Removal of Azo Dyes from Water on a Large Scale Using a Low-Cost and Eco-Friendly Adsorbent
Abstract
1. Introduction
2. Materials and Methods
2.1. Non-Conventional and Low-Cost Adsorbent
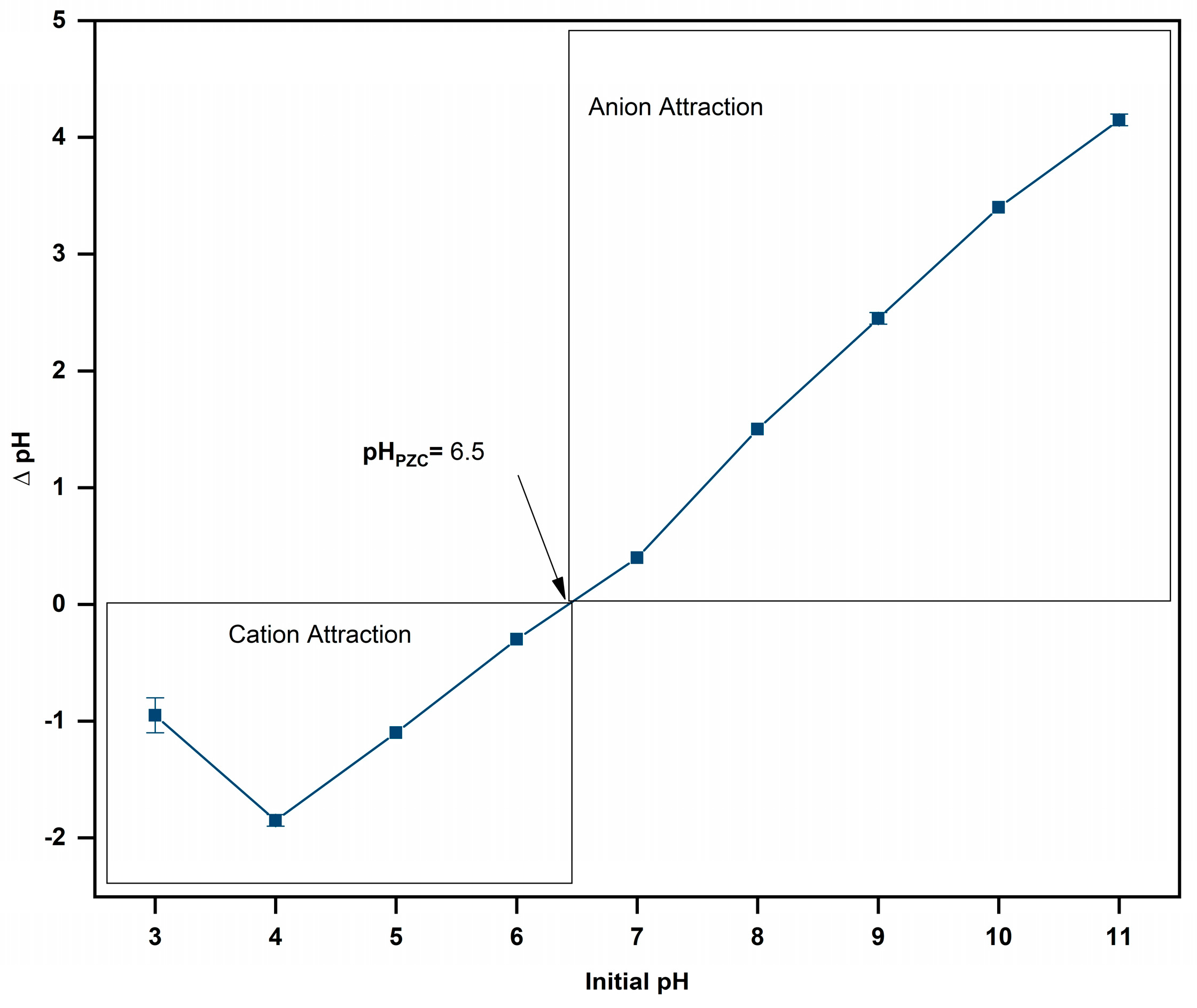
2.2. Determination of Point Zero Charge
2.3. Azo Dye Solution (Pollutant)
2.4. Adsorbent Dose Effect
2.5. Adsorption Studies
2.6. Isotherm Models of Adsorption
2.7. Kinetic Models
2.8. Characterization of Luffa cylindrica
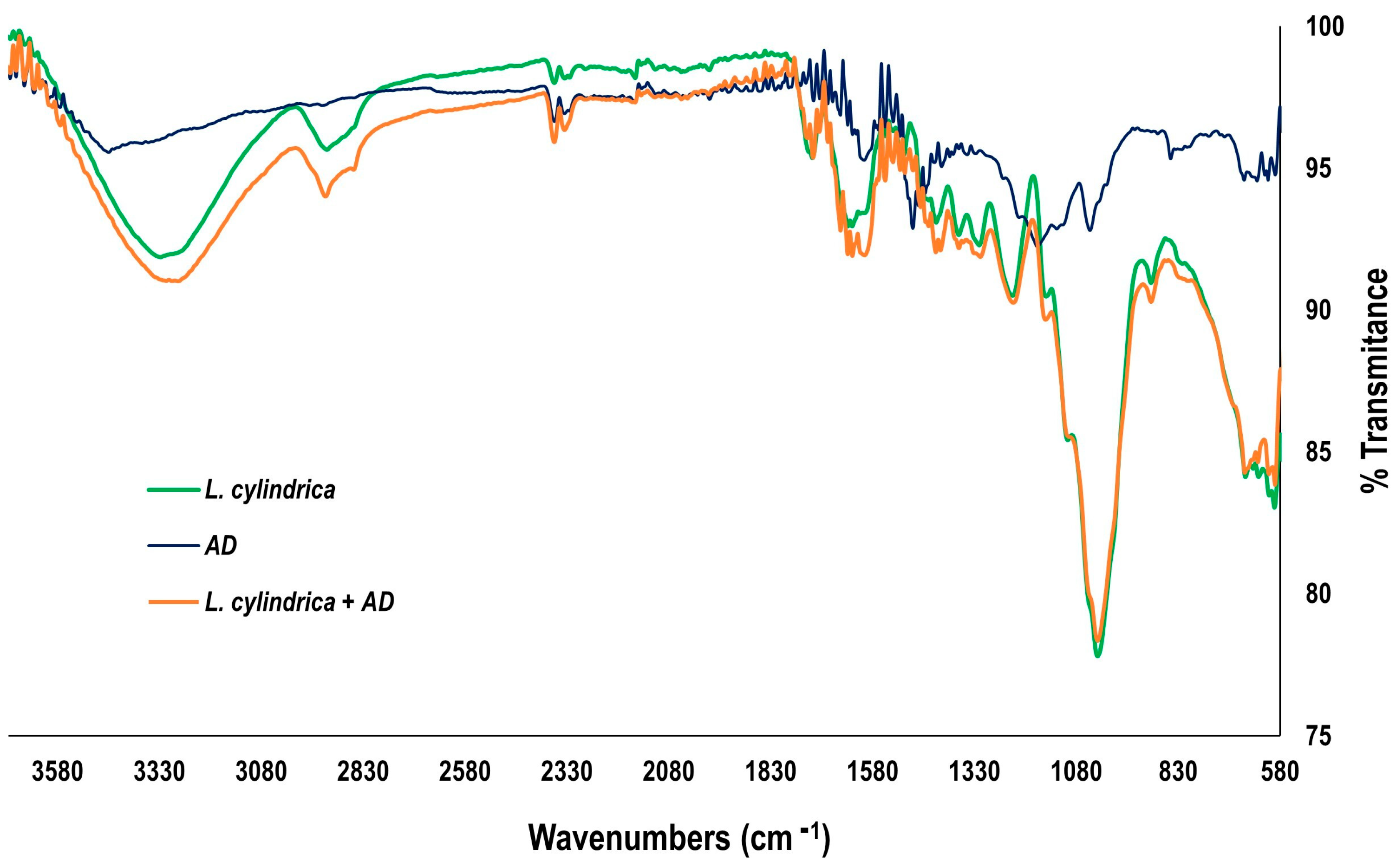
2.8.1. FTIR Spectroscopy
2.8.2. FESEM Image of Natural Adsorbent
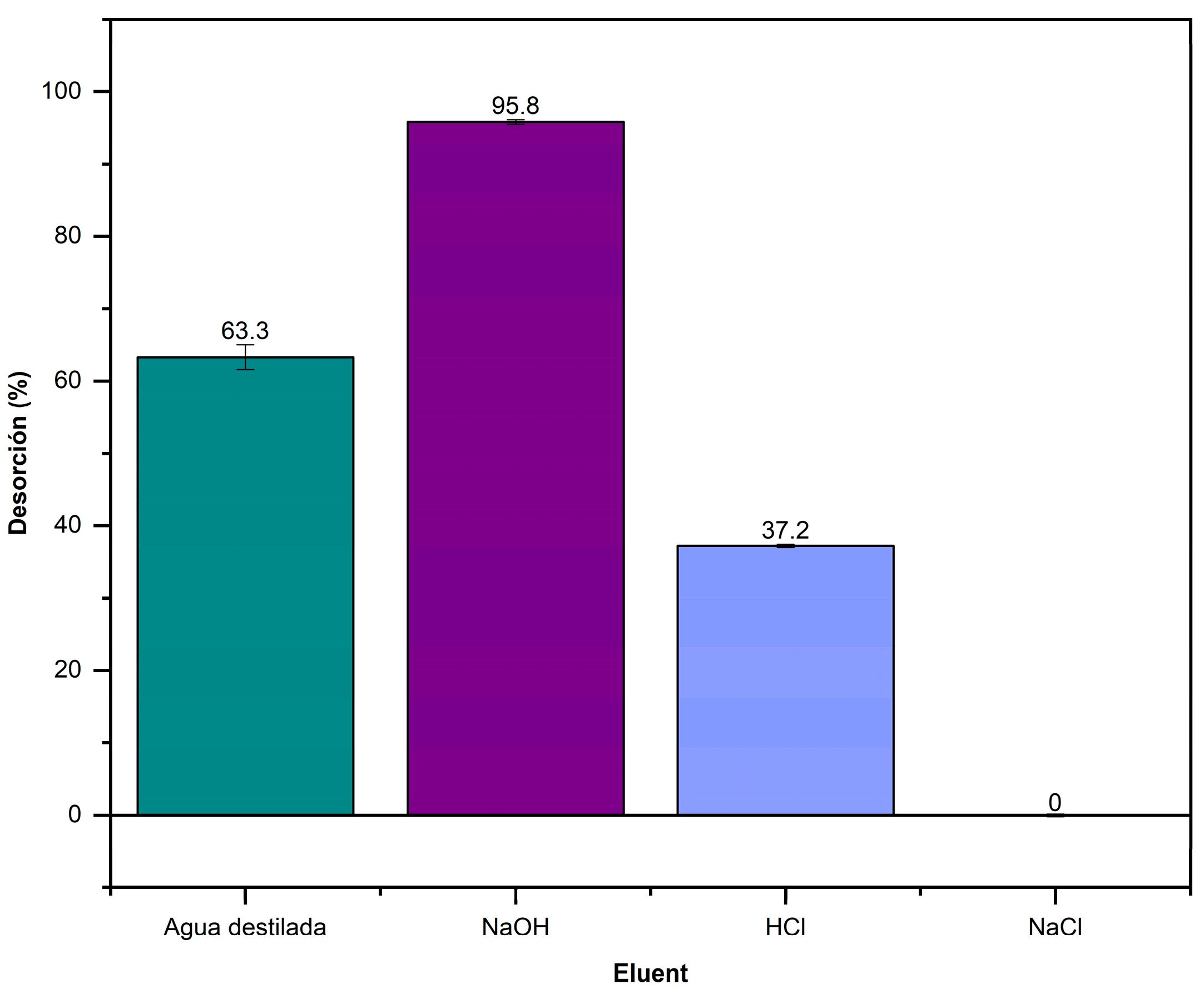
2.9. Desorption Experiments
3. Results
3.1. Point Zero Charge Determination
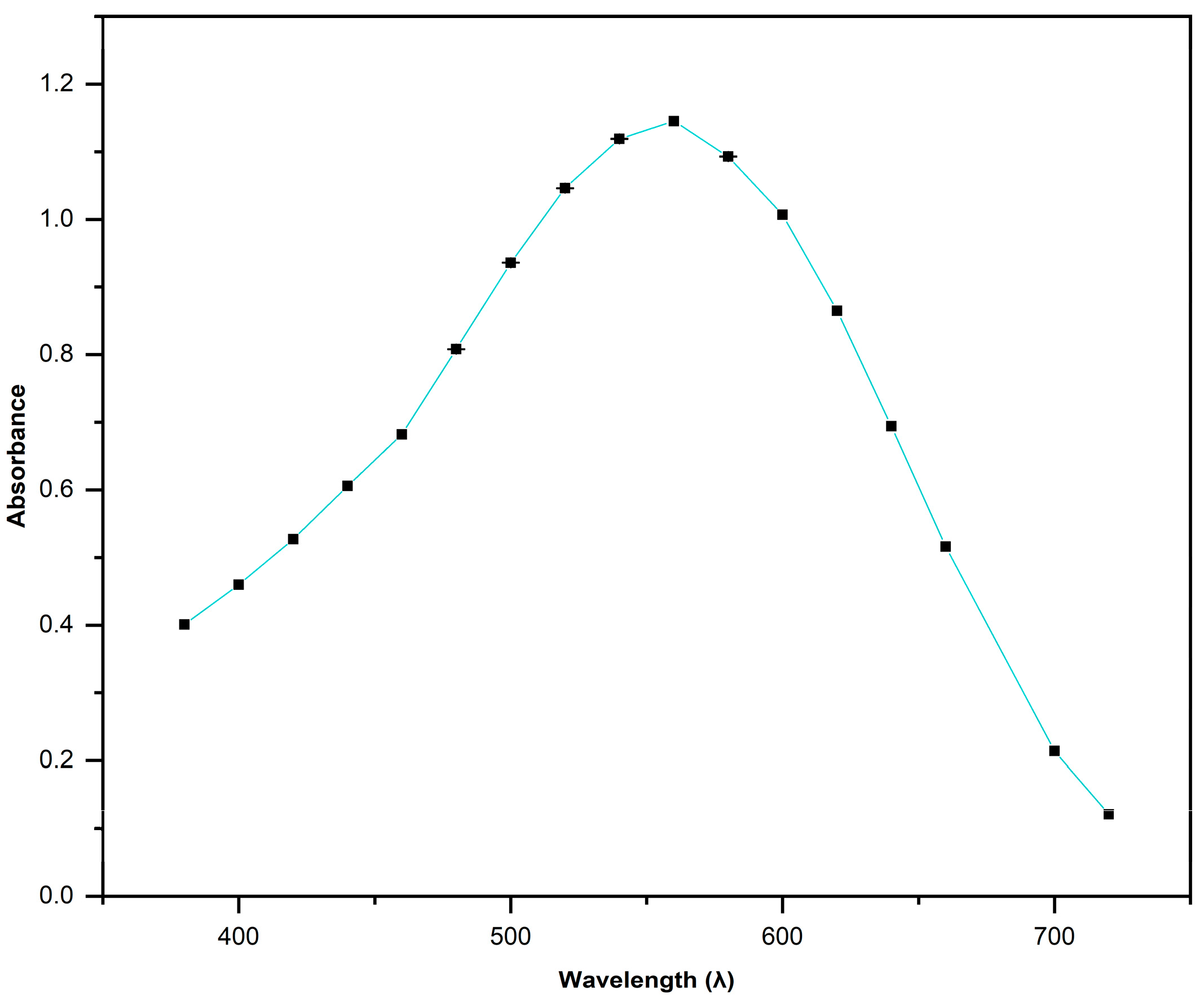
3.2. UV-VIS Spectroscopy of Azo Dye
3.3. Effect of Adsorbent Doses
3.4. Adsorption Study
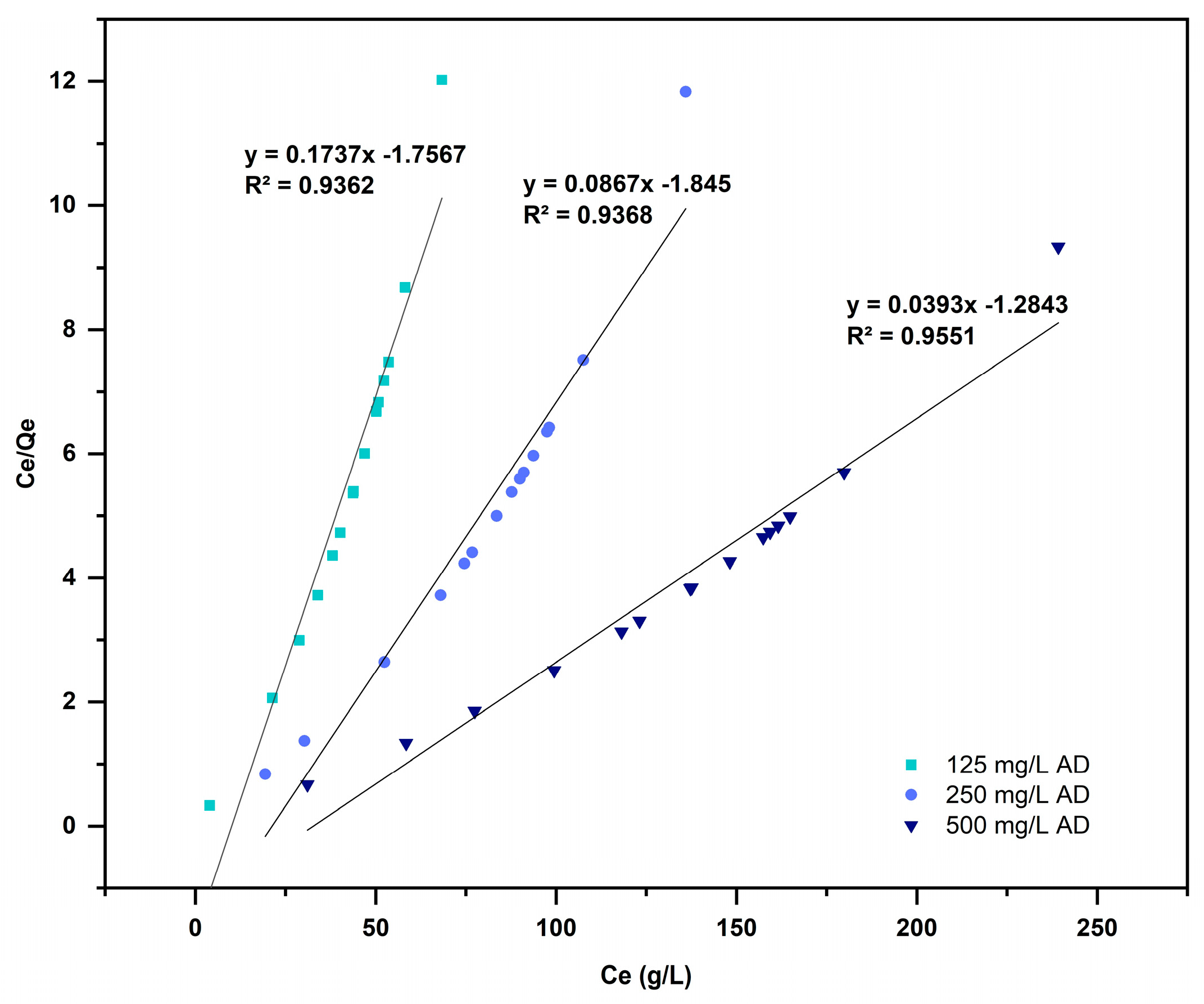
3.5. Isotherm of Adsorption
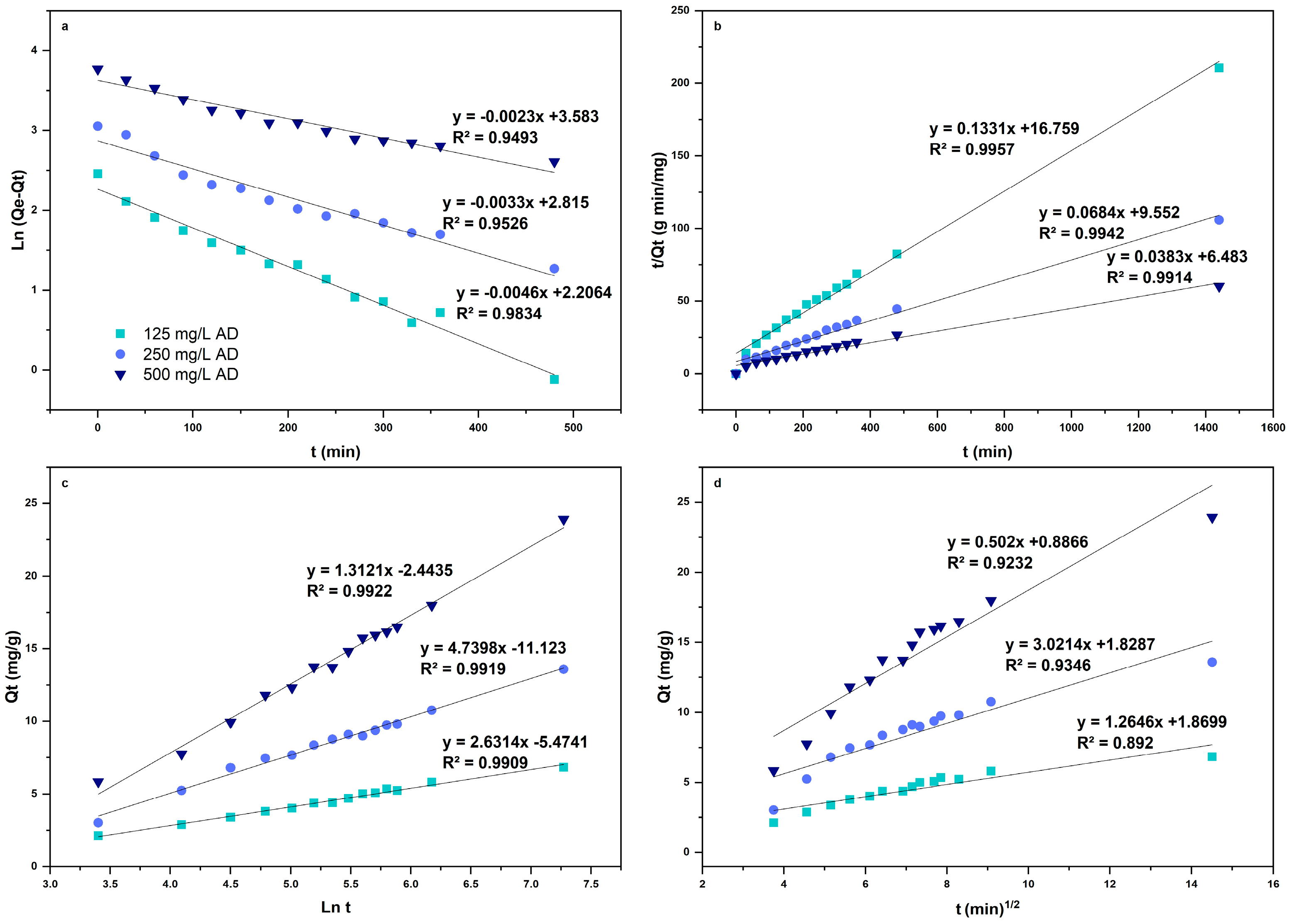
3.6. Adsorption Kinetics
3.7. Characterization of L. cylindrica Before and After Adsorption of AD
3.7.1. FTIR Analysis Spectroscopy
3.7.2. FESEM Natural Adsorbent Analysis
3.8. Desorption Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Azo dye |
| BOD | Biological oxygen demand |
| COD | Chemical oxygen demand |
| TSS | Total suspended solids |
| NaOH | Sodium hydroxide |
| HCl | Hydrogen chloride |
| KNO3 | Potassium nitrate |
| pH | Hydrogen potential |
| M | Molarity |
| UV-VIS | Ultraviolet–visible |
| FTIR | Fourier transformed infrared |
| FE-SEM | Field emission scanning electron microscope |
| Point charge zero | |
| Initial concentration | |
| Equilibrium concentration | |
| Maximum adsorption capacity |
References
- Münzel, T.; Hahad, O.; Daiber, A.; Landrigan, P.J. Soil and water pollution and human health: What should cardiologists worry about? Cardiovasc. Res. 2023, 119, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Babuji, P.; Thirumalaisamy, S.; Duraisamy, K.; Periyasamy, G. Human health risks due to exposure to water pollution: A review. Water 2023, 15, 2532. [Google Scholar] [CrossRef]
- Mishra, R.K. Freshwater availability and its global challenge. BJMAS 2023, 4, 1–78. [Google Scholar] [CrossRef]
- Riffat, R.; Husnain, T. Fundamentals of Wastewater Treatment and Engineering, 2nd ed.; CRC Press: Abingdon, UK, 2022. [Google Scholar] [CrossRef]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Romanholo Ferreira, L.F.; Bilal, M.; Chandra, R.; Bharagava, R.N. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Khadir, A.; Negarestani, M.; Kheradmand, A.; Azad, A.; Sillanpää, M. The Utilization of Biomaterials for Water Purification: Dyes, Heavy Metals, and Pharmaceuticals. In Novel Materials for Dye-Containing Wastewater Treatment. Sustainable Textiles: Production, Processing, Manufacturing & Chemistry; Muthu, S.S., Khadir, A., Eds.; Springer: Singapore, 2021; pp. 27–58. [Google Scholar] [CrossRef]
