The Role of Sustainable Lithium Processing in Renewable Energy Development: A Comprehensive Review and the Potential of Kazakhstan Deposits
Abstract
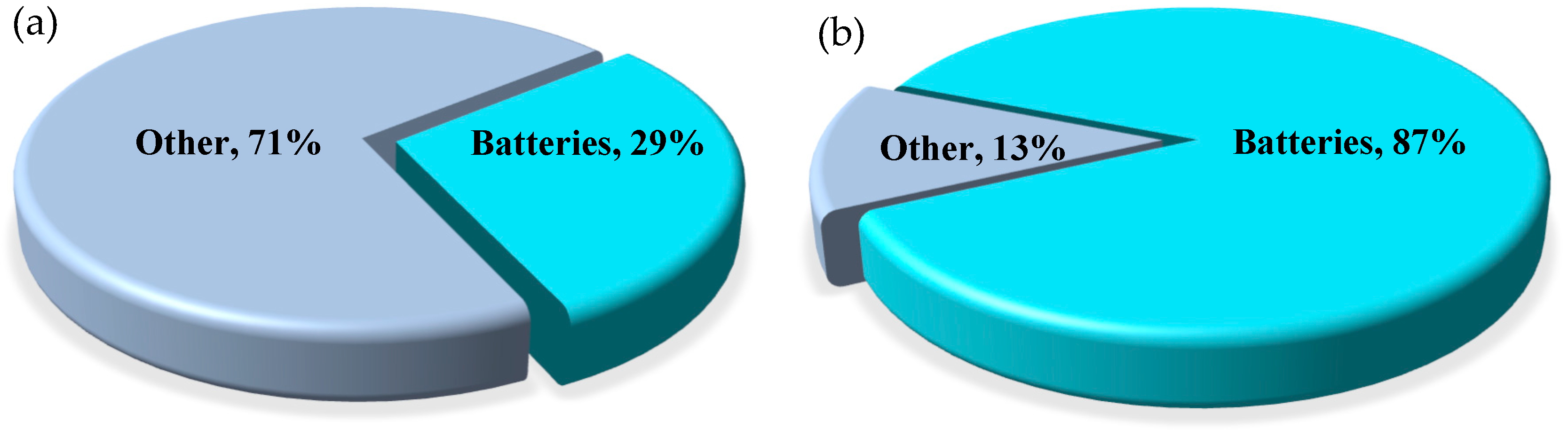
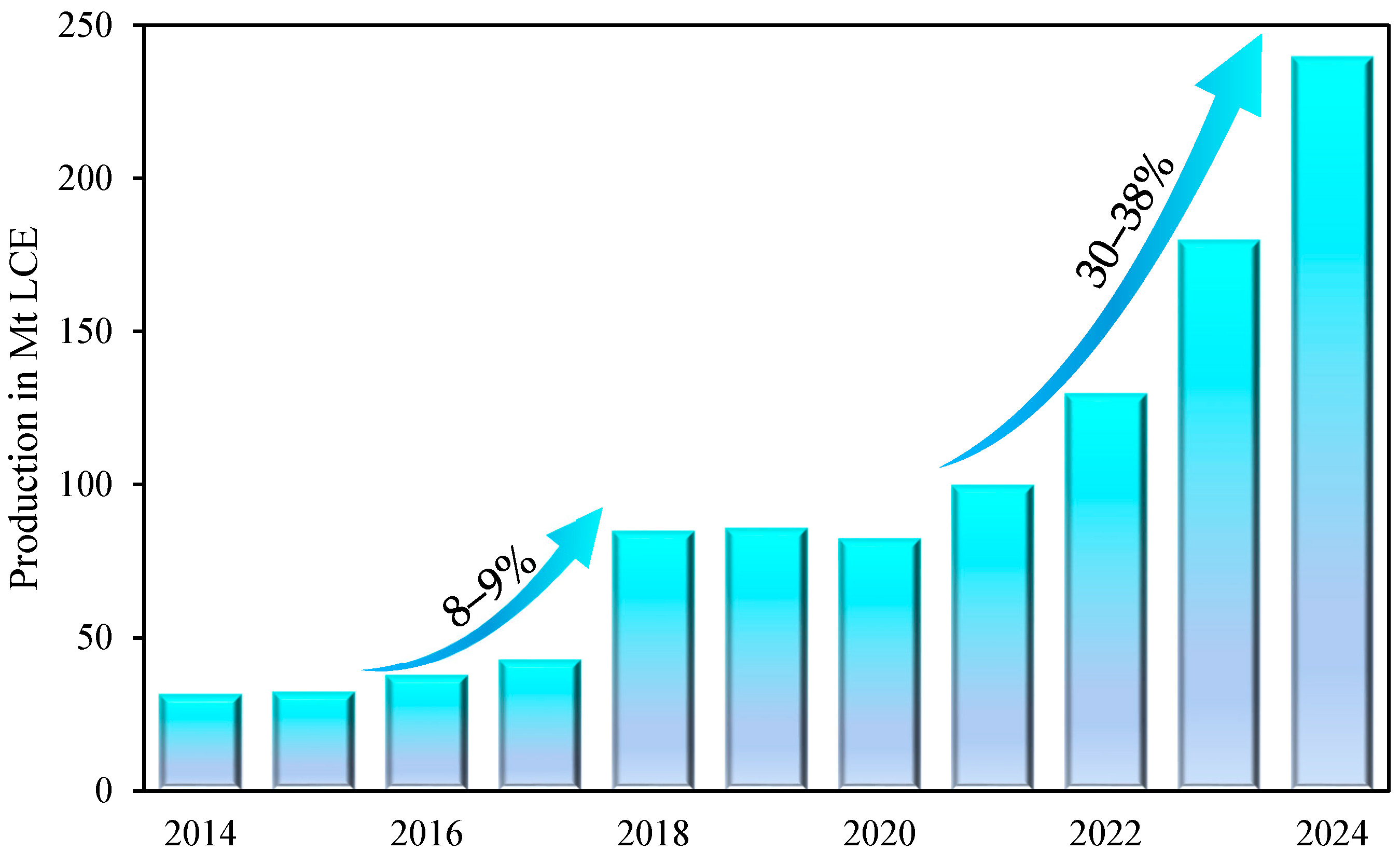
1. Introduction
Lithium’s Nature, Abundance, and Economically Important Deposits
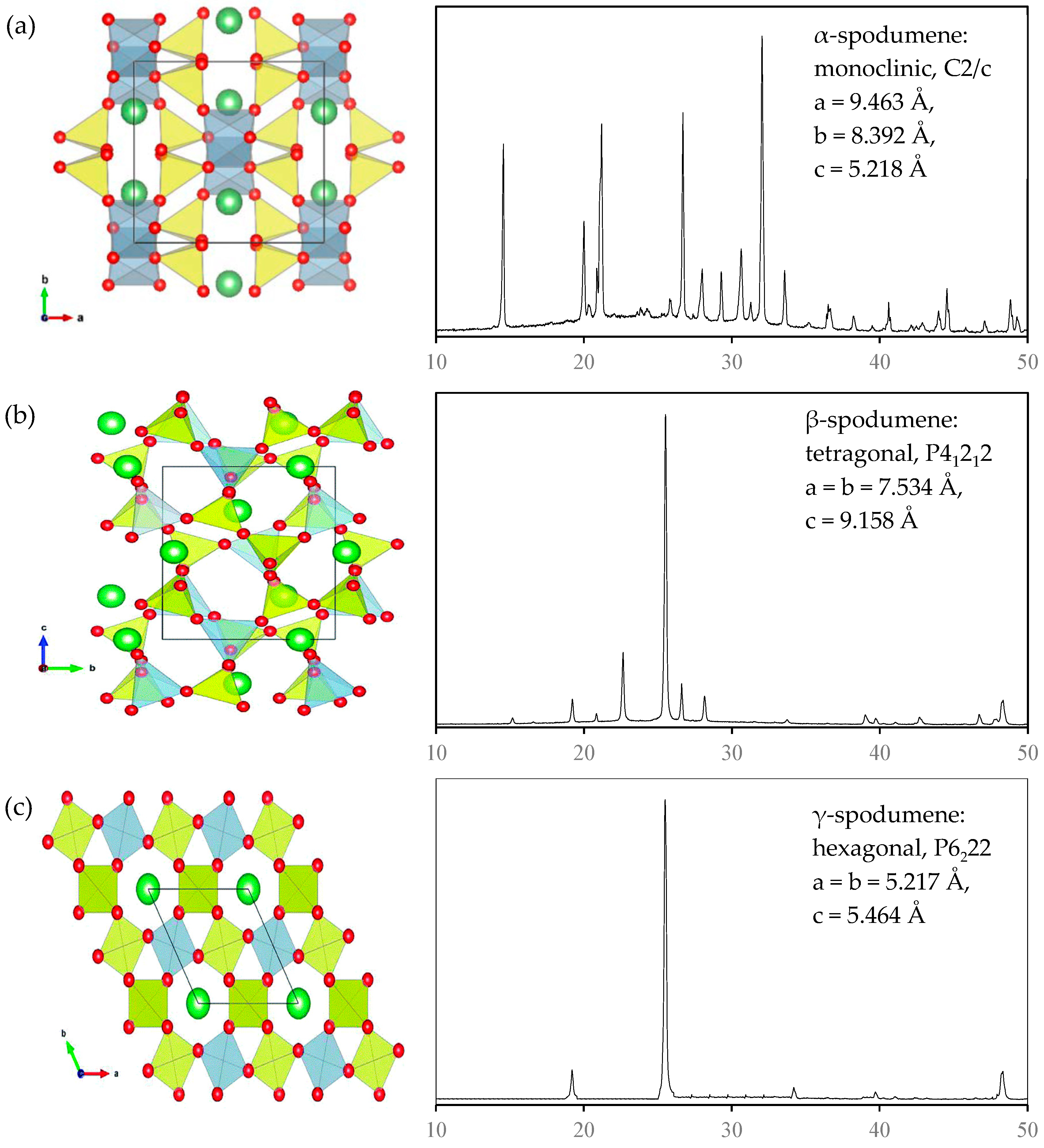
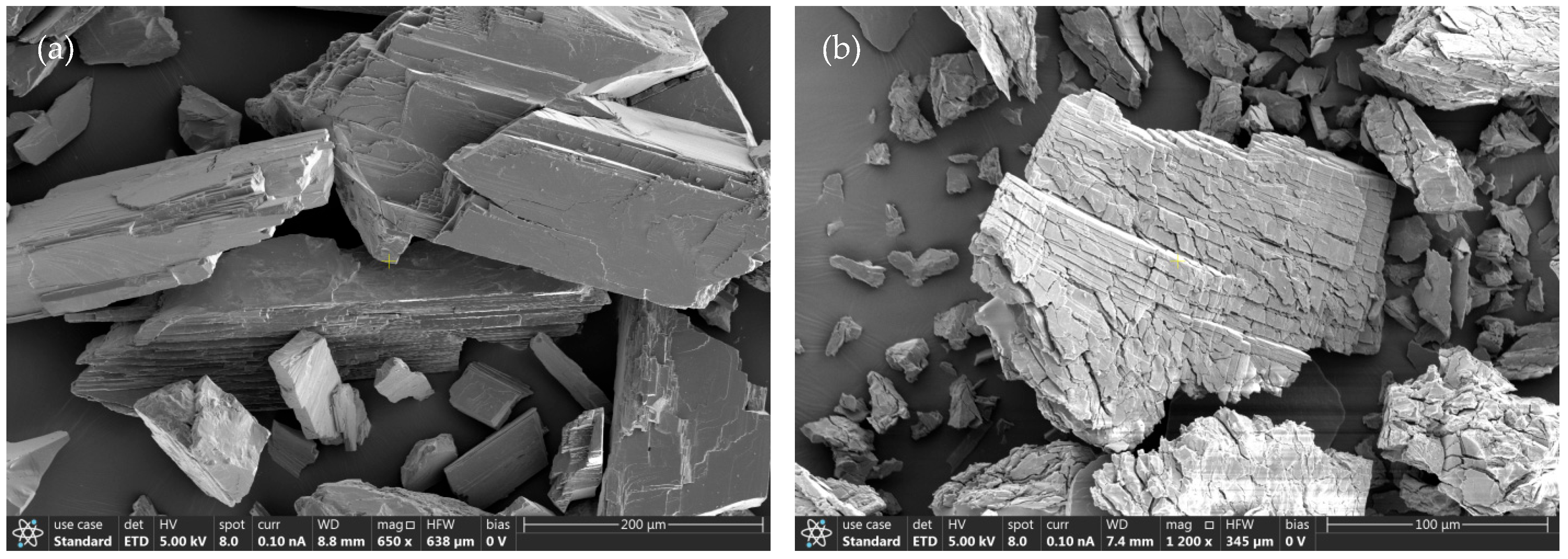
2. Mineralogy of Spodumene: Cleavage, Crystal Habit, and Lithium Coordination
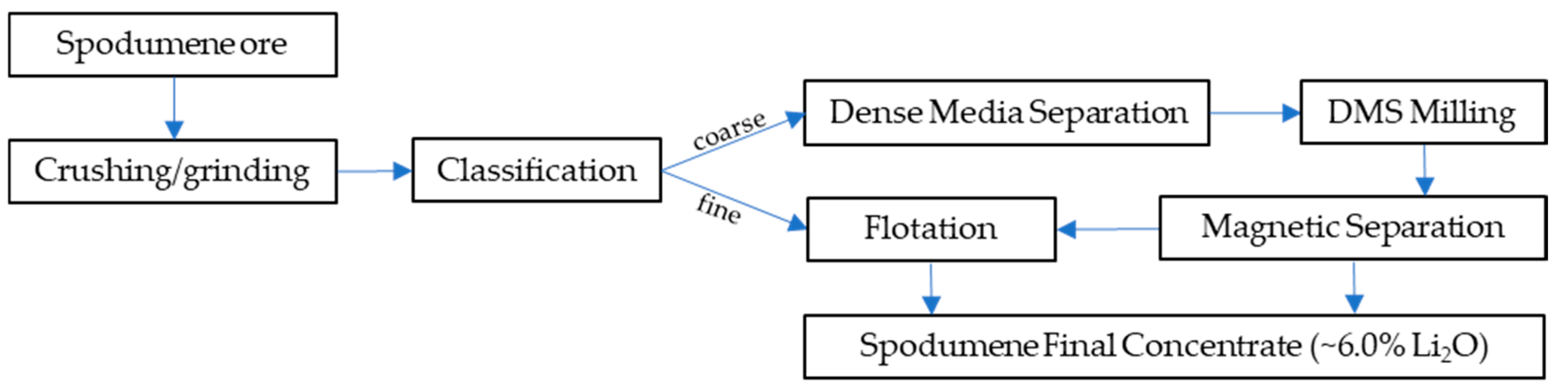
3. Beneficiation of Spodumene
3.1. Dense Media Separation
3.2. Pre-Flotation Treatment
3.3. De-Sliming
3.4. Magnetic Separation
3.5. Flotation
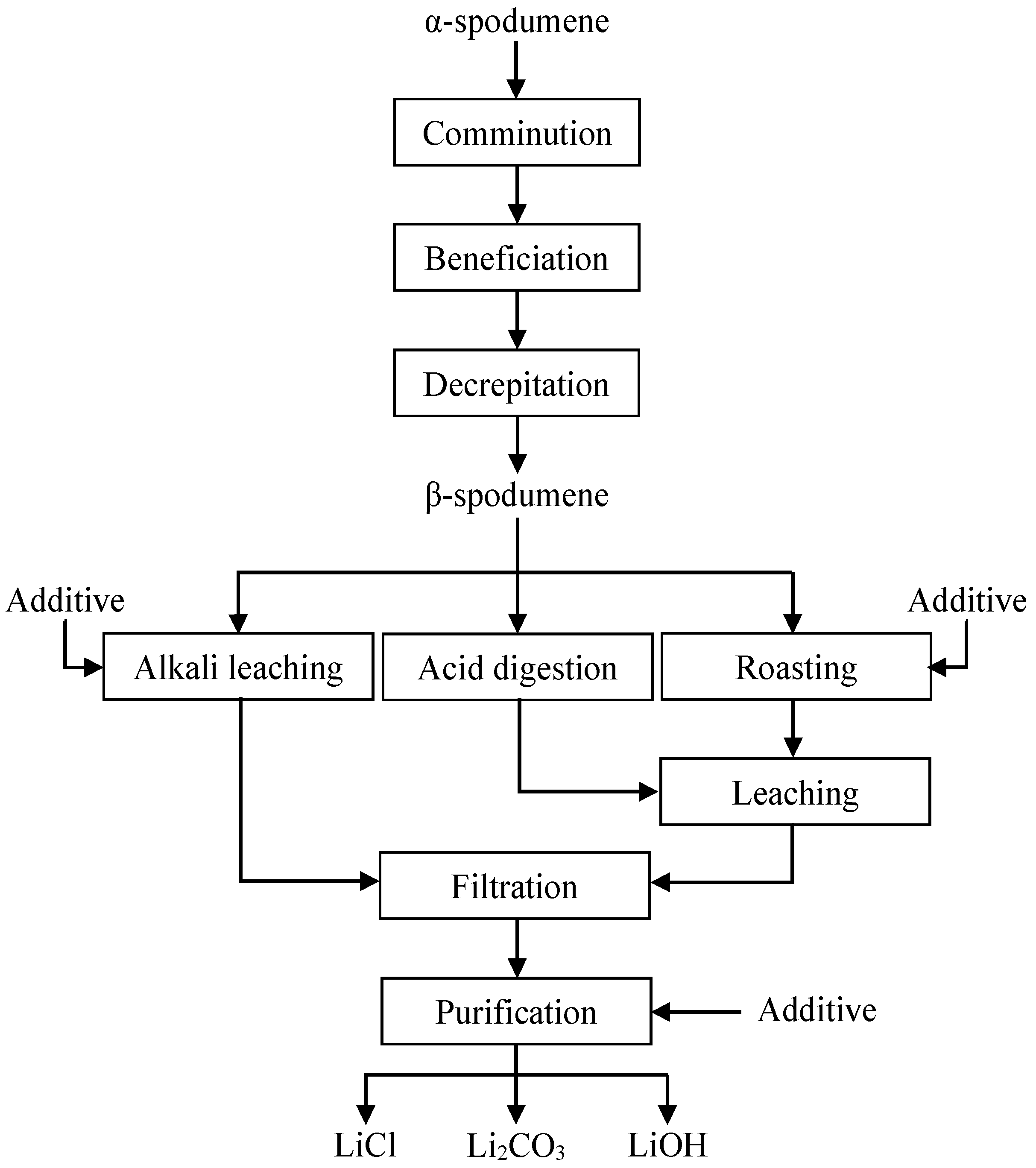
4. Extraction of Lithium from Spodumene
4.1. Decrepitation
4.1.1. Sulfuric Acid Roasting
4.1.2. Hydrochloric Acid and Nitric Acid Method
4.1.3. Alkali Leaching Method
4.1.4. Chlorination Process
4.2. Alkali Roasting Method
4.3. Lime Roasting Process
4.4. Sulfate Roasting
4.5. Fluorination Processing
4.6. Biohydrometallurgical Approaches for Lithium Extraction
4.7. Electrochemical Leaching Methods
4.8. Lithium Recovery from Spent Batteries via Hydrometallurgical Methods
4.9. Environmental Trade-Offs of Lithium Processing Technologies
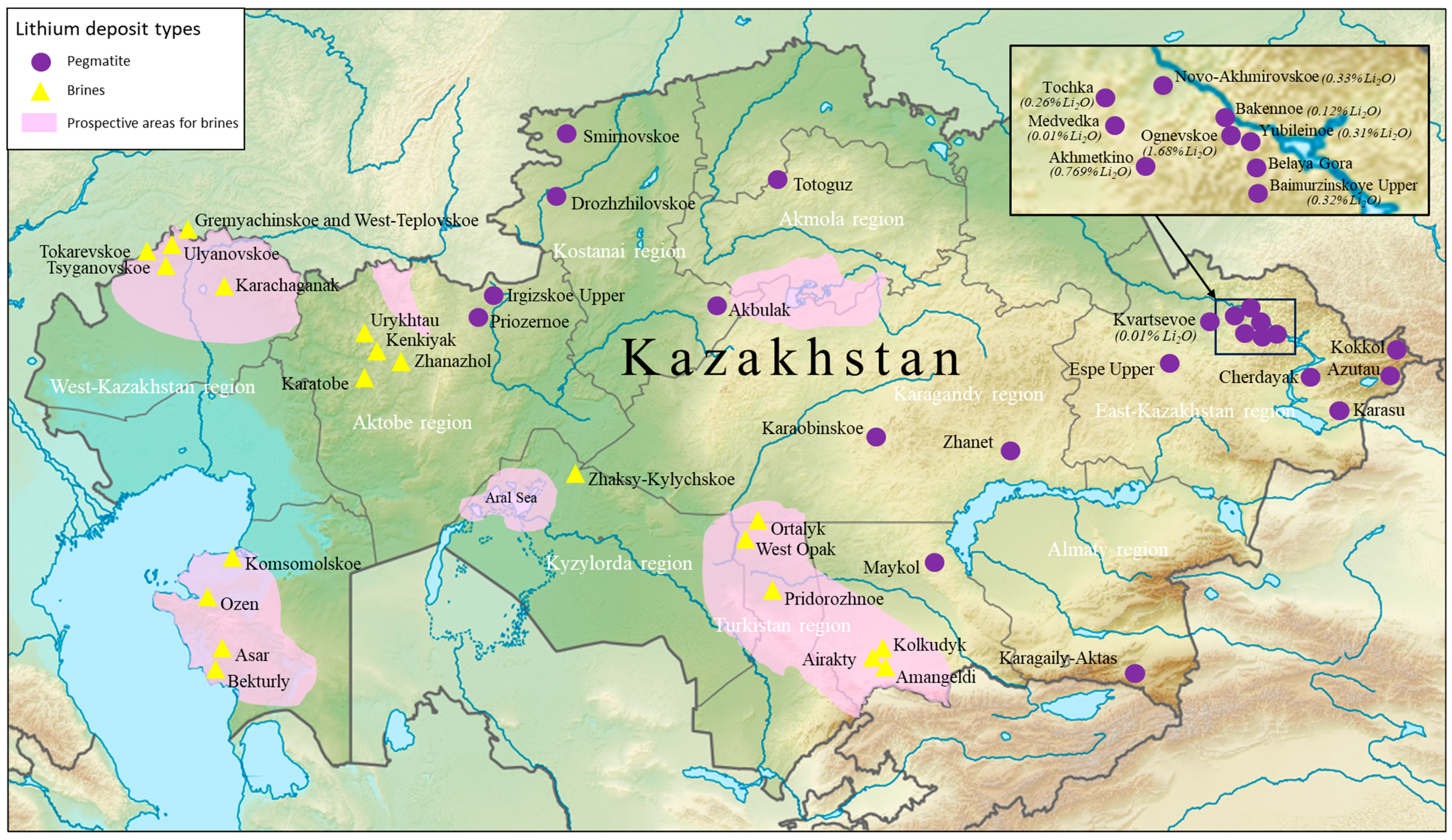
5. Kazakhstan’s Potential, Viability, and Sustainability Considerations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- IEA. Net Zero by 2050; IEA: Paris, France, 2021; Available online: https://www.iea.org/reports/net-zero-by-2050 (accessed on 25 January 2024).
- Sagzhanov, D.; Ito, J.; Altansukh, B.; Godirilwe, L.L.; Haga, K.; Takasaki, Y.; Shibayama, A. Lithium Ore Beneficiation: Sustainable Approaches for Efficient Recovery of Lithium from a Low-Grade Spodumene Ore. J. Sustain. Metall. 2025, 11, 754–772. [Google Scholar] [CrossRef]
