Prepared of Titanate as Pb (II) Adsorbent from SCR Waste Catalyst by Sub-Molten Salt Method: A Sustainable Strategy for Hazardous Waste Recycling and Heavy Metal Remediation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Adsorbent Materials
2.2. Preparation of Pb(II) Solution
2.3. Experimental Methods
2.4. Equipment and Reagents
3. Results
3.1. Characterization Analysis of Waste SCR Catalyst Materials and Adsorbent Materials
3.1.1. SEM and EDS Analysis
3.1.2. Particle Size Distribution and BET Analysis
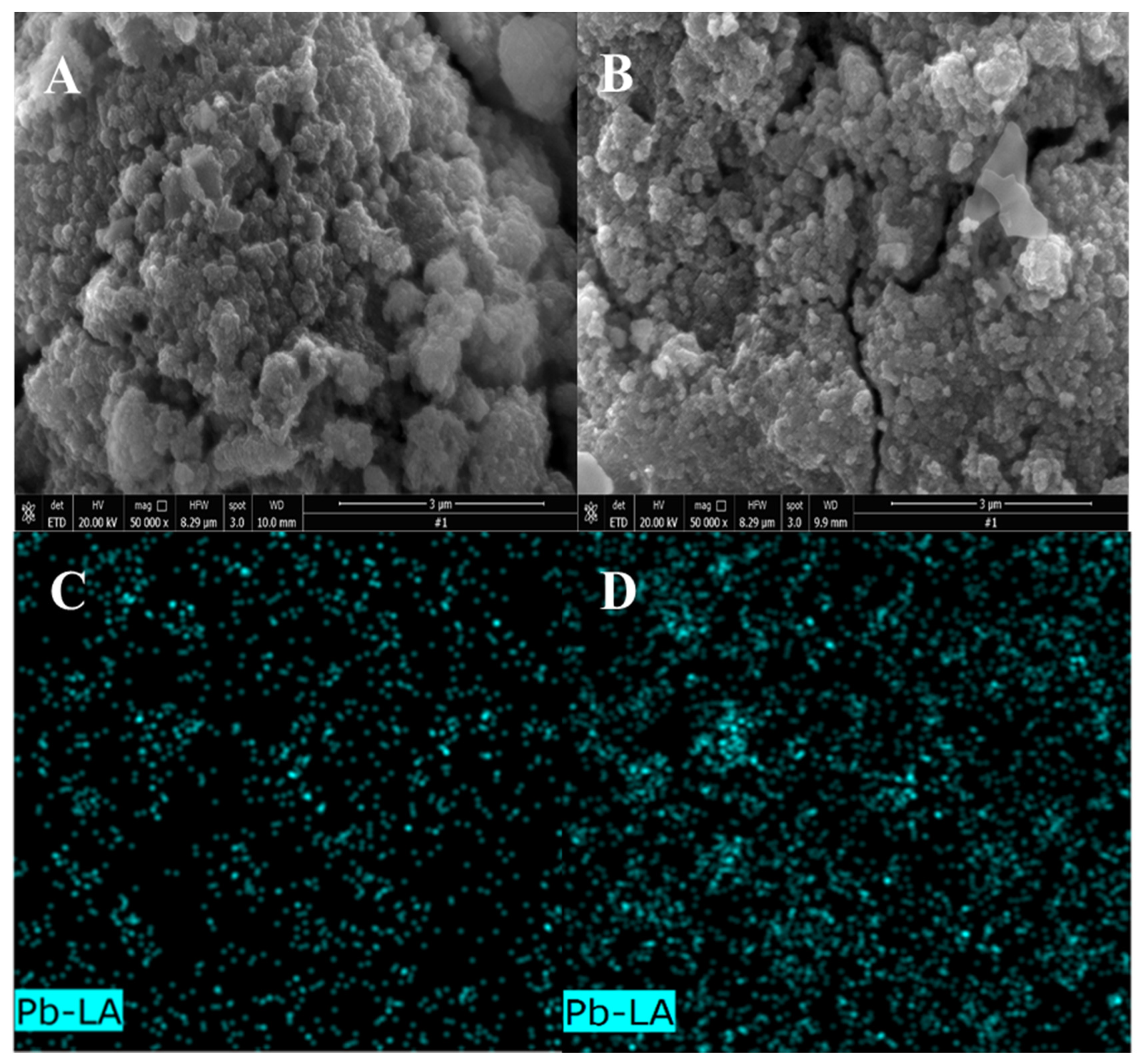
3.1.3. Local SEM and EDS Analysis of Adsorption Materials
3.2. Effect of Different Factors on the Pb(II) Adsorption Process
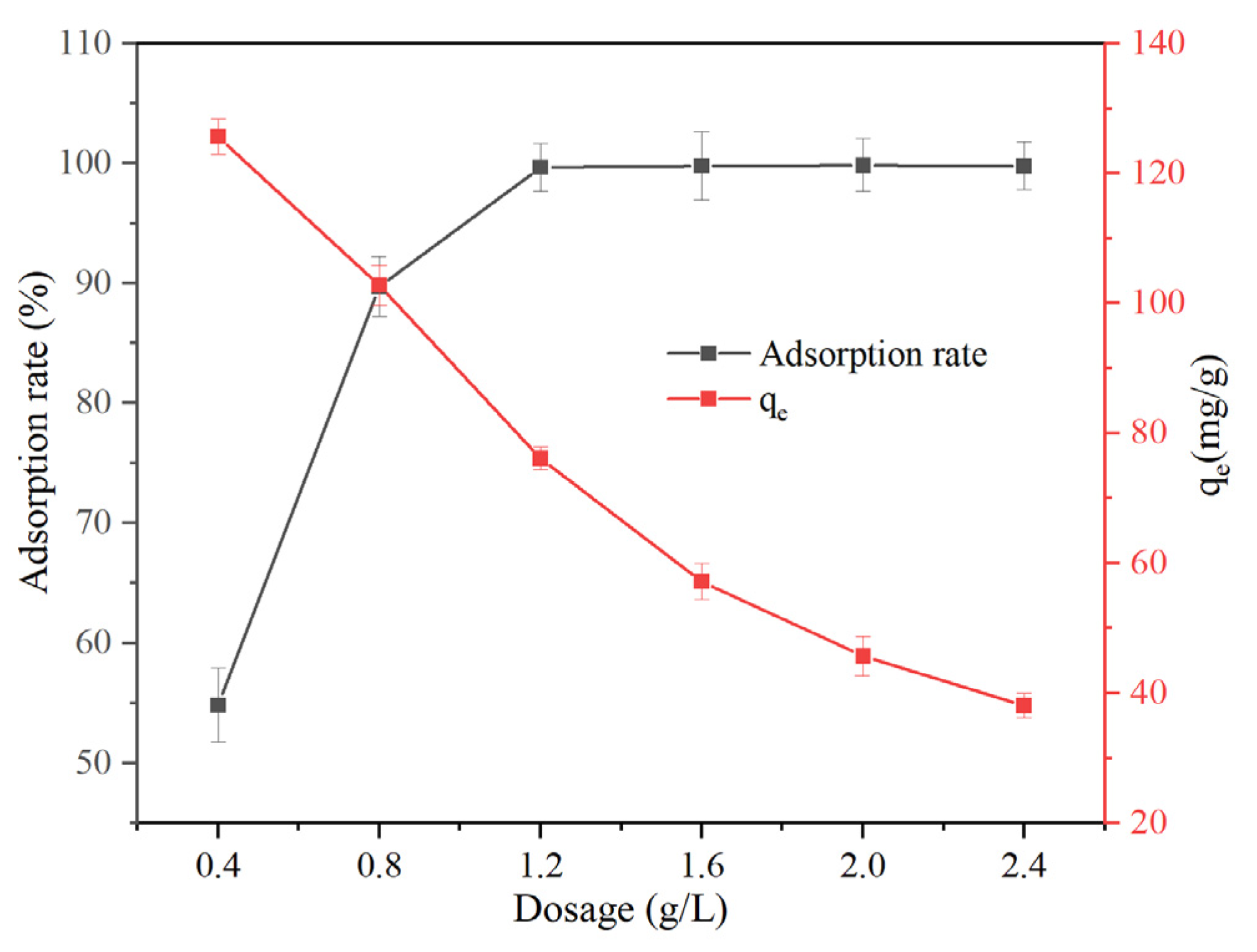
3.2.1. Influence of Adsorption Material Dosage on the Pb(II) Adsorption Process
3.2.2. Influence of Contact Time on the Pb(II) Adsorption Process
3.2.3. Influence of Initial Concentration on the Pb(II) Adsorption Process
