1. Introduction
Approximately 2.4 billion tires are produced and sold worldwide each year [
1], ranging from public transport vehicles to industrial machinery. It has been identified that one of the main problems related to the mass production of tires is the large amount of waste produced; a significant proportion (around 50%) of the total tires produced ends their useful life as unrecoverable waste [
2]. This issue is exacerbated by the non-biodegradable nature of tires, which take between 800 and 1000 years to decompose [
3]. The improper disposal of waste tires (WTs) poses a significant risk to public health and the environment. Accumulated WTs can become breeding grounds for disease vectors [
4], as stagnant water promotes mosquito proliferation, facilitating the spread of diseases like dengue, Zika, and chikungunya. They also attract rodents and insects, increasing the risk of infections such as leptospirosis, hantavirus, and salmonellosis [
5]. Additionally, WTs are highly flammable and pose a serious fire hazard to surrounding populations [
6]. This situation highlights a significant environmental challenge, as the massive disposal of tires has direct negative consequences for natural ecosystems and environmental quality. The improper disposal of non-recyclable waste can lead to soil and water contamination, as well as the release of toxic compounds and pollutants into the atmosphere, thereby contributing to climate change and other environmental problems [
4].
To face these problems, innovative and sustainable approaches are needed to man-age this waste and mitigate its adverse effects. Various technologies and methods exist to harvest and reuse WTs, including mechanical recycling, reuse in infrastructure, gasification, and incineration [
7]. Mechanical recycling involves shredding tires into crumb rubber for secondary products; however, it does not recover energy or valuable chemicals, limiting resource efficiency. Additionally, reusing tires in infrastructure such as roadbeds or retaining walls offers a practical solution but is limited by its inability to extract energy or high-value materials, often being considered a low-value application. Gasification and incineration are thermal processes that can convert WTs into energy, but both are often criticized for their high operating temperatures, lower selectivity, and potential emission of harmful pollutants [
7]. In contrast, emerging thermochemical processes such as pyrolysis offer more sustainable alternatives by maximizing resource recovery, reducing dependence on fossil fuels, and lowering the overall environmental impact. These processes can extract both energy and valuable materials, aligning with circular economy principles [
8]. Compared to incineration, WT pyrolysis is considered a more environmentally friendly and economically competitive method, generating three valuable products: fuel gas, oil, and carbon [
9,
10,
11]. Additionally, pyrolysis meets three essential principles of solid waste management: volume reduction, resource recovery, and pollutant emission mitigation. The high energy intensity of WTs has also encouraged their reuse through thermochemical processes, especially pyrolysis, which enables the generation of alternative fuels and materials, contributing to energy independence and environmental sustainability [
8].
Despite being a relatively good method for recycling used tires, the conventional pyrolysis of used tires is subject to certain limitations due to its dependence on impurities such as sulfur in the obtained pyrolytic oil [
12]. This issue can limit the direct use of pyrolytic oil in engines or refineries without further treatment [
8]. To overcome these challenges, catalytic pyrolysis has gained attention as an approach to enhance the efficiency and selectivity of the process. Various types of catalysts, including natural zeolite, have been used to improve the quantity and quality of oils from tires in catalytic pyrolysis [
12,
13]. Strong solid acid catalysts like zeolites facilitate thermal decomposition at relatively lower temperatures, shifting product distribution toward lighter, more valuable hydrocarbons in the boiling range of fuel oil. This makes catalytic pyrolysis more economically viable and environmentally sound by improving product quality and reducing energy consumption [
14]. The distribution of pyrolytic products typically consists of gas (20%), liquid (35%), carbon black (33%), and metallic residue (12%) by mass [
15]. However, such proportions vary depending on factors such as the type of pyrolysis reactor [
16], the operating conditions [
17] (temperature, pressure, residence time, carrier gas (N
2 or CO
2), particle size, and catalyst), and other factors such as the original composition of the tire [
18]. In this sense, a key factor in the present research is the use of zeolites as catalysts, whether commercial or synthesized. This is because some researchers have stated that the addition of zeolite catalysts in the WT pyrolysis process promotes a change in the yield of pyrolytic products [
14,
19], so the appropriate catalyst can be chosen according to the purpose of the research. For instance, Razzaq and Majeed [
20] found that the effects of ZSM-5 and HY zeolite catalyst were a decreased oil yield and an improved gas yield. Olazar et al. [
21] concluded that the catalyst has a significant effect on product fraction distribution and composition, which are very sensitive to the shape selectivity of the zeolite catalyst in the range of 425–500 °C. Suhartono et al. [
19] obtained oil and char weight ratios of 36.63% and 47.91%, respectively, at 450 °C in a tubular reactor. The use of zeolites has proven effective in reducing the formation of undesirable compounds, such as sulfur-containing species, in the liquid fraction. This improvement may enhance the commercial value of the pyrolytic oil and decrease the need for extensive post-treatment.