- Nadaroglu, H.; Cicek, S.; Onem, H.; & Gungor, A.A. The Investigation of Removing Direct Blue 15 Dye from Wastewater Using Magnetic Luffa sponge NPs. In Iron Ores and Iron Oxide Materials; Shatokha, V., Ed.; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Berradi, M.; Hsissou, R.; Khudhair, M.; Assouag, M.; Cherkaoui, O.; El Bachiri, A.; El Harfi, A. Textile finishing dyes and their impact on aquatic environs. Heliyon 2019, 5, e02711. [Google Scholar] [CrossRef]
- Rawat, D.; Mishra, V.; Sharma, R.S. Detoxification of azo dyes in the context of environmental processes. Chemosphere 2016, 155, 591–605. [Google Scholar] [CrossRef]
- Srinivasan, A.; Viraraghavan, T. Decolorization of dye wastewaters by biosorbents: A review. J. Environ. Manag. 2010, 91, 1915–1929. [Google Scholar] [CrossRef]
- Tkaczyk-Wlizło, A.; Mitrowska, K.; Błądek, T. Quantification of twenty pharmacologically active dyes in water samples using UPLC-MS/MS. Heliyon 2022, 8, e09331. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Ziolo, L.F.; Gaviria-Restrepo, L.F.; Agudelo, E.A.; Cardona-Gallo, S.A. Technologies for the removal of dyes and pigments present in wastewater. A review. Dyna 2015, 82, 118–126. [Google Scholar] [CrossRef]
- Li, W.; Mu, B.; Yang, Y. Feasibility of industrial-scale treatment of dye wastewater via bio-adsorption technology. Bioresour. Technol. 2019, 277, 157–170. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef] [PubMed]
- Sonune, A.; Ghate, R. Developments in wastewater treatment methods. Desalination 2004, 167, 55–63. [Google Scholar] [CrossRef]
- Muthu, S.S.; Khadir, A. Novel Materials for Dye-Containing Wastewater Treatment; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Natrayan, L.; Niveditha, V.R.; Nadh, V.S.; Srinivas, C.; Dhanraj, J.A.; Saravanan, A. Application of response surface and artificial neural network optimization approaches for exploring methylene blue adsorption using luffa fiber treated with sodium chlorite. J. Water Proc. Eng. 2024, 58, 104778. [Google Scholar] [CrossRef]
- Valladares-Cisneros, M.G.; Valerio-Cárdenas, C.; de la Cruz-Burelo, P.; Melgoza-Alemán, R.M. Adsorbentes no-convencionales, alternativas sustentables para el tratamiento de aguas residuales. RIUM 2017, 16, 55–73. [Google Scholar] [CrossRef]
- Torres, E. Biosorption: A review of the latest advances. Processes 2020, 8, 1584. [Google Scholar] [CrossRef]
- Arivumani, V.; Singh, V.; Geetha, C.; Senthilkumar, C. Activated rice husk biochar for azo dye removal: Batch adsorption, kinetics, and thermodynamic studies. Glob. NEST 2024, 26, 05498. [Google Scholar] [CrossRef]
