The Use of Collagen Hydrolysate from Chromium Waste in the Optimization of Leather Retanning
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- Roksol STE (producer: PCC Exol SA, Brzeg Dolny, Poland)—a fatliquoring agent composed of a mixture of anionic emulsifiers and aliphatic compounds containing carboxyl groups. The product is characterized by a pH of 5.5–7.4 in a 10% aqueous emulsion and an active substance content ranging from 45% to 60%. When applied to leather, it functions as a lubricant, facilitating the relative movement of collagen fibers, which imparts the desired functional properties to the leather, such as softness and elasticity.
- Melamine (producer: Grupa Azoty Zakłady Azotowe, Puławy, Poland)—an aromatic compound from the amine group, classified as a triazine derivative and a cyanamide trimer. When introduced into the leather structure, it primarily functions as a filler in areas with a looser fibrous arrangement, such as the flanks and groin regions. Melamine exhibits the ability to form weak non-covalent bonds with functional groups of collagen as well as with other reagents used in the chemical processing of leather.
- Chrome shavings (producer: tannery in Radom, Poland)—solid leather waste generated during the shaving of chrome-tanned leather, characterized by a moisture content of 43% and a chromium oxide (Cr2O3) content of 3.7% relative to dry mass. In this study, chrome shavings were utilized as a raw material for the production of collagen hydrolysate.
- Semi-finished wet-blue leather (producer: tannery in Radom, Poland)—chrome-tanned leather after the shaving process, with a thickness of 1.4–1.6 mm, moisture content of 47%, and chromium oxide (Cr2O3) content of 3.8% relative to dry mass.
- Collagen hydrolysate—a product obtained through the acid hydrolysis of chrome-tanned leather shavings (the detailed procedure for collagen hydrolysate preparation is provided below). When applied to the leather structure, it exhibits a retanning effect by binding to functional groups within the collagen matrix, as well as a filling effect by occupying voids in areas with a looser fibrous structure.
- Semi-finished crust leather—dry leather obtained after the retanning and fatliquoring of the wet-blue semi-finished product.
2.1.1. Obtaining Collagen Hydrolysate
- first, 100 g of chrome-tanned leather shavings and 250 mL of water were added to a 1000 mL three-necked flask,
- a thermometer, mechanical stirrer, and reflux condenser were placed in the necks of the flask,
- the stirrer and cold-water flow through the reflux condenser were turned on, and the mixture in the flask was heated to 100 °C using a heating mantle,
- after reaching the desired temperature, 10 mL of concentrated H2SO4 was slowly added to the reaction mixture along the walls of the flask in 5 portions every 5 min and stirring and heating were continued for another 40 min (a total of 60 min from the addition of the first portion of sulfuric acid),
- then, after lowering the temperature of the reaction mixture to 80 °C in the same reactor, precipitation of Cr(OH)3 was started by adding 50 mL of 10% Ca(OH)2 suspension for about 30 min,
- after precipitation, the resulting mixture was left for 24 h to allow the chromium hydroxide precipitate to settle,
- the mixture was then filtered using a vacuum filtration system with a Büchner funnel.
2.1.2. Retanning of the Wet-Blue Semi-Finished Product
2.2. Methods
2.2.1. An Organoleptic Evaluation
2.2.2. Measurement of pH
2.2.3. Determination of Chromium Content
2.2.4. Determination of Dry Mass
2.2.5. Determination of Density
- dc—density of the tested liquid,
- m1—mass of an empty pycnometer,
- m2—mass of the pycnometer with the tested liquid,
- m3—mass of the pycnometer with distilled water,
- 0.9982—water density (g/cm3).
- A detailed description of the procedure is included in the PN-EN ISO 2811-1:2016-04 standard.
2.2.6. Determination of Dynamic Viscosity
- η—dynamic viscosity,
- t—time of a ball falling (s),
- dk—ball density (kg/m3),
- dc—density of test fluid (kg/m3),
- KS—constant of the ball used (9.03 × 10−9 Pa·m3/kg).
