Impact of Urban Greenspace Pattern Dynamics on Plant Diversity: A Case Study in Yangzhou, China
Abstract
1. Introduction
2. Data Acquisition and Research Methods
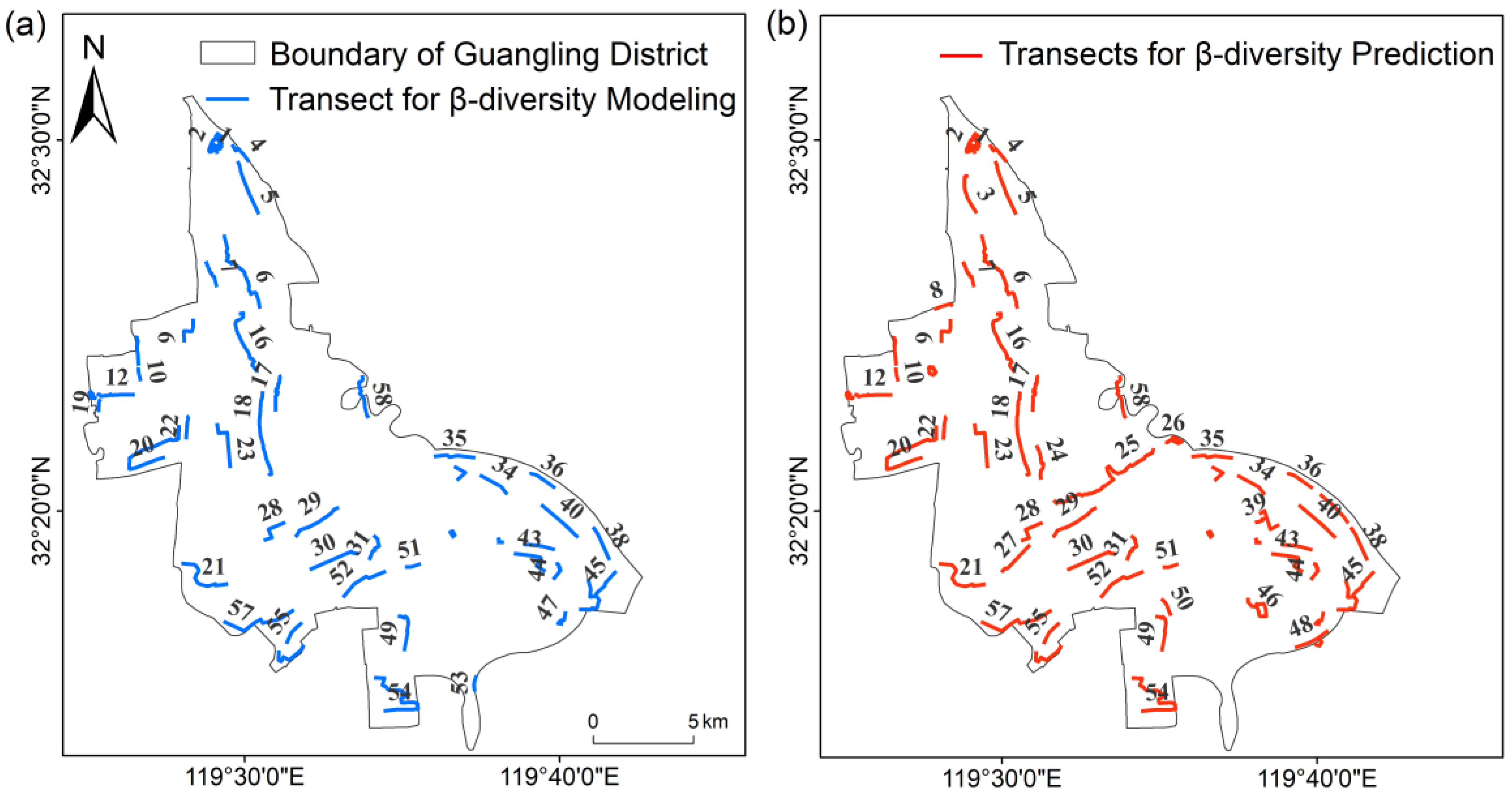
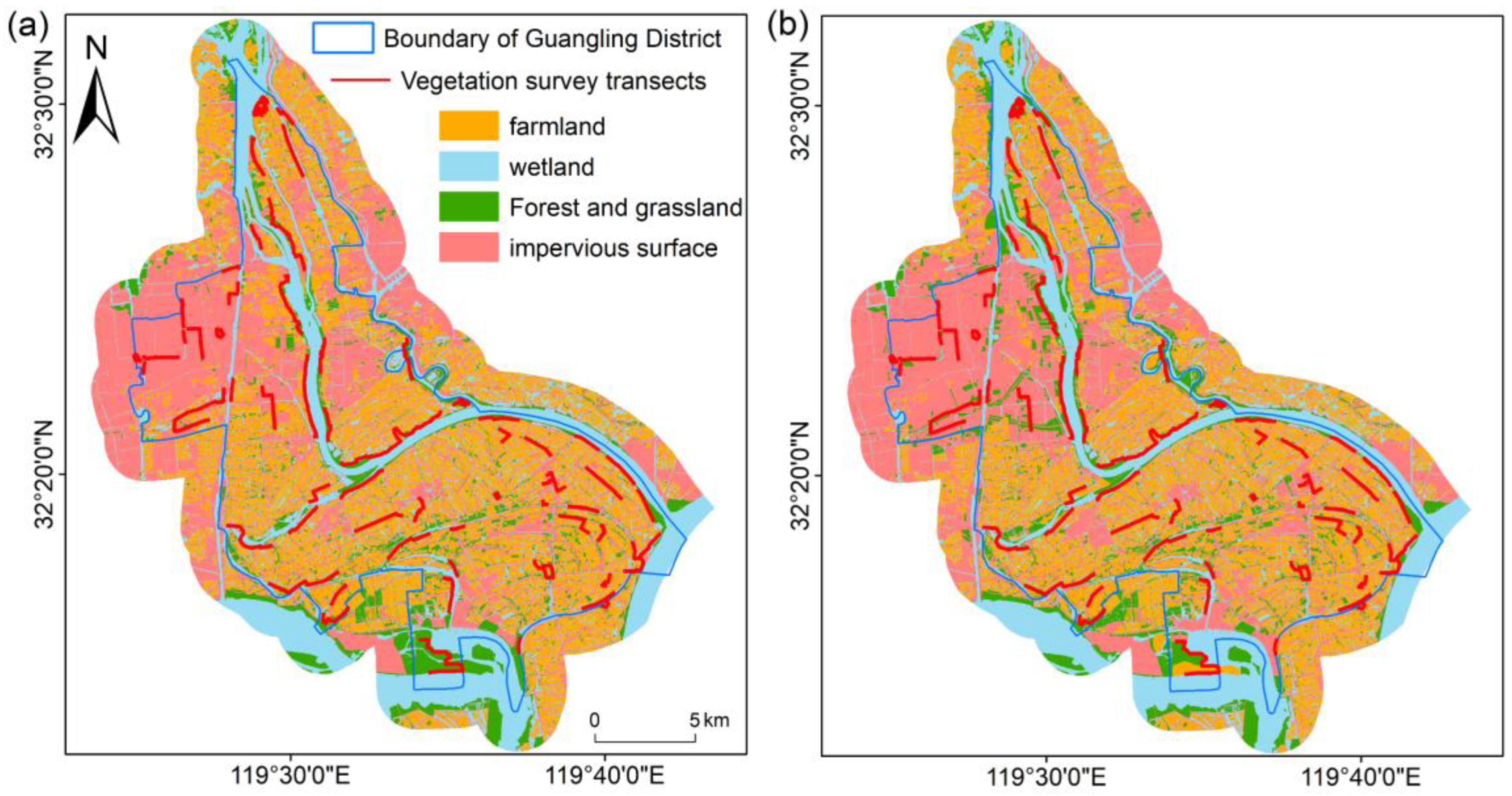
2.1. Research Area
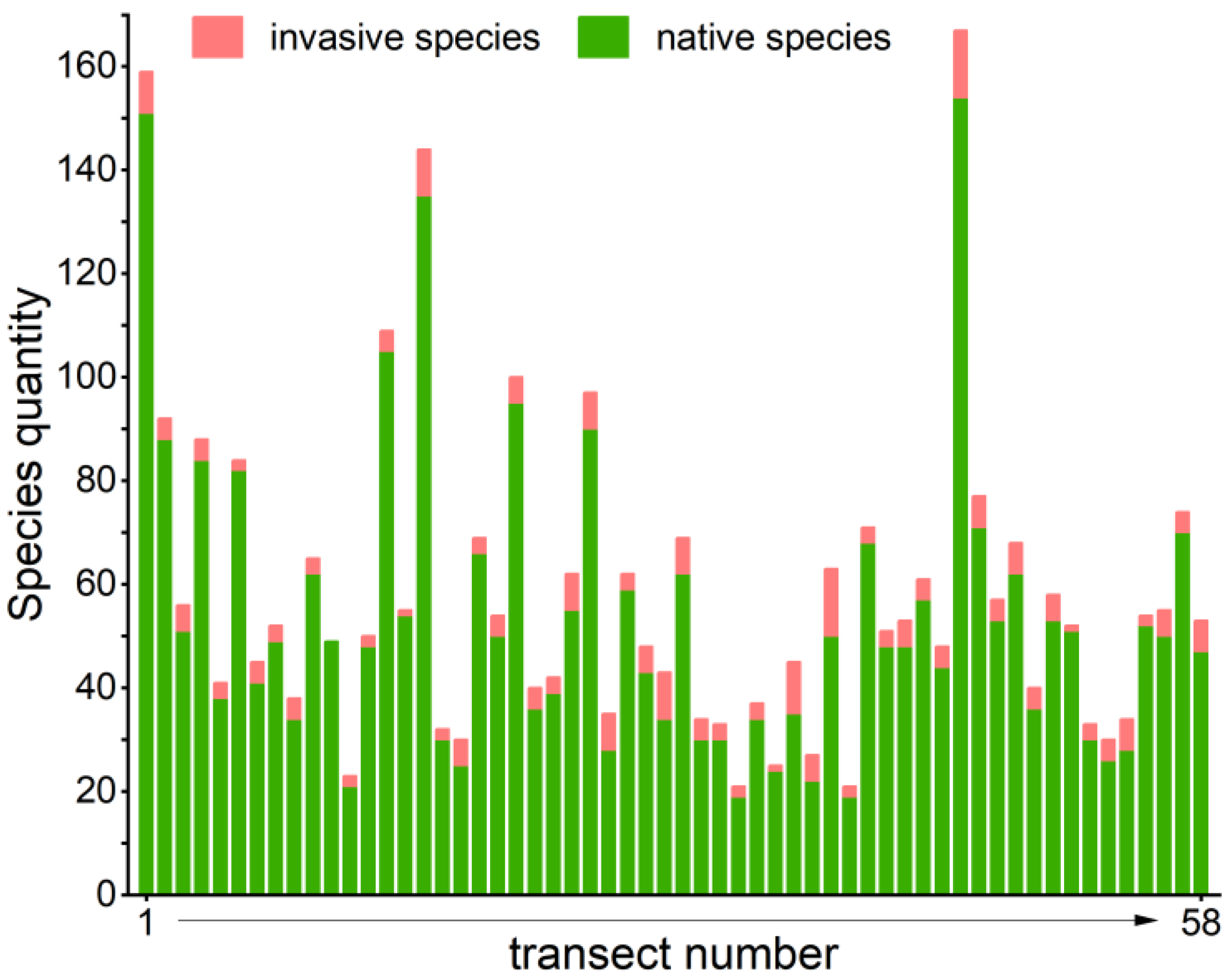
2.2. Biodiversity Index
2.3. Greenspace Pattern Data
2.4. Statistical Analysis
3. Results
3.1. Greenspace Components and Plant Species Composition
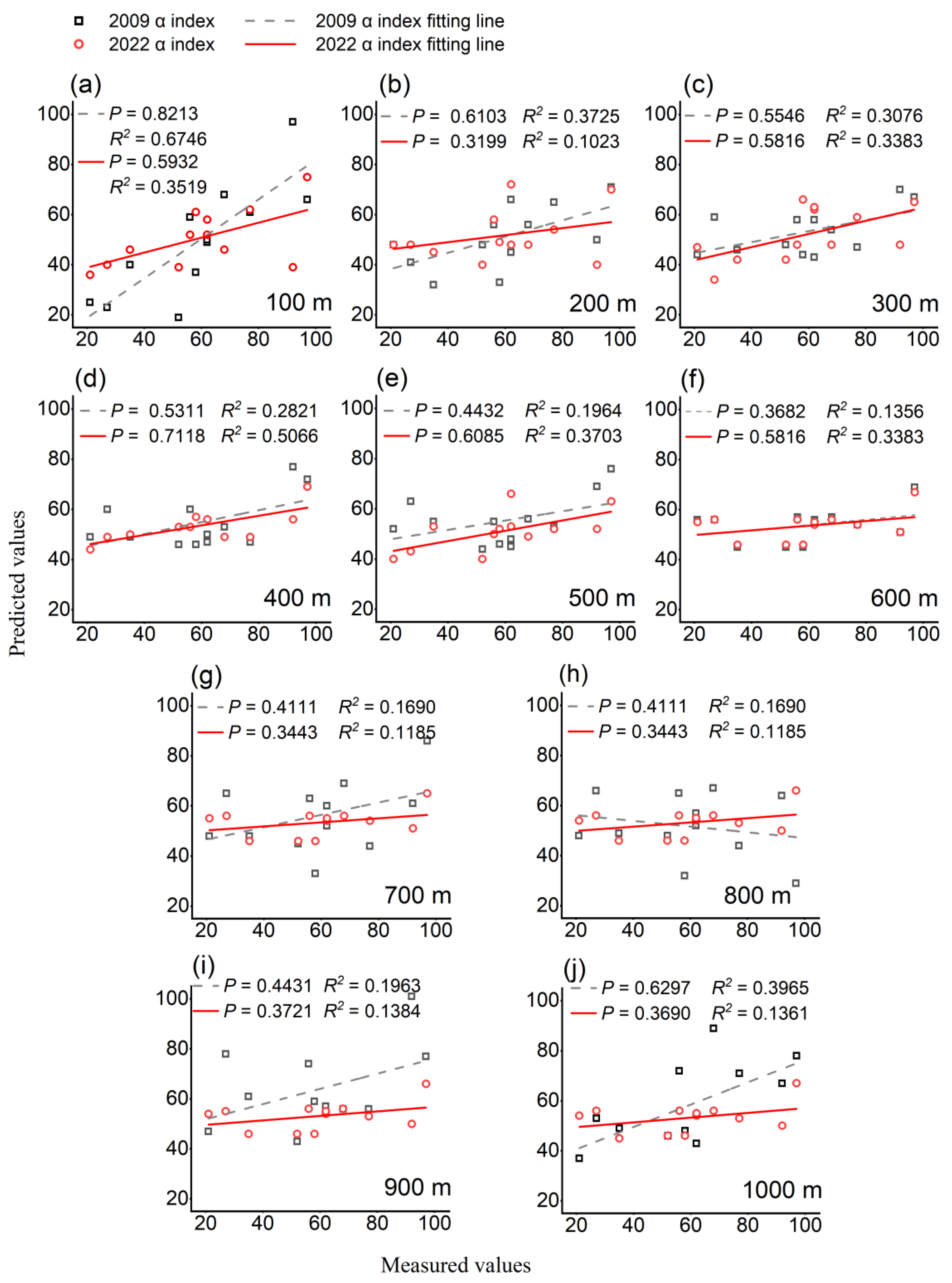
3.2. Landscape Indices with Significant Effects on Plant Diversity
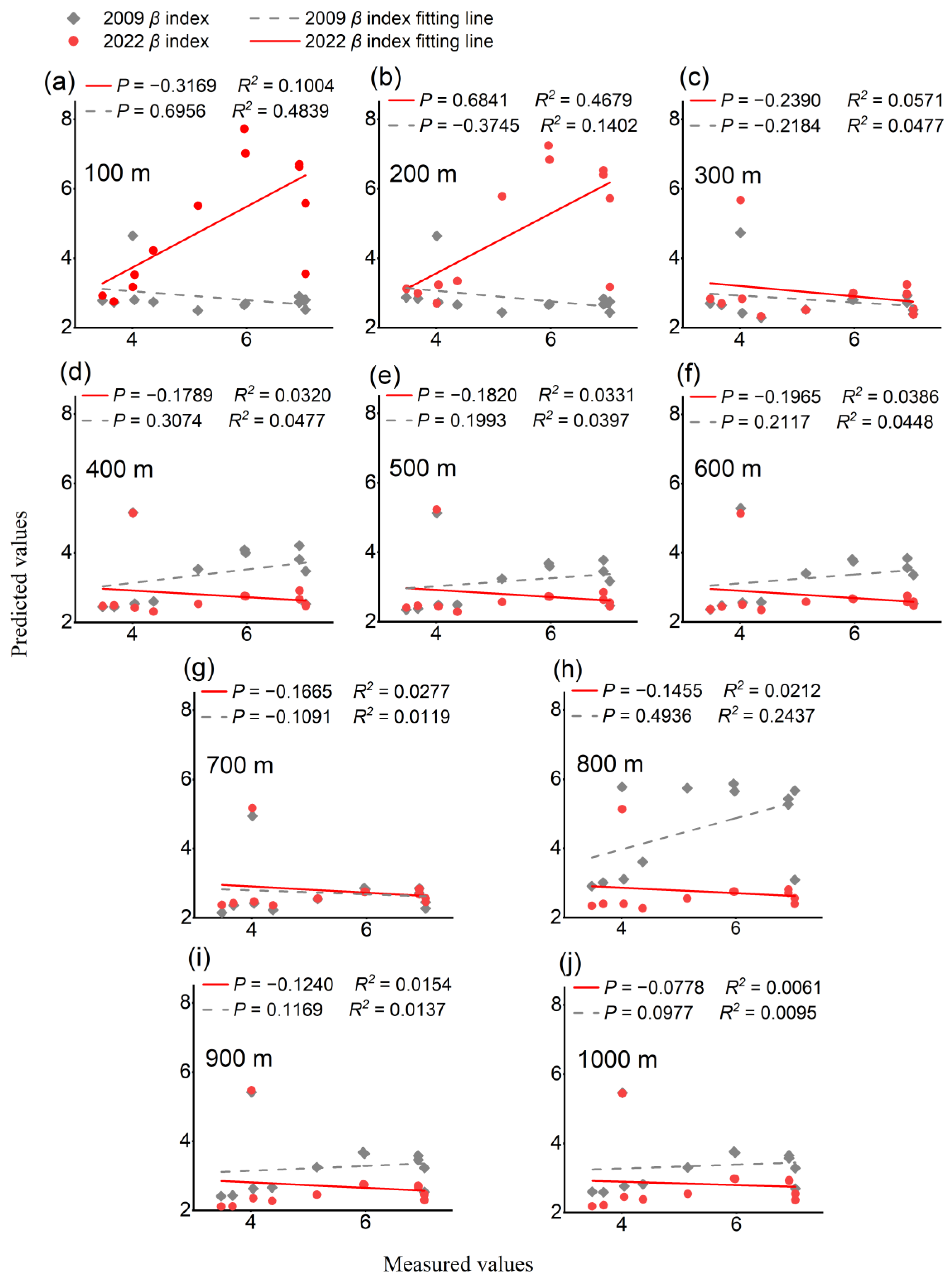
3.3. Impact of Greenspace Pattern on Plant Diversity
4. Discussion
4.1. Legacy and Scale Effects of Greenspace Pattern
4.2. Differences in Green Spatial Pattern and Plant Groups
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nilon, C.H.; Aronson, M.F.J. Routledge Handbook of Urban Biodiversity; Routledge: New York, NY, USA, 2024. [Google Scholar]
- Haase, D. Global Urbanisation, Biodiversity and Ecosystem Services: Challenges and Opportunities; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Khatri, A.; Bustamante, D.; Ruta, M. BiodiverCities by 2030: Transforming Cities’ Relationship with Nature. World Economic Forum. Available online: https://www.weforum.org/publications/biodivercities-by-2030-transforming-cities-relationship-with-nature/ (accessed on 27 March 2024).
- Shafer, H.B. Urban biodiversity arks. Nat. Sustain. 2018, 1, 725–727. [Google Scholar] [CrossRef]
- Sandifer, P.A.; Sutton-Grier, A.E.; Ward, B.P. Exploring connections among nature, biodiversity, ecosystem services, and human health and well-being: Opportunities to enhance health and biodiversity conservation. Ecosyst. Serv. 2015, 12, 1–15. [Google Scholar] [CrossRef]
- Goddard, M.A.; Dougill, A.J.; Benton, T.G. Scaling up from gardens: Biodiversity conservation in urban environments. Trends Ecol. Evol. 2010, 25, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Aronson, M.F.J.; Sorte, F.A.L.; Nilon, C.H.; Katti, M.; Goddard, M.A.; Lepczyk, C.A.; Warren, P.S.; Williams, N.S.G.; Cilliers, S.; Clarkson, B.; et al. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. R. Soc. B 2014, 281, 20133330. [Google Scholar] [CrossRef]
- Ives, C.D.; Lentini, P.E.; Threlfall, C.G.; Ikin, K.; Shanahan, D.F.; Garrard, G.E.; Bekessy, S.A.; Fuller, R.A.; Mumaw, L.; Rayner, L.; et al. Cities are hotspots for threatened species. Global Ecol. Biogeogr. 2016, 25, 117–126. [Google Scholar] [CrossRef]
