Biomes Affect Baking Properties and Quality Parameters of Different Wheat Genotypes
Abstract
1. Introduction
2. Materials and Methods
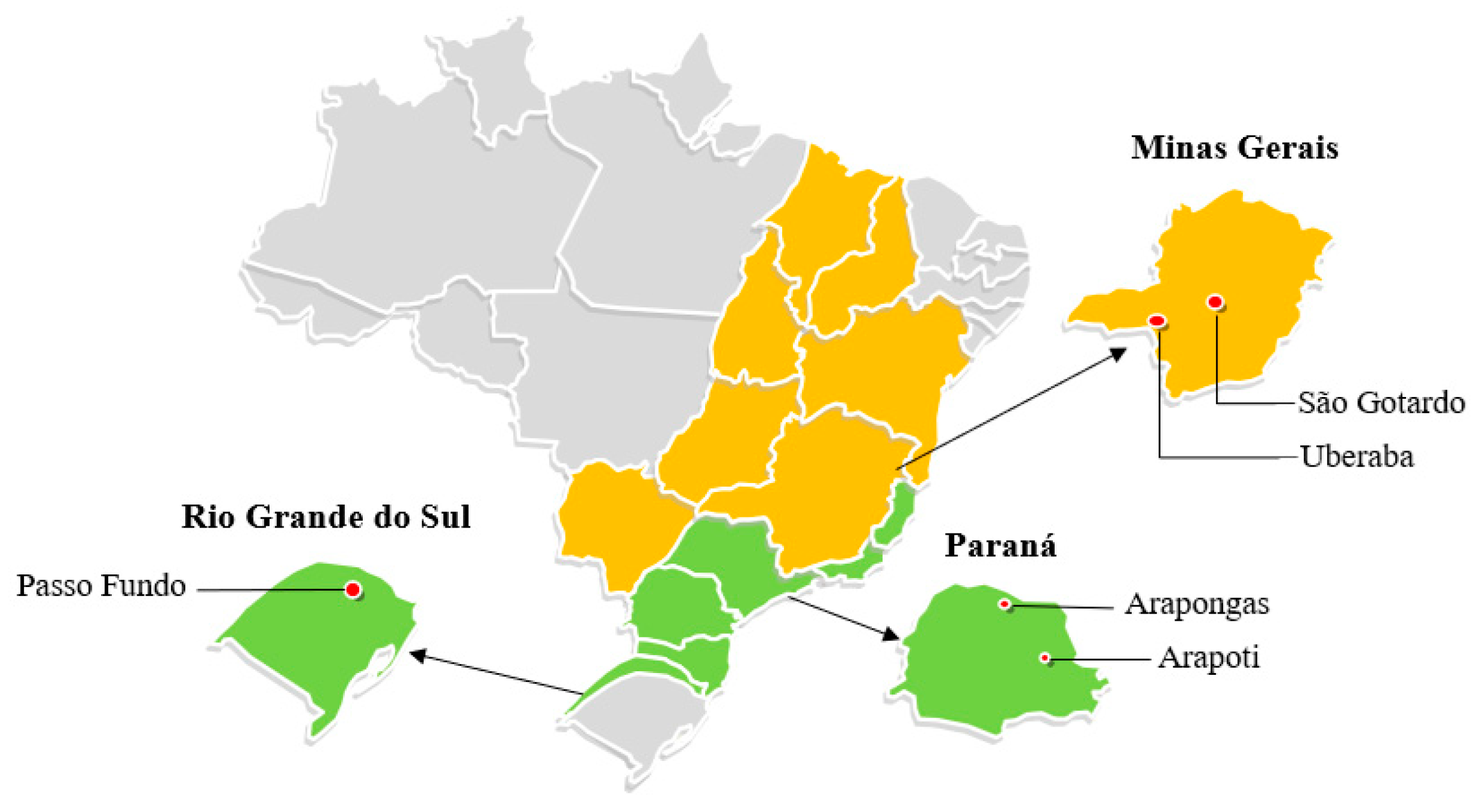
2.1. Sampling and Crop Management
2.2. Chemical Composition
2.3. Wheat Defects
2.4. Test Weight and Thousand-Grain Weight
2.5. Colorimetric Profile
2.6. Solvent Retention Capacity
2.7. Hagberg Falling Number and Alveograph
2.8. Statistical Analysis
3. Results
3.1. Chemical Composition
3.2. Wheat Defects
3.3. Test Weight and Thousand-Grain Weight
3.4. Colorimetric Profile
| Environment | Genotipes | |||||
|---|---|---|---|---|---|---|
| Destak | 1403 | Madrepérola | Senna | Feroz | Guardião | |
| Burned grain (%) | ||||||
| Arapoti | 0.16 ± 0.01 Db | 0.33 ± 0.01 Ca | 0.12 ± 0.01 Cc | 0.00 ± 0.00 Ed | 0.35 ± 0.07 Ba | 0.00 ± 0.00 Dd |
| Arapongas | 0.12 ± 0.01 Db | 0.05 ± 0.01 Dc | 0.15 ± 0.01 Cb | 0.05 ± 0.01 Dc | 0.00 ± 0.00 Dd | 0.57 ± 0.05 Ba |
| Passo Fundo | 0.28 ± 0.01 Ca | 0.00 ± 0.00 Dd | 0.18 ± 0.01 Cb | 0.11 ± 0.01 Cc | 0.15 ± 0.01 Cb | 0.09 ± 0.00 Cc |
| Uberaba | 0.58 ± 0.03 Bc | 0.55 ± 0.02 Bd | 0.53 ± 0.04 Bd | 0.66 ± 0.01 Bb | 0.35 ± 0.01 Be | 0.52 ± 0.04 Ba |
| São Gotardo | 1.48 ± 0.03 Ac | 0.98 ± 0.01 Ae | 2.56 ± 0.08 Ac | 8.98 ± 0.02 Aa | 0.89 ± 0.01 Af | 5.03 ± 0.03 Ab |
| Grain damaged by insects (%) | ||||||
| Arapoti | 0.22 ± 0.01 Bb | 0.26 ± 0.02 Cb | 0.22 ± 0.01 Cb | 0.07 ± 0.03 Bc | 0.03 ± 0.00 Dc | 0.00 ± 0.00 Dd |
| Arapongas | 0.11 ± 0.01 Ca | 0.00 ± 0.00 Dc | 0.12 ± 0.01 Da | 0.04 ± 0.00 Bb | 0.02 ± 0.01 Db | 0.10 ± 0.00 Ca |
| Passo Fundo | 0.11 ± 0.01 Cb | 0.00 ± 0.00 Dc | 0.49 ± 0.01 Ba | 0.00 ± 0.00 Cc | 0.15 ± 0.01 Cb | 0.10 ± 0.00 Cb |
| Uberaba | 0.08 ± 0.01 Cd | 0.55 ± 0.02 Ba | 0.05 ± 0.00 Ee | 0.16 ± 0.01 Ac | 0.23 ± 0.00 Bb | 0.23 ± 0.01 Bb |
| São Gotardo | 1.48 ± 0.03 Ab | 0.98 ± 0.01 Ac | 2.51 ± 0.16 Aa | 0.00 ± 0.00 Ce | 0.43 ± 0.04 Ae | 0.53 ± 0.01 Ad |
| Shrunken grain (%) | ||||||
| Arapoti | 1.75 ± 0.07 Bb | 1.49 ± 0.01 Bc | 1.20 ± 0.01 Dd | 2.49 ± 0.01 Ba | 0.78 ± 0.04 Ce | 2.52 ± 0.02 Ca |
| Arapongas | 9.15 ± 0.07 Aa | 3.15 ± 0.07 Ad | 4.96 ± 0.04 Ac | 7.49 ± 0.02 Ab | 3.48 ± 0.04 Ad | 3.63 ± 0.18 Ad |
