Microclimate of Pedunculate Oak (Quercus robur L.) Sustainable Managed Forest Stands—A Study of Air and Soil Temperatures in Shelterwood Cutting
Abstract
1. Introduction
2. Materials and Methods
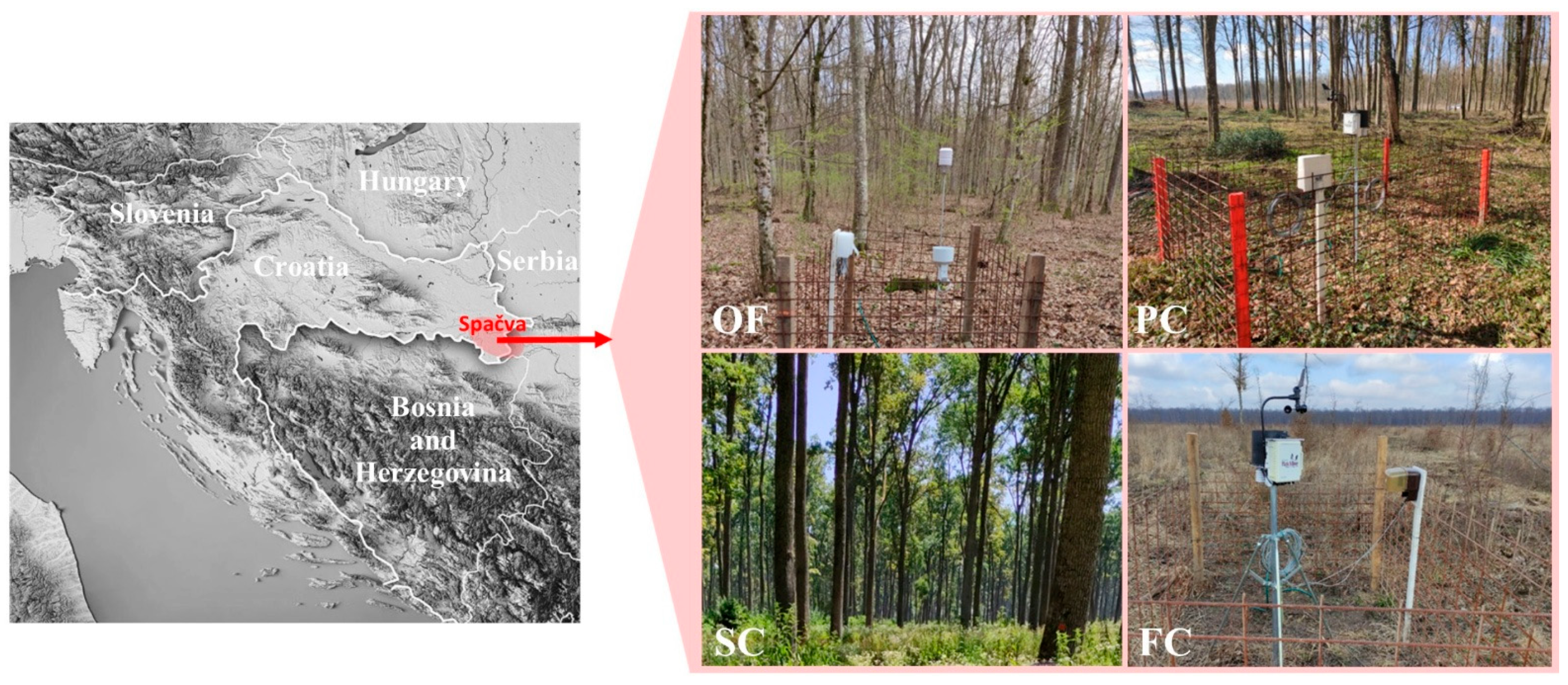
2.1. Research Area
2.2. Microclimatic Measurements
2.3. Statistical Processing of the Data
3. Results
3.1. Air Temperature
3.2. Soil Temperature
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vukelić, J. Šumska Vegetacija Hrvatske; Šumarski Fakultet Sveučilišta u Zagrebu—Državni Zavod za Zaštitu Prirode: Zagreb, Croatia, 2012; 403p. [Google Scholar]
- Matić, S.; Anić, I.; Oršanić, M. Forest management in floodplain forests. In Floodplain Forests of the Temperate Zone of Europe; Klimo, E., Ed.; Lesnicka Práce, s.r.o., Publishing House for Forestry: Kostelec nad Černymi lesy, Czech Republic, 2008; 623p, pp. 231–283. ISBN 978-80-87154-16-8. [Google Scholar]
- Ritter, E.; Dalsgaard, L.; Einhorn, S.K. Light, temperature and soil moisture regimes following gap formation in a semi-natural beech-dominated forest in Denmark. For. Ecol. Manag. 2005, 206, 15–33. [Google Scholar] [CrossRef]
- Ugarković, D.; Tikvić, I.; Popić, K.; Malnar, J.; Stankić, I. Microclimate and natural regeneration of forest gaps as a consequence of silver fir (Abies alba Mill) dieback. Šumarski List 2018, 5–6, 235–245. [Google Scholar]
- von Arx, G.; Dobbertin, M.; Rebetez, M. Spatio-temporal effects of forest canopy on understory microclimate in a long-term experiment in Switzerland. Agric. For. Meteorol. 2012, 166–167, 144–155. [Google Scholar] [CrossRef]
- Barry, R.G.; Blanken, P.D. Microclimate and Local Climate; Cambridge University Press: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- De Frenne, P.; Lenoir, J.; Luoto, M.; Scheffers, B.R.; Zellweger, F.; Aalto, J.; Ashcroft, M.B.; Christiansen, D.M.; Decocq, G.; De Pauw, K.; et al. Forest microclimates and climate change: Importance, drivers and future research agenda. Glob. Change Biol. 2021, 27, 2279–2297. [Google Scholar] [CrossRef] [PubMed]
- Aussenac, G. Interactions between forest stands and microclimate: Ecophysiological aspects and consequences for silviculture. Ann. For. Sci. 2000, 57, 287–301. [Google Scholar] [CrossRef]
- Bramer, I.; Anderson, B.J.; Bennie, J.; Bladon, A.J.; De Frenne, P.; Hemming, D.; Hill, R.A.; Kearney, M.R.; Körner, C.; Korstjens, A.H.; et al. Advances in monitoring and modelling climate at ecologically relevant scales. In Next Generation Biomonitoring: Part 1 Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 2018; pp. 101–161. [Google Scholar] [CrossRef]