- Beninde, J.; Veith, M.; Hochkirch, A. Biodiversity in cities needs space: A meta-analysis of factors determining intra-urban biodiversity variation. Ecol. Lett. 2015, 18, 581–592. [Google Scholar] [CrossRef]
- Zhao, H.X.; Wang, S.F.; Meng, F.; Niu, M.J.; Luo, X.L. Green space pattern changes and its driving mechanism: A case study of Nanjing metropolitan area. Acta Ecologica Sin. 2020, 40, 7861–7872. [Google Scholar] [CrossRef]
- Schrammeijer, E.A.; Malek, Ž.; Verburg, P.H. Mapping demand and supply of functional niches of urban green space. Ecol. Indic. 2022, 140, 109031. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; Aronson, M.F.J.; Evans, K.L.; Goddard, M.A.; Lerman, S.B. Biodiversity in the City: Fundamental Questions for Understanding the Ecology of Urban Green Spaces for Biodiversity Conservation. BioScience 2017, 67, 799–807. [Google Scholar] [CrossRef]
- Guo, Y.T.; Li, Y.Y. Construction paths of plant communities in urban green space based on biodiversity. Landsc. Archit. 2022, 29, 59–63. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, Q.C.; Zhang, Y.Y.; Chen, X.Y. Biodiversity at disequilibrium: Updating conservation strategies in cities. Trends Ecol. Evol. 2022, 37, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.S.; Fan, M.; Peng, Y.; Xin, J.X.; Peng, N.Y. Effects of landscape pattern dynamics on plant species and functional diversity in Hunshandak Sandland. Biodivers. Sci. 2023, 31, 23048. [Google Scholar] [CrossRef]
- Chaudron, C.; Perronne, R.; Bonthoux, S.; Di Pietro, F. A stronger influence of past rather than present landscape structure on present plant species richness of road-field boundaries. Acta Oecol. 2018, 92, 85–94. [Google Scholar] [CrossRef]
- Bürgi, M.; Östlund, L.; Mladenoff, D.J. Legacy effects of human land use: Ecosystems as time-lagged systems. Ecosystems 2017, 20, 94–103. [Google Scholar] [CrossRef]
- Ziter, C.; Graves, R.A.; Turner, M.G. How do land use legacies affect ecosystem services in United States cultural landscapes? Landsc. Ecol. 2017, 32, 2205–2218. [Google Scholar] [CrossRef]
- Ameztegui, A.; Coll, L.; Brotons, L.; Ninot, J.M. Land-use legacies rather than climate change are driving the recent upward shift of the mountain tree line in the Pyrenees. Global Ecol. Biogeogr. 2016, 25, 263–273. [Google Scholar] [CrossRef]
- Garbarino, M.; Morresi, D.; Urbinati, C.; Malandra, F.; Motta, R.; Sibona, E.M.; Vitali, A.; Weisberg, P.J. Contrasting land use legacy effects on forest landscape dynamics in the Italian Alps and the Apennines. Landscape Ecol. 2020, 35, 2679–2694. [Google Scholar] [CrossRef]