| Passo Fundo | 0.99 ± 0.01 Ce | 1.36 ± 0.01 Cd | 3.03 ± 0.01 Ba | 2.20 ± 0.01 Cc | 0.55 ± 0.00 Df | 2.80 ± 0.00 Bb |
| Uberaba | 0.38 ± 0.01 Db | 0.25 ± 0.00 Dc | 0.21 ± 0.01 Ec | 0.09 ± 0.00 Ed | 0.51 ± 0.01 Da | 0.33 ± 0.01 Eb |
| São Gotardo | 0.25 ± 0.00 Ec | 0.26 ± 0.01 Dc | 1.47 ± 0.04 Cb | 1.46 ± 0.05 Db | 1.65 ± 0.07 Ba | 1.42 ± 0.03 Db |
| Test weight (kg·hL−1) | ||||||
| Arapoti | 80.00 ± 0.13 Aa | 79.09 ± 0.43 Ab | 77.05 ± 0.34 Bc | 79.35 ± 0.30 Ab | 78.20 ± 0.53 Bb | 78.50 ± 0.22 Bb |
| Arapongas | 77.63 ± 0.21 Bc | 79.28 ± 0.24 Aab | 78.64 ± 0.10 Bb | 76.41 ± 0.26 Bd | 80.12 ± 0.04 Aa | 80.60 ± 0.39 Aa |
| Passo Fundo | 77.03 ± 0.25 Bc | 79.33 ± 0.19 Aa | 78.05 ± 0.53 Bb | 79.34 ± 0.67 Aa | 79.42 ± 0.119 Aa | 79.74 ± 0.38 Aa |
| Uberaba | 79.76 ± 0.41 Aa | 80.12 ± 0.16 Aa | 78.52 ± 0.16 Bb | 75.92 ± 0.51 Bd | 75.32 ± 0.18 Cd | 76.79 ± 0.51 Bc |
| São Gotardo | 80.68 ± 0.18 Aa | 80.92 ± 0.16 Aa | 80.10 ± 0.42 Aa | 79.29 ± 0.11 Aa | 79.95 ± 0.27 Aa | 81.04 ± 0.11 Aa |
| Thousand-grain weight (g) | ||||||
| Arapoti | 35.14 ± 0.41 Ab | 31.89 ± 0.39 Ac | 38.40 ± 0.22 Ab | 41.80 ± 0.18 Aa | 36.80 ± 0.33 Ab | 44.79 ± 0.38 Aa |
| Arapongas | 33.41 ± 0.52 Ab | 30.14 ± 0.78 Ab | 34.85 ± 0.25 Bb | 33.15 ± 0.68 Bb | 35.04 ± 0.33 Ab | 43.64 ± 0.34 Aa |
| Passo Fundo | 28.68 ± 0.47 Cc | 27.18 ± 0.37 Bc | 28.99 ± 0.37 Cc | 39.01 ± 0.39 Aa | 32.66 ± 0.19 Bb | 41.16 ± 0.14 Aa |
| Uberaba | 30.80 ± 0.27 Bb | 28.37 ± 0.42 Ab | 29.79 ± 0.10 Cb | 38.01 ± 0.32 Aa | 32.95 ± 0.20 Bb | 39.85 ± 0.54 Aa |
| São Gotardo | 33.72 ± 0.43 Ab | 32.09 ± 0.42 Ab | 33.95 ± 0.42 Bb | 37.46 ± 0.22 Ab | 34.23 ± 0.14 ABb | 40.41 ± 0.87 Aa |
| Value L* | ||||||
| Arapoti | 88.77 ± 2.83 Aa | 85.20 ± 6.16 Aa | 92.70 ± 1.27 Aa | 86.44 ± 3.23 Aa | 90.05 ± 2.29 Aa | 89.87 ± 6.32 Aa |
| Arapongas | 89.64 ± 0.92 Aa | 90.25 ± 1.48 Aa | 88.56 ± 4.61 Aa | 89.43 ± 5.02 Aa | 91.99 ± 1.98 Aa | 91.71 ± 0.88 Aa |
| Passo Fundo | 89.41 ± 1.89 Aa | 88.11 ± 0.59 Aa | 92.65 ± 4.61 Aa | 86.72 ± 3.41 Aa | 85.74 ± 3.91 Aa | 86.28 ± 6.25 Aa |
| Uberaba | 87.85 ± 1.08 Aa | 89.25 ± 1.50 Aa | 89.96 ± 1.47 Aa | 88.15 ± 3.33 Aa | 89.52 ± 1.52 Aa | 85.87 ± 4.49 Aa |
| São Gotardo | 88.50 ± 1.78 Aa | 91.17 ± 3.85 Aa | 86.46 ± 4.17 Aa | 84.62 ± 0.57 Aa | 85.60 ± 0.85 Aa | 85.91 ± 3.18 Aa |