- IEA. The Role of Critical Minerals in Clean Energy Transitions; IEA: Paris, France, 2021; Available online: https://www.iea.org/reports/the-role-of-critical-minerals-in-clean-energy-transitions (accessed on 4 April 2024).
- U.S. Geological Survey. Mineral Commodity Summaries 2025; U.S. Geological Survey: Reston, VA, USA, 2025; p. 212.
- Liu, Y.; Ma, B.; Lü, Y.; Wang, C.; Chen, Y. A review of lithium extraction from natural resources. Int. J. Miner. Metall. Mater. 2023, 30, 209–224. [Google Scholar] [CrossRef]
- IEA. Global EV Outlook 2024; IEA: Paris, France, 2024; Available online: https://www.iea.org/reports/global-ev-outlook-2024 (accessed on 15 July 2023).
- IEA. Electricity 2025; IEA: Paris, France, 2025; Available online: https://www.iea.org/reports/electricity-2025 (accessed on 30 March 2025).
- Li, H.; Eksteen, J.; Kuang, G. Recovery of lithium from mineral resources: State-of-the-art and perspectives—A review. Hydrometallurgy 2019, 189, 105129. [Google Scholar] [CrossRef]
- Goto, M.; Okumura, K.; Nakagawa, S.; Inaba, Y.; Matsuura, H.; Nakaya, H.; Katayama, K. Nuclear and thermal feasibility of lithium-loaded high temperature gas-cooled reactor for tritium production for fusion reactors. Fusion Eng. Des. 2018, 136, 357–361. [Google Scholar] [CrossRef]
- Youssef, A.; Anwar, R.; Bashter, I.I.; Amin, E.A.; Reda, S.M. Neutron yield as a measure of achievement nuclear fusion using a mixture of deuterium and tritium isotopes. Phys. Scr. 2022, 97, 085601. [Google Scholar] [CrossRef]
- Khan, M.H.; Tucci, V.; Lamberti, P.; Longo, R.; Guadagno, L. Lithium-Based Batteries in Aircraft. Eng. Proc. 2025, 90, 39. [Google Scholar]
- Water Resources Division, U.S. Geological Survey. Mineral Commodity Summaries 2015; U.S. Geological Survey: Reston, VA, USA, 2015; p. 199.