- Jung, K.-W.; Choi, B.H.; Hwang, M.-J.; Choi, J.-W.; Lee, S.-H.; Chang, J.-S.; Ahn, K.-H. Adsorptive removal of anionic azo dye from aqueous solution using activated carbon derived from extracted coffee residues. J. Clean. Prod. 2017, 166, 360–368. [Google Scholar] [CrossRef]
- Tran, T.T.H.; Vu, N.T.; Pham, T.N.; Nguyen, X.T. Ability to Remove Azo Dye from Textile Dyeing Wastewaters of Carbonaceous Materials Produced from Bamboo Leaves. In Novel Materials for Dye-containing Wastewater Treatment. Sustainable Textiles: Production, Processing, Manufacturing & Chemistry; Muthu, S.S., Khadir, A., Eds.; Springer: Singapore, 2021; pp. 185–208. [Google Scholar] [CrossRef]
- Tomczak, E.; Tosik, P. Waste plant material as a potential adsorbent of a selected azo dye. Chem. Process Eng. 2017, 38, 283–294. [Google Scholar] [CrossRef][Green Version]
- Akdemir, M.; Isik, B.; Cakar, F.; Cankurtaran, O. High-performing natural materials (Leonurus cardiaca): Dye biosorption studies and statistical analysis. Biomass Convers. Biorefin. 2023, 13, 14281–14299. [Google Scholar] [CrossRef]
- Daneshvar, E.; Vazirzadeh, A.; Niazi, A.; Sillanpää, M.; Bhatnagar, A. A Comparative study of methylene blue biosorption using different modified brown, red, and green macroalgae–Effect of pretreatment. Chem. Eng. J. 2017, 307, 435–446. [Google Scholar] [CrossRef]
- Fathana, H.; Rahmi; Adlim, M.; Lubis, S.; Iqhrammullah, M. Sugarcane bagasse-derived cellulose as an eco-friendly adsorbent for azo dye removal. IIETA 2023, 18, 11–20. [Google Scholar] [CrossRef]
- Benjelloun, M.; Miyah, Y.; Bouslamti, R.; Nahali, L.; Mejbar, F.; Lairini, S. The fast-efficient adsorption process of the toxic dye onto shell powders of walnut and peanut: Experiments, equilibrium, thermodynamic, and regeneration studies. Chem. Afr. 2022, 5, 375–393. [Google Scholar] [CrossRef]
- Huynh, P.T.; Nguyen, D.K.; Duong, B.N.; Nguyen, P.H.; Dinh, V.P. Methylene Orange and Methyl Blue Adsorption Behavior on Pine Leaves Biomass (Pinus kesiya). Res. Sq. 2023, 1, 1–20. [Google Scholar] [CrossRef]
- Abdul Rahim, A.R.; Mohsin, H.M.; Chin, K.B.; Johari, K.; Saman, N. Promising low-cost adsorbent from desiccated coconut waste for removal of Congo red dye from aqueous solution. Water Air Soil Poll. 2021, 232, 357. [Google Scholar] [CrossRef]
- Salih, S.J.; Kareem, A.S.; Anwer, S.S. Adsorption of anionic dyes from textile wastewater utilizing raw corncob. Heliyon 2022, 8, e10092. [Google Scholar] [CrossRef]
- Ma, J.; Hou, L.; Li, P.; Zhang, S.; Zheng, X. Modified fruit pericarp as an effective biosorbent for removing azo dye from aqueous solution: Study of adsorption properties and mechanisms. Environ. Eng. Res. 2022, 27, 200634. [Google Scholar] [CrossRef]
- Dinçer, A.; Sevildik, M.; Aydemir, T. Optimization, isotherm, and kinetics studies of azo dye adsorption on eggshell membrane. Int. J. Chem. Technol. 2019, 3, 52–60. [Google Scholar] [CrossRef]
