Abstract
Sustainability urgently needs to be achieved in urban green infrastructure. Maintaining and restoring biodiversity are critical for developing an urban ecosystem more resilient to abiotic and biotic stresses. The biodiversity of urban green spaces is vital as it reduces the risks associated with climate change (diseases and pests), improves the resilience of the urban ecosystem, and enhances citizens’ well-being. Urban green areas can provide important ecosystem services necessary for achieving prosperity, urban well-being, and the One Health paradigm at various scales. Urban green areas can serve as corridors and stepping stones between the rural environments surrounding cities, increasing their connections and reducing the risk of ecological traps. The conservation and restoration of biodiversity are strategies to increase ecosystem services. In this context, this review aims to analyze the possible contribution of ornamental plants to urban biodiversity, investigating the available knowledge and the gaps that need to be filled. Plants chosen for their esthetic functions are often allogamous species, characterized by showy flowers that attract fauna for pollination, thus helping insects and other fauna survive. If not invasive, these plants can actively contribute to biodiversity in the urban environment and to human well-being. Choosing suitable species and methods that favor plant communities and sustainable maintenance practices improves biodiversity and the ecosystem services that ornamental plants provide.
1. Introduction
Cities face complex challenges, such as climate change, population growth, decreasing human well-being, and the need to implement the One Health paradigm. Consequently, many city administrators and residents are looking for new and innovative strategies to improve urban sustainability [1]. Among these strategies, the priority is to enhance green infrastructure (GI). In fact, thanks to the contribution of plants, GI can provide benefits called ecosystem services (ESs), which are fundamental to improving the sustainability of cities [2]. Therefore, studying the characteristics of GI has become a research priority in cities worldwide [3].
Biodiversity and its conservation have been the focus of numerous political initiatives. At the Convention on Biological Diversity (CBD), all parties agreed to dedicate efforts to the conservation of biodiversity in urban areas [4]. The International Network Urban Biodiversity and Design (URBIO) was established in 2008 to promote the conservation of urban biodiversity through ongoing dialog with the CBD [5]. The United Nations, alongside other previous biodiversity conservation initiatives, has recently published a report titled “State of Finance for Nature in Cities 2024” through the United Nations Environment Programme (UNEP) [6]. One of the objectives of the European Biodiversity Strategy for 2030 is to reverse the trend of diminishing natural habitats in urban areas [7]. City biodiversity conservation is also a focus of Local Governments for Sustainability (ICLEI) [8].
Biodiversity is regarded as a crucial indicator of the environmental health of cities, as key species reflect the viability of biotic communities and the overall urban ecosystem [9].
The capacity of urban green infrastructure (UGI) to provide ESs is a key indicator used to understand which green spaces contribute to the sustainability of a city [10]. ESs provided by plants in cities include air purification, climate regulation, mitigation of extreme temperatures, and intellectual stimulation [2]. Furthermore, plants improve the technical and acoustic characteristics of buildings, ensuring significant optimization of rainwater management and creating biodiversity [11]. The development and maintenance of sustainable urban landscapes are challenging tasks that different stakeholders and scientists must address [12].
Urban greening in synergy with the dynamic development of urban planning encourages innate human biophilia, which is based on our affinity with nature: biophilia forces urban planners and policymakers to seek sustainable forms of urban green spaces to ensure the well-being of city residents [13]. GI, at the same time, should also be ensured to compensate for biodiversity loss and biotic homogenization [14]. The use of native plants could bring residents closer to nature and create a sense of belonging. Many native plants can be employed as ornamental plants in complex garden compositions, as they do not require high maintenance. A combination of native and exotic ornamental plants can create a more attractive plant community [15]. Their strong anthropic effect on the urban environment can also create considerable changes compared with the effects of the natural surrounding environment [16].
Urban parks, particularly those with large spatial extensions, generate considerable plant diversity capital, vegetation cover patterns with predominantly spontaneous natural vegetation, facilities for nature and sports recreation, and social services [17]. To support biodiversity and provide ESs in urban parks, a “biodiversity-friendly” planning approach based on geobotanical data is needed, where both the perception of biodiversity and the acceptance of green space management are involved in the proposed solutions and decisions [16].
GI functions vary depending on the discipline in which the term is applied; according to Murkin et al. [18], its key aspect is the formation of green corridors or networks of green spaces that wind through the urban environment and connect rural and urban areas. The intended role of GI is to provide a range of ESs in both urban and rural contexts [19], namely cultural, regulation, maintenance, and provisioning services. Jerome et al. [20] suggest that among the factors that influence the quality of GI for people, proximity to residential areas and whether the habitats form an ecological network are the most important in defining connectivity between GI.
Sustainability, biodiversity, and low maintenance are the keywords underlying the current trends in landscape design [21]. However, to promote natural management in urban green spaces, determining the types of spontaneous plants that naturally grow there is essential, in terms of functional types and life cycles. Owing to high population density and resource demands, cities represent a critical scenario in the search for sustainable development solutions that meet the needs of society without overexploiting resources [1]. Despite biodiversity in urban landscapes, as well as ornamental urban plants, having previously been investigated, the interaction between these two topics has not yet been the focus of scientific studies, and the scientific literature is definitively scarcely reviewed. By exploring the relationship between biodiversity and sustainability in the unique conditions featured by urban landscapes, we come closer to achieving the United Nations’ global sustainable development goals [22].
The socio-ecological approach to environmental management paves the way for greater incorporation of biodiversity into urban planning and sustainability, while also considering cultural attitudes towards urban ecosystems [23].
To ensure adequate access to ESs of the urban forest, the 3–30–300 rule of thumb has been proposed, which means that everyone should see at least three trees from their home, 30 percent tree cover should be guaranteed in all neighborhoods, and all residents should have a park within 300 m of where they live [24].
Future research and planning in nature conservation and sustainable development of urban industrial areas should fully account for biological diversity in cities [25]. With the progression of climate change becoming more concerning [26], numerous studies have investigated the capacity of green spaces or infrastructure to modify the urban environment so that the effects of climate change can be mitigated [18].
Although ornamental plants are widely used in UGI, little is known about the contribution that they can offer in terms of biodiversity while maintaining their contributions as ornamental pieces, and above all, this contribution is not often considered. However, what an “ornamental plant” is should be adequately defined in relation to increased ecological awareness. While the risk associated with the spread of exotic ornamental plants, also in an urban context, is widely recognized and studied [27], the positive role of ornamental plants and how to improve this role deserves more attention. In this context, this review aims to analyze the available knowledge on the contribution of ornamental plants to urban biodiversity and to critically analyze the gaps that must be filled to better understand how to increase urban biodiversity and the use of ornamental plants. Gathering these data can be helpful for different types of end users, such as those involved in basic and applied research, as well as urban green area planning and management.
2. Methodology and Literature Research
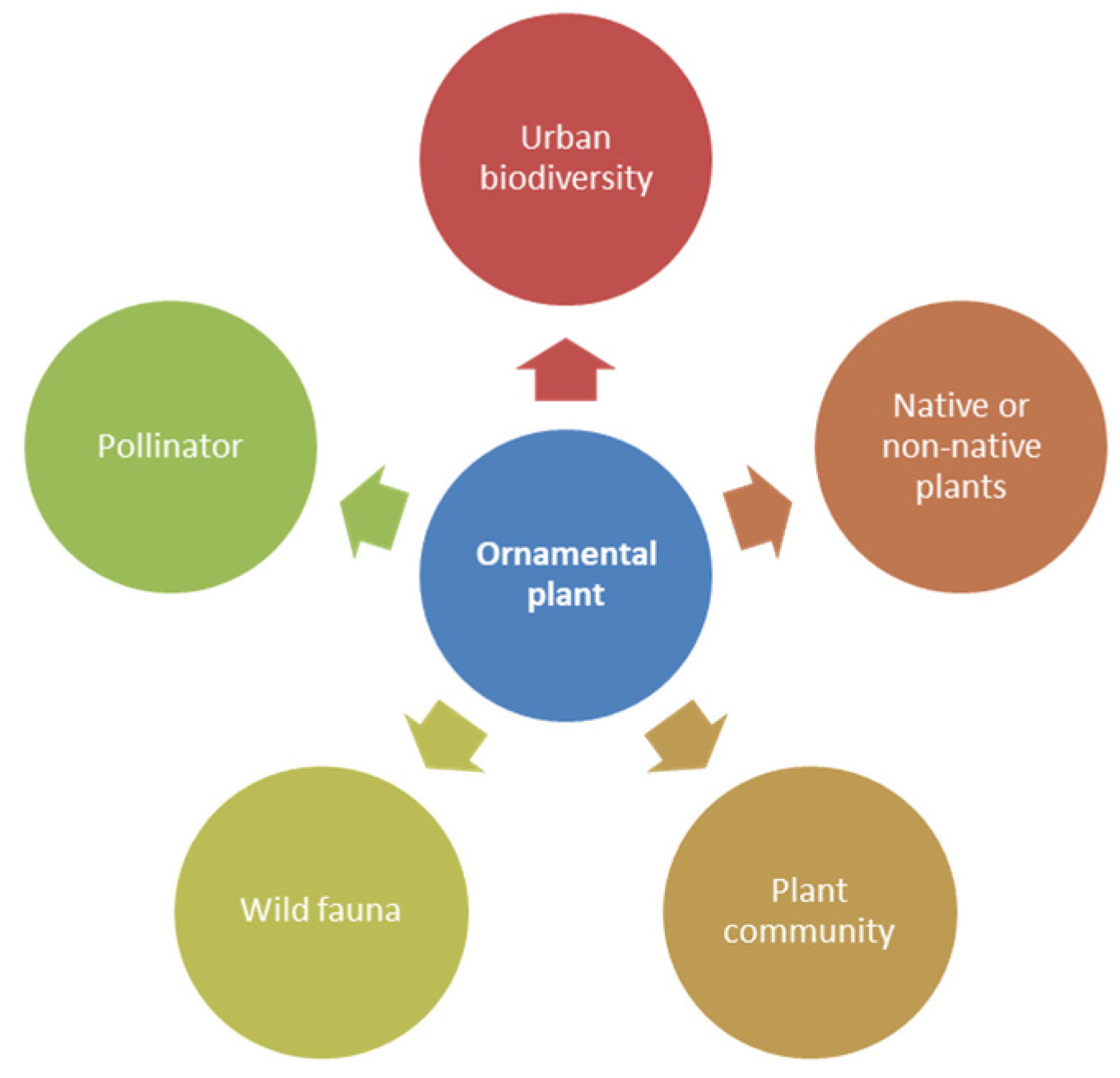
The aim of this literature review was to investigate the contribution of ornamental plants to biodiversity in urban areas, trying to quantify their role and to understand whether, and to what extent, the choice of species and particular cultivation practices could increase biodiversity. To this end, a thematic approach was adopted to identify and select the relevant literature. The search was conducted in Web of Science, Scopus, and Google Scholar. In particular, a deductive approach was used to collate previous research, organizing the results according to the following themes: (1) definitions of ornamental plants; (2) the relationship between biodiversity and urban environments; (3) the contribution of native and or non-native plants; (4) the role of plant communities; (5) the effects of ornamental plants on the diversity of wild fauna; and (6) the relationships among ornamental plants, pollinators, and other type of wild fauna. The main criterion was the functions of ornamental plants (Figure 1).
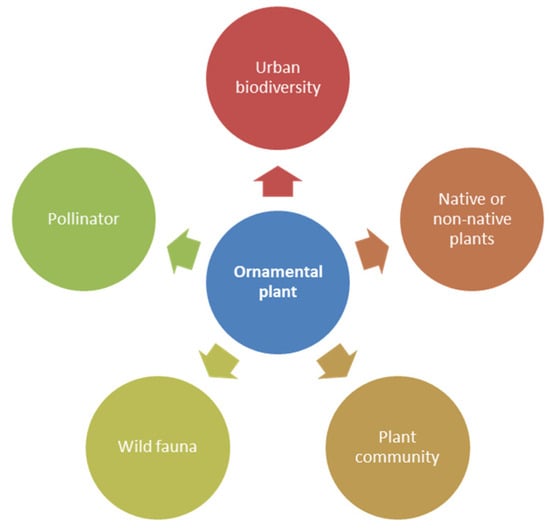
Figure 1.
Predetermined themes used for this literature review.
Considering the multifunctional meanings of “ornamental”, linked not only to the esthetic showiness of plants but also to their environmental functions [28], the keywords that were used included “ornamental plants”, “urban ecosystem”, “ecosystem services”, “biodiversity”, “native” and “non-native” plants, “plant communities”, “pollinators”, “flowering meadows”, and “trees”. The search terms were always used with the Boolean operator “and”, between the searched terms and ornamental plant, as we wanted to strictly associate the various terms with the object of our attention. The inclusion criteria for the articles were (1) publications written in English or with at least an abstract written in English, and (2) scientific articles, books, and political documents. The results were used to understand the contribution of ornamental plants and to formulate suggestions on strategies to enhance biodiversity in urban areas.
3. The Link Between the Biodiversity and Functioning of Urban Ecosystems
A possible link exists between biodiversity, green areas, and increased sustainability, which is also enshrined in legislation in several countries. For example, in Indonesia, the government ratified Law No. 26/2007 as a commitment to sustainability; this law ensures that cities must provide open green spaces covering at least 30% of urbanized areas [29]. From a conservation perspective, interestingly, biodiversity plays an important role in human survival; however, questions remain regarding how and under what circumstances biodiversity contributes to sustainability. According to Behm [22], the mechanistic links between biodiversity and sustainability still need to be explored, particularly in urban landscapes.
Due to unsustainable consumption of resources, it is believed that we are facing the sixth mass extinction on Earth [30]. According to Behm [22], biodiversity influences the functioning of the ecosystem, and ESs increase the sustainability of the landscape.
The sustainability of a landscape is based on its capacity to provide ESs and is influenced by the biological, physical, and social components that make up the landscape itself [31]. The positive correlations commonly observed between biodiversity (measured as species richness) and ecosystem functioning are explained via several mechanisms, including niche complementarity, selection effect, and functional redundancy [32]. Species-rich assemblages can stabilize ecosystem functions in the face of disturbances that cause species extinction. Most experimental studies on biodiversity and ecosystem functioning have been conducted in natural and agricultural landscapes [33] and therefore may have limited transferability to urban landscapes [34]. In other cases, similar studies failed to include the effects of cultural services [35].
Urban environments host, in addition to native ornamental species, non-native ornamental and generalist species that can tolerate and better exploit urban conditions [22]. Non-native ornamental species with different levels of ecosystem functioning could alter “natural” patterns of functional redundancy and influence ecosystem resilience [22]. The predominance of generalist species could cause greater functional redundancy than in rural landscapes (natural and agricultural). Non-native and ornamental species could influence the capacity for niche complementarity, since plant communities are strongly influenced by humans and do not always give rise to assemblages of species with a shared co-evolutionary history; niche complementarity may be less likely to be a mechanism that maintains ecosystem functioning [36].
In cities, seed wind dispersal may be reduced, as buildings stop wind, zoochory can be affected by the limited presence of animals [37], and species turnover could occur at very different rates compared with that in rural landscapes [38]. Urban landscapes also exhibit higher spatial heterogeneity in species composition than rural landscapes, especially when considering human-managed GI [22]. On the other hand, the demand for ESs is higher in urban landscapes than in rural ones because of the high population density. The urban population also places great importance on the regulation of ESs directly related to quality of life (e.g., pollution control, heat reduction, etc.) [39]. Furthermore, some ESs related to the cultural sphere, such as improved well-being, better mental health, and reduced petty crime [40,41], are not very evident in rural areas, so not much experimental evidence exists on how and if biodiversity can support these ESs [22].