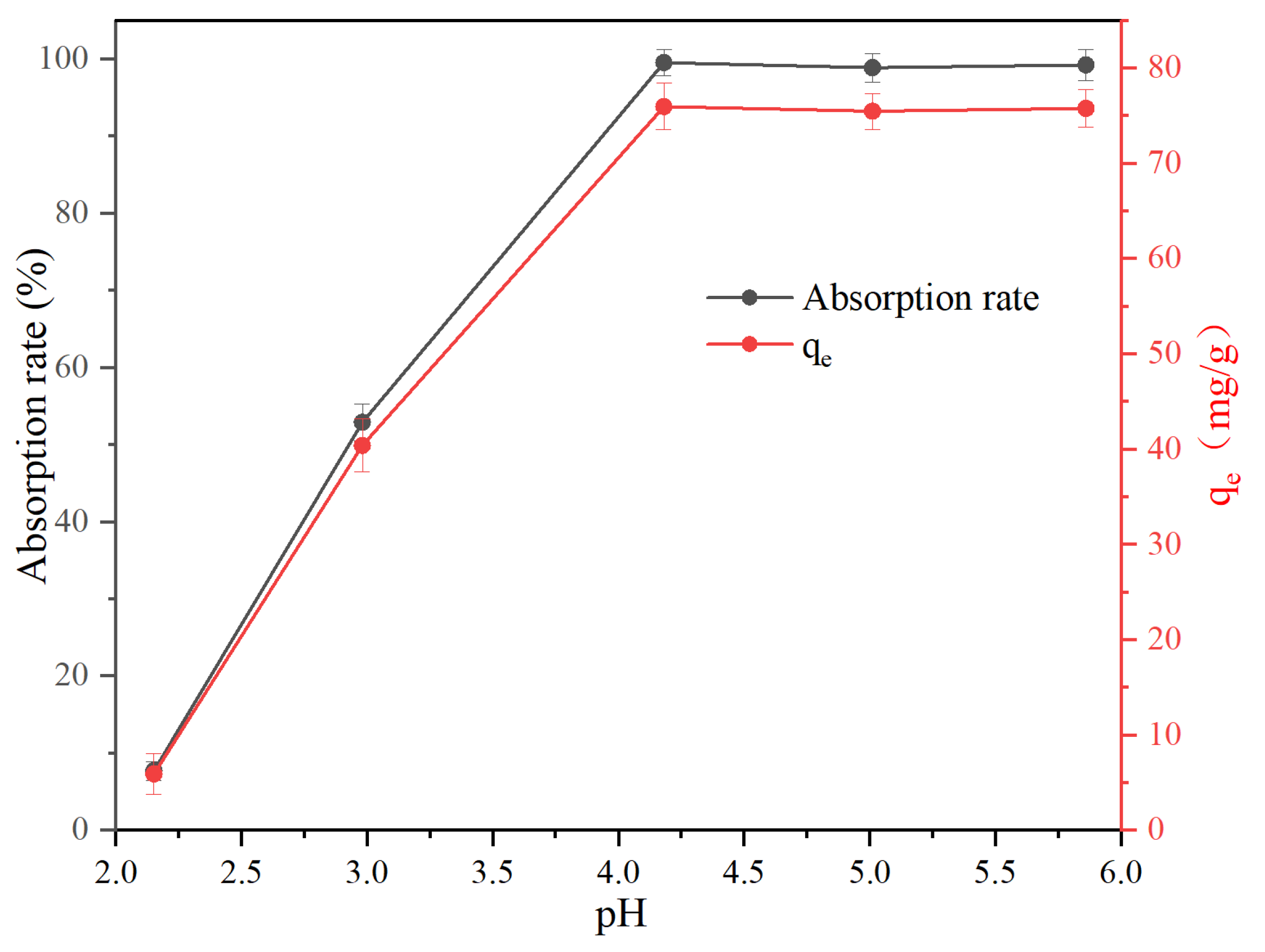
3.2.4. Influence of pH on the Pb(II) Adsorption Process
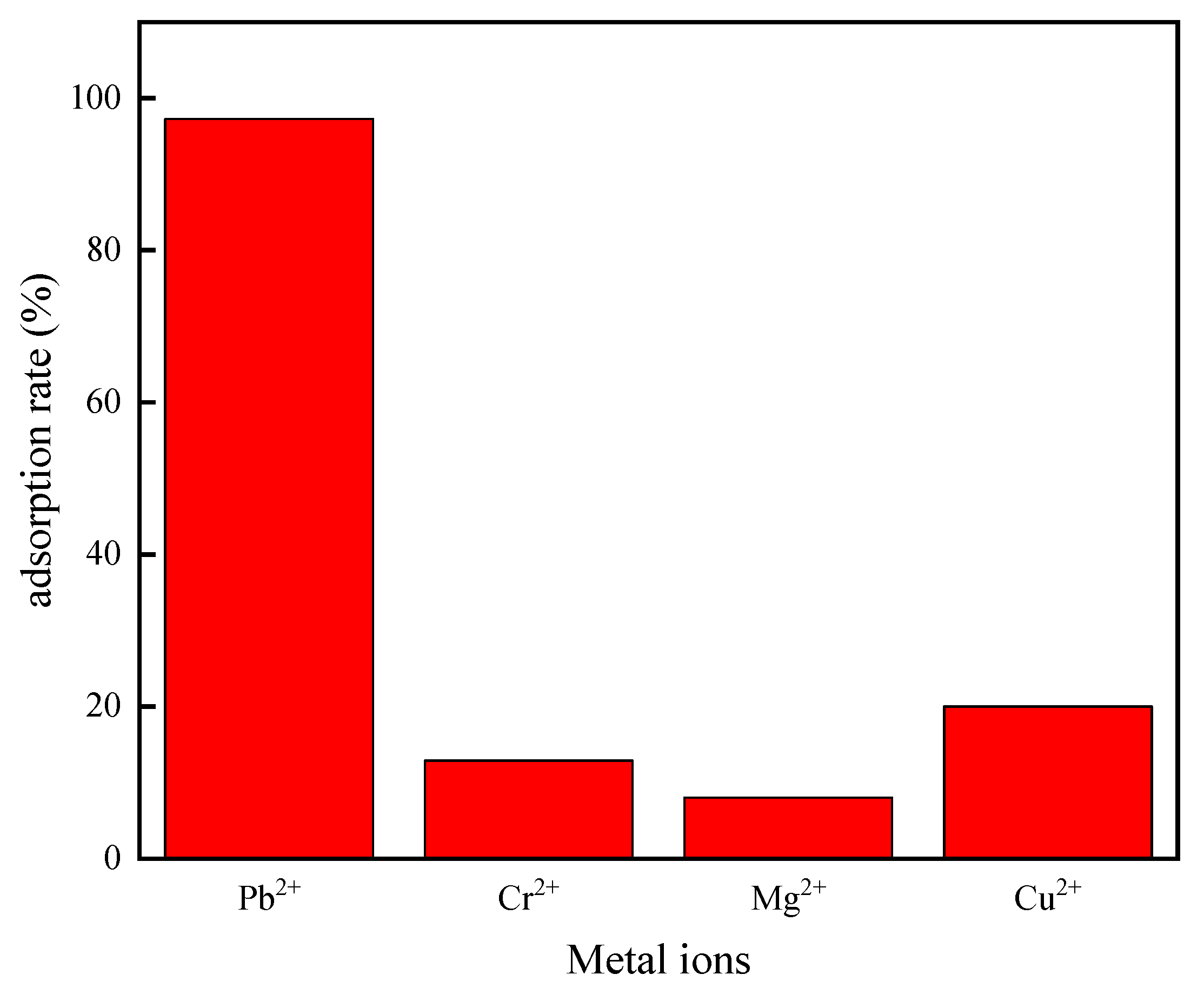
3.3. Competitive Adsorption Performance of Adsorption Materials
4. Discussion
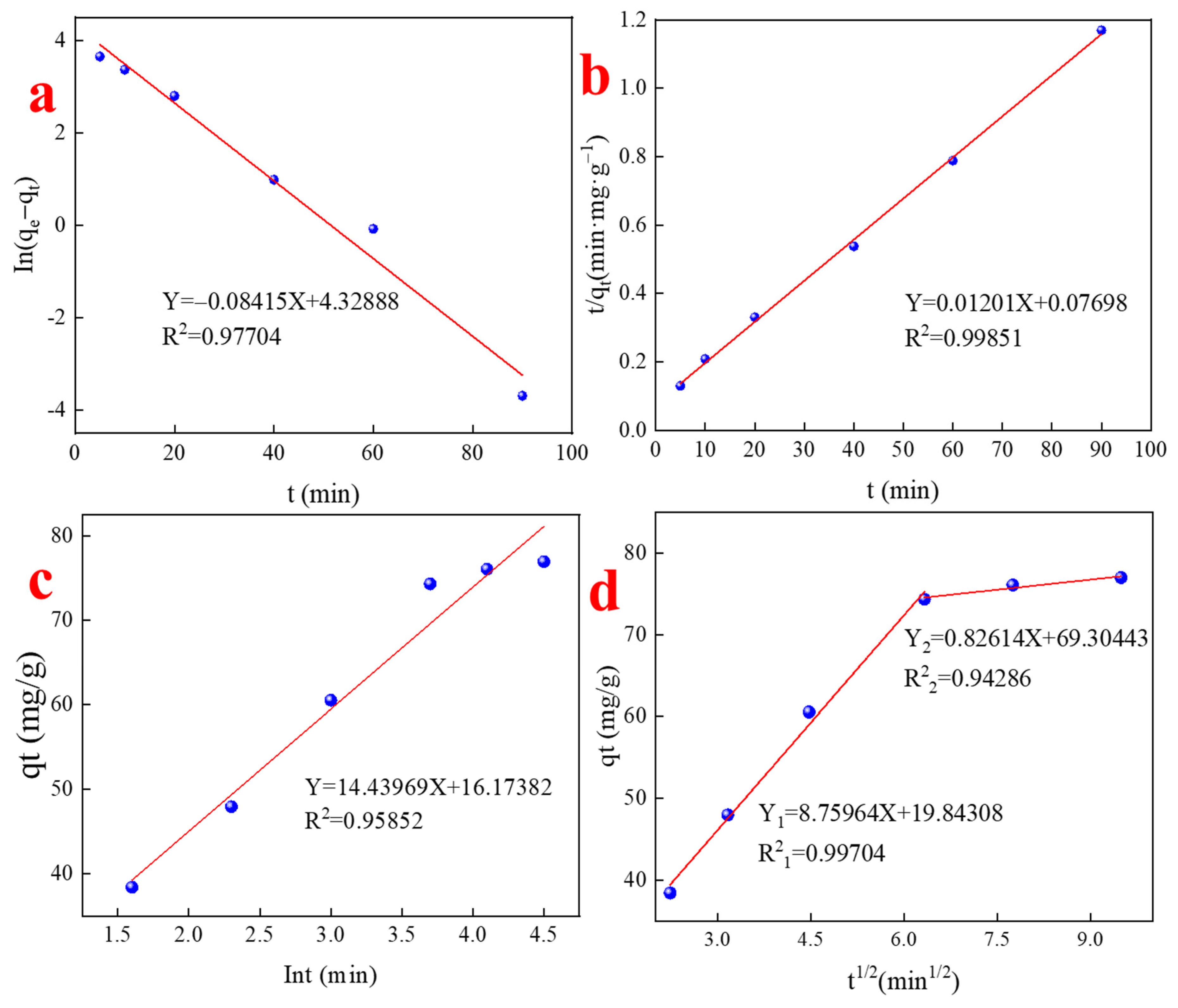
4.1. Adsorption Kinetics for Pb(II) Adsorption
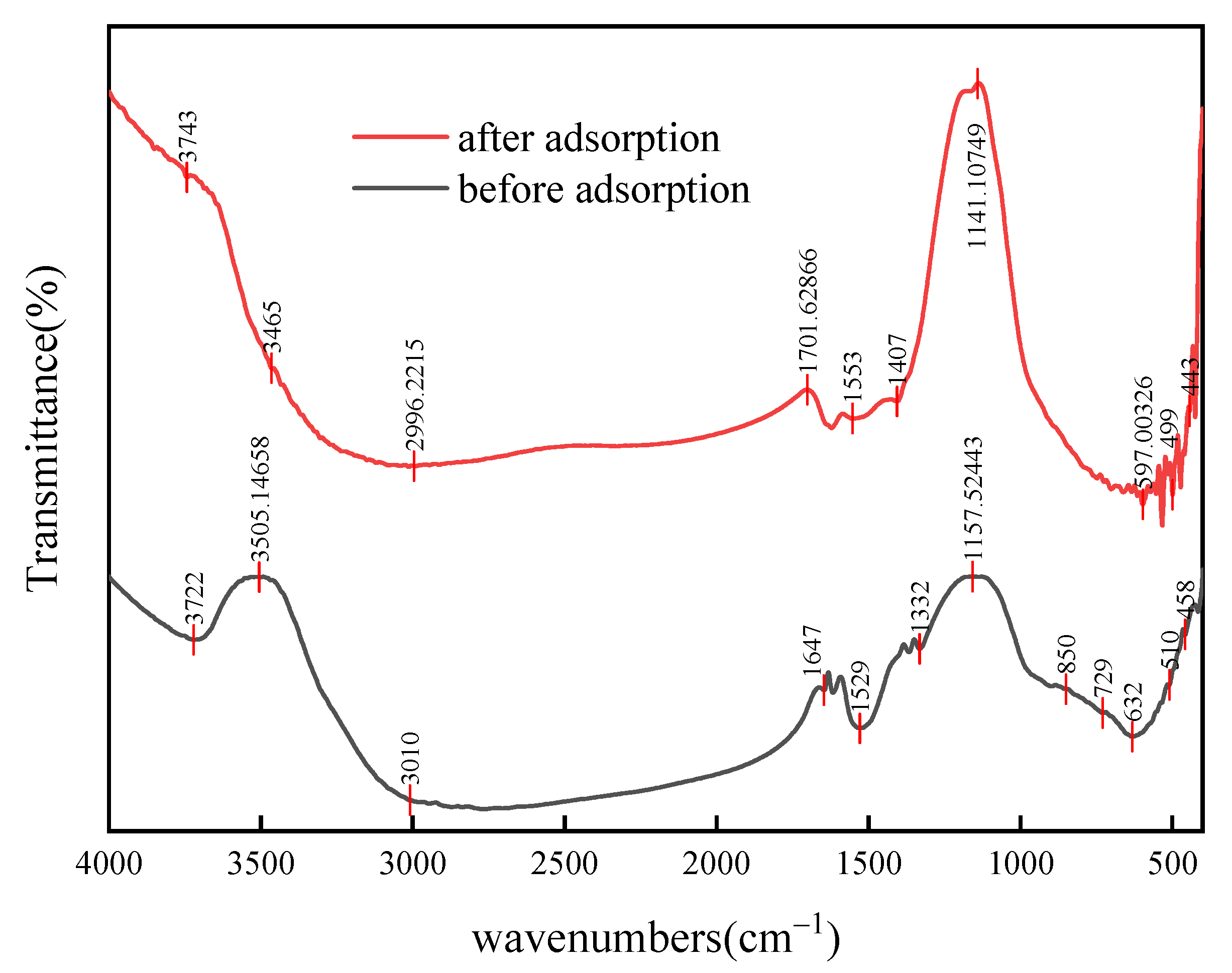
4.2. Characterization Analysis
4.3. Adsorption Mechanism
5. Conclusions
- (1)
- Under the conditions of adsorbent dosage of 1.2 g/L, initial Pb(II) concentration of 100 mg/L, contact time of 60 min, and pH = 4, the adsorbent material achieved a near-complete adsorption of Pb(II), with the adsorption rate as high as 99.65%, and the adsorption capacity as high as 76.08 mg/g. The results show that the SCR catalyst for concentrated alkali leaching is a kind of good and highly efficient adsorbent material.
- (2)
- The kinetic study showed that the quasi-second-order kinetic model revealed that the adsorption of Pb by the adsorbent material was mainly controlled by chemisorption. The intraparticle diffusion model was used to fit the data, and the relationship between the adsorbed amount of Pb and t1/2 could be fitted by two straight lines with different slopes without passing through the origin, indicating that the adsorption process was not influenced by a single diffusion factor.