- Hosseinzehi, M.; Khatebasreh, M.; Dalvand, A. Modeling of Reactive Black 5 azo dye adsorption from aqueous solution on activated carbon prepared from poplar sawdust using response surface methodology. Int. J. Environ. Anal. Chem. 2022, 102, 6970–6987. [Google Scholar] [CrossRef]
- Caicho-Caranqui, J.; Vivanco, G.; Egas, D.A.; Cristina Chuya-Sumba, C.; Guerrero, V.H.; Ramirez-Cando, L.; Reinoso, C.; De Sousa, F.B.; Leon, M.; Ochoa-Herrera, V.; et al. Non-modified cellulose fibers for toxic heavy metal adsorption from water. Adsorption 2025, 31, 18. [Google Scholar] [CrossRef]
- Boudechiche, N.; Mokaddem, H.; Sadaoui, Z.; Trari, M. Biosorption of cationic dye from aqueous solutions onto lignocellulosic biomass (Luffa cylindrica): Characterization, equilibrium, kinetic and thermodynamic studies. Int. J. Ind. Chem. 2016, 7, 167–180. [Google Scholar] [CrossRef]
- Oun, A.A.; Kamal, K.H.; Farroh, K.; Ali, E.F.; Hassan, M.A. Development of fast and high-efficiency sponge-gourd fibers (Luffa cylindrica)/hydroxyapatite composites for removal of lead and methylene blue. Arab. J. Chem. 2021, 14, 103281. [Google Scholar] [CrossRef]
- Aranda-Figueroa, M.; Rodríguez-Torres, A.; Rodríguez, A.; Bolio-López, G.; Salinas-Sánchez, D.; Arias-Atayde, D.; Romero, R.J.; Valladares-Cisneros, M.G. Removal of Azo Dyes from Water Using Natural Luffa cylindrica as a Non-Conventional Adsorbent. Molecules 2024, 29, 1954. [Google Scholar] [CrossRef] [PubMed]
- Faraj, M.; Erhayem, M. Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich isotherm studies of equilibrium sorption of Methylene Blue from Aqueous Solution onto Mulberry tree (Morus nigra L) roots powder. JOPAS 2018, 17, 100–107. [Google Scholar] [CrossRef]
- Faraj, M.; Erhayem, M.; Mohamed, R. Kinetics and Thermodynamic Study for Adsorption of Methylene Blue onto Mulberry Tree (Morus nigra L) Roots Powder. JOPAS 2019, 18, 1–6. [Google Scholar] [CrossRef]
- Chen, X. Modeling of experimental adsorption isotherm data. Information 2015, 6, 14–22. [Google Scholar] [CrossRef]
- Emene, A.U.; Edyvean, R.; Akpan, U.G. Light-assisted adsorption of methylene blue onto Luffa cylindrica. Desalin. Water Treat. 2021, 216, 379–388. [Google Scholar] [CrossRef]
- Henini, G.; Laidani, Y.; Souahi, F. Study of the kinetics and thermodynamics of adsorption of Red Bemacid on the cords of Luffa cylindrica. Desalin. Water Treat. 2016, 57, 3741–3749. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Villabona-Ortíz, Á.; Gonzalez-Delgado, Á.D. Adsorption of azo-anionic dyes in a solution using modified coconut (Cocos nucifera) mesocarp: Kinetic and equilibrium study. Water 2021, 13, 1382. [Google Scholar] [CrossRef]
- Zanella, O.; Tessaro, I.C.; Féris, L.A. Desorption-and decomposition-based techniques for the regeneration of activated carbon. Chem. Eng. Technol. 2014, 37, 1447–1459. [Google Scholar] [CrossRef]