Ecosystem Services Related to Biodiversity
Biodiversity is a service provided by ecosystems that simultaneously increases the level of ES and is an essential component of nature-based solutions (NbS). Biodiversity contributes to human security, resilience, health, and freedom of choice and action [42]. Biodiversity and ecosystem functioning are linked [43], as demonstrated for natural and urban systems [44]. Therefore, the provision of ES and the ability to promote the health and well-being of citizens from a one-health perspective depend on urban biodiversity.
Given the strong human influence on urban green spaces, understanding the natural and human-controlled processes that alter urban biodiversity is essential for its conservation and the provision of associated services [45]. Soil is a central element of urban green spaces, their diversity and functioning [44]. Urban soil consists of various types, with human influence being the primary factor in their genesis [46].
The interaction between soil and plants is relevant because plant communities influence soil functioning through biogeochemical cycles [47]. Urban soils and plants are primary contributors to essential ESs, such as biodiversity maintenance, air quality, flood mitigation, climate regulation, and food production [48]. Urban soil properties may be altered by anthropogenic disturbance, which can lead to discontinuous ground cover and, ultimately, a deterioration of soil quality, characterized by a decrease in nutrients and soil fauna and an increase in mineralization processes [49].
Analyzing the relationship between biodiversity and ESs in Mediterranean cities, Molina et al. [46] noted that plant communities make good descriptors of urban habitats and indicators of ecosystem functions and services. The degree of soil disturbance strongly influences plant communities and the ESs they provide. To achieve better ecological services, such as biodiversity conservation, soil carbon storage, soil biota, nutrient cycling, and water regulation, urban green spaces should be managed, and spontaneous vegetation should be maintained without soil compaction. In this regard, perennial herbaceous plants are related to a higher carbon content in soil and a rapid and high demand for nutrients [46]. GI design, management, and accessibility can synergically be drivers of biodiversity improvement in urban NbSs (Figure 2).
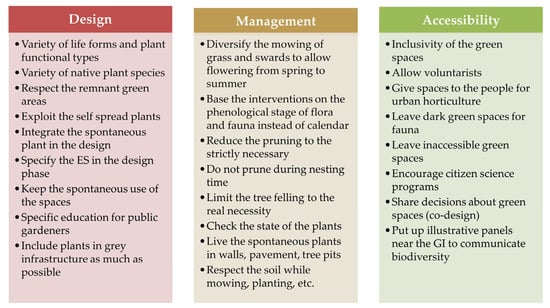
Figure 2.
How to implement NbSs to improve biodiversity in cities; people’s involvement is part of NbS implementation, and people need to accept a naturalistic level of maintenance.
The concept of ES emphasizes the value of ecological functions, which often provide direct benefits to human populations in terms of physical health as well as economic and social benefits [50]. Urban greenery, as a major provider of ESs, consists of different green elements in cities, e.g., individual trees in streets and gardens, tree canopies of different layers, lawns and grasses, bushes and shrubs, flower beds, and ornamental plant arrangements. These “basic units” are part of multi-structural green areas, e.g., green corridors; parks and gardens; wild nature spaces; urban forests and community woodlands; cemeteries; allotments; playgrounds; brownfields; derelict and despoiled land; or, to a lesser extent, built-up urban structural units (green roofs and walls).
A biodiversity-related psychological benefit threshold has been observed, suggesting an innate genetic affinity for high biodiversity. Meng et al. [51] emphasize the benefits of stress recovery due to high-value ecological environments, attesting to a close connection between human and ecological health. Interestingly, research has demonstrated that visitors’ perception of and satisfaction with urban green spaces may be linked to biodiversity. In fact, citizens tend to enjoy immersing themselves in more biodiverse urban green spaces [52], although their overall perception may also be influenced by their attitudes toward nature [46].
Ren et al. [53], analyzing the city of Qingdao as a case study, evaluated the relationship between plant diversity and biogenic volatile organic compound (BVOC) emissions from urban green spaces, trying to find a solution to increase biodiversity and reduce BVOC emissions at the same time. Their investigation found that the species and phylogenetic diversity of urban trees were 22% and 16% lower, respectively, than those in rural forests in this region and that urban areas had a higher BVOC emission intensity than their rural surroundings. By introducing 11 selected tree species, species and phylogenetic diversity were expected to increase by 15% and 11%, respectively, and by introducing selected low-BVOC emitting tree species, 34% of these emissions could be reduced [53]. Moreover, biodiverse herbaceous assemblages are more effective at capturing pollutants than mowed meadows [54].
Weiskopf et al. [55] reviewed studies that examined the relationship between biodiversity and ecosystem functioning in urban areas. Based on 70 studies, such relationships are more positive than negative in urban areas, especially for pollination, nutrient cycling, and retention. Surprisingly, the positive and negative relationships between biodiversity and biomass production and storage were not statistically different, perhaps because of the extensive management of plants in urban areas. However, the number of studies and geographical coverage of this review demonstrated that studies are still insufficient to provide a general predictive framework for the positive influence that biodiversity has on ecosystem functioning [55].
4. Green Infrastructure in Urban Environments
4.1. GI Characteristics
More than 50% of the world’s population currently lives in urban areas, defined as areas with a population of 10,000 or more, and this share is expected to reach nearly 70% by 2050 [56]. In Europe alone, urban ecosystems increased by 7.1% between 2000 and 2018 [57]. Cities also face complex sustainability challenges, such as climate change, population growth, and declining human well-being.
Urban environments are complex and highly modified socio-ecological systems with a wide range of ecological and social factors. Cities are characterized by high populations, alien species, pollution, high temperatures, and impervious surfaces (e.g., concrete) that influence biodiversity in numerous ways [58]. In this context, urban green spaces, including parks, gardens, abandoned lots, and wastelands, are habitats for numerous wild species and are therefore a major source of biodiversity within cities [59], providing important social and ecological benefits [46].
Although urban green spaces often have a primary non-ecological purpose, such as esthetics or human recreation [60], they can provide a range of environmental and ecological benefits (e.g., improved air quality, noise reduction, increased biodiversity, and heat island reduction) [2], making them multifunctional spaces [61,62]. Research has shown that urban areas are home to many native species and are important for the conservation of threatened species [63], reinforcing the role of cities as suitable habitats for nature conservation. Urban ecosystems represent nature that most people encounter regularly, as most people live in cities, which gives green spaces special importance for human well-being and biodiversity conservation [64]. Urban green areas can also provide great opportunities for ecological and environmental education [65]. For most people, their only real encounter with diverse lifeforms occurs in their own backyard or suburban neighborhood. Thus, engaging children and adults in learning about the species that surround them and the role they play in the ecosystem is important [66].
4.2. Contribution of GI to Biodiversity
The recognition of the social value of urban ecosystems has led to a greater emphasis on connecting people with nature through conservation in urban areas [67] and an understanding of its central role in global policies on ecosystem restoration (e.g., UN Decade of Ecosystem Restoration https://www.decadeonrestoration.org/generation-restoration-cities, accessed on 12 February 2025). However, the highly fragmented nature of the urban landscape presents barriers to the natural recolonization of restored sites for many species, and active translocations may be needed to overcome the obstacles to their dispersal [68]. While areas of natural or semi-natural vegetation have received more attention, inadequate attention has been paid to the biodiversity provided by informal or unmanaged urban green spaces [69]. Cities display different kinds of green areas according to Heywood [69], with some reported in Figure 3. The fourth group of vegetation cover reported in Figure 3 is the result of different land uses and their intensities of use and maintenance, thus providing different ESs [70].

Figure 3.
Different kinds of green areas in cities.
The fact that cities are mosaics of diverse habitats [71] means that urban biodiversity is relatively high [72]. Specific types of green spaces, in addition to marginal and uncultivated environments, such as urban green alleys [10], cemeteries [73], extensively managed turfs, and riverbanks [69], can contribute to enriching urban biodiversity and serve as refuges for rare plant taxa and endangered species [74].
Urban GI, or the network of green spaces in cities, provides ESs that are important for urban sustainability; for this reason, cities are increasingly redeveloping underused alleys into GI to enhance the capacity for ESs (food provision, habitat for pollinators, regulation of anthropogenic noise, and regulation of air temperature) [10]. An in-depth analysis was conducted on the green alleys of Montreal (Canada), and the results showed that vegetative ground cover is strongly associated with habitat for pollinators, thus highlighting its role as an indicator of this service. However, no correlations were found between the vegetation in the alleys and the air temperature or the anthropogenic noise, unlike the results from previous studies [75,76], suggesting an opportunity for further research [10].
Green roofs and living walls are NbSs that can offer a valid contribution to biodiversity, as they become additional spaces for plants (novel ecosystems). Their different climatic conditions from those in urban parks can cause shifts in flowering periods, which can extend the season during which pollinating insects visit, which is a valuable time. Vegetation rich in life forms (forbs, graminoids, and succulents) can also improve the ESs of green roofs in terms of climate mitigation, reduction in gas emissions [77], and biodiversity [78]. Vegetation survival, diversity, size, and flowering are also influenced by substrate depth [79].
Among GI, green roofs can improve urban biodiversity as they host diverse vegetation structures, offering more opportunities for foraging and support for animals, and increasing the connectivity between urban and rural habitats. To improve such services, the density, distribution, and mutual distance between different green roofs should be considered in an integrated manner during urban planning [80].
Due to rapid development in megacities, cemeteries are becoming major land use components in the urban landscape. These green spaces are extremely important for plant diversity, and old urban cemeteries serve as a refuge for rare plant taxa and endangered species. Some threatened plant species that are extinct or critically endangered in adjacent areas are found in relatively undisturbed habitats [73].
The urban environment becomes increasingly important for sustaining biodiversity, which has become seriously threatened by the combined effects of intensive agriculture, agro-technical measures, and pesticide use [45,81]. A significant issue is the extinction and rapid decline of pollinator populations [82], especially of wild bees (Hymenoptera and Apoidea). These insects are regarded as the most effective pollinators, as they are responsible for pollinating nearly 80% of flowering plants [83]. In this context, botanical gardens, parks, and garden squares play vital roles in the conservation of biodiversity [83].
Unmanaged urban green spaces, brownfields, road or railway verges, river edges, and abandoned lots play important roles in biodiversity, as they are spontaneously vegetated and relatively undisturbed. Management practices have been identified as having the most common negative impact on diversity, whereas factors such as vegetation, site age, distance from the city center, and habitat diversity exert positive influences. The maintenance regimes adopted can, therefore, significantly influence biodiversity and its conservation [84].
The floristic composition of green spaces changes from the periphery towards the city center; the richness of non-native species increases as one moves from the rural periphery to the urban core, with approximately 30–50% of plant species in the urban core being non-native, while the number of native species decreases [85]. Similarly, under conditions of low or moderate levels of urban development (e.g., suburbanization), species richness can increase [86]. Increased species numbers in suburban landscapes result from high habitat heterogeneity, high numbers of introduced species, socioeconomic factors, and altered disturbance regimes [87]. On the other hand, Hope et al. [88] reported that species richness in Phoenix, Arizona, a desert city, increases with urbanization due to human influences, such as irrigation and ornamental gardening.
Seitz et al. [89] surveyed plants growing in 18 community gardens in Berlin, Germany, to study the diversity of cultivated and wild plant species. They found that the number of wild and cultivated plant species in gardens was high, especially for wild plant species. This suggests that community gardens may represent important habitats for plant diversity, along with playing a role in urban food provision. Finally, a positive relationship was found between the number of cultivated and wild species, which highlights that community gardens represent a unique urban ecosystem in which land sharing between cultivated and wild flora may occur [89].
5. The Contribution of Ornamental Plants to Biodiversity
5.1. The Definition of Ornamental Plants
Vegetation in urban environments loses its natural characteristics and reflects human preferences [90], including a preference for ornamental plants with high esthetic value, in spite of their origin. Numerous reasons exist for studying biodiversity in urban areas, among which esthetic and ethical considerations must not be overlooked. Humans are inherently drawn to nature and its living organisms; this innate “biophilia”, as Wilson [91] noted, compels us to engage with diverse lifeforms. Being surrounded by plants and animals creates a sense of peace and tranquility [92]. This sense of gratification increases if we surround ourselves with “more beautiful” plants: ornamental plants. The visual characteristics of ornamental plants have a significant beneficial effect on human well-being, such as reducing blood pressure [93]. Biodiversity can enhance the feeling of well-being and a sense of belonging for citizens [94].
Ornamental plants are distinguished by the showiness of their shapes, the color of their leaves/flowers, and their use in gardening and landscaping. Ornamental plants are important living components in public and private gardens, and if chosen appropriately, they can provide important ESs.
Ornamental plants have been defined in various ways. Generally, they are characterized as plants intentionally cultivated for their esthetic qualities, such as the beauty of their form and colors; their foliage, flowers, and fruits; and, when available, their fragrance. These plants are utilized in gardening, landscaping, as well as interior design. The esthetic value of ornamental plants is linked to their ability to please individuals immersed in landscapes adorned with them [95]. Additionally, it is well established that ornamental plants can have positive effects on both human psychological and physiological well-being [96]. Similarly, wild plant communities can be perceived as esthetically pleasing [97]. They also play a significant role in promoting and preserving biodiversity [98] and improving the esthetic attractiveness of both indoor and outdoor environments [99]. Ethnobotany defines the relation between plants and humans; as the use and cultivation of plants has endured the times and remains strong, this relation has been defined as “mutualistic” [100]. However, the preference for plants and thus their ornamentality are related to certain cultures and time periods; for example, while Victorian plant hunters collected exotic plants around the world to fill their huge and decorative greenhouses, William Robinson celebrated the beauty of naturality and wilderness in gardens and John Ruskin considered hybridized plants to be ugly [101]. Additionally, today, ornamental plants face the challenges of providing ESs and, at the same time, adapting to stresses and disturbance, so the criteria should be updated [28].
5.2. Native and Non-Native Ornamental Plants
Healthy urban vegetation contributes significantly to environmental improvement, leading to lower temperatures and the sequestration of pollutants, with a positive impact on human health [2]. One of the reasons for the mistrust of ornamental plants and their low consideration for the provision of ESs is linked to the fact that non-native plants, which may be considered potentially invasive, are often used for ornamental purposes. The ability to colonize new areas is a characteristic of both native and non-native plants, and green lists of non-invasive ornamentals are therefore the subject of studies [102].
The search for traditional forms has often led to the selection of non-native plants for esthetic purposes. This is evident in studies that have analyzed the types of plants used in green spaces, even in tropical and subtropical environments that are home to native plants of significant esthetic value. Thus, da Silva Mouga et al. [103], analyzing plants growing in green spaces in the State of Santa Caterina in southern Brazil, found 201 species of ornamentals, with a slight majority (109 species) of non-native plants. In addition, a survey conducted in the urban area of the Province of Northwest Guanacaste in Costa Rica, a nation that is characterized by the showiness of their native flora, found an equivalence between non-native and native plants [104]. In a biodiversity study conducted in Metro Cebu in the Philippines, approximately 95% of the species were found to be non-native in urban parks, while only 5% were native [105]. In Colombia, a predominance of non-native species was found within 143 spaces in the city of Medellín. Of the 198 species found, 158 are non-native; of these species, 93 are mentioned in the literature as species with a potential risk of invasion, stinging, or toxicity [106].
While on the one hand, ornamental plants are considered essential components of urban landscapes [107], as they improve the esthetic appearance of cities and contribute to ecological balance and ESs, ensuring habitat for other living beings, pollution control, and climate regulation [2], on the other hand, their alien origin is feared. Global horticultural trade has stimulated the introduction of non-native species that sometimes have a significant capacity to adapt and proliferate in new environments, to the point of being classified as “invasive ornamental plants” [108]. Although native plants are recommended because they can adapt to pedoclimatic conditions, present a low risk of invasion, and help conserve genetic resources [109], often in the urban environment, the conditions are clearly different from those of the local natural environment.