- Calisaya-Azpilcueta, D.; Herrera-Leon, S.; Cisternas, L.A. Current and Future Global Lithium Production Till 2025. Open Chem. Eng. J. 2020, 14, 36–51. [Google Scholar] [CrossRef]
- Sovacool, B.K.; Ali, S.H.; Bazilian, M.; Radley, B.; Nemery, B.; Okatz, J.; Mulvaney, D. Sustainable minerals and metals for a low-carbon future. Science 2020, 367, 30–33. [Google Scholar] [CrossRef]
- Habib, K.; Hansdóttir, S.T.; Habib, H. Critical metals for electromobility: Global demand scenarios for passenger vehicles, 2015–2050. Resour. Conserv. Recycl. 2020, 154, 104603. [Google Scholar] [CrossRef]
- Baylis, R. Evaluating and Forecasting the Lithium Market from a Value Perspective; Roskill Information Services Ltd.: Las Vegas, NV, USA, 2013. [Google Scholar]
- Sustainability, D.; et Minières, B.d.R.G. Study on the Review of the List of Critical Raw Materials: Non-Critical Raw Materials Factsheets; Publications Office of the European Union: Luxembourg, 2017. [Google Scholar]
- Latunussa, C.; Georgitzikis, K.; Torres de Matos, C.; Grohol, M.; Eynard, U.; Wittmer, D.; Mancini, L.; Unguru, M.; Pavel, C.; Carrara, S. European Commission, Study on the EU’s list of Critical Raw Materials, Factsheets on Critical Raw Materials; Publications Office of the European Union: Luxembourg, 2020; Available online: https://rmis.jrc.ec.europa.eu/uploads/CRM_2020_Factsheets_critical_Final.pdf (accessed on 9 September 2023).
- Rezaee, M.; Han, S.; Sagzhanov, D.; Vaziri Hassas, B.; Slawecki, T.M.; Agrawal, D.; Akbari, H.; Mensah-Biney, R. Microwave-assisted calcination of spodumene for efficient, low-cost and environmentally friendly extraction of lithium. Powder Technol. 2021, 397, 116992. [Google Scholar] [CrossRef]
- Salakjani, N.K.; Singh, P.; Nikoloski, A.N. Production of Lithium—A Literature Review. Part 2. Extraction from Spodumene. Miner. Process. Extr. Metall. Rev. 2021, 42, 268–283. [Google Scholar] [CrossRef]
- Salakjani, N.K.; Singh, P.; Nikoloski, A.N. Production of Lithium—A Literature Review Part 1: Pretreatment of Spodumene. Miner. Process. Extr. Metall. Rev. 2019, 41, 335–348. [Google Scholar] [CrossRef]
- Gibson, C.E.; Aghamirian, M.; Grammatikopoulos, T.A. A review: The benefication of lithium minerals from hard rock deposits. Miner. Eng. 2017, 69, 18–37. [Google Scholar]
- Salakjani, N.K.; Singh, P.; Nikoloski, A.N. Acid roasting of spodumene: Microwave vs. conventional heating. Min. Eng. 2019, 138, 161–167. [Google Scholar] [CrossRef]
- Xiong, J.; He, L.; Zhao, Z. Lithium extraction from high-sodium raw brine with Li0.3FePO4 electrode. Desalination 2022, 535, 115822. [Google Scholar] [CrossRef]
- Ding, T.; Zheng, M.; Peng, S.; Lin, Y.; Zhang, X.; Li, M. Lithium extraction from salt lakes with different hydrochemical types in the Tibet Plateau. Geosci. Front. 2022, 14, 101485. [Google Scholar] [CrossRef]
- Han, S.; Sagzhanov, D.; Pan, J.; Vaziri Hassas, B.; Rezaee, M.; Akbari, H.; Mensah-Biney, R. Direct Extraction of Lithium from α-Spodumene by Salt Roasting–Leaching Process. ACS Sustain. Chem. Eng. 2022, 10, 13495–13504. [Google Scholar] [CrossRef]
- Tadesse, B.; Makuei, F.; Albijanic, B.; Dyer, L. The beneficiation of lithium minerals from hard rock ores: A review. Miner. Eng. 2019, 131, 170–184. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Tripathy, S.K.; Nayak, A.; Hembrom, K.C.; Dey, S.; Rath, R.K.; Mohanta, M.K. Beneficiation of lithium bearing pegmatite rock: A review. Miner. Process. Extr. 2022, 45, 1–27. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, Y.; Liu, J.; Li, Y.; Wang, X.; Shumin, Z. Research status of spodumene flotation: A review. Miner. Process. Extr. 2021, 42, 321–334. [Google Scholar] [CrossRef]
- Retamal, J.I.; Robles, P.A.; Quezada, G.R.; Jeldres, R.I. Molecular Design and Spodumene Flotation—A Review. Int. J. Mol. Sci. 2024, 25, 3227. [Google Scholar] [CrossRef] [PubMed]
- Cook, B.K.; Gibson, C.E. A Review of Fatty Acid Collectors: Implications for Spodumene Flotation. Minerals 2023, 13, 212. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.; Mankhand, T. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review. Hydrometallurgy 2014, 150, 192–208. [Google Scholar] [CrossRef]
- Choubey, P.K.; Kim, M.-s.; Srivastava, R.R.; Lee, J.-c.; Lee, J.-Y. Advance review on the exploitation of the prominent energy-storage element: Lithium. Part I: From mineral and brine resources. Miner. Eng. 2016, 89, 119–137. [Google Scholar] [CrossRef]
- Fosu, A.Y.; Kanari, N.; Vaughan, J.; Chagnes, A. Literature review and thermodynamic modelling of roasting processes for lithium extraction from spodumene. Metals 2020, 10, 1312. [Google Scholar] [CrossRef]
- Yelatontsev, D.; Mukhachev, A. Processing of lithium ores: Industrial technologies and case studies—A review. Hydrometallurgy 2021, 201, 105578. [Google Scholar] [CrossRef]
- Karrech, A.; Azadi, M.R.; Elchalakani, M.; Shahin, M.A.; Seibi, A.C. A review on methods for liberating lithium from pegmatities. Miner. Eng. 2020, 145, 106085. [Google Scholar] [CrossRef]
- Konhauser, K.; Ehrlich, H.; Kappler, A.; Newmann, D. Geomicrobial interactions with silicon. In Ehrlich’s Geomicrobiology, 6th ed.; CRC Press, Taylor & Frances Group: Boca Raton, FL, USA, 2016; pp. 237–255. [Google Scholar]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Dessemond, C.; Lajoie-Leroux, F.; Soucy, G.; Laroche, N.; Magnan, J.-F. Spodumene: The Lithium Market, Resources and Processes. Minerals 2019, 9, 334. [Google Scholar] [CrossRef]
- Vikström, H.; Davidsson, S.; Höök, M. Lithium availability and future production outlooks. Appl. Energy 2013, 110, 252–266. [Google Scholar] [CrossRef]
- Grew, E.S. The minerals of lithium. Elem. Int. Mag. Mineral. Geochem. Petrol. 2020, 16, 235–240. [Google Scholar] [CrossRef]
- Bowell, R.J.; Lagos, L.; Camilo, R.; Declercq, J. Classification and characteristics of natural lithium resources. Elements 2020, 16, 259–264. [Google Scholar] [CrossRef]
- Alexeev, S.; Alexeeva, L.; Vakhromeev, A. Brines of the Siberian platform (Russia): Geochemistry and processing prospects. Appl. Geochem. 2020, 117, 104588. [Google Scholar] [CrossRef]
- Kelly, J.C.; Wang, M.; Dai, Q.; Winjobi, O. Energy, greenhouse gas, and water life cycle analysis of lithium carbonate and lithium hydroxide monohydrate from brine and ore resources and their use in lithium ion battery cathodes and lithium ion batteries. Resour. Conserv. Recycl. 2021, 174, 105762. [Google Scholar] [CrossRef]
- Absametov, M.K.; Boyarko, G.Y.; Dutova, E.M.; Bolsunovskaya, L.M.; Itemen, N.M.; Chenzybaev, D.B. Lithium capacity of Kazakhstan mineral resource base. Bull. Tomsk. Polytech. Univ. Geo Assets Eng. 2024, 335, 141–154. [Google Scholar] [CrossRef]
- Shaw, R.A. Global Lithium (Li) Mines, Deposits and Occurrences; British Geological Survey: Keyworth, UK, 2021.
