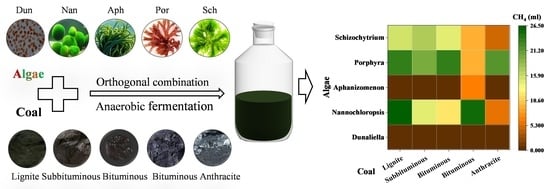
Substrate Composition Effects on the Microbial Enhancement of Biogenic Methane Production from Coal
Abstract
1. Introduction
2. Materials and Methods
2.1. Coal Samples and Microorganisms
2.2. Culture Media
2.3. Experimental Procedure
2.4. Test Methods for Coal Samples and Microorganisms
2.5. Statistical Analysis Methods
3. Results
3.1. Composition of Coal Samples and Microorganisms
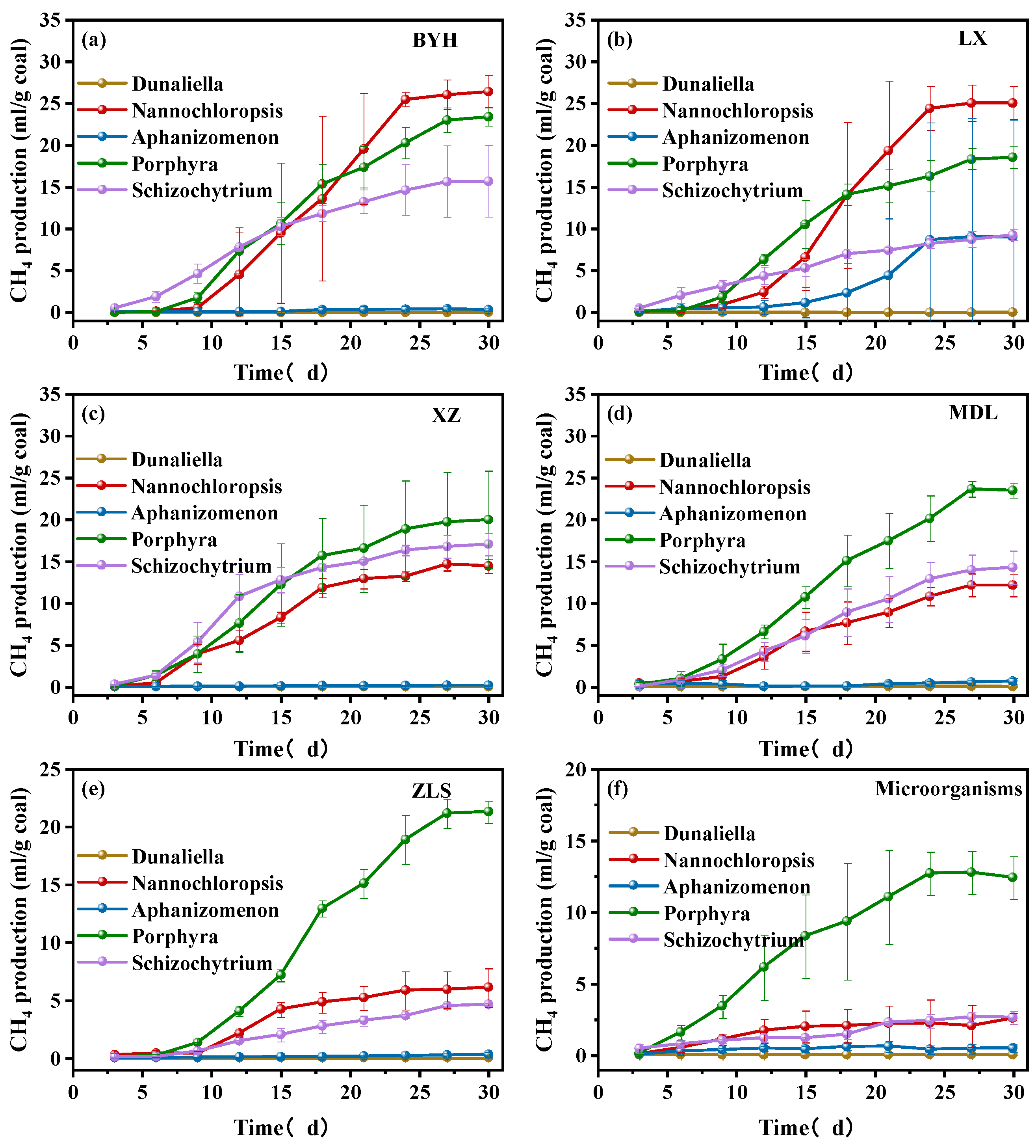
3.2. Methane Production
3.3. Statistical Analysis
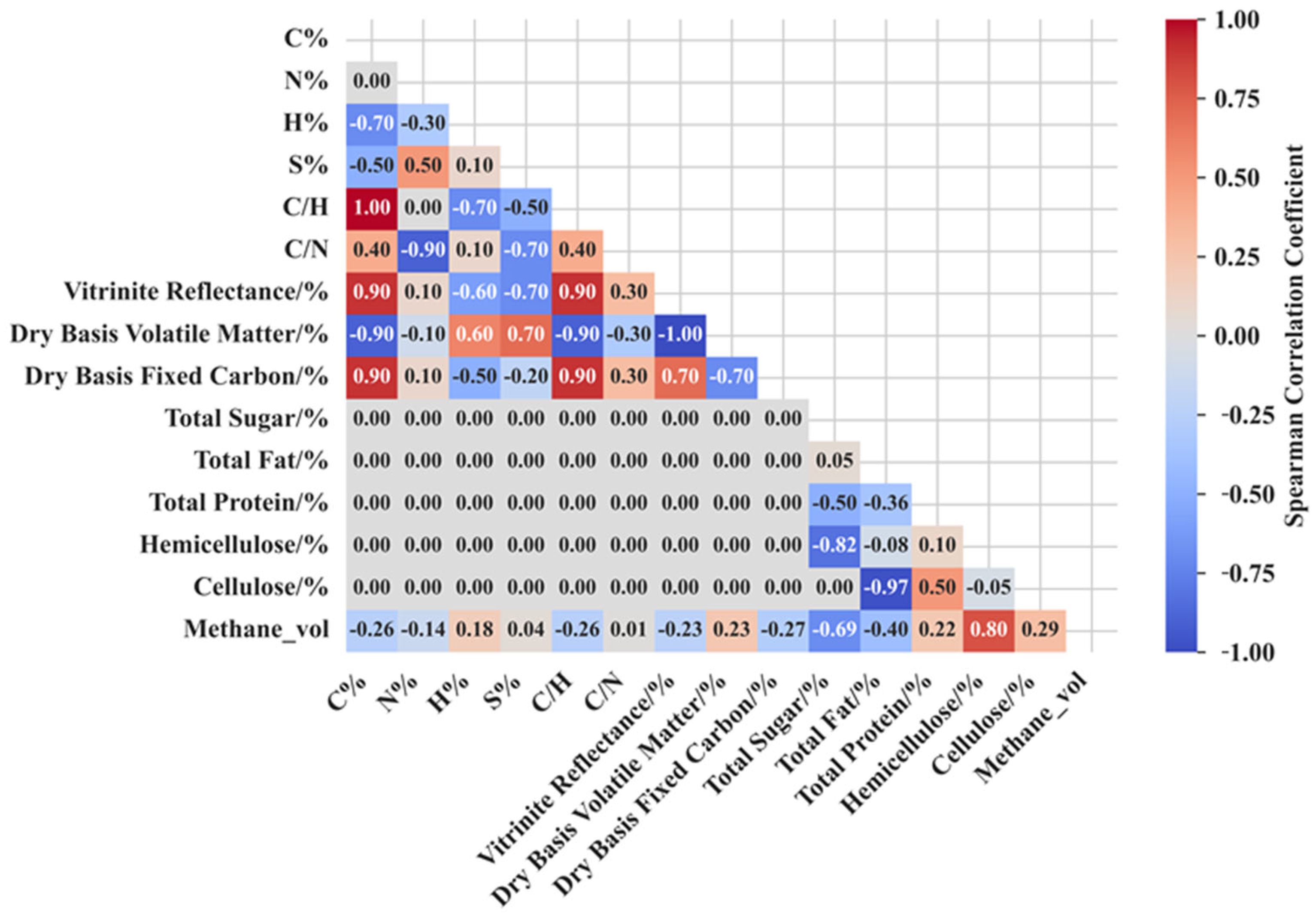
3.3.1. Spearman Correlation Analysis
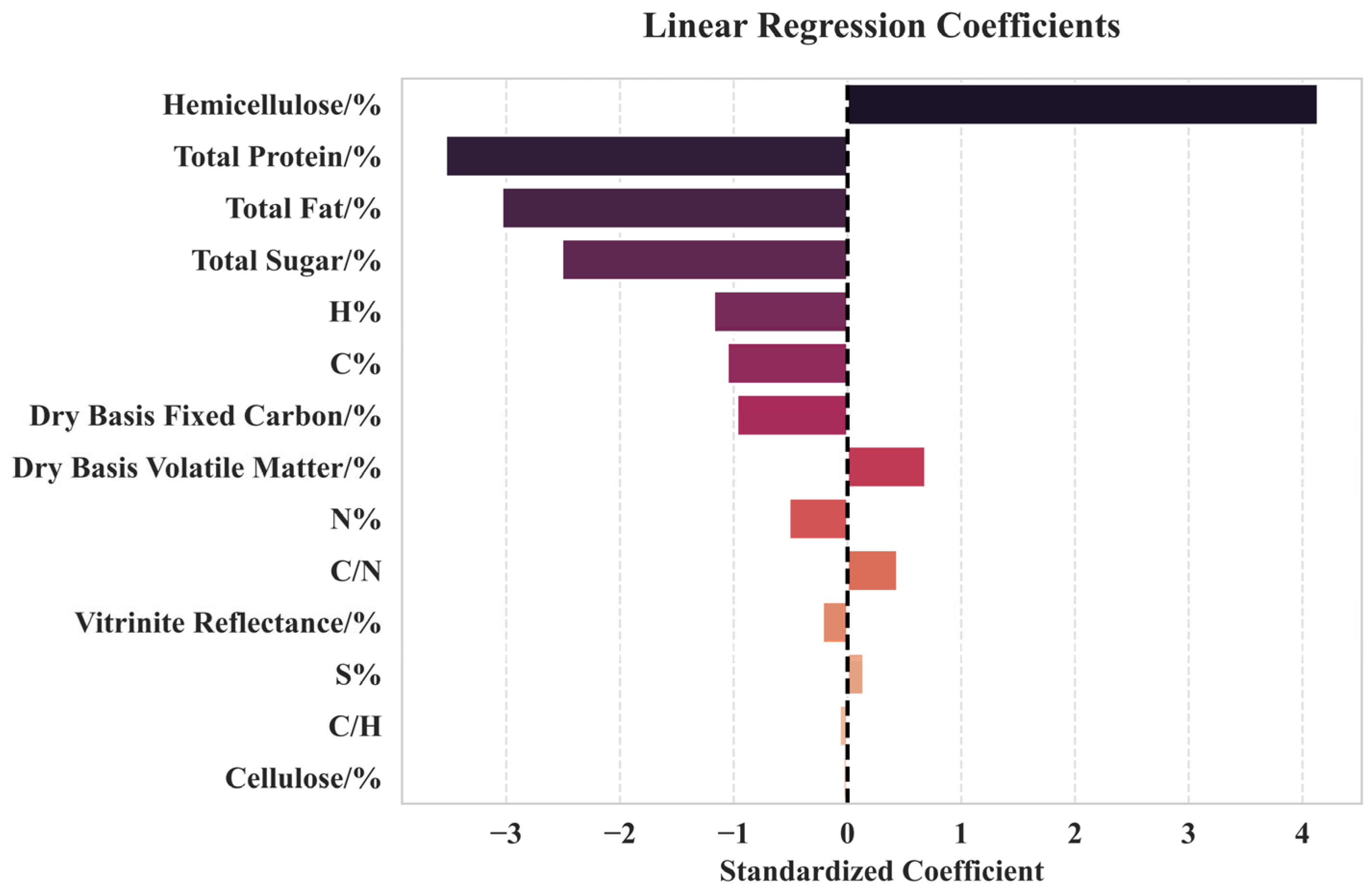
3.3.2. Multiple Linear Regression Analysis
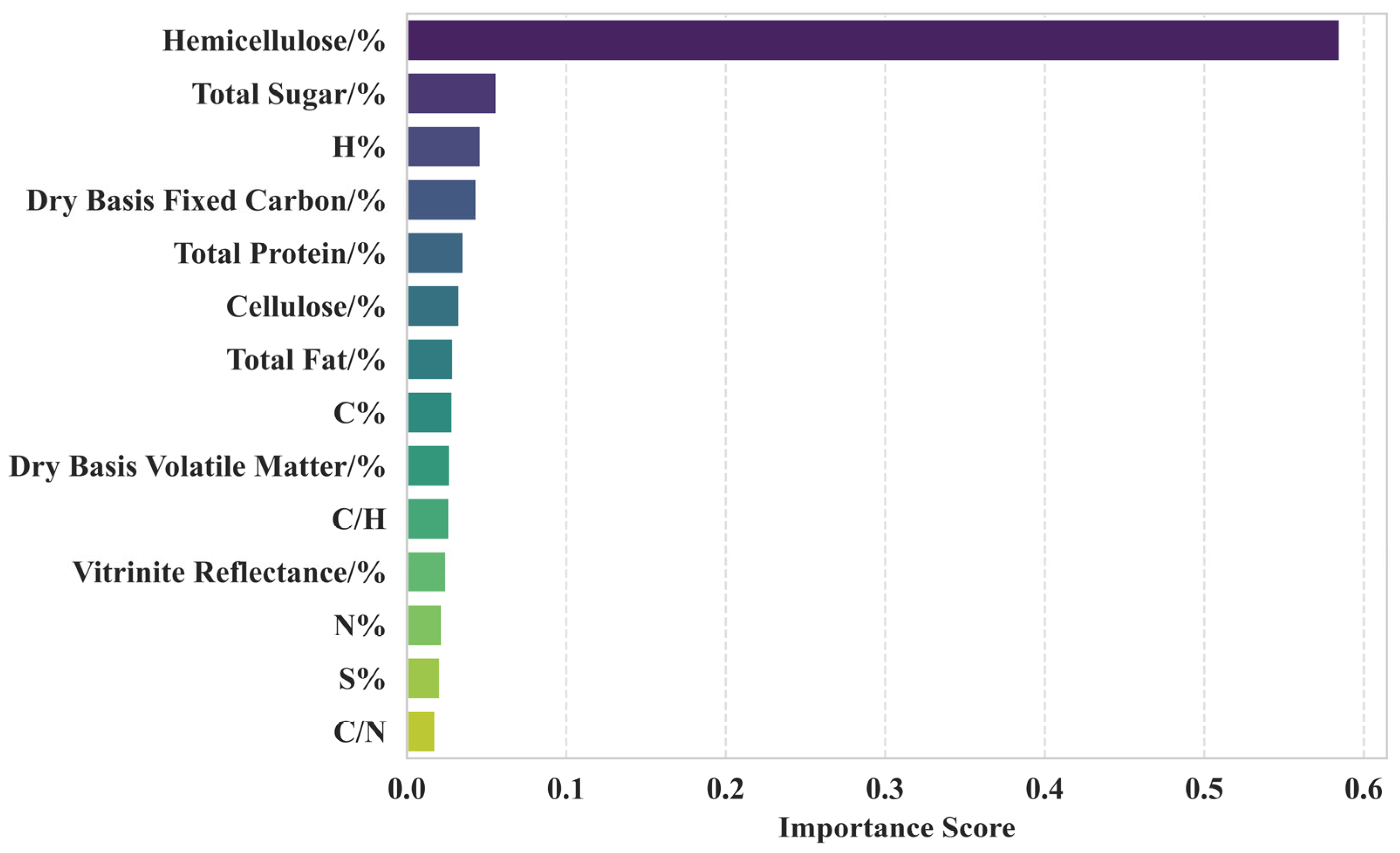