If, on the one hand, ornamental plants can support wild biodiversity, on the other hand, they can sometimes be detrimental to other wild organisms. Indeed, a significant portion of the world’s naturalized flora originates from cultivation in domestic and botanical gardens for horticultural purposes [110]. Non-native trees planted as street furniture can escape from cultivation, exhibiting a higher frequency of offspring production, particularly among species originating from similar climates, as seen in a central European city [111]. Similarly, non-woody alien ornamental plants thrive in public green spaces and private gardens. Additional species may establish themselves in the future if climatic conditions become more favorable [112]. The extent to which these species may negatively impact urban biodiversity remains poorly understood, despite documented cases of urban sites threatened by invasive alien plants [113]. Some of these sites are also renowned for their remarkable biodiversity. However, it is well established that certain non-native ornamental species can easily spread into natural environments, resulting in significant negative effects on native biodiversity, either directly, indirectly, or both [114]. Similar scenarios, in which native biodiversity is adversely affected by alien plants, could also occur in urban areas.
When selecting a species, considering its ability to thrive in challenging and disturbed environments, such as urban areas, as well as its tolerance to various stresses, particularly drought, which is increasingly prevalent in Mediterranean regions, along with pollution and disturbance, is essential. Some non-native plants, especially succulents, are well adapted to tolerate water shortages. On the other hand, invasiveness is connected to the plant’s ability to produce numerous dispersal units (seeds, fruits, propagules, pads, and branches) [Kalanchoë × houghtonii D.B.Ward, Cenchrus setaceus (Forssk.) Morrone, Nassella trichotoma (Nees) Hack. ex Arechav.] and to colonize nearby and distant areas, or to generate sprouts [e.g., Ailanthus altissima (Mill.) Swingle; Acacia dealbata Link; Broussonetia papyrifera (L.) L’Hér. ex Vent.; Yucca gloriosa L.].
Some non-native species can provide important resources for native species. For example, in Davis, California, 29 of the 32 native butterflies reproduce on non-native plants [115].
Selecting the plants used in landscape design is a critical point in modern strategies to reduce the economic and ecological costs of managing green spaces and the risks associated with invasions [12].
Approximately 406,700 plant species exist on Earth, of which 85,000 to 99,000 possess ornamental value [116]. Ornamental plants are characterized by the shape and color of their leaves and flowers, as well as their use in gardening and landscaping. To list the ornamental plant species for bees present in the State of Santa Catarina (SC), southern Brazil, a survey of the forage resources registered in the State was conducted. Great botanical diversity was found, demonstrating the possibilities of urban foraging and ornamental characteristics from a sustainability perspective. Many of the ornamental plants characterized by showy flowers and requiring pollinators are essential for the maintenance of pollinators in urban areas [103]. Particularly favored by both wild and domestic bees are species from the families Fabaceae, Asteraceae, Solanaceae, Rosaceae, and Lamiaceae [103]. These families are often among the most prevalent in ornamental greenery [117].
The diversity and abundance of pollinators are directly affected by human activities. Urban environments can offer refugia to pollinators. Poole et al. [118] found that enhancing green public spaces has positive effects on key pollinator groups and can help mitigate the impacts of urbanization. Non-native ornamental plants also play a role in enhancing green spaces for pollinators while maintaining their recreational functions [118]. In this context, effective management is crucial for providing suitable habitats. Therefore, avoiding pruning and mowing during flowering time is essential.
5.3. Ornamental Plants and Biodiversity
To promote sustainability in the urban environment, the biodiversity of the used plants needs to be increased, and plants at the bottom of the food chain need to be identified. Spontaneous flora, which includes plants of high esthetic value, can be grown sustainably and generally show high ecological plasticity and resistance to local biotic and abiotic stress factors [74].
The biodiversity of urban green spaces is important because it can reduce the risks associated with the presence of parasites and diseases, and the extreme conditions caused by global change [119]. Biodiversity can also improve the resilience of the ESs provided by green spaces. The “Santamour rule of thumb” 10/20/30 was proposed for managing and improving biodiversity; according to this rule, UGI should not include more than 10% of a plant species, 20% of a genus, and 30% of a single botanical family [120]. A mixed-planted meadow composed of 14 perennial ornamental flower species, including Iris tectorum Maxim., Iris lactea Pall., and Patrinia scabiosifolia Link, established in a demolition site with sewage-contaminated soil in Beijing, was compared with a meadow established with I. tectorum alone. The mixed-plant meadow produced a visually appealing landscape and dynamic seasonal enrichment, significantly increasing the content of soil total nitrogen (TN) and organic matter (SOM) by 1.99- and 1.21-fold, respectively. TN was positively correlated with the soil microbial α-diversity and community structure. Therefore, the results suggest a better contribution of mixed-plant meadows in restoring degraded urban soils by influencing the soil microbial community and improving the ecological service function [121].
6. Strategies to Increase the Contribution of Ornamental Plants to Biodiversity
6.1. Biodiverse-Friendly Urban Landscape Design
With climate change, which is probably the biggest threat to our planet, we are witnessing unprecedented biodiversity loss and growing challenges to human health. The need to prioritize UGI has never been greater [122]. Urban greening should adopt solutions that meet the requirements of the EU Biodiversity Strategy for 2030, which involves biodiversity enhancement [16].
Globally, biodiversity loss and ecosystem degradation require the formation of new concepts, ecological restoration, and rehabilitation, aiming to improve ecosystem functions, services, and biodiversity conservation in cities. To facilitate the restoration and rehabilitation of new urban ecosystems, established, species-rich, and well-functioning urban ecosystems should be used [123].
To support biodiversity and provide ESs in urban parks, we need “biodiversity-friendly” planning approaches based on the results of specially programmed geobotanical research. This research should incorporate both the perception of biodiversity and acceptance of green space management in the proposed solutions and decisions [16].
In addition to the high taxonomic diversity of plants, the dominant types of functional plants are likely to differ between urban and rural areas [124]. In cities, the effect of urbanization on plant functional types and their traits has been studied predominantly in residual natural areas with wild-growing species [125]; however, how functional groups differ between cultivated and natural areas is less clear. The dominance of specific functional types, such as graminoid species over woody ones, has implications for many ecosystem functions and services [126] but has yet to be fully studied in cultivated urban areas, particularly in those with warmer climates.
Urban plants can be classified as species that grow spontaneously, i.e., species that grow and reproduce without human intervention, and cultivated species that are planted and managed by humans. All plants in these two groups can be native or not and contribute to biodiversity [127,128]. Cultivated species can even escape and grow spontaneously [129]. Although crop species have long been recognized as important components of urban ecosystems [130], many studies of urban plants have focused on residual pockets of uncultivated vegetation, with native species and non-native species that can regenerate naturally [131]. Studies of crop species have focused on residential gardens as important contributors to GI [132] or as a source of invasive species [133]. Few studies have focused on the communities and functional ecology of crop vegetation [128]. Such studies have found that residents’ preferences for particular types of plants are reflected in the types [134] and number of plants present in private yards and neighborhoods [135].
6.2. Urbanization as a Driver of Biodiversity
Urbanization is a global driver of biodiversity. Cities are recognized centers of high plant diversity [14] and host many non-native plant species [136]. The underlying causes of high diversity in cities are numerous [137] and potentially include the presence of weeds [138], the location of cities in areas that were highly diverse before urbanization [139], and the importation of cultivated plants [87].
In urban areas, trees, gardens, and residential courtyards contribute significantly to plant biodiversity. However, the consequences and mechanisms of plant cultivation on biodiversity remain uncertain [140].
Urban green spaces, therefore, contain a unique biodiversity that depends on the choices made and the management of green areas. To increase biodiversity, the use of spontaneous plants could be increased, including plants of high esthetic value, which can be grown in an ecologically compatible way owing to some of their biological and cultural characteristics (ecological plasticity, high resistance, etc.). However, urbanization generally has a negative influence on the diversity of local species [74]. Introducing resilient plant varieties into urban landscapes improves the ecological balance and, simultaneously, the overall quality of urban environments, both for human inhabitants and wild animals [93]. Ecological restoration projects within urban environments seek to increase the number of suitable habitats available within cities, thereby improving the capacity of the urban environment to support native biodiversity [141].
The focus of biodiversity is not only on native species but also on non-native species. Considering opinions on the introduction of non-native plantings, a UK study [142] indicated that over 75% of survey participants favored the introduction of climate-adapted non-native species into public parks and gardens if these species were better suited to future climatic conditions than the species currently in use. The main driver of public acceptance was the need to adapt to climate change, thereby reducing the need for irrigation. Indeed, climate change has already led to entire biomes moving towards higher altitudes as temperatures rise [143]. Plant species commonly utilized in UGI design at specific latitudes, such as Quercus robur L. in the UK, are becoming increasingly unsuitable for their intended purposes. Therefore, exploring other climate zones, such as Greece and other regions of southeastern Europe, has become essential for species such as Quercus cerris L. to create a resilient future environment [142].
As a network of multifunctional parks, green and blue spaces, and features such as green roofs and walls, UGI can provide NbSs to increase biodiversity and mitigate and adapt to climate change [144], while also improving esthetics [145] and recreational opportunities [146] to support human health and well-being [147]. Biodiversity conservation and enhancement are co-benefits that can be easily achieved [79]. The most successful schemes consist of creating different zones that serve different functions, such as recreation areas, biodiversity spots, or sites where the main purpose is to provide esthetically pleasing plant compositions. In the city of Lyon, France, the Division of Green Spaces manages sites throughout the city, such as “natural spaces”, prioritizing biodiversity and limiting public access; “living spaces”, prioritizing public use and recreation; and “flowering spaces”, where managers strive to generate “a wow effect” through vibrant, colorful planting [122,145].
6.3. Factors Increasing Biodiversity
An element that contributes to increasing biodiversity is residents’ income, as urban plant biodiversity generally increases with the income of inhabitants of a given neighborhood [148]. The financial resources needed to invest in garden maintenance and the purchase of purely ornamental species depend on the economic status of individual owners [149].
The design of urban green spaces according to ecological, climatic, and social criteria requires appropriate indicators and evaluation strategies that maintain the multiple benefits that urban green spaces offer [150]. The planning, decision-making, and maintenance of urban green spaces in cities are increasingly based on the ecosystem function assessment approach [151]. Cities play an important role in conserving global biodiversity, particularly through UGI planning and management. However, UGI management is subject to a complex array of social, cultural, and economic factors, including governance, economics, social networks, stakeholders’ individual preferences, and social constraints [152].
Homeowners’ associations and municipalities increasingly require a certain percentage of native trees and shrubs as part of any new landscaping installation. This requirement is linked to the belief that native plants are more suitable than exotic species because of their adaptability to local pedo-climatic conditions and their ability to enhance ecosystem biodiversity. In contrast, non-native trees and shrubs are labeled as harmful to biodiversity, mainly because they are often improperly assimilated, becoming invasive species. In an attempt to establish the veracity of this statement, Chalker-Scott [153] analyzed, in a review, the effects of native and non-native woody species on the stability of the urban landscape, measured by the biodiversity of associated plants, birds, insects, reptiles, and mammals. Most studies have demonstrated that parameters other than species nativity have a greater influence on the biodiversity of these groups. The authors concluded that adopting a more practical and evidence-based approach to enhance the biodiversity of the urban landscape rather than limiting the list of plants to only native trees and shrubs, was a more appropriate strategy [153].
Tartaglia and Aronson [154] compared native and exotic plants and systematically reviewed the literature following the PRISMA guidelines. They tried to understand whether a difference exists between native and non-native plants in supporting faunal biodiversity; in the provision of urban ESs; and in terms of survival, growth, and fitness. A total of 165 articles were analyzed. Of these articles, 120 supported the superiority of native plants across various parameters, 57 demonstrated mixed effects, 56 showed no significant differences related to the origin of the plants, and 26 indicated better performance by non-native plants. Nevertheless, the evidence strongly supports the conclusion that native plants support a greater abundance and diversity of fauna. This review suggests that native plants provide multiple ecosystem functions, proving to be superior to exotic plant species [154].
Green roofs are NbSs that can significantly contribute to biodiversity. In particular, extensive green roofs are challenging environments for plant growth because of their extreme substrate temperatures, high light intensity, limited water availability, and limited substrate depth [155]. These conditions modify the flowering phenology of species, which occurs earlier than that of plants growing on the ground. This earlier flowering period provides a habitat for pollinators at the beginning of the season, presumably due to the availability of resources offered by the plants present on the roof [156]. Non-native plants are visited by pollinators in a similar manner to that of native plants. In contrast, hybrid cultivars are visited less frequently. Shrubs and herbaceous perennials show a high frequency of pollinator visitation compared with other growth forms [156]. Sedums were also found to be visited by wild bees in May on extensive green roofs free of any maintenance intervention [157].
Wild plant resources contribute to biodiversity, especially native wild ornamental plants (WOPs); they express a considerable potential to improve the esthetic attractiveness of landscapes and promote sustainable landscape design practices. The cultivation of WOPs could contribute not only to environmental protection and the preservation of native plant species but also to the advancement of ornamental horticulture. It offers genotypes that are tolerant to abiotic stresses [158] and are suitable for sustainable landscape design [159], as well as reduces the impact of phytochemicals in the nursery industry.
6.4. The Contribution of Plant Communities and Associations
Urban areas are typically characterized by a reduced presence of wild species and vegetation communities, primarily due to ecosystem degradation [160]. Despite this unfavorable situation, enhancing urban green spaces to increase their contribution to biodiversity shows significant potential. Cities can maintain natural areas in their immediate surroundings and within their centers in certain cases, which can help to conserve biodiverse habitats and protect rare species [161]. Some of these species may possess ornamental potential [162]. Together with the habitats in which they thrive, they can serve as destinations for urban ecotourism [163]. Other semi-natural urban green spaces, often called informal green spaces (IGSs), may present esthetic value for cities [164,165]. Furthermore, these areas are recognized for their rich biodiversity [164], particularly when compared with other parts of the city. In urban IGSs hosting wild vegetation with the presence of trees, at the taxa level, bats, native birds, beetles, and bugs occupancy were positively correlated with understory volume [166].
Two significant ecological weaknesses of urban areas are the loss of vegetated surfaces and high fragmentation of green spaces [167]. These issues have serious negative effects on urban biodiversity [168], particularly affecting animal movement [169]. Therefore, it is evident that implementing new green corridors in urban areas, which may also incorporate ornamental species, improves the connectivity between green spaces. This enhancement facilitates wildlife movement and allows for colonization of new areas. From an urban decor perspective, corridors that enhance connections between fragmented green spaces also contribute esthetic value to the city itself [170]. The role of ornamental plants in enhancing connectivity is evident in some animal species. Research has demonstrated that private gardens can help to reduce habitat fragmentation [171]. Additionally, their ability to host variegated biodiversity has been assessed by the engagement of citizens [172]. Fragmentation can be mitigated through careful urban planning, which enhances or preserves connectivity between green patches. Using the least-cost path method, Kong et al. [173] identified potential corridors in a large urban area in China, leading to the development of a green network based on graph theory and gravity modeling. In addition to ecological corridors, connectivity between green spaces can be enhanced using stepping stones, which can incorporate ornamental plants. In theory, every green space that is not part of a continuous ecological corridor can function as a stepping stone for urban connectivity.