- (3)
- The adsorption of Pb by the Pb(II) adsorbent mainly occurs on the flaky surface of the adsorbent material, and the adsorption products are substances containing CO32− and OH-. The paper proposes a new approach for the high-value utilization of waste SCR catalysts that incorporates innovative waste treatment methods and provides a practical framework for improving the environmental sustainability of waste SCR catalysts. In addition, it effectively realizes the value of lead recovery and disposal.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, L.; Guo, Y.; Liu, Z.; Feng, P.; Li, Z. An investigation on the catalytic characteristic of NOx reduction in SCR systems. J. Taiwan Inst. Chem. Eng. 2019, 99, 53–59. [Google Scholar] [CrossRef]
- Kumar, M.S.; Alphin, M.S.; Manigandan, S.; Vignesh, S.; Vigneshwaran, S.; Subash, T. A review of comparison between the traditional catalyst and zeolite catalyst for ammonia-selective catalytic reduction of NOx. Fuel 2023, 344, 128125. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, D.; He, F.; Cao, Z.; Wang, B. Study of leaching mechanism and kinetics of vanadium and tungsten on the process of recovery spent SCR catalyst by sodium roasted. Mater. Rep. 2021, 35, 429–433. [Google Scholar]
- China Environmental Protection Industry Association Desulfurization and Denitration Committee. China Development Report on Desulfurization and Denitration Industries in 2014; China Environmental Protection Industry: Beijing, China, 2015; Volume 12, pp. 4–23. [Google Scholar]
- Wang, J.-Z.; Du, H.; Olayiwola, A.; Liu, B.; Gao, F.; Jia, M.-L.; Wang, M.-H.; Gao, M.-L.; Wang, X.-D. Recent advances in the recovery of transition metals from spent hydrodesulfurization catalysts. Tungsten 2021, 3, 305–328. [Google Scholar] [CrossRef]
- Dai, Z. Research on Heavy Metal Leaching Characteristics of Waste SCR Catalyst and Its Harmless Treatment. Master’s Thesis, Huazhong University of Science and Technology, Wuhan, China, 2018. [Google Scholar]
- Wang, Q. Research on chemical hazardous waste incineration and flue gas treatment process. Chem. Des. Newsl. 2022, 48, 188–190. [Google Scholar]
- Xue, Q. Analysis of hazardous waste incineration and disposal technology. Chem. Manag. 2022, 44–46. [Google Scholar]
- Wu, T.; Liu, X.; Xi, W. Study on factors affecting acid dissolution rate in titanium separation from waste denitrification catalyst. Mod. Chem. Ind. 2018, 38, 115–117. [Google Scholar]
- Kim, J.W.; Lee, W.G.; Hwang, I.S.; Lee, J.Y.; Han, C. Recovery of tungsten from spent selective catalytic reduction catalysts by pressure leaching. J. Ind. Eng. Chem. 2015, 28, 73–77. [Google Scholar] [CrossRef]
- Choi, I.-H.; Moon, G.; Lee, J.-Y.; Jyothi, R.K. Hydrometallurgical processing of spent selective catalytic reduction (SCR) catalyst for recovery of tungsten. Hydrometallurgy 2018, 178, 137. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, W.; Yang, J.; Jia, L.; Li, L.; Cao, H.; Guo, X. Cd2+ and Zn2+ fluorescence turn-on sensing and the subsequent detection of S2− by a quinol imide based sensor in water and living cells with application in the combinational logic gate. Tetrahedron 2022, 121, 132916. [Google Scholar] [CrossRef]
- Wei, Y.; Li, D.; Qiao, J.; Guo, X. Research progress on disposal and recovery of waste SCR denitrification catalysts. Sci. Technol. Rev. 2024, 42, 62–72. [Google Scholar]
- Wang, J.; Guo, P.; Li, X.; Zhu, J.; Reinert, T.; Heitmann, J.; Spemann, D.; Vogt, J.; Flagmeyer, R.-H.; Butz, T. Source identification of lead pollution in the atmosphere of Shanghai City by analyzing single aerosol particles (SAP). Environ. Sci. Technol. 2000, 34, 1900–1905. [Google Scholar] [CrossRef]