- Decocq, G.; Aubert, M.; Dupont, F.; Alard, D.; Saguez, R.; Wattez-Franger, A.; Foucault, B.D.; Delelis-Dusollier, A.; Bardat, J. Plant diversity in a managed temperate deciduous forest: Understorey response to two silvicultural systems. J. Appl. Ecol. 2004, 41, 1065–1079. [Google Scholar] [CrossRef]
- Bonan, G.B. Ecological Climatology: Concepts and Applications, 3rd ed.; Cambridge University Press: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Lin, H.; Chen, Y.; Song, Q.; Fu, P.; Cleverly, J.; Magliulo, V.; Law, B.E.; Gough, C.M.; Hörtnagl, L.; Di Gennaro, F.; et al. Quantifying deforestation and forest degradation with thermal response. Sci. Total Environ. 2017, 607–608, 1286–1292. [Google Scholar] [CrossRef]
- Ehbrecht, M.; Schall, P.; Ammer, C.; Seidel, D. Quantifying stand structural complexity and its relationship with forest management, tree species diversity and microclimate. Agric. For. Meteorol. 2017, 242, 1–9. [Google Scholar] [CrossRef]
- De Frenne, P.; Verheyen, K. Weather stations lack forest dana. Science 2016, 351, 234. [Google Scholar] [CrossRef]
- Chen, J.Q.; Saunders, S.C.; Crow, T.R.; Naiman, R.J.; Brosofske, K.D.; Mroz, G.D.; Brookshire, B.L.; Franklin, J.F. Microclimate in forest ecosystem and landscape ecology—Variations in local climate can be used to monitor and compare the effects of different management regimes. BioScience 1999, 49, 288–297. [Google Scholar] [CrossRef]
- Binkley, D.; Giardina, C. Why do tree species affect soils? The warp and woof of tree-soil interactions. Biogeochemistry 1998, 42, 89–106. [Google Scholar] [CrossRef]
- Tranquillini, W. Physiological Ecology of the Alpine Timberline: Tree Existence at High Altitude with Special Reference to the European Alps, Ecological Studeies; Springer: Berlin, Germany; New York, NY, USA, 1979; 140p. [Google Scholar] [CrossRef]
- Denslow, J.S. Tropical rain forest gaps and tree species diversity. Annu. Rev. Ecol. Evol. Syst. 1987, 18, 431–451. [Google Scholar] [CrossRef]
- Potter, B.E.; Teclaw, R.M.; Zasada, J.C. The impact of forest structure on near-ground temperatures during two years of contrasting temperature extremes. Agric. For. Meteorol. 2001, 106, 331–336. [Google Scholar] [CrossRef]
- Cindrić, Ž. Komparativna Mikroklimatska Istraživanja u Šumskim Fitocenozama na Području Spačve; Elaborat Šumarski Institut: Jastrebarsko, Hrvatska, 1975; 67p. [Google Scholar]
