Effectiveness of Grafting in Enhancing Salinity Tolerance of Tomato (Solanum lycopersicum L.) Using Novel and Commercial Rootstocks in Soilless Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
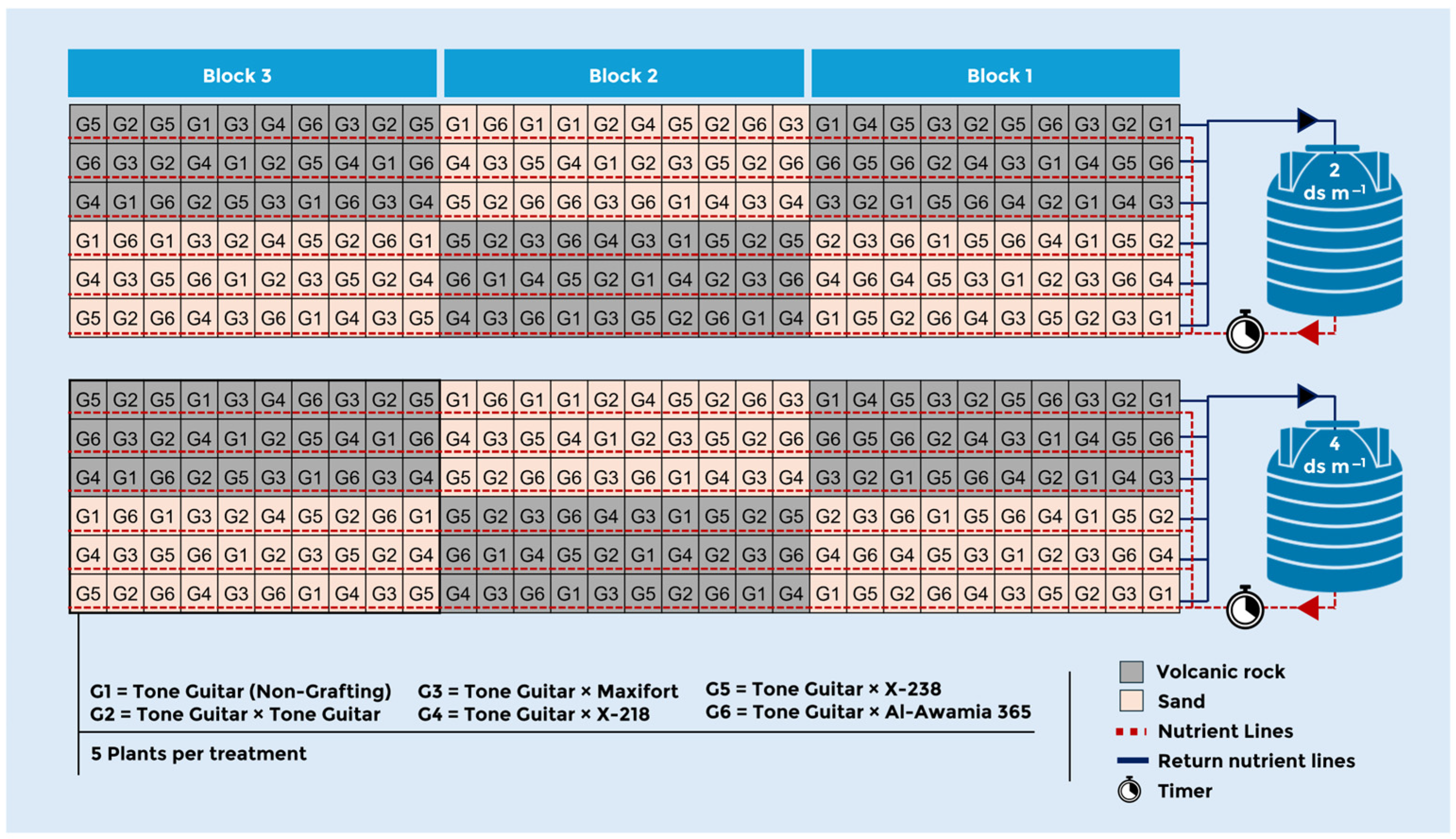
2.2. Experimental Design
2.3. Grafting of the Plants
2.4. Experimental Site Preparation and Plant Transplanting
2.5. Nutrient and Irrigation Water Solutions
2.6. The Measurements
2.6.1. Physicochemical Properties of Tomato Fruits
2.6.2. Chemical Content of Tomato Leaves
2.6.3. Yield and Water Productivity (WP)
2.7. Data Analysis
3. Results
3.1. Physicochemical Characteristics of Tomato Fruits
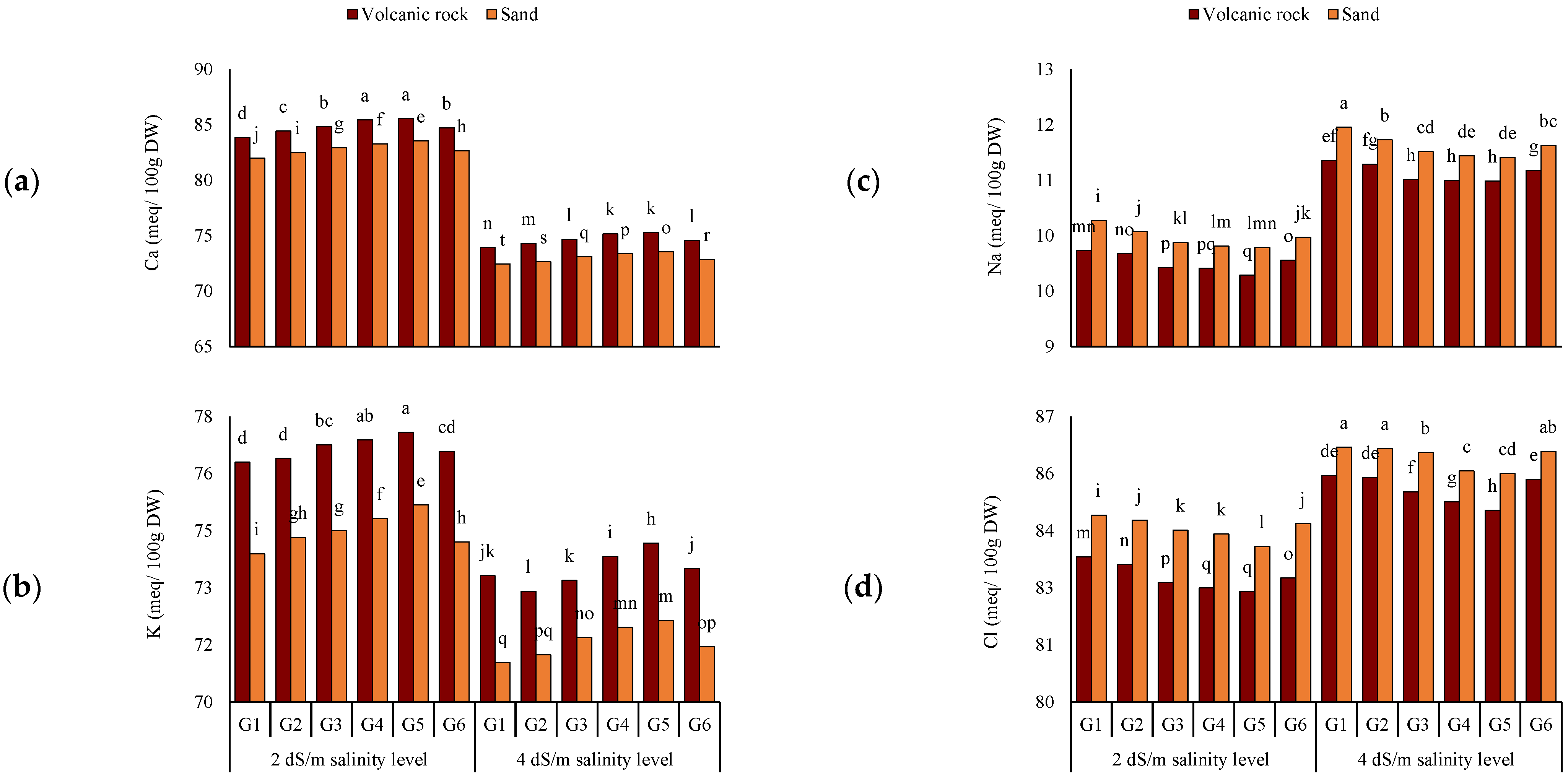
3.2. Chemical Content of Tomato Leaves
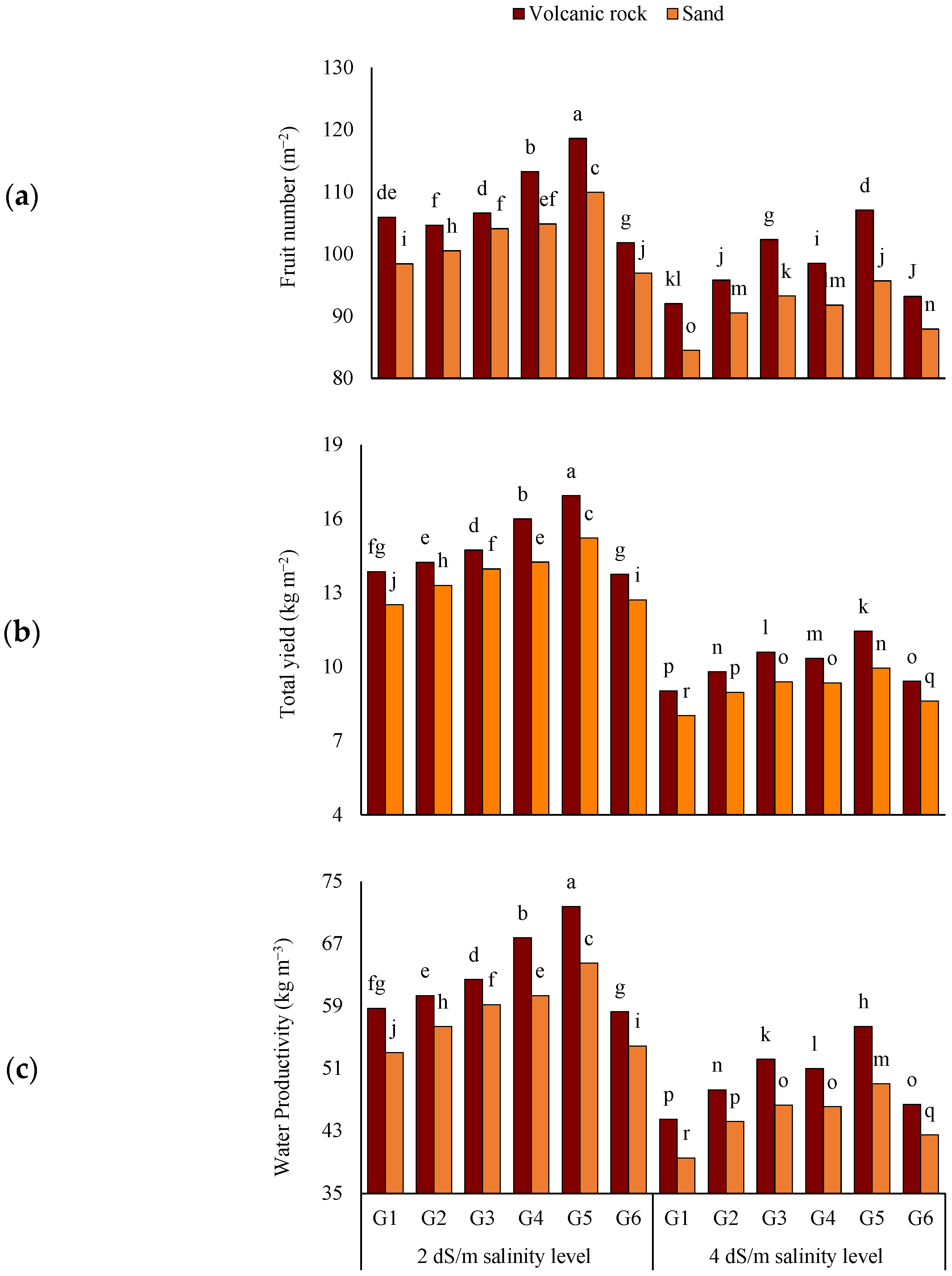
3.3. Yield and Water Productivity of Tomato Plants
3.4. Pearson’s Correlation Coefficient Between All the Studied Parameters Under Different Salinity Levels
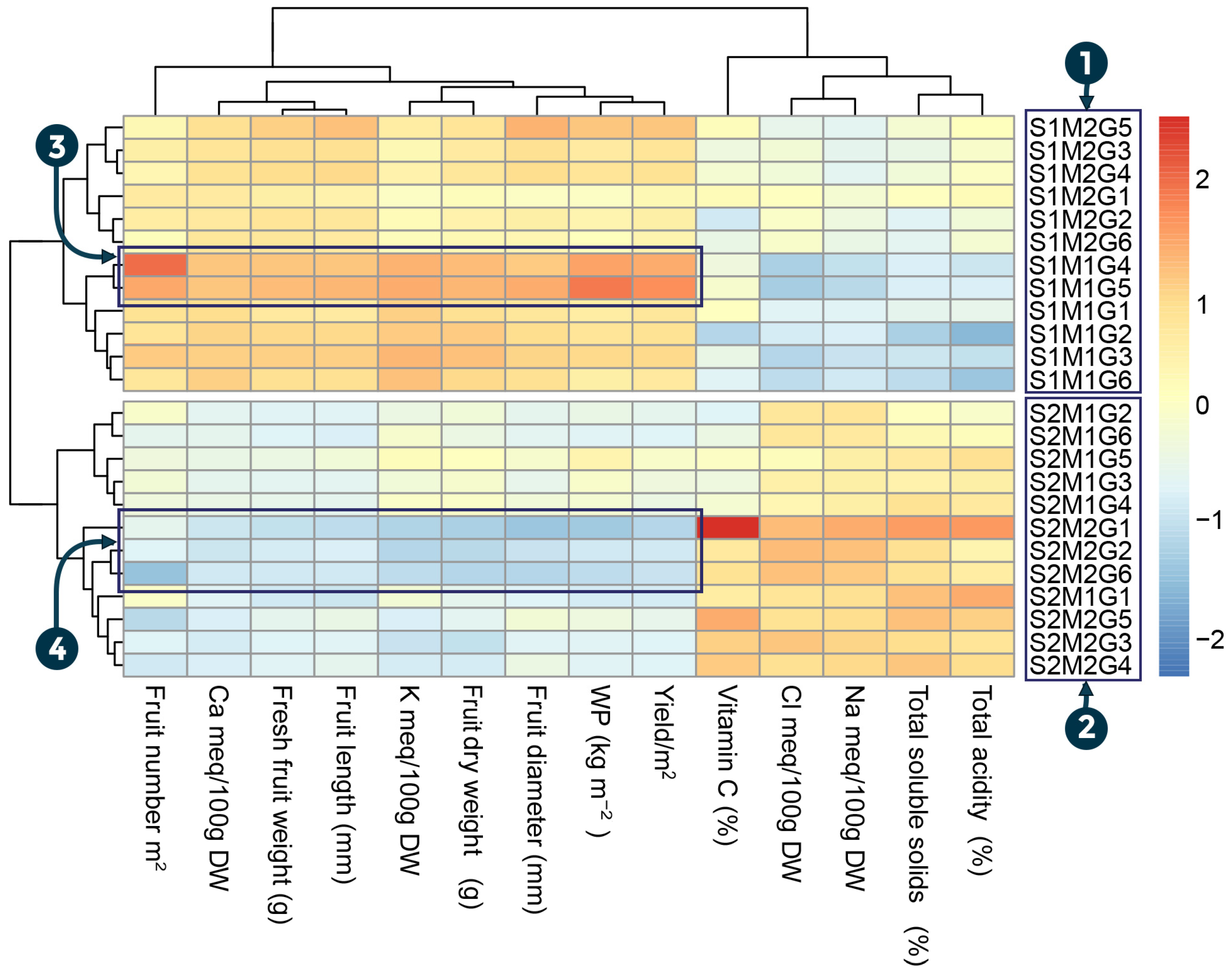
3.5. Heatmap Analysis of Measured Traits in Tomato Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aydin, A. Effects of grafting with wild tomato (Solanum pimpinellifolium and Solanum habrochaites) rootstocks on growth and leaf mineral accumulation in salt stress. Hortic. Environ. Biotechnol. 2024, 65, 785–801. [Google Scholar] [CrossRef]
- Sholi, N.J. Effect of salt stress on seed germination, plant growth, photosynthesis and ion accumulation of four tomato cultivars. Am. J. Plant Physiol. 2012, 7, 269–275. [Google Scholar] [CrossRef]
- Ali, M.; Kamran, M.; Abbasi, G.H.; Saleem, M.H.; Ahmad, S.; Parveen, A.; Malik, Z.; Afzal, S.; Ahmar, S.; Dawar, K.M. Melatonin-induced salinity tolerance by ameliorating osmotic and oxidative stress in the seedlings of two tomato (Solanum lycopersicum L.) cultivars. J. Plant Growth Regul. 2021, 40, 2236–2248. [Google Scholar] [CrossRef]
- FAOSTAT. FAOSTAT Statistics Database. Available online: https://www.fao.org/faostat/en/#home (accessed on 30 April 2025).
- Ministry of Environment, Water and Agriculture (MEWA). Census Book Ministry of Agriculture. 2023. Available online: https://www.mewa.gov.sa/ar/InformationCenter/Researchs/Reports/Pages/default.aspx (accessed on 30 April 2025).
- Department of Economic and Social Affairs. World Population Prospects 2022: Summary of Results. UN DESA/POP/2022/TR/NO. 3; United Nations: New York, NY, USA, 2022. Available online: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/wpp2022_summary_of_results.pdf (accessed on 30 April 2025).