- Sharrow, S.H. Soil compaction by grazing livestock in silvopastures as evidenced by changes in soil physical properties. Agroforest Syst. 2007, 71, 215–223. [Google Scholar] [CrossRef]
- Aber, J.D.; Ollinger, S.V.; Dricoll, C.T. Modeling nitrogen saturation in forest ecosystems in response to landuse and atmospheric deposition. Ecol. Model. 1997, 101, 61–78. [Google Scholar] [CrossRef]
- Mclauchlan, K. The Nature and Longevity of Agricultural Impacts on Soil Carbon and Nutrients: A Review. Ecosystems 2006, 9, 1364–1382. [Google Scholar] [CrossRef]
- Tordoni, E.; Casolo, V.; Bacaro, G.; Martini, F.; Rossi, A.; Boscutti, F. Climate and landscape heterogeneity drive spatial pattern of endemic plant diversity within local hotspots in South-Eastern Alps. Perspect. Plant Ecol. 2020, 43, 125512. [Google Scholar] [CrossRef]
- Newman, E.A.; Kennedy, M.C.; Falk, D.A.; McKenzie, D. Scaling and complexity in landscape ecology. Front Ecol. Evol. 2019, 7, 293. [Google Scholar] [CrossRef]
- Fahrig, L.; Watling, J.I.; Arnillas, C.A.; Arroyo-Rodriguez, V.; Jorger-Hickfang, T.; Muller, J.; Pereira, H.M.; Riva, F.; Rosch, V.; Seibold, S.; et al. Resolving the SLOSS dilemma for biodiversity conservation: A research agenda. Biol. Rev. 2022, 97, 99–114. [Google Scholar] [CrossRef]
- Alignier, A.; Baudry, J. Is plant temporal beta diversity of field margins related to changes in management practices? Acta Oecol. 2016, 75, 1–7. [Google Scholar] [CrossRef]
- Li, H.D.; Ma, W.B.; Zhang, L.J.; Lü, Y.J.; Liu, C.W.; Zhao, L.J. Synergistic Governance of Urban Ecology and Environment: From the Perspectives of Ecological Resilience and Synergistic Efficiency. J. Ecol. Rural. Environ. 2023, 39, 1096–1102. [Google Scholar] [CrossRef]
- Whittaker, R.H. Evolution and Measurement of Species Diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Global Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Baselga, A. Multiple site dissimilarity quantifies compositional heterogeneity among several sites, while average pairwise dissimilarity may be misleading. Ecography 2013, 36, 124–128. [Google Scholar] [CrossRef]
- Prevedello, J.A.; Vieira, M.V. Does the type of matrix matter? A quantitative review of the evidence. Biodivers Conserv. 2010, 19, 1205–1223. [Google Scholar] [CrossRef]
- Toit, M.J.D.; Kotze, D.J.; Cilliers, S.S. Landscape history, time lags and drivers of change: Urban natural grassland remnants in potchefstroom, South Africa. Landsc. Ecol. 2016, 31, 2133–2150. [Google Scholar] [CrossRef]