2.2.7. Softness
2.2.8. Shrinkage Temperature
2.2.9. Water Vapor Permeability
2.2.10. Water Absorption
2.2.11. Mechanical Properties
2.2.12. Statistical Analysis
3. Results and Discussion
4. Conclusions
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lasek, W. Kolagen–Chemia i Wykorzystanie; WNT: Warszawa, Poland, 1978. [Google Scholar]
- Sundar, V.J.; Muralidharan, C.; Mandal, A.B. Eco-benign stabilization of skin protein-Role of Jatropha curcas oil as a co-tanning agent. Ind. Crops Prod. 2013, 47, 227–231. [Google Scholar] [CrossRef]
- Hao, D.; Wang, X.; Liang, S.; Yue, O.; Liu, X.; Hao, D.; Dang, X. Sustainable leather making-An amphoteric organic chrome-free tanning agents based on recycling waste leather. Sci. Total Environ. 2023, 867, 161531. [Google Scholar] [CrossRef] [PubMed]
- Świetlik, R. Obciążenie środowiska regionu radomskiego chromem z przemysłu garbarskiego. Ochr. Powietrza Probl. Odpadów. 2001, 35, 29–33. [Google Scholar]
- Sathish, M.; Madhan, B.; Rao, J.R. Leather solid waste: An eco-benign raw material for leather chemical preparation–A circular economy example. Waste Manag. 2019, 87, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Rosu, L.; Varganici, C.D.; Crudu, A.M.; Rosu, D.; Bele, A. Ecofriendly wet-white leather vs. conventional tanned wet-blue leather. A photochem. approach. J. Clean. Prod. 2018, 177, 708–720. [Google Scholar] [CrossRef]
- Hu, J.; Xiao, Z.; Zhou, R.; Deng, W.; Wang, M.; Ma, S. Ecological utilization of leather tannery waste with circular economy model. J. Clean. Prod. 2011, 19, 221–228. [Google Scholar] [CrossRef]