- Leridon, H. Population mondiale: Vers une explosion ou une implosion? Popul. Soc. 2020, 573, 1–4. [Google Scholar] [CrossRef]
- Velazquez-Gonzalez, R.S.; Garcia-Garcia, A.L.; Ventura-Zapata, E.; Barceinas-Sanchez, J.D.O.; Sosa-Savedra, J.C. A review on hydroponics and the technologies associated for medium-and small-scale operations. Agriculture 2022, 12, 646. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. The State of Food Security and Nutrition in the World: Building Climate Resilience for Food Security and Nutrition; Food and Agriculture Organization, World Food Programe and World Health Organization: Rome, Italy, 2018. [Google Scholar]
- Fedoroff, N.V. Food in a future of 10 billion. Agric. Food Secur. 2015, 4, 11. [Google Scholar] [CrossRef]
- Huang, X.; Swain, D.L.; Hall, A.D. Future precipitation increase from very high resolution ensemble downscaling of extreme atmospheric river storms in California. Sci. Adv. 2020, 6, eaba1323. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, P.; Kumar, A.; Kyriacou, M.C.; Colla, G.; Rouphael, Y. Grafting tomato as a tool to improve salt tolerance. Agronomy 2020, 10, 263. [Google Scholar] [CrossRef]
- Zörb, C.; Geilfus, C.M.; Dietz, K.J. Salinity and crop yield. Plant Biol. 2019, 21, 31–38. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Foolad, M. Recent advances in genetics of salt tolerance in tomato. Plant Cell Tissue Org. Cult. 2004, 76, 101–119. [Google Scholar] [CrossRef]
- Rao, E.S.; Kadirvel, P.; Symonds, R.C.; Ebert, A.W. Relationship between survival and yield related traits in Solanum pimpinellifolium under salt stress. Euphytica 2013, 190, 215–228. [Google Scholar] [CrossRef]
- Hasegawa, P.M. Sodium (Na+) homeostasis and salt tolerance of plants. Environ. Exp. Bot. 2013, 92, 19–31. [Google Scholar] [CrossRef]
- Abbasi, H.; Jamil, M.; Haq, A.; Ali, S.; Ahmad, R.; Malik, Z.; Parveen, Z. Salt stress manifestation on plants, mechanism of salt tolerance and potassium role in alleviating it: A review. Zemdirb. Agric. 2016, 103, 229–238. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Alqardaeai, T.; Alharbi, A.; Alenazi, M.; Alomran, A.; Elfeky, A.; Osman, M.; Obadi, A.; Aldubai, A.; Ortiz, N.R.; Melino, V.J. Effect of Tomato Grafting onto Novel and Commercial Rootstocks on Improved Salinity Tolerance and Enhanced Growth, Physiology, and Yield in Soilless Culture. Agronomy 2024, 14, 1526. [Google Scholar] [CrossRef]