- Perring, M.P.; Bernhardt-Römermann, M.; Baeten, L.; Midolo, G.; Blondeel, H.; Depauw, L.; Landuyt, D.; Maes, S.L.; De Lombaerde, E.; Carón, M.M.; et al. Global environmental change effects on plant community composition trajectories depend upon management legacies. Glob. Change Biol. 2018, 24, 1722–1740. [Google Scholar] [CrossRef]
- Duan, M.C.; Liu, Y.H.; Li, X.; Wu, P.L.; Hu, W.H.; Zhang, F.; Shi, H.L.; Yu, Z.R.; Baudry, J. Effect of present and past landscape structures on the species richness and composition of ground beetles (Coleoptera: Carabidae) and spiders (Araneae) in a dynamic landscape. Landsc. Urban Plan. 2019, 192, 103649. [Google Scholar] [CrossRef]
- He, Y. Study on Urban Vegetation Change and Its Influencing Factors in China Under the Background of Rapid Urbanization. Ph.D. Thesis, Nanjing University of Information Science and Technology, Nanjing, China, 2023. [Google Scholar]
- Nassauer, J.I.; Raskin, J. Urban vacancy and land use legacies: A frontier for urban ecological research, design, and planning. Landsc. Urban Plan. 2014, 125, 245–253. [Google Scholar] [CrossRef]
- Burke, I.C.; Lauenroth, W.K.; Coffin, D.P. Soil organic matter recovery in semiarid grasslands: Implications for the conservation reserve program. Ecol. Appl. 1995, 5, 793–801. [Google Scholar] [CrossRef]
- Compton, J.E.; Boone, R.D.; Motzkin, G.; Foster, D.R. Soil carbon and nitrogen in a pine-oak sand plain in central Massachusetts: Role of vegetation and land-use history. Oecologia 1998, 116, 536–542. [Google Scholar] [CrossRef]
- Sork, V.L.; Smouse, P.E. Genetic analysis of landscape connectivity in tree populations. Landsc. Ecol. 2006, 21, 821–836. [Google Scholar] [CrossRef]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef]
- Brudvig, L.A.; Damschen, E.I. Land-use history, historical connectivity, and land management interact to determine longleaf pine woodland understory richness and composition. Ecography 2010, 34, 257–266. [Google Scholar] [CrossRef]
- O’Connor, T.G. Local extinction in perennial grasslands: A life-history approach. Am. Nat. 1991, 137, 753–773. [Google Scholar] [CrossRef]
- Vellend, M. The Theory of Ecological Communities (MPB-57); Princeton University Press: Princeton, NJ, USA, 2020. [Google Scholar]
- Fahrig, L.; Arroyo-Rodríguez, V.; Bennett, J.R.; Boucher-Lalonde, V.; Cazetta, E.; Currie, D.J.; Eigenbrod, F.; Ford, A.T.; Harrison, S.P.; Jaeger, J.A.G.; et al. Is habitat fragmentation bad for biodiversity? Biol. Conserv. 2019, 230, 179–186. [Google Scholar] [CrossRef]