- Kesler, S.E.; Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Everson, M.P.; Wallington, T.J. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- Bishimbayeva, G.; Zhumabayeva, D.; Zhanabayeva, A.; Nalibayeva, A.; Abdikalykov, E.; Bakenov, Z.B. Prospects for creating a full cycle of lithium production in Kazakhstan—From ore processing to lithium batteries. Ser. Chem. Technol. 2020, 5, 38–45. [Google Scholar] [CrossRef]
- Tabelin, C.B.; Dallas, J.; Casanova, S.; Pelech, T.; Bournival, G.; Saydam, S.; Canbulat, I. Towards a low-carbon society: A review of lithium resource availability, challenges and innovations in mining, extraction and recycling, and future perspectives. Miner. Eng. 2021, 163, 106743. [Google Scholar] [CrossRef]
- Peiro, L.T.; Mendez, G.V.; Ayres, R.U. Lithium: Sources, Production, Uses, and Recovery Outlook. JOM 2013, 65, 986–996. [Google Scholar] [CrossRef]
- Flexer, V.; Baspineiro, C.F.; Galli, C.I. Lithium recovery from brines: A vital raw material for green energies with a potential environmental impact in its mining and processing. Sci. Total Environ. 2018, 639, 1188–1204. [Google Scholar] [CrossRef]
- Wietelmann, U.; Steinbild, M. Lithium and Lithium Compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2014; pp. 1–38. [Google Scholar]
- Mohr, S.H.; Mudd, G.M.; Giurco, D. Lithium resources and production: Critical assessment and global projections. Minerals 2012, 2, 65–84. [Google Scholar] [CrossRef]
- Huang, T.; Fu, X.; Ge, L.; Zou, F.; Hao, X.; Yang, R.; Xiao, R.; Fan, J. The genesis of giant lithium pegmatite veins in Jiajika, Sichuan, China: Insights from geophysical, geochemical as well as structural geology approach. Ore Geol. Rev. 2020, 124, 103557. [Google Scholar] [CrossRef]
- Christmann, P.; Gloaguen, E.; Labbé, J.-F.; Melleton, J.; Piantone, P. Global lithium resources and sustainability issues. In Lithium Process Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–40. [Google Scholar]
- Vivoda, V.; Bazilian, M.D.; Khadim, A.; Ralph, N.; Krame, G. Lithium nexus: Energy, geopolitics, and socio-environmental impacts in Mexico’s Sonora project. Energy Res. Soc. Sci. 2024, 108, 103393. [Google Scholar] [CrossRef]
- Annikova, I.Y.; Vladimirov, A.; Smirnov, S.; Oitseva, T.; Mikheev, E.; Jes, E.; Travin, A.; D’yachkov, B.; Maslov, V.; Gertner, I. Geology and mineralogy of the Novo-Akhmirovskoe deposit of lithium topaz-zinnwaldite granites (East Kazakhstan). Lithosphere 2019, 304–326. (In Russian) [Google Scholar] [CrossRef]
- U.S. Department of the Interior; U.S. Geological Survey. Mineral Commodity Summaries; U.S. Geological Survey: Reston, VA, USA, 2023; p. 210.[Green Version]
- Nurpeissova, A.; Seipiyev, A. Preparation of Battery-Grade Lithium Composites from Local Spodumene. In Proceedings of the 242nd Electrochemical Society Meeting, Atlanta, GA, USA, 9–13 October 2022; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2022. [Google Scholar][Green Version]
- Leontiev, L.N. Formation of Late Hercynian Rare-Metal-Bearing Grantes and Rare-Metal Belts of the Irtysh Region; Nedra Publishers: Moscow, Russia, 1969; p. 164. [Google Scholar][Green Version]
- Samoilov, V.; Borsuk, A.; Kulenova, N. Industrial methods for the integrated processing of minerals that contain beryllium and lithium. Metallurgist 2009, 53, 53–56. [Google Scholar] [CrossRef]
- Gavrilenko, O.D.; Zimanovskaya, N.A. Geochemical Features of Lithium in Narym; Bulletin D; Serikbayev East Kazakhstan Technical University: Oskemen, Kazakhstan, 2015; pp. 3–9. (In Russian) [Google Scholar][Green Version]
- Mataybaeva, I.E. Regularities of Formation, Conditions of Placement and Forecast-Search Criteria for Assessing the Prospects of Deposits of Rare Metals and Rare Earths of East Kazakhstan. Ph.D. Thesis, Ust-Kamenogorsk, Kazakhstan, 2017; p. 147. Available online: https://www.geokniga.org/books/21728 (accessed on 22 June 2025). (In Russian).[Green Version]
- Stepanenko, N.I.; Pankratova, N.L.; Dyusembaeva, K.S.; Maylyanova, E.N. The geological structure and prospects of ore-bearing Upper Irgiz ore field (West Kazakhstan). In News of the Academy of Sciences of the Republic of Kazakhstan; Series of Geology and Technical Sciences; National academy of sciences of the Republic of Kazakhstan: Almaty, Kazakhstan, 2016; Volume 1, pp. 34–41. Available online: http://www.geolog-technical.kz/en/archive/%E2%84%961.html (accessed on 22 June 2025). (In Russian)
- Letnikov, F. Topaz granites in northern Kazakhstan. Petrology 2008, 16, 319–334. [Google Scholar] [CrossRef]
- Kopobaeva, A.N. Investigation of Patterns of Distribution of Rare Elements (Be, W, Mo) in Rocks of Central Kazakhstan. Ph.D. Thesis, Karaganda Technical University, Karaganda, Kazakhstan, 2020. [Google Scholar][Green Version]
- Zabotina, M.V. Ore formation conditions at the Drozhilovsky rare metal deposit, Kazakhstan. Metallog. Anc. Mod. Ocean. 2020, 1, 65–69. (In Russian) [Google Scholar]
- Kembaev, M.K. Forms of Finding Rare Earths in the Weathering Crusts of Deposits in Northern Kazakhstan and Their 3D Models. Ph.D. Thesis, Kazakh National Research Technical University, Almaty, Kazakhstan, 2017. [Google Scholar][Green Version]
- Absametov, M.K.; Murtazin, E.Z.; Kan, S.M.; Isabekov, R.B.; Shagarova, L.V. Industrial Waters and Assessment of Pollution of the Oil and gas Environment of the Regions of Kazakhstan; Satbayev University Publisher: Almaty, Kazakhstan, 2017; p. 128. (In Russian) [Google Scholar][Green Version]
- Seitmuratova, E.Y.; Baratov, R.; Arshamov, Y.K.; Dautbekov, D.; Seytzhanov, S.A. Lithium and gold content in salt domes and saline lands of Western and Southern Kazakhstan. Nauk. Visnyk Natsionalnoho Hirnychoho Universytetu 2023, 1, 10–19. [Google Scholar] [CrossRef]
- Gebreyohannes, B.G.; del Rosario Alberto, V.; Yimam, A.; Woldetinsae, G.; Tadesse, B. Alternative beneficiation of tantalite and removal of radioactive oxides from Ethiopian Kenticha pegmatite–spodumene ores. Int. J. Miner. Metall. Mater. 2017, 24, 727–735. [Google Scholar] [CrossRef]
- Gao, T.-m.; Fan, N.; Chen, W.; Dai, T. Lithium extraction from hard rock lithium ores (spodumene, lepidolite, zinnwaldite, petalite): Technology, resources, environment and cost. China Geol. 2023, 6, 137–153. [Google Scholar]
- Hongyun, D.; Shengwen, Z.; Yuxin, L.; Weifa, P.; Shaojun, Z. Study and optimization of lithium extraction from amblygonite by the sulfuric acid method. Nonferrous Met. Sci. Eng. 2022, 13, 35–43. [Google Scholar]
- Losey, A.; Rakovan, J.; Hughes, J.M.; Francis, C.A.; Dyar, M.D. Structural variation in the lithiophilite–triphylite series and other olivine-group structures. Can. Mineral. 2004, 42, 1105–1115. [Google Scholar] [CrossRef]
- Al-Ani, T.; Ahtola, T. Mineralogy of Spodumene Pegmatites, Kaustinen, Western Finland; Geological Survey of Finland, Report; Southern Finland Office: Helsinki, Finland, 2008; Volume 19, pp. 1–48. Available online: https://tupa.gtk.fi/raportti/arkisto/m19_2323_2008_61.pdf (accessed on 25 January 2025).[Green Version]
- Bulatovic, S.M. Beneficiation of lithium ores. In Handbook of Flotation Reagents: Chemistry, Theory and Practice, Volume 3: Flotation of Industrial Minerals; Elsevier: Amsterdam, The Netherlands, 2014; pp. 41–56. [Google Scholar][Green Version]
- He, G.-C.; Xiang, H.-M.; Jiang, W.; Kang, Q.; Chen, J.-H. First-principles theory on electronic structure and floatability of spodumene. Rare Met. 2014, 33, 742–748. [Google Scholar] [CrossRef]
- Salakjani, N.K.; Singh, P.; Nikoloski, A.N. Mineralogical transformations of spodumene concentrate from Greenbushes, Western Australia. Part 1: Conventional heating. Miner. Eng. 2016, 98, 71–79. [Google Scholar] [CrossRef]
- Moore, R.L.; Mann, J.P.; Montoya, A.; Haynes, B.S. In situ synchrotron XRD analysis of the kinetics of spodumene phase transitions. Phys. Chem. Chem. Phys. 2018, 20, 10753–10761. [Google Scholar] [CrossRef]
- Dessemond, C.; Soucy, G.; Harvey, J.-P.; Ouzilleau, P. Phase Transitions in the α–γ–β Spodumene Thermodynamic System and Impact of γ-Spodumene on the Efficiency of Lithium Extraction by Acid Leaching. Minerals 2020, 10, 519. [Google Scholar] [CrossRef]
- Salakjani, N.K.; Nikoloski, A.N.; Singh, P. Mineralogical transformations of spodumene concentrate from Greenbushes, Western Australia. Part 2: Microwave heating. Miner. Eng. 2017, 100, 191–199. [Google Scholar] [CrossRef]
- Li, C.-T. The crystal structure of LiAlSi2O6 III (high-quartz solid solution). Z. Krist.-Cryst. Mater. 1968, 127, 327–348. [Google Scholar] [CrossRef]
- Kotsupalo, N.P.; Menzheres, L.T.; Ryabtsev, A.D.; Boldyrev, V.V. Mechanical activation of α-spodumene for further processing into lithium compounds. Theor. Found. Chem. Eng. 2010, 44, 503–507. [Google Scholar] [CrossRef]
- Buerger, M.J. The stuffed derivatives of the silica structures. Am. Mineral. J. Earth Planet. Mater. 1954, 39, 600–614. [Google Scholar]
- Welsch, A.; Murawski, D.; Prekajski, M.; Vulic, P.; Kremenovic, A. Ionic conductivity in single-crystal LiAlSi2O6: Influence of structure on lithium mobility. Phys. Chem. Miner. 2015, 42, 413–420. [Google Scholar] [CrossRef]
- Abdullah, A.A.; Oskierski, H.C.; Altarawneh, M.; Senanayake, G.; Lumpkin, G.; Dlugogorski, B. Phase transformation mechanism of spodumene during its calcination. Miner. Eng. 2019, 140, 105883. [Google Scholar] [CrossRef]
- Peltosaari, O.; Tanskanen, P.; Heikkinen, E.P.; Fabritius, T. α→γ→β phase transformation of spodumene with hybrid microwave and conventional furnaces. Miner. Eng. 2015, 82, 54–60. [Google Scholar] [CrossRef]
- Gamage McEvoy, J.; Thibault, Y.; Duguay, D. Investigating Exchange Efficiencies of Sodium and Magnesium to Access Lithium from β-Spodumene and Li-Stuffed β-Quartz (γ-Spodumene). Crystals 2024, 14, 988. [Google Scholar] [CrossRef]
- Munoz, J. Stability relations of LiAlSi2O6 at high pressures. Mineral. Soc. Am. Spec. Pap. 1969, 2, 203–209. [Google Scholar]
- Ullrich, A.; Schranz, W.; Miletich, R. The nonlinear anomalous lattice elasticity associated with the high-pressure phase transition in spodumene: A high-precision static compression study. Phys. Chem. Miner. 2009, 36, 545–555. [Google Scholar] [CrossRef]
- Yonghua, D.; Lishi, M.; Ping, L.; Yong, C. First-principles calculations of electronic structures and optical, phononic, and thermodynamic properties of monoclinic α-spodumene. Ceram. Int. 2017, 43, 6312–6321. [Google Scholar] [CrossRef]
- Legault-Seguin, E.; Mohns, C.; Rylatt, M.; Lakefield, O. Dense medium separation–An effective and robust pre-concentration technology. In Proceedings of the 48th Annual Canadian Mineral Processors Operators Conference, Ottawa, ON, Canada, 19–21 January 2016; Volume 44, pp. 1–32. [Google Scholar]
- Gibson, C.E.; Aghamirian, M.; Grammatikopoulos, T.; Smith, D.L.; Bottomer, L. The Recovery and Concentration of Spodumene Using Dense Media Separation. Minerals 2021, 11, 649. [Google Scholar] [CrossRef]
- Wills, B.; Finch, J. Chapter 11-Dense Medium Separation (DMS). In Wills’ Mineral Processing Technology; Butterworth-Heinemann: Boston, MA, USA, 2016. [Google Scholar]
- Kundu, T.; Rath, S.S.; Das, S.K.; Parhi, P.K.; Angadi, S.I. Recovery of lithium from spodumene-bearing pegmatites: A comprehensive review on geological reserves, beneficiation, and extraction. Powder Technol. 2022, 415, 118142. [Google Scholar] [CrossRef]
- Manser, R.M. Handbook of Silicate Flotation; Warren Spring Laboratory: Stevenage, UK, 1975. [Google Scholar]
- Aghamirian, M.; Mohns, C.; Grammatikopoulos, T.; Imeson, D.; Pearse, G. An overview of spodumene beneficiation. In Proceedings of the 44th Annual Meeting of the Canadian Mineral Processors, Ottawa, ON, Canada, 1 January 2012. [Google Scholar]
- Marion, C.; Williams, H.; Langlois, R.; Kökkılıç, O.; Coelho, F.; Awais, M.; Rowson, N.; Waters, K. The potential for dense medium separation of mineral fines using a laboratory Falcon Concentrator. Miner. Eng. 2017, 105, 7–9. [Google Scholar] [CrossRef]
- Munson, G.A.; Clarke, F.F. Mining and concentrating spodumene in the Black Hills, South Dakota. AIME Trans. 1955, 202, 1041–1045. [Google Scholar]
- Redeker, I.H. Concentration of Spodumene from North Carolina Pegmatite Ores; The American Institute of Mining, Metallurgical, and Petroleum Engineers: San Ramon, CA, USA, 1979. [Google Scholar]
- Amarante, M.; De Sousa, A.B.; Leite, M.M. Processing a spodumene ore to obtain lithium concentrates for addition to glass and ceramic bodies. Miner. Eng. 1999, 12, 433–436. [Google Scholar] [CrossRef]
- Cook, B.K.; Aghamirian, M.; Li, H.; Nabiri, N.; Nottingham, J.; Quinn, J.; Gunning, C.; Gibson, C.E.; Brindle, P. Production of Spodumene Concentrate from the North Carolina Piedmont Lithium Project. In Conference of Metallurgists; Springer International Publishing: Cham, Switzerland, 2022; pp. 979–992. [Google Scholar]
- Grewal, I.; Lundt, M.; Wong, D.; Tse, W. Recent Developments in Preconcentration Using Dense Media Separation. 2016. Available online: https://www.911metallurgist.com/blog/wp-content/uploads/2016/05/Dense-Media-Separation.pdf (accessed on 16 August 2023).
- Lundt, M.; Grewal, I. Dense Media Separation—A Valuable Process for Preconcentration. In Proceedings of the Metallurgical Plant Design and Operating Strategies—World’s Best Practice (MetPlant 2017), Perth, Western Australia, 11–12 September 2017; The Australasian Institute of Mining and Metallurgy: Melbourne, Australia, 2017; pp. 122–130. [Google Scholar]
- Oliazadeh, M.; Aghamirian, M.; Ali, S.; Legault, E.; Gibson, C. Flowsheet Development for Benefication of Lithium Minerals from Hard Rock Deposits. In Extraction 2018; Springer: Berlin/Heidelberg, Germany, 2018; pp. 2293–2307. [Google Scholar]
- Cerny, P.; Ercit, T.S.; Vanstone, P.T. Petrology and Mineralization of the Tanco Rare-Element Pegmatite, Southeastern Manitoba; Field Trip Guidebook; Geological Association of Canada—Mineralogical Association of Canada: St. John’s, NL, Canada, 1996; Volume 63, Available online: https://www.researchgate.net/publication/270758183_Petrology_and_Mineralization_of_the_Tanco_Rare-Element_Pegmatite_Southeastern_Manitoba_Field_Trip_A3?channel=doi&linkId=54b3ddb50cf26833efcfd8be&showFulltext=true (accessed on 22 June 2025).