- Azhar Zakir, M.J.; Ramalingam, S.; Balasubramanian, P.; Rathinam, A.; Sreeram, K.J.; Rao, J.R.; Nair, B.U. Innovative material from paper and pulp industry for leather processing. J. Clean. Prod. 2015, 104, 436–444. [Google Scholar] [CrossRef]
- Taylor, M.M.; Lee, J.; Bumanlag, L.P.; Latona, R.J.; Brown, E.M. Biopolymers produced from gelatin and whey protein concentrate using polyphenols. J. Am. Leather Chem. Assoc. 2014, 109, 82–88. [Google Scholar]
- Taylor, M.M.; Bumanlag, L.P.; Brown, E.M.; Liu, C.K. Biopolymers produced from gelatin and chitosan using polyphenols. J. Am. Leather Chem. Assoc. 2015, 110, 392–400. [Google Scholar]
- Taylor, M.M.; Marmer, W.N.; Brown, E.M.; Rabinovich, D.; Cot, J.; Shelly, D.; Levy, J. Characterization of biopolymers prepared from gelatin and sodium caseinate for potential use in leather processing. J. Am. Leather Chem. Assoc. 2005, 100, 149–159. [Google Scholar]
- Taylor, M.M.; Bumanlag, L.P.; Marmer, W.N.; Brown, E.M. Use of enzymatically modified gelatin and casein as fillers in leather processing. J. Am. Leather Chem. Assoc. 2006, 101, 169–178. [Google Scholar]
- Taylor, M.M.; Marmer, W.N.; Brown, E.M. Preparation and characterization of biopolymers derived from enzymatically modified gelatin and whey. J. Am. Leather Chem. Assoc. 2006, 101, 235–248. [Google Scholar]
- Li, R.; Ren, L.; Chen, L.; Liu, H.; Qiang, T. New materials-based on gelatin coordinated with zirconium or aluminum for ecological retanning. Int. J. Biol. Macromol. 2024, 261, 129922. [Google Scholar] [CrossRef]
- Ramalingam, S.; Sreeram, K.J.; Rao, J.R.; Nair, B.U. Hybrid composites: Amalgamation of proteins with polymeric phenols as a multifunctional material for leather processing. RSC Adv. 2015, 5, 33221–33232. [Google Scholar] [CrossRef]