- Qu, M.J.; Ababaike, N.; Zou, X.G.; Zhao, H.; Zhu, W.L.; Wang, J.M.; Li, J.W. Influence of geographic distance and environmental factors on beta diversity of plants in the Alxa gobi region in northern. China Biodivers. Sci. 2022, 30, 22029. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, K.; Liu, Q.; He, Q.; Van de Koppel, J.; Teng, S.N.; Miao, X.; Liu, M.; Bertness, M.D.; Xu, C. Long-distance facilitation of coastal ecosystem structure and resilience. Proc. Natl. Acad. Sci. USA 2022, 119, e2123274119. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 2016. [Google Scholar]
- Chase, J.M.; Blowes, S.A.; Knight, T.M.; Gerstner, K.; May, F. Ecosystem decay exacerbates biodiversity loss with habitat loss. Nature 2020, 584, 238–243. [Google Scholar] [CrossRef]
- Rösch, V.; Tscharntke, T.; Scherber, C.; Batáry, P. Landscape composition, connectivity and fragment size drive effects of grassland fragmentation on insect communities. J. Appl. Ecol. 2013, 50, 387–394. [Google Scholar] [CrossRef]
- Fan, M.; Lu, Y.T.; Wang, Z.H.; Huang, Y.Q.; Peng Yu Shang, J.X.; Zhang, Y. Effects of patch pattern on plant diversity and functional traits in center Hunshandak Sandland. Chin. J. Plant Ecol. 2022, 46, 51–61. [Google Scholar] [CrossRef]
- Riva, F.; Fahrig, L. Landscape-scale habitat fragmentation is positively related to biodiversity, despite patch-scale ecosystem decay. Ecol. Lett. 2023, 26, 268–277. [Google Scholar] [CrossRef]
- Moreira, R.A.; Fernandes, G.W.; Collevatti, R.G. Fragmentation and spatial genetic structure in Tabebuia ochracea (Bignoniaceae) a seasonally dry Neotropical tree. Forest Ecol. Manag. 2009, 258, 2690–2695. [Google Scholar] [CrossRef]
- Tscharntke, T.; Grass, I.; Wanger, T.C.; Westphal, C.; Batáry, P. Beyond organic farming harnessing biodiversity-friendly landscapes. Trends Ecol. Evol. 2021, 36, 919–930. [Google Scholar] [CrossRef]
- Tamme, R.; Hiiesalu, I.; Laanisto, L.; Szava-Kovats, R.; Pärtel, M. Environmental heterogeneity, species diversity and co-existence at different spatial scales. J. Veg. Sci. 2010, 21, 796–801. [Google Scholar] [CrossRef]
- Lasky, J.R.; Keitt, T.H. Reserve size and fragmentation alter community assembly, diversity, and dynamics. Am. Nat. 2013, 182, 142–160. [Google Scholar] [CrossRef]

| Landscape Index | Ecological Significance | Abbr |
|---|---|---|