- Patel, H. Review on solvent desorption study from the exhausted adsorbent. J. Saudi Chem. Soc. 2021, 25, 101302. [Google Scholar] [CrossRef]
- Hong, T.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Applications of infrared spectroscopy in polysaccharide structural analysis: Progress, challenge, and perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef] [PubMed]
- Javier-Astete, R.; Jimenez-Davalos, J.; Zolla, G. Determination of hemicellulose, cellulose, holocellulose and lignin content using FTIR in Calycophyllum spruceanum (Benth.) K. Schum. and Guazuma crinita Lam. PLoS ONE 2021, 16, e0256559. [Google Scholar] [CrossRef] [PubMed]
- Mbarek, W.B.; Daza, J.; Escoda, L.; Fiol, N.; Pineda, E.; Khitouni, M.; Suñol, J.J. Removal of reactive black 5 azo Dye from aqueous solutions by a combination of reduction and natural adsorbents processes. Metals 2023, 13, 474. [Google Scholar] [CrossRef]
- Jawad, A.H.; Ngoh, Y.S.; Radzun, K.A. Utilization of watermelon (Citrullus lanatus) rinds as a natural low-cost biosorbent for adsorption of methylene blue: Kinetic, equilibrium and thermodynamic studies. J. Taibah Univ. Sci. 2018, 12, 371–381. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Tsoutsa, E.K.; Katsoyiannis, I.A.; Kyzas, G.Z. Simultaneous removal of anionic and cationic dyes on quaternary mixtures by adsorption onto banana, orange, and pomegranate peels. Colloids Surf. A 2024, 685, 133176. [Google Scholar] [CrossRef]
- Rauf, A.; Mahmud, T.; Ashraf, C.M.; Rehman, R. Sorptive Removal of Alizarin Yellow-R Dye from the Water Using Fibers of Luffa cylindrica Sponge. Int. J. Environ. Sustain. 2016, 12, 31. [Google Scholar] [CrossRef]
- Villa, F.A. Determinación del punto de carga cero y punto isoeléctrico de dos residuos agrícolas y su aplicación en la remoción de colorantes. RIAA 2013, 4, 27–36. [Google Scholar] [CrossRef]
- Ye, C.; Hu, N.; Wang, Z. Experimental investigation of Luffa cylindrica as a natural sorbent material for the removal of a cationic surfactant. J. Taiwan Inst. Chem. Eng. 2013, 44, 74–80. [Google Scholar] [CrossRef]
- Ng, H.W.; Lee, L.Y.; Chan, W.L.; Gan, S.; Chemmangattuvalappil, N. Luffa acutangula peel as an effective natural biosorbent for malachite green removal in aqueous media: Equilibrium, kinetic and thermodynamic investigations. Desalin. Water Treat. 2016, 57, 7302–7311. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Pashalidis, I. The application of oxidized carbon derived from Luffa cylindrica for caffeine removal. Equilibrium, thermodynamic, kinetic and mechanistic analysis. J. Mol. Liq. 2019, 296, 112078. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A. Preparation, characterization, and adsorption studies of the chemically modified Luffa aegyptica peel as a potential adsorbent for the removal of malachite green from aqueous solution. J. Mol. Liq. 2019, 274, 317–327. [Google Scholar] [CrossRef]