- Sagzhanov, D.; Ito, J.; Altansukh, B.; Godirilwe, L.L.; Jeon, S.; Haga, K.; Shibayama, A. Beneficiation of Low-Grade Lithium Ores from Eastern Kazakhstan by Dense Media Separation (DMS) and Froth Flotation. In Rare Metal Technology 2024; TMS 2024. The Minerals, Metals & Materials Series; Springer: Cham, Switzerland. [CrossRef]
- Chu, H.; Chen, L.; Lu, D.; Wang, Y.; Zheng, X. Ultrasonic pretreatment of spodumene with different size fractions and its influence on flotation. Ultrason. Sonochem. 2022, 82, 105889. [Google Scholar] [CrossRef] [PubMed]
- Parapari, P.S.; Irannajad, M.; Mehdilo, A. Effect of acid surface dissolution pretreatment on the selective flotation of ilmenite from olivine and pyroxene. Int. J. Miner. Process. 2017, 167, 49–60. [Google Scholar] [CrossRef]
- Irannajad, M.; Nuri, O.S.; Mehdilo, A. Surface dissolution-assisted mineral flotation: A review. J. Environ. Chem. Eng. 2019, 7, 103050. [Google Scholar] [CrossRef]
- Irannajad, M.; Mehdilo, A.; Nuri, O.S. Influence of microwave irradiation on ilmenite flotation behavior in the presence of different gangue minerals. Sep. Purif. Technol. 2014, 132, 401–412. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, G.; Zhang, L.; Lu, D.; Wang, L.; Zhao, Y.; Zheng, H. Surface dissolution of spodumene and its role in the flotation concentration of a spodumene ore. Miner. Eng. 2018, 125, 120–125. [Google Scholar] [CrossRef]
- Zhu, G.; Zhao, Y.; Zheng, X.; Wang, Y.; Zheng, H.; Lu, D. Surface features and flotation behaviors of spodumene as influenced by acid and alkali treatments. Appl. Surf. Sci. 2020, 507, 145058. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, X.; Li, E.; Wang, Y.; Miller, J.D. Wetting characteristics of spodumene surfaces as influenced by collector adsorption. Miner. Eng. 2019, 130, 117–128. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.; Zhu, G.; Cao, Y. Effects of dissolution by alkali treatment on anisotropic surface properties and flotation behavior of spodumene. Colloids Surf. A Physicochem. Eng. Asp. 2023, 675, 132088. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, N.; Chu, H.; Zheng, X.; Lu, D.; Zheng, H. Surface dissolution behavior and its influences on the flotation separation of spodumene from silicates. Sep. Sci. Technol. 2021, 56, 1407–1417. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Y.; Zhang, L. Effect of spodumene leaching with sodium hydroxide on its flotation. Physicochem. Probl. Miner. Process. 2015, 51, 745–754. [Google Scholar]
- Moon, K.S.; Fuerstenau, D.W. Surface crystal chemistry in selective flotation of spodumene (LiAl[SiO3]2) from other aluminosilicates. Int. J. Miner. Process. 2003, 72, 11–24. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, Y.; Liu, X.; Yu, F.; Lu, D. The cleavage and surface properties of wet and dry ground spodumene and their flotation behavior. Appl. Surf. Sci. 2015, 357, 333–339. [Google Scholar] [CrossRef]
- Luo, L.; Xu, L.; Shi, X.; Meng, J.; Liu, R. Microscale insights into the influence of grinding media on spodumene micro-flotation using mixed anionic/cationic collectors. Int. J. Min. Sci. Technol. 2022, 32, 171–179. [Google Scholar] [CrossRef]
- Chu, H.; Chen, L.; Lu, D.; Wang, Y.; Zheng, X.; Zhu, G. Ultrasound application in alkaline pretreatment process of spodumene to improve particle floatability. Int. J. Min. Sci. Technol. 2023, 33, 883–891. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, B.; Yang, S.; Yang, Z.; Luo, X.; Zhou, H. Comprehensive Recovery of Lithium, Tantalum and Feldspar from Granite Pegmatite Spodumene Ore in Yichun of Jiangxi. Conserv. Util. Miner. Resour. 2022, 42, 30–37. [Google Scholar]
- Bale, M.; May, A. Processing of ores to produce tantalum and lithium. Miner. Eng. 1989, 2, 299–320. [Google Scholar] [CrossRef]
- Phiri, T.; Tepa, C.; Nyati, R. Effect of Desliming on Flotation Response of Kansanshi Mixed Copper Ore. J. Miner. Mater. Charact. Eng. 2019, 7, 193. [Google Scholar] [CrossRef]
- Ansari, M. Fine Particle Processing—A Difficult Problem for Mineral Engineers. 1997. Available online: https://eprints.nmlindia.org/2867 (accessed on 1 March 2025).
- Wang, W.; Pan, M.; Duan, C.; Jiang, H.; Zhao, Y.; Lu, H. Dry deep screening of spodumene and its mineral processing technology. Miner. Eng. 2022, 179, 107445. [Google Scholar] [CrossRef]
- Banks, M.K.; McDaniel, W.; Sales, P. A method for Concentration oi North Carolina Spodumene Ores. Trans. AIME 1953, 181–186. [Google Scholar]
- Norragn, D.; Mankaso, M. Bench Scale and Pilot Plant Test for Magnetic Cocentartion Circuit Design. Miner. Process. Plant Desing/Practice and Control Proc. 2002, 1, 176–200. [Google Scholar]
- Maguran, D.; Dupere, M.; Gagnon, R.; Anson, J.; Boyd, A.; Gravel, A. NI 43-101 Technical Report on the Estimate to Complete for the Whabouchi Lithium Mine and Shawinigan Electrochemical Plant; Nemaska Project; Nemaska Lithium Inc.: James Bay, QC, Canada, 2019; p. 129. [Google Scholar]
- McVay, T.L.; Browning, J.S. Flotation of Spodumene from Pegmatites of Cleveland County, NC; US Department of the Interior, Bureau of Mines: Washington, DC, USA, 1962.
- Jirestig, J.A.; Forssberg, K. Dispersion of flotation concentrates before magnetic separation. Miner. Eng. 1994, 7, 1505–1516. [Google Scholar] [CrossRef]
- Siame, E. Recovery of Lithium from China Clay Waste Using a Combination of Froth Flotation, Magnetic Separation, Roasting and Leaching. Ph.D. Thesis, University of Exeter, Exeter, UK, 2011. [Google Scholar]
- Wills, B.A.; Finch, J. Froth flotation. In Wills’ Mineral Processing Technology (7th Edition): An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery; Butterworth-Heinemann: Oxford, UK, 2006; pp. 267–2344. [Google Scholar]
- Xie, R.; Zhu, Y.; Liu, J.; Li, Y. Flotation behavior and mechanism of α-bromododecanoic acid as collector on the flotation separation of spodumene from feldspar and quartz. J. Mol. Liq. 2021, 336, 116303. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, Y.; Liu, J.; Li, Y. The flotation behavior and adsorption mechanism of a new cationic collector on the separation of spodumene from feldspar and quartz. Sep. Purif. Technol. 2021, 264, 118445. [Google Scholar] [CrossRef]
- Shu, K.; Xu, L.; Wu, H.; Tang, Z.; Luo, L.; Yang, J.; Xu, Y.; Feng, B. Selective flotation separation of spodumene from feldspar using sodium alginate as an organic depressant. Sep. Purif. Technol. 2020, 248, 117122. [Google Scholar] [CrossRef]
- Cook, B.K.; Aghamirian, M.; Gibson, C.E. Optimization of spodumene flotation with a fatty acid collector. Miner. Eng. 2023, 204, 108412. [Google Scholar] [CrossRef]
- Xu, L.; Peng, T.; Tian, J.; Lu, Z.; Hu, Y.; Sun, W. Anisotropic surface physicochemical properties of spodumene and albite crystals: Implications for flotation separation. Appl. Surf. Sci. 2017, 426, 1005–1022. [Google Scholar] [CrossRef]
- Norman, J.; Gieseke, E.W. Beneficiation of Spodumene Rock by Froth Flotation; The American Institute of Mining, Metallurgical, and Petroleum Engineers: San Ramon, CA, USA, 1942; Volume 4. [Google Scholar]
- Coghill, W.H.; Clemmer, J.B. Soap flotation of the nonsulfides. Trans. AIME 1934, 112, 449. [Google Scholar]
- Taggart, A.F. Flotation—Application to Nonmetallics. Eng. Min. J. 1936, 137, 90–91. [Google Scholar]
- Dietrich, W.F.; Engel, A.L.; Guggenheim, M.; Rice, A.C.; Yerkes, L.A.; Davis, C.W. Progress Reports—Metallurgical Division: Ore-Testing Studies; U.S. Department of the Interior, Bureau of Mines: Washington, DC, USA, 1937.
