 1.92 | |
| MSS | 361 | 8.73 | 351.77 | 2.42 | |
| AC+MSS I | 270 | 6.78 | 262.97 | 2.51 | |
| AC+MSS II | 540 | 7.38 | 532.12 | 1.37 | |
| AB+MSS I | 358 | 8.62 | 349.63 | 2.41 | |
| AB+MSS II | 717 | 8.79 | 707.71 | 1.23 | |
| CV, % | 62.00 | 21.72 | 63.82 | 28.69 | |
| Al | Ct | 0 | 912 | −912 | n/a |
| AC | 1,405,826 | 548 | 1,405,278 | 0.04 | |
| AB | 1,248,877 | 478 | 1,248,399 | 0.04 | |
| MSS | 385,917 | 1081 | 384,836 | 0.28 | |
| AC+MSS I | 895,871 | 968 | 894,903 | 0.11 | |
| AC+MSS II | 1,791,743 | 993 | 1,790,749 | 0.06 | |
| AB+MSS I | 817,397 | 924 | 816,473 | 0.11 | |
| AB+MSS II | 1,634,793 | 921 | 1,633,872 | 0.06 | |
| CV, % | 60.00 | 26.00 | 60.00 | 86.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonkiewicz, J.; Kołodziej, B.; Bryk, M.; Kądziołka, M.; Pełka, R.; Koliopoulos, T. Sustainable Management of Bottom Ash and Municipal Sewage Sludge as a Source of Micronutrients for Biomass Production. Sustainability 2025, 17, 7493. https://doi.org/10.3390/su17167493
Antonkiewicz J, Kołodziej B, Bryk M, Kądziołka M, Pełka R, Koliopoulos T. Sustainable Management of Bottom Ash and Municipal Sewage Sludge as a Source of Micronutrients for Biomass Production. Sustainability. 2025; 17(16):7493. https://doi.org/10.3390/su17167493
Chicago/Turabian StyleAntonkiewicz, Jacek, Beata Kołodziej, Maja Bryk, Magdalena Kądziołka, Robert Pełka, and Tilemachos Koliopoulos. 2025. "Sustainable Management of Bottom Ash and Municipal Sewage Sludge as a Source of Micronutrients for Biomass Production" Sustainability 17, no. 16: 7493. https://doi.org/10.3390/su17167493
APA StyleAntonkiewicz, J., Kołodziej, B., Bryk, M., Kądziołka, M., Pełka, R., & Koliopoulos, T. (2025). Sustainable Management of Bottom Ash and Municipal Sewage Sludge as a Source of Micronutrients for Biomass Production. Sustainability, 17(16), 7493. https://doi.org/10.3390/su17167493