- Prpić, B. Posljedice promjene šumske fitoklime u ekosustavu poplavne šume hrasta lužnjaka. In Zbornik Simpozija Sto Godina Znanstvenog i Organiziranog Pristupa Šumarstvu Jugoistočne Slavonije; JAZU, Centar za znanstveni rad Vinkovci: Vinkovci, Hrvatska, 1975. [Google Scholar]
- Medvedović, J. Mikroklima staništa hrasta lužnjaka. In Hrast lužnjak (Quercus robur L.) u Hrvatskoj, HAZU i Hrvatske šume p.o. Zagreb, 167–212, Zagreb; Klepac, D., Ed.; HAZU—Centar za znanstveni rad Vinkovci: Vinkovci, Zagreb, 1996; pp. 83–89. [Google Scholar]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest tree and stands under severe drought: A review of ecophysiological responses, adaptation processes and longterm consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Deshayes, M.; Guyon, D.; Jeanjean, H.; Stach, N.; Jolly, A.; Hagolle, O. The contribution of remote sensing to the assessment of drought effects in forest ecosystems. Ann. For. Sci. 2006, 63, 579–595. [Google Scholar] [CrossRef]
- De Frenne, P.; Zellweger, F.; Rodriguez-Sanchez, F.; Scheffers, B.R.; Hylander, K.; Luoto, M.; Vellend, M.; Verheyen, K.; Lenoir, J. Global buffering of temperatures under forest canopies. Nat. Ecol. Evol. 2019, 3, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Rauš, Đ. Vegetacijski i sinekološki odnosi šuma u bazenu Spačva. Glas. Šumske Pokuse Ann. Pro Exp. For. 1975, 18, 225–346. Available online: https://repozitorij.sumfak.unizg.hr/en/islandora/object/sumfak:2405 (accessed on 28 January 2025).
- Škvorc, Ž.; Cestarić, D.; Franjić, J.; Krstonošić, D.; Sever, K.; Guzmić, M. Dinamika šumske vegetacije spačvanskog bazena u posljednjih četrdeset godina. In Zbornik Skupa Šume Hrasta Lužnjaka u Promijenjenim Stanišnim i Gospodarskim Uvjetima; HAZU: Zagreb, Croatia, 2009; pp. 75–101. [Google Scholar]
- Dubravac, T.; Dekanić, S. Struktura i dinamika sječe suhih i odumirućih stabala hrasta lužnjaka u Spačvanskom bazenu od 1996. do 2006. godine. Šumarski List 2009, 133, 391–405. [Google Scholar]
- SpecWare 9 Pro and Basic Updates | Spectrum Technologies. Available online: https://www.specmeters.com/software (accessed on 1 February 2025).
- Šegota, T.; Filipčić, A. Klimatologija za Geografe; Školska knjiga Zagreb: Zagreb, Hrvatska, 1996; 471p. [Google Scholar]
- Hyndman, R.; Athanasopoulos, G.; Bergmeir, C.; Caceres, G.; Chhay, L.; O’Hara-Wild, M.; Petropoulos, F.; Razbash, S.; Wang, E.; Yasmeen, F. Forecast: Forecasting Functions for Time Series and Linear Models. R Package Version 8.23.0. 2024. Available online: https://pkg.robjhyndman.com/forecast/ (accessed on 1 February 2025).