| Value a* | ||||||
| Arapoti | −0.25 ± 0.13 Aa | −0.43 ± 0.09 Ba | −0.88 ± −1.05 Aa | −0.96 ± 0.07 Ba | −0.39 ± 0.10 Aa | −0.48 ± 0.08 Ba |
| Arapongas | −0.58 ± 0.07 Bc | −0.92 ± 0.06 Cd | −1.05 ± 0.07 Ad | −0.31 ± 0.10 Ab | −0.31 ± 0.04 Ab | −0.05 ± 0.07 Aa |
| Passo Fundo | −0.75 ± 0.06 Ca | −1.14 ± 0.03 Cb | −1.25 ± 0.04 Ab | −1.10 ± 0.12 Bb | −0.87 ± 0.05 Ca | −0.75 ± 0.04 Ca |
| Uberaba | −0.75 ± 0.05 Ca | −0.86 ± 0.27 Ca | −1.25 ± 0.07 Ac | −1.32 ± 0.05 Bc | −1.08 ± 0.07 Db | −1.08 ± 0.08 Db |
| São Gotardo | −0.29 ± 0.11 Aa | −0.19 ± 0.08 Aa | −1.33 ± 0.07 Ad | −0.44 ± 0.10 Ab | −0.53 ± 0.02 Bb | −0.89 ± 0.06 Cc |
| Value b* | ||||||
| Arapoti | 13.18 ± 0.79 Ba | 13.12 ± 1.03 Ba | 8.06 ± 0.47 Ac | 10.58 ± 1.40 Ab | 11.35 ± 0.09 Aab | 12.27 ± 0.25 Aa |
| Arapongas | 12.68 ± 0.50 Ba | 13.94 ± 0.65 Ba | 9.52 ± 0.71 Ab | 11.68 ± 0.43 Aa | 11.30 ± 0.42 Aa | 12.56 ± 0.54 Aa |
| Passo Fundo | 12.20 ± 0.52 Bb | 13.94 ± 0.29 Ba | 9.23 ± 1.03 Ac | 10.68 ± 0.73 Ab | 11.19 ± 0.82 Ab | 12.65 ± 0.72 Ab |
| Uberaba | 12.02 ± 0.84 Ba | 14.68 ± 1.17 ABa | 9.80 ± 0.60 Ab | 12.03 ± 0.50 Aa | 12.88 ± 0.79 Aa | 13.10 ± 0.78 Aa |
| São Gotardo | 14.01 ± 0.40 Aab | 15.12 ± 0.68 Aa | 10.70 ± 1.43 Ac | 12.33 ± 0.47 Ab | 12.92 ± 0.62 Ab | 13.13 ± 0.24 Ab |
3.5. Solvent Retention Capacity
3.6. Hagberg Falling Number
3.7. Alveography
4. Discussions
4.1. Chemical Composition
4.2. Wheat Defects
4.3. Test Weight and Thousand-Grain Weight
4.4. Colorimetric Profile
4.5. Solvent Retention Capacity
4.6. Hagberg Falling Number
4.7. Alveography
4.8. Global Overview of Differences in Baking Properties and Quality Parameters of Wheat Genotypes Cultivated in the Atlantic Forest and Savannah Biomes by Multivariate Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EMBRAPA. Wheat, a Crop to Go Down in History. 2022. Available online: https://www.embrapa.br/busca-de-noticias/-/noticia/77085844/trigo-uma-safra-para-ficar-na-historia (accessed on 13 March 2023).
- SOSMA. Food Production in the Atlantic Forest. 2022. Available online: https://cms.sosma.org.br/wp-content/uploads/2022/11/SOSMA_Produ%C3%A7%C3%A3o-de-Alimentos-na-Mata-Atl%C3%A2nticadigital.pdf (accessed on 23 June 2023).