- Afsar, A.; Bugra, O.; Gulumser, G.; Aslan, A. A study on usability of collagen hydrolysate along with oxazolidine in leather processing. Tekst. Konfeksiyon 2010, 20, 37–40. [Google Scholar]
- Cantera, C.S.; Goya, L.; Mingo, R. Collagen hydrolysate: ‘Soluble skin’ applied in post-tanning processes part 1: Characterisation. J. Soc. Leather Technol. Chem. 2000, 84, 29–37. [Google Scholar]
- Cantera, C.S.; Martegani, J.; Esterelles, G.; Vergara, J. Collagen hydrolysate: ‘Soluble skin’ applied in post-tanning processes Part 2: Interaction with acrylic retanning agents. J. Soc. Leather Technol. Chem. 2002, 86, 195–202. [Google Scholar]
- Gargano, M.; Florio, C.; Sannia, G.; Lettera, V. From leather wastes to leather: Enhancement of low quality leather using collagen recovered from leather tanned wastes. Clean. Technol. Environ. Policy 2023, 25, 3065–3074. [Google Scholar] [CrossRef]
- Liu, X.; Yue, O.; Wang, X.; Hou, M.; Zheng, M.; Jiang, H. Preparation and application of a novel biomass-based amphoteric retanning agent with the function of reducing free formaldehyde in leather. J. Clean. Prod. 2020, 65, 121796. [Google Scholar] [CrossRef]
- Sancakli, A.; Ismar, E.; Arican, F.; Polat, O.; Basaran, B.; Mizan, A. Utilization of collagen wastes as bioretanning agent and effects on the mechanical properties of leather. Tekst. Konfeksiyon 2023, 33, 330–336. [Google Scholar] [CrossRef]
- Żarłok, J.; Śmiechowski, K.; Kowalska, M. Hygienic properties of leather finished with formulations containing collagen hydrolysate obtained by acid hydrolysis. J. Soc. Leather Technol. Chem. 2015, 99, 297–301. [Google Scholar]