Among the three main byproducts obtained from waste tire pyrolysis, carbon black stands out for its wide industrial applicability and superior quality compared to commercial variants such as semi-reinforced carbon, thanks to the characteristics conferred by the pyrolytic process [
22,
23]. The quality of this solid residue depends on operational variables such as temperature, residence time, and pressure. In research on WT pyrolysis, the influence of parameters such as temperature, tire type, and the presence of a catalyst and residence time, which determine the quality of the solid product obtained, has been studied [
16,
24]. In addition to carbon black, the other pyrolysis products also have valuable uses: the pyrolytic oil can be upgraded into diesel-like fuels or used directly in industrial burners, while the syngas, composed mainly of CO, H
2, CH
4, and other light hydrocarbons, can be recovered for energy generation or to fuel the pyrolysis process itself, enhancing energy efficiency. Likewise, the steel residues can be recovered and recycled in the metallurgical industry, contributing to the circular economy [
14]. Moreover, pyrolytic carbon black can be further treated to produce activated carbon, a porous material with high surface area and broad applicability, including water and air purification, energy storage, pollutant removal, gas treatment, and even medical uses [
25]. However, the presence of inorganic compounds, particularly ZnO and other metals like Fe, Al, Ca, and Mg, along with high sulfur content, can limit its direct application [
26,
27,
28]. The presence of these substances together with the high sulfur content limits the solid byproduct, so its use is often restricted [
28]. The carbon content of the pyrolytic sediments was the highest, up to 86% by weight, followed by its sulfur and nitrogen contents [
28]. Pyrolysis carbons obtained at higher temperatures (700–800 °C) had a lower sulfur content. A lower temperature seemed to cause a high sulfur retention due to the relatively high volatility of sulfur at higher temperatures [
29]. From what was reviewed, demineralization is highly recommended, since the removal of minerals promotes the creation of new mesopores and micropores, where these pores act as active sites in a subsequent activation [
30]. Likewise, as part of the proposed methodology, a chemical activation of the carbon residue will be carried out. In the chemical activation process, various chemical reagents are used to minimize the activation time and temperature, making chemical activation more effective than physical activation. However, corrosiveness and the generation of high pH values are the most considerable drawbacks of this process. Although ZnCl
2, NaOH, HNO
3, H
2SO
4, and H
3PO
4 are applied for the activation of carbon residue from tire pyrolysis, K
2CO
3 and KOH are commonly used due to their effective technical performance in increasing the surface area and microvolume [
31]. KOH was chosen due to its high efficiency in improving porosity and developing a highly microporous structure. KOH promotes the removal of impurities and the expansion of the pore network, which significantly increases the surface area and microvolume of the activated carbon. Furthermore, its use minimizes the generation of hazardous waste compared to other activators such as ZnCl
2, which can generate toxic waste, or H
3PO
4, which requires extensive washing to remove phosphorus residues. Compared to NaOH, KOH shows greater efficiency in creating well-developed pores and better thermal stability during activation, thus optimizing the final quality of the activated carbon [
31].
This study addresses a current research gap in the field of waste tire valorization by exploring the catalytic pyrolysis pathway not only as a means for energy recovery but also for the generation of value-added materials. The novelty of this work lies in the strategic use of zeolite-based catalysts, particularly the use of a synthesized zeolite derived from natural pozzolan, as a means to enhance the quality of the carbonaceous residue obtained during WT catalytic pyrolysis. This residue, typically considered a low-value byproduct, is here revalorized into high-quality activated carbon through chemical activation. The specific aim of this study is to evaluate the potential of producing activated carbons with enhanced surface and chemical properties from the solid fraction resulting from the catalytic pyrolysis of WTs. For this purpose, two zeolitic catalysts (a commercial one and a synthesized one) were thoroughly characterized in terms of textural, morphological, and crystalline properties to assess their influence during the pyrolysis process. Subsequently, the carbonaceous residues or waste tire carbon (WTC) obtained from the catalytic pyrolysis were characterized to understand how catalyst type and pyrolysis temperature affect their potential as precursors for activation. Finally, the WTC and the activated carbons were evaluated with respect to their surface area, porosity, burn-off, cation exchange capacity, and morphology, to determine their suitability for environmental or industrial applications in terms of their adsorption capacity. This integrated approach allows a direct link between catalyst design and the development of high-performance activated carbon materials, contributing to the circular economy and the sustainable management of tire waste. It also addresses a key limitation of conventional pyrolysis by proposing a route that maximizes the utility of solid byproducts through the catalyst-driven enhancement of their properties.