- Sall, M.L.; Diaw, A.K.D.; Gningue-Sall, D.; Aaron, S.E.; Aaron, J.-J. Toxic heavy metals: Impact on the environment and human health, and treatment with conducting organic polymers, a review. Environ. Sci. Pollut. Res. 2020, 27, 29927–29942. [Google Scholar] [CrossRef]
- Qin, G.; Niu, Z.; Yu, J.; Li, Z.; Ma, J.; Xiang, P. Soil heavy metal pollution and food safety in China: Effects, sources and removing technology. Chemosphere 2021, 267, 129205. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; McBride, M.B.; Xia, H.; Li, N.; Li, Z. Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Sci. Total Environ. 2009, 407, 1551–1561. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, Z.; Hills, C.; Xue, G.; Tyrer, M. Precipitation of heavy metals from wastewater using simulated flue gas: Sequent additions of fly ash, lime and carbon dioxide. Water Res. 2009, 43, 2605–2614. [Google Scholar] [CrossRef]
- Jokar, M.; Mirghaffari, N.; Soleimani, M.; Jabbari, M. Preparation and characterization of novel bio ion exchanger from medicinal herb waste (chicory) for the removal of Pb2+ and Cd2+ from aqueous solutions. J. Water Process Eng. 2019, 28, 88–99. [Google Scholar] [CrossRef]
- Kárászová, M.; Bourassi, M.; Gaálová, J. Membrane removal of emerging contaminants from water: Which kind of membranes should we use. Membranes 2020, 10, 305. [Google Scholar] [CrossRef]
- Vaudevire, E.; Radmanesh, F.; Kolkman, A.; Vughs, D.; Cornelissen, E.; Post, J.; van der Meer, W. Fate and removal of trace pollutants from an anion exchange spent brine during the recovery process of natural organic matter and salts. Water Res. 2019, 154, 34–44. [Google Scholar] [CrossRef]
- Rodriguez, A.Z.; Wang, H.; Hu, L.; Zhang, Y.; Xu, P. Treatment of produced water in the permian basin for hydraulic fracturing: Comparison of different coagulation processes and innovative filter media. Water 2020, 12, 770. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, P.; Peng, W.; Tang, J. Research progress on modification methods of pomelo peel as biological adsorbent. South For. Sci. 2022, 50, 61–66+72. [Google Scholar]
- Vo, T.S.; Hossain, M.M.; Jeong, H.M.; Kim, K. Heavy metal removal applications using adsorptive membranes. Nano Converg. 2020, 7, 36. [Google Scholar] [CrossRef]
- Adornado, A.P.; Soriano, A.N.; Orfiana, O.N.; Pangon, M.B.J.; Nieva, A.D. Simulated biosorption of Cd (II) and Cu (II) in single and binary metal systems by water hyacinth (Eichhornia crassipes) using Aspen adsorption. ASEAN J. Chem. Eng. 2017, 16, 21–43. [Google Scholar] [CrossRef]
- Wang, B.-J.; Xie, Q.; Sha, Y.-T.; Liu, J.-C.; Liang, D.-C. Research progress of activated carbon preparation. Mod. Agric. Sci. Technol. 2024, 40, 118–122+127. [Google Scholar]
- Chen, X.; Duan, F.; Yu, X.; Xie, Y.; Wang, Z.; El-Baz, A.; Ni, B.J.; Ni, S.Q. One-stage anammox and thiocyanate-driven autotrophic denitrification for simultaneous removal of thiocyanate and nitrogen: Pathway and mechanism. Water Res. 2024, 265, 122268. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, Z.; Yuan, B.; Zhi, S.; Zhang, Y.; Li, J.; Wu, A. Ultralight and superhydrophobic perfluorooctyltrimethoxysilane modified biomass carbonaceous aerogel for oil-spill remediation. Chem. Eng. Res. Des. 2021, 174, 71–78. [Google Scholar] [CrossRef]
- Chengsheng, S.; Dali, Z.; Lei, Y.; Jiabei, Z.; Can, L.; Zhi-Gang, C. Recovery TiO2 and sodium titanate nanowires as Cd (II) adsorbent from waste V2O5-WO3/TiO2 selective catalytic reduction catalysts by Na2CO3-NaCl-KCl molten salt roasting method. J. Taiwan Inst. Chem. Eng. 2018, 88, 226–233. [Google Scholar]
- Luo, J.; Wenjuan, G.; Guiqing, Z. High pressure leaching of tungsten and vanadium with sodium carbonate from spent SCR denitration catalysts. Rare Met. Cem Carbides 2015, 43, 1–6. [Google Scholar]