- Atkinson, B. The Climate near the Ground, 6th ed.; Geiger, R., Aron, R.H., Todhunter, P., Eds.; Rowman and Littlefield Publishers: Lanham, MD, USA, 2003; p. xviii +584. ISBN 0-7425-1857-4. [Google Scholar]
- Meeussen, C.; Govaert, S.; Vanneste, T.; Haesen, S.; Van Meerbeek, K.; Bollmann, K.; Brunet, J.; Calders, K.; Cousins, S.A.O.; Diekmann, M.; et al. Drivers of carbon stocks in forest edges across Europe. Sci. Total Environ. 2021, 759, 143497. [Google Scholar] [CrossRef]
- Wubet, T.; Christ, S.; Schöning, I.; Boch, S.; Gawlich, M.; Schnabel, B.; Fischer, M.; Buscot, F. Differences in soil fungal communities between European beech (Fagus sylvatica L.) dominated forests are related to soil and understory vegetation. PLoS ONE 2012, 7, e47500. [Google Scholar] [CrossRef]
- von Arx, G.; Graf Pannatier, E.; Thimonier, A.; Rebetez, M.; Gilliam, F. Microclimate in forests with varying leaf area index and soil moisture: Potential implications for seedling establishment in a changing climate. J. Ecol. 2013, 101, 1201–1213. [Google Scholar] [CrossRef]
- Ashcroft, M.; Gollan, J. The sensitivity of topoclimatic models to fine-scale microclimatic variability and the relevance for ecological studies. Theor. Appl. Climatol. 2013, 114, 281–289. [Google Scholar] [CrossRef]
- De Frenne, P.; Rodríguez-Sánchez, F.; Coomes, D.A.; Baeten, L.; Verstraeten, G.; Vellend, M.; Bernhardt-Römermann, M.; Brown, C.D.; Brunet, J.; Cornelis, J.; et al. Microclimate moderates plant responses to macroclimate warming. Proc. Natl. Acad. Sci. USA 2013, 110, 18561–18565. [Google Scholar] [CrossRef] [PubMed]
- Gaudio, N.; Gendre, X.; Saudreau, M.; Seigner, V.; Balandier, P. Microclimate in Mediterranean pine forests: What is the influence of the shrub layer? Agric. For. Meteorol. 2020, 282–283, 107856. [Google Scholar] [CrossRef]
- Kovács, B.; Tinya, F.; Németh, C.; Ódor, P. Unfolding the effects of different forestry treatments on microclimate in oak forests: Results of a 4-yr experiment. Ecol. Appl. 2020, 30, e02043. [Google Scholar] [CrossRef] [PubMed]
- Blonder, B.; Both, S.; Coomes, D.A.; Elias, D.; Jucker, T.; Kvasnica, J.; Majalap, N.; Malhi, Y.S.; Milodowski, D.; Riutta, T.; et al. Extreme and highly heterogeneous microclimates in selectively logged tropical forests. Front. For. Glob. Change 2018, 1, 5. [Google Scholar] [CrossRef]
- Thom, D.; Ammer, C.; Annighöfer, P.; Aszalós, R.; Dittrich, S.; Hagge, J.; Keeton, W.S.; Kovacs, B.; Krautkrämer, O.; Müller, J.; et al. Regeneration in European beech forests after drought: The effects of microclimate, deadwood and browsing. Eur. J. For. Res. 2023, 291, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Örlander, G. Shading reduces both visible and invisible frost damage to Norway spruce seedlings in the field. Forestry 1993, 66, 27–36. [Google Scholar] [CrossRef]
- Liechty, H.O.; Holmes, M.J.; Reed, D.D.; Mroz, G.D. Changes in microclimate after stand conversion in two northern hardwoods stands. For. Ecol. Manag. 1992, 50, 253–264. [Google Scholar] [CrossRef]