3.3.3. Random Forest Analysis
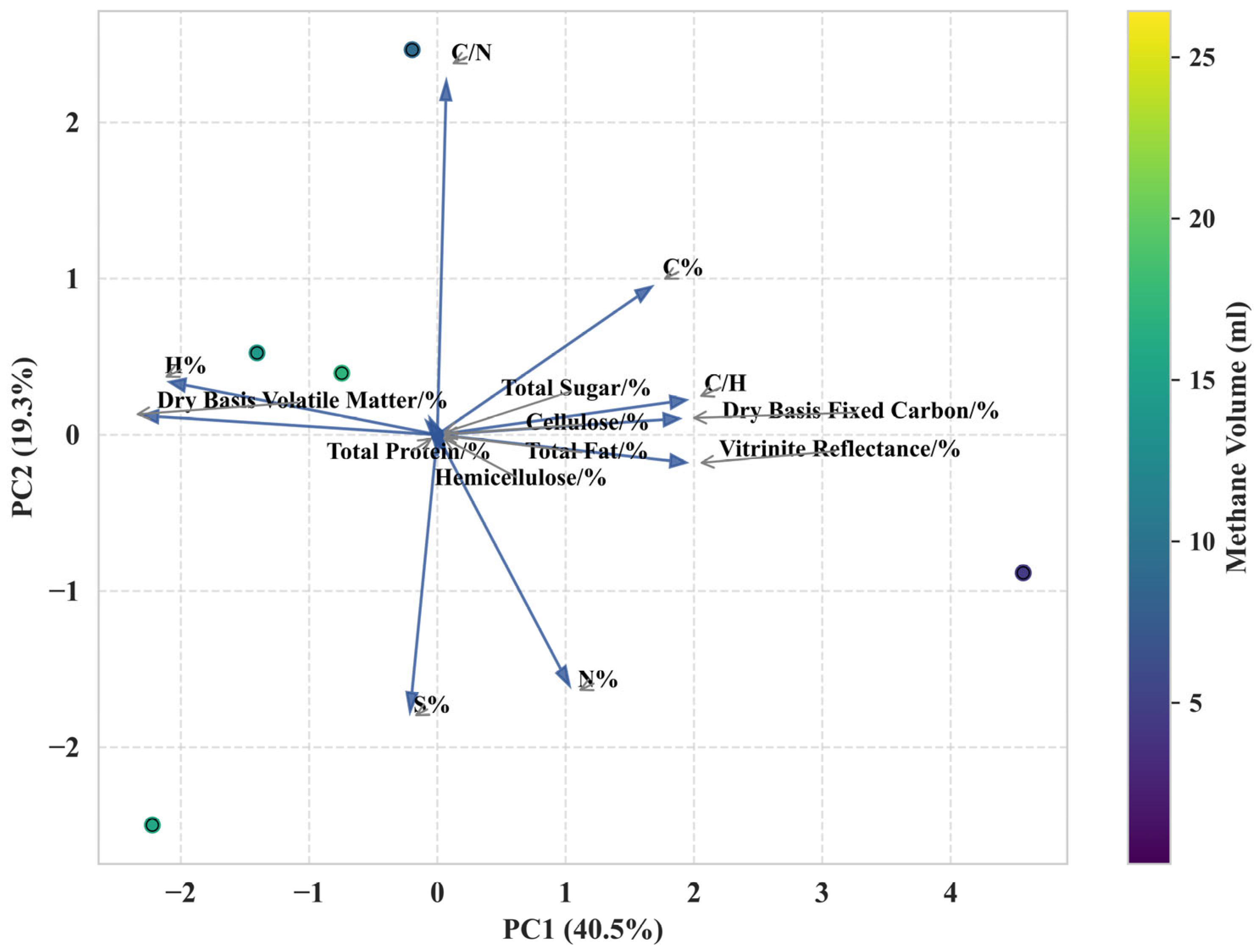
3.3.4. PCA Analysis
4. Discussion
4.1. Critical Components Affecting Methane Production
4.2. Components Weakly Correlated with Methane Production
4.3. Limitations and Research Implications
5. Conclusions
- (1)
- The highest methane-producing combination was that of BYH coal and Nannochloropsis, with a methane production of 26.43 mL, which was significantly higher than the other combinations tested. The lowest methane-producing combination was XZ coal and Dunaliella, with a methane production of only 0.03 mL. Coal performed the best among all algal combinations, and the microorganism that efficiently promoted methane production from coal was Nannochloropsis.