- Massa, D.; Incrocci, L.; Maggini, R.; Carmassi, G.; Campiotti, C.; Pardossi, A. Strategies to decrease water drainage and nitrate emission from soilless cultures of greenhouse tomato. Agric. Water Manag. 2010, 97, 971–980. [Google Scholar] [CrossRef]
- Fu, S.; Chen, J.; Wu, X.; Gao, H.; Lü, G. Comprehensive evaluation of low temperature and salt tolerance in grafted and rootstock seedlings combined with yield and quality of grafted tomato. Hortic. 2022, 8, 595. [Google Scholar] [CrossRef]
- Abdeldym, E.A.; El-Mogy, M.M.; Abdellateaf, H.R.; Atia, M.A. Genetic characterization, agro-morphological and physiological evaluation of grafted tomato under salinity stress conditions. Agronomy 2020, 10, 1948. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, P.; Chaudhari, S.; Edelstein, M. Tomato grafting: A global perspective. HortScience 2017, 52, 1328–1336. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Jawad, R.; Kumar, P.; Rea, E.; Cardarelli, M. The effectiveness of grafting to improve NaCl and CaCl2 tolerance in cucumber. Sci. Hortic. 2013, 164, 380–391. [Google Scholar] [CrossRef]
- Etehadnia, M.; Waterer, D.R.; Tanino, K.K. The method of ABA application affects salt stress responses in resistant and sensitive potato lines. J. Plant Growth Regul. 2008, 27, 331–341. [Google Scholar] [CrossRef]
- Di Gioia, F.; Signore, A.; Serio, F.; Santamaria, P. Grafting improves tomato salinity tolerance through sodium partitioning within the shoot. HortScience 2013, 48, 855–862. [Google Scholar] [CrossRef]
- Martinez-Rodriguez, M.; Santa-Cruz, A.; Estan, M.; Caro, M.; Bolarin, M. Influence of rootstock in the tomato response to salinity. In Proceedings of the International Symposium on Techniques to Control Salination for Horticultural Productivity 573, Leuven, Belgium, 7–10 November 2002. [Google Scholar]
- Conti, V.; Parrotta, L.; Romi, M.; Del Duca, S.; Cai, G. Tomato biodiversity and drought tolerance: A multilevel review. Int. J. Mol. Sci. 2023, 24, 10044. [Google Scholar] [CrossRef] [PubMed]
- Szymański, J.; Bocobza, S.; Panda, S.; Sonawane, P.; Cárdenas, P.D.; Lashbrooke, J.; Kamble, A.; Shahaf, N.; Meir, S.; Bovy, A. Analysis of wild tomato introgression lines elucidates the genetic basis of transcriptome and metabolome variation underlying fruit traits and pathogen response. Nat. Genet. 2020, 52, 1111–1121. [Google Scholar] [CrossRef]