- Al-Zawahreh, K.; Barral, M.T.; Al-Degs, Y.; Paradelo, R. Comparison of the sorption capacity of basic, acid, direct, and reactive dyes by compost in batch conditions. J. Environ. Manag. 2021, 294, 113005. [Google Scholar] [CrossRef]
- Shoaib, A.G.; El Nemr, A.; Ramadan, M.S.; Masoud, M.S.; El Sikaily, A. Composite fabrication and characterization of crosslinked polyaniline/Pterocladia capillacea-activated carbon for adsorption of direct blue-86 dye from water. Polym. Bull. 2023, 80, 10393–10428. [Google Scholar] [CrossRef]
- Kumar, D.; Gupta, S.K. Green synthesis of novel biochar from Abelmoschus esculentus seeds for direct blue 86 dye removal: Characterization, RSM optimization, isotherms, kinetics, and fixed bed column studies. Environ. Pollut. 2023, 337, 122559. [Google Scholar] [CrossRef] [PubMed]
- Zua, M.Z.; Mustafa, M.R.; Abd Manan, T.S.; Ibrahim, N. Direct Blue 86 Textile Dye Removal from Aqueous Solution Using Rice Husk-based Adsorbent. ASEAN J. Sci. Technol. Dev. 2023, 40, 44–248. [Google Scholar] [CrossRef]
- Mahmoud, A.S. Effect of nano bentonite on direct yellow 50 dye removal; Adsorption isotherm, kinetic analysis, and thermodynamic behavior. Prog. React. Kinet. Mech. 2022, 47, 14686783221090377. [Google Scholar] [CrossRef]
- Ismail, L.F.; Sallam, H.B.; Farha, S.A.; Gamal, A.M.; Mahmoud, G.E. Adsorption behaviour of direct yellow 50 onto cotton fiber: Equilibrium, kinetic and thermodynamic profile. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 131, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Konicki, W.; Pełech, I.; Mijowska, E.; Jasińska, I. Adsorption of anionic dye Direct Red 23 onto magnetic multi-walled carbon nanotubes-Fe3C nanocomposite: Kinetics, equilibrium, and thermodynamics. Chem. Eng. J. 2012, 210, 87–95. [Google Scholar] [CrossRef]
- Alexandre, J.I.D.S.; Santos Neto, S.M.D.; Coutinho, A.P.; Melo, T.D.A.T.D.; Gonçalves, E.A.P.; Gondim, M.V.S.; Antonino, A.C.D.; Rabelo, A.E.C.D.G.D.C.; Oliveira, A.L.D. Sorption of the Direct Black 22 dye in alluvial soil. Rev. Amb. Água 2020, 15, e2483. [Google Scholar] [CrossRef]
- Zaruma, P.; Proal, J.; Hernández, I.C.; Salas, H.I. Los colorantes textiles industriales y tratamientos óptimos de sus efluentes de agua residual: Una breve revisión. Revista de la Facultad de Ciencias Químicas 2018, 19, 38–47. [Google Scholar]
- El Nemr, A.; El Sadaawy, M.M.; Khaled, A.; El Sikaily, A. Adsorption of the anionic dye Direct Red 23 onto new activated carbons developed from Cynara cardunculus: Kinetics, equilibrium and thermodynamics. Blue Biotechnol. J. 2014, 3, 121–142. [Google Scholar]