- (2)
- The hemicellulose content was a determinant of methane production, with each 1% increase in hemicellulose content increasing methane production by 7.76 mL/g coal. Total sugar and total fat decreased the efficiency of methane production.
- (3)
- The fermentation experiment preferred a combination of low-rank coal with a specular group reflectance <0.5%, volatile matter > content >35%, hemicellulose content >4.5%, and total sugar content <20%. Higher-order coal with a specular group reflectance >2.5%, volatile fraction <10%, and high-sugar microorganisms with total sugar > content >60% are not recommended.
- (4)
- The results of this study provide a basis for the application design of microorganism-enhanced methane production from coal; however, future studies need to be combined with microbial communities to improve the accuracy of the model predictions and further reveal the key regulators of the coal–microorganism co-fermentation system.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bao, Y.; Ju, Y.; Huang, H.; Yun, J.; Guo, C. Potential and constraints of biogenic methane generation from coals and mudstones from huaibei coalfield, eastern China. Energy Fuels 2019, 33, 287–295. [Google Scholar] [CrossRef]
- Davis, K.J.; Gerlach, R. Transition of biogenic coal-to-methane conversion from the laboratory to the field: A review of important parameters and studies. Int. J. Coal Geol. 2018, 185, 33–43. [Google Scholar] [CrossRef]
- Su, X.; Zhao, W.; Xia, D.; Hou, S.; Fu, H.; Zhou, Y. Experimental study of advantages of coalbed gas bioengineering. J. Nat. Gas Sci. Eng. 2022, 102, 104585. [Google Scholar] [CrossRef]
- Xia, D.; Zhang, H.; Hong, J.; Su, X.; Liu, X. Variation in endogenous trace elements during methane generation from different coal ranks. Energy Fuels 2017, 31, 12168–12173. [Google Scholar] [CrossRef]
- Huang, Z.; Sednek, C.; Urynowicz, M.A.; Guo, H.; Wang, Q.; Fallgren, P.; Jin, S.; Jin, Y.; Igwe, U.; Li, S. Low carbon renewable natural gas production from coalbeds and implications for carbon capture and storage. Nat. Commun. 2017, 8, 568. [Google Scholar] [CrossRef]
- Bao, Y.; Hao, Y.; Guo, Z.; Hu, Y.; Li, D. Experimental research on biogenic methane generation pathway and microflora constraints based on in-situ gas-water-microbe. Fuel 2024, 377, 132828. [Google Scholar] [CrossRef]
- Ponnudurai, V.; Kumar, P.S.; Muthuvelu, K.S.; Velmurugan, S.; Subhani, S.; Arumugam, L.; Rajarathinam, R. Investigation on future perspectives of ex-situ biogenic methane generation from solid waste coal and coal washery rejects. Fuel 2022, 318, 123497. [Google Scholar] [CrossRef]
- Fallgren, P.H.; Jin, S.; Zeng, C.; Ren, Z.; Lu, A.; Colberg, P.J.S. Comparison of coal rank for enhanced biogenic natural gas production. Int. J. Coal Geol. 2013, 115, 92–96. [Google Scholar] [CrossRef]
- Colosimo, F.; Thomas, R.; Lloyd, J.R.; Taylor, K.G.; Boothman, C.; Smith, A.D.; Lord, R.; Kalin, R.M. Biogenic methane in shale gas and coal bed methane: A review of current knowledge and gaps. Int. J. Coal Geol. 2016, 165, 106–120. [Google Scholar] [CrossRef]
- Fuertez, J.; Boakye, R.; Mclennan, J.; Adams, D.J.; Sparks, T.D.; Gottschalk, A. Developing methanogenic microbial consortia from diverse coal sources and environments. J. Nat. Gas Sci. Eng. 2017, 46, 637–650. [Google Scholar] [CrossRef]
- Welte, C.U. A microbial route from coal to gas. Science 2016, 354, 184. [Google Scholar] [CrossRef] [PubMed]
- Aramaki, N.; Tamamura, S.; Ueno, A.; Badrul, A.A.K.M.; Murakami, T.; Tamazawa, S.; Yamaguchi, S.; Aoyama, H.; Kaneko, K. Experimental investigation on the feasibility of industrial methane production in the subsurface environment via microbial activities in northern Hokkaido, Japan—A process involving subsurface cultivation and gasification. Energy Conv. Manag. 2017, 153, 566–575. [Google Scholar] [CrossRef]