2. Materials and Methods
2.1. Materials
The catalysts used were a commercial zeolite and a synthesized one. Both materials were sieved to maintain a particle size of 106 μm using a No. 140 mesh sieve [
32], and dried in a Venticell 222 oven (MMM Group, Berlin, Germany) at 100 °C for 24 h prior to use. The commercial zeolite was a ZSM-5 CBV 524G from the Zeolyst International brand (Kansas City, MO, USA). The synthesized zeolite, referred to in this investigation as PZ2, was made from pozzolan, a natural precursor extracted in the city of Arequipa. This region is characterized by a high availability of pozzolanic material due to the surrounding volcanoes, making it an abundant and cost-effective raw material for zeolite synthesis. The pozzolan used was characterized by X-ray fluorescence (XRF) using a Rigaku dispersive fluorescence equipment, model NEX QC + QuantEZ (Austin, TX, USA), identifying high contents of silicon and aluminum oxides in the material (SiO
2—77.90%; Al
2O
3—15.10%; K
2O—3.65%; Fe
2O
3—1.33%; CaO—1.30%; TiO
2—0.265%; Si/Al—9.10%; Others—0.46%), which indicates that this precursor is suitable for zeolite synthesis [
33].
The waste tires (WTs) were obtained from a retreading plant in Arequipa. The company provided pre-shredded tires of approximately 5 cm, which were further processed using a Poling plastic shredder to achieve a particle size that passed through a No. 10 mesh sieve (2 mm), in accordance with ASTM E11 [
32]. Subsequently, the obtained tire particles were dried in a Venticell 222 oven at 120 °C for 24 h.
For the carbon activation process, KOH from the HiMedia brand was used as the activating agent, and HCl from the J.T. Baker brand was used for washing the activated carbon.
2.2. Preparation of the Synthesized Catalyst PZ2
One of the catalysts used in this study for WT pyrolysis was synthesized via the alkaline fusion/hydrothermal method with concentrated NaOH, based on the procedure described by Mamani et al. [
34], using pozzolan as the precursor.