- Crudu, M.; Deacon, M.; Pruneanu, M.; Constantin, A.M.; GURĂU, D. Experiments to obtain new bioproducts based on protein extracts from wet-white leather waste intended for the total or partial replacement of phenol formaldehyde resins in wet finishing of leather. Leather Footwear J. 2019, 19, 177–188. [Google Scholar] [CrossRef]
- Puhazhselvan, P.; Pandi, A.; Sujiritha, P.B.; Antony, G.S.; Jaisankar, S.N.; Ayyadurai, N.; Saravanan, P.; Kamini, N.R. Recycling of tannery fleshing waste by a two step process for preparation of retanning agent. Process Saf. Environ. Prot. 2022, 157, 59–67. [Google Scholar] [CrossRef]
- Ammasi, R.; Victor, J.S.; Chellan, R.; Chellappa, M. Amino acid enriched proteinous wastes: Recovery and reuse in leather making. Waste Biomass Valorization 2020, 11, 5793–5807. [Google Scholar] [CrossRef]
- Castell, J.C.; Fabregat, C.; Sorolla, S.; Solano, D.; Olle, L.; Bacardit, A. Optimizing a sustainable and innovative wet-White Process with Tara tannins. J. Am. Leather Chem. Assoc. 2011, 106, 278–286. [Google Scholar]
- Kleeman, W. Experimentelle Parameteroptimierungsmethode. Plaste Kautschunk 1964, 11, 723. [Google Scholar]
- Janas, S.; Kowalska, M. Accuracy of Drying Selected Products Using a Moisture Analyzer Method Based on Infrared Radiation. Metrol. Meas. Syst. 2023, 30, 305–321. [Google Scholar] [CrossRef]
- Śmiechowski, K.; Żarłok, J.; Kowalska, M. The Relationship Between Water Vapour Permeability and Softness for Leathers Produced in Poland. J. Soc. Leather Technol. Chem. 2014, 94, 259–263. [Google Scholar]
- Kowalska, M.; Paździor, M.; Śmiechowski, K. Wykorzystanie programu komputerowego opartego na metodzie Klemmana jako narzędzia pozwalającego uzyskać stabilną emulsję spożywczą®. The of the computer program based on Kleeman’s method as a tool to obtain a stable food emulsion®. Postępy Tech. Przetwórstwa Spożywczego 2014, 1, 41–46. [Google Scholar]