- Frary, A.; Göl, D.; Keleş, D.; Ökmen, B.; Pınar, H.; Şığva, H.Ö.; Yemenicioğlu, A.; Doğanlar, S. Salt tolerance in Solanum pennellii: Antioxidant response and related QTL. BMC Plant Biol. 2010, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, E.; Martínez, J.-P.; Benahmed, H.; Hichri, I.; Dobrev, P.I.; Motyka, V.; Quinet, M.; Lutts, S. Phytohormone profiling in relation to osmotic adjustment in NaCl-treated plants of the halophyte tomato wild relative species Solanum chilense comparatively to the cultivated glycophyte Solanum lycopersicum. Plant Sci. 2017, 258, 77–89. [Google Scholar] [CrossRef]
- Voutsela, S.; Yarsi, G.; Petropoulos, S.A.; Khan, E.M. The effect of grafting of five different rootstocks on plant growth and yield of tomato plants cultivated outdoors and indoors under salinity stress. Afr. J. Agric. Res. 2012, 7, 5553–5557. [Google Scholar]
- Yang, H.; Shukla, M.K.; Du, T. Assessment of plant mineral nutrition concentrations of tomato irrigated with brackish water and RO concentrate. J. Plant Nutr. 2023, 46, 4639–4656. [Google Scholar] [CrossRef]
- Rosadi, R.B.; Senge, M.; Suhandy, D.; Tusi, A. The effect of EC levels of nutrient solution on the growth, yield, and quality of tomatoes (Solanum lycopersicum) under the hydroponic system. J. Agric. Eng. Biotechnol. 2014, 2, 7. [Google Scholar] [CrossRef]
- Al-Harbi, A.; Al-Omran, A.; Alqardaeai, T.; Abdel-Rassak, H.; Alharbi, K.; Obadi, A.; Saad, M. Grafting affects tomato growth, productivity, and water use efficiency under different water regimes. J. Agric. Sci. Technol. 2018, 20, 1227–1241. [Google Scholar]
- Djidonou, D.; Zhao, X.; Simonne, E.H.; Koch, K.E.; Erickson, J.E. Yield, water-, and nitrogen-use efficiency in field-grown, grafted tomatoes. HortScience 2013, 48, 485–492. [Google Scholar] [CrossRef]
- Lee, J.-M.; Kubota, C.; Tsao, S.; Bie, Z.; Echevarria, P.H.; Morra, L.; Oda, M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Bie, Z.; Nawaz, M.A.; Huang Yuan, H.Y.; Lee JungMyung, L.J.; Colla, G. Introduction to vegetable grafting. In Vegetable Grafting: Principles and Practices; CABI: Wallingford, UK, 2017; pp. 1–21. [Google Scholar]
- Khah, E.; Kakava, E.; Mavromatis, A.; Chachalis, D.; Goulas, C. Effect of grafting on growth and yield of tomato (Lycopersicon esculentum Mill.) in greenhouse and open-field. J. Appl. Hortic. 2006, 8, 3–7. [Google Scholar] [CrossRef]
- Vu, N.-T.; Xu, Z.-H.; Kim, Y.-S.; Kang, H.-M.; Kim, I.-S. Effect of nursery environmental condition and different cultivars on survival rate of grafted tomato seedling. Acta Hortic. 2014, 1037, 765–770. [Google Scholar]
- Shah, A.; Shah, S.; Muneer, S.; Rehman, M. Comparison of two nutrient solution recipes for growing spinach crop in a non-circulating hydroponic system. Sarhad J. Agric. 2009, 25, 405–418. [Google Scholar]