Avifauna in urban areas is an important indicator of environmental health, and it is evident that the availability of food, sheltering, and nesting sites plays a crucial role in supporting the presence of birds in these contexts and in influencing their composition [174]. Ornamental plants, both esthetically pleasing and species that create communities of esthetic value, play significant roles in attracting birds to urban environments [175]. Due to the heavy effect of urbanization, the few bird species that tolerate the conditions that are experienced in cities fall into two categories: “urban exploiters” and “suburban adapters” [176]. The distribution of these animals in cities exhibits a heterogeneous pattern that depends on various factors that are largely independent of plant presence and primarily related to urban density. Indeed, an increase in urban avian richness has been observed along a gradient from the most to the least urbanized areas [177]. Similarly, a significantly lower species density was identified when urbanized areas were compared with non-urban areas [131]. However, it is also possible to implement strategies to enhance the presence of avifauna in cities. As expected, research has demonstrated that bird richness is positively correlated with the size of urban green spaces [178]. Thus, vegetated patches, which act as stepping stones, can enhance the presence of birds in urban contexts. The availability of nesting sites is one of the major factors influencing the presence of birds in cities. Trees, either wild or planted, can serve this purpose [179]. Street trees alone contributed positively to bird numbers, as seen in the American continent [180], particularly when native species and tree species richness were higher. In contrast, cultivated lawns, squares, and gardens are frequently mostly composed of generalist species [180]. Depending on the bird groups, a nesting preference on ornamental plants has been observed with passerines favoring Melia azedarach L. and Hibiscus syriacus L. in Valls (Catalonia, Spain) [181]. In an urban park in China, more bird species were observed in areas with shrubs than in those with trees or lawns. Moreover, the richness, diversity, and density of woody plant species have been proven to be beneficial for bird communities, suggesting that urban green spaces should be designed to prioritize the highest possible plant species diversity and complexity [182]. Birds that are frequent in urban areas exhibit a diverse diet that may include insects, nectar, fruits, and grains; they may also consume fish or adopt an omnivorous diet. Ornamental trees, either native or non-native, can provide foraging opportunities for birds [183]. Additionally, those that produce suitable flowers can provide nourishment for nectarivorous birds [184]. As shrubs positively affect insects, they can indirectly increase the presence of insectivorous birds in urban areas [185]. The establishment of both green roofs and walls has been shown to enhance the presence of birds, which utilize these structures for feeding, shelter, and nesting [186]. In Los Angeles, a higher number of birds were observed to feed on small private yards planted with native ornamental species than on those planted with non-native species and managed as turf [187]. The planning of private and public gardens has been proven to be a significant factor influencing urban bird diversity in North America. Key elements include plant diversity, the use of native plants instead of non-native plants, connectivity to other green spaces, and nearby natural habitats [188].
In addition to birds, certain species of Chiroptera also inhabit urban areas [189] where trees can provide essential roosting sites [190]. Both native and non-native fruit trees, whether wild or planted, can serve as significant food sources for fruit bats [191]. Similarly to the case for birds, enhancing the presence of insects through specific urban vegetation and maintaining trees in open green spaces can indirectly promote the presence of insectivorous bats [166,192]. Interestingly, a positive relationship has also been observed between green roofs and insectivorous bats in Europe, particularly when suitable habitats are available nearby [186].
A series of complex interactions has evolved between plants and various groups of insects. Plants can provide numerous services to insects that can either be beneficial to plants or act as pests for both plants and humans. Cultivated ornamental plants have been shown to attract pollinators in urban environments. Marquardt et al. [193] observed in a German city that bees were the most abundant visitors to flower beds. They also noted significant differences in the pollinators visiting the plants depending on the species and cultivars. Similar results were obtained by Palmersheim et al. [194]; however, interestingly, they discovered that insects were more attracted to wild native species and perennials than to annuals. In another study, slightly different results emerged, with honeybees showing no preference among the plant groups, whereas herbivores favored wild species [195]. Conversely, no significant increase in beneficial insect richness was observed following the addition of wild species to urban gardens in New York City [196]. In addition to introducing flowering plants that attract insects, the design of urban green spaces may play a crucial role in enhancing the presence of these animals in cities. These considerations have been thoroughly reviewed by Schueller et al. [197]. In the case of pollinators, a key factor to consider is flowering time. As expected, a longer flowering period increased the number of pollinators that could benefit from pollen and nectar. Native and non-native plant species, which also possess ornamental potential, can provide progressive blooming throughout the year. Several key factors can either enhance or decrease insect presence in green spaces, including the frequency of mowing, management practices (such as the application of herbicides and pesticides, and pruning), density of flowering plants, spatial arrangement of flowers, layout of plants within the green space, availability of nesting sites, connectivity between green spaces and surrounding natural habitats, and overall size of the green space. Additionally, cicadas, which are relatively common insects in urban areas, significantly contribute to the summer soundscape; their population density is primarily influenced by the availability of host plants [198]. Ornamental plants and crops can unintentionally serve as vectors for pest introduction in urban areas [199]. This has occurred, among others, with Buxus sempervirens L., some alien ornamental palms from Argentina, and Phoenix spp. with their respective pests: Cydalima perspectalis (Walker, 1859), Paysandisia archon (Burmeister, 1880), and Rhynchophorus ferrugineus (Olivier, 1790). These pests can pose a significant threat to both cultivated and native plants. It is important to note that regarding perceptions of insects in urban areas, people often exhibit mixed feelings towards these organisms. Indeed, it has been hypothesized that urbanization and the resulting loss of knowledge about natural history have contributed to an increased aversion to insects [200]. However, positive sentiments towards insects have also emerged from surveys, particularly among gardeners [201]. Overall, insects can undeniably provide ecological disservices, considering the range of beneficial ecosystem services they provide is equally important [202].
Woody plant species can support epiphytic organisms such as lichens and bryophytes at various heights. In addition to environmental factors, tree species are crucial for the establishment of epiphytic communities [203]. Urban trees contribute to various attributes, including canopy cover, temperature regulation, moisture retention, bark roughness, and bark acidity, which create suitable conditions for the colonization of these epiphytes [204].
Once ecological corridors and stepping stones are integrated into the city perimeter, the need for effective management becomes essential to maximize their biodiversity benefits. Examples of these best practices have been reviewed by Gregory et al. [205].
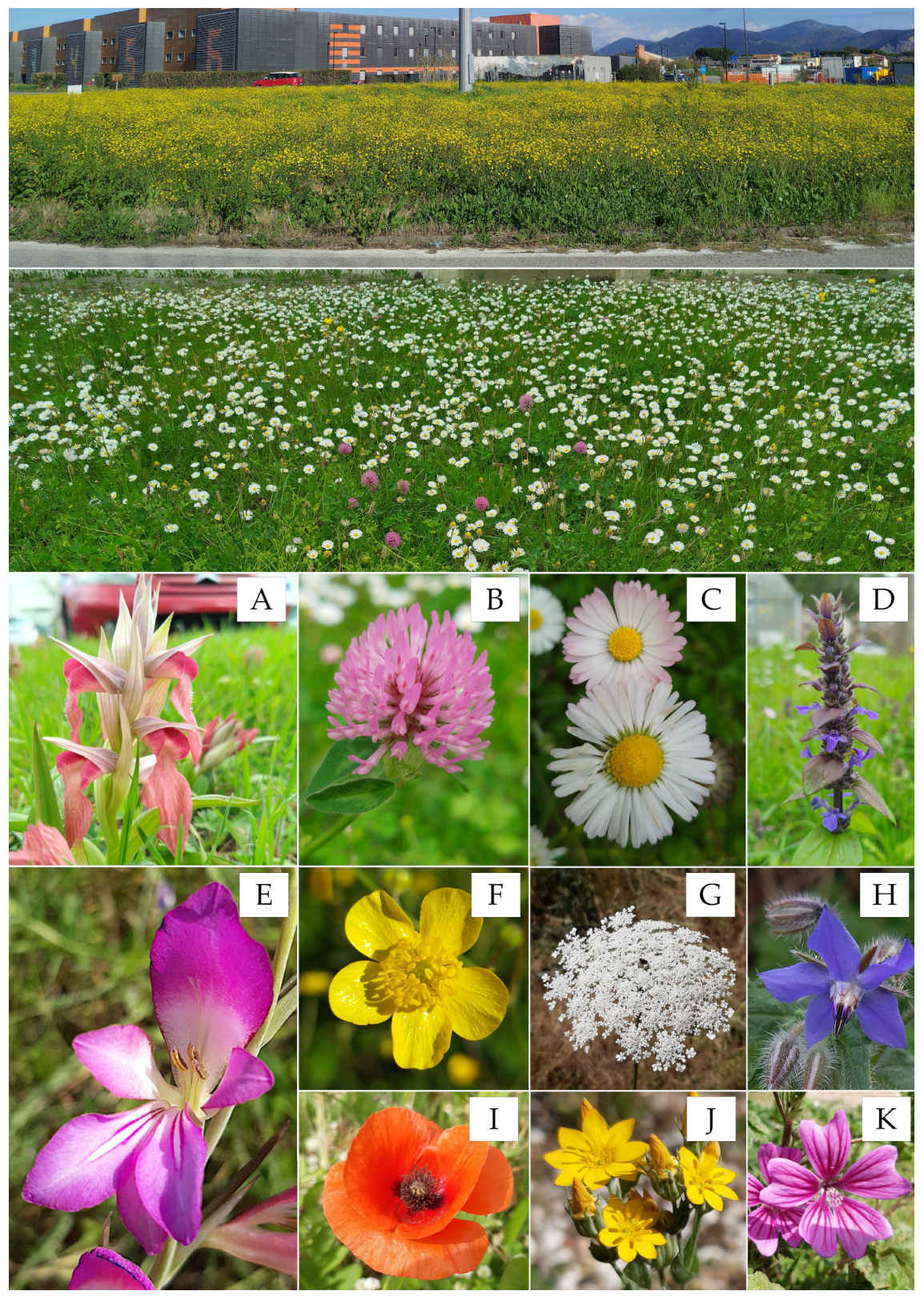
Species-rich herbaceous communities such as grasslands, steppes, meadows, and pastures have high biodiversity value (Figure 4).
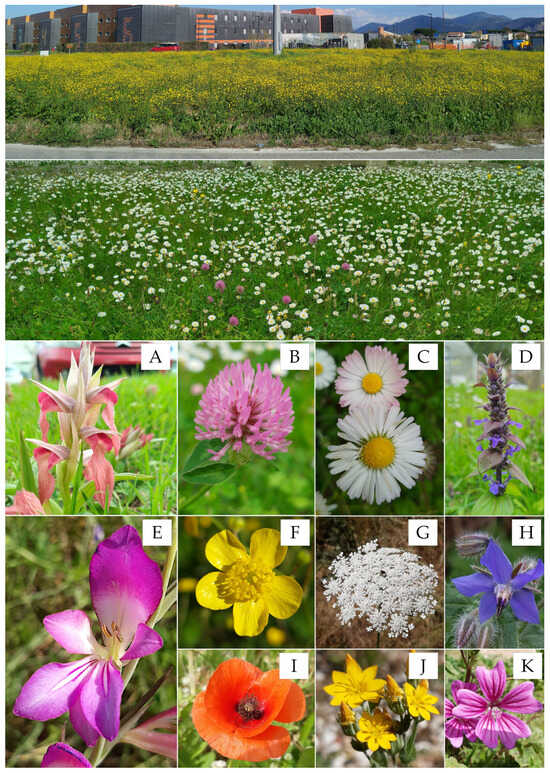
Figure 4.
Two types of wild meadows are common in Mediterranean urban areas, featuring plants visited by pollinators. Roundabout totally colonized by two species of buttercups in full bloom, and the meadow dominated by common daisies. The bottom images show some common species in Mediterranean urban meadows that attract pollinators. From top to bottom and from left to right: (A) Serapias neglecta De Not.; (B) Trifolium pratense L. subsp. pratense; (C) Bellis perennis L.; (D) Ajuga reptans L.; (E) Gladiolus italicus Mill.; (F) Ranunculus velutinus Ten.; (G) Daucus carota L. subsp. carota; (H) Borago officinalis L.; (I) Papaver rhoeas L.; (J) Blackstonia acuminata (W.D.J.Koch & Ziz) Domin; (K) Malva sylvestris L. Photos by VL.
There is considerable interest stemming from the loss of these complex ecosystems and their associated biodiversity due to intensive agriculture, abandonment of pastures, pollution, and climate change. These habitats can be used as models, in terms of landscape management and plant community composition, which can be successfully repeated in anthropized areas to mitigate the negative effects of human activities in the city and improve the biotic components. The idea is to revegetate degraded urban soil with esthetically pleasing wildflower meadows while increasing biodiversity, creating a habitat, and conserving the local flora at low management costs [206].
Benvenuti [207] conducted a survey to evaluate whether a rural landscape could adapt to an urban environment through the use of wildflowers on green roofs. As expected, each group of plants had different flowering periods, during which the highest rates of pollinator visits were observed (domestic and solitary bees, bumblebees, Lepidoptera, both hoverflies and bumblebees, and Diptera). Agronomic management, which consists of mowing senescing vegetation, is of fundamental importance in ensuring the balance between planting spring and summer flowering species. The lack of prevalent species has been highlighted using several biodiversity indices. In contrast, early and late flowering species (geophytes) did not require vegetation management. Although wildflowers present critical aspects in terms of canopy dynamics, due to the periodic senescence of vegetation, they were found to be a valid tool for improving the biodiversity and landscape of the Mediterranean urban ecosystem.
The effectiveness of wildflower meadows in increasing biodiversity is related to the composition of the seed mix and its adaptation to local conditions, which depends on the number of species, flower abundance, plant species diversity, and vegetation structure [208]. The use of a wide range of species in urban wildflower meadows with different life forms and survival strategies promotes biodiversity and improves the establishment capabilities of the plant community [206]. However, the best approach is to choose species that grow wild in spontaneous associations, as they have adapted to coexist using a habitat template approach [77]. Native species are recommended because they have adapted to specific climatic and soil conditions, minimizing the risk of invasion and helping maintain genetic diversity [109]. Plant species selection should consider the species’ ability to survive in stressful and/or disturbed environments, the long flowering period afforded by the species mix, different plant functional types and life forms [205], germination characteristics [209], and the establishment performance of the plant species [210]. Adopting seed mixes of annual, biennial, and perennial species offers the benefits of different life strategies. Using species with different flowering periods prolongs the enjoyment that people derive from them and the resources available to pollinators [79,211]. Research on wildflowers has also focused on increasing pollinators and bees in agroecosystems [212]. Some of the native species listed in studies on seed production for pasture restoration [213] can be used in wildflower meadows in urban areas, although the soil and climate conditions are different because of the extreme temperatures of cities under the effects of climate change. Planting meadows in human-generated landscapes, whether they are urban or rural areas, compensates for the loss of habitat for pollinators and the food web, provides opportunities for the conservation of many plant species, and offers the opportunity to enjoy natural and citizen science programs. Meadow vegetation provides many ESs that are now more necessary than ever for a healthy life on the planet. The methods used to establish wildflower meadows have been extensively studied in Northern Europe and America and, more recently, in different climatic regions, such as the Mediterranean [71].
Suitable management can promote grassland biodiversity. To investigate this aspect, an experiment has been conducted since 2010 to compare three models of urban grassland management: ornamental grassland, urban grassland, and permaculture. The results showed that human decisions and activities influenced species composition, and 46 plant taxa were found: 12 in ornamental grasslands, 24 in urban grasslands, and 31 in permaculture grasslands. Therefore, the latter appears to be the best in terms of biodiversity and soil moisture content. Increasing grassland management intensity results in lower plant diversity (Figure 5) [214].

Figure 5.
Effects of mowing during the flowering season in urban green spaces with wild species in full bloom. The most represented species are: Coleostephus myconis (L.) Cass. ex Rchb.f., Papaver rhoeas L. and Sulla coronaria (L.) B.H.Choi & H.Ohashi. Photos taken a week apart in May 2023. Photos by VL.
Reducing management intensity allows the plant species richness in urban lawns to increase, as demonstrated in the green spaces at the National Research Council Area of Research in Pisa, where over 180 plant species were recorded with more spaced-out mowings (Table S1). Of those species, the majority are represented by native and entomogamous species, so they offer the right habitat for many pollinators, but management practices should take note of this aspect and stop mowing during the flowering period. Further research should be conducted to determine the number of spontaneously colonizing and potentially ornamental species in urban lawns.
