The Chemical Analysis of Wild Thyme Variability for the Enhanced Production of Bioactive Compounds and Agro-Ecosystem Sustainability in the Mountains of Pistoia (Italy)
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Wild Thyme and Its Maintenance in Pots
2.2. Morphological Analysis
2.3. Terpene Identification and Quantification by Gas Chromatography Coupled to Mass Spectrometry (GC-MS) Analyses
2.4. In Vitro Micropropagation of Wild Thyme Plants
2.5. Statistical Analysis
3. Results
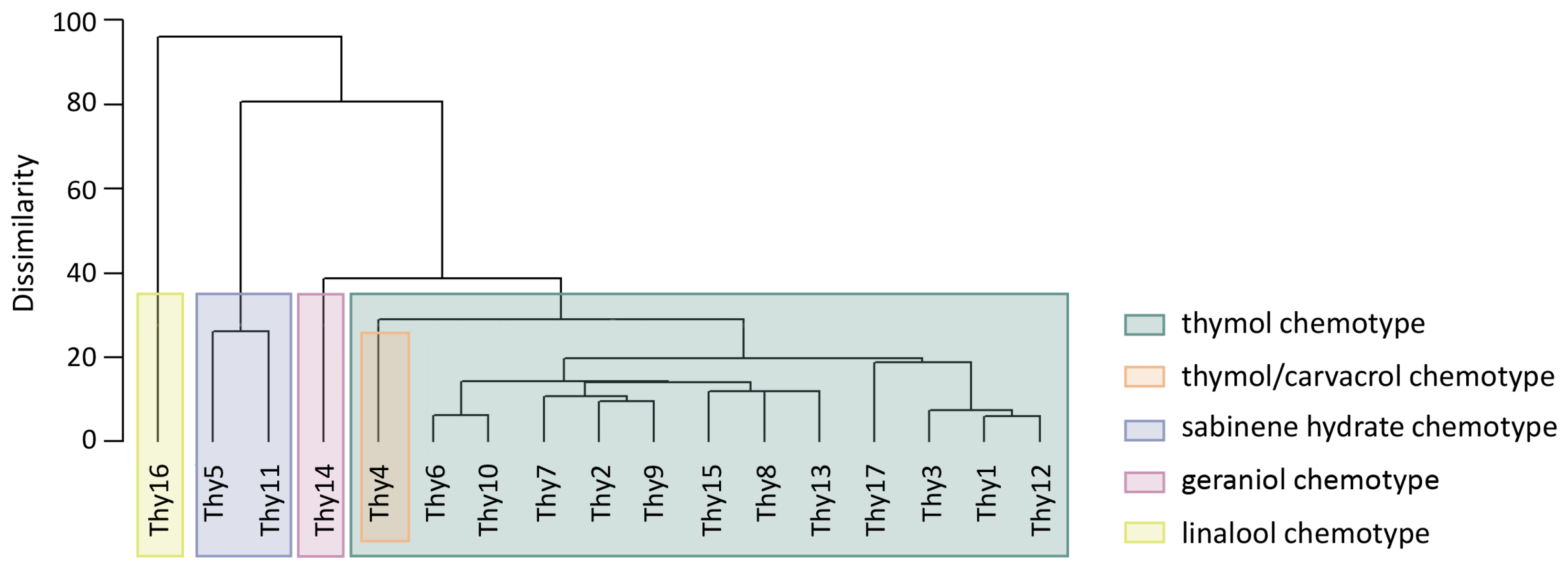
3.1. Chemical and Morphological Characterization of Wild Thyme Plants
3.2. Chemotype Stability and Optimal Harvesting Season
3.3. In Vitro Maintenance of High-Value Wild Thyme Chemotypes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, N.; Ray, R.L.; Sargani, G.R.; Ihtisham, M.; Khayyam, M.; Ismail, S. Current Progress and Future Prospects of Agriculture Technology: Gateway to Sustainable Agriculture. Sustainability 2021, 13, 4883. [Google Scholar] [CrossRef]
- Diyaolu, C.O.; Folarin, I.O. The Role of Biodiversity in Agricultural Resilience: Protecting Ecosystem Services for Sustainable Food Production. Int. J. Res. Publ. Rev. 2024, 5, 1560–1573. [Google Scholar] [CrossRef]
- Jago, S.; Borrell, J.S. Agrobiodiversity Conservation Enables Sustainable and Equitable Land Sparing. Trends Ecol. Evol. 2024, 39, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Skaltsa, H.; Konstantopoulou, M. Medicinal and Aromatic Plants (MAPs): The Connection between Cultivation Practices and Biological Properties. Agronomy 2022, 12, 3108. [Google Scholar] [CrossRef]
- Nyaupane, S.; Mainali, R.P.; Joshi, T.; Duwal, R. Plant-Based Agro-Biodiversity Solutions for Reducing Agrochemical Use and Effects. In One Health Implications of Agrochemicals and Their Sustainable Alternatives. Sustainable Development and Biodiversity; Springer Nature Singapore Pte Ltd.: Singapore, 2023; pp. 545–563. [Google Scholar]
- Rodino, S.; Butu, M. Herbal Extracts—New Trends in Functional and Medicinal Beverages. In Functional and Medicinal Beverages: Volume 11: The Science of Beverages; Academic Press: Cambridge, MA, USA, 2019; pp. 73–108. [Google Scholar] [CrossRef]
- Grigore-Gurgu, L.; Dumitrașcu, L.; Aprodu, I. Aromatic Herbs as a Source of Bioactive Compounds: An Overview of Their Antioxidant Capacity, Antimicrobial Activity, and Major Applications. Molecules 2025, 30, 1304. [Google Scholar] [CrossRef]
- Salaria, D.; Rolta, R.; Lal, U.R.; Dev, K.; Kumar, V. A Comprehensive Review on Traditional Applications, Phytochemistry, Pharmacology, and Toxicology of Thymus serpyllum. Indian J. Pharmacol. 2023, 55, 385–394. [Google Scholar] [CrossRef]