- Gioia, F.D.; Serio, F.; Buttaro, D.; Ayala, O.; Santamaria, P. Influence of rootstock on vegetative growth, fruit yield and quality in ‘Cuore di Bue’, an heirloom tomato. J. Hortic. Sci. Biotechnol. 2010, 85, 477–482. [Google Scholar] [CrossRef]
- Williams, S. Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Agricultural Chemists: Washington, WA, USA, 1995. [Google Scholar]
- Estefan, G. Methods of Soil, Plant, and Water Analysis: A Manual for the West Asia and North Africa Region; International Center for Agricultural Research in the Dry Areas: Beirut, Lebanon, 2013. [Google Scholar]
- Chapman, H.D.; Pratt, P.F. Methods of analysis for soils, plants and waters. Soil Sci. 1962, 93, 68. [Google Scholar] [CrossRef]
- Yeo, A.; Kramer, D.; Liuchli, A.; Gullasch, J. Ion distribution in salt-stressed mature Zea mays roots in relation to ultrastructure and retention of sodium. J. Exp. Bot. 1977, 28, 17–29. [Google Scholar] [CrossRef]
- Alshami, A.K.; El-Shafei, A.; Al-Omran, A.M.; Alghamdi, A.G.; Louki, I.; Alkhasha, A. Responses of tomato crop and water productivity to deficit irrigation strategies and salinity stress in greenhouse. Agronomy 2023, 13, 3016. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics, a Biometrical Approach; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]
- Giannakoula, A.E.; Ilias, I. The effect of water stress and salinity on growth and physiology of tomato (Lycopersicon esculentum Mil.). Arch. Biol. Sci. 2013, 65, 611–620. [Google Scholar] [CrossRef]
- Khanbabaloo, N.; Seyed Hajizadeh, H.; Behtash, F. Effects of salinity on taste quality and biochemical traits of four tomato varieties (Solanum lycopersicum) grown under hydroponic conditions. J. Hortic. Postharvest Res. 2018, 1, 15–26. [Google Scholar]
- Obadi, A.; Alharbi, A.; Alomran, A.; Alghamdi, A.G.; Louki, I.; Alkhasha, A.; Alqardaeai, T. Enhancement in Tomato Yield and Quality Using Biochar Amendments in Greenhouse under Salinity and Drought Stress. Plants 2024, 13, 1634. [Google Scholar] [CrossRef]
- Parvin, K.; Ahamed, K.U.; Islam, M.M.; Haque, M.N.; Hore, P.K.; Siddik, M.A.; Roy, I. Reproductive behavior of tomato plant under saline condition with exogenous application of calcium. Mid. East J. Sci. Res. 2015, 23, 2920–2926. [Google Scholar]
- Agius, C.; von Tucher, S.; Rozhon, W. The effect of salinity on fruit quality and yield of cherry tomatoes. Horticulturae 2022, 8, 59. [Google Scholar] [CrossRef]