| Number of Patch | The total number of patches of a certain patch type in the landscape | NP * |
| Patch Density | Describe the fragmentation of the landscape, the higher the value, the more severe the fragmentation | PD * |
| Edge Density | Total edge length of landscape divided by total landscape area | ED * |
| Patch Richness Density | Number of patches per unit area | PRD |
| Percentage of Landscape | Proportion of patch types that make up the landscape, which could determine the dominant landscape types | PLAND * |
| Landscape Shape Index | Patch variability in the landscape | LSI * |
| Shannon’s Diversity Index | The more abundant the patch type, the more severe the fragmentation, the higher the SHDI value | SHDI |
| Shannon’s Evenness Index | The uniformity of the distribution of patch types in the landscape | SHEI |
| Type | 2009 | 2022 |
|---|---|---|
| Cropland area | 91.09 km2 | 80.51 km2 |
| Wetland area | 60.98 km2 | 58.29 km2 |
| Forest and grassland area | 24.83 km2 | 29.13 km2 |
| Impervious surfaces area | 88.46 km2 | 97.44 km2 |
| Urban built-up area | 60.83 km2 | 67.39 km2 |
| Greenspace rate in built-up area | 33.40% | 42.07% |
| Landscape Types | Major Plant Species |
|---|---|
| Cropland | Citrus medica, Prunus armeniaca, Setaria viridi, Cayratia japonica, Plantago asiatica, Justicia procumbens, Eleusine indica, Avena fatua, Geranium wilfordii, Equisetum hyemale, Stellaria media, Metaplexis japonica, Solidago canadensis, Bidens pilosa, Erigeron annuu, Phytolacca americana, etc. |
| Wetland | Taxodium distichum var. imbricarium, Phragmites australis, Arundo donax, Acorus calamus, Hydrocharis dubia, Hydrilla verticillata, Lemna minor, Eichhornia crassipes, Musa basjoo, Thalia dealbata, Salvinia natans, Nelumbo nucifera, Alternanthera philoxeroides, etc. |
| Forest and grassland | Cunninghamia lanceolata, Robinia pseudoacacia, Eriobotrya japonic, Prunus armeniaca, Ziziphus jujuba, Celtis sinensis, Ulmus pumila, Broussonetia papyrifera, Quercus fabri, Pterocarya stenoptera, Sapium sebiferum, Populus euramevicana, etc. |
| Impervious surfaces | Ginkgo biloba, Metasequoia glyptostroboides, Cedrus deodara, Magnolia denudate, Chimonanthus praecox, Chimonanthus subavenium, Chimonanthus camphora, Ophiopogon bodinieri, Buxus sinica, Ligustrum lucidum, Viburnum macrocephalum f. keteleeri, etc. |
| Time Scale/a | Spatial Scale/m | Landscape Indices Significantly Correlated with α-Diversity | Landscape Indices Significantly Correlated with β-Diversity |
|---|---|---|---|