- Zhao, J.; Wu, Q.; Tang, Y.; Zhou, J.; Guo, H. Tannery wastewater treatment: Conventional and promising processes, an updated 20-year review. J. Leather Sci. Eng. 2022, 4, 10. [Google Scholar] [CrossRef]
- Sadhukhan, J.; Dugmore, T.I.J.; Matharu, A.; Martinez-Hernandez, E.; Aburto, J.; Rahman, P.K.S.M.; Lynch, J. Perspectives on “Game Changer” Global Challenges for Sustainable 21st Century: Plant-Based Diet, Unavoidable Food Waste Biorefining, and Circular Economy. Sustainability 2020, 12, 1976. [Google Scholar] [CrossRef]
- Chojnacka, K.; Skrzypczak, D.; Mikula, K.; Witek-Krowiak, A.; Izydorczyk, G.; Kuligowski, K.; Bandrów, P.; Kułażyński, M. Progress in sustainable technologies of leather wastes valorization as solutions for the circular economy. J. Clean. Prod. 2021, 313, 127902. [Google Scholar] [CrossRef]
- Czarnecki, M.; Wrzesińska-Jędrusiak, E.; Konkol, I.; Świerczek, L.; Postawa, K.; Kułażyński, M.; Myczko, A. Management of Tanning Waste from Leather Processing by Anaerobic Digestion Using a Dynamic Method on a Semi-Technical Scale. Sustainability 2024, 16, 9501. [Google Scholar] [CrossRef]
- Wrzesińska-Jędrusiak, E.; Czarnecki, M.; Kazimierski, P.; Bandrów, P.; Szufa, S. The Circular Economy in the Management of Waste from Leather Processing. Energies 2023, 16, 564. [Google Scholar] [CrossRef]
- Myjak, W.; Mendrycka, M. Otrzymywanie kolagenu ze skór cielęcych. Przemysł Chem. 2015, 94, 169–173. [Google Scholar]
- Shanthi, C.; Banerjee, P.; Chandra Babu, N.; Rajakumar, G. Recovery and characterization of protein hydrolysate from chrome shavings by microbial degradation. J. Am. Leather Chem. Assoc. 2013, 108, 231–239. [Google Scholar]