- Oliveira, C.E.d.S.; Zoz, T.; Seron, C.d.C.; Boleta, E.H.M.; Lima, B.H.d.; Souza, L.R.R.; Pedrinho, D.R.; Matias, R.; Lopes, C.d.S.; Oliveira Neto, S.S.d. Can saline irrigation improve the quality of tomato fruits? Agron. J. 2022, 114, 900–914. [Google Scholar] [CrossRef]
- Islam, M.M.; Jahan, K.; Sen, A.; Urmi, T.A.; Haque, M.M.; Ali, H.M.; Siddiqui, M.H.; Murata, Y. Exogenous application of calcium ameliorates salinity stress tolerance of tomato (Solanum lycopersicum L.) and enhances fruit quality. Antioxidants 2023, 12, 558. [Google Scholar] [CrossRef]
- Al-Gaadi, K.A.; Tola, E.; Madugundu, R.; Zeyada, A.M.; Alameen, A.A.; Edrris, M.K.; Edrees, H.F.; Mahjoop, O. Response of leaf photosynthesis, chlorophyll content and yield of hydroponic tomatoes to different water salinity levels. PLoS ONE 2024, 19, e0293098. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, M.; Abdeldaym, E. Effect of grafting and different EC levels of saline irrigation water on growth, yield and fruit quality of tomato (Lycopersicon esculentum) in greenhouse. Plant Arch. 2019, 19, 3021–3027. [Google Scholar]
- Yang, H.; Du, T.; Mao, X.; Ding, R.; Shukla, M.K. A comprehensive method of evaluating the impact of drought and salt stress on tomato growth and fruit quality based on EPIC growth model. Agric. Water Manag. 2019, 213, 116–127. [Google Scholar] [CrossRef]
- Ahmed, N.B. Effect of Grafting Two Hybrids on Two Rootstocks of Tomato cultivated in Green House Under Salinity Stress; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar]
- Turhan, A.; Ozmen, N.; Serbeci, M.; Seniz, V. Effects of grafting on different rootstocks on tomato fruit yield and quality. Hortic. Sci. 2011, 38, 142–149. [Google Scholar] [CrossRef]
- Imam, F.; un nisa Memon, N.; Leghari, M.H.; Sheikh, S.A. Optimizing Tomato Grafts for Improved Growth, Yield and Fruit Quality. J. Appl. Res. Plant Sci. 2024, 5, 12–18. [Google Scholar] [CrossRef]
- Dorin, S.; Doltu, M.; DrĂGhici, E.M.; Bogoescu, M.I. Effect of grafting on tomato fruit quality. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 1246–1251. [Google Scholar]
- Al-Harbi, A.; Hejazi, A.; Al-Omran, A. Responses of grafted tomato (Solanum lycopersiocon L.) to abiotic stresses in Saudi Arabia. Saudi J. Biol. Sci. 2017, 24, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Semiz, G.D.; Suarez, D.L. Tomato salt tolerance: Impact of grafting and water compositionon yield and ion relations. Turk. J. Agric. For. 2015, 39, 876–886. [Google Scholar] [CrossRef]
