Abstract
In the context of climate policy, measures are being taken around the world to reduce pollution. These have been intensified in the areas of transport, industry, and energy, with the goal of zero emissions. The role of the biogas plant in energy transition and as a waste treatment plant for disposal is very important. This article describes research on a dynamic anaerobic digestion (AD) process plant. The subject of this study was leather shavings, which is a problematic waste. The research presented here is intended to demonstrate the decomposition of the flesh in the process, to confirm its biogas yield, and to evaluate the biological and technical parameters of the process. High biochemical stability was achieved for each of the tests evaluated, and no specific technical requirements were demonstrated. The only technical aspect to be addressed during operation was sedimentation, which can be solved by preparing the mixture earlier or by changing the mixing method. This made it reasonable to investigate the material further in the context of an industrial project. The characteristics of protein degradation in the AD process resulted in a high methane content in the biogas, above 65%. It was also observed that the long conditioning time of biogas in the gas cushion favourably affected the proportion of methane in biogas. Analytical results confirmed 77.5% methane content, which was a very good result. This paper presents the results of a surprising effect of chromium, primarily Cr (III), on the performance of anaerobic digestion.
1. Introduction
With energy transition being intensively implemented in most parts of the world, research is underway to improve the performance of energy technologies. This includes sources, storage, and the development of energy transport systems (power grids). The overarching aim of these activities is to reduce emissions of gases with greenhouse gas potential, thereby reducing the anthropogenic impact on climate change. The EU, USA, China, Japan, India, and South Africa account for approximately 87% of global greenhouse gas (GHG) (CO2 equivalent) emissions and, currently, trends in these countries vary [1,2,3,4]. However, these countries have issued documents setting out their climate policies and declare their climate neutrality by 2050–2070 [5]. The energy sector is responsible for significant global greenhouse gas emissions. The IPCC’s Technology-Specific Cost and Performance Parameters Report [6] was prepared to determine the impact of particular energy generation, conversion, and storage technology. It shows that the median commonly used energy sources are lowest for offshore wind, wind on shore, and nuclear power. According to the IEA report [7], electricity production from biogas is burdened by emissions of 320 . Unfortunately, this analysis does not considernumerous beneficial factors, such as avoided CO2 emissions, reduced emissions of other greenhouse gases, and energy efficiency, which is high for controllable energy sources. This is probably due to the high complexity of the technology, and the complexity of using the process versus not using it. Each plant, especially if operating with other systems, must be approached on an individual basis.
Biogas plants can be divided into wastewater, agricultural, and industrial. Their economic and environmental effect depends primarily on the substrate supply chain, its origin, the efficiency in the anaerobic digestion, and its avoided emission factor [8]. Several pieces of the literature were analysed that presented life cycle assessment (LCA) results for diverse types of biogas plants. They described cases where biogas plants have a beneficial environmental effect for livestock facilities [9], for the management of vegetable agricultural waste [10,11], and for wastewater facilities [12]. By processing the substrate in anaerobic digestion processes, the following is achieved: improved nutrient availability for plants [13,14,15,16], reduced nitrogen oxides and methane emissions from the digestate as a fertiliser relative to raw slurry or manure [17], elimination of disease-causing microorganisms and plant fungus, and reduced amounts of some environmentally hazardous substances [18]. Therefore, the prominent level of average emissions should be considered in the context of a closed-loop economy, not only in the context of an energy source [9,17,18,19,20].
According to the Act of 17 August 2023 amending the Renewable Energy Sources Act and Certain Other Acts [21], which was drafted based on the recommendation of the committee of 14 March 2023—energy storage, the basis for a decarbonised and secure EU energy system (2023/C 103/01) [22]—the energy storage is any isolated device or set of devices for storing energy in any form not producing emissions that are a burden on the environment, in such a way that it can be at least partially recovered. A similar definition is proposed in the article by Twitchell et al. [23]. It follows that any energy source that provides dispatchability can be an energy store, i.e., it can be switched on and off. This means that, in the case of some energy storages, they are recharged through fuel supply or on-site generation. A detailed review of the technology and needs for energy storage was conducted in the monographs by Nozari et al. [24] and Zhang et al. [25].
A biogas CHP plant (combined heat and power) fits into both definitions of energy storage. It is a unit of energy that has a high availability, so it is a special case of energy source and energy storage in the form of a single plant [26,27].The vast majority of biogas plants are combined with cogeneration plants that convert the generated biogas into electricity and heat. Inside the tank, biomass is processed, which is often classified as waste. Therefore, the loading of the energy store, which is a biogas tank or gas cushion over the fermenting biomass, is conducted by supervising the process and ensuring a continuous supply of substrate [28]. The parameters of a biogas plant as an energy store can be easily developed, for example, by using compression systems or systems for cooling biogas and storing it in a compressed or liquefied form [29].
Hybrid energy systems or integrated agro-energy systems, of which an agricultural biogas plant is an essential component, are frequently designed and constructed on an industrial scale. Thanks to its multifunctionality, processes can be conveniently combined to allow a symbiosis effect and therefore an improved energy balance. The most common RES hybrid solution is a hybrid plant consisting of photovoltaics and an agricultural biogas plant [30,31,32]. There are also many other methods of using solar and wind energy for useful hybrid energy. The most common for this purpose is hydrogen, which, as a gaseous fuel, can complement very well biogas or biomethane production [33].
An interesting study was presented in the article by Mendeck et al. [30]. The aim of the study was to develop such a system operation model where100% renewable energy could be managed. The function of the emergency energy source was performed by an internal combustion engine, which was switched on when it was not possible to supply consumers with renewable energy. The results of the study were strongly dependent on the location analysed.
Another solution for the cooperation of these two energy sources is to allocate the excess energy for biogas upgrading. An interesting hybrid system was created based on the separation of biogas to power the engine and to process it into biomethane. This allowed a favourable economic and environmental effect [31].
Agro-energy systems often require specific forms of energy or factors. In addition to electricity and water, heat, cooling, gaseous fuel, solid fuel, fertilizer, bedding material, and many forms of energy are often required on farms. Numerous studies confirm the validity of combining the characteristics of different energy sources applicable to agriculture. An exceptionally good example of this is the research on trigeneration systems conducted by W. Gazda and W. Stanek [34].They proved that remarkably high energy savings are possible (about 40%) and that the environmental effect is favourable, providing a reduction in GHG emissions of about 60% relative to equipment operating independently. Other studies [35] show that the cooperation of a biogas plant with an organic Rankine cycle system improves the annual electricity production by about 10%.
In addition to energy and environmental benefits, biogas plants also provide numerous benefits related to the utilisation of organic waste [36,37,38] or the treatment of organic production residues [39,40].
As part of the MIZDRA 2.0 project, research was conducted to develop a rational management of leather shavings. The anaerobic digestion (AD) studies conducted took into account many aspects that are not considered in standard biogas studies. The dynamic method of anaerobic digestion also makes it possible to evaluate the mixing method used, the behaviour of the feedstock in the individual phases of AD, and the deposition of inhibitors contained in the substrate in the process tank. In this article, the material studied, the inoculum, the laboratory plant, and the semi-technical plant are presented in detail. The dynamic method is a commonly used substrate biogas method among agricultural biogas plants, food processing, and wastewater management.
2. Materials and Methods
2.1. Goal and Scope
The aim of this research was to test the effective management of leather shavings, which is a waste product of the leather industry. It was conducted within the project “Development of technologies for rational management of leather shavings from leather processing (MIZDRA 2.0)” co-financed by the National Centre for Research and Development from the Smart Development Operational Programme by three scientific units and a company. This project investigated processes such as methane fermentation, fertilisation [41,42], drying, pelleting, briquetting, pyrolysis, incineration [43], membrane filtration of process leachates [44], and others.
2.2. Substrate Processing Levels
Leather shavings are an exceedingly difficult waste to process. An extensive description of the process to obtain shavings is described within publication [44], using the example of the tannery from which the analysed material originated.