- Żarłok, J. Zastosowanie hydrolizatu kolagenowego do dogarbowania półproduktu wet-white. Przegląd Włókienniczy–Włókno Odzież Skóra 2024, 5, 20–24. [Google Scholar] [CrossRef]

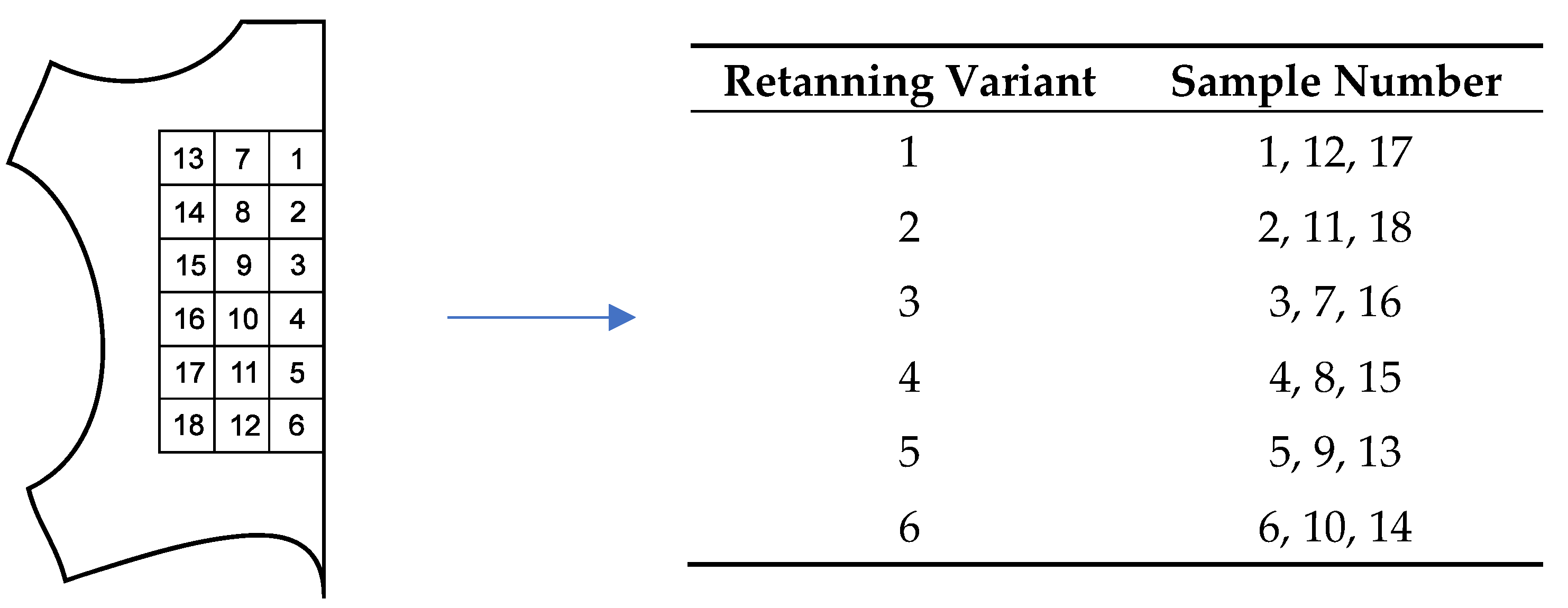

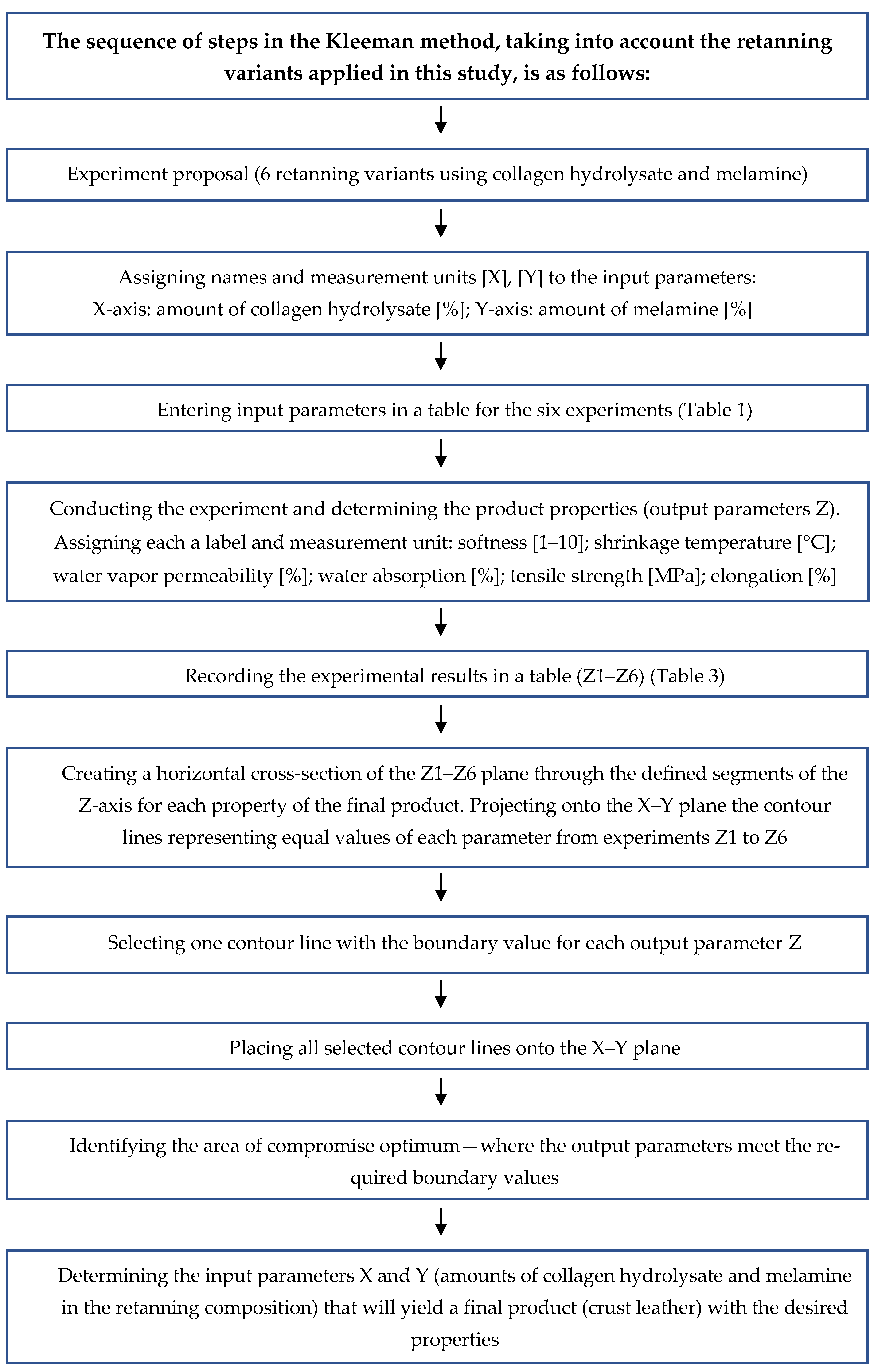

| Retanning Variant | Amount of Collagen Hydrolysate (X) [g] | Amount of Melamine (Y) [g] |
|---|---|---|
| 1 | 25 | 2.5 |
| 2 | 35 | 2.5 |
| 3 | 30 | 3.0 |
| 4 | 30 | 3.5 |
| 5 | 35 | 4.0 |
| 6 | 25 | 4.0 |
| Operation | Type of Agent | Amount [%] | Temp [°C] | Times [min] | pH/Comments |
|---|---|---|---|---|---|
| Washing I | Water | 200 | 30 | 10 | Pour out the bath |
| Washing II | Water | 200 | 35 | 10 | Pour out the bath |
| Neutralization | Water Sodium bicarbonate | 150 1, 3 | 35 | 40 | Bromocresol green pH = 5.0–5.2 |
| Washing | Water | 200 | 35 | 10 | Pour out the bath |
| Rettaning | Water Melamine Collagen hydrolysate | 150 X: 2.5/3.0/3.5/4.0 Y: 25/30/35 | 35 | 90 | |
| Fatliquoring | Roksol STE Formic acid | 10 1 | 60 2 × 10 +20 | Add emulsifier with hot water Pour out the bath | |
| Washing | Water | 200 | 20 | 10 | Pour out the bath |
| Determined Metal | Cr |
|---|---|
| Wavelength [nm] | 357.9 |
| Slit width [nm] | 0.2 |
| Property | Value of Parameter |
|---|---|
| Color of solution | Yellow |
| Odor | No odor |
| Density [g/cm3] | 1.04 ± 0.08 |
| Dynamic viscosity [mPa·s] | 1.98 ± 0.15 |
| Dry mass [%] | 12.9 ± 0.5 |
| pH | 7.5 ± 0.2 |
| Content of chromium [mg Cr/L] | 3.05 ± 0.25 |
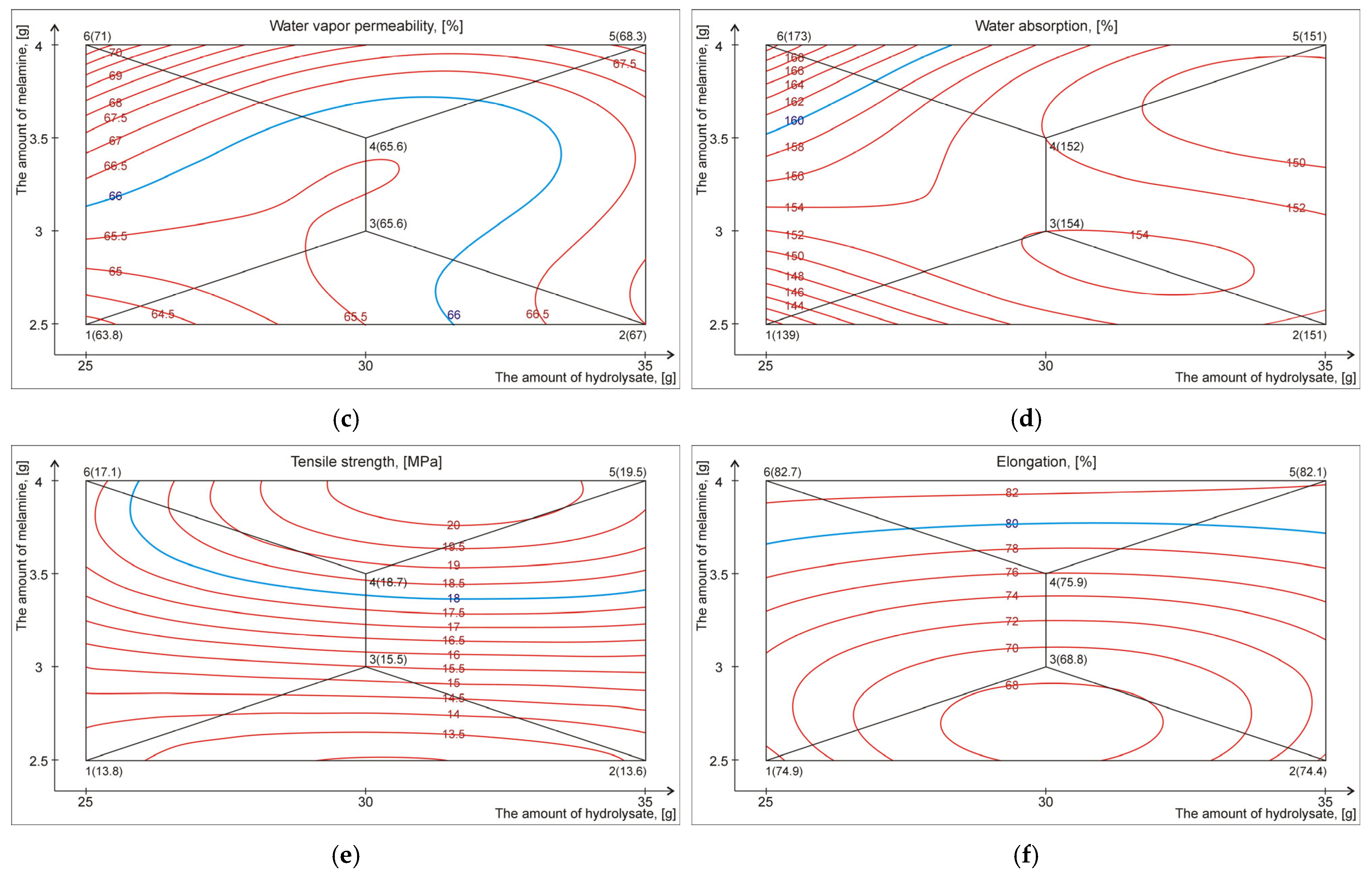
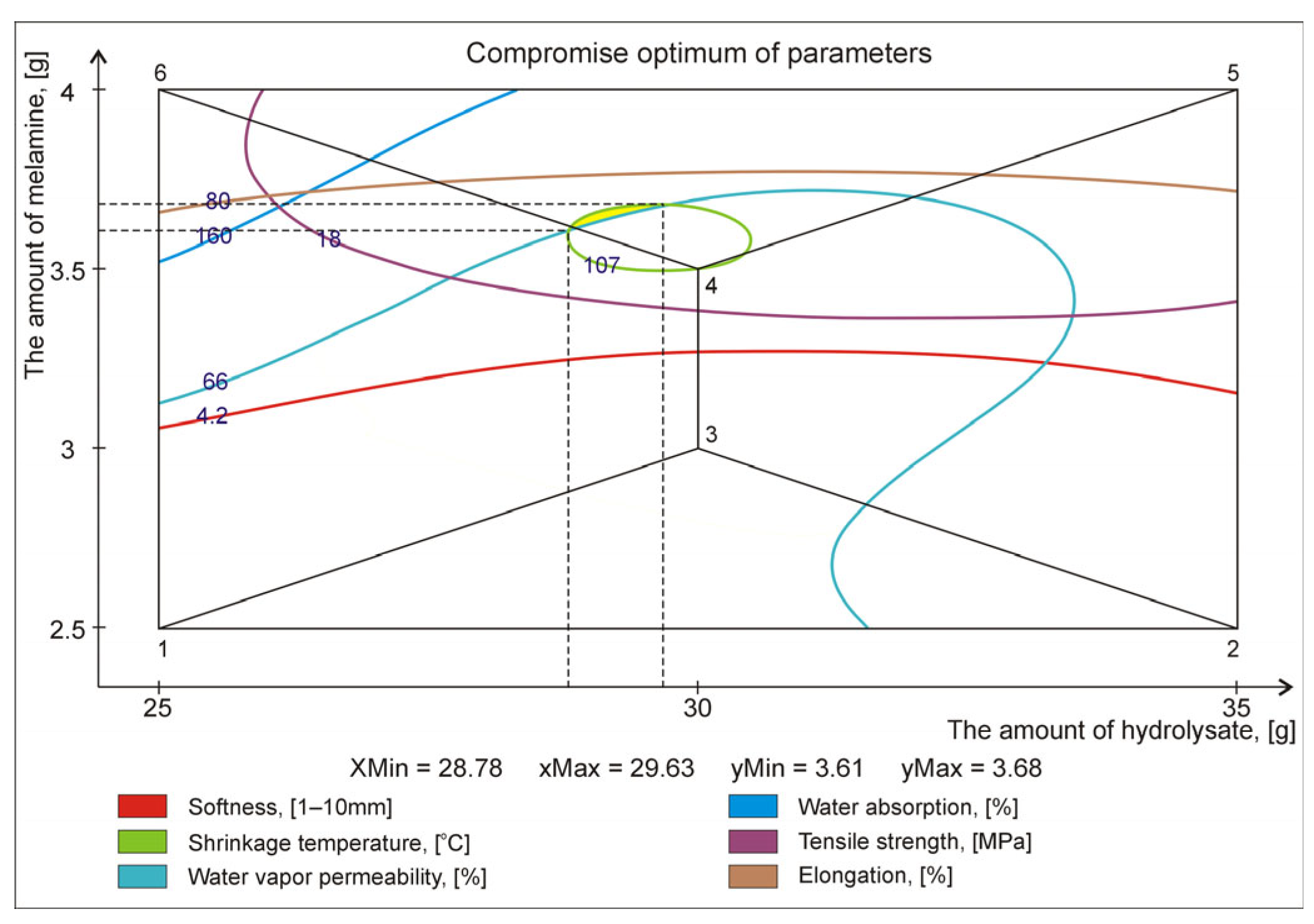