- Alhujaily, A.; Yu, H.; Zhang, X.; Ma, F. Adsorptive removal of anionic dyes from aqueous solutions using spent mushroom waste. Appl. Water Sci. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Piccin, J.S.; Cadaval, T.R.S.A.; De Pinto, L.A.A.; Dotto, G.L. Adsorption isotherms in liquid phase: Experimental, modeling, and interpretations. In Adsorption Processes for Water Treatment and Purification; Bonilla-Petriciolet, A., Mendoza-Castillo, D.I., Reynel-Ávila, H.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 19–51. [Google Scholar] [CrossRef]
- El-Hendawy, A.N.A.; Alexander, A.J.; Andrews, R.J.; Forrest, G. Effects of activation schemes on porous, surface and thermal properties of activated carbons prepared from cotton stalks. JAAP 2008, 82, 272–278. [Google Scholar] [CrossRef]
- Kesraoui, A.; Moussa, A.; Ali, G.B.; Seffen, M. Biosorption of alpacide blue from aqueous solution by lignocellulosic biomass: Luffa cylindrica fibers. Environ. Sci. Pollut. Res. 2016, 23, 15832–15840. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.; Montanher, S.; Rollemberg, M. Removal of textile dyes by sorption on low-cost sorbents. A case study: Sorption of reactive dyes onto Luffa cylindrica. Desalin. Water Treat. 2011, 25, 54–64. [Google Scholar] [CrossRef]
- Altınışık, A.; Gür, E.; Seki, Y. A natural sorbent, Luffa cylindrica for the removal of a model basic dye. J. Hazard. Mater. 2010, 179, 658–664. [Google Scholar] [CrossRef]
- Cao, S.; Zhu, R.; Wu, D.; Su, H.; Liu, Z.; Chen, Z. How hydrogen bonding and π–π interactions synergistically facilitate mephedrone adsorption by bio-sorbent: An in-depth microscopic scale interpretation. Environ. Pollut. 2024, 342, 123044. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, S.; Xi, C.; Su, H.; Chen, Z. A new nanocomposite assembled with metal organic framework and magnetic biochar derived from pomelo peels: A highly efficient adsorbent for ketamine in wastewater. J. Environ. Chem. Eng. 2021, 9, 106207. [Google Scholar] [CrossRef]
- Chen, T.; Li, M.; Liu, J. π–π stacking interaction: A nondestructive and facile means in material engineering for bioapplications. Cryst. Growth Des. 2018, 18, 2765–2783. [Google Scholar] [CrossRef]







| Dye | Adsorbent | (mg/g) | Reference |
|---|---|---|---|
| Acid Brown | Rice husk biochar b | 10.4 | [20] |
| Acid Orange 7 | Extracted coffee residues biochar b | 122.5 | [21] |
| Alizarin Red S | Bamboo leaves biochar c | 394.4 | [22] |
| Brilliant Green | 343.9 | ||
| Direct Orange 26 | Sunflower husks c | 11.0 | [23] |
| Basic Red 2 | Leonurus cardiaca leaves c | 714.0 | [24] |
| Methylene Blue | Nizamuddinia zanardinii a | 142.1 | [25] |
| Gracilaria parvispora a | 87.8 | ||
| Ulva fasciata a | 143.9 | ||
| Sugarcane bagasse b | 66.4 | [26] | |
| Rice husk biochar b | 11.8 | [20] | |