- Lindh, M.; Fagerström, M.; Gustafsson, L. Microclimate changes following different intensities of forest harvesting in a boreal forest. For. Ecol. Manag. 2019, 453, 117624. [Google Scholar] [CrossRef]
- Ewers, R.M.; Banks-Leite, C. Fragmentation impairs the microclimate buffering effect of tropical forests. PLoS ONE 2013, 8, e58093. [Google Scholar] [CrossRef] [PubMed]
- Groot, A.; Carlson, D.W. Influence of shelter on night temperatures, frost damage, and bud break of white spruce seedlings. Can. J. For. Res. 1996, 26, 1531–1538. [Google Scholar] [CrossRef]
- Marsden, B.J.; Lieffers, V.J.; Zwiazek, J.J. The effect of humidity on photosynthesis and water relations of white spruce seedlings during the early establishment phase. Can. J. For. Res. 1996, 26, 1015–1021. [Google Scholar] [CrossRef]
- Muscolo, A.; Settineri, G.; Romeo, F.; Mallamaci, C. Soil Biodiversity as Affected by Different Thinning Intensities in a Pinus laricio Stand of Calabrian Apennine, South Italy. Forests 2021, 12, 108. [Google Scholar] [CrossRef]
- Waldron, R.M.; Kolabinski, V.S. Uniform Shelterwood Cutting and Scarifying in White Spruce- Trembling Aspen Stands to Induce Natural White Spruce Regeneration, Manitoba and Saskatchewan. Canada-Manitoba Partnership Agreement in Forestry Miscellaneous Report. 1994. Available online: https://ostrnrcan-dostrncan.canada.ca/handle/1845/225529 (accessed on 25 February 2025).
- Man, R.; Lieffers, V.J. Seasonal photosynthetic responses to light and temperature in white spruce (Picea glauca) seedlings planted under an aspen (Populus tremuloides) canopy and in the open. Tree Physiol. 1997, 17, 437–444. [Google Scholar] [CrossRef]
- Lenoir, J.; Hattab, T.; Pierre, G. Climatic microrefugia under anthropogenic climate change: Implications for species redistribution. Ecography 2017, 40, 253–266. [Google Scholar] [CrossRef]




| Old Forest (OF) | Preparatory Cut (PC) | Seed Cut (SC) | Final Cut (FC) | |
|---|---|---|---|---|
| Coordinates | 19.023; 45.020 | 18.874; 44.947 | 18.911; 45.063 | 19.018; 45.012 |
| Altitude (m) | 80–81 | 82–84 | 82–84 | 79–81 |
| Soil type | Gley | Luvisol | Gley | Gley |
| Age (years) | 131 | 132 | 152 | 4 |
| Average tree diameter (cm) | 59 | 60 | 61 | 1–5 |
| Average tree height (m) | 38 | 37 | 35 | 0.6–0.7 |
| Cut intensity (%) | 0 | 38.26 | 43.66 | 51.21 |
| Density (N ha−1) | 354 | 316 | 256 | 64,915 |
| Basal Area (m2 ha−1) | 32.43 | 31.36 | 28.19 | 26.87 |
| Volume (m3 ha−1) | 525 | 294 | 459 | 405 |
| Pedunculate oak (%) | 64.81 | 44 | 69.81 | 56.83 |
| Hornbeam (%) | 29.12 | 3 | 3.19 | 29.84 |
| Narrow leaved-ash (%) | 4.21 | 31 | 25.6 | 11.15 |
| Season | Stage | Temperature (°C) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Air | Soil | ||||||||
| Min | Max | Mean | St. Dev. | Min | Max | Mean | St. Dev. | ||
| Spring | OF | 3.28 | 29.97 | 15.03 | 5.67 | 4.8 | 18.6 | 11.41 | 3.21 |