- Zhu, L.; Yao, Q.; Diao, W.; Huang, Z.; Li, X. Bioelectrochemically enhanced biomethane production from low-rank coal using multiple microbial strains. J. Clean. Prod. 2024, 442, 141028. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, Z.; Guo, H.; Li, Z.; Huang, Z.; Urynowicz, M. Enhancement of biogenic methane production from coal using supercritical CO2. J. Supercrit. Fluids 2023, 201, 106023. [Google Scholar] [CrossRef]
- Huang, Z.; Urynowicz, M.A.; Colberg, P.J.S. Stimulation of biogenic methane generation in coal samples following chemical treatment with potassium permanganate. Fuel 2013, 111, 813–819. [Google Scholar] [CrossRef]
- Webster, J.; Lee, M.; Gurba, L.W.; Manefield, M.; Thomas, T. The effect of oxidative treatment on soluble compounds from australian coal. Fuel 2019, 257, 116071. [Google Scholar] [CrossRef]
- Haq, S.R.; Tamamura, S.; Ueno, A.; Tamazawa, S.; Aramaki, N.; Murakami, T.; Alam, A.K.M.B.; Igarashi, T.; Kaneko, K. Biogenic methane generation using solutions from column reactions of lignite with hydrogen peroxide. Int. J. Coal Geol. 2018, 197, 66–73. [Google Scholar] [CrossRef]
- Piao, D.; Song, Y.; Kim, D. Bioelectrochemical enhancement of biogenic methane conversion of coal. Energies 2018, 11, 2577. [Google Scholar] [CrossRef]
- Gao, L.; Feng, X.; Zhang, Y.; Guo, H.; Mu, X.; Huang, Z.; Urynowicz, M. Methane production from the biodegradation of lignite with different sizes by mixed fungi-methanogen microflora. FEMS Microbiol. Lett. 2024, 371, fnae037. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, J.; Duan, K.; Cheng, Y.; Guo, H.; Huang, Z.; Urynowicz, M.; Ali, M.I. The succession of microorganisms and organics in the process of methane generation by co-degradation of anthracite and rice straw. Int. J. Energy Res. 2022, 46, 15116–15126. [Google Scholar] [CrossRef]
- Xiao, D.; Peng, S.; Wang, E. Fermentation enhancement of methanogenic archaea consortia from an illinois basin coalbed via dol emulsion nutrition. PLoS ONE 2015, 10, e124386. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Deng, Z.; Wang, B.; Yu, Z. Coal-straw co-digestion-induced biogenic methane production: Perspectives on microbial communities and associated metabolic pathways. Sci. Rep. 2024, 14, 26554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, Z.; Zhang, D.; Medunic, G.; Urynowicz, M.; Liu, F.; Guo, H.; Haider, R.; Ali, M.I.; Jamal, A.; et al. Enhancement of biomethane production by huminite-enriched lignite pretreated with hydrogen peroxide. Int. J. Coal Geol. 2023, 274, 104284. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Hao, Z.; Ding, D.; Liu, Z. Clean utilization of lignite to produce biomethane by optimizing the microbial community. Energy 2023, 262, 125533. [Google Scholar] [CrossRef]
- Fallgren, P.H.; Zeng, C.; Ren, Z.; Lu, A.; Ren, S.; Jin, S. Feasibility of microbial production of new natural gas from non-gas-producing lignite. Int. J. Coal Geol. 2013, 115, 79–84. [Google Scholar] [CrossRef]
- Mcleish, A.G.; Vick, S.H.W.; Grigore, M.; Pinetown, K.L.; Midgley, D.J.; Paulsen, I.T. Adherent microbes in coal seam environments prefer mineral-rich and crack-associated microhabitats. Int. J. Coal Geol. 2021, 234, 103652. [Google Scholar] [CrossRef]
- Li, B.; Guo, H.; Chen, Z.; Xu, Q.; Xia, D.; Lv, J.; Yu, H. Metabolism mechanisms of biogenic methane production by synergistic biodegradation of lignite and guar gum. Sci. Total Environ. 2024, 946, 174085. [Google Scholar] [CrossRef]