- Passam, H.C.; Karapanos, I.C.; Bebeli, P.J.; Savvas, D. A review of recent research on tomato nutrition, breeding and post-harvest technology with reference to fruit quality. Eur. J. Plant Sci. Biotechnol. 2007, 1, 1–21. [Google Scholar]
- Thakur, S.; Sinha, A.; Singh, D.-V.; Mandial, A. Impact of Saline Irrigation Water on Yield and Quality of Tomato (Solanum lycopersicum L.). Chem. Sci. Rev. Lett. 2022, 11, 81–83. [Google Scholar]
- Sun, K.; Hunt, K.; Hauser, B.A. Ovule abortion in Arabidopsis triggered by stress. Plant Physiol. 2004, 135, 2358–2367. [Google Scholar] [CrossRef]
- Roy, P.R.; Tahjib-Ul-Arif, M.; Polash, M.A.S.; Hossen, M.Z.; Hossain, M.A. Physiological mechanisms of exogenous calcium on alleviating salinity-induced stress in rice (Oryza sativa L.). Physiol. Mol. Biol. Plants 2019, 25, 611–624. [Google Scholar] [CrossRef]
- Al-Harbi, A.R.; Al-Omran, A.M.; Alenazi, M.M.; Wahb-Allah, M.A. Salinity and deficit irrigation influence tomato growth, yield and water use efficiency at different developmental stages. Int. J. Agric. Biol. 2015, 17, 241–250. [Google Scholar]
- Çebİ, Ü.K.; Özer, S.; Altıntaș, S.; Öztürk, O.; Yurtseven, E. Effect of different irrigation levels and irrigation water salinity on water use efficiency and yield of tomato grown in greenhouse. J. Agric. Food Sci. 2018, 22, 33–46. [Google Scholar]
- Madugundu, R.; Al-Gaadi, K.A.; Tola, E.; Patil, V.C.; Sigrimis, N. The Impact of Salinity and Nutrient Regimes on the Agro-Morphological Traits and Water Use Efficiency of Tomato under Hydroponic Conditions. Appl. Sci. 2023, 13, 9564. [Google Scholar] [CrossRef]
- Gaion, L.A.; Braz, L.T.; Carvalho, R.F. Grafting in vegetable crops: A great technique for agriculture. Int. J. Veg. Sci. 2018, 24, 85–102. [Google Scholar] [CrossRef]
- Kumar, P.; Rouphael, Y.; Cardarelli, M.; Colla, G. Vegetable grafting as a tool to improve drought resistance and water use efficiency. Front. Plant Sci. 2017, 8, 1130. [Google Scholar] [CrossRef]
- Giuffrida, F.; Martorana, M.; Leonardi, C. How sodium chloride concentration in the nutrient solution influences the mineral composition of tomato leaves and fruits. HortScience 2009, 44, 707–711. [Google Scholar] [CrossRef]
- Wang, X.; Geng, S.; Ma, Y.; Shi, D.; Yang, C.; Wang, H. Growth, photosynthesis, solute accumulation, and ion balance of tomato plant under sodium-or potassium-salt stress and alkali stress. Agron. J. 2015, 107, 651–661. [Google Scholar] [CrossRef]