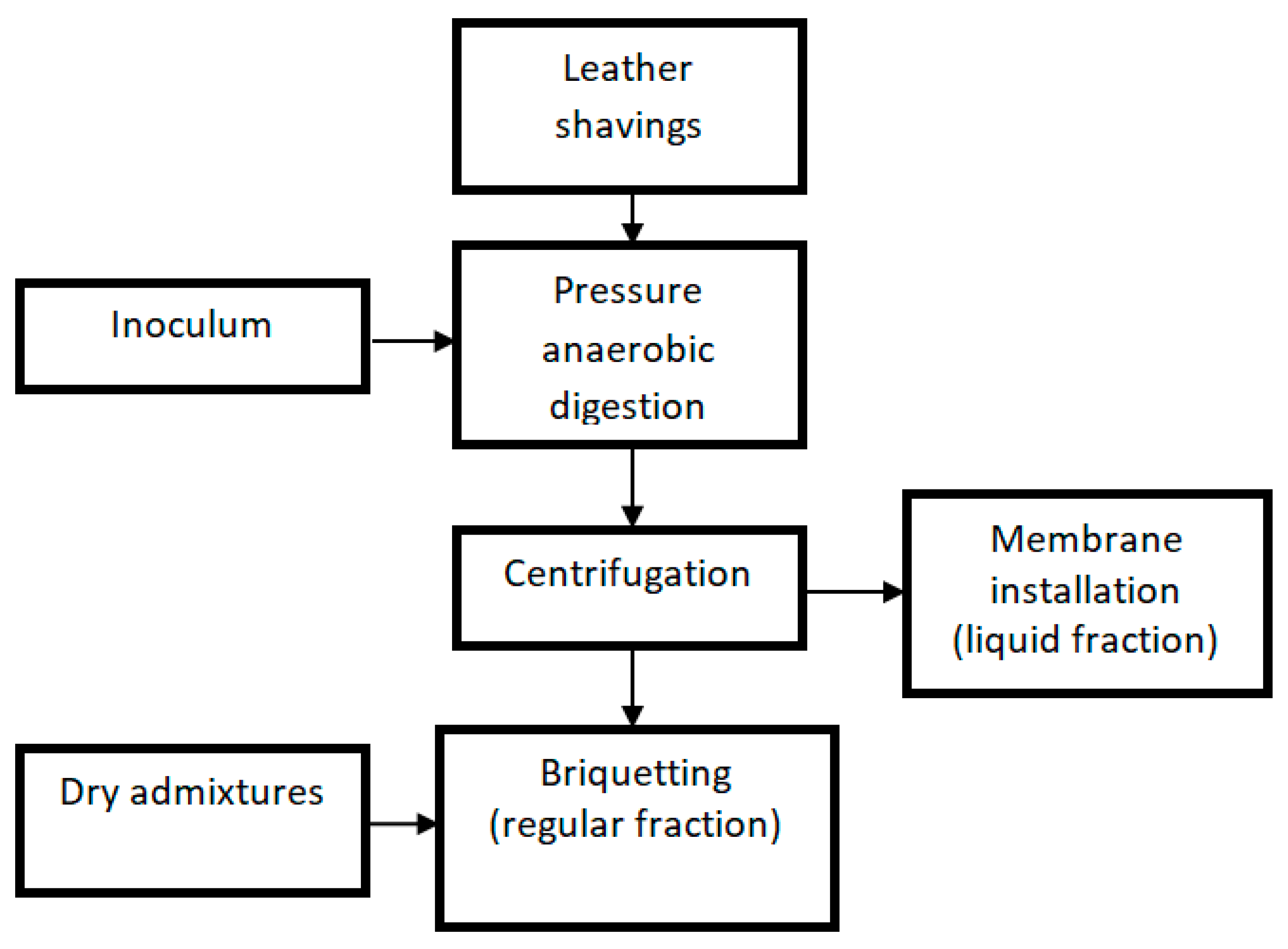
As illustrated in Figure 1, equipment was purchased as part of the project to provide a processing line for the waste. The operation of this system would produce gaseous biofuel (biogas), solid biofuel (briquette), and a small amount of liquid fraction with an increased chemical oxygen demand (COD)value (concentrate) and a large amount of liquid fraction with a reduced COD value (permeate). However, our work primarily focused on enhancing the AD of leather waste.
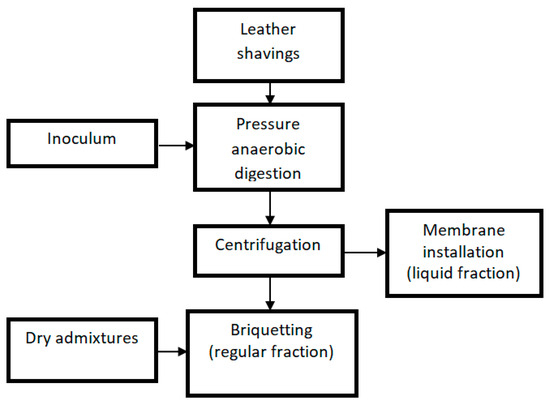
Figure 1.
Scheme of the technological system for the processing of leather shavings.
2.3. Leather Wastes (White and Blue)
Basic physical analysis confirmed that it was a solid loose material with a moisture content that was dependent on time and storage conditions. The dry matter of the substrate was determined by the drying method at 105 °C, according to the method in PN-ISO 6496:2002 [45]. When the leather waste was in direct contact with air, the material reached an absorption–desorption equilibrium of 24–29% moisture content. In the case of material not exposed to air, the water content of the material was approximately 45–57%. The bulk density of the test material was strongly dependent on its moisture content. For material received directly from the manufacturer, it was 121 kg/m3.
The fraction of the material was highly variable. Some of it was dusty, while most of the mass was in the form of shavings (long chips) up to 150 mm in length. In addition, the material was visibly fibrous, elastic, soft, and moderately strong.
Due to the origin of the material, much of the content of the leather waste was protein and organic nitrogen. Blue leather waste can contain significant amounts of chromium, which, at the right concentration, is an inhibitor of the fermentation process. A detailed description of the material and the processes to which it is subjected is presented in the article by E. Wrzesińska-Jędrusiak et al. [44].
2.4. Inoculum
A co-substrate was selected as the inoculum for the fermentation process, the composition of which could include enzymes that degraded the difficult test material. In order to confirm this hypothesis, an additional study was carried out, which is described further in Section 2.5. The inoculum was wastewater that had been fermented in an industrial biogas plant. The material was in a liquid state, with a water content of more than 97%. For the purposes of this study, the density of the sludge was taken as a value of 1 . According to the Regulation of the Ministry of the Environment, Natural Resources and Forestry, waste from the fleshing process was classified under the number 04 01 01. Sewage sludge was also classified as waste under 19 08 05 [46]. This classification complies with the European waste catalogue of 18 December 2014 (2014/955/EU) [47]. It was developed in accordance with the requirements of the Waste Act, taking into account the new requirements for waste prevention programmes set out in Directive (EU) 2018/851) [48], here in after referred to as “Directive 2018/851”. Consequently, two organic wastes for which no further treatment or management route is foreseen are possible within the scope of the ongoing research.
2.5. Mixture of Leather Shavings and Inoculum
The mixture of these materials had variable properties depending on the retention time. At the beginning of the mixture formation, leather shavings were subjected to a flotation process. Depending on the proportions adopted, the flotation period varied. After a few days, sedimentation took place. This time depended, among other things, on the fraction of the material, as was demonstrated in a simple study. The same mixture was prepared as was added each day to the digester for anaerobic digestion, i.e., a mixture of 3 litres of inoculum and 0.08 kg of leather shavings with a moisture content of 45–50%. Two trials were prepared. The first used non-mixed leather shavings, the second one contained mixed leather shavings. The flotation period was noticeably shorter for the mixed leather shavings (about 4 days) than for the non-mixed ones (about 7 days). This was due to the better availability of water for the material, which accelerated the soaking process of the material and consequently faster sedimentation.
This study lasted for 60 days. This simple test was designed to see whether the inoculum contained enzymes that broke down proteins and other chemicals found in the leather shavings. The second, much more important reason, was to check the flotation and sedimentation of the wet mizdra. Implementing a methodology of soaking the leather shavings for several days before dosing it into the process avoided the formation of a dross that prevented the biogas from escaping into the gas cushion.
2.6. Static Anaerobic Digestion Process According to DIN 38314
In order to determine the biogas yield of leather shavings, a laboratory-scale AD process was carried out in accordance with DIN 38 414 [49]. In order to be able to determine this value, it was also necessary to determine the biogas yield of the wastewater that was used as the inoculum in this study. The static method of testing provided a reference value for the anaerobic digestion process on a semi-technical scale using the dynamic method.
The fermentation station comprised six 1 dm3 glass reactors placed in a water bath. The bath was equipped with an electric heater and a pump to ensure constant temperature distribution throughout its volume. The heater was switched on by a signal received from a thermostat based on the measurement of the water temperature. The gas was discharged into a gas cushion bound by the liquid contained in the column around the gas escape channel. Based on the difference in the level of the liquid displaced by the biogas, the volume of biogas produced was read. Fermentation was carried out at a temperature of 39 °C. Due to the low biogas yield, no analysis of the biogas composition was carried out. The results of the study are presented in Table 1.

Table 1.
Biogas yield from AD according to DIN 38414.
2.7. Research Facility for Biogas Production
This research was conducted on a semi-technical scale system (shown in Figure 2), with a process tank with a capacity of 140 L (active 60–120 L). The most important component of the system was the process tank—the digester. Inside it, the anaerobic digestion process took place. It had a heating jacket whose purpose was to maintain the temperature inside the process tank. A heating system, consisting of an electric heater and a circulation pump, was responsible for heating the water in the heating jacket.

Figure 2.
Visualisation of laboratory biogas plant.