An acid pre-treatment was performed to remove impurities. After crushing and sieving, 50 g of the pozzolan was mixed with 250 mL of 1 M HCl and stirred magnetically at 90 °C for 2 h. The solid was then filtered, rinsed three times with deionized water, and dried at 110 °C for 24 h. The acid-treated material was mechanically mixed with powdered sodium hydroxide in a 1.2:1 weight ratio (NaOH/material). This mixture was fused in a muffle furnace at 550 °C for 1 h, then cooled to room temperature, and ground to a fine powder. The fused powder was mixed with water at a 1:5 weight ratio and stirred at room temperature for 3 h. The resulting mixture was transferred to a Teflon-lined stainless-steel autoclave and subjected to hydrothermal treatment under static conditions at 90 °C for 12 h. Finally, the solid was cooled, filtered, and washed with 0.5 M HCl until the pH was below 9. The filtered solid was dried at 105 °C for 12 h to remove residual moisture [
34].
2.3. Characterization of the Commercial Catalyst and the Synthesized Catalyst
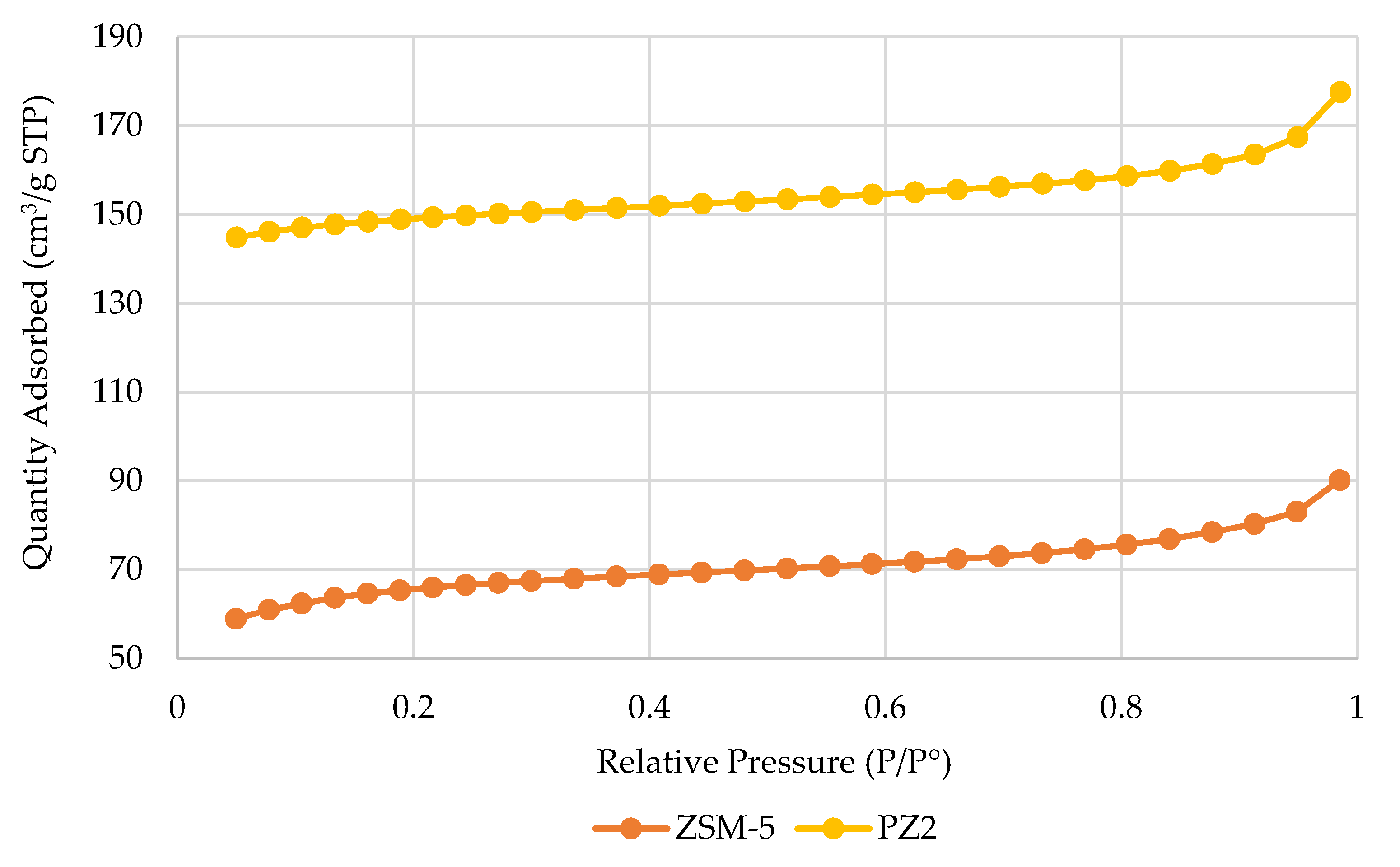
The textural characterization of the samples was conducted using the Brunauer–Emmett–Teller (BET) method with nitrogen gas as the adsorbate, employing a Gemini VII 2390 surface area analyzer (Norcross, GA, USA), under an evacuation rate of 1000 mmHg·min
−1 and an equilibration time of 5 s [
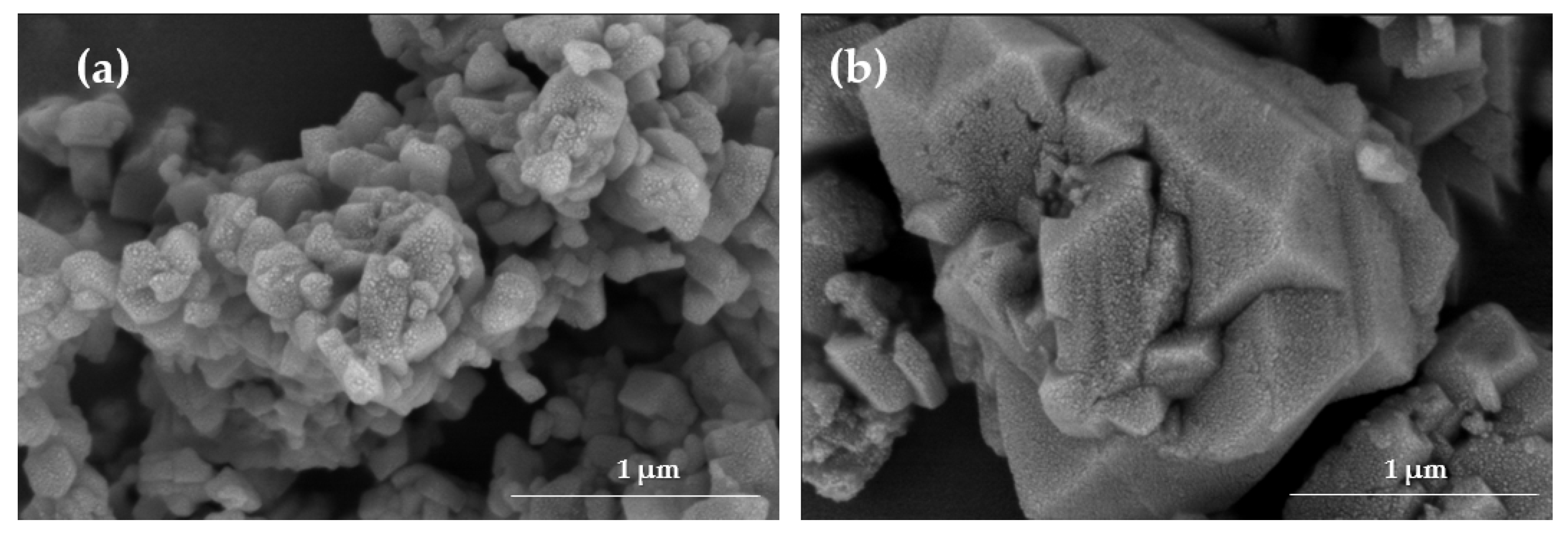
34]. Morphological analysis was carried out via scanning electron microscopy (SEM) using a Hitachi SU8230 scanning electron microscope (Hitachi High-Tech, Tokyo, Japan) with backscattered electrons, acquiring high-resolution images at a scale of 1 µm to evaluate the surface structure of the zeolites [
34]. The crystalline structure of the samples was characterized via X-ray diffraction (XRD) using a Rigaku Miniflex 600 diffractometer (Tokyo, Japan), operated at 40 kV and 15 mA. Data were collected over a 2θ range of 3° to 90° [
34], and the diffraction patterns were processed using Origin 2025 software.