- Falconer, S. Pretreatment of mineral surfaces for froth flotation. Trans. Metall. Soc. AIME 1949, 184, 247–255. [Google Scholar]
- Xu, L.; Hu, Y.; Wu, H.; Tian, J.; Liu, J.; Gao, Z.; Wang, L. Surface crystal chemistry of spodumene with different size fractions and implications for flotation. Sep. Purif. Technol. 2016, 169, 33–42. [Google Scholar] [CrossRef]
- Xu, L.; Jiao, F.; Jia, W.; Pan, Z.; Hu, C.; Qin, W. Selective flotation separation of spodumene from feldspar using mixed anionic/nonionic collector. Colloids Surf. A Physicochem. Eng. Asp. 2020, 594, 124605. [Google Scholar] [CrossRef]
- Menéndez, M.; Vidal, J.; Toraño, J.; Gent, M. Optimisation of spodumene flotation. Eur. J. Miner. Process. Environ. Prot. 2004, 4, 130–135. [Google Scholar]
- Yu, F.; Wang, Y.; Zhang, L.; Zhu, G. Role of oleic acid ionic− molecular complexes in the flotation of spodumene. Miner. Eng. 2015, 71, 7–12. [Google Scholar] [CrossRef]
- Zhu, G.; Cao, Y.; Wang, Y.; Wang, X.; Miller, J.D.; Lu, D.; Zheng, X. Surface chemistry features of spodumene with isomorphous substitution. Miner. Eng. 2020, 146, 106139. [Google Scholar] [CrossRef]
- Quezada, G.R.; Toledo, P.G. Complexation of alkali and alkaline-earth metal cations at spodumene-saltwater interfaces by molecular simulation: Impact on oleate adsorption. Minerals 2020, 11, 12. [Google Scholar] [CrossRef]
- Yu, F.-S.; Wang, Y.-H.; Wang, J.-M.; Xie, Z.-F.; Zhang, L. First-principle investigation on mechanism of Ca ion activating flotation of spodumene. Rare Met. 2014, 33, 358–362. [Google Scholar] [CrossRef]
- Jie, Z.; Weiqing, W.; Jing, L.; Yang, H.; Qiming, F.; Hong, Z. Fe (III) as an activator for the flotation of spodumene, albite, and quartz minerals. Miner. Eng. 2014, 61, 16–22. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Wang, W.; Zhang, J.; Yan, W.; Deng, J.; Feng, Q.; Huang, Y. The effects of Ca (II) and Mg (II) ions on the flotation of spodumene using NaOL. Miner. Eng. 2015, 79, 40–46. [Google Scholar] [CrossRef]
- Yongbing, Z.; Hepeng, Z.; Yijun, C.; Xianping, L.; Fanxin, X.; Boyuan, Z.; Siqi, Y. Activation mechanism of calcium hydrolysate on the spodumene surface and its effect on the adsorption of collector. Miner. Eng. 2021, 174, 107221. [Google Scholar] [CrossRef]
- Hu, Z.; Sun, C. Effects and mechanism of different grinding media on the flotation behaviors of Beryl and Spodumene. Minerals 2019, 9, 666. [Google Scholar] [CrossRef]
- Pashkevich, D.; Li, R.; Waters, K. Temperature and climate-induced fluctuations in froth flotation: An overview of different ore types. Can. Metall. Q. 2023, 62, 511–548. [Google Scholar] [CrossRef]
- Zhenfu, X.; Yuhua, W.; Fushun, Y.; Zijun, T.; Guangli, Z. Reviews of flotation research on pegmatite spodumene ores. Chin. J. Rare Met. 2013, 37, 641–649. [Google Scholar]
- Wang, Y.; Zhu, G.; Yu, F.; Lu, D.; Wang, L.; Zhao, Y.; Zheng, H. Improving spodumene flotation using a mixed cationic and anionic collector. Physicochem. Probl. Miner. Process. 2018, 54, 567–577. [Google Scholar]
- Fuerstenau, D. Zeta potentials in the flotation of oxide and silicate minerals. Adv. Colloid Interface Sci. 2005, 114, 9–26. [Google Scholar] [CrossRef]
- Fuerstenau, D.; Fuerstenau, M. The flotation of oxide and silicate minerals. In The Principles of Flotation; King, R.P., Ed.; Southern African Institute of Mining and Metallurgy: Johannesburg, South Africa, 1982; pp. 109–158. [Google Scholar]
- Tian, J.; Xu, L.; Deng, W.; Jiang, H.; Gao, Z.; Hu, Y. Adsorption mechanism of new mixed anionic/cationic collectors in a spodumene-feldspar flotation system. Chem. Eng. Sci. 2017, 164, 99–107. [Google Scholar] [CrossRef]
- Filippov, L.; Farrokhpay, S.; Lyo, L.; Filippova, I. Spodumene flotation mechanism. Minerals 2019, 9, 372. [Google Scholar] [CrossRef]
- Somasundaran, P.; Fuerstenau, D. Mechanisms of alkyl sulfonate adsorption at the alumina-water interface1. J. Phys. Chem. 1966, 70, 90–96. [Google Scholar] [CrossRef]
- Zhang, R.; Somasundaran, P. Advances in adsorption of surfactants and their mixtures at solid/solution interfaces. Adv. Colloid Interface Sci. 2006, 123, 213–229. [Google Scholar] [CrossRef]
- Fuerstenau, D.; Colic, M. Self-association and reverse hemimicelle formation at solid–water interfaces in dilute surfactant solutions. Colloids Surf. A Physicochem. Eng. Asp. 1999, 146, 33–47. [Google Scholar] [CrossRef]
- Fuerstenau, D.W.; Jia, R. The adsorption of alkylpyridinium chlorides and their effect on the interfacial behavior of quartz. Colloids Surf. A Physicochem. Eng. Asp. 2004, 250, 223–231. [Google Scholar] [CrossRef]
- Vanjara, A.K.; Dixit, S.G. Formation of mixed aggregates at the alumina-aqueous surfactant solution Interface. Langmuir 1995, 11, 2504–2507. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, Y.; Li, Y.; Han, Y. Flotation behavior and mechanism of a new mixed collector on separation of spodumene from feldspar. Colloids Surf. A Physicochem. Eng. Asp. 2020, 599, 124932. [Google Scholar] [CrossRef]
- Xu, L.; Wu, H.; Dong, F.; Wang, L.; Wang, Z.; Xiao, J. Flotation and adsorption of mixed cationic/anionic collectors on muscovite mica. Miner. Eng. 2013, 41, 41–45. [Google Scholar] [CrossRef]
- Rao, K.H.; Forssberg, K. Mixed collector systems in flotation. Int. J. Miner. Process. 1997, 51, 67–79. [Google Scholar]
- Rai, B.; Sathish, P.; Tanwar, J.; Moon, K.; Fuerstenau, D. A molecular dynamics study of the interaction of oleate and dodecylammonium chloride surfactants with complex aluminosilicate minerals. J. Colloid Interface Sci. 2011, 362, 510–516. [Google Scholar] [CrossRef]
- Bian, Z.; Zhang, H.; Ye, J.; Ning, Z. Flotation behavior of oleate and dodecylamine as mixed collector for recovery of lithium and rubidium from low-grade spodumene tailings: Experiment, Characterization and DFT calculation. Appl. Surf. Sci. 2023, 638, 158117. [Google Scholar] [CrossRef]
- Wu, H.; Tian, J.; Xu, L.; Fang, S.; Zhang, Z.; Chi, R. Flotation and adsorption of a new mixed anionic/cationic collector in the spodumene-feldspar system. Miner. Eng. 2018, 127, 42–47. [Google Scholar] [CrossRef]
- Shu, K.; Xu, L.; Wu, H.; Peng, L.; Xu, Y.; Luo, L.; Yang, J.; Tang, Z. In situ adsorption of mixed collectors BHA/DDA in spodumene-feldspar flotation system. Sep. Purif. Technol. 2020, 251, 117325. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, Y.; Liu, J.; Wang, X.; Li, Y. Differential collecting performance of a new complex of decyloxy-propyl-amine and α-bromododecanoic acid on flotation of spodumene and feldspar. Miner. Eng. 2020, 153, 106377. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Tian, J.; Wu, H.; Yang, Y.; Zeng, X.; Wang, Z.; Wang, J. Selective flotation separation of spodumene from feldspar using new mixed anionic/cationic collectors. Miner. Eng. 2016, 89, 84–92. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.; Li, Y. Interaction between cationic and anionic surfactants: Detergency and foaming properties of mixed systems. J. Surfactants Deterg. 2014, 17, 881–888. [Google Scholar] [CrossRef]
- Pan, A.; Rakshit, S.; Sahu, S.; Bhattacharya, S.C.; Moulik, S.P. Synergism between anionic double tail and zwitterionic single tail surfactants in the formation of mixed micelles and vesicles, and use of the micelle templates for the synthesis of nano-structured gold particles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 644–654. [Google Scholar] [CrossRef]
- Valdiviezo, E.; Oliveira, J. Synergism in aqueous solutions of surfactant mixtures and its effect on the hydrophobicity of mineral surfaces. Miner. Eng. 1993, 6, 655–661. [Google Scholar] [CrossRef]
- Wang, L.; Liu, R.; Hu, Y.; Sun, W. Adsorption of mixed DDA/NaOL surfactants at the air/water interface by molecular dynamics simulations. Chem. Eng. Sci. 2016, 155, 167–174. [Google Scholar] [CrossRef]
- Lucassen-Reynders, E.; Lucassen, J.; Giles, D. Surface and bulk properties of mixed anionic/cationic surfactant systems i. equilibrium surface tensions. J. Colloid Interface Sci. 1981, 81, 150–157. [Google Scholar] [CrossRef]
- Bendaouia, A.; Qassimi, S.; Boussetta, A.; Benzakour, I.; Benhayoun, A.; Amar, O.; Bourzeix, F.; Baïna, K.; Cherkaoui, M.; Hasidi, O. Hybrid features extraction for the online mineral grades determination in the flotation froth using Deep Learning. Eng. Appl. Artif. Intell. 2024, 129, 107680. [Google Scholar] [CrossRef]
- Costa, A.C.; Campos, F.V.; Araujo, L.R.; Torres, L.C.; Braga, A.P. Deep architecture for silica forecasting of a real industrial froth flotation process. Eng. Appl. Artif. Intell. 2022, 115, 105196. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, Z.; Xie, Y.; Gao, X.; Chen, Q.; Gui, W. Long short-term memory-based grade monitoring in froth flotation using a froth video sequence. Miner. Eng. 2021, 160, 106677. [Google Scholar] [CrossRef]
- Margarido, F.; Vieceli, N.; Durão, F.; Guimarães, C.; Nogueira, C. Minero-metallurgical processes for lithium recovery from pegmatitic ores= Processos minero-metalúrgicos para a recuperação de lítio de minérios pegmatíticos. Comun. Geol. 2014, 101, 795–798. [Google Scholar]
- Rioyo, J.; Tuset, S.; Grau, R. Lithium Extraction from Spodumene by the Traditional Sulfuric Acid Process: A Review. Miner. Process. Extr. 2022, 43, 97–106. [Google Scholar] [CrossRef]
- Ellestad, R.B.; Milne, L.K. Method of Extracting Lithium Values from Spodumene Ores. U.S. Patent 2516109A, 25 July 1950. [Google Scholar]
- Berdikulova, F.A.; Serikbayeva, A.K.; Tabylganov, M.T.; Syrlybekkyzy, S.; Suleimenova, B.S. Methods for lithium-bearing raw materials processing. J. Chem. Technol. Metall. 2022, 57, 1220. [Google Scholar]
- Abdullah, A.A. Thermal Treatment of Spodumene (LiAlSi2O6) for Lithium Extraction. Ph.D. Thesis, Murdoch University, Perth, Australia, 2019. [Google Scholar]
- Zhang, Z.; Xu, C.; Cheng, G.; Lau, E. Towards carbon neutrality: A comprehensive study on the utilization and resource recovery of coal-based solid wastes. Int. J. Coal Sci. Technol. 2025, 12, 34. [Google Scholar] [CrossRef]
- Subasinghe, H.; Rezaee, M. Direct lithium extraction from α-Spodumene using NaOH roasting and water leaching. Chem. Eng. J. 2025, 505, 159661. [Google Scholar] [CrossRef]
- Kuang, G.; Chen, Z.B.; Guo, H.; Li, M.H. Lithium Extraction Mechanism from α-Spodumene by Fluorine Chemical method. Adv. Mater. Res. 2012, 524, 2011–2016. [Google Scholar] [CrossRef]
- Guo, H.; Kuang, G.; Wang, H.; Yu, H.; Zhao, X. Investigation of enhanced leaching of lithium from α-spodumene using hydrofluoric and sulfuric acid. Minerals 2017, 7, 205. [Google Scholar] [CrossRef]
- Spektor, K.; Fischer, A.; Haussermann, U. Crystallization of LiAlSiO4 glass in hydrothermal environments at gigapascal pressures–dense hydrous aluminosilicates. Inorg. Chem. 2016, 55, 8048–8058. [Google Scholar] [CrossRef]
- Iezzi, G.; Bromiley, G.D.; Cavallo, A.; Das, P.P.; Karavassili, F.; Margiolaki, I.; Stewart, A.A.; Tribaudino, M.; Wright, J.P. Solid solution along the synthetic LiAlSi2O6-LiFeSi2O6 (spodumene-ferri-spodumene) join: A general picture of solid solutions, bond lengths, lattice strains, steric effects, symmetries, and chemical compositions of Li clinopyroxenes. Am. Mineral. 2016, 101, 2498–2513. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, T.; He, L.; Zhao, Z.; Liu, X. A promising approach for directly extracting lithium from α-spodumene by alkaline digestion and precipitation as phosphate. Hydrometallurgy 2019, 189, 105141. [Google Scholar] [CrossRef]
- Aylmore, M.G.; Merigot, K.; Rickard, W.D.; Evans, N.J.; McDonald, B.J.; Catovic, E.; Spitalny, P. Assessment of a spodumene ore by advanced analytical and mass spectrometry techniques to determine its amenability to processing for the extraction of lithium. Miner. Eng. 2018, 119, 137–148. [Google Scholar] [CrossRef]
- Fraas, F.; Ralston, O.C. Beneficiation of Spodumene by Decrepitation; US Department of the Interior, Bureau of Mines: Washington, DC, USA, 1937; Volume 3336.
- Hevia, R.P.; Gil, C.; Carda, J.B. Mineral Treatment of Spodumene in the Ceramic Industry. Characterisation Andtechnological Use. 2006. Available online: https://www.qualicer.org/recopilatorio/ponencias/pdfs/0632130e.pdf (accessed on 31 March 2023).
- Peltosaari, O.; Tanskanen, P.; Hautala, S.; Heikkinen, E.-P.; Fabritius, T. Mechanical enrichment of converted spodumene by selective sieving. Miner. Eng. 2016, 98, 30–39. [Google Scholar] [CrossRef]
- Nazir, M.K.; Dyer, L.; Tadesse, B.; Albijanic, B.; Kashif, N. Effect of calcination on coarse gangue rejection of hard rock lithium ores. Sci. Rep. 2022, 12, 12963. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.K.; Dyer, L.; Tadesse, B.; Albijanic, B.; Kashif, N. Flotation performance of calcined spodumene. Adv. Powder Technol. 2022, 33, 103772. [Google Scholar] [CrossRef]
- Garrett, D.E. Handbook of Lithium and Natural Calcium Chloride; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Gasafi, E.; Pardemann, R. Processing of spodumene concentrates in fluidized-bed systems. Miner. Eng. 2020, 148, 106205. [Google Scholar] [CrossRef]
- Botto, I.; Arazi, C. Kinetics of polymorphic transformation of spodumene I into spodumene II. Bol. Soc. Esp. Ceram. Vidr. 1975, 14, 433–440. [Google Scholar]
- White, G.D.; McVay, T.N. Some Aspects of the Recovery of Lithium from Spodumene; Oak Ridge National Laboratory (ORNL): Oak Ridge, TN, USA, 1958. [Google Scholar]
- Gasalla, H.J.; Pereira, E. Activation-deactivation mechanisms in spodumene samples. Solid State Ion. 1990, 42, 1–6. [Google Scholar] [CrossRef]
- Clark, D.E.; Folz, D.C.; West, J.K. Processing materials with microwave energy. Mater. Sci. Eng. A 2000, 287, 153–158. [Google Scholar] [CrossRef]
- Rezaee, M.; Hassas, B.V.; Akbari, H.; Agrawal, D.; Slawecki, T. Process for Exctraction of Lithium. WO Patent WO2021/168210A1, 26 August 2021. Available online: https://patents.google.com/patent/WO2021168210A1/en?oq=WO+2021%2f168210 (accessed on 22 June 2025).