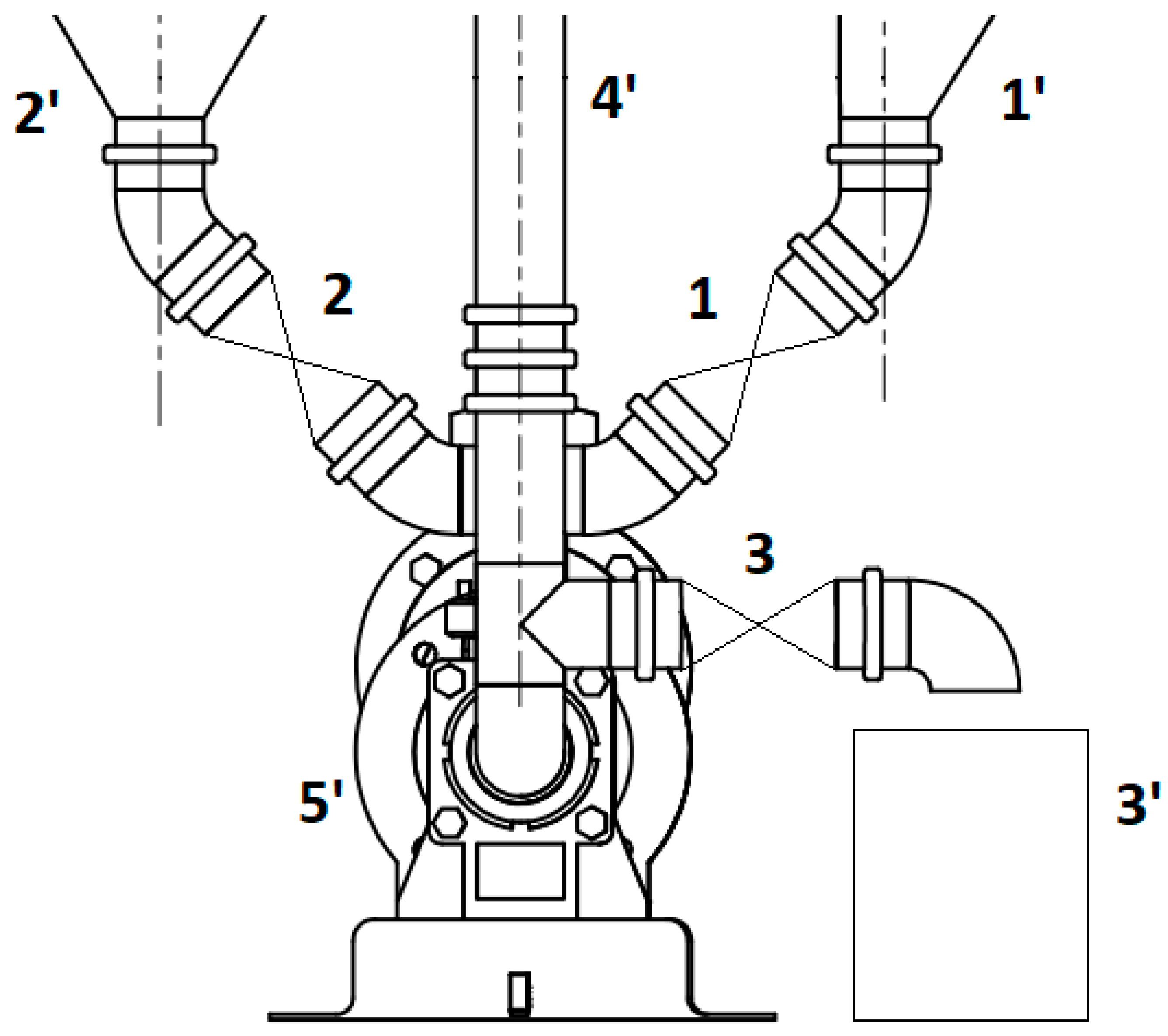
A screw pump (5′), driven by a geared electric motor, was designed for dosing, pouring, and mixing the substrate. The motor was electrically connected to an inverter, which allowed the speed of the motor to be varied and, therefore, the speed of dosing, pouring, and mixing. Figure 3 shows a schematic of the material distribution node between the pre-tank (1′), process tank (2′), and digester (3′) and mixing by taking the lower contents of the digester and feeding through a channel (4′) into its upper part.
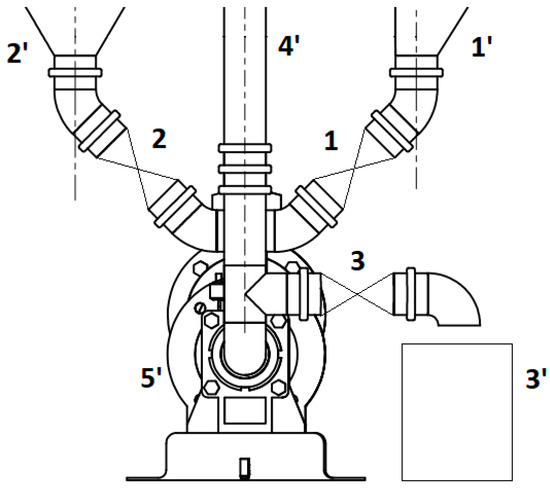
Figure 3.
Material distribution unit (1′—pre-tank, 2′—process tank, 3′—digestate tank, 4′—top channel, 5′—screw pump, 1–3—ball solenoid valves).
The research installation operated in a semi-automatic mode. All settings were made from the computer (except for the biogas analysis). The operational maintenance by the research team members involved securing the substrate supply to the tank and emptying the digestate tank. Most of the results were collected automatically in a .csv file; only the reading of the biogas produced and the electricity consumed by the entire system was performed manually. The actuators of the control system were a pump, ball valves (1, 2, 3), a pneumatically operated flap valve, a thermostat, and an inverter. Together with the prepared plant operating algorithm, it was the solenoid valves that determined the implementation of pouring, topping up, or mixing. Table 2 shows the solenoid valve settings, where these, together with the prepared plant algorithm, were responsible for the implementation of pouring, topping up, or mixing.

Table 2.
Solenoid valve settings for various pump operations (C—close, O—open).
The plant, with an appropriate safety factor, was designed to operate at an overpressure of 0.3 MPa. It has several safety features to ensure safe operation. These included a safety valve (which opens when the pressure exceeds 0.3 MPa), a dry-run sensor (which shuts down the pump when the flow is interrupted), a non-return valve (which prevents the fermentation pulp from flowing back), and other safeguards that were incorporated into the programme for when alarm conditions occur for selected process parameter values.
The unit was equipped with sensors for process temperature (in the axis of the tank, at a depth of approx. 70 cm), water jacket temperature, pressure (in the upper bottom), liquid level (in the upper bottom), a gas meter, and an energy meter. Periodically, a biogas analysis was performed using an external device. A manual ball valve was installed below the process vessel isolation to allow sampling for biotechnological analysis of the substrate and process control. The other parameters of the DTR F120 plant are shown in Table 3.

Table 3.
Digester specifications.
2.8. Dynamic AD Process
A basic study was carried out, the results of which are included in Table 4. The pH of the digester pulp was regularly checked using a pHmeter adapted for wastewater and aggressive organic matter [50].

Table 4.
Process parameters for anaerobic fermentation of leather shavings.
A mesophilic AD process was carried out in the described plant. During the biotechnological start-up of the plant, material properties were observed that had not been investigated in the basic analysis. Once the material properties were known, the constant operating parameters shown in Table 4 were selected.
Based on the assumed HRT time, a daily dose of a mixture consisting of 3 kg of inoculum and 80 g of leather shavings was calculated. For the process, the water content of leather shavings was a critical issue. The organic dry matter content of the test material depended on this, which affected the organic load inside the digester. The higher the dry matter content and thus the organic dry matter in the leather shavings, the higher the FOS/TAC, and this also affected other biological and chemical factors [51]. An attempt was made to keep the water content of the leather shaving constant at each stage of the process at around 45%. Although the HRT value was dependent on the daily dosage, which was a value that varied to some extent from case to case, the HRT was classified as a constant value. Its value fluctuated within a narrow range. Table 5 shows the values of the variables of the consecutive research trials.

Table 5.
Variable parameters of the anaerobic digestion process of leather shavings.
In test 1, a batch of a mixture of inoculum and ground leather shavings was prepared, and this was then fed into the process tank. In test 2, the method of dosing the substrate into the pre-tank was changed. The prepared mixture had an approximate 5-day conditioning time with occasional stirring to effectively soak the leather shavings to start their sedimentation. This procedure was crucial for the uniform dosing of leather shavings into the digester; its flotation resulted in only inoculum being pumped, as shown in the scheme in Figure 2. In addition, the mixing of shavings was discontinued. The clapper valve limit pressure was also increased to 0.1 MPa overpressure. Test 3 was conducted to determine the effect of the addition of glycerine, which was expected to increase the organic load and biogas growth and to determine the effect of fat decomposition on the biogas composition. Subsequently (test 4), the organic load was increased to test the possibility of recovering part of the solid fraction in the digestate and to determine the maximum load. Due to the possible inhibitor content of the blue leather shavings, they were fermented last. Test 5 aimed to primarily verify the effect of chromium on the anaerobic digestion process. In each case, after the HRT considered to be the time for the digester contents to stabilise for at least 14 days, the biogas yield and its relevant parameters were examined and the results were then approximated to the full HRT time. A similar methodology should not be adopted for static (batch) fermentations, as different biogas yields were observed each day. In the following, it was confirmed that the daily biogas yield in the systems studied was almost constant.
2.9. Biogas Measurements
There were several sources of measurement error in the biogas production. The most significant of these resulted from minor leaks in the path between the clapper solenoid valve and the gas meter, changes in the volume of the gas cushion during substrate pouring and topping up, evaporation of water from the fermenting biomass, and sensitivity and measurement inertia of the electronic manometer.