- Phondani, P.C.; Bhatt, I.D.; Negi, V.S.; Kothyari, B.P.; Bhatt, A.; Maikhuri, R.K. Promoting Medicinal Plants Cultivation as a Tool for Biodiversity Conservation and Livelihood Enhancement in Indian Himalaya. J. Asia Pac. Biodivers. 2016, 9, 39–46. [Google Scholar] [CrossRef]
- Greff, B.; Sáhó, A.; Lakatos, E.; Varga, L. Biocontrol Activity of Aromatic and Medicinal Plants and Their Bioactive Components against Soil-Borne Pathogens. Plants 2023, 12, 706. [Google Scholar] [CrossRef]
- Erdogan, O.; Celik, A.; Zeybek, A. In Vitro Antifungal Activity of Mint, Thyme, Lavender Extracts and Essential Oils on Verticillium Dahliae Kleb. Fresenius Environ. Bull. 2016, 25, 4856–4862. [Google Scholar]
- Gholijani, N.; Gharagozloo, M.; Kalantar, F.; Ramezani, A.; Amirghofran, Z. Modulation of Cytokine Production and Transcription Factors Activities in Human Jurkat t Cells by Thymol and Carvacrol. Adv. Pharm. Bull. 2015, 5, 653–660. [Google Scholar] [CrossRef]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and Medicinal Uses of Terpenes. In Medicinal Plants: From Farm to Pharmacy; Springer International Publishing: Cham, Switzerland, 2019; pp. 333–359. ISBN 9783030312695. [Google Scholar]
- Sateriale, D.; Forgione, G.; De Cristofaro, G.A.; Facchiano, S.; Boscaino, F.; Pagliuca, C.; Colicchio, R.; Salvatore, P.; Paolucci, M.; Pagliarulo, C. Towards Green Strategies of Food Security: Antibacterial Synergy of Essential Oils from Thymus vulgaris and Syzygium aromaticum to Inhibit Escherichia coli and Staphylococcus aureus Pathogenic Food Isolates. Microorganisms 2022, 10, 2446. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, M.M.; Martel, A.B.; Strugnell, C.A. Environmental Factors Regulate Plant Secondary Metabolites. Plants 2023, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Lin, G.; Liu, L.; Zhou, P.; Shao, Y.; Dai, S.; Sang, M.; Jiang, Z.; Liu, C.; Wu, Q. Comparison of Pulegone and Estragole Chemotypes Provides New Insight Into Volatile Oil Biosynthesis of Agastache Rugosa. Front. Plant Sci. 2022, 13, 850130. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, Y.; Zhu, L.; Liu, N.; Bai, H.; Sun, G.; Zhang, J.; Shi, L. Chromosome-Level Assembly and Analysis of the Thymus Genome Provide Insights into Glandular Secretory Trichome Formation and Monoterpenoid Biosynthesis in Thyme. Plant Commun. 2022, 3, 100413. [Google Scholar] [CrossRef]
- Menicucci, F.; Crisci, A.; Tarraf, W.; Santini, C.; Ieri, F.; Cencetti, G.; Michelozzi, M.; Ienco, A.; Palagano, E. Exploring Wild Thymus sp. (L.) Chemotypes across Pistoia Mountains Provides Thyme Essential Oil and Hydrolate Inhibiting Fungal Growth on Paper. Fitoterapia 2025, 182, 106418. [Google Scholar] [CrossRef]