- Huo, Y.; Chang, Z.; Li, W.; Liu, S.; Dong, B. Reuse and valorization of vanadium and tungsten from waste V2O5–WO3/TiO2 SCR catalyst. Waste Biomass Valorization 2015, 6, 159–165. [Google Scholar] [CrossRef]
- Li, S.-C.; Kang, C.-S.; Kim, S.-C.; Kim, C.-J.; Kang, C.-J. The extraction of Ta, Nb and rare earths from fergusonite by using KOH sub-molten salt leaching. Hydrometallurgy 2021, 201, 105358. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, M.; Tan, Y. Preparation of porous nanoporous barium strontium titanate based on straw template method and its adsorption performance on heavy metals. J. Chem. 2010, 68, 1641–1648. [Google Scholar]

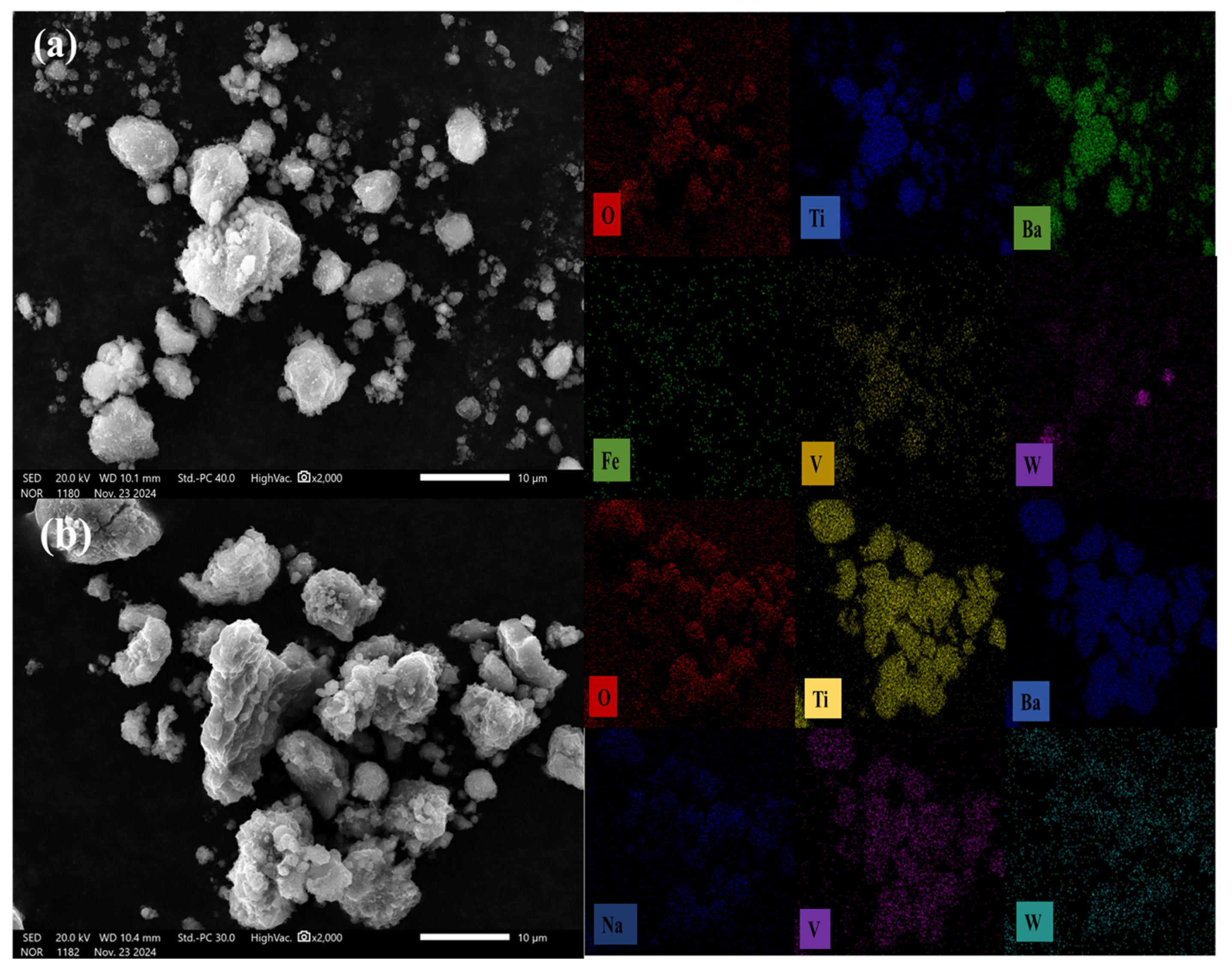





| Ti | O | Na | W | Ba | Si | Ca | |
|---|---|---|---|---|---|---|---|
| SCR catalyst raw materials | 47.21 | 40.10 | 0.07913 | 4.325 | 2.613 | 1.609 | 1.376 |
| Adsorbent materials | 46.53 | 41.69 | 5.301 | 0.128 | 2.211 | 0.8909 | 1.917 |
| EDS Quantitative Results Element | Waste SCR Catalyst Materials | Adsorbent Materials |
|---|---|---|
| O | 47.58 | 39.45 |
| Ti | 28.12 | 31.00 |
| Fe | 5.47 | 5.96 |
| Al | 0.86 | 0.74 |
| Si | 1.83 | 1.64 |
| W | 5.99 | 1.30 |
| Na | 0.00 | 6.37 |
| Ca | 0.08 | 2.22 |
| Ba | 8.90 | 11.22 |
| Materials | Specific Surface Areas |
|---|---|
| waste SCR catalysts | 52.825 |
| adsorbent materials | 5.393 |
| EDS Quantitative Results Element | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| O | 15.03 | 26.23 | 30.99 | 30.00 |
| Ti | 55.38 | 50.64 | 42.40 | 44.20 |
| Fe | 2.86 | 3.26 | 4.18 | 2.28 |
| Na | 2.02 | 3.77 | 5.99 | 5.86 |
| W | 1.05 | 0.28 | 2.64 | 0.90 |
| V | 0.00 | 0.00 | 0.61 | 0.22 |
| Si | 0.64 | 0.95 | 1.18 | 1.04 |
| Ca | 2.25 | 2.57 | 1.99 | 2.43 |
| Ba | 19.95 | 11.81 | 9.02 | 12.35 |
| Models | Parameters | ||
|---|---|---|---|
| Pseudo-first-order | k1 (min−1) | qe (mg/g) | R2 |
| 0.08415 | 75.8593 | 0.97704 | |
| Pseudo-second-order | k2 (g·mg−1·min−1) | qe (mg/g) | R2 |
| 0.001874 | 83.2639 | 0.99851 | |
| Elovich equation | α (g·mg−1·min−1) | β (mg/g) | R2 |
| 0.21535 | 14.43969 | 0.95852 | |
| Intraparticle diffusion | ki (g·mg−1·min−1) k1 = 8.75964 k2 = 0.82614 | C (mg/g) 19.84308 69.30443 | R2 0.99704 0.94286 |
| Element | Before Adsorption | After Adsorption |
|---|---|---|
| Pb | 1.86 | 24.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, L.; Yuan, W.; Yu, M.; Niu, H.; Mubula, Y.; Xu, K.; Zhu, Z. Prepared of Titanate as Pb (II) Adsorbent from SCR Waste Catalyst by Sub-Molten Salt Method: A Sustainable Strategy for Hazardous Waste Recycling and Heavy Metal Remediation. Sustainability 2025, 17, 4823. https://doi.org/10.3390/su17114823
Zeng L, Yuan W, Yu M, Niu H, Mubula Y, Xu K, Zhu Z. Prepared of Titanate as Pb (II) Adsorbent from SCR Waste Catalyst by Sub-Molten Salt Method: A Sustainable Strategy for Hazardous Waste Recycling and Heavy Metal Remediation. Sustainability. 2025; 17(11):4823. https://doi.org/10.3390/su17114823
Chicago/Turabian StyleZeng, Ling, Weiquan Yuan, Mingming Yu, Heyue Niu, Yusupujiang Mubula, Kun Xu, and Zhehan Zhu. 2025. "Prepared of Titanate as Pb (II) Adsorbent from SCR Waste Catalyst by Sub-Molten Salt Method: A Sustainable Strategy for Hazardous Waste Recycling and Heavy Metal Remediation" Sustainability 17, no. 11: 4823. https://doi.org/10.3390/su17114823
APA StyleZeng, L., Yuan, W., Yu, M., Niu, H., Mubula, Y., Xu, K., & Zhu, Z. (2025). Prepared of Titanate as Pb (II) Adsorbent from SCR Waste Catalyst by Sub-Molten Salt Method: A Sustainable Strategy for Hazardous Waste Recycling and Heavy Metal Remediation. Sustainability, 17(11), 4823. https://doi.org/10.3390/su17114823