| 2009 | 100 | NP, LSI, NPw, NPf, NPi, LSIf, LSIi | PLANDi |
| 200 | NP, NPw, NPf, LSIf, LSIi | PLANDw, PLANDi | |
| 300 | NP, NPw, NPf, NPi, LSIf, LSIi | PRD, SHDI, SHEI, PLANDw, PLANDi | |
| 400 | NPw, NPf, LSIf | PRD, SHDI, SHEI, PLANDw, PLANDi | |
| 500 | NPw, NPf, LSIf | SHDI, SHEI, PLANDw, PLANDi | |
| 600 | LSIf | SHDI, SHEI, PLANDw, PLANDi | |
| 700 | LSIf | SHDI, SHEI, PLANDw, PLANDi | |
| 800 | – | SHDI, SHEI, PLANDw, PLANDi | |
| 900 | – | SHDI, SHEI, PLANDw, PLANDi | |
| 1000 | – | SHDI, SHEI, PLANDw, PLANDi | |
| 2022 | 100 | NP, LSI, NPc, NPw, NPf, LSIc, LSIf, LSIi | PLANDi |
| 200 | NP, NPc, NPw, NPf, LSIc, LSIi | SHDI, PLANDi | |
| 300 | NP, NPw, NPf | SHDI, PLANDw, PLANDi | |
| 400 | NPw, NPf | SHDI, SHEI, PLANDw, PLANDi | |
| 500 | NPw | SHDI, SHEI, PLANDw, PLANDi | |
| 600 | – | SHDI, SHEI, PLANDw, PLANDi | |
| 700 | – | SHDI, SHEI, PLANDw, PLANDi | |
| 800 | – | SHDI, SHEI, PLANDw, PLANDi | |
| 900 | – | SHDI, SHEI, PLANDw, PLANDi | |
| 1000 | – | SHDI, SHEI, PLANDw, PLANDi |
| Plant Diversity Index | Time Scale/a | Spatial Scale/m | Stepwise Regression Model | R2 | Adjusted R2 |
|---|---|---|---|---|---|
| α-diversity | 2009 | 100 | y = 27.366 + 15.255LSIi − 0.074ED − 11.568LSI + 0.521PLANDc + 4.127LSIf | 0.544 | 0.468 |
| 200 | y = 46.874 + 1.193NPf − 0.152EDf | 0.293 | 0.253 | ||
| 300 | y = 43.899 + 5.628LSIf − 0.22EDf − 0.491PDc | 0.353 | 0.298 | ||
| 400 | y = 42.672 + 5.222LSIf − 0.248PD − 0.191EDf | 0.332 | 0.275 | ||
| 500 | y = 34.571 + 4.574LSIf − 1.710PDf | 0.295 | 0.256 | ||
| 600 | y = 77.489 − 18.997PRD | 0.129 | 0.105 | ||
| 700 | y = 47.739 + 3.173LSIi − 0.133ED − 0.633PLANDf | 0.322 | 0.266 | ||
| 800 | y = 53.254 + 2.967LSIi − 0.15ED − 0.777PLANDf | 0.327 | 0.274 | ||
| 900 | y = 56.181 − 1.015PLANDf + 1.048PLANDw + 2.794LSIf − 64.023SHEI | 0.386 | 0.319 | ||
| 1000 | y = 0.601PLANDi + 6.023LSIi − 7.354LSI + 0.434NPi + 1.934LSIf | 0.91 | 0.898 | ||
| 2022 | 100 | y = 35.936 + 1.303NPf | 0.305 | 0.284 | |
| 200 | y = 37.533 + 0.564NPw | 0.226 | 0.202 | ||
| 300 | y = 56.593 + 1.546NPf − 5.532LSIf | 0.283 | 0.238 | ||
| 400 | y = 52.185 + 0.558NPf − 0.224PD | 0.192 | 0.143 | ||
| 500 | y = 27.830 + 0.250NPw + 0.904PLANDf | 0.213 | 0.166 | ||
| 600 | y = 73.758 − 16.251PRD | 0.096 | 0.071 | ||
| 700 | y = 75.101 − 21.472PRD | 0.096 | 0.071 | ||
| 800 | y = 76.426 − 27.560PRD | 0.096 | 0.071 | ||
| 900 | y = 77.732 − 34.567PRD | 0.096 | 0.071 | ||
| 1000 | y = 79.951 − 43.970PRD | 0.099 | 0.074 | ||