- Cunha, J.E.F.; Bravo, J.V.M. Effects of environmental protection policies on fragile areas of a watershed occupied by agriculture in the Brazilian Savannah. J. Environ. Manag. 2022, 319, 115695. [Google Scholar] [CrossRef]
- Oliveira, M.E.A.S.; Alves, T.O.; Gutkoski, L.C.; Miranda, M.Z.; Ferreira, M.S.L.; Takeiti, C.Y. Brazilian Cerrado wheat: Technological quality of genotypes grown in tropical locations. J. Food Proces. Preserv. 2022, 46, e16228. [Google Scholar] [CrossRef]
- Mwiinga, B.; Sibiya, J.; Kondwakwenda, A.; Musvosvi, C.; Chigeza, G. Genotype x environment interaction analysis of soybean (Glycine max (L.) Merrill) grain yield across production environments in Southern Africa. Field Crops 2020, 256, 107922. [Google Scholar] [CrossRef]
- Cho, S.; Kang, C.; Ko, H.S.; Baik, B.; Cho, K.; Park, C.S. Influence of protein characteristics and the proportion of gluten on end-use quality in Korean wheat cultivars. J. Integr. Agric. 2018, 17, 1706–1719. [Google Scholar] [CrossRef]
- BRAZIL. Normative Instruction; Ministry of Agriculture, Livestock and Supply: Brasília, Brazil, 2010. Available online: https://sistemasweb.agricultura.gov.br/sislegis/action/detalhaAto.do?method=visualizarAtoPortalMapa&chave=358389789 (accessed on 20 January 2023).
- BRAZIL. Rules for Seed Analysis; Ministry of Agriculture, Livestock and Supply: Brasília, Brazil, 2009; 399; SNDA/DNDV/CLAV. [Google Scholar]
- Bettge, A.; Morris, C.; De Macon, V.L.; Kidwell, K. Adaptation of AACC Method 56-11, Solvent Retention Capacity, for use as a first-generation selection tool for cultivar development. Cereal Chem. 2002, 79, 670–674. [Google Scholar] [CrossRef]
- AACC. Approved Methods of Analysis, 11th ed.; Cereals and Grains Association: Eagan, MN, USA, 2010; Available online: https://www.cerealsgrains.org/resources/methods/Pages/default.aspx (accessed on 23 June 2023).
- Zorb, C.; Ludewig, U.; Hawkesford, M.J. Perspective on wheat yield and quality with reduced nitrogen supply. Trends Plant Sci. 2018, 23, 1029–1037. [Google Scholar] [CrossRef]
- Shi, K.; Yin, T.; Zhu, Y.; Liu, B.; Tang, L.; Cao, W.; Liu, L. Estimating the effect of low-temperature stress on the spatial distribution patterns of protein in wheat grains. J. Cereal Sci. 2022, 105, 103461. [Google Scholar] [CrossRef]
- Manstretta, V.; Morcia, C.; Terzi, V.; Rossi, V. Germination of Fusarium graminearum ascospores and wheat infection are affected by dry periods and by temperature and humidity during dry periods. Phytopathology 2016, 106, 216–313. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, A.K.; Gupta, S.K.; Kaur, N. Effect of sowing time on protein quality and starch pasting characteristics in wheat (Triticum aestivum L.) genotypes grown under irrigated and rain-fed conditions. Food Chem. 2010, 122, 559–565. [Google Scholar] [CrossRef]
- Ramos, A.H.; Timm, N.S.; Ferreira, C.D. Effects of the intensification of soybean defects: Consequences on the physicochemical, technological, protein and oil properties. Eur. Food Res. Technol. 2021, 247, 1277–1289. [Google Scholar] [CrossRef]
- Figueiredo, A.S.T.; Resende, J.T.V.; Morales, R.G.F.; Meert, L.; Rizzardi, D.A. Influence of moisture grain wheat about quantitative and qualitative losses during mechanised harvesting. Ambiência 2013, 9, 349–357. [Google Scholar] [CrossRef]