| Shells Powders of Walnut and Peanut b | 67.4 | [27] | |
| 101.4 | |||
| Pine leaves c | 140.8 | [28] | |
| Congo Red | Desiccated coconut waste b | 0.07 | [29] |
| Methyl Red | Raw corncob b | 4.3 | [30] |
| Methyl Orange | Lychee and longan pericarps b | 349.4 | [31] |
| Raw corncob b | 7.5 | [30] | |
| Pine leaves c | 136.9 | [28] | |
| Reactive Black 5 | Eggshell membrane b | 333.3 | [32] |
| Poplar sawdust b | 0.8 | [33] | |
| Reactive Red 195 | Eggshell membrane b | 76.9 | [32] |
| Reactive Red | Rice husk biochar b | 11.8 | [20] |
| Reactive Violet 5 | Nannochloropsis a | 115.0 | [33] |
| [mg/L] | (mg/g) |
|---|---|
| 125 | 5.76 |
| 250 | 11.51 |
| 500 | 25.45 |
| AD [mg/L] | 125 | 250 | 500 | |
|---|---|---|---|---|
| Pseudo-first-order model | R² | 0.983 | 0.953 | 0.949 |
| k1 (min−1) | 0.005 | 0.003 | 0.002 | |
| , exp (mg/g) | 6.705 | 14.325 | 31.566 | |
| , cal (mg/g) | 9.083 | 16.693 | 35.981 | |
| Pseudo-second-order model | R² | 0.997 | 0.994 | 0.991 |
| k2 (g min/mg) | 0.001 | 0.000 | 0.000 | |
| , exp (mg/g) | 6.705 | 14.325 | 31.566 | |
| , cal (mg/g) | 7.310 | 14.620 | 26.110 | |
| Intraparticle diffusion model | R² | 0.904 | 0.892 | 0.935 |
| 0.485 | 1.265 | 3.021 | ||
| C | 0.983 | 1.870 | 1.829 | |
| Elovich model | R² | 0.989 | 0.991 | 0.992 |
| β (g/mg) | 0.780 | 0.380 | 0.211 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aranda-Figueroa, M.G.; Romero, R.J.; Rodríguez, M.; Rodríguez-Torres, A.; Rodríguez, A.; Bolio-López, G.I.; Arias-Ataide, D.M.; Torres-Islas, Á.; Valladares-Cisneros, M.G. Removal of Azo Dyes from Water on a Large Scale Using a Low-Cost and Eco-Friendly Adsorbent. Sustainability 2025, 17, 4816. https://doi.org/10.3390/su17114816
Aranda-Figueroa MG, Romero RJ, Rodríguez M, Rodríguez-Torres A, Rodríguez A, Bolio-López GI, Arias-Ataide DM, Torres-Islas Á, Valladares-Cisneros MG. Removal of Azo Dyes from Water on a Large Scale Using a Low-Cost and Eco-Friendly Adsorbent. Sustainability. 2025; 17(11):4816. https://doi.org/10.3390/su17114816
Chicago/Turabian StyleAranda-Figueroa, Ma. Guadalupe, Rosenberg J. Romero, Mario Rodríguez, Adriana Rodríguez-Torres, Alexis Rodríguez, Gloria Ivette Bolio-López, Dulce María Arias-Ataide, Álvaro Torres-Islas, and Maria Guadalupe Valladares-Cisneros. 2025. "Removal of Azo Dyes from Water on a Large Scale Using a Low-Cost and Eco-Friendly Adsorbent" Sustainability 17, no. 11: 4816. https://doi.org/10.3390/su17114816
APA StyleAranda-Figueroa, M. G., Romero, R. J., Rodríguez, M., Rodríguez-Torres, A., Rodríguez, A., Bolio-López, G. I., Arias-Ataide, D. M., Torres-Islas, Á., & Valladares-Cisneros, M. G. (2025). Removal of Azo Dyes from Water on a Large Scale Using a Low-Cost and Eco-Friendly Adsorbent. Sustainability, 17(11), 4816. https://doi.org/10.3390/su17114816