To estimate the crystallinity, the ‘Peak Analyzer’ tool within Origin was employed. Specifically, the ‘Calculate Peak Area’ function was used to determine both the area under the crystalline peaks and the total diffracted area, which corresponds to the sum of the crystalline and amorphous areas. Crystallinity was calculated using the following equation [
35]:
where C is the crystallinity, A
c represents the area of the crystalline peaks, and A
a is the area attributed to the amorphous phase in the XRD diffractogram.
2.4. Pyrolysis of Waste Tires (WTs)
The WT pyrolysis process was conducted using a YUDIAN tubular furnace with a double heating zone. The nitrogen (N2) flow rate was regulated with a Cole-Parmer controller.
According to Lewandowski et al. [
16], key factors influencing the quality of the resulting waste tire char (WTC) include the operating temperature and the presence of catalysts. Accordingly, six different carbon samples were obtained using 10 g of WT for each run; only the temperature (in °C) and the presence or absence of zeolite catalyst varied.
Table 1 presents these variables along with the respective sample codes.
To perform the tests, the procedure described below was followed. First, the tube furnace was turned on, and the heating was programmed according to the set temperature, either 450 °C or 575 °C, maintaining a heating ramp of 10 °C per minute. Subsequently, the previously determined amount of WT and zeolite (used as a catalyst) was weighed using an analytical balance, and the sample was placed in an alumina crucible. Simultaneously, the mass flow controller was set to establish a nitrogen gas flow rate of 250 mL/min, ensuring an oxygen-free and inert atmosphere.
The cooling system was then placed inside an insulating container filled with liquid nitrogen. Once the tube furnace reached the desired temperature, the crucible containing the WT and zeolite was inserted into the quartz tube, maintaining the set temperature for 30 min. A lid and the cooling system were installed at both ends of the quartz tube, ensuring proper nitrogen flow. After the established time, the crucible was removed to allow it to cool to room temperature. It was necessary to wait until the quartz tube and the tubular furnace reached a temperature of 250 °C to properly collect the liquid products from both the cooling system and the quartz tube. The quartz tube was then carefully removed and left to cool for approximately 15 min or until it reached room temperature.
The pyrolysis and subsequent chemical activation processes were performed using the same laboratory installation, which was equipped to operate under controlled thermal conditions and a nitrogen atmosphere.
Figure 1 shows the experimental setup used for both pyrolysis and activation stages.
Finally, the liquid products were collected from the cooling system located in the flask with liquid nitrogen. Additionally, the solid residues from the crucible, the liquids obtained from the quartz tube, and the waxes generated in the cooling system were weighed. Thorough cleaning of both the quartz tube and the cooling system was then conducted to ensure that they were in optimal condition for subsequent experiments.
Once all the byproducts, the solid fraction (WTC), the liquid fraction (pyrolytic oil), and the gases (n-c gas), were collected and weighed, their respective yields were determined based on the initial mass of the waste tire. The calculation method and the statistical treatment of the yield data are detailed below.
The yield of the obtained byproducts, solid char (WTC), pyrolytic oil, and non-condensable gas, was calculated based on the mass ratio between the recovered product and the initial mass of tire waste fed into the reactor, using the following equation:
where m
product is the mass (in grams) of the recovered product (WTC, oil, or gas), and m
tire is the initial mass (in grams) of tire residue. Each experimental condition was performed in triplicate.
The average yield (
) was then calculated as follows:
where
is the mean value,
represents the yield value obtained in each repetition, and
n = 3 (total number of repetitions).
And the corresponding standard error of the mean was determined using the equation:
where
n = 3 is the number of replicates. The results are presented as mean ± standard error.
2.5. Chemical Activation of WTC with KOH
A portion of the obtained WTC was chemically activated using KOH as a reagent, with the objective of increasing its surface area and adsorption capacity. For this process, the WTC was manually mixed with KOH pellets using a mortar. The WTC: KOH weight ratio was 1:6, based on research by Sirinwaranon et al. [
36], who determined that this ratio significantly increased the surface area of the activated carbon. The mixture was then placed in an Alsint alumina crucible.