- Karaog-lu, M.M.; Aydeniz, M.; Kotancilar, G.H.; Gerçelaslan, K.E.A. A comparison of the functional characteristics of wheat stored as grain with wheat stored in spike form. Int. J. Food Sci. Technol. 2010, 45, 38–47. [Google Scholar] [CrossRef]
- Li, Y.F.; Wu, Y.; Hernandez-Espinosa, N.; Peña, R.J. Heat and drought stress on durum wheat: Responses of genotypes, yield, and quality parameters. J. Cereal Sci 2013, 57, 398–404. [Google Scholar] [CrossRef]
- Javed, A.; Ahmad, N.; Ahmed, J.; Hameed, A.; Ashra, M.A.A.; Zafar, S.A.; Maqbool, A.; Al-Amrah, H.; Alatawi, H.A.; Al- Harbi, M.S.; et al. Grain yield, chlorophyll and protein contents of elite wheat genotypes under drought stress. J. King Saud Univ.–Sci. 2022, 34, 102279. [Google Scholar] [CrossRef]
- Schaarschmidt, S.; Fauhl-Hassek, S. The Fate of Mycotoxins During the Processing of Wheat for Human Consumption. Food Sci. Food Saf. 2018, 17, 556–593. [Google Scholar] [CrossRef]
- Timm, N.S.; Ramos, A.H.; Ferreira, C.D.; Rios, A.O.; Zambiazi, R.C.; Oliveira, M. Influence of germ storage from different corn genotypes on technological properties and fatty acid, tocopherol, and carotenoid profiles of oil. Eur. Food Res. Technol. 2021, 247, 1449–1460. [Google Scholar] [CrossRef]
- Patwa, N.; Penning, B.W. Genetics of a diverse soft winter wheat population for pre-harvest sprouting, agronomic, and flour quality traits. Front. Plant Sci. 2023, 14, 1137808. [Google Scholar] [CrossRef] [PubMed]
- Garcia-santamaria, G.; Hua, D.; Sneller, C. Quantitative trait loci associated with soft wheat quality in a cross of good by moderate quality parents. PeerJ 2018, 6, e4498. [Google Scholar] [CrossRef]
- Nörnberg, M.L.; Bortolotti, C.B.; Minella, E.; Laerte Nörnberg, J.L. Characterization and rheological potential of barley and mixed flours. Braz. J. Dev. 2022, 8, 10289. [Google Scholar] [CrossRef]
- Pelegrin, A.J.; Carvalho, I.R.; Ferrari, M.; Nardino, M.; Szareski, V.I.J.; Meira, D.; Oliveira, A.C. Evaluation of solvent retention capacity of wheat (Triticum aestivum L.) flour depending on genotype and different timing of nitrogenous fertilizer application. Afr. J. Agric. Res. 2016, 11, 4389–4394. [Google Scholar]
- Pike, P.R.; Macritchie, F. Protein composition and quality of some new hard white winter wheats. Crop Sci. 2004, 44, 173–176. [Google Scholar] [CrossRef]
- Gutkoski, L.C.; Pagnussatt, F.A.; Spier, F.; Pedó, I. Effect of damaged starch content on the production of semi-hard biscuits. Scie. Techn. Food 2007, 27, 119–124. [Google Scholar] [CrossRef]
- Delcour, J.A.; Hoseney, R.C. Principles of Cereal Science and Technology, 3rd ed.; AACC: St. Paul, MN, USA, 2010. [Google Scholar]
- Dong, K.; Hao, C.Y.; Wang, A.L.; Cai, M.H.; Yan, Y.M. Characterization of HMW glutenin subunits in bread and tetraploid wheats by reserved-phase highperformance liquid chromatography. Cereal Res. Commun. 2009, 37, 65–73. [Google Scholar] [CrossRef]



| Environment | Genotipes | |||||
|---|---|---|---|---|---|---|
| Destak | 1403 | Madrepérola | Senna | Feroz | Guardião | |
| Protein (%) | ||||||
| Arapoti | 15.70 ± 0.16 Ab | 14.18 ± 0.03 Ac | 16.82 ± 0.17 Aa | 16.02 ± 0.30 Aa | 17.39 ± 0.09 Aa | 15.05 ± 0.20 Ab |