| PC | −1.6 | 33.4 | 14.42 | 6.82 | 4.1 | 19.3 | 11.06 | 4.56 | |
| SC | −3.4 | 31.7 | 14.07 | 7.09 | 4.8 | 18.3 | 12.02 | 3.27 | |
| FC | −4.7 | 33.8 | 14.11 | 8.17 | 4.6 | 22.3 | 13.53 | 4.3 | |
| Summer | OF | 5.55 | 35.16 | 20.68 | 5.79 | 13.6 | 21.6 | 18.59 | 1.97 |
| PC | 7.3 | 38.2 | 21.6 | 6.33 | 14.4 | 22.4 | 19.65 | 1.87 | |
| SC | 5.9 | 36.4 | 21.18 | 6.47 | 14.9 | 22.1 | 19.21 | 1.71 | |
| FC | 1.6 | 40.2 | 21.21 | 9.15 | 14.7 | 25.8 | 21.39 | 2.88 | |
| Fall | OF | −5.8 | 25.9 | 7.07 | 5.69 | 3.9 | 16.2 | 9.26 | 3.09 |
| PC | −4.6 | 27.5 | 7.4 | 5.69 | 4.8 | 17 | 9.8 | 3.13 | |
| SC | −7.2 | 28.3 | 6.94 | 6.08 | 4.3 | 16.7 | 9.55 | 3.45 | |
| FC | −8.9 | 30.6 | 6.54 | 6.96 | 3 | 17 | 8.59 | 3.75 | |
| Winter | OF | −11.73 | 23.16 | 3.51 | 6.05 | 1.3 | 5.9 | 3.98 | 1.1 |
| PC | −9.6 | 23.1 | 3.83 | 5.84 | 1.5 | 9.1 | 4.51 | 1.43 | |
| SC | −13.4 | 23.5 | 3.21 | 6.27 | 0.7 | 9.7 | 4 | 1.8 | |
| FC | −15.9 | 24 | 2.67 | 7.17 | 0.1 | 8.3 | 3.2 | 1.76 | |
| Growing season | OF | 3.29 | 35.16 | 18.06 | 6.38 | 5.6 | 21.6 | 15.4 | 4.16 |
| PC | −1.6 | 38.2 | 18.04 | 7.5 | 4.1 | 22.4 | 16.29 | 4.93 | |
| SC | −3.4 | 36.4 | 17.64 | 7.68 | 5.9 | 22.1 | 16.04 | 4.08 | |
| FC | −5.9 | 40.2 | 17.61 | 9.2 | 5.3 | 25.8 | 17.85 | 5.01 | |
| Annual | OF | −11.73 | 35.16 | 11.23 | 8.98 | 1.3 | 21.6 | 10.7 | 5.89 |
| PC | −9.6 | 38.2 | 11.53 | 9.24 | 1.5 | 22.4 | 10.96 | 6.29 | |
| SC | −13.4 | 36.4 | 11.06 | 9.51 | 0.7 | 22.1 | 11.07 | 6.18 | |
| FC | −15.9 | 40.2 | 10.81 | 10.57 | 0.1 | 25.8 | 11.53 | 7.56 | |
| Old Forest (OF) | Preparatory Cut (PC) | Seed Cut (SC) | Final Cut (FC) | |
|---|---|---|---|---|
| Icy | 2 | 0 | 2 | 14 |
| Cold | 102 | 96 | 122 | 139 |
| Warm | 47 | 49 | 58 | 49 |
| Hot | 29 | 45 | 42 | 69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popić, K.; Tafro, A.; Baričević, D.; Šapić, I.; Tikvić, I.; Ugarković, D. Microclimate of Pedunculate Oak (Quercus robur L.) Sustainable Managed Forest Stands—A Study of Air and Soil Temperatures in Shelterwood Cutting. Sustainability 2025, 17, 5106. https://doi.org/10.3390/su17115106
Popić K, Tafro A, Baričević D, Šapić I, Tikvić I, Ugarković D. Microclimate of Pedunculate Oak (Quercus robur L.) Sustainable Managed Forest Stands—A Study of Air and Soil Temperatures in Shelterwood Cutting. Sustainability. 2025; 17(11):5106. https://doi.org/10.3390/su17115106
Chicago/Turabian StylePopić, Krešimir, Azra Tafro, Dario Baričević, Irena Šapić, Ivica Tikvić, and Damir Ugarković. 2025. "Microclimate of Pedunculate Oak (Quercus robur L.) Sustainable Managed Forest Stands—A Study of Air and Soil Temperatures in Shelterwood Cutting" Sustainability 17, no. 11: 5106. https://doi.org/10.3390/su17115106
APA StylePopić, K., Tafro, A., Baričević, D., Šapić, I., Tikvić, I., & Ugarković, D. (2025). Microclimate of Pedunculate Oak (Quercus robur L.) Sustainable Managed Forest Stands—A Study of Air and Soil Temperatures in Shelterwood Cutting. Sustainability, 17(11), 5106. https://doi.org/10.3390/su17115106