Plant species composition and vegetation cover are often positively associated with the diversity and abundance of fauna. Non-native plants contribute substantially to garden plant diversity, and evidence from some studies has shown that their functional attributes may also benefit other species. In contrast, intensive management practices, including frequent mowing, fertilizer and pesticide application, and landscaping for a more formal and “tidier” garden appearance, have often been associated with reduced biodiversity. However, the findings vary between studies, for example, in relation to the impact of mowing frequency on lawn diversity. Several studies have identified the importance of connectivity between gardens and other GI for species dispersal and ecosystem functioning. Threats to garden biodiversity include the conversion of gardens into impervious surfaces, reductions in plot size, and the replacement of plants [215]. Habitat connectivity is a successful strategy for biodiversity [216].
English et al. [217] analyzed a network of remnant grasslands along a gradient from urban to rural areas of Los Angeles, CA, USA. Across this gradient, α and β diversity patterns were assessed during the growing season. They noted that the α diversity (i.e., species richness) of native plants in remnant grasslands decreased in urban landscapes, mainly due to the loss of native species. However, at intermediate scales in unmanaged parks and green spaces, an increase in β diversity, and thus the difference in species composition between two or more distinct communities, was observed in more urbanized locations, likely due to the uneven dominance of exotic species in urban locations, whereas non-native and native species were common across all plots in rural locations. Conservation that considers α and β diversity can promote a virtuous cycle in which the promotion and protection of biodiversity simultaneously reduce the negative effects of invasion.
6.5. Ornamental Plants and Pollinators
Erickson et al. [218] used statistical modeling analyses to explore the overall and relative attractiveness of cultivars to bee, fly, beetle, wasp, and butterfly taxa and to understand how community and landscape dynamics influence pollinator visitation to ornamental plants. The study reported that (i) annual ornamentals made a limited contribution to ecological community stability, whereas perennial ornamentals did not, and (ii) ornamental cultivars showed significant within- and between-gender variations in pollinator attractiveness. The latter is influenced by numerous factors, including landscape and community context, and the artificial selection of phenotypic flower traits. Ornamental plant taxa used in managed landscapes to increase overall floral resource availability, therefore, provide foraging resources throughout the season and increase habitat connectivity. Furthermore, some ornamental taxa can help to build more resilient and stable ecological communities in human-modified environments.
From this perspective, some ornamental plants can provide long-lasting supplementary foraging resources for generalist pollinator communities [219]. Landscape plant diversity is related to the abundance and diversity of pollinator visitors, and planting schemes should consider cultivar effects, landscape plant diversity, floral phenology, and their contribution to a stable ecological community when choosing plants [218].
In the face of pollinator decline, cities can act as refugia and biodiversity conservation habitats, managing floral resources in public green spaces. Ornamental plants could play an important role in attracting pollinators because they are part of urban floral management. However, specific knowledge on the response to drought is lacking, and whether entomogamous ornamental plants will be suitable pollinators in future climates remains unknown. The main objective of a study conducted by Quinanzoni et al. [220] was to determine the covariation in floral traits of ornamental plant species and the effects of drought on them. Drought affected flowering-related morphological traits more than the other floral traits, with an average decrease of 28% in flower height, 35% in flower area, and 58% in flower number. Ornamental plants appeared attractive to different pollinator morphotypes depending on the number of floral units, nectar sugar concentration, or nectar tube depth, with most visits being made by hymenopterans. These results should encourage green space managers to select urban plants based on their functional characteristics and to adapt their plant choice to climate change [220].
Urban flower-rich habitats have been shown to be particularly valuable for urban pollinators although the ability of ornamental plants to support urban pollinator communities has not been well documented. In one study, flowerbeds in 13 different urban test sites planted with identical sets of ornamental garden plants were compared. Pollinators’ visit patterns were observed during the summer. A total of 10,565 pollinators were recorded over the two years of the trial, with wild bees (>50%, excluding bumblebees) being the most abundant pollinator group. The results showed that the assortment of ornamental plants was visited by many urban pollinators for pollen and nectar and that the abundance and composition of pollinators varied significantly within the tested ornamental plants [193].
Different strategies are used to improve biodiversity through urban meadow management and, hence, the relationship between plants and pollinators (Figure 6).

Figure 6.
How to increase the biodiversity of green areas with urban meadows, adapted from Fekete et al. [221].
7. Conclusions
Biodiversity is a key factor in a well-functioning urban environment. Proper functioning of ecosystems enables cities to adapt to the many challenges related to climate change, pollution, the overpopulation of parasites, and diseases in urban green spaces. Therefore, reducing the risk associated with the presence of parasites and diseases and the extreme conditions influenced by global change becomes extremely important. Biodiversity can also improve the resilience of the ESs provided via green spaces. The different characteristics of GI present in cities are essential for increasing biodiversity. Thus, marginal spaces, which are remnants of nature that have not been disturbed by human intervention or have only been disturbed a little bit, play important roles as refuge areas for threatened species. Additionally, NbSs, such as green facades, roof gardens, rain gardens, and container gardens, can contribute to increasing urban biodiversity.
Ornamental plants are appreciated for their “wow effect”, and often, non-native plants are chosen due to the showiness of their traits and their exoticism. Therefore, we must recognize, without prejudiced attitudes, that these plants can actively contribute to biodiversity in the urban environment and to human well-being. Many of these showy-flowered species are insect-pollinated and contribute significantly to the food chain that underpins biodiversity. The contribution of plants living in communities, association meadows, urban forests, and so on is therefore very relevant to increasing biodiversity.
Selecting ornamental species that are well suited to specific urban pedoclimatic conditions and preserving naturally occurring, visually appealing plant communities are essential factors that can enhance the wild urban biodiversity of green spaces. Management plays a crucial role in the conservation of urban biodiversity via pruning, mowing, and the use of fertilizers and pesticides, with all agronomical practices being drivers of improvements or decreases in biodiversity. Consequently, the data presented in this review may prove valuable to those responsible for planning and managing urban green spaces, as well as to policymakers. Furthermore, given that much still needs to be learned about the relationships between ornamental plants and urban biodiversity, the information reported here may also benefit scholars engaged in basic research in the field of urban ecology.
Attention must be paid, and research should move towards determining biodiversity-friendly practices capable of enhancing the ESs of ornamental plants.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/su17094061/s1. Table S1: List of species spontaneously colonizing the lawns at the National Research Council Area of Research in Pisa, Italy.
Author Contributions
Conceptualization, D.R. and F.B.; methodology, S.T., D.R., V.L., L.L. and F.B.; formal analysis, S.T., D.R., V.L., L.L. and F.B.; writing—original draft preparation, S.T., D.R., V.L., L.L. and F.B.; writing—review and editing, S.T., D.R., V.L. and F.B.; supervision, D.R. and F.B. All authors have read and agreed to the published version of the manuscript.
Funding
Project funded under the National Recovery and Resilience Plan (NRRP), Mission 4 Component 2 Investment 1.4—Call for tender No. 3138 of 16 December 2021, rectified by Decree n.3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union—NextGenerationEU; Award Number: Project code CN_00000033, Concession Decree No. 1034 of 17 June 2022 adopted by the Italian Ministry of University and Research, CUP, B83C22002930006 Project title “National Biodiversity Future Center—NBFC”.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Elmqvist, T.; Andersson, E.; Frantzeskaki, N.; McPhearson, T.; Olsson, P.; Gaffney, O.; Takeuchi, K.; Folke, C. Sustainability and resilience for transformation in the urban century. Nat. Sustain. 2019, 2, 267–273. [Google Scholar] [CrossRef]
- Francini, A.; Romano, D.; Toscano, S.; Ferrante, A. The contribution of ornamental plants to urban ecosystem services. Earth 2022, 3, 1258–1274. [Google Scholar] [CrossRef]
- Vila-Ruiz, C.P.; Meléndez-Ackerman, E.; Santiago-Bartolomei, R.; Garcia-Montiel, D.; Lastra, L.; Figuerola, C.E.; Fumero-Caban, J. Plant species richness and abundance in residential yards across a tropical watershed: Implications for urban sustainability. Ecol. Soc. 2014, 19, 22. [Google Scholar] [CrossRef]
- Secretariat of the Convention on Biological Diversity. Convention on Biological Diversity: Text and Annexes; Secretariat for the Convention on Biological Diversity: Montreal, QC, Canada, 2011; Available online: https://www.cbd.int/doc/legal/cbd-en.pdf (accessed on 17 March 2025).
- Müller, N.; Kamada, M. URBIO: An introduction to the International Network in Urban Biodiversity and Design. Landsc. Ecol. Eng. 2011, 7, 1–8. [Google Scholar] [CrossRef]
- United Nations Environment Programme. State of Finance for Nature in Cities 2024. From Grey to Green: Better Data to Finance Nature in Cities: Nairobi, Kenya. 2024. Available online: https://wedocs.unep.org/handle/20.500.11822/46453;jsessionid=D52D4680F6BAE5FCE38BA496ACEAB771 (accessed on 17 March 2025).
- Maes, J.; Quaglia, A.; Guimarães Pereira, Â.; Tokarski, M.; Zulian, G.; Marando, F.; Schade, S. BiodiverCities: A Roadmap to Enhance the Biodiversity and Green Infrastructure of European Cities by 2030; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar]
- Frantzeskaki, N.; Buchel, S.; Spork, C.; Ludwig, K.; Kok, M.T.J. The multiple roles of ICLEI: Intermediating to innovate urban biodiversity governance. Ecol. Econ. 2019, 164, 106350. [Google Scholar] [CrossRef]
- Hancock, T. Indicators of environmental health in the urban setting. Can. J. Public. Health. 2002, 93, S45–S51. [Google Scholar] [CrossRef]
- Dade, M.C.; Richmond, I.C.; Rieb, J.T.; Crockett, E.T.; Hutt-Taylor, K.; Sinno, S.; Benessaiah, K.; Destrempes, C.; Hamilton, J.; Izadi, F.; et al. Testing a rapid assessment approach for estimating ecosystem services capacity in urban green alleys. Urban For. Urban Green. 2024, 99, 128472. [Google Scholar] [CrossRef]
- Bendiouis, F.; Aboura, R.; Ainad Tabet, M. Characterization of the biodiversity of ornamental flora in the urban perimeter of the city of Tlemcen (Northwest of Algeria). Biodivers. J. 2022, 13, 25–35. [Google Scholar] [CrossRef]
- Mircea, D.M.; Boscaiu, M.; Sestras, R.E.; Sestras, A.F.; Vicente, O. Abiotic stress tolerance and invasive potential of ornamental plants in the Mediterranean area: Implications for sustainable landscaping. Agronomy 2024, 15, 52. [Google Scholar] [CrossRef]
- Beatley, T. Handbook of Biophilic City Planning & Design; Island Press: Washington, DC, USA, 2016; p. 312. [Google Scholar]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Kühn, N. Intentions for the unintentional. Spontaneous vegetation as the basis for innovative planting design in urban areas. J. Landsc. Archit. 2006, 1, 46–53. [Google Scholar] [CrossRef]
- Borysiak, J.; Stępniewska, M. Perception of the vegetation cover pattern promoting biodiversity in urban parks by future greenery managers. Land 2022, 11, 341. [Google Scholar] [CrossRef]
- Breuste, J. The Urban Nature Concept—Of What Urban Green Consists of. In Making Green Cities—Concepts, Challenges and Practice; Breuste, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 16–48. [Google Scholar]
- Murkin, K.; Shiode, N.; Shiode, S.; Kidd, D. Biodiversity and the recreational value of green infrastructure in England. Sustainability 2023, 15, 2915. [Google Scholar] [CrossRef]
- European Union. Green Infrastructure (GI)—Enhancing Europe’s Natural Capital. 2013. Available online: https://www.eea.europa.eu/policy-documents/green-infrastructure-gi-2014-enhancing (accessed on 10 February 2025).