| Arapongas | 13.75 ± 0.10 Ba | 12.90 ± 0.11 Ba | 13.37 ± 0.24 Ba | 12.89 ± 0.08 Ca | 13.94 ± 0.07 Ca | 13.03 ± 0.03 Ba |
| Passo Fundo | 14.86 ± 0.46 ABab | 13.56 ± 0.30 Bb | 13.53 ± 0.18 Bb | 14.10 ± 0.12 Bb | 15.08 ± 0.10 Ba | 13.58 ± 0.12 Bb |
| Uberaba | 15.12 ± 0.01 Aa | 14.03 ± 0.01 Ab | 14.06 ± 0.10 Bb | 15.96 ± 0.06 Aa | 15.10 ± 0.08 Ba | 14.95 ± 0.04 Aa |
| São Gotardo | 13.15 ± 0.03 Bb | 12.47 ± 0.03 Bc | 12.85 ± 0.26 Bc | 14.71 ± 0.03 Ba | 13.50 ± 0.05 Cb | 13.81 ± 0.07 Bb |
| Lipid (%) | ||||||
| Arapoti | 1.96 ± 0.03 Aa | 1.65 ± 0.06 Bb | 1.65 ± 0.12 Bb | 1.69 ± 0.05 Ab | 1.60 ± 0.04 Ab | 1.26 ± 0.07 Cc |
| Arapongas | 1.51 ± 0.06 Cb | 1.70 ± 0.05 Ba | 1.42 ± 0.01 Bb | 1.48 ± 0.05 Bb | 1.58 ± 0.07 Ab | 1.45 ± 0.01 Bb |
| Passo Fundo | 1.51 ± 0.04 Ca | 1.77 ± 0.04 Ba | 1.57 ± 0.07 Ba | 1.61 ± 0.06 Ba | 1.65 ± 0.08 Aa | 1.51 ± 0.01 Ba |
| Uberaba | 1.84 ± 0.02 Aa | 1.88 ± 0.02 Aa | 1.91 ± 0.04 Aa | 1.74 ± 0.02 Ab | 1.60 ± 0.08 Ab | 1.59 ± 0.03 Ac |
| São Gotardo | 1.67 ± 0.03 Ba | 1.65 ± 0.01 Ba | 1.57 ± 0.01 Ba | 1.65 ± 0.05 Ba | 1.51 ± 0.04 Aa | 1.44 ± 0.00 Ba |
| Starch (%) | ||||||
| Arapoti | 57.99 ± 0.30 Ca | 52.92 ± 0.13 Cc | 51.39 ± 0.20 Dd | 55.29 ± 0.80 BCb | 52.65 ± 0.53 Cc | 53.48 ± 0.21 Cc |
| Arapongas | 55.27 ± 0.14 CDa | 53.75 ± 0.18 Cb | 53.47 ± 0.24 Cb | 54.14 ± 0.23 Cab | 53.98 ± 0.66 Cb | 55.63 ± 0.74 Ba |
| Passo Fundo | 53.19 ± 0.13 Bb | 53.51 ± 0.25 Cb | 54.28 ± 0.30 Cb | 57.47 ± 0.32 Ba | 53.78 ± 0.07 Cb | 56.90 ± 1.91 Ba |
| Uberaba | 59.53 ± 0.13 Ba | 59.55 ± 0.13 Ba | 59.15 ± 0.11 Ba | 59.41 ± 0.22 Aa | 57.97 ± 0.11 Bb | 59.75 ± 0.74 Aa |
| São Gotardo | 62.44 ± 0.32 Aa | 62.29 ± 0.18 Aa | 61.46 ± 0.29 Aab | 60.20 ± 0.08 Aa | 60.32 ± 0.13 Aa | 60.46 ± 0.12 Aa |
| Ashes (%) | ||||||
| Arapoti | 1.41 ± 0.12 Ce | 1.49 ± 0.03 Cd | 1.64 ± 0.02 Bb | 1.60 ± 0.03 Cc | 1.70 ± 0.03 Ba | 1.65 ± 0.01 Bb |
| Arapongas | 1.59 ± 0.09 Ba | 1.47 ± 0.03 Ce | 1.67 ± 0.02 Ab | 1.56 ± 0.01 Dc | 1.58 ± 0.00 Da | 1.54 ± 0.01 Ed |
| Passo Fundo | 1.59 ± 0.02 Bb | 1.53 ± 0.02 Bd | 1.65 ± 0.02 Ba | 1.55 ± 0.03 Dc | 1.65 ± 0.02 Ca | 1.56 ± 0.02 Dc |
| Uberaba | 1.70 ± 0.02 Ad | 1.63 ± 0.02 Ae | 1.64 ± 0.02 Be | 1.84 ± 0.02 Aa | 1.77 ± 0.02 Ab | 1.74 ± 0.02 Ac |
| São Gotardo | 1.59 ± 0.01 Bc | 1.53 ± 0.01 Be | 1.68 ± 0.05 Ab | 1.70 ± 0.04 Ba | 1.56 ± 0.02 Ed | 1.61 ± 0.02 Cc |
| Fibers (%) | ||||||
| Arapoti | 2.50 ± 0.01 Cc | 2.61 ± 0.05 Ab | 2.66 ± 0.02 Bb | 2.70 ± 0.05 Ab | 2.53 ± 0.03 Cc | 2.85 ± 0.02 Aa |
| Arapongas | 2.69 ± 0.01 Ba | 2.66 ± 0.02 Aab | 2.75 ± 0.01 Aa | 2.64 ± 0.02 Ab | 2.64 ± 0.03 Bb | 2.78 ± 0.08 Aa |
| Passo Fundo | 2.76 ± 0.03 Aa | 2.59 ± 0.05 Ab | 2.74 ± 0.04 Aa | 2.64 ± 0.07 Aab | 2.76 ± 0.06 Aa | 2.74 ± 0.09 Aa |
| Uberaba | 2.51 ± 0.03 Cb | 2.40 ± 0.06 Bc | 2.49 ± 0.07 Cb | 2.46 ± 0.02 Bc | 2.45 ± 0.01 Dc | 2.64 ± 0.03 Ba |
| São Gotardo | 2.24 ± 0.03 Dc | 2.11 ± 0.01 Cd | 2.28 ± 0.02 Dc | 2.51 ± 0.04 Ba | 2.38 ± 0.06 Db | 2.57 ± 0.02 Ba |
| Environment | Genotipes | |||||
|---|---|---|---|---|---|---|
| Destak | 1403 | Madrepérola | Senna | Feroz | Guardião | |
| SRC—Water (%) | ||||||
| Arapoti | 94.14 ± 15.01 Ca | 105.51 ± 3.48 Aa | 105.20 ± 3.22 Ba | 88.44 ± 0.93 Ca | 105.61 ± 1.61 Aa | 103.53 ± 7.45 Aa |