- Penner, T.J.; Foght, J.M.; Budwill, K. Microbial diversity of western canadian subsurface coal beds and methanogenic coal enrichment cultures. Int. J. Coal Geol. 2010, 82, 81–93. [Google Scholar] [CrossRef]
- Barnhart, E.P.; Davis, K.J.; Varonka, M.; Orem, W.; Cunningham, A.B.; Ramsay, B.D.; Fields, M.W. Enhanced coal-dependent methanogenesis coupled with algal biofuels: Potential water recycle and carbon capture. Int. J. Coal Geol. 2017, 171, 69–75. [Google Scholar] [CrossRef]
- Zhang, J.; Park, S.Y.; Liang, Y.; Harpalani, S. Finding cost-effective nutrient solutions and evaluating environmental conditions for biogasifying bituminous coal to methane ex situ. Appl. Energy 2016, 165, 559–568. [Google Scholar] [CrossRef]
- Davis, K.J.; Lu, S.P.; Barnhart, E.P.; Parker, A.E.; Fields, M.W.; Gerlach, R. Type and amount of organic amendments affect enhanced biogenic methane production from coal and microbial community structure. Fuel 2018, 211, 600–608. [Google Scholar] [CrossRef]
- Smith, H.J.; Schweitzer, H.D.; Barnhart, E.P.; Orem, W.; Gerlach, R.; Fields, M.W. Effect of an algal amendment on the microbial conversion of coal to methane at different sulfate concentrations from the powder river basin, USA. Int. J. Coal Geol. 2021, 248, 103860. [Google Scholar] [CrossRef]
- Priyadarshani, I.; Rath, B. Commercial and industrial applications of micro algae—A review. J. Algal Biomass Util. 2012, 3, 89–100. [Google Scholar]
- Zhu, L.; Yao, Q.; Huang, Z.; Li, X.; Ma, Z. Biogenic methane generation from lignite coal at different temperatures. Gas Sci. Eng. 2023, 117, 205016. [Google Scholar] [CrossRef]
- ASTM D7582; Standard Test Methods for Proximate Analysis of Coal and Coke by Macro Thermogravimetric Analysis. ASTM: West Conshohocken, PA, USA, 2015.
- SRM 1632d; Trace Elements in Coal (Bituminous). NIST: Gaithersburg, MD, USA, 2014.
- ISO 11760; Classification of Coals. ISO: Geneva, Switzerland, 2018.
- Liu, Z.; Ma, C.Q.; Gao, C.; Xu, P. Efficient utilization of hemicellulose hydrolysate for propionic acid production using propionibacterium acidipropionici. Bioresour. Technol. 2012, 114, 711–714. [Google Scholar] [CrossRef]
- Xie, D.D.; Sun, Y.Q.; Lei, Y.A. Effect of glucose levels on carbon flow rate, antioxidant status, and enzyme activity of yeast during fermentation. J. Sci. Food. Agric. 2022, 102, 5333–5347. [Google Scholar] [CrossRef]
- Ma, S.S.; Wang, H.L.; Li, J.X.; Fu, Y.; Zhu, W.B. Methane production performances of different compositions in lignocellulosic biomass through anaerobic digestion. Energy 2019, 189, 116190. [Google Scholar] [CrossRef]
- Tomasini, M.; Faber, M.D.; Ferreira-Leitao, V.S. Sequential production of hydrogen and methane using hemicellulose hydrolysate from diluted acid pretreatment of sugarcane straw. Int. J. Hydrogen Energy 2023, 48, 9971–9987. [Google Scholar] [CrossRef]
- Hao, J.H.; de Los Reyes, F.L.; He, X. Fat; oil, and grease (fog) deposits yield higher methane than fog in anaerobic co-digestion with waste activated sludge. J. Environ. Manag. 2020, 268, 110708. [Google Scholar] [CrossRef]
- Aboudi, K.; Alvarez-Gallego, C.J.; Romero-García, L.I. Influence of total solids concentration on the anaerobic co-digestion of sugar beet by-products and livestock manures. Sci. Total Environ. 2017, 586, 438–445. [Google Scholar] [CrossRef]
- Strapoc, D.; Mastalerz, M.; Dawson, K.; Macalady, J.; Callaghan, A.V.; Wawrik, B.; Turich, C.; Ashby, M. Biogeochemistry of microbial coal-bed methane. Annu. Rev. Earth Planet. Sci. 2011, 39, 617–656. [Google Scholar] [CrossRef]
- Platt, G.A.; Davis, K.J.; Schweitzer, H.D.; Smith, H.J.; Fields, M.W.; Barnhart, E.P.; Gerlach, R. Algal amendment enhances biogenic methane production from coals of different thermal maturity. Front. Microbiol. 2023, 14, 1097500. [Google Scholar] [CrossRef] [PubMed]