Therefore, its value was determined in three ways, followed by the calculation of the mean value and calculation of the standard deviation. The first was based on reading the value of the gas meter, which measured the vented biogas in an analogue manner. The second was based on measuring the pressure prevailing in the gas cushion, considering the volume of the gas cushion. The last considered the pressure difference between successive measurements before and after the clapper solenoid valve was opened, multiplying the resulting difference by the volume of the gas cushion.
In order to check the composition of the biogas, the gas path was altered using manual ball valves. As a result, when overpressure occurred and the clapper valve was opened, biogas was vented from the digester through the gas meter up to the biogas sample bag. The biogas sample was then sealed and transported to an external laboratory, which used the Biogas Analyser 5000GE produced by Geotech subsidiary LandTec in Dexter Bishop Circle West (Dexter, MI, USA). This instrument is designed to determine the biogas content in terms of its four main components: methane, carbon dioxide, oxygen, and hydrogen sulphide. Each of these gases has a particularly significant role in the context of operating a biogas plant. The level of methane determines the calorific value of the biogas produced and is the most important product of the process. Carbon dioxide is an inert gas for the fermentation process and can be partially converted to methane in the digester. The oxygen content of biogas is an undesirable factor; methanogenic bacteria should function in a strictly anaerobic environment [52]. Hydrogen sulphide is a gas whose main combustion product is sulphuric acid. As in many plants, the fuel produced is converted in the internal combustion engine into electricity and heat, and it is imperative that hydrogen sulphide is removed prior to the combustion process [53,54].
The biogas analysis lasted 30–60 s. During this time, the analyser’s pump was running, taking in the tested gas and stabilising the displayed values close to the actual biogas composition.
2.10. Digestate Analysis
The analysis of digestate had important implications for the context of this entire study. Its main objective was to manage the waste and further process it into different forms. Preliminary biogas yield studies on a laboratory scale concluded that leather shavings would not provide optimal amounts of biogas. Hence, in line with the circular economy, it was decided to use two materials classified as waste and produce a product from them by anaerobic digestion. Ammonium nitrogen was determined according to the methodology contained in PN-C-04576-15:1975 [55], while total nitrogen was determined using the Kjeldahl method [55]. Volatile organic acids (FOSs) were determined according to the method of PN-75/C-04616/04 [56].
3. Results and Discussion
After some corrective adjustments, it was possible to run the process and monitor it. Fixed operating parameters and variable parameters such as organic load, process overpressure, and feedstock were assumed. On this basis, the effects on other parameters, especially energy and operating parameters, were determined.
Biotechnological monitoring is an important part of the fermentation process. Each of the parameters (Table 6) must have a specific value to provide the right conditions for microorganisms to live and to ensure the right amount of organic material as food.

Table 6.
Digester content test results.
The organic load should vary between 0.2 and 0.4 of the FOS/TAC ratio. If the value of 0.4 is exceeded, the digester will be overloaded with dry organic matter, the reaction rate will be inhibited by a change in pH, and, as a result, the biogas yield of the mixture will be lower or the process will take longer [54,57]. In the present study, safe conditions were provided for the growth of anaerobic bacteria, and a higher organic loading of the process tank was possible. This was determined according to the methodology presented in the study of N. Nagao et al. [58].
A clear increase in ammoniacal nitrogen, FOS, and TAC values was observed after changing the test material from white leather shavings (tests 1–4) to blue leather shavings (test 5). In the case of an increase in ammoniacal nitrogen, this had an effect related to the increase in pH and alkaline potential. By applying the same dose in test 4 as in test 5, the FOS/TAC ratio remained unchanged. Despite the invariability of the FOS/TAC ratio, the marked increase in volatile fatty acids indicated a possible faster saturation of methanogenic microorganisms and, therefore, also a lower maximum organic load that could be applied to the anaerobic digestion of blue leather shavings compared with white leather shavings. The maximum organic load with which it would be possible to load the reactor would therefore be limited by the formation of salts (reaction of fatty acids with carbonates), which, once the value was exceeded, would inhibit the process of anaerobic decomposition [59,60].
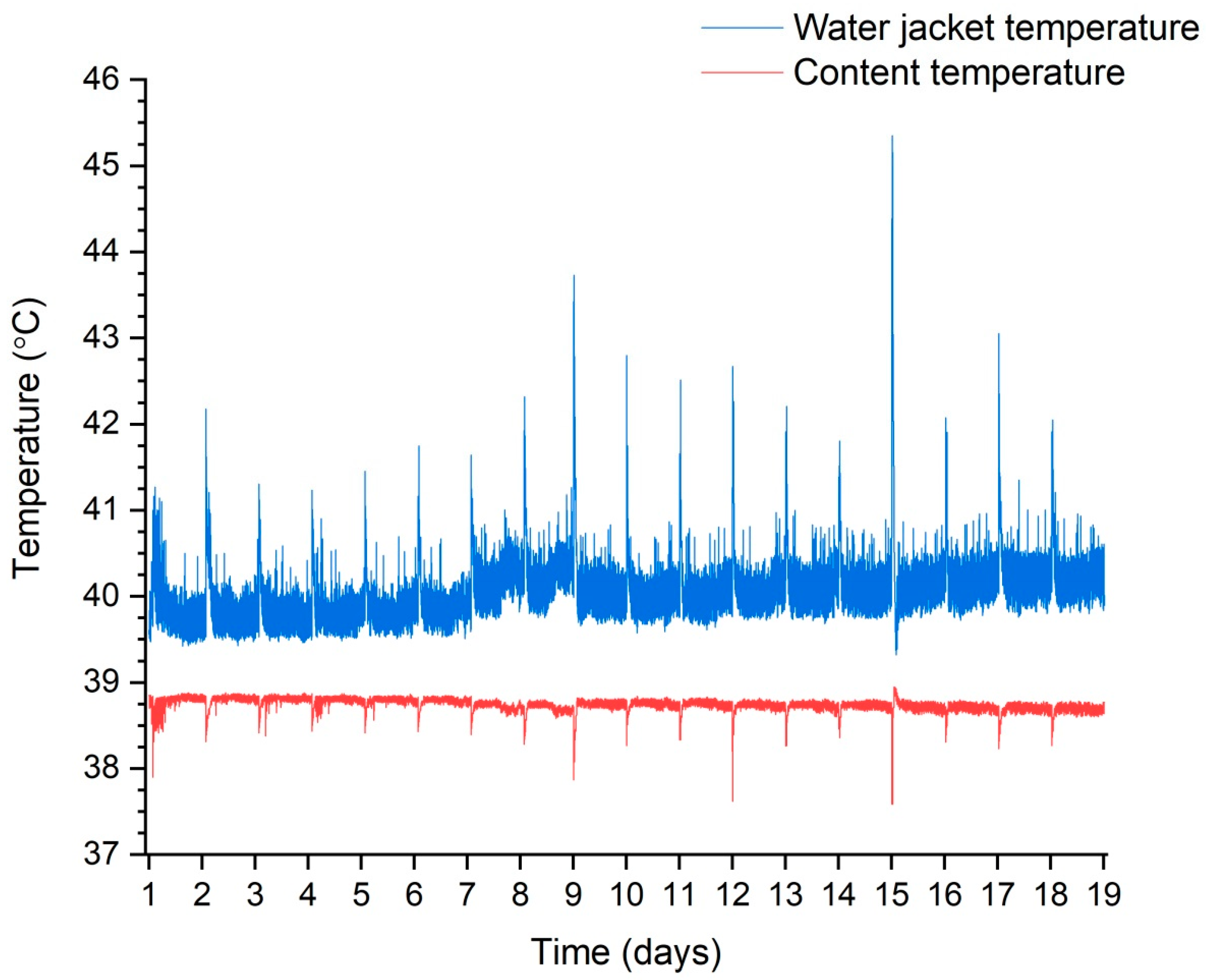
The process temperature was controlled continuously and dynamically. Figure 4 shows how the temperature inside the process tank was maintained during one of the test trials by controlling the electric heater in the heating jacket circuit. The temperature significantly decreased every 24 h following the end of fresh substrate dosing. The process of reheating to 38.3 °C usually took about 15 min. It shows that, for more than 99% of the time, the content temperature was between 38 and 39 °C.
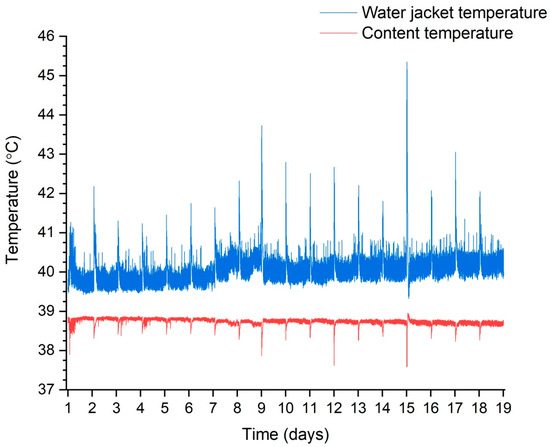
Figure 4.
Temperature maintenance during the process using trial 3 as an example.