| Arapongas | 125.61 ± 1.61 Aa | 88.28 ± 0.97 Bc | 128.00 ± 3.78 Aa | 122.76 ± 5.38 Aa | 93.96 ± 0.05 Ab | 98.12 ± 4.86 Ab |
| Passo Fundo | 134.56 ± 6.98 Aa | 88.66 ± 4.33 Bc | 87.50 ± 1.22 Dc | 107.03 ± 1.54 Bb | 104.15 ± 2.61 Ab | 98.28 ± 1.60 Ab |
| Uberaba | 117.86 ± 0.15 Ba | 103.28 ± 10.76 Ab | 92.38 ± 1.29 Cb | 81.14 ± 0.22 Dc | 94.24 ± 6.54 Ab | 109.59 ± 5.07 Ab |
| São Gotardo | 102.95 ± 5.73 Ca | 94.53 ± 0.99 Ab | 92.88 ± 0.57 Cb | 95.52 ± 2.46 Cb | 85.42 ± 1.28 Bc | 109.33 ± 5.66 Aa |
| SRC—Sodium carbonate (%) | ||||||
| Arapoti | 103.73 ± 2.08 Aa | 135.16 ± 1.25 Aa | 114.07 ± 1.89 B Aa | 114.90 ± 1.78 Aa | 140.94 ± 4.90 Aa | 119.52 ± 0.34 Aa |
| Arapongas | 110.30 ± 4.69 C Aa | 103.73 ± 0.82 C Aa | 129.15 ± 1.75 Aa | 134.84 ± 10.19 Aa | 117.31 ± 134.57 Aa | 126.53 ± 0.71 Aa |
| Passo Fundo | 125.55 ± 3.24 Aa | 106.53 ± 2.94 Aa | 108.60 ± 4.07 Aa | 128.47 ± 0.77 Aa | 126.72 ± 5.09 Aa | 122.89 ± 3.21 Aa |
| Uberaba | 108.54 ± 1.75 Aa | 112.36 ± 2.66 Aa | 106.05 ± 1.69 Aa | 106.51 ± 0.10 Aa | 124.28 ± 0.45 Aa | 131.79 ± 1.49 Aa |
| São Gotardo | 114.66 ± 0.50 Aa | 107.81 ± 0.95 Aa | 117.59 ± 0.01 Aa | 117.46 ± 0.46 Aa | 107.86 ± 1.22 Aa | 141.65 ± 13.25 Aa |
| SRC—Sucrose (%) | ||||||
| Arapoti | 105.63 ± 11.68 Aa | 127.84 ± 4.19 Aa | 108.61 ± 79.94 Aa | 108.73 ± 11.58 Aa | 105.09 ± 1.38 Aa | 107.30 ± 3.77 Aa |
| Arapongas | 119.25 ± 13.95 Aa | 113.57 ± 6.33 Aa | 125.02 ± 32.33 Aa | 123.60 ± 3.74 Aa | 108.58 ± 3.01 Aa | 109.95 ± 5.42 Aa |
| Passo Fundo | 128.59 ± 10.88 Aa | 104.63 ± 15.95 Aa | 100.98 ± 1.61 Aa | 226.56 ± 145.61 Aa | 111.78 ± 0.46 Aa | 121.15 ± 0.22 Aa |
| Uberaba | 116.12 ± 1.52 Aa | 125.18 ± 1.61 Aa | 102.76 ± 2.30 Aa | 93.21 ± 1.80 Aa | 121.73 ± 9.11 Aa | 127.59 ± 12.53 Aa |
| São Gotardo | 129.48 ± 3.11 Aa | 107.91 ± 7.87 Aa | 115.04 ± 0.69 Aa | 110.58 ± 10.85 Aa | 94.11 ± 2.74 Aa | 129.72 ± 4.66 Aa |
| SRC—Lactic acid (%) | ||||||
| Arapoti | 135.50 ± 8.70 Dc | 163.12 ± 3.66 Bb | 182.37 ± 10.51 Ba | 182.62 ± 7.70 Aa | 179.59 ± 5.42 Aa | 146.08 ± 9.53 Ab |
| Arapongas | 202.06 ± 0.88 Aa | 179.06 ± 5.25 Bb | 205.09 ± 8.68 Aa | 153.88 ± 5.83 Bb | 161.16 ± 1.36 Ab | 166.08 ± 9.53 Ab |
| Passo Fundo | 186.48 ± 7.10 Ba | 168.14 ± 4.13 Bb | 181.50 ± 0.67 Ba | 152.65 ± 0.41 Bb | 152.70 ± 1.61 Bb | 166.54 ± 0.27 Ab |
| Uberaba | 163.72 ± 9.22 Ba | 159.07 ± 1.83 Ca | 132.73 ± 1.39 Db | 173.56 ± 6.06 Aa | 182.49 ± 10.27 Aa | 158.51 ± 0.60 Aa |
| São Gotardo | 147.63 ± 1.51 Cc | 201.38 ± 1.40 Aa | 159.29 ± 0.98 Cc | 188.50 ± 5.13 Ab | 172.12 ± 13.04 Ab | 153.53 ± 5.50 Ac |
| Hagberg Falling number (s) | ||||||
| Arapoti | 477.50 ± 9.19 Aa | 400.50 ± 36.06 Bb | 447.50 ± 4.95 Ab | 483.00 ± 15.56 Aa | 396.50 ± 14.85 Bb | 407.00 ± 12.73 Ab |
| Arapongas | 457.50 ± 7.78 Aa | 426.50 ± 9.19 Bb | 360.50 ± 0.71 Bc | 336.50 ± 6.36 Cd | 336.00 ± 1.41 Cd | 374.00 ± 15.56 Bc |
| Passo Fundo | 454.00 ± 9.90 Aa | 371.00 ± 15.56 Cc | 354.50 ± 13.44 Bc | 402.00 ± 1.41 Bb | 397.00 ± 9.90 Bb | 400.00 ± 11.31 Ab |
| Uberaba | 444.00 ± 49.50 Ab | 559.50 ± 34.65 Aa | 343.50 ± 7.78 Bc | 445.50 ± 16.26 Bb | 463.00 ± 16.97 Ab | 427.00 ± 19.80 Ab |
| São Gotardo | 442.00 ± 26.87 Aa | 451.50 ± 20.51 Ba | 460.00 ± 24.04 Aa | 427.00 ± 19.80 Ba | 366.50 ± 28.99 Bb | 444.50 ± 2.12 Aa |
| Tenacity (mm) | ||||||