The WTC: KOH mixture was subsequently subjected to a pyrolysis process in a tubular furnace at a reaction temperature of 700 °C for 2 h, with a heating rate of 10 °C/min. The process was carried out under a N2 atmosphere with a flow rate of 150 mL/min. Upon completion of activation, the furnace was shut down, and the equipment was allowed to cool to room temperature, maintaining the N2 flow throughout. Once cooled, the crucible was removed and weighed to determine the degree of activation (burn-off).
The coding for the activated carbons resulting from the precursor WTCs obtained previously is presented in
Table 2.
The burn-off or weight percent of dry ash-free basis (daf) was calculated using the following equation [
37]:
where
w1 and
w2 represent the carbonized mass (daf) before and after the activation process, respectively.
Note: daf refers to the mass of the sample excluding both moisture and ash content, providing a more accurate measurement of the organic matter involved in the reaction [
37].
Subsequently, the activated carbon was washed with 5 M HCl and stirred for 12 h. After stirring, the carbon was filtered and washed with ultrapure water until a neutral pH was achieved. Finally, the resulting sample was dried in an oven at 100 °C for 3 days. The experimental procedure for obtaining KOH-activated carbons is presented in the flowchart shown in
Figure 2.
2.6. Cation Exchange Capacity Test by the Calcium Chloride Method
A 5 g sample of the activated carbon was weighed and placed in an Erlenmeyer flask, then 20 cc of 1 N CaCl2 was added and stirred for 5 min. The resulting mixture was filtered and the filtrate discarded. The solid residue was washed with 20 cc of distilled water, repeating the process three times and collecting the filtrates, to which 2–3 drops of oxalic acid were added. If a white precipitate formed, the washing process continued until a clear filtrate was obtained, indicating a negative calcium reaction.
The solid was then taken along with the filter paper and placed in a new Erlenmeyer flask, to which 20 cc of 1 N KCl was added and stirred for 5 min. The contents were then filtered, and 5 cc of the filtrate was transferred to another Erlenmeyer flask, along with 1 cc of 4 N NaOH and enough distilled water to fill one-third of the flask. A pinch of murexide indicator was added, and a titration with 0.02 N ethylenediaminetetraacetic acid (EDTA) was performed until the color changed from pinkish-red to violet or lilac, recording the volume used to perform the corresponding calculation.
To calculate the cation exchange capacity (CEC), the following calculations must be considered [
38]:
- (1)
Calcium (Ca) concentration, in milliequivalents per liter (meq/L), is calculated as:
where
X1 is the calcium concentration in the extract in units of meq/L,
a is the volume of EDTA spent from the filtrate in mL and
b is the volume taken from the filtrate in mL.
- (2)
Calculation of the amount of Ca extracted with 20 mL of KCl solution:
where X
2 is the amount of Ca extracted in meq, 20 mL is the KCl solution previously added, and 1000 mL is the conversion factor from mL to L.
- (3)
CEC extrapolated to 100 g of soil:
where X
3 is CEC in meq/100 g of soil, 100 g is a standard mass reference, and c is the actual mass of soil used in g.
According to the International System of Units (SI), the unit of CEC is cmolc/kg of activated carbon, which is the equivalent of meq/100 g. The CEC classification is presented in
Table 3.