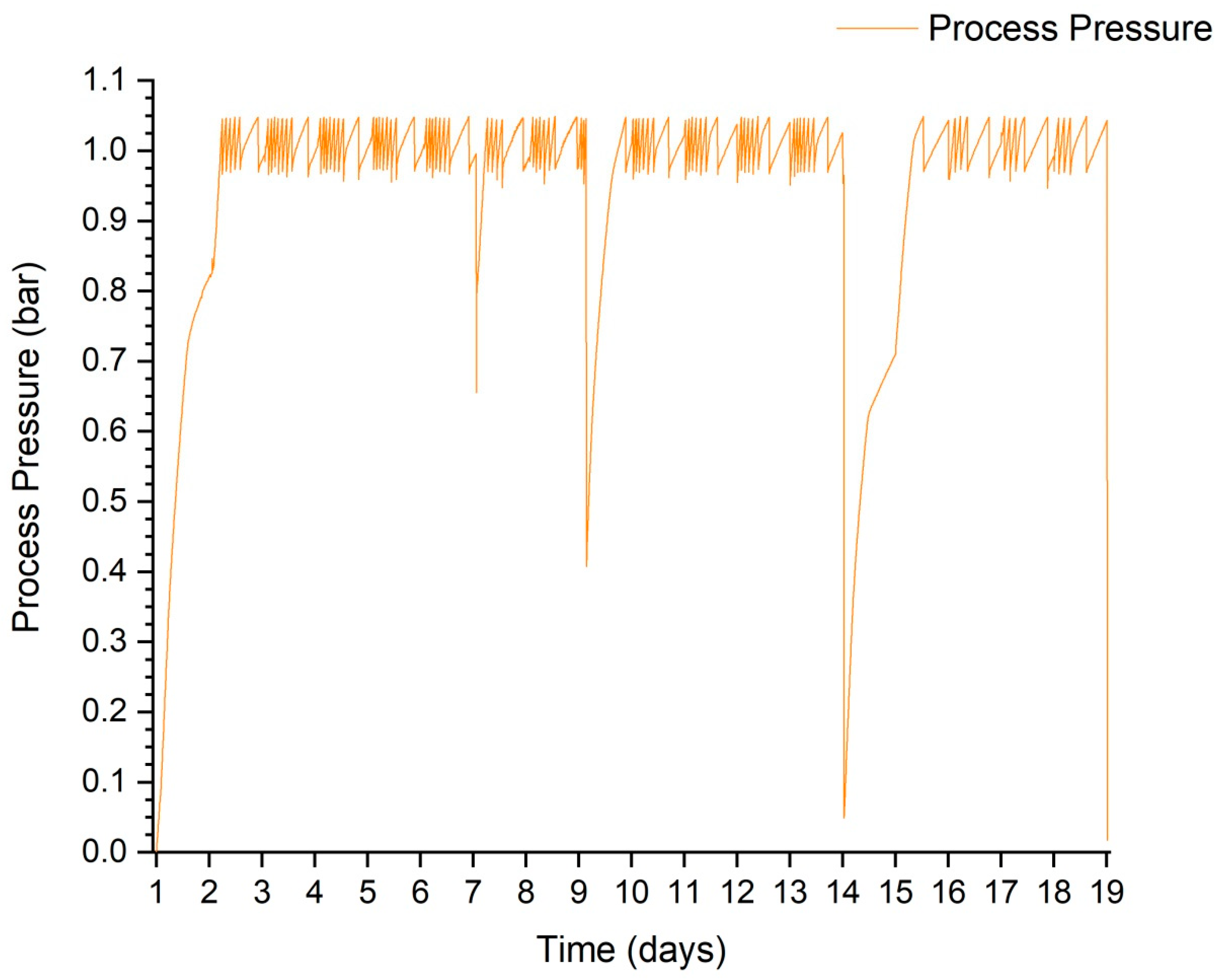
The pressure inside the tank was measured every 5 min. This sampling allowed its value to be tracked as accurately as possible, limiting the amount of data that would be processed in the case of 1 min measurements. Its value allowed biogas production to be observed throughout the anaerobic digestion stage. Figure 5 shows the pressure waveform during test 3. This graph shows very well the effect of feeding the digester and the influence of a temporary reduction in the temperature of the contents on the subsequent pressure rise. Most of the time, the pressure in the gas cushion fluctuated between 0.095 and 0.105 MPa. The lowering of the pressure to a lower value was due to the need to take a sample of the biogas to analyse its composition.
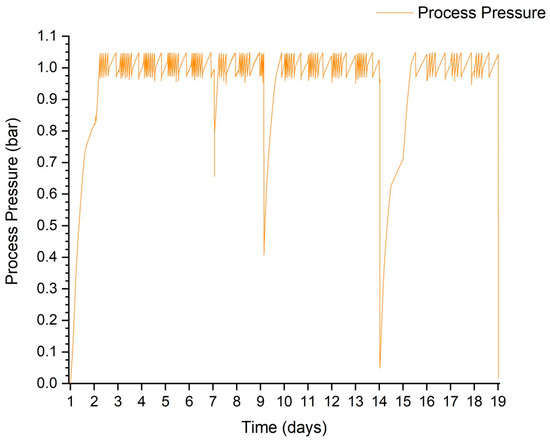
Figure 5.
Course of pressure fluctuations during the process using trial 3 as an example.
Table 7 shows the results for the biogas yield. The biogas yield of pure leather shavings was calculated from the difference in biogas yields of the other components of the mixture, considering their total weight in the digester and dividing by the total weight of leather shavings in the digester. Although a biologically/chemically stable inoculum was applied, which had already been decomposed, its low biogas yield was intended to facilitate calculations. Even though the biogas yield was too low in the first two tests to determine the biogas yield of leather shavings before the study began after a 3-month biotechnological start-up, this was probably due to insufficient feeding of the methanogenic microorganisms, and this hindered their further growth. On this basis, it was concluded that the leather shavings were not sufficiently decomposed. In subsequent studies, the total biogas yield exceeded that of the inoculum and glycerol under laboratory conditions; hence, it was possible to determine the biogas yield value of the leather shavings.

Table 7.
Biogas yield results.
The methane production rate (MPR) is a key parameter comparing the ability of substrates to produce methane. Biomethane potential tests (MPRs) are used to determine the amount of methane that can be produced from organic materials in order to design various components of full-scale biogas plants. Achieving the highest possible value for the HRT and organic loading used provides the most reliable information in terms of demand-scale biogas plant design. If its value is in the range of 0–0.3, then the feedstock mixture will have a low yield and low profitability for the construction of a biogas plant processing the mixture [61].
The results of the anaerobic digestion tests of the leather shavings proved to be surprising. Contrary to initial assumptions and attempts to ferment blue leather shavings using a static method, it was shown that blue leather shavings had many times the biogas yield of white leather shavings. The processes to which blue leather shavings were subjected during the leather processing meant that they contained high concentrations of heavy metals. It was expected that it was the heavy metals that determined its low performance on a laboratory scale. Comparing the above results with the Szewalski Fluid-Flow Machinery Institute, PASci scientists working on exactly the same material, it was confirmed that for, similar samples using the dynamic method, higher biogas yields were achieved than for white leather shavings. A significant amount of the chromium in the material was chromium (III), which may be a macronutrient that stimulates the process. The effect of chromium is extensively described in the article by Chojnacka et al. [62].There may be several reasons for the surprising results. It is possible that, for the large-scale mixture of inoculum and substrate used, the blue flesh was much better decomposed than for another inoculum that was used on a small scale. Another reason for this may be the metal content that occurred during the process. Studies show that up to certain concentrations of zinc, cadmium, nickel, and lead it is possible to improve fermentation conditions [63,64,65,66]. It is possible that the key to the results was the influence of both of these factors.