- Jerome, G.; Sinnett, D.; Burgess, S.; Calvert, T.; Mortlock, R. A framework for assessing the quality of green infrastructure in the built environment in the UK. Urban For. Urban Green. 2019, 40, 174–182. [Google Scholar] [CrossRef]
- Ochoa, J.; Muñoz, M.; Vicente, M.J.; Martínez-Sánchez, J.J.; Franco, J.A. Native ornamental species for urban landscaping and xero-gardening in semi-arid environments. Acta Hortic. 2010, 881, 425–428. [Google Scholar] [CrossRef]
- Behm, J.E. Is biodiversity needed for sustainability? A spotlight on urban landscapes. Am. J. Bot. 2020, 107, 703–706. [Google Scholar] [CrossRef]
- Hassall, C. The ecology and biodiversity of urban ponds. Wiley Interdiscip. Rev. Water 2014, 1, 187–206. [Google Scholar] [CrossRef]
- Konijnendijk, C.C. Evidence-based guidelines for greener, healthier, more resilient neighbourhoods: Introducing the 3–30–300 rule. J. For. Res. 2023, 34, 821–830. [Google Scholar] [CrossRef]
- Bush, J.; Doyon, A. Building urban resilience with nature-based solutions: How can urban planning contribute? Cities 2019, 95, 102483. [Google Scholar] [CrossRef]
- Zittis, G.; Almazroui, M.; Alpert, P.; Ciais, P.; Cramer, W.; Dahdal, Y.; Fnais, M.; Francis, D.; Hadjinicolaou, P.; Howari, F.; et al. Climate change and weather extremes in the Eastern Mediterranean and Middle East. Rev. Geophys. 2022, 60, e2021RG000762. [Google Scholar] [CrossRef]
- Kowarik, I. 7 Urban Ornamentals Escaped. In Crop Ferality and Volunteerism; Gressel, J., Ed.; CRC Press Taylor & Francis: Boca Raton, FL, USA, 2005; pp. 97–121. [Google Scholar]
- Savé, R. What is stress and how to deal with it in ornamental plants? Acta Hortic. 2009, 813, 241–254. [Google Scholar] [CrossRef]
- Arifin, H.S.; Nakagoshi, N. Landscape ecology and urban biodiversity in tropical Indonesian cities. Landsc. Ecol. Eng. 2011, 7, 33–43. [Google Scholar] [CrossRef]
- Turvey, S.T.; Crees, J.J. Extinction in the Anthropocene. Curr. Biol. 2019, 29, R982–R986. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. 2013. Landscape sustainability science: Ecosystem services and human well-being in changing landscapes. Landsc. Ecol. 2013, 28, 999–1023. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin Iii, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- van der Plas, F.; Allan, E.; Fischer, M.; Alt, F.; Arndt, H.; Binkenstein, J.; Blaser, S.; Blüthgen, N.; Böhm, S.; Hölzel, N.; et al. Towards the development of general rules describing landscape heterogeneity–multifunctionality relationships. J. Appl. Ecol. 2019, 56, 168–179. [Google Scholar] [CrossRef]
- Schwarz, N.; Moretti, M.; Bugalho, M.N.; Davies, Z.G.; Haase, D.; Hack, J.; Hof, A.; Melero, Y.; Pett, T.J.; Knapp, S. Understanding biodiversity-ecosystem service relationships in urban areas: A comprehensive literature review. Ecosyst. Serv. 2017, 27, 161–171. [Google Scholar] [CrossRef]
- Ziter, C. The biodiversity–ecosystem service relationship in urban areas: A quantitative review. Oikos 2016, 125, 761–768. [Google Scholar] [CrossRef]
- Flombaum, P.; Aragón, R.; Chaneton, E.J. A role for the sampling effect in invaded ecosystems. Oikos 2017, 126, 1229–1232. [Google Scholar] [CrossRef]
- Gelmi-Candusso, T.A.; Hämäläinen, A.M. Seeds and the city: The interdependence of zoochory and ecosystem dynamics in urban environments. Front. Ecol. Evol. 2019, 7, 41. [Google Scholar] [CrossRef]
- Thuring, C.E.; Dunnett, N.P. Persistence, loss and gain: Characterizing mature green roof vegetation by functional composition. Landsc. Urban Plan. 2019, 185, 228–236. [Google Scholar] [CrossRef]
- Martín-López, B.; Iniesta-Arandia, I.; García-Llorente, M.; Palomo, I.; Casado-Arzuaga, I.; García-del Amo, D.; Gómez-Baggethun, E.; Oteros-Rozas, E.; Palacios-Agundez, I.; Willaarts, B.; et al. Uncovering ecosystem service bundles through social preferences. PLoS ONE 2012, 7, e38970. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.C.; South, E.C.; Branas, C.C.; Richmond, T.S.; Wiebe, D.J. The association between urban tree cover and gun assault: A case-control and case-crossover study. Am. J. Epidemiol. 2017, 186, 289–296. [Google Scholar] [CrossRef] [PubMed]
- South, E.C.; Hohl, B.C.; Kondo, M.C.; MacDonald, J.M.; Branas, C.C. Effect of greening vacant land on mental health of community-dwelling adults: A cluster randomized trial. JAMA Netw. Open 2018, 1, e180298. [Google Scholar] [CrossRef]
- Carlos, C.; Simon, H.; Mc Michael, A. Millennium Ecosystem Assessment (MA), Ecosystems and Human Well-Being; World Resources Institute: Washington, DC, USA; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Gonzalez, A.; Germain, R.M.; Srivastava, D.S.; Filotas, E.; Dee, L.E.; Gravel, D.; Thompson, P.L.; Isbell, F.; Wang, S.; Kefi, S.; et al. Scaling-up biodiversity-ecosystem functioning research. Ecol. Lett. 2020, 23, 757–776. [Google Scholar] [CrossRef]
- Schittko, C.; Onandia, G.; Bernard-Verdier, M.; Heger, T.; Jeschke, J.M.; Kowarik, I.; Maass, S.; Joshi, J. Biodiversity maintains soil multifunctionality and soil organic carbon in novel urban ecosystems. J. Ecol. 2022, 110, 916–934. [Google Scholar] [CrossRef]
- Dearborn, D.C.; Kark, S. Motivations for conserving urban biodiversity. Conserv. Biol. 2010, 24, 432–440. [Google Scholar] [CrossRef]
- Molina, J.A.; Martín-Sanz, J.P.; Casermeiro, M.Á.; Quintana, J.R. Spontaneous urban vegetation as an indicator of soil functionality and ecosystem services. Appl. Veg. Sci. 2023, 26, e12827. [Google Scholar] [CrossRef]
- Freschet, G.T.; Violle, C.; Roumet, C.; Garnier, É. Interactions between soil and vegetation: Structure of plant communities and soil functioning. In Soils as a Key Component of the Critical Zone: Ecology; Lemanceau, P., Blouin, M., Eds.; ISTE Editions: London, UK, 2018; pp. 83–104. [Google Scholar]
- Blanchart, A.; Séré, G.; Cherel, J.; Warot, G.; Stas, M.; Consalès, J.N.; Morel, J.L.; Schwartz, C. Towards an operational methodology to optimize ecosystem services provided by urban soils. Landsc. Urban Plan. 2018, 176, 1–9. [Google Scholar] [CrossRef]
- Tresch, S.; Moretti, M.; Le Bayon, R.-C.; Mäder, P.; Zanetta, A.; Frey, D.; Fliessbach, A. A gardener’s influence on urban soil quality. Front. Environ. Sci. 2018, 6, 25. [Google Scholar] [CrossRef]
- Ahern, J. Green infrastructure for cities: The spatial dimension. In Cities of the Future: Towards Integrated Sustainable Water and Landscape Management; Novotny, V., Brown, P., Eds.; IWA Publishing: London, UK, 2007; pp. 267–283. [Google Scholar]
- Meng, L.; Li, S.; Zhang, X. Assessing biodiversity’s impact on stress and affect from urban to conservation areas: A virtual reality study. Ecol. Indic. 2024, 158, 111532. [Google Scholar] [CrossRef]
- Southon, G.E.; Jorgensen, A.; Dunnett, N.; Hoyle, H.; Evans, K.L. Perceived species-richness in urban green spaces: Cues, accuracy and well-being impacts. Landsc. Urban Plan. 2018, 172, 1–10. [Google Scholar] [CrossRef]
- Ren, Y.; Ge, Y.; Ma, D.; Song, X.; Shi, Y.; Pan, K.; Qu, Z.; Guo, P.; Han, W.; Chang, J. Enhancing plant diversity and mitigating BVOC emissions of urban green spaces through the introduction of ornamental tree species. Urban For. Urban Green. 2017, 27, 305–313. [Google Scholar] [CrossRef]
- Przybysz, A.; Popek, R.; Stankiewicz-Kosyl, M.; Zhu, C.Y.; Małecka-Przybysz, M.; Maulidyawati, T.; Mikowska, K.; Deluga, D.; Griżuk, K.; Sokalski-Wieczorek, J.; et al. Where trees cannot grow–Particulate matter accumulation by urban meadows. Sci. Total Environ. 2021, 785, 147310. [Google Scholar] [CrossRef]
- Weiskopf, S.R.; Lerman, S.B.; Isbell, F.; Lyn Morelli, T. Biodiversity promotes urban ecosystem functioning. Ecography 2024, 2024, e07366. [Google Scholar] [CrossRef]
- United Nations. The World Population Prospects 2019: Highlights. New York: United Nations. Available online: https://population.un.org/wpp/assets/Files/WPP2019_10KeyFindings.pdf (accessed on 10 February 2025).
- Petersen, J.E.; Mancosu, E.; King, S. Ecosystem extent accounts for Europe. Ecosyst. Serv. 2022, 57, 101457. [Google Scholar] [CrossRef]
- Todd, P.A.; Heery, E.C.; Loke, L.H.; Thurstan, R.H.; Kotze, D.J.; Swan, C. Towards an urban marine ecology: Characterizing the drivers, patterns and processes of marine ecosystems in coastal cities. Oikos 2019, 128, 1215–1242. [Google Scholar] [CrossRef]
- Zara, L.; Tordoni, E.; Castro-Delgado, S.; Colla, A.; Maccherini, S.; Marignani, M.; Panepinto, F.; Trittoni, M.; Bacaro, G. Cross-taxon relationships in Mediterranean urban ecosystem: A case study from the city of Trieste. Ecol. Indic. 2021, 125, 107538. [Google Scholar] [CrossRef]
- Wilcox, M.D. Auckland’s Remarkable Urban Forest; Auckland Botanical Society: Auckland, New Zeland, 2012; p. 347. [Google Scholar]
- European Environment Agency. Who Benefits from Nature in Cities? Social Inequalities in Access to Urban Green and Blue Spaces Across Europe. 2023. Available online: https://www.eea.europa.eu/publications/who-benefits-from-nature-in (accessed on 10 February 2025).
- Roberts, M.; Glenk, K.; McVittie, A. Urban residents value multi-functional urban greenspaces. Urban For. Urban Green. 2022, 74, 127681. [Google Scholar] [CrossRef]
- Soanes, K.; Lentini, P.E. When cities are the last chance for saving species. Front. Ecol. Environ. 2019, 17, 225–231. [Google Scholar] [CrossRef]
- Soga, M.; Gaston, K.J. Extinction of experience: The loss of human–nature interactions. Front. Ecol. Environ. 2016, 14, 94–101. [Google Scholar] [CrossRef]
- Berkowitz, A.R.; Nilon, C.H.; Hollweg, K.S. Understanding Urban Ecosystems: A New Frontier for Science and Education; Springer: New York, NY, USA, 2003. [Google Scholar]
- Szlavecz, K.; Warren, P.; Pickett, S. Biodiversity on the urban landscape. In Human Population: Its Influences on Biological Diversity; Cincotta, R.P., Gorenflo, L.J., Eds.; Springer: Berlin, Germany, 2011; pp. 75–101. [Google Scholar]
- Spotswood, E.N.; Beller, E.E.; Grossinger, R.; Grenier, J.L.; Heller, N.E.; Aronson, M.F. The biological deserts fallacy: Cities in their landscapes contribute more than we think to regional biodiversity. BioScience 2021, 71, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Clinton, S.M.; Hartman, J.; Macneale, K.H.; Roy, A.H. Stream macroinvertebrate reintroductions: A cautionary approach for restored urban streams. Freshw. Sci. 2022, 41, 507–520. [Google Scholar] [CrossRef]
- Heywood, V.H. The nature and composition of urban plant diversity in the Mediterranean. Flora Mediterr. 2017, 27, 195–220. [Google Scholar] [CrossRef]
- Breuste, J.; Schnellinger, J.; Qureshi, S.; Faggi, A. Urban Ecosystem services on the local level: Urban green spaces as providers. Ekol. Bratisl. 2013, 32, 290–304. [Google Scholar] [CrossRef]
- Bretzel, F.; Vannucchi, F.; Pezzarossa, B.; Paraskevopoulou, A.; Romano, D. Establishing wildflower meadows in anthropogenic landscapes. Front. Hortic. 2023, 2, 1248785. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, J.; Qian, Y.; Pickett, S.T.; Li, W.; Han, L. The rapid but “invisible” changes in urban greenspace: A comparative study of nine Chinese cities. Sci. Total Environ. 2018, 627, 1572–1584. [Google Scholar] [CrossRef]
- Yılmaz, H.; Ku¸sak, B.; Akkemik, Ü. The role of Aşiyan Cemetery (İstanbul) as a green urban space from an ecological perspective and its importance in urban plant diversity. Urban For. Urban Green. 2018, 33, 92–98. [Google Scholar] [CrossRef]
- Ilie, D.; Cosmulescu, S. Spontaneous plant diversity in urban contexts: A review of its impact and importance. Diversity 2023, 15, 277. [Google Scholar] [CrossRef]
- Duncan, J.M.A.; Boruff, B.; Saunders, A.; Sun, Q.; Hurley, J.; Amati, M. Turning down the heat: An enhanced understanding of the relationship between urban vegetation and surface temperature at the city scale. Sci. Total Environ. 2019, 656, 118–128. [Google Scholar] [CrossRef]
- Liu, O.Y.; Russo, A. Assessing the contribution of urban green spaces in green infrastructure strategy planning for urban ecosystem conditions and services. Sustain. Cities Soc. 2021, 68, 102772. [Google Scholar] [CrossRef]
- Lundholm, J.T.; Richardson, P.J. Mini-review: Habitat analogues for reconciliation ecology in urban and industrial environments. J. Appl. Ecol. 2010, 47, 966–975. [Google Scholar] [CrossRef]
- Vannucchi, F.; Buoncristiano, A.; Scatena, M.; Caudai, C.; Bretzel, F. Low productivity substrate leads to functional diversification of green roof plant assemblage. Ecol. Eng. 2022, 176, 106547. [Google Scholar] [CrossRef]
- Hoyle, H.; Norton, B.; Dunnett, N.; Richards, P.; Russell, J.; Warren, P. Plant species or flower colour diversity? Identifying the drivers of public and invertebrate response to designed annual meadows. Landsc. Urban Plan. 2018, 180, 103–113. [Google Scholar] [CrossRef]
- Wang, Y.; Niemelä, J.; Kotze, D.J. The delivery of cultural ecosystem services in urban forests of different landscape features and land use contexts. People Nat. 2022, 4, 1369–1386. [Google Scholar] [CrossRef]
- Sanderson, E.W.; Huron, A. Conservation in the city. Conserv. Biol. 2011, 25, 421–423. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Honchar, G.Y.; Gnatiuk, A.M. Urban ornamental plants for sustenance of wild bees (Hymenoptera, Apoidea). Plant Introd. 2020, 85–86, 93–108. [Google Scholar] [CrossRef]
- Rupprecht, C.D.; Byrne, J.A.; Garden, J.G.; Hero, J.M. Informal urban green space: A trilingual systematic review of its role for biodiversity and trends in the literature. Urban For. Urban Green. 2015, 14, 883–908. [Google Scholar] [CrossRef]
- Dunn, C.P.; Heneghan, L. Composition and diversity of urban vegetation. In Urban Ecology: Patterns, Processes and Applications; Niemeliä, J., Ed.; Oxford University Press: Oxford, UK, 2011; pp. 103–134. [Google Scholar]
- McKinney, M.L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- Kowarik, I. Novel urban ecosystems, biodiversity, and conservation. Environ. Pollut. 2011, 159, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Hope, D.; Gries, C.; Zhu, W.X.; Fagan, W.F.; Redman, C.L.; Grimm, N.B.; Nelson, A.L.; Martin, C.; Kinzig, A. Socioeconomics drive urban plant diversity. Proc. Natl. Acad. Sci. USA 2003, 100, 8788–8792. [Google Scholar] [CrossRef] [PubMed]
- Seitz, B.; Buchholz, S.; Kowarik, I.; Herrmann, J.; Neuerburg, L.; Wendler, J.; Winker, L.; Egerer, M. Land sharing between cultivated and wild plants: Urban gardens as hotspots for plant diversity in cities. Urban Ecosyst. 2022, 25, 927–939. [Google Scholar] [CrossRef]