- Huang, Y.; Zhang, T.-a.; Dou, Z.; Han, G.; Cao, Y.; Hou, C. Decomposition mechanism of a mixed rare earth concentrate with sodium hydroxide in the microwave heating process. Miner. Eng. 2019, 132, 220–227. [Google Scholar] [CrossRef]
- Le, T.; Ju, S.; Ravindra, A.; Li, X.; Wang, Q. Effect of microwave roasting on aluminum extraction from diasporic bauxite-sodium carbonate-calcium hydroxide mixtures. JOM 2019, 71, 831–837. [Google Scholar] [CrossRef]
- Bishimbayeva, G.; Zhumabayeva, D.; Zhandayev, N.; Nalibayeva, A.; Shestakov, K.; Levanevsky, I.; Zhanabayeva, A. Technological Improvement Lithium Recovery Methods from Primary Resources. Orient. J. Chem. 2018, 34, 2762. [Google Scholar] [CrossRef]
- Clarke, G.M. Lithium-ion batteries raw material considerations. Chem. Eng. Prog. 2013, 109, 44–52. [Google Scholar]
- Colton, J.W. Recovery of Lithium from Complex Silicates; ACS Publications: Washington, DC, USA, 1957. [Google Scholar]
- Borges, P.H.; Santos, F.A.; Milikic, N.; A Barsante, C. Lithium Aluminosilicate Residue as Raw Material in the Production of Sustainable Concrete Masonry Units: A Brazilian Case. Open Constr. Build. Technol. J. 2016, 10, 418–430. [Google Scholar] [CrossRef]
- Kuang, G.; Liu, Y.; Li, H.; Xing, S.Z.; Li, F.J.; Guo, H. Extraction of lithium from beta-spodumene using sodium sulfate solution. Hydrometallurgy 2018, 177, 49–56. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, B.; Lv, Y.; Wang, C.; Chen, Y. An effective method for directly extracting lithium from α-spodumene by activated roasting and sulfuric acid leaching. J. Ind. Eng. Chem. 2023, 122, 540–550. [Google Scholar] [CrossRef]
- Sharma, Y. Processing of Lithium Containing Material. U.S. Patent 20150152523A1, 4 June 2015. [Google Scholar]
- Hunwick, R. Recovery of Lithium from Silicate Minerals. U.S. Patent 20170175228A, 20 November 2018. [Google Scholar]
- Chen, Y.; Tian, Q.Q.; Chen, B.Z.; Shi, X.C.; Liao, T. Preparation of lithium carbonate from spodumene by a sodium carbonate autoclave process. Hydrometallurgy 2011, 109, 43–46. [Google Scholar] [CrossRef]
- Chubb, P.A. Treatment of Lithium Ores. U.S. Patent 3073673A, 15 January 1963. [Google Scholar]
- Li, G.Q.; Cui, F.H. Method for Extracting Lithium Salt from Spodumene Using Lime Activation Under Pressure Leaching. Patent No. CN109437251A, 11 December 2018. [Google Scholar]
- Anovitz, L.; Blencoe, J.; Palmer, D. Method of Extracting Lithium. U.S. Patent 20060171869A1, 3 March 2006. [Google Scholar]
- Xing, P.; Wang, C.; Zeng, L.; Ma, B.; Wang, L.; Chen, Y.; Yang, C. Lithium Extraction and Hydroxysodalite Zeolite Synthesis by Hydrothermal Conversion of α-Spodumene. ACS Sustain. Chem. Eng. 2019, 7, 9498–9505. [Google Scholar] [CrossRef]
- Qiu, S.; Liu, C.; Yu, J. Conversion from α-spodumene to intermediate product Li2SiO3 by hydrothermal alkaline treatment in the lithium extraction process. Miner. Eng. 2022, 183, 107599. [Google Scholar] [CrossRef]
- Grasso, M.L.; Gonzalez, J.A.; Gennari, F.C. Lithium extraction from β-LiAlSi2O6 using Na2CO3 through thermal reaction. Miner. Eng. 2022, 176, 107349. [Google Scholar] [CrossRef]
- Fosu, A.Y.; Kanari, N.; Bartier, D.; Vaughan, J.; Chagnes, A. Novel extraction route of lithium from α-spodumene by dry chlorination. RSC Adv. 2022, 12, 21468–21481. [Google Scholar] [CrossRef]
- Barbosa, L.I.; Valente, G.; Orosco, R.P.; Gonzalez, J.A. Lithium extraction from beta-spodumene through chlorination with chlorine gas. Miner. Eng. 2014, 56, 29–34. [Google Scholar] [CrossRef]
- Barbosa, L.I.; Valente, N.; Gonzalez, J.A. Kinetic study on the chlorination of β-spodumene for lithium extraction with Cl2 gas. Thermochim. Acta 2013, 557, 61–67. [Google Scholar] [CrossRef]
- Peterson, J.A.; Gloss, G.H. Lithium Values Recovery Process. U.S. Patent 2893828A, 7 July 1959. [Google Scholar]
- Alhadad, M.F.; Oskierski, H.C.; Chischi, J.; Senanayake, G.; Dlugogorski, B.Z. Lithium extraction from β-spodumene: A comparison of keatite and analcime processes. Hydrometallurgy 2023, 215, 105985. [Google Scholar] [CrossRef]
- Gabra, G.; Torma, A.; Olivier, C. Pressure leaching of beta-spodumene by sodium chloride. Can. Metall. Q. 1975, 14, 355–359. [Google Scholar] [CrossRef]
- Barbosa, L.I.; Gonzalez, J.A.; Ruiz, M.D. Extraction of lithium from beta-spodumene using chlorination roasting with calcium chloride. Thermochim. Acta 2015, 605, 63–67. [Google Scholar] [CrossRef]
- Ni, C.; Liu, C.; Liu, J.; Wang, J.; Liang, Y.; Sun, W.; Zhong, H.; He, Z. Thermochemically driven crystal phase transfer via mechanical activation-assisted chlorination roasting toward the selective extraction of lithium from spodumene. J. Ind. Eng. Chem. 2024, 138, 632–640. [Google Scholar] [CrossRef]
- Medina, L.F.; El-Naggar, M.M. An alternative method for the recovery of lithium from spodumene. Metall. Trans. B 1984, 15. [Google Scholar] [CrossRef]
- El-Naggar, M.; Medina, L.; Espinola, A. The reaction between spodumene and tachyhydrite. Metall. Trans. B 1988, 19, 663–668. [Google Scholar] [CrossRef]
- Zelikman, A.N.; Kreĭn, O.G.E.; Samsonov, G.V. Metallurgy of Rare Metals; Israel Program for Scientific Translations: Jerusalem, Israel, 1966. [Google Scholar]
- Yan, Q.; Li, X.; Wang, Z.; Wu, X.; Wang, J.; Guo, H.; Hu, Q.; Peng, W. Extraction of lithium from lepidolite by sulfation roasting and water leaching. Int. J. Miner. Process. 2012, 110, 1–5. [Google Scholar] [CrossRef]
- Chagnes, A.; Swiatowska, J. Lithium Process Chemistry: Resources, Extraction, Batteries, and Recycling; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Lee, S. Extraction of Lithium from Spodumene by Alkali Fusion. Master’s Thesis, Seoul National Universit, Seoul, Republic of Korea, 2018. [Google Scholar]
- Pechini, R. Method of Preparing Lead and Alkaline Earth Titanates and Niobates and Coating Method Using the Same to Form a Capacitor. U.S. Patent 3330697A, 26 August 1963. [Google Scholar]
- Ncube, T.; Oskierski, H.; Senanayake, G.; Dlugogorski, B.Z. Two-step reaction mechanism of roasting spodumene with potassium sulfate. Inorg. Chem. 2021, 60, 3620–3625. [Google Scholar] [CrossRef]
- Hayes, E.T.; Williams, F.P.; Sternberg, W.M. Production of Lithium Chloride from Spodumene. U.S. Patent 2533246A, 12 December 1950. [Google Scholar]
- Rosales, G.D.; Resentera, A.C.; Gonzalez, J.A.; Wuilloud, R.G.; Rodriguez, M.H. Efficient extraction of lithium from β-spodumene by direct roasting with NaF and leaching. Chem. Eng. Res. Des. 2019, 150, 320–326. [Google Scholar] [CrossRef]
- Rodriguez, M.H.; Rosales, G.D.; Maria del Carmen, R. Process for Obtaining Lithium from Aluminosilicates and Intermediate Compounds. U.S. Patent 10259719B2, 16 April 2019. [Google Scholar]
- Resentera, A.C.; Rosales, G.D.; Esquivel, M.R.; Rodriguez, M.H. Thermal and structural analysis of the reaction pathways of α-spodumene with NH4HF2. Thermochim. Acta 2020, 689, 178609. [Google Scholar] [CrossRef]
- Wilson, M.; Cabrera, J.; Zou, Y. The process and mechanism of alkali—Silica reaction using fused silica as the reactive aggregate. Adv. Cem. Res. 1994, 6, 117–125. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Y.; Ma, B.; Wang, C.; Chen, Y. Strengthening extraction of lithium and rubidium from activated α-spodumene concentrate via sodium carbonate roasting. J. Ind. Eng. Chem. 2023, 123, 248–259. [Google Scholar] [CrossRef]
- Grasso, M.L.; Castro, F.; Gonzalez, J.A.; Gennari, F.C. Obtaining Li2CO3 from β-LiAlSi2O6 by solid state reaction with NaOH. Miner. Eng. 2023, 201, 108214. [Google Scholar] [CrossRef]
- Lileev, I.; Sakun, L.S.; Gucova, I. Reaction of hydrated lithium dialuminate with sodium hydroxide solutions. Russ. J. Inorg. Chem. 1968, 13, 213–217. [Google Scholar]
- Fu, W.; Meng, L.; Qu, J. Sintering Mechanism and Leaching Kinetics of Low-Grade Mixed Lithium Ore and Limestone. Metals 2024, 14, 1075. [Google Scholar] [CrossRef]
- Su, H.; Ju, J.; Zhang, J.; Yi, A.; Lei, Z.; Wang, L.; Zhu, Z.; Qi, T. Lithium recovery from lepidolite roasted with potassium compounds. Miner. Eng. 2020, 145, 106087. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, J.; Wen, X.; Pu, J.; Wang, Y.; Yuan, D. Extraction of lithium from lepidolite by sulfate process. Inorg. Chem. Ind. 2014, 46, 41–44. [Google Scholar]
- Suharyanto, A.; Lalasari, L.; Mubarok, M. Decomposition of spodumene mineral in granitic rocks from South Kalimantan-Indonesia by potassium sulphate. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; p. 012044. [Google Scholar]
- Guo, H.; Lv, M.; Kuang, G.; Wang, H. Enhanced lithium extraction from α-spodumene with fluorine-based chemical method: A stepwise heat treatment for fluorine removal. Miner. Eng. 2021, 174, 107246. [Google Scholar] [CrossRef]
- Rosales, G.D.; Resentera, A.C.; Braga, P.F.; Esquivel, M.R.; Rodriguez, M.H. Simple process for lithium extraction from α-spodumene with potassium fluoride: Modeling and optimization. Chem. Eng. Res. Des. 2023, 191, 319–324. [Google Scholar] [CrossRef]
- Rosales, G.D.; Ruiz, M.D.; Rodriguez, M.H. Novel process for the extraction of lithium from beta-spodumene by leaching with HF. Hydrometallurgy 2014, 147, 1–6. [Google Scholar] [CrossRef]
- Rosales, G.D.; Ruiz, M.C.; Rodriguez, M.H. Study of the extraction kinetics of lithium by leaching β-spodumene with hydrofluoric acid. Minerals 2016, 6, 98. [Google Scholar] [CrossRef]
- Resentera, A.C.; Rosales, G.D.; Esquivel, M.R.; Rodriguez, M.H. Acid dissolution of LiF/(NH4)3AlF6 mixtures obtained in the fluorination of α-spodumene with NH4HF2: Modeling and optimization. Chem. Eng. Res. Des. 2023, 200, 388–395. [Google Scholar] [CrossRef]
- Resentera, A.C.; Esquivel, M.R.; Rodriguez, M.H. Low-temperature lithium extraction from α-spodumene with NH4HF2: Modeling and optimization by least squares and artificial neural networks. Chem. Eng. Res. Des. 2021, 167, 73–83. [Google Scholar] [CrossRef]
- Karavaĭko, G.; Krutsko, V.; Mel’nikova, E.; Avakian, Z.; IuI, O. Role of microorganisms in the destruction of spodumene. Mikrobiologiia 1980, 49, 547–551. [Google Scholar]