| Retanning Variant | Properties of Crust Leather (Output Parameters) | |||||
|---|---|---|---|---|---|---|
| Softness [1–10 mm] | Shrinkage Temp. [°C] | Water Vapor Permeability [%] | Water Absorption [%] | Tensile Strength [MPa] | Maximum Elongation [%] | |
| 1 | 4.0 ± 0.2 | 108 ± 2 | 63.8 ± 3.6 | 139 ± 12 | 13.8 ± 3.8 | 74.9 ± 8.9 |
| 2 | 4.0 ± 0.3 | 107 ± 1 | 67.0 ± 4.1 | 151 ± 18 | 13.6 ± 2.5 | 74.4 ± 7.4 |
| 3 | 4.1 ± 0.1 | 108 ± 2 | 65.6 ± 4.3 | 154 ± 15 | 15.5 ± 3.3 | 68.8 ± 7.8 |
| 4 | 4.3 ± 0.2 | 107 ± 2 | 65.6 ± 3.4 | 152 ± 16 | 18.7 ± 2.7 | 75.9 ± 6.4 |
| 5 | 4.6 ± 0.2 | 111 ± 1 | 68.3 ± 2.8 | 151 ± 10 | 19.5 ± 4.0 | 82.1 ± 9.0 |
| 6 | 4.7 ± 0.3 | 110 ± 1 | 71.0 ± 3.8 | 173 ± 21 | 17.1 ± 1.9 | 82.7 ± 8.1 |
| Wet-blue | 5.3 ± 0.2 | 105 ± 3 | 67.9 ± 4.0 | 142 ± 13 | 13.3 ± 2.2 | 91.2 ± 7.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarlok, J.; Kowalska, M.; Szakiel, J. The Use of Collagen Hydrolysate from Chromium Waste in the Optimization of Leather Retanning. Sustainability 2025, 17, 7912. https://doi.org/10.3390/su17177912
Zarlok J, Kowalska M, Szakiel J. The Use of Collagen Hydrolysate from Chromium Waste in the Optimization of Leather Retanning. Sustainability. 2025; 17(17):7912. https://doi.org/10.3390/su17177912
Chicago/Turabian StyleZarlok, Jan, Małgorzata Kowalska, and Jerzy Szakiel. 2025. "The Use of Collagen Hydrolysate from Chromium Waste in the Optimization of Leather Retanning" Sustainability 17, no. 17: 7912. https://doi.org/10.3390/su17177912
APA StyleZarlok, J., Kowalska, M., & Szakiel, J. (2025). The Use of Collagen Hydrolysate from Chromium Waste in the Optimization of Leather Retanning. Sustainability, 17(17), 7912. https://doi.org/10.3390/su17177912