- Acar, C.; Acar, H.; Eroğlu, E. Evaluation of ornamental plant resources to urban biodiversity and cultural changing: A case study of residential landscapes in Trabzon city (Turkey). Build. Environ. 2007, 42, 218–229. [Google Scholar] [CrossRef]
- Wilson, E.O. Biophilia; Harvard University Press: Cambridge, MA, USA, 1984; p. 176. [Google Scholar]
- Frumkin, H. Beyond toxicity: Human health and the natural environment. Am. J. Prev. Med. 2001, 20, 234–240. [Google Scholar] [CrossRef]
- Kisvarga, S.; Horotán, K.; Wani, M.A.; Orlóci, L. Plant responses to global climate change and urbanization: Implications for sustainable urban landscapes. Horticulturae 2023, 9, 1051. [Google Scholar] [CrossRef]
- Taylor, L.; Hochuli, D.F. Creating better cities: How biodiversity and ecosystem functioning enhance urban residents’ wellbeing. Urban Ecosyst. 2015, 18, 747–762. [Google Scholar] [CrossRef]
- Singh, H.P. Landscape gardening for ecological and aesthetic gains. In Floriculture and Landscape Gardening; Malhotra, S.K., Ram, L., Eds.; Central Institute of Horticulture: Nagaland, India, 2017; pp. 1–10. [Google Scholar]
- Rihn, A.L.; Knuth, M.J.; Behe, B.K.; Hall, C.R. Benefit information’s impact on ornamental plant value. Horticulturae 2023, 9, 740. [Google Scholar] [CrossRef]
- Li, X.-P.; Fan, S.-X.; Kühn, N.; Dong, L.; Hao, P.-Y. Residents’ ecological and aesthetical perceptions toward spontaneous vegetation in urban parks in China. Urban For. Urban Green. 2019, 44, 126397. [Google Scholar] [CrossRef]
- Ciftcioglu, G.C.; Ebedi, S.; Abak, K. Evaluation of the relationship between ornamental plants–based ecosystem services and human wellbeing: A case study from Lefke Region of North Cyprus. Ecol. Indic. 2019, 102, 278–288. [Google Scholar] [CrossRef]
- Gabellini, S.; Scaramuzzi, S. Evolving consumption trends, marketing strategies, and governance settings in ornamental horticulture: A grey literature review. Horticulturae 2022, 8, 234. [Google Scholar] [CrossRef]
- Wilson, A.; Kendal, D.; Moore, J.L. Humans and ornamental plants: A mutualism? Ecopsychology 2016, 8, 257–263. [Google Scholar] [CrossRef]
- Tunnard, C. Gardens in the Modern Landscape: A Facsimile of the Revised 1948 Edition; University of Pennsylvania Press: Philadelphia, PA, USA, 2014; p. 184. [Google Scholar]
- Dehnen-Schmutz, K. Determining non-invasiveness in ornamental plants to build green lists. J. Appl. Ecol. 2011, 48, 1374–1380. [Google Scholar] [CrossRef]
- da Silva Mouga, D.M.D.; Feretti, V.; de Sena, J.C.; Warkentin, M.; dos Santos, A.K.G.; Ribeiro, C.L. Ornamental bee plants as foraging resources for urban bees in Southern Brazil. Agric. Sci. 2015, 6, 365. [Google Scholar] [CrossRef]
- Frankie, G.W.; Vinson, S.B.; Rizzardi, M.A.; Griswold, T.L.; Coville, R.E.; Grayum, M.H.; Martinez, L.E.S.; Foltz-Sweat, J.; Pawelek, J.C. Relationships of bees to host ornamental and weedy flowers in urban Northwest Guanacaste Province, Costa Rica. J. Kans. Entomol. Soc. 2013, 86, 325–351. [Google Scholar] [CrossRef]
- Flores, P.M.C.; Fernandez, A.I.; Orozco, K.J.U.; Endino, R.M.C.; Picardal, J.P.; Garces, J.J.C. Ornamental plant diversity, richness and composition in urban parks: Studies in Metro Cebu, Philippines. Environ. Exp. Biol. 2020, 18, 183–192. [Google Scholar] [CrossRef]
- Restrepo, L.A.V.; Villa, M.H. Contemporary ornamental gardens: Trans-nationalisation, landscaping and biodiversity. An exploratory study in Medellín, Colombia. Rev. Fac. Nac. Agron. Medellin 2015, 68, 7557–7568. [Google Scholar] [CrossRef]
- Kellert, S.R. Birthright: People and Nature in the Modern World; Yale University Press: New Haven, CT, USA, 2012; p. 242. [Google Scholar]
- Le Maitre, D.C.; Van Wilgen, B.W.; Gelderblom, C.; Bailey, C.; Chapman, R.A.; Nel, J. Invasive alien trees and water resources in South Africa: Case studies of the costs and benefits of management. For. Ecol. Manag. 2002, 160, 143–159. [Google Scholar] [CrossRef]
- Gann, G.D.; McDonald, T.; Walder, B.; Aronson, J.; Nelson, C.R.; Jonson, J.; Hallett, J.G.; Eisenberg, C.; Guariguata, M.R.; Liu, J.; et al. International principles and standards for the practice of ecological restoration. Restor. Ecol. 2019, 27 (Suppl. 1), S1–S46. [Google Scholar] [CrossRef]
- van Kleunen, M.; Essl, F.; Pergl, J.; Brundu, G.; Carboni, M.; Dullinger, S.; Early, R.; González-Moreno, P.; Groom, Q.J.; Hulme, P.E.; et al. The changing role of ornamental horticulture in alien plant invasions. Biol. Rev. 2018, 93, 1421–1437. [Google Scholar] [CrossRef]
- Čeplová, N.; Lososová, Z.; Kalusová, V. Urban ornamental trees: A source of current invaders; a case study from a European City. Urban Ecosyst. 2017, 20, 1135–1140. [Google Scholar] [CrossRef]
- Mayer, K.; Haeuser, E.; Dawson, W.; Essl, F.; Kreft, H.; Pergl, J.; Pyšek, P.; Weigelt, P.; Winter, M.; Lenzner, B.; et al. Naturalization of ornamental plant species in public green spaces and private gardens. Biol. Invasions 2017, 19, 3613–3627. [Google Scholar] [CrossRef]
- Potgieter, L.J.; Gaertner, M.; Irlich, U.M.; O′Farrell, P.J.; Stafford, L.; Vogt, H.; Richardson, D.M. Managing Urban Plant Invasions: A Multi-Criteria Prioritization Approach. Environ. Manag. 2018, 62, 1168–1185. [Google Scholar] [CrossRef] [PubMed]
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P.; et al. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Shapiro, A.M. The Californian urban butterfly fauna is dependent on alien plants. Divers. Distrib. 2002, 8, 31–40. [Google Scholar] [CrossRef]
- Chowdhuri, T.K.; Deka, K. Biodiversity and Conservation of Ornamental Crops. In Conservation and Utilization of Horticultural Genetic Resources; Rajasekharan, P., Rao, V., Eds.; Springer: Singapore, 2019; pp. 139–216. [Google Scholar] [CrossRef]
- Toscano, S.; Tribulato, A.; Romano, D. The biodiversity of Sicilian traditional gardens. Acta Hortic. 2018, 1215, 263–266. [Google Scholar] [CrossRef]
- Poole, O.; Costa, A.; Kaiser-Bunbury, C.N.; Shaw, R.F. Pollinators respond positively to urban green space enhancements using wild and ornamental flowers. Insect Conserv. Divers. 2024, 18, 16–28. [Google Scholar] [CrossRef]
- Cable, J.; Barber, I.; Boag, B.; Ellison, A.R.; Morgan, E.R.; Murray, K.; Pascoe, E.L.; Sait, S.M.; Wilson, A.J.; Booth, M. Global change, parasite transmission and disease control: Lessons from ecology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160088. [Google Scholar] [CrossRef]
- Kendal, D.; Dobbs, C.; Lohr, V.I. Global patterns of diversity in the urban forest: Is there evidence to support the 10/20/30 rule? Urban For. Urban Green. 2014, 13, 411–417. [Google Scholar] [CrossRef]
- Qiao, H.; Wu, L.; Li, C.; Yuan, T.; Gao, J. Microbial perspective on restoration of degraded urban soil using ornamental plants. J. Environ. Manag. 2024, 359, 120920. [Google Scholar] [CrossRef]
- Hoyle, H.E.; Sant’Anna, C.G. Rethinking ‘future nature’ through a transatlantic research collaboration: Climate-adapted urban green infrastructure for human wellbeing and biodiversity. Landsc. Res. 2023, 48, 460–476. [Google Scholar] [CrossRef]
- Klaus, V.H.; Kiehl, K. A conceptual framework for urban ecological restoration and rehabilitation. Basic Appl. Ecol. 2021, 52, 82–94. [Google Scholar] [CrossRef]
- Knapp, S.; Dinsmore, L.; Fissore, C.; Hobbie, S.E.; Jakobsdottir, I.; Kattge, J.; King, J.Y.; Klotz, S.; McFadden, J.P.; Cavender-Bares, J. Phylogenetic and functional characteristics of household yard floras and their changes along an urbanization gradient. Ecology 2012, 93, 83–98. [Google Scholar] [CrossRef]
- Williams, N.S.G.; Hahs, A.K.; Vesk, P.A. Urbanisation, plant traits and the composition of urban floras. Perspect. Pl. Ecol. Evol. Syst. 2015, 17, 78–86. [Google Scholar] [CrossRef]
- Díaz, S.; Cabido, M. Vive la différence: Plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 2001, 16, 646–655. [Google Scholar] [CrossRef]
- Pearse, W.D.; Cavender-Bares, J.; Hobbie, S.E.; Avolio, M.L.; Bettez, N.; Roy Chowdhury, R.; Darling, L.E.; Groffman, P.M.; Grove, J.M.; Hall, S.J.; et al. Homogenization of plant diversity, composition, and structure in North American urban yards. Ecosphere 2018, 9, e02105. [Google Scholar] [CrossRef]
- Wang, H.-F.; Qureshi, S.; Knapp, S.; Friedman, C.R.; Hubacek, K. A basic assessment of residential plant diversity and its ES and disservices in Beijing, China. Appl. Geogr. 2015, 64, 121–131. [Google Scholar] [CrossRef]
- Reichard, S.H.; White, P. Horticulture as a pathway of invasive plant introductions in the United States. BioScience 2001, 51, 103–113. [Google Scholar] [CrossRef]
- Whitney, G.G.; Adams, S.D. Man as a maker of new plant communities. J. Appl. Ecol. 1980, 17, 431–448. [Google Scholar] [CrossRef]
- Aronson, M.F.J.; Handel, S.N.; La Puma, I.P.; Clemants, S.E. Urbanization promotes non-native woody species and diverse plant assemblages in the New York metropolitan region. Urban Ecosyst. 2015, 18, 31–45. [Google Scholar] [CrossRef]
- Cameron, R.W.F.; Blanuša, T.; Taylor, J.E.; Salisbury, A.; Halstead, A.J.; Henricot, B.; Thompson, K. The domestic garden—Its contribution to urban green infrastructure. Urban For. Urban Green. 2012, 11, 129–137. [Google Scholar] [CrossRef]
- Dehnen-Schmutz, K.; Touza, J.; Perrings, C.; Williamson, M. The horticultural trade and ornamental plant invasions in Britain. Conserv. Biol. 2007, 21, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Kendal, D.; Williams, K.J.H.; Williams, N.S.G. Plant traits link people’s plant preferences to the composition of their gardens. Landsc. Urban Plan. 2012, 105, 34–42. [Google Scholar] [CrossRef]
- Avolio, M.L.; Pataki, D.E.; Trammell, T.L.E.; Endter-Wada, J. Biodiverse cities: The nursery industry, homeowners, and neighborhood differences drive urban tree composition. Ecol. Monogr. 2018, 88, 259–276. [Google Scholar] [CrossRef]
- Kelcey, J.G.; Müller, N. Plants and Habitats of European Cities; Springer: New York, NY, USA, 2009; pp. XVII, 685. [Google Scholar] [CrossRef]
- Luck, G.W. A review of the relationships between human population density and biodiversity. Biol. Rev. Camb. Philos. Soc. 2007, 82, 607–645. [Google Scholar] [CrossRef]
- Kowarick, I. On the role of alien species in urban flora and vegetation. In Plant Invasions—General Aspects and Special Problems; Pysek, P., Prach, K., Rejmanek, M., Wade, M., Eds.; SPB Academic Publishing: Amsterdam, The Netherlands, 2008; pp. 85–103. [Google Scholar]
- Kühn, I.; Brandl, R.; Klotz, S. The flora of German cities is naturally species rich. Evol. Ecol. Res. 2004, 6, 749–764. Available online: https://www.evolutionary-ecology.com/issues/v06n05/jjar1629.pdf (accessed on 30 January 2025).
- Avolio, M.; Pataki, D.E.; Jenerette, G.D.; Pincetl, S.; Clarke, L.W.; Cavender-Bares, J.; Gillespie, T.W.; Hobbie, S.E.; Larson, K.L.; McCarthy, H.R.; et al. Urban plant diversity in Los Angeles, California: Species and functional type turnover in cultivated landscapes. Plants People Planet 2020, 2, 144–156. [Google Scholar] [CrossRef]
- Gobster, P.H. Urban ecological restoration. Nat. Cult. 2010, 5, 227–230. [Google Scholar] [CrossRef]
- Hoyle, H.; Hitchmough, J.D.; Jorgensen, A. Attractive, climate-adapted and sustainable? Public perception of non-native planting in the designed urban landscape. Landsc. Urban Plan. 2017, 164, 49–63. [Google Scholar] [CrossRef]
- Scheffers, B.R.; De Meester, L.; Bridge, T.C.; Hoffmann, A.A.; Pandolfi, J.M.; Corlett, R.T.; Butchart, S.H.; Pearce-Kelly, P.; Kovacs, K.M.; Dudgeon, D.; et al. The broad footprint of climate change from genes to biomes to people. Science 2016, 354, aaf7671. [Google Scholar] [CrossRef]
- Demuzere, M.; Orru, H.; Orru, K.; Heidrich, O.; Olazabal, E.; Geneletti, D.; Bhave, A.G.; Mittal, N.; Feliu, E.; Faehnle, M. Mitigating and adapting to climate change: Multi-functional and multi-scale assessment of green urban infrastructure. J. Environ. Manag. 2014, 146, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, H.; Hitchmough, J.D.; Jorgensen, A. All about the ‘wow factor’? The relationships between aesthetics, restorative effect and perceived biodiversity in designed urban planting. Landsc. Urban Plan. 2017, 164, 109–123. [Google Scholar] [CrossRef]
- Fischer, L.K.; Honold, J.; Botzat, A.; Brinkmeyer, D.; Cvejić, R.; Delshammar, T.; Elands, B.; Haase, D.; Kabisch, N.; Karle, S.J.; et al. Recreational ecosystem services in European cities: Sociocultural and geographical contexts matter for park use. Ecosyst. Serv. 2018, 31, 455–467. [Google Scholar] [CrossRef]
- Hartig, T.; Mitchell, R.; de Vries, S.; Frumkin, H. Nature and Health. Annu. Rev. Public. Health 2014, 35, 207–228. [Google Scholar] [CrossRef]
- Cocks, M. Biocultural diversity: Moving beyond the realm of ‘indigenous’ and ‘local’ people. Hum. Ecol. 2006, 34, 185–200. [Google Scholar] [CrossRef]
- Drake, L.; Lawson, L.J. Results of a US and Canada community garden survey: Shared challenges in garden management amid diverse geographical and organizational contexts. Agric. Hum. Values 2015, 32, 241–254. [Google Scholar] [CrossRef]
- Mörtberg, U.; Goldenberg, R.; Kalantari, Z.; Kordas, O.; Deal, B.; Balfors, B.; Cvetkovic, V. Integrating ecosystem services in the assessment of urban energy trajectories—A study of the Stockholm Region. Energy Policy 2017, 100, 338–349. [Google Scholar] [CrossRef]
- Hansen, R.; Frantzeskaki, N.; McPhearson, T.; Rall, E.; Kabisch, N.; Kaczorowska, A.; Pauleit, S. The uptake of the ecosystem services concept in planning discourses of European and American cities. Ecosyst. Serv. 2015, 12, 228–246. [Google Scholar] [CrossRef]
- Aronson, M.F.J.; Lepczyk, C.A.; Evans, K.L.; Goddard, M.A.; Lerman, S.B.; MacIvor, J.S.; Nilon, C.H.; Vargo, T. Biodiversity in the city: Key challenges for urban green space management. Front. Ecol. Environ. 2017, 15, 189–196. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Nonnative, noninvasive woody species can enhance urban landscape biodiversity. Arboric. Urban For. 2015, 41, 173–186. [Google Scholar] [CrossRef]
- Tartaglia, E.S.; Aronson, M.F. Plant native: Comparing biodiversity benefits, ecosystem services provisioning, and plant performance of native and non-native plants in urban horticulture. Urban Ecosyst. 2024, 27, 2587–2611. [Google Scholar] [CrossRef]
- Leotta, L.; Toscano, S.; Romano, D. Which plant species for green roofs in the mediterranean environment? Plants 2023, 12, 3985. [Google Scholar] [CrossRef] [PubMed]