- Kotsinis, V.; Dritsoulas, A.; Ntinokas, D.; Giannakou, I.O. Nematicidal Effects of Four Terpenes Differ among Entomopathogenic Nematode Species. Agriculture 2023, 13, 1143. [Google Scholar] [CrossRef]
- Jarić, S.; Mitrović, M.; Pavlović, P. Review of Ethnobotanical, Phytochemical, and Pharmacological Study of Thymus serpyllum L. Evid.-Based Complement. Altern. Med. 2015, 2015, 101978. [Google Scholar] [CrossRef]
- Asadollahi-Baboli, M.; Aghakhani, A. Headspace Adsorptive Microextraction Analysis of Oregano Fragrance Using Polyaniline-Nylon-6 Nanocomposite, GC-MS, and Multivariate Curve Resolution. Int. J. Food Prop. 2015, 18, 1613–1623. [Google Scholar] [CrossRef]
- Bakó, C.; Balázs, V.L.; Kerekes, E.; Kocsis, B.; Nagy, D.U.; Szabó, P.; Micalizzi, G.; Mondello, L.; Krisch, J.; Pethő, D.; et al. Flowering Phenophases Influence the Antibacterial and Anti-Biofilm Effects of Thymus vulgaris L. Essential Oil. BMC Complement. Med. Ther. 2023, 23, 168. [Google Scholar] [CrossRef]
- Dina, E.; Vontzalidou, A.; Cheilari, A.; Bagatzounis, P.; Agapidou, E.; Giannenas, I.; Grigoriadou, K.; Aligiannis, N. Sustainable Use of Greek Herbs By-Products, as an Alternative Source of Biologically Active Ingredients for Innovative Products. Front. Nutr. 2022, 9, 867666. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents—Myth or Real Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef] [PubMed]
- Micucci, M.; Protti, M.; Aldini, R.; Frosini, M.; Corazza, I.; Marzetti, C.; Mattioli, L.B.; Tocci, G.; Chiarini, A.; Mercolini, L.; et al. Thymus vulgaris L. Essential Oil Solid Formulation: Chemical Profile and Spasmolytic and Antimicrobial Effects. Biomolecules 2020, 10, 860. [Google Scholar] [CrossRef]
- Menicucci, F.; Palagano, E.; Raio, A.; Cencetti, G.; Luchi, N.; Ienco, A.; Michelozzi, M. Plant Sampling for Production of Essential Oil and Evaluation of Its Antimicrobial Activity In Vitro. Methods Mol. Biol. 2022, 2536, 475–493. [Google Scholar] [PubMed]
- Menicucci, F.; Pizzo, B.; Salvadori, B.; Chelazzi, L.; Ienco, A.; Palagano, E. Antifungal Activity of Carvacrol-Based Solids and Their Effects on Whatman and Kraft Paper. Int. Biodeterior. Biodegrad. 2024, 195, 105894. [Google Scholar] [CrossRef]
- Maggioni, L.; Maxted, N.; Engels, J. The European Cooperative Programme for Plant Genetic Resources (ECPGR) and Planta Europa: An Opportunity For Synergies. Plant Europa News, 2008 Winter. Available online: https://www.ecpgr.org/fileadmin/templates/ecpgr.org/upload/NW_and_WG_UPLOADS/MAP_Descriptors/Thymus_vulgaris_DRAFT_DESCRIPTOR_LIST_FINAL.pdf (accessed on 7 March 2024).