2.7. Characterization of Waste Tire Carbon (WTC) and Activated Carbons
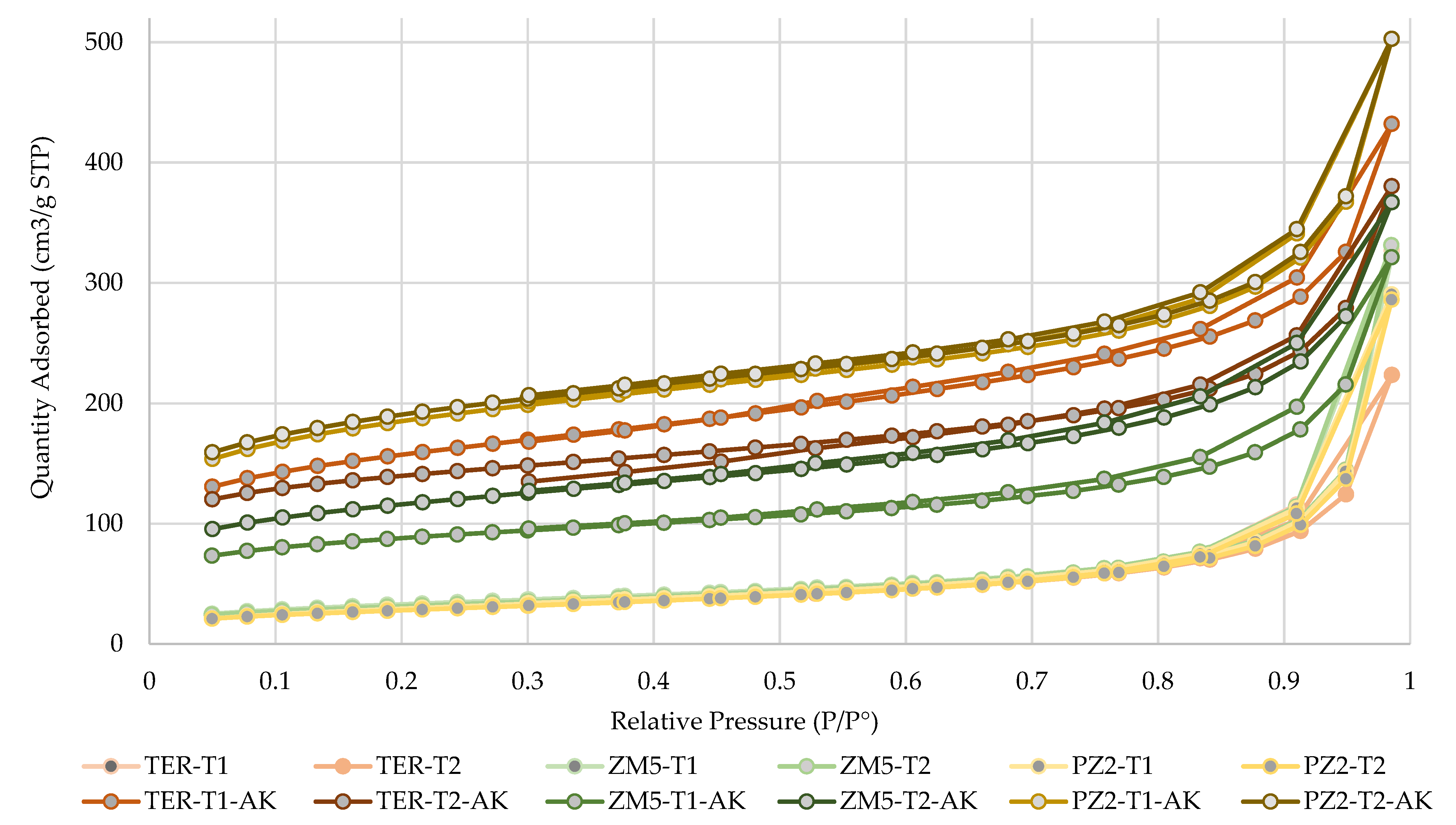
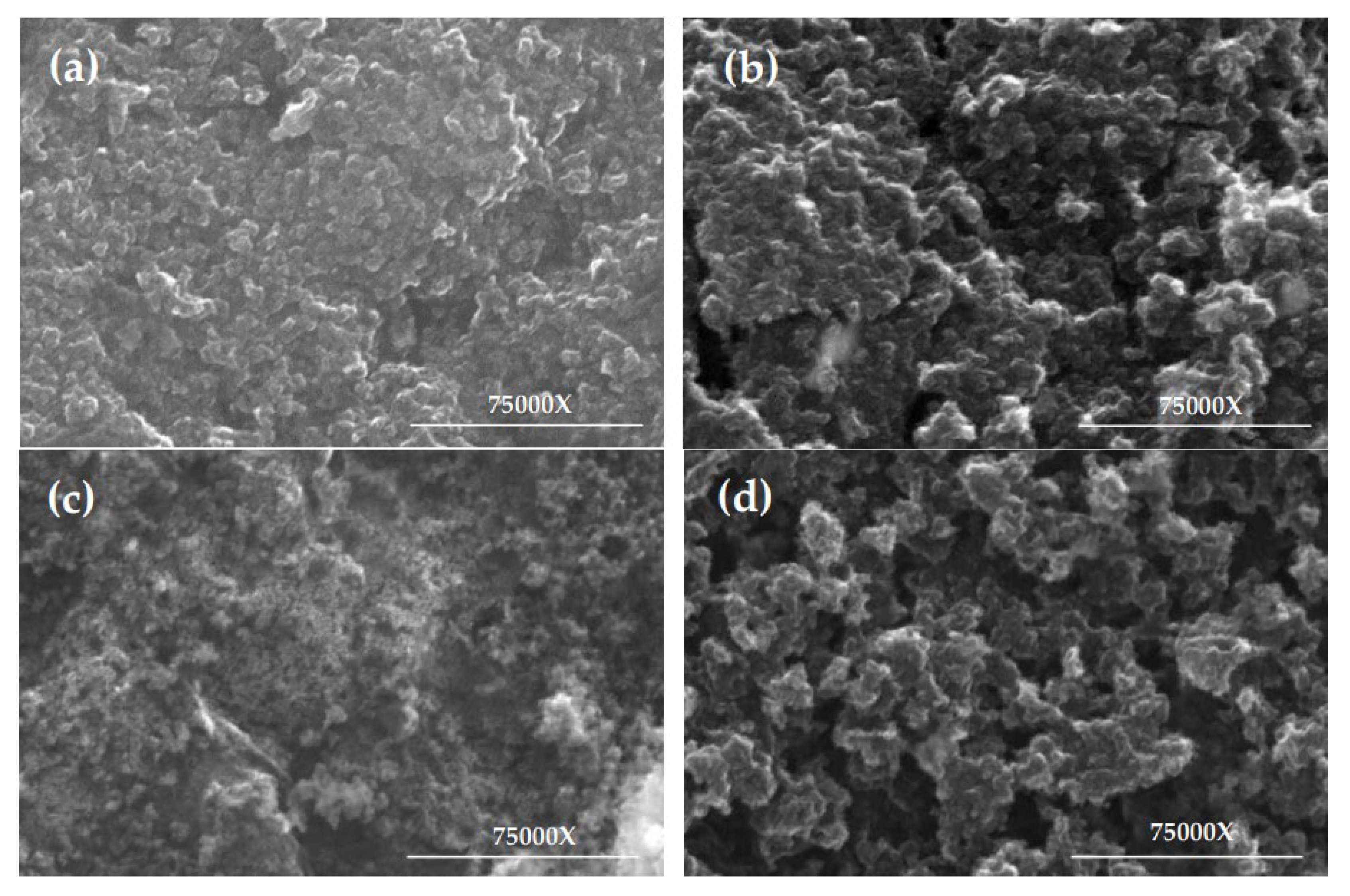
The textural characterization of the samples was conducted using the Brunauer–Emmett–Teller (BET) method with nitrogen gas as the adsorbate, employing a Gemini VII 2390 surface area analyzer, under an evacuation rate of 1000 mmHg·min
−1 and an equilibration time of 5 s [
34]. Morphological analysis was carried out via scanning electron microscopy (SEM) using a Hitachi SU8230 scanning electron microscope (Hitachi High-Tech, Tokyo, Japan), acquiring high-resolution images at a scale of 75,000× to evaluate the surface structure of the zeolites [
34].
The whole experimental procedure is summarized in the flowchart presented in
Figure 3.
4. Conclusions
The performance of WTC in pyrolysis remains stable (35.57–38.97%) without significant variations due to temperature or catalyst presence, indicating that increasing the temperature or including catalysts is not necessary to maximize carbon production, thereby reducing costs and energy consumption. Moreover, pyrolytic oil and gas yields exhibit an inverse relationship under the applied temperature conditions, with catalysts promoting cracking, though without drastic variations. Additionally, the structural stability of catalysts influences product distribution: more ordered structures facilitate cracking, while less ordered structures lead to variations in byproducts. These findings support the optimization of operating conditions based on the desired product.
According to the characterization of WTC and the activated carbons, in terms of sur-face area and volume, the best results were obtained for PZ2-T1-AK and PZ2-T2-AK, with BET surface areas of 608.65 m2/g and 624.37 m2/g, respectively. Furthermore, PZ2-T2-AK achieved a micropore volume of 0.1337 cm3/g and a micropore