- Guidi, M.A. Investigation of Flowering Phenology, Pollinator and Invertebrate Biodiversity Value on Urban Green Roofs and an Evaluation of Ornamental Horticulture Crops for Pollinator Value. Master’s Thesis, Colorado State University, Fort Collins, CO, USA, 2023. [Google Scholar]
- Vannucchi, F.; Pini, R.; Scatena, M.; Benelli, G.; Canale, A.; Bretzel, F. Deinking sludge in the substrate reduces the fertility and enhances the plant species richness of extensive green roofs. Ecol. Eng. 2018, 116, 87–96. [Google Scholar] [CrossRef]
- Leotta, L.; Toscano, S.; Ferrante, A.; Romano, D.; Francini, A. New strategies to increase the abiotic stress tolerance in woody ornamental plants in Mediterranean climate. Plants 2023, 12, 2022. [Google Scholar] [CrossRef]
- Gupta, A.; Naorem, A.; Prasad, M.G.; Khuraijam, J.S. Using wild plant species in ornamental horticulture: A possible future. In Ornamental Horticulture: Latest Cultivation Practices and Breeding Technologies; Bhargava, B., Kumar, P., Verma, V., Eds.; Springer: Singapore, 2024; pp. 259–273. [Google Scholar] [CrossRef]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global change and the ecology of cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef]
- Ong, T.W.; Lin, B.B.; Lucatero, A.; Cohen, H.; Bichier, P.; Egerer, M.H.; Danieu, A.; Jha, S.; Philpott, S.M.; Liere, H. Rarity begets rarity: Social and environmental drivers of rare organisms in cities. Ecol. Appl. 2022, 32, e2708. [Google Scholar] [CrossRef]
- De Pascale, S.; Romano, D. Potential use of wild plants in floriculture. Acta Hortic. 2019, 1240, 87–98. [Google Scholar] [CrossRef]
- Weaver, D. Mass and urban ecotourism: New manifestations of an old concept. Tour. Recreat. Res. 2005, 30, 19–26. [Google Scholar] [CrossRef]
- Rupprecht, C.D.D.; Byrne, J.A. Informal urban greenspace: A typology and trilingual systematic review of its role for urban residents and trends in the literature. Urban For. Urban Green. 2014, 13, 597–611. [Google Scholar] [CrossRef]
- Gawryszewska, B.J.; Łepkowski, M.; Pietrych, Ł.; Wilczyńska, A.; Archiciński, P. The structure of beauty: Informal green spaces in their users’ eyes. Sustainability 2024, 16, 1619. [Google Scholar] [CrossRef]
- Threlfall, C.G.; Mata, L.; Mackie, J.A.; Hahs, A.K.; Stork, N.E.; Williams, N.S.G.; Livesley, S.J. Increasing biodiversity in urban green spaces through simple vegetation interventions. J. Appl. Ecol. 2017, 54, 1874–1883. [Google Scholar] [CrossRef]
- Li, F.; Zheng, W.; Wang, Y.; Liang, J.; Xie, S.; Guo, S.; Li, X.; Yu, C. Urban green space fragmentation and urbanization: A spatiotemporal perspective. Forests 2019, 10, 333. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; Aronson, M.F.J.; Evans, M.K.L.; Goddard, A.; Lerman, S.B.; MacIvor, J.S. Biodiversity in the city: Fundamental questions for understanding the ecology of urban green spaces for biodiversity conservation. BioScience 2017, 67, 799–807. [Google Scholar] [CrossRef]
- Aziz, H.A.; Rasidi, M.H. The role of green corridors for wildlife conservation in urban landscape: A literature review. IOP Conf. Ser. Earth Environ. Sci. 2014, 18, 012093. [Google Scholar] [CrossRef]
- Ahern, J. Greenways as a planning strategy. Landsc. Urban Plan. 1995, 33, 131–155. [Google Scholar] [CrossRef]
- App, M.; Strohbach, M.W.; Schneider, A.-K.; Schröder, B. Making the case for gardens: Estimating the contribution of urban gardens to habitat provision and connectivity based on hedgehogs (Erinaceus europaeus). Landsc. Urban Plan. 2022, 220, 104347. [Google Scholar] [CrossRef]
- Beumer, C.; Martens, P. Biodiversity in my (back)yard: Towards a framework for citizen engagement in exploring biodiversity and ecosystem services in residential gardens. Sustain. Sci. 2015, 10, 87–100. [Google Scholar] [CrossRef]
- Kong, F.H.; Yin, H.W.; Nakagoshi, N.; Zong, Y.G. Urban green space network development for biodiversity conservation: Identification based on graph theory and gravity modeling. Landsc. Urban Plan. 2010, 95, 16–27. [Google Scholar] [CrossRef]
- Patankar, S.; Jambhekar, R.; Suryawanshi, K.R.; Nagendra, H. Which traits influence bird survival in the city? A review. Land 2021, 10, 92. [Google Scholar] [CrossRef]
- Idilfitri, S.; Sulaiman, S.; Salleh, N.S. Role of ornamental plants for bird community’ habitats in urban parks. Procedia Soc. Behav. Sci. 2014, 153, 666–677. [Google Scholar] [CrossRef]
- Blair, R.B. Land use and avian species diversity along an urban gradient. Ecol. Appl. 1996, 6, 506–519. [Google Scholar] [CrossRef]
- Batáry, P.; Kurucz, K.; Suarez-Rubio, M.; Chamberlain, D.E. Non-linearities in bird responses across urbanization gradients: A meta-analysis. Glob. Chang. Biol. 2018, 24, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Leveau, L.M.; Ruggiero, A.; Matthews, T.J.; Bellocq, M.I. A global consistent positive effect of urban green area size on bird richness. Avian Res. 2019, 10, 30. [Google Scholar] [CrossRef]
- Reynolds, S.J.; Ibáñez-Álamo, J.D.; Sumasgutner, P.; Mainwaring, M.C. Urbanisation and nest building in birds: A review of threats and opportunities. J. Ornithol. 2019, 160, 841–860. [Google Scholar] [CrossRef]
- Pena, J.C.; Martello, F.; Ribeiro, M.C.; Armitage, R.A.; Young, R.J.; Rodrigues, M. Street trees reduce the negative effects of urbanization on birds. PLoS ONE 2017, 12, e0174484. [Google Scholar] [CrossRef]
- Marlès Magre, J.; Boada Juncà, M.; Campanera, J.M.; Bach Pagès, A.; Ruiz Mallén, I.; Maneja Zaragoza, R.; Sánchez Mateo, S.; Pallarès Barberà, M.; Barriocanal Lozano, C. How Urban Green Management Is Influencing Passerine Birds’ Nesting in the Mediterranean: A case study in a Catalan city. Urban For. Urban Green. 2019, 41, 221–229. [Google Scholar] [CrossRef]
- Yang, G.; Xu, J.; Wang, Y.; Wang, X.; Pei, E.; Yuan, X.; Li, H.; Ding, Y.; Wang, Z. Evaluation of microhabitats for wild birds in a Shanghai urban area park. Urban For. Urban Green. 2015, 14, 246–254. [Google Scholar] [CrossRef]
- Wood, E.M.; Esaian, S. The importance of street trees to urban avifauna. Ecol. Appl. 2020, 30, e02149. [Google Scholar] [CrossRef]
- French, K.; Major, R.; Hely, K. Use of native and exotic garden plants by suburban nectarivorous birds. Biol. Conserv. 2005, 121, 545–559. [Google Scholar] [CrossRef]
- Pellissier, V.; Cohen, M.; Boulay, A.; Clergeau, P. Birds are also sensitive to landscape composition and configuration within the city centre. Landsc. Urban Plan. 2012, 104, 181–188. [Google Scholar] [CrossRef]
- Pearce, H.; Walters, C.L. Do green roofs provide habitat for bats in urban areas? Acta Chiropterol. 2012, 14, 469–478. [Google Scholar] [CrossRef]
- Smallwood, N.L.; Wood, E.M. The ecological role of native-plant landscaping in residential yards to birds during the nonbreeding period. Ecosphere 2022, 14, e4360. [Google Scholar] [CrossRef]
- Cerra, J.F.; Crain, R. Urban birds and planting design: Strategies for incorporating ecological goals into residential landscapes. Urban Ecosyst. 2016, 19, 1823–1846. [Google Scholar] [CrossRef]
- Jung, K.; Threlfall, C.G. Trait-Dependent Tolerance of Bats to Urbanization: A Global Meta-Analysis. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181222. [Google Scholar] [CrossRef]
- Corlett, R.T. Interactions between birds, fruit bats and exotic plants in urban Hong Kong, South China. Urban Ecosyst. 2005, 8, 275–283. [Google Scholar] [CrossRef]
- Nunes, H.; Rocha, F.L.; Cordeiro-Estrela, P. Bats in urban areas of Brazil: Roosts, food resources and parasites in disturbed environments. Urban Ecosyst. 2017, 20, 953–969. [Google Scholar] [CrossRef]
- Callas, M.; Lumsden, L.F.; Rendall, A.R.; Yokochi, K. More trees and fewer roads: The importance of local and landscape features for insectivorous bats in open urban green spaces. Wildl. Res. 2024, 51, WR23079. [Google Scholar] [CrossRef]
- Marquardt, M.; Kienbaum, L.; Kretschmer, L.A.; Penell, A.; Schweikert, K.; Ruttensperger, U.; Rosenkranz, P. Evaluation of the importance of ornamental plants for pollinators in urban and suburban areas in Stuttgart, Germany. Urban Ecosyst. 2020, 24, 811–825. [Google Scholar] [CrossRef]
- Palmersheim, M.C.; Schürch, R.; O’Rourke, M.E.; Slezak, J.; Couvillon, M.J. If you grow it, they will come: Ornamental plants impact the abundance and diversity of pollinators and other flower-visiting insects in gardens. Horticulturae 2022, 8, 1068. [Google Scholar] [CrossRef]
- Lerch, D.; Blüthgen, N.; Mody, K. Home sweet home: Evaluation of native versus exotic plants as resources for insects in urban green spaces. Ecol. Solut. Evid. 2024, 5, e12380. [Google Scholar] [CrossRef]
- Matteson, K.C.; Langellotto, G.A. Small scale additions of native plants fail to increase beneficial insect richness in urban gardens. Insect. Conserv. Divers. 2011, 4, 89–98. [Google Scholar] [CrossRef]
- Schueller, S.K.; Li, Z.; Bliss, Z.; Roake, R.; Weiler, B. How informed design can make a difference: Supporting insect pollinators in cities. Land 2023, 12, 1289. [Google Scholar] [CrossRef]
- Kim, T.E.; Oh, S.-Y.; Chang, E.; Jang, Y. Host availability hypothesis: Complex interactions with abiotic factors and predators may best explain population densities of cicada species. Anim. Cells Syst. 2014, 18, 143–153. [Google Scholar] [CrossRef]
- Patoka, J.; Bláha, M.; Kalous, L.; Vrabec, V.; Buřič, M.; Kouba, A. Potential pest transfer mediated by international ornamental plant trade. Sci. Rep. 2016, 6, 25896. [Google Scholar] [CrossRef]
- Fukano, Y.; Soga, M. Why do so many modern people hate insects? The urbanization–disgust hypothesis. Sci. Total Environ. 2021, 777, 146229. [Google Scholar] [CrossRef]
- Vanderstock, A.; Grandi-Nagashiro, C.; Kudo, G.; Latty, T.; Nakamura, S.; White, T.E.; Soga, M. For the love of insects: Gardening grows positive emotions (biophilia) towards invertebrates. J. Insect Conserv. 2022, 26, 751–762. [Google Scholar] [CrossRef]
- Noriega, J.A.; Hortal, J.; Azcárate, F.M.; Berg, M.P.; Bonada, N.; Briones, M.J.; Del Toro, I.; Goulson, D.; Ibanez, S.; Landis, D.A.; et al. Research trends in ecosystem services provided by insects. Basic Appl. Ecol. 2018, 26, 8–23. [Google Scholar] [CrossRef]
- Żołnierz, L.; Fudali, E.; Szymanowski, M. Epiphytic Bryophytes in an urban landscape: Which factors determine their distribution, species richness, and diversity? A case study in Wroclaw, Poland. Int. J. Environ. Res. Public. Health 2022, 19, 6274. [Google Scholar] [CrossRef]
- Jung, N.J.; Eyster, H.N.; Chan, K.M.A. Re-envisioning urban landscapes: Lichens, liverworts, and mosses coexist spontaneously with us. Front. Ecol. Environ. 2025, 23, e2836. [Google Scholar] [CrossRef]
- Gregory, A.; Spence, E.; Beier, P.; Garding, E. Toward best management practices for ecological corridors. Land 2021, 10, 140. [Google Scholar] [CrossRef]
- Bretzel, F.; Vannucchi, F.; Romano, D.; Malorgio, F.; Benvenuti, S.; Pezzarossa, B. Wildflowers: From conserving biodiversity to urban greening—A review. Urban For. Urban Green. 2016, 20, 428–436. [Google Scholar] [CrossRef]
- Benvenuti, S. Wildflower green roofs for urban landscaping, ecological sustainability and biodiversity. Landsc. Urban Plan. 2014, 124, 151–161. [Google Scholar] [CrossRef]
- Haaland, C.; Gyllin, M. Sown wildflower strips–a strategy to enhance biodiversity and amenity in intensively used agricultural areas. In The importance of biological interactions in the study of biodiversity; López-Pujol, J., Ed.; InTech: Rijeka, Croatia, 2011; pp. 155–172. [Google Scholar]
- Bhatt, A.; Bhat, N.R.; Santo, A.; Phartyal, S.S. Influence of temperature, light and salt on the germination of Deverra triradiata seeds. Seed Sci. Technol. 2019, 47, 25–31. [Google Scholar] [CrossRef]
- Scheper, J.; Bukovinszky, T.; Huigens, M.E.; Kleijn, D. Attractiveness of sown wildflower strips to flower-visiting insects depends on seed mixture and establishment success. Basic Appl. Ecol. 2021, 56, 401–415. [Google Scholar] [CrossRef]
- Fernandes, M.P.; Matono, P.; Almeida, E.; Pinto-Cruz, C.; Belo, A.D. Sowing wildflower meadows in Mediterranean peri-urban green areas to promote grassland diversity. Front. Ecol. Evol. 2023, 11, 1112596. [Google Scholar] [CrossRef]
- Karamaouna, F.; Kati, V.; Volakakis, N.; Varikou, K.; Garantonakis, N.; Economou, L.; Birouraki, A.; Markellou, E.; Liberopoulou, S.; Edwards, M. Ground cover management with mixtures of flowering plants to enhance insect pollinators and natural enemies of pests in olive groves. Agric. Ecosyst. Environ. 2019, 274, 76–89. [Google Scholar] [CrossRef]
- Kiehl, K.; Kirmer, A.; Shaw, N. (Eds.) Guidelines for Native Seed Production and Grassland Restoration; Scholars Publishing: Cambridge, UK, 2014; p. 315. [Google Scholar]
- Winkler, J.; Pasternak, G.; Sas, W.; Hurajová, E.; Koda, E.; Vaverková, M.D. Nature-Based Management of Lawns—Enhancing Biodiversity in Urban Green Infrastructure. Appl. Sci. 2024, 14, 1705. [Google Scholar] [CrossRef]
- Delahay, R.J.; Sherman, D.; Soyalan, B.; Gaston, K.J. Biodiversity in residential gardens: A review of the evidence base. Biodivers. Conserv. 2023, 32, 4155–4179. [Google Scholar] [CrossRef]
- Minor, E.S.; Anderson, E.C.; Belaire, J.A.; Garfinkel, M.; Smith, A.D. Urban green infrastructures and ecological networks for urban biodiversity conservation. In Urban Biodiversity. From Research to Practice; Ossola, A., Niemelä, J., Eds.; Routledge: London, UK, 2018; pp. 186–199. [Google Scholar]
- English, J.; Barry, K.E.; Wood, E.M.; Wright, A.J. The effect of urban environments on the diversity of plants in unmanaged grasslands in Los Angeles, United States. Front. Ecol. Evol. 2022, 10, 921472. [Google Scholar] [CrossRef]
- Erickson, E.; Patch, H.M.; Grozinger, C.M. Herbaceous perennial ornamental plants can support complex pollinator communities. Sci. Rep. 2021, 11, 17352. [Google Scholar] [CrossRef]
- Erickson, E.; Adam, S.; Russo, L.; Wojcik, V.; Patch, H.M.; Grozinger, C.M. More than meets the eye? The role of annual ornamental flowers in supporting pollinators. Environ. Entomol. 2020, 49, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Quinanzoni, M.; Marcolet, D.; Michelot-Antalik, A. Drought response and urban-pollinator attractiveness of ornamental plant species. Basic Appl. Ecol. 2024, 78, 1–13. [Google Scholar] [CrossRef]
- Fekete, R.; Valkó, O.; Fischer, L.K.; Deák, B.; Klaus, V.H. Ecological restoration and biodiversity-friendly management of urban grasslands–A global review on the current state of knowledge. J. Environ. Manag. 2024, 368, 122220. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).