- Rezza, I.; Salinas, E.; Calvente, V.; Benuzzi, D.; de Tosetti, M.I.S. Extraction of lithium from spodumene by bioleaching. Lett. Appl. Microbiol. 1997, 25, 172–176. [Google Scholar] [CrossRef]
- Vandevivere, P.; Welch, S.; Ullman, W.; Kirchman, D.L. Enhanced dissolution of silicate minerals by bacteria at near-neutral pH. Microb. Ecol. 1994, 27, 241–251. [Google Scholar] [CrossRef]
- Kirk, R.D.; Newsome, L.; Falagan, C.; Hudson-Edwards, K.A. Bioleaching of lithium from jadarite, spodumene, and lepidolite using Acidiothiobacillus ferrooxidans. Front. Microbiol. 2024, 15, 1467408. [Google Scholar] [CrossRef]
- Yin, Z.; Lu, X.; Liu, H.; Li, J.; Chen, J.; Zhang, D. Effect of organic acids on the dissolution of spodumene and preliminary study on microbial lithium extraction. In Proceedings of the Goldschmidt 2023 Conference, Lyon, France, 14 July 2023. [Google Scholar]
- Yuan, H.; Li, M.; Cui, L.; Wang, L.; Cheng, F. Electrochemical extraction technologies of lithium: Development and challenges. Desalination 2024, 598, 118419. [Google Scholar] [CrossRef]
- Haddad, A.Z.; Cha, H.; McDonough, L.; Dun, C.; Pohlman, G.; Urban, J.J.; Kostecki, R. Electrochemical lithium extraction from hectorite ore. Commun. Chem. 2024, 7, 1–8. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, F. Direct Extraction of Lithium from α-Phase Spodumene By Electrochemical Leaching. In Proceedings of the Electrochemical Society Meeting (PRiME 2024), Honolulu, HI, USA, 6–11 October 2024; p. 2017. [Google Scholar]
- Dong, Q.; Gang, H.; Xu, J.; Li, Z.; Wang, Z. The technologies of electrochemical lithium extraction process from lithium-containing solutions. J. Exp. Theor. Anal. 2024, 2, 91–102. [Google Scholar] [CrossRef]
- Asadi Dalini, E.; Karimi, G.; Zandevakili, S.; Goodarzi, M. A review on environmental, economic and hydrometallurgical processes of recycling spent lithium-ion batteries. Miner. Process. Extr. 2021, 42, 451–472. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Z. Hydrometallurgically recycling spent lithium-ion batteries. In Recycling of Spent Lithium-Ion Batteries: Processing Methods and Environmental Impacts; Springer: Cham, Switzerland, 2019; pp. 27–55. [Google Scholar]
- Ekberg, C.; Petranikova, M. Lithium batteries recycling. In Lithium Process Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 233–267. [Google Scholar]
- Doose, S.; Mayer, J.K.; Michalowski, P.; Kwade, A. Challenges in ecofriendly battery recycling and closed material cycles: A perspective on future lithium battery generations. Metals 2021, 11, 291. [Google Scholar] [CrossRef]
- Qing, J.; Wu, X.; Zeng, L.; Guan, W.; Cao, Z.; Li, Q.; Wang, M.; Zhang, G.; Wu, S. Novel approach to recycling of valuable metals from spent lithium-ion batteries using hydrometallurgy, focused on preferential extraction of lithium. J. Clean. Prod. 2023, 431, 139645. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Zhou, T.; Liu, D.; Hu, H.; Fan, S. Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries. Waste Manag. 2015, 38, 349–356. [Google Scholar] [CrossRef]
- Guan, J.; Xiao, H.; Lou, X.; Guo, Y.; Luo, X.; Li, Y.; Yan, C.; Yan, X.; Gao, G.; Yuan, H. Enhanced hydrometallurgical recovery of valuable metals from spent lithium-ion batteries by mechanical activation process. ES Energy Environ. 2018, 1, 80–88. [Google Scholar] [CrossRef]
- Wang, H.; Huang, K.; Zhang, Y.; Chen, X.; Jin, W.; Zheng, S.; Zhang, Y.; Li, P. Recovery of lithium, nickel, and cobalt from spent lithium-ion battery powders by selective ammonia leaching and an adsorption separation system. ACS Sustain. Chem. Eng. 2017, 5, 11489–11495. [Google Scholar] [CrossRef]
- Li, H.; Chen, Y.; Cui, Z.; Su, Y.; Liu, Z.; Dong, X.; Wang, Z.L.; Tang, W. One-step green hydrometallurgical recycling of spent lithium-ion batteries’ cathode. J. Hazard. Mater. 2025, 484, 136769. [Google Scholar] [CrossRef]
- Mousavinezhad, S.; Fahimi, A.; Sharifian, S.; Vahidi, E. Sustainable lithium production from sedimentary rock deposits: Carbon reduction and EV synergies. Resour. Conserv. Recycl. 2025, 218, 108271. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, P.; Li, W.; Zhang, Q.; Chen, W.-Q.; Feng, D. Environmental impacts of lithium supply chains from Australia to China. Environ. Res. Lett. 2024, 19, 094035. [Google Scholar] [CrossRef]
- Peiseler, L.; Schenker, V.; Schatzmann, K.; Pfister, S.; Wood, V.; Schmidt, T. Carbon footprint distributions of lithium-ion batteries and their materials. Nat. Commun. 2024, 15, 10301. [Google Scholar] [CrossRef]
- World Bank. Kazakhstan Mining Sector Diagnostic; World Bank Group: Washington, DC, USA, 2023; Available online: https://documents1.worldbank.org/curated/en/099081823001539573/pdf/P17674501063760b08b290a4ae6547845d.pdf (accessed on 7 June 2025).
- Rentsch, L.; Martin, G.; Bertau, M.; Höck, M. Lithium Extracting from Zinnwaldite: Economical Comparison of an Adapted Spodumene and a Direct-Carbonation Process. Chem. Eng. Technol. 2018, 41, 975–982. [Google Scholar] [CrossRef]
- Alhadad, M.F. Production of Lithium Chemicals from Spodumene Using Novel Leaching Processes. Ph.D. Thesis, Murdoch University, Murdoch, Australia, 2022. [Google Scholar]

| Mineral Name | General Formula | Li2O Content (wt%) | Reference |
|---|---|---|---|
| Spodumene | LiAlSi2O6 | 4–7 | [32] |
| Petalite | LiAlSi4O10 | 4.9 | [52] |
| Lepidolite | K(Li,Al)3(Si,Al)4O10(F,OH)2 | 4–8 | [50] |
| Zinnwaldite | KLiFe2+Al(AlSi3)O10(F,OH)2 | 2–5 | [72] |
| Amblygonite | (Li,Na)Al(PO4)(F,OH) | 7.4 | [73] |
| Eucryptite | LiAlSiO4 | 11.9 | [49] |
| Jadarite | LiNaB3SiO7(OH) | 7.3 | [27] |
| Hectorite | Na0.3(Mg,Li)3Si4O10(OH)2 | 1–3 | [42] |
| Triphylite | Li(Fe,Mn,)PO4 | 9.5 | [74] |
| Lithiophilite | Li(Mn,Fe)PO4 | 9.5 | [74] |
| Collectors | Adsorption Mechanism | Li2O, % | Rec., % | Ref. |
|---|---|---|---|---|
| Single Collectors | ||||
| DAA, NaOL, BHA, DTAC naphthenic soap and fatty acid | Poor selectivity and low recovery. | - | - | [29,118,160,170] |
| Mixed collectors (nonionic/nonionic, ionic/ionic and ionic/nonionic) | ||||
| DAA/NaOL (200 g/t, 9:1 ratio) | The mixture collector acted on the mineral surface mainly through physical adsorption, reducing the thickness of the multilayer adsorbed layer of DDA on the mineral surface. The composition of DDA and NaOL was easier for static electricity to absorb on the mineral surface by increasing the polarity of functional groups. | 5.10 | 62.99 | [171] |
| NaOL/TTPC 0.4 mM, 5:1 ratio, pH = 4.0 | The adsorption of this mixed collector on spodumene was shown to involve chemical interaction and electrostatic attraction. | 4.6 | 88 | [172] |
| NaOL/DTAC 0.4 mM, 9:1 ratio, pH = 8.5 | NaOL first reacts with aluminum sites on the mineral surfaces, followed by co-adsorption of DTAC, forming an electroneutral complex due to NaOL’s chemisorption. | - | 82.15 | [160] |
| BHA/DDA 0.4 mM, 6:1 ratio, pH = 8–9 | BHA initially reacts with aluminum sites on the mineral surface, followed by DDA, forming an electroneutral complex through chemisorption of BHA. A lower value of CMC and polarity result in higher adsorption. | - | 88.31 | [173] |
| DPA/α-BDDA 14.3 mg/L, 1:1 ratio, pH = 4.5 | The formation of halogen bonds between DPA and α-BDDA results in the creation of supermolecules. The adsorption of DPA-BDDA on spodumene was facilitated by both electrostatic attraction and chemisorption, enhancing spodumene’s recovery in the flotation process. | 7.61 | 82.14 | [174] |
| HPA/DIDA 57.14 mg/L, 1:3 ratio | HPA adsorbed on the spodumene surface mainly through hydrogen bonds, while DIDA adsorbed through chemisorption on both spodumene and feldspar surfaces. | - | 85.61 | [167] |
| Process | Roasting | Leaching | Status | Ref. | |||
|---|---|---|---|---|---|---|---|
| Additive | Temp. °C | Time, h | Reagent | Results, % | |||
| Decrepitation + acid | H2SO4 | 175-250 | ~1 | H2O | 98 | Commercialized | [123] |
| -- | 108–170 | 6–10 | HCl/HNO3 | 95 | Commercialized | [184,217] | |
| Decrepitation + alkali leaching | - | 225 | 1 | Na2CO3/NaOH | 90–94 | Commercialized | [215] |
| Decrepitation + chlorination | CaCl2/Cl2 | 900 | 2 | H2O | 90 | Commercialized | [232,233] |
| KCl/NaCl | 1000 | ~1 | H2O/HCl | 97 | Commercialized | [229] | |
| KCl/NaOH | 93 | Experimental | [230] | ||||
| NH4Cl | 250–750 | - | H2O | 98 | Experimental | [236] | |
| Alkali roasting | Na2CO3/NaOH | 400–850 | 1 | 99 | Experimental | [26,239] | |
| Lime roasting | CaCO3/CaO | 100–1050 | 1 | H2O | 92 | Commercialized | [240] |
| Sulfation | K2SO4 | 870 | H2O | 90 | Experimental | [241] | |
| CaSO4 + CaCO3 | 1150 | ~3 | H2O | 90 | Commercialized | [242] | |
| Na2SO4 + NaOH | 200–300 | - | Na2SO4 + CaO | 93 | Commercialized | [215] | |
| Fluorination | NaF/KF | 600 | 2 | HF | 90 | Commercialized | [243,244] |
| HF/H2SO4 | 100 | 3 | H2O | 95 | Experimental | [192] | |
| - | - | ~0.5 | NH4HF2 | 93 | Experimental | [245] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagzhanov, D.; Godirilwe, L.L.; Altansukh, B.; Takasaki, Y.; Shibayama, A. The Role of Sustainable Lithium Processing in Renewable Energy Development: A Comprehensive Review and the Potential of Kazakhstan Deposits. Sustainability 2025, 17, 5903. https://doi.org/10.3390/su17135903
Sagzhanov D, Godirilwe LL, Altansukh B, Takasaki Y, Shibayama A. The Role of Sustainable Lithium Processing in Renewable Energy Development: A Comprehensive Review and the Potential of Kazakhstan Deposits. Sustainability. 2025; 17(13):5903. https://doi.org/10.3390/su17135903
Chicago/Turabian StyleSagzhanov, Daulet, Labone L. Godirilwe, Batnasan Altansukh, Yasushi Takasaki, and Atsushi Shibayama. 2025. "The Role of Sustainable Lithium Processing in Renewable Energy Development: A Comprehensive Review and the Potential of Kazakhstan Deposits" Sustainability 17, no. 13: 5903. https://doi.org/10.3390/su17135903
APA StyleSagzhanov, D., Godirilwe, L. L., Altansukh, B., Takasaki, Y., & Shibayama, A. (2025). The Role of Sustainable Lithium Processing in Renewable Energy Development: A Comprehensive Review and the Potential of Kazakhstan Deposits. Sustainability, 17(13), 5903. https://doi.org/10.3390/su17135903