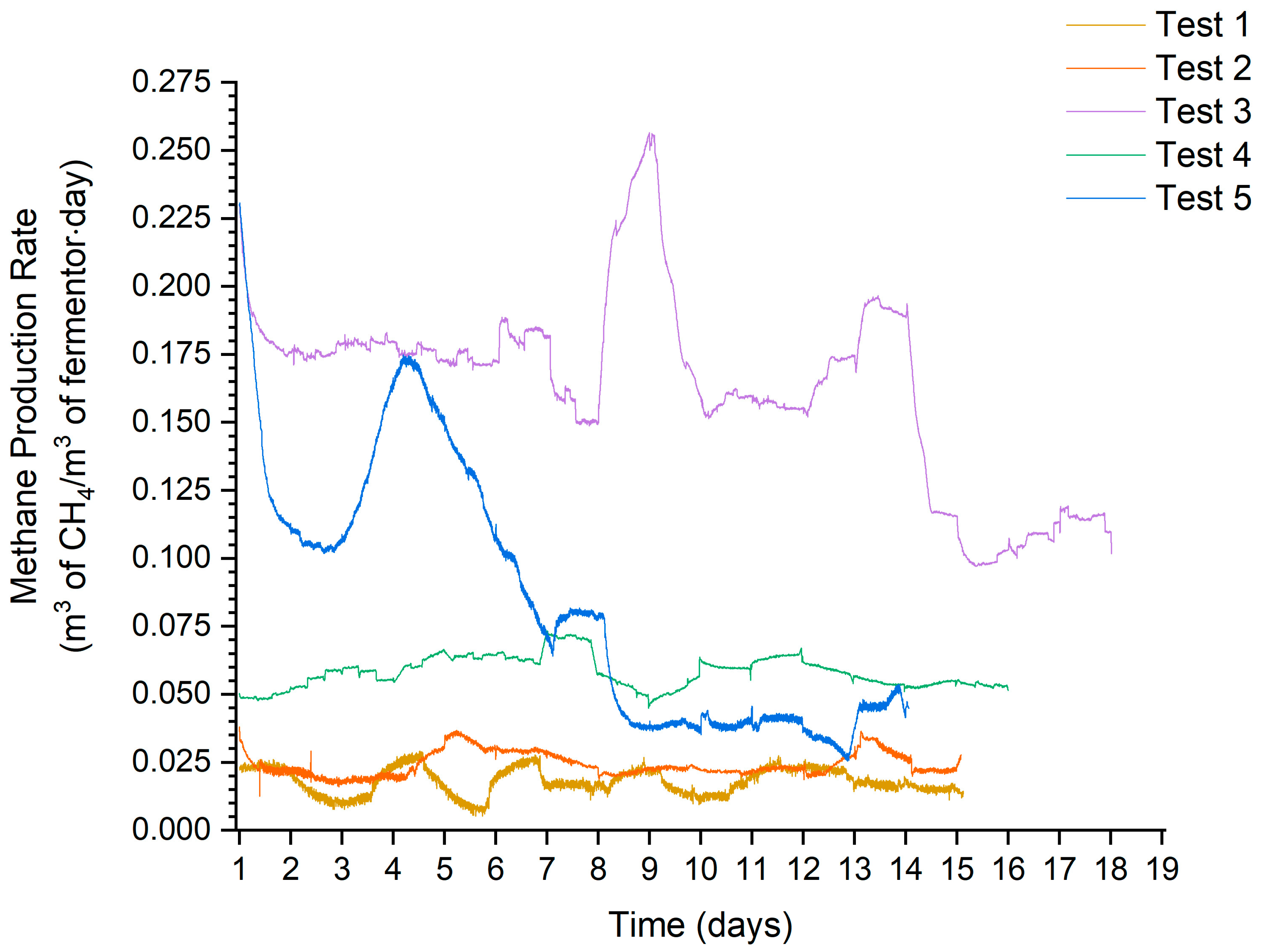
The following characteristics (Figure 6) show the process flows for the individual anaerobic digestion tests. This made it possible to observe the stability of biogas production. The stability of biogas production is also shown in the form of standard deviation values in Table 6.
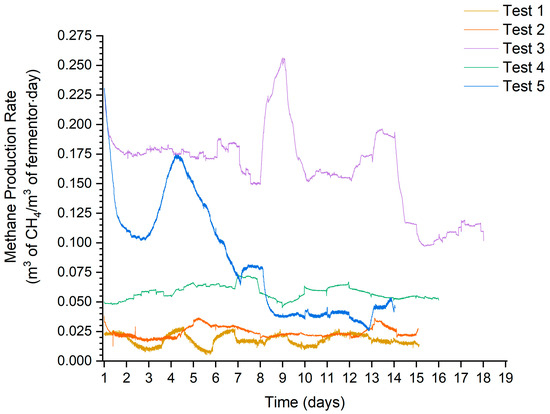
Figure 6.
MPR time course characteristics.
The way the mix was dosed, and therefore through the substrate buffer, made additional hesitation visible on the run. This was due to the dosing of an uneven mixture. Particularly strong fluctuations could be observed in the MPR run for test 3. This was related to the presence of glycerine, which had an extremely high biogas yield relative to the other substrates. The resulting peak indicates that more glycerine was added on a given day than in the process steps where these peaks were not present.
For each of the five tests, two analytical biogas tests were conducted. The Table 8 shows the averaged measurements.

Table 8.
Composition of biogas results.
Remarkably high methane results were obtained in the biogas composition for tests 1 and 2. This was due to two important process aspects. The first was due to protein decomposition, characterised by higher methane yields in biogas relative to the decomposition of sugars and fats in anaerobic digestion. The second direct reason for this was the low biogas production, and this was related to the long biogas conditioning time in the digester. This favoured the synthesis of hydrogen and carbon dioxide to methane, thereby lowering the proportion of CO2 in the biogas. Higher hydrogen production would allow even higher methane values to be achieved.
Laboratory and semi-technical plants are characterised by a high energy consumption per unit of energy produced. The daily methane production in this study for the individual tests ranged from 0.017 to 0.157 . Prior to the additional insulation measures, the plant consumed approximately 7.67 . After housing the outlet of the heating system containing the heater, energy consumption decreased to between 5.62 and 6.18 . It was observed that more than 90 per cent of the energy supplied to the system covered the thermal energy requirement. The ambient conditions in the test laboratory were 18–22 °C.
Table 9 shows the properties of digestate. The following parameters made it possible to assess the possibility of its further use and to assess the environmental risk that the product may pose.

Table 9.
Digestate test results of leather shaving and sludge digestion process.
The use of enzymatic hydrolysis makes it possible to significantly increase the biogas yield. Importantly, the enzyme decomposing leather shavings is not an acid, which could cause problems for the further management of digestate. It is a set of organisms classified as fungi, which can work under normal conditions. In the research discussed in the article by E. Wrzesińska-Jędrusiak et al. [44], just 1 mL of the preparation was sufficient to decompose 10g of leather shavings and increase its biogas yield to 248 Nm3·Mg−1 while maintaining a high methane concentration in the biogas. The enzymatic hydrolysis lasted 24 h.
This research also proved that it is possible to load the tank with organic matter of approximately 4.5–5 kg ODM·Mg−1·d−1. Given the annual waste production of 1892 mg per year, it is possible to design an industrial-scale biogas plant that could simultaneously process leather shavings and slurry.
4. Conclusions
The properties of leather shavings can cause a number of technological problems (low water content, fraction of fairly enormous size, elasticity, and high protein content, variable properties depending on the degree of decomposition). In order to recognise the full process cycle and determine the process kinetics inside the tank with the presented material, several months of tests were carried out to eliminate the main technological problems. These tests succeeded in bringing the plant to strictly controlled and stable operating conditions.
This study showed that the breakdown of proteins in the anaerobic digestion process and the long conditioning time of the biogas in the digester makes methane the main component of biogas. The addition of glycerine to the process confirmed this, as a decrease in methane relative to the total biogas produced was observed.
The main objective of this study was the utilisation of residues from the leather tanning process. The analysis of digestate confirmed that leather shavings are highly degradable under the influence of methanogenic microorganisms. This significantly reduced the volume of waste by changing the bulk density from 121 kg/m3 of leather shavings to 1.007–1.02 mg/m3 of digestate.
Considering the biotechnological factors signalling underfeeding of the digester for test 4, it was possible to achieve a significantly higher MPR, despite the low biogas yield of the leather shavings. Attempts to maximise the organic loading of the process tank and to shorten the HRT should be the subject of further research to determine the potential of operating biogas plants treating liquid biomass with the addition of leather shavings. It is important that the fermented liquid biomass (inoculum) has a higher biogas yield. It is also advisable to see if the process will run just as efficiently at a lower temperature but with a lower energy input. It was concluded that the DTR F120 digestion plant model presented in this paper is a good starting model and that the tests carried out on it have similar characteristics to industrial plants like temperature maintenance, pump operation, and biotechnology supervision. Interesting directions in the development of the presented research may also be the study of isolated hydrolysis as the first phase of anaerobic fermentation, the study of leather shavings after enzymatic hydrolysis on a semi-technical scale, or the study of other inoculating co-substrates. These lines of research will help demonstrate that anaerobic processing may be the right direction of development on the way to industrial application.