| Domain | Phylum | Class | Order | Family | Genus | Microorganism Sample for Experiment |
|---|---|---|---|---|---|---|
| Plantae | Chlorophyta | Chlorophyceae | Chlamydomonadales | Dunaliellaceae | Dunaliella | Dunaliella |
| Chromista | Heterokontophyta | Eustigmatophyceae | Eustigmatales | Monodopsidaceae | Nannochloropsis | Nannochloropsis |
| Bacteria | Cyanobacteria | Cyanophyceae | Nostocales | Aphanizomenonaceae | Aphanizomenon | Aphanizomenon |
| Plantae | Rhodophyta | Bangiophyceae | Bangiales | Bangiaceae | Porphyra | Porphyra |
| Eukaryote | Bigyra | Labyrinthulea | Thraustochytrida | Thraustochytriidae | Schizochytrium | Schizochytrium |
| Sample | Moisture, % (ar) | Ash, % (d) | Volatile Matter, % (d) | Fixed Carbon, % (d) | Dry Basis, % (d) | Volatile Matter, % (ad) | Volatile Matter, % (daf) | Fixed Carbon, % (ad) | Fixed Carbon, % (daf) |
|---|---|---|---|---|---|---|---|---|---|
| BYH | 16.43 | 9.59 | 35.59 | 38.39 | 11.47 | 42.59 | 48.11 | 45.94 | 51.89 |
| XZ | 7.41 | 4.50 | 30.14 | 57.95 | 4.86 | 32.55 | 34.22 | 62.59 | 65.78 |
| MDL | 5.20 | 21.65 | 29.53 | 43.62 | 22.84 | 31.15 | 40.37 | 46.01 | 59.63 |
| LX | 11.90 | 9.87 | 29.26 | 51.76 | 11.20 | 30.05 | 33.84 | 58.75 | 66.16 |
| ZLS | 2.67 | 9.92 | 7.06 | 80.35 | 10.19 | 7.25 | 8.08 | 82.56 | 91.92 |
| Sample | C, % | N, % | H, % | S, % | C/H | C/N |
|---|---|---|---|---|---|---|
| BYH | 46.74 | 1.09 | 3.93 | 0.99 | 11.89 | 42.88 |
| XZ | 62.59 | 0.84 | 4.11 | 0.67 | 15.23 | 74.51 |
| MDL | 60.42 | 0.93 | 4.44 | 0.29 | 13.61 | 64.97 |
| LX | 65.16 | 0.67 | 3.67 | 0.21 | 17.74 | 97.25 |
| ZLS | 73.81 | 1.26 | 2.88 | 0.61 | 25.63 | 58.58 |
| Sample | Average Reflectivity, % | Reflectivity, % |
|---|---|---|
| BYH | 0.250 | 0.25 to <0.30 |
| XZ | 0.459 | 0.45 to <0.50 |
| MDL | 0.504 | 0.50 to <0.55 |
| LX | 0.762 | 0.75 to <0.80 |
| ZLS | 2.671 | 2.65 to <2.70 |
| Microorganism | Total Sugar, % | Total Protein, % | Total Fat, % | Hemicellulose, % | Cellulose, % |
|---|---|---|---|---|---|
| Dunaliella | 69.5 | 0.4 | 4 | 0.08 | 0.04 |
| Nannochloropsis | 12 | 37.4 | 3.2 | 4.51 | 0.18 |
| Aphanizomenon | 17.2 | 50.5 | 2.86 | 0.08 | 4.85 |
| Porphyra | 45.3 | 21.8 | 2.24 | 0.67 | 5.03 |
| Schizochytrium | 16.1 | 17.2 | 2.86 | 1.52 | 3.43 |
| Sample | Dunaliella/mL | Nannochloropsis/mL | Aphanizomenon/mL | Porphyra/mL | Schizochytrium/mL |
|---|---|---|---|---|---|
| BYH | 0.03 | 26.43 | 0.33 | 23.43 | 15.7 |
| XZ | 0.01 | 14.44 | 0.18 | 19.98 | 17.05 |
| MDL | 0.03 | 12.16 | 0.65 | 23.49 | 14.29 |
| LX | 0.03 | 25.08 | 6.53 | 9.05 | 9.27 |
| ZLS | 0.02 | 6.08 | 0.29 | 21.28 | 4.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Diao, W.; Gong, C.; Wang, H.; Zhu, P.; Liu, Y. Substrate Composition Effects on the Microbial Enhancement of Biogenic Methane Production from Coal. Sustainability 2025, 17, 4953. https://doi.org/10.3390/su17114953
Zhu L, Diao W, Gong C, Wang H, Zhu P, Liu Y. Substrate Composition Effects on the Microbial Enhancement of Biogenic Methane Production from Coal. Sustainability. 2025; 17(11):4953. https://doi.org/10.3390/su17114953
Chicago/Turabian StyleZhu, Liu, Wangjie Diao, Chenyao Gong, Haihan Wang, Peilin Zhu, and Yi Liu. 2025. "Substrate Composition Effects on the Microbial Enhancement of Biogenic Methane Production from Coal" Sustainability 17, no. 11: 4953. https://doi.org/10.3390/su17114953
APA StyleZhu, L., Diao, W., Gong, C., Wang, H., Zhu, P., & Liu, Y. (2025). Substrate Composition Effects on the Microbial Enhancement of Biogenic Methane Production from Coal. Sustainability, 17(11), 4953. https://doi.org/10.3390/su17114953