area of 257.53 m2/g. The CEC values for these carbons were remarkably high, ranging from 19.2 cmolc/kg (PZ2-T2-AK) to 21.0 cmolc/kg (PZ2-T1-AK), along with burn-off levels that indicate high material durability. When compared with activated carbons from other studies, those derived from PZ2 as a precursor exhibited superior physical properties. These properties are closely related to adsorption processes, particularly SPSA and SBET, which are critical parameters for evaluating the quality and performance of activated carbons. It is also important to highlight that chemical activation using KOH at the designated temperature contributed to achieving materials with desirable properties, suggesting that these activated carbons not only meet the requirements for adsorption applications but also present new opportunities for the development of highly efficient materials. Additionally, morphological analysis via SEM revealed that PZ2-T1-AK and PZ2-T2-AK possess a well-distributed and accessible structure, optimizing the mesoporous network due to the influence of the PZ2 zeolite, which is key for adsorption applications.
This study underscores the potential of waste tires for the production of activated carbons, promoting sustainable waste management and the development of functional materials for environmental remediation. Furthermore, it opens the door to exploring various catalysts and activation techniques to further enhance these carbons and expand the reuse of industrial waste in advanced adsorption materials, contributing to sustainability and the circular economy. For future research, it is recommended to explore different catalysts and activation strategies to develop even more efficient activated carbons, broadening their applicability in adsorption processes and reinforcing the valorization of industrial waste through the production of advanced materials.