Author Contributions
Conceptualization, M.C., E.W.-J. and A.M.; methodology, M.C. and E.W.-J.; software, K.P.; validation, M.C., E.W.-J., I.K. and L.Ś.; formal analysis, M.C. and K.P.; investigation, M.C. and E.W.-J.; resources, E.W.-J. and A.M.; data curation, M.C.; writing—original draft preparation, M.C.; writing—review and editing, M.C., E.W.-J., I.K., L.Ś., K.P., M.K. and A.M.; visualization, M.C.; supervision, M.C., E.W.-J., M.K. and A.M.; project administration, E.W.-J., M.K. and A.M.; funding acquisition, E.W.-J., M.K. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
Work carried out under the project nr POIR.04.01.04-00-0071/20–00 co-financed by the European Regional Development Fund, entitled “Development of technologies for rational management of bovine shavings from leather processing (MIZDRA 2.0)” co-financed by the National Centre for Research and Development from the Smart Development Operational Program, Action 4.1.4 “Application Projects”. Beneficiaries: the Szewalski Institute of Fluid-Flow Machinery Polish Academy of Sciences, Wrocław University of Science and Technology, Institute of Technology and Life Sciences/Poznań, BADER Polska sp. z o.o.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
Author Marek Kulazynski was employed by Innovation and Implementation Company Ekomotor Ltd. Other authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Liu, D.; Guo, X.; Xiao, B. What causes growth of global green housegas emissions? Evidence from 40 countries. Sci. Total Environ. 2019, 661, 750–766. [Google Scholar] [CrossRef] [PubMed]
- Lamb, W.F.; Wiedmann, T.; Pongratz, J.; Andrew, R.; Crippa, M.; Olivier, J.G.J.; Wiedenhofer, D.; Mattioli, G.; Khourdajie, A.A.; House, J.; et al. A review of trends and drivers of greenhouse gas emissions by sector from 1990 to 2018. Environ. Res. Lett. 2021, 16, 073005. [Google Scholar] [CrossRef]
- Balogh, J.M. The impacts of agricultural development and trade on CO2 emissions? Evidence from the Non-European Unioncountries. Environ. Sci. Policy 2022, 137, 99–108. [Google Scholar] [CrossRef]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Hauck, J.; Olsen, A.; Peters, G.P.; Peters, W.; Pongratz, J.; Sitch, S.; et al. Global Carbon Budget 2020. Earth Syst. Sci. Data 2020, 12, 3269–3340. [Google Scholar] [CrossRef]
- Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.W.; Trisos, C.; Romero, J.; Aldunce, P.; Barrett, K.; Blanco, G.; et al. 2023: Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023. [Google Scholar] [CrossRef]
- Schloemer, S.; Bruckner, T.; Fulton, L.; Hertwich, E.; McKinnon, A.; Perczyk, D.; Roy, J.; Schaeffer, R.; Sims, R.; Smith, P.; et al. Annex III: Technology-specific cost and performance parameters. In Climate Change 2014: Mitigation of Climate Change: Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; pp. 1329–1356. Available online: https://abdn.pure.elsevier.com/en/publications/annex-iii-technology-specific-cost-and-performance-parameters (accessed on 28 August 2023).
- Bruckner, T.; Bashmakov, I.A.; Mulugetta, Y.; Chum, H.; De la Vega Navarro, A.; Edmonds, J.; Faaij, A.; Fungtammasan, B.; Garg, A.; Hertwich, E.; et al. Chapter 7—Energy Systems; Cambridge University Press: Cambridge, UK, 2014; Available online: http://www.ipcc.ch/pdf/assessment-report/ar5/wg3/ipcc_wg3_ar5_chapter7.pdf (accessed on 28 August 2023).
- Al-Wahaibi, A.; Osman, A.I.; Al-Muhtaseb, A.H.; Alqaisi, O.; Baawain, M.; Fawzy, S.; Rooney, D.W. Techno-economic evaluation of biogas production from food waste via anaerobic digestion. Sci. Rep. 2020, 10, 15719. [Google Scholar] [CrossRef]
- Eggemann, L.; Rau, F.; Stolten, D. The ecological potential of manure utilisation in small-scale biogas plants. Appl. Energy 2023, 331, 120445. [Google Scholar] [CrossRef]
- Bacenetti, J.; Sala, C.; Fusi, A.; Fiala, M. Agricultural anaerobic digestion plants: What LCA studies pointed out and what can be done to make them more environmentally sustainable. Appl. Energy 2016, 179, 669–686. [Google Scholar] [CrossRef]
- Bojarski, W.; Pulka, J.; Szewczyk, P.; Jasiński, T.; Jasiński, J.; Czekała, W. Waste as substrates for agricultural biogas plants: A case study from Poland. J. Water Land Dev. 2023, 45–50. [Google Scholar] [CrossRef]
- Zawartka, P.; Burchart-Korol, D.; Blaut, A. Model of Carbon Footprint Assessment for the Life Cycle of the System of Wastewater Collection, Transport and Treatment. Sci. Rep. 2020, 10, 5799. [Google Scholar] [CrossRef]
- Schnürer, A. Biogas Production: Microbiology and Technology. Adv. Biochem. Eng. Biotechnol. 2016, 156, 195–234. [Google Scholar] [CrossRef] [PubMed]
- Ahlberg-Eliasson, K.; Nadeau, E.; Levén, L.; Schnürer, A. Production efficiency of Swedish farm-scale biogas plants. Biomass Bioenergy 2017, 97, 27–37. [Google Scholar] [CrossRef]
- Neumann, P.; Pesante, S.; Venegas, M.; Vidal, G. Developments in pre-treatment methods to improve anaerobic digestion of sewage sludge. Rev. Environ. Sci. Biotechnol. 2016, 15, 173–211. [Google Scholar] [CrossRef]
- Nordell, E.; Björn, A.; Waern, S.; Yekta, S.S.; Sundgren, I.; Moestedt, J. Thermal post-treatment of digestate in order to increase biogas production with simultaneous pasteurization. J. Biotechnol. 2022, 344, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Villarroel-Schneider, J.; Höglund-Isaksson, L.; Mainali, B.; Martí-Herrero, J.; Cardozo, E.; Malmquist, A.; Martin, A. Energyself-sufficiency and greenhousegas emission reductions in Latin American dairy farms through massive implementation of biogas – based solutions. Energy Convers. Manag. 2022, 261, 115670. [Google Scholar] [CrossRef]
- Tavera, C.G.; Raab, T.; Holguin Trujillo, L. Valorization o fbiogas digestate as organic fertilizer for closing the loop on the economic viability to develop biogas projects in Colombia. Clean. Circ. Bioecon. 2023, 4, 100035. [Google Scholar] [CrossRef]
- Andersen, L.; Lamp, A.; Dieckmann, C.; Baetge, S.; Schmidt, L.-M.; Kaltschmitt, M. Biogas plants as key units of biorefinery concepts: Options and their assessment. J. Biotechnol. 2018, 283, 130–139. [Google Scholar] [CrossRef]
- Manogaran, M.D.; Shamsuddin, R.; Yusoff, M.H.M.; Lay, M.; Siyal, A.A. A review on treatment processes of chicken manure. Clean. Circ. Bioecon. 2022, 2, 100013. [Google Scholar] [CrossRef]
- Ustawa z Dnia 20 Lutego 2015r. o Odnawialnych Źródłach Energii, n.d. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20150000478 (accessed on 28 August 2023).
- ZALECENIE KOMISJI z Dnia 14 Marca 2023r.—Magazynowanie Energii—Podstawa Zdekarbonizowanego I Bezpiecznego Systemu Energetycznego UE (2023/C103/01). 2023. Available online: https://sip.lex.pl (accessed on 2 September 2023).
- Twitchell, J.; DeSomber, K.; Bhatnagar, D. Defining long duration energy storage. J. Energy Storage 2023, 60, 105787. [Google Scholar] [CrossRef]
- Nozari, M.H.; Yaghoubi, M.; Jafarpur, K.; Mansoori, G.A. Development of dynamic energy storage hub concept: A comprehensive literature review of multi storage systems. J. Energy Storage 2022, 48, 103972. [Google Scholar] [CrossRef]
- Zhang, D.; Shafiullah, G.M.; Das, C.K.; Wong, K.W. A systematic review of optimal planning and deployment of distributed generation and energy storage systems in power networks. J. Energy Storage 2022, 56, 105937. [Google Scholar] [CrossRef]
- Singh, R.; Singh, R.P.; Singh, R. Biogas driven multi generation integrated with simultaneous charging-discharging type thermal energy storage system. Energy Convers. Manag. 2022, 270, 116234. [Google Scholar] [CrossRef]
- Klimek, K.E.; Wrzesińska-Jedrusiak, E.; Kapłan, M.; Łaska-Zieja, B. Management of biomass of selected grape leaves varieties in the process of methane fermentation. J. Water Land Dev. 2022, 17–27. [Google Scholar] [CrossRef]
- Su, X.; Shao, X.; Geng, Y.; Tian, S.; Huang, Y. Optimization of feedstock and insulating strategies to enhance biogas production of solar-assisted biodigester system. Renew. Energy 2022, 197, 59–68. [Google Scholar] [CrossRef]
- Rehman, A.; Zhang, B.; Qyyum, M.A.; Zhuqiang, Y.; Haider, J. Improvement potential detection of integrated biomethane liquefaction and liquidair energy storage system. J. Energy Storage 2023, 66, 107455. [Google Scholar] [CrossRef]
- Mendecka, B.; Chiappini, D.; Tribioli, L.; Cozzolino, R. A biogas –solar based hybrid off-grid power plant with multiple storages for United States commercial buildings. Renew. Energy 2021, 179, 705–722. [Google Scholar] [CrossRef]