| β-diversity | 2009 | 100 | y = 1.385 + 0.048PLANDi | 0.721 | 0.696 |
| 200 | y = 1.593 + 0.044PLANDi | 0.747 | 0.723 | ||
| 300 | y = 2.762 + 0.034PLANDi − 0.033PLANDw | 0.853 | 0.823 | ||
| 400 | y = 2.155 + 0.033PLANDi − 0.027PDw + 0.068LSIw − 0.035LSIi − 0.003EDw | 0.989 | 0.981 | ||
| 500 | y = 1.834 + 0.038PLANDi − 0.039PDw + 0.049LSIw − 0.028LSIi | 0.984 | 0.977 | ||
| 600 | y = 1.749 + 0.04PLANDi − 0.046PDw + 0.058LSIw − 0.038LSI | 0.990 | 0.985 | ||
| 700 | y = 2.824 + 0.036PLANDi − 0.038PLANDw − 0.087PDw + 0.006EDw + 0.038PDf | 0.991 | 0.984 | ||
| 800 | y = 11.334 − 0.014PLANDw − 0.088PDw − 43.569SHDI + 0.136LSI + 51.425SHEI − 0.113LSIc + 0.002NPc | 0.996 | 0.991 | ||
| 900 | y = 6.358 − 3.554SHEI − 0.027PLANDw − 0.047PDw + 0.022LSIw + 0.013PLANDi | 0.987 | 0.978 | ||
| 1000 | y = 8.484 − 3.427SHDI − 0.035PLANDw − 0.041PDw + 0.016LSIw − 1.740PRD | 0.981 | 0.968 | ||
| 2022 | 100 | y = 1.629 + 0.035NPi | 0.511 | 0.462 | |
| 200 | y = 1.629 + 0.022NPi | 0.427 | 0.37 | ||
| 300 | y = 2.617 + 0.051PLANDi − 0.07PLANDf | 0.819 | 0.783 | ||
| 400 | y = 2.714 + 0.034PLANDi − 0.036PLANDw | 0.867 | 0.841 | ||
| 500 | y = 2.720 + 0.034PLANDi − 0.035PLANDw | 0.885 | 0.862 | ||
| 600 | y = 2.763 + 0.032PLANDi − 0.035PLANDw | 0.88 | 0.855 | ||
| 700 | y = 2.946 + 0.034PLANDi − 0.026PLANDw − 0.035PLANDf | 0.897 | 0.863 | ||
| 800 | y = 3.103 + 0.033PLANDi − 0.028PLANDw − 0.043PLANDf | 0.902 | 0.87 | ||
| 900 | y = 7.988 − 5.167SHEI − 0.025PLANDw | 0.928 | 0.913 | ||
| 1000 | y = 7.964 − 5.031SHEI − 0.026PLANDw | 0.926 | 0.912 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Li, H.; Wang, N.; Yao, G.; Li, Z.; Yan, S. Impact of Urban Greenspace Pattern Dynamics on Plant Diversity: A Case Study in Yangzhou, China. Sustainability 2025, 17, 5416. https://doi.org/10.3390/su17125416
Li H, Li H, Wang N, Yao G, Li Z, Yan S. Impact of Urban Greenspace Pattern Dynamics on Plant Diversity: A Case Study in Yangzhou, China. Sustainability. 2025; 17(12):5416. https://doi.org/10.3390/su17125416
Chicago/Turabian StyleLi, Hui, Haidong Li, Nan Wang, Guohui Yao, Zhonglin Li, and Shouguang Yan. 2025. "Impact of Urban Greenspace Pattern Dynamics on Plant Diversity: A Case Study in Yangzhou, China" Sustainability 17, no. 12: 5416. https://doi.org/10.3390/su17125416
APA StyleLi, H., Li, H., Wang, N., Yao, G., Li, Z., & Yan, S. (2025). Impact of Urban Greenspace Pattern Dynamics on Plant Diversity: A Case Study in Yangzhou, China. Sustainability, 17(12), 5416. https://doi.org/10.3390/su17125416