| Arapoti | 119.50 ± 2.52 Bb | 117.00 ± 10.12 Bb | 64.25 ± 3.00 Ac | 102.50 ± 9.94 Bb | 97.00 ± 3.61 Bb | 160.25 ± 5.29 Ba |
| Arapongas | 91.00 ± 8.08 Ba | 98.00 ± 3.79 Ca | 58.75 ± 0.00 Ac | 80.00 ± 2.00 Bab | 70.50 ± 9.17 Cb | 103.50 ± 3.51 Da |
| Passo Fundo | 118.00 ± 7.37 Ba | 123.00 ± 9.29 ABa | 46.25 ± 1.53 Cb | 97.25 ± 1.00 Ba | 127.50 ± 6.24 Aa | 102.25 ± 10.69 Da |
| Uberaba | 112.75 ± 5.03 Bc | 125.50 ± 1.73 Bc | 56.75 ± 1.15 Ad | 138.75 ± 0.58 Ab | 125.75 ± 1.53 Ac | 203.25 ± 1.53 Aa |
| São Gotardo | 128.50 ± 1.53 Ab | 136.25 ± 6.11 Ab | 51.25 ± 0.58 Bd | 106.25 ± 4.16 Bc | 125.00 ± 3.06 Ab | 141.67 ± 0.58 Ca |
| Extensibility (mm) | ||||||
| Arapoti | 83.75 ± 4.62 Aa | 69.25 ± 4.93 Aa | 103.50 ± 13.20 Ba | 97.25 ± 15.95 Aa | 93.50 ± 6.24 Aa | 50.50 ± 3.51 Cb |
| Arapongas | 89.50 ± 10.44 Aa | 66.75 ± 8.62 Ab | 69.00 ± 14.43 Cab | 112.25 ± 5.86 Aa | 107.75 ± 14.36 Aa | 65.75 ± 5.20 Bb |
| Passo Fundo | 77.25 ± 10.15 Abc | 43.75 ± 1.53 Bd | 84.50 ± 2.08 Cb | 122.50 ± 5.51 Aa | 117.50 ± 13.00 Aa | 61.00 ± 4.04 Bc |
| Uberaba | 89.00 ± 2.89 Ac | 70.50 ± 7.23 Ac | 154.00 ± 5.51 Aa | 99.50 ± 2.31 Ab | 78.75 ± 1.73 Bc | 48.50 ± 3.46 Cd |
| São Gotardo | 89.25 ± 2.08 Ac | 67.50 ± 1.15 Ad | 141.50 ± 6.03 Aa | 108.75 ± 4.62 Ab | 85.50 ± 6.24 ABc | 83.00 ± 2.65 Ac |
| Strength of gluten (10−4 J) | ||||||
| Arapoti | 313.50 ± 19.30 Ba | 270.25 ± 28.01 Bc | 218.50 ± 2.00 Bc | 305.25 ± 58.64 Cb | 331.50 ± 20.07 Ba | 306.75 ± 18.15 Bb |
| Arapongas | 263.00 ± 36.69 Ca | 229.00 ± 27.15 Ca | 154.50 ± 4.55 Ba | 291.75 ± 11.15 Ca | 249.75 ± 46.87 Ba | 229.00 ± 4.93 Ca |
| Passo Fundo | 278.25 ± 44.19 Cc | 210.75 ± 19.97 Cc | 149.25 ± 3.21 Bd | 349.00 ± 2.34 Bb | 441.00 ± 18.72 Aa | 204.00 ± 24.34 Cc |
| Uberaba | 320.50 ± 13.43 Bb | 276.75 ± 23.76 Bc | 248.75 ± 11.72 Ac | 416.50 ± 3.61 Aa | 323.00 ± 6.03 Bb | 364.00 ± 15.04 Ab |
| São Gotardo | 362.25 ± 3.79 Aa | 312.50 ± 8.39 Ab | 219.00 ± 8.89 Bc | 355.75 ± 2.31 Ba | 316.50 ± 3.61 Bb | 358.00 ± 7.55 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, L.A.; Cañizares, L.d.C.C.; Meza, S.L.R.; Timm, N.d.S.; Valério, I.P.; Lovegrove, A.; Coradi, P.C.; Oliveira, M.d. Biomes Affect Baking Properties and Quality Parameters of Different Wheat Genotypes. Sustainability 2025, 17, 5236. https://doi.org/10.3390/su17125236
Rodrigues LA, Cañizares LdCC, Meza SLR, Timm NdS, Valério IP, Lovegrove A, Coradi PC, Oliveira Md. Biomes Affect Baking Properties and Quality Parameters of Different Wheat Genotypes. Sustainability. 2025; 17(12):5236. https://doi.org/10.3390/su17125236
Chicago/Turabian StyleRodrigues, Larissa Alves, Lázaro da Costa Corrêa Cañizares, Silvia Leticia Rivero Meza, Newiton da Silva Timm, Igor Pirez Valério, Alison Lovegrove, Paulo Carteri Coradi, and Maurício de Oliveira. 2025. "Biomes Affect Baking Properties and Quality Parameters of Different Wheat Genotypes" Sustainability 17, no. 12: 5236. https://doi.org/10.3390/su17125236
APA StyleRodrigues, L. A., Cañizares, L. d. C. C., Meza, S. L. R., Timm, N. d. S., Valério, I. P., Lovegrove, A., Coradi, P. C., & Oliveira, M. d. (2025). Biomes Affect Baking Properties and Quality Parameters of Different Wheat Genotypes. Sustainability, 17(12), 5236. https://doi.org/10.3390/su17125236