- Su, B.; Wang, H.; Zhang, X.; He, H.; Zheng, J. Using photovoltaic thermal technology to enhance biomethane generation via biogas up grading in anaerobic digestion. Energy Convers. Manag. 2021, 235, 113965. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, M.; Liu, Y.; Wang, D.; Zhuang, Z.; Quan, M. Energy, exergy, and economic analysis of a centralized solar and biogas hybrid heating system for rural areas. Energy Convers. Manag. 2023, 276, 116591. [Google Scholar] [CrossRef]
- Ceran, B. The concept of use of PV/WT/FC hybrid power generation system for smoothing the energy profile of the consumer. Energy 2019, 167, 853–865. [Google Scholar] [CrossRef]
- Gazda, W.; Stanek, W. Energy and environmental assessment of integrated biogas trigeneration and photovoltaic plant as more sustainable industrial system. Appl. Energy 2016, 169, 138–149. [Google Scholar] [CrossRef]
- Baccioli, A.; Ferrari, L.; Vizza, F.; Desideri, U. Potential energy recovery by integrating an ORC in a biogas plant. Appl. Energy 2019, 256, 113960. [Google Scholar] [CrossRef]
- Dutta, N.; Giduthuri, A.T.; Khan, M.U.; Garrison, R.; Ahring, B.K. Improved valorization of sewage sludge in the circular economy by anaerobic digestion: Impact of an innovative pretreatment technology. Waste Manag. 2022, 154, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Risberg, K.; Cederlund, H.; Pell, M.; Arthurson, V.; Schnürer, A. Comparative characterization of digestate versus pig slurry and cow manure—Chemical composition and effects on soil microbial activity. Waste Manag. 2017, 61, 529–538. [Google Scholar] [CrossRef]
- Li, Y.; Jia, X.; Li, X.; Liu, P.; Zhang, X.; Guo, M. Study on the potential of sludge-derived humic acid as energy storage material. Waste Manag. 2023, 162, 55–62. [Google Scholar] [CrossRef]
- de la Casa, J.A.; Bueno, J.S.; Castro, E. Recycling of residues from the olive cultivation and olive oil production process for manufacturing of ceramic materials. A comprehensive review. J. Clean. Prod. 2021, 296, 126436. [Google Scholar] [CrossRef]
- Buller, L.S.; Sganzerla, W.G.; Lima, M.N.; Muenchow, K.E.; Timko, M.T.; Forster-Carneiro, T. Ultrasonicpretreatmentofbrewers’spentgrainsforanaerobicdigestion:Biogasproductionforasustainableindustrialdevelopment. J. Clean. Prod. 2022, 355, 131802. [Google Scholar] [CrossRef]
- Kuligowski, K.; Cenian, A.; Konkol, I.; Świerczek, L.; Chojnacka, K.; Izydorczyk, G.; Skrzypczak, D.; Bandrów, P. Application of Leather Waste Fractions and Their Biochars as Organic Fertilisers for Ryegrass Growth: Agri-Environmental Aspects and Plants Response Modelling. Energies 2023, 16, 3883. [Google Scholar] [CrossRef]
- Mikula, K.; Konieczka, M.; Taf, R.; Skrzypczak, D.; Izydorczyk, G.; Moustakas, K.; Kułażyński, M.; Chojnacka, K.; Witek-Krowiak, A. Tannery waste as are new able source of nitrogen for production of multi component fertilizers with biostimulating properties. Environ. Sci. Pollut. Res. 2023, 30, 8759–8777. [Google Scholar] [CrossRef]
- Turzyński, T.; Januszewicz, K.; Kazimierski, P.; Kardaś, D.; Hercel, P.; Szymborski, J.; Niewiadomski, J. The role of additives in improving the flammability and calorific value of leather shavings and the binding of chromium compounds in ash. Waste Manag. 2023, 163, 52–60. [Google Scholar] [CrossRef]
- Wrzesińska-Jędrusiak, E.; Czarnecki, M.; Kazimierski, P.; Bandrów, P.; Szufa, S. The Circular Economy in the Management of Waste from Leather Processing. Energies 2023, 16, 564. [Google Scholar] [CrossRef]
- PN-ISO 6496:2002; Determination of Moisture and Content of Other Volatile Substances. Polish Accreditation Center: Warsaw, Polish, 2022.
- Rozporządzenie Ministra Ochrony Środowiska, Zasobów Naturalnych I Leśnictwa z Dnia 24 Grudnia 1997r. w Sprawie Klasyfikacji Odpadów., n.d. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU19971621135 (accessed on 28 August 2023).
- Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Establishing a European Chemicals Agency, Amending Directive 1999/45/E Cand Repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as Well as Council Directive 76/769/EE Cand Commission Directives 91/155/EEC, 93/67/EEC, 93/105/E Cand2000/21/EC, 2006. Available online: http://data.europa.eu/eli/reg/2006/1907/oj/eng (accessed on 2 September 2023).
- Directive (EU) 2018/851 of the European Parliament and of the Council of 30 May 2018 Amending Directive 2008/98/EC on Waste—Official Journal of the EU L 150, 14.06.2018, p. 109. Available online: https://eur-lex.europa.eu/eli/dir/2018/851/oj (accessed on 2 September 2023).
- German Standard Methods for the Examination of Water, Waste Waterand Sludge—Sludge and Sediments (GroupS)—Part 17: Determination of the Organically Bound Halogens Amen able to Extraction (EOX) (S17). 2017. Available online: https://www.scribd.com/document/155645690/E-DIN-38414-17-E-EOX (accessed on 28 August 2024).
- PN-EN ISO 10390:2022-09; Soil, Treated Biowaste and Sludge—Determination of pH. Polish Accreditation Center: Warsaw, Polish, 2020.
- Bi, S.; Qiao, W.; Xiong, L.; Ricci, M.; Adani, F.; Dong, R. Effects of organic loading rate on anaerobic digestion of chicken manure under mesophilic and thermophilic conditions. Renew. Energy 2019, 139, 242–250. [Google Scholar] [CrossRef]
- PN-EN 25663:2001; Water Quality—Determination of Kjeldahl Nitrogen—Method after Mineralization with Selenium. Polish Accreditation Center: Warsaw, Poland, 2020.
- Huertas, J.K.; Quipuzco, L.; Hassanein, A.; Lansing, S. Comparing Hydrogen Sulfide Removal Efficiency in a Field–Scale Digester Using Micro aeration and Iron Filters. Energies 2020, 13, 4793. [Google Scholar] [CrossRef]
- Fu, S.; Lian, S.; Angelidaki, I.; Guo, R. Micro-aeration: An attractive strategy to facilitate anaerobic digestion. Trends Biotechnol. 2023, 41, 714–726. [Google Scholar] [CrossRef]
- PN-C-04576-15:1975; Waterand Wastewater—Tests for Nitrogen Compounds—Determination of Ammoniacal Nitrogen in Sewage Sludge. Polish Accreditation Center: Warsaw, Poland, 1975.
- PN-75/C-04616/04; Water and Sewage—Special Tests of Sludge—Determination of Volatile Organic Acids in Sewage Sludgeand Filtrate Waters by Steam Distillation Method. Polish Accreditation Center: Warsaw, Poland, 1991.
- Duan, N.; Zhang, D.; Lin, C.; Zhang, Y.; Zhao, L.; Liu, H.; Liu, Z. Effect of organic loading rate on anaerobic digestion of pig manure: Methane production, mass flow, reactors cale and heating scenarios. J. Environ. Manag. 2019, 231, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Nagao, N.; Tajima, N.; Kawai, M.; Niwa, C.; Kurosawa, N.; Matsuyama, T.; Yusoff, F.M.; Toda, T. Maximum organic loading rate for the single-stage wet anaerobic digestion of food waste. Bioresour. Technol. 2012, 118, 210–218. [Google Scholar] [CrossRef]
- Kogut, P.; Piekarski, J.; Ignatowicz, K. Politechnika Koszalińska, Rozruch instalacji biogazowej z wykorzystaniem osaduzaszczepowego. In Annual Set The Environment Protection; 2014; Volume 16, pp. 534–545. Available online: https://www.ros.edu.pl/images/roczniki/2014/pp_2014_01_34.pdf (accessed on 28 August 2024).
- Magrel, L. Metodyka Oceny Efektywności Procesu Fermentacji Metanowej Wybranych Osadów Ściekowych. Politechnika Białostocka, Białystok, Poland, 2002. Available online: https://pbc.biaman.pl/dlibra/publication/374/edition/548 (accessed on 26 September 2023).
- Holliger, C.; Fruteaude Laclos, H.; Hack, G. Methane Production of Full-Scale Anaerobic Digestion Plants Calculated from Substrate’s Biomethane Potentials Compares Well with the One Measured On-Site. Front. Energy Res. 2017, 5, 12. [Google Scholar] [CrossRef]
- Chojnacka, K.; Skrzypczak, D.; Mikula, K.; Witek-Krowiak, A.; Izydorczyk, G.; Kuligowski, K.; Bandrów, P.; Kułażyński, M. Progress in sustainable technologies of leather wastes valorization a ssolutions for the circular economy. J. Clean. Prod. 2021, 313, 127902. [Google Scholar] [CrossRef]
- Altaş, L. Inhibitory effect of heavy metals on methane-producing anaerobic granular sludge. J. Hazard. Mater. 2009, 162, 1551–1556. [Google Scholar] [CrossRef]
- Romero-Güiza, M.; Vila, J.; Mata-Alvarez, J.; Chimenos, J.; Astals, S. The role of additives on anaerobic digestion: A review. Renew. Sustain. Energy Rev. 2016, 58, 1486–1499. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, Y.; Wang, L.; Zhang, L.; Dai, L. Ecophysiological characteristics and biogas production of cadmium-contaminated crops. Bioresour. Technol. 2013, 146, 628–636. [Google Scholar] [CrossRef]
- Kadam, R.; Khanthong, K.; Jang, H.; Lee, J.; Park, J. Occurrence, Fate, and Implications of Heavy Metals during Anaerobic Digestion: A Review. Energies 2022, 15, 8618. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).