- Bellumori, M.; Michelozzi, M.; Innocenti, M.; Congiu, F.; Cencetti, G.; Mulinacci, N. An Innovative Approach to the Recovery of Phenolic Compounds and Volatile Terpenes from the Same Fresh Foliar Sample of Rosmarinus officinalis L. Talanta 2015, 131, 81–87. [Google Scholar] [CrossRef]
- Mendes, M.D.; Cristina Figueiredo, A.; Margarida Oliveira, M.; Trindade, H. Essential Oil Production in Shoot Cultures versus Field-Grown Plants of Thymus Caespititius. Plant Cell Tissue Organ Cult. (PCTOC) 2013, 113, 341–351. [Google Scholar] [CrossRef]
- Faisal, M.; Zamzami, E.M. Sutarman Comparative Analysis of Inter-Centroid K-Means Performance Using Euclidean Distance, Canberra Distance and Manhattan Distance. J. Phys. Conf. Ser. 2020, 1566, 12112. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric Multivariate Analyses of Changes in Community Structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Clarke, K.; Green, R. Statistical Design and Analysis for a “biological Effects” Study. Mar. Ecol. Prog. Ser. 1988, 46, 213–226. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package; CRAN: Contributed Packages; 2001. Available online: https://cran.r-project.org/package=vegan (accessed on 4 February 2025).
- Liu, Q.; Sun, X.; Wu, W.; Liu, Z.; Fang, G.; Yang, P. Agroecosystem Services: A Review of Concepts, Indicators, Assessment Methods and Future Research Perspectives. Ecol. Indic. 2022, 142, 109218. [Google Scholar] [CrossRef]
- Širvaitytė, J.; Šiugždaitė, J.; Valeika, V.; Dambrauskiene, E. Application of Essential Oils of Thyme as a Natural Preservative in Leather Tanning. Proc. Est. Acad. Sci. 2012, 61, 220. [Google Scholar] [CrossRef]
- Halat, D.H.; Krayem, M.; Khaled, S.; Younes, S. A Focused Insight into Thyme: Biological, Chemical, and Therapeutic Properties of an Indigenous Mediterranean Herb. Nutrients 2022, 14, 2104. [Google Scholar] [CrossRef]
- Valev, T.S.; Dobreva, K.Z.; Dimov, M.D. Phytochemical Characterization of Different Varieties of Thyme. Bulg. Chem. Commun. 2024, 56, 133–137. [Google Scholar] [CrossRef]
- Sadowska, U.; Kopeć, A.; Kourimska, L.; Zarubova, L.; Kloucek, P. The Effect of Drying Methods on the Concentration of Compounds in Sage and Thyme. J. Food Process Preserv. 2017, 41, e13286. [Google Scholar] [CrossRef]
- Pérez-Sánchez, R.; Ubera, J.L.; Lafont, F.; Gálvez, C. Composition and Variability of the Essential Oil in Thymus zygis from Southern Spain. J. Essent. Oil Res. 2008, 20, 192–200. [Google Scholar] [CrossRef]
- Bigdeloo, M.; Hadian, J.; Nazeri, V. Composition of Essential Oil Compounds from Different Populations of Thymus Caramanicus Jalas. J. Appl. Res. Med. Aromat. Plants 2017, 7, 95–98. [Google Scholar] [CrossRef]
- Etri, K.; Pluhár, Z. Exploring Chemical Variability in the Essential Oils of the Thymus Genus. Plants 2024, 13, 1375. [Google Scholar] [CrossRef] [PubMed]
- Najar, B.; Pistelli, L.; Ferri, B.; Angelini, L.G.; Tavarini, S. Crop Yield and Essential Oil Composition of Two Thymus Vulgaris Chemotypes along Three Years of Organic Cultivation in a Hilly Area of Central Italy. Molecules 2021, 26, 5109. [Google Scholar] [CrossRef]
- Figueiredo, M.C.C.; Passos, A.R.; Hughes, F.M.; dos Santos, K.S.; da Silva, A.L.; Soares, T.L. Reproductive Biology of Physalis angulata L. (Solanaceae). Sci. Hortic. 2020, 267, 109307. [Google Scholar] [CrossRef]
- Verma, R.; Chauhan, A.; Verma, R.; Yadav, A. Seasonal Variation in Essential Oil Content and Composition of Thyme, Thymus Serpyllum l. Cultivated in Uttarakhand Hills. Indian J. Pharm. Sci. 2011, 73, 233. [Google Scholar] [CrossRef]
- Kamatou, G.P.; Viljoen, A.M. Linalool—A Review of a Biologically Active Compound of Commercial Importance. Nat. Prod. Commun. 2008, 3, 1183–1192. [Google Scholar] [CrossRef]
- Chen, W.; Viljoen, A.M. Geraniol—A Review of a Commercially Important Fragrance Material. S. Afr. J. Bot. 2010, 76, 643–651. [Google Scholar] [CrossRef]
- Wise, M.L.; Rorrer, G.L.; Polzin, J.J.; Croteau, R. Biosynthesis of Marine Natural Products: Isolation and Characterization of a Myrcene Synthase from Cultured Tissues of the Marine Red Alga Ochtodes Secundiramea. Arch. Biochem. Biophys. 2002, 400, 125–132. [Google Scholar] [CrossRef]
- Escobar, A.; Pérez, M.; Romanelli, G.; Blustein, G. Thymol Bioactivity: A Review Focusing on Practical Applications. Arab. J. Chem. 2020, 13, 9243–9269. [Google Scholar] [CrossRef]
- Bao, T.; Kimani, S.; Li, Y.; Li, H.; Yang, S.; Zhang, J.; Wang, Q.; Wang, Z.; Ning, G.; Wang, L.; et al. Allelic Variation of Terpene Synthases Drives Terpene Diversity in the Wild Species of the Freesia Genus. Plant Physiol. 2023, 192, 2419–2435. [Google Scholar] [CrossRef]
- Qiao, Z.; Hu, H.; Shi, S.; Yuan, X.; Yan, B.; Chen, L. An Update on the Function, Biosynthesis and Regulation of Floral Volatile Terpenoids. Horticulturae 2021, 7, 451. [Google Scholar] [CrossRef]
- Ghosh, D.; Chaudhary, N.; Uma Kumari, K.; Singh, J.; Tripathi, P.; Meena, A.; Luqman, S.; Yadav, A.; Chanotiya, C.S.; Pandey, G.; et al. Diversity of Essential Oil-Secretory Cells and Oil Composition in Flowers and Buds of Magnolia sirindhorniae and Its Biological Activities. Chem. Biodivers. 2021, 18, e2000750. [Google Scholar] [CrossRef]
- Détár, E.; Zámbori-Németh, É.; Gosztola, B.; Harmath, A.; Ladányi, M.; Pluhár, Z. Ontogenesis and Harvest Time Are Crucial for High Quality Lavender—Role of the Flower Development in Essential Oil Properties. Ind. Crops Prod. 2021, 163, 113334. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santini, C.; Bonetti, D.; Della Maggiora, L.; Tarraf, W.; Menicucci, F.; Ieri, F.; Crisci, A.; Cencetti, G.; Ienco, A.; Palagano, E. The Chemical Analysis of Wild Thyme Variability for the Enhanced Production of Bioactive Compounds and Agro-Ecosystem Sustainability in the Mountains of Pistoia (Italy). Sustainability 2025, 17, 5073. https://doi.org/10.3390/su17115073
Santini C, Bonetti D, Della Maggiora L, Tarraf W, Menicucci F, Ieri F, Crisci A, Cencetti G, Ienco A, Palagano E. The Chemical Analysis of Wild Thyme Variability for the Enhanced Production of Bioactive Compounds and Agro-Ecosystem Sustainability in the Mountains of Pistoia (Italy). Sustainability. 2025; 17(11):5073. https://doi.org/10.3390/su17115073
Chicago/Turabian StyleSantini, Costanza, Daniele Bonetti, Lorenzo Della Maggiora, Waed Tarraf, Felicia Menicucci, Francesca Ieri, Alfonso Crisci, Gabriele Cencetti, Andrea Ienco, and Eleonora Palagano. 2025. "The Chemical Analysis of Wild Thyme Variability for the Enhanced Production of Bioactive Compounds and Agro-Ecosystem Sustainability in the Mountains of Pistoia (Italy)" Sustainability 17, no. 11: 5073. https://doi.org/10.3390/su17115073
APA StyleSantini, C., Bonetti, D., Della Maggiora, L., Tarraf, W., Menicucci, F., Ieri, F., Crisci, A., Cencetti, G., Ienco, A., & Palagano, E. (2025). The Chemical Analysis of Wild Thyme Variability for the Enhanced Production of Bioactive Compounds and Agro-Ecosystem Sustainability in the Mountains of Pistoia (Italy). Sustainability, 17(11), 5073. https://doi.org/10.3390/su17115073