- Parra-Terraza, S.; Angulo-Castro, A.; Sánchez-Peña, P.; Valdez-Torres, J.B.; Rubio-Carrasco, W. Effect of Cl− and Na+ ratios in nutrient solutions on tomato (Solanum lycopersicum L.) yield in a hydroponic system. Rev. Chapingo Ser. Hortic. 2022, 28, 67–78. [Google Scholar] [CrossRef]
- Houimli, S.I.M.; Denden, M.; Mouhandes, B.D. Effects of 24-epibrassinolide on growth, chlorophyll, electrolyte leakage and proline by pepper plants under NaCl-stress. EurAsian J. Biosci. 2010, 4, 96–104. [Google Scholar] [CrossRef]
- Hossain, M.S.; Hasanuzzaman, M.; Sohag, M.M.H.; Bhuyan, M.B.; Fujita, M. Acetate-induced modulation of ascorbate: Glutathione cycle and restriction of sodium accumulation in shoot confer salt tolerance in Lens culinaris Medik. Physiol. Mol. Biol. Plants 2019, 25, 443–455. [Google Scholar] [CrossRef]
- Malone, M.; Andrews, J. The distribution of xylem hydraulic resistance in the fruiting truss of tomato. Plant Cell Environ. 2001, 24, 565–570. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Leonardi, C.; Bie, Z. Role of grafting in vegetable crops grown under saline conditions. Sci. Hortic. 2010, 127, 147–155. [Google Scholar] [CrossRef]
- Wang, Q.; Men, L.; Gao, L.; Tian, Y. Effect of grafting and gypsum application on cucumber (Cucumis sativus L.) growth under saline water irrigation. Agric. Water Manag. 2017, 188, 79–90. [Google Scholar] [CrossRef]
- Razali, R.; Bougouffa, S.; Morton, M.J.; Lightfoot, D.J.; Alam, I.; Essack, M.; Arold, S.T.; Kamau, A.A.; Schmöckel, S.M.; Pailles, Y. The genome sequence of the wild tomato Solanum pimpinellifolium provides insights into salinity tolerance. Front. Plant Sci. 2018, 9, 390082. [Google Scholar] [CrossRef] [PubMed]
- Vaca, L.E.M.; Torres, O.G.V.; Lugo, S.L.; Cortes, M.A.; Ruiz, N.L.; Patiño, M.L.D. New Materials Optimization Process in Tomato. MRS Online Proc. Libr. 2015, 1767, 127–132. [Google Scholar] [CrossRef]
- Ortega Martínez, L.D.; Martínez Valenzuela, C.; Ocampo Mendoza, J.; Sandoval Castro, E.; Pérez Armendáriz, B. Efficiency of substrates in soil and hydroponic system for greenhouse tomato production. Rev. Mex. Cienc. Agríc. 2016, 7, 643–653. [Google Scholar]
- Benko, B.; Uher, S.F.; Radman, S.; Opačić, N. Hydroponic Production Systems in Greenhouses. In Climate Smart Greenhouses-Innovations and Impacts; IntechOpen: London, UK, 2023. [Google Scholar]




| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) Fruit diameter (mm) | 0.95 | 0.93 | 0.83 | 0.01 | −0.35 | −0.34 | 0.10 | 0.91 | 0.91 | 0.77 | 0.70 | −0.73 | −0.82 | |
| (2) Fruit length (mm) | 0.91 | 0.98 | 0.79 | −0.05 | −0.33 | −0.33 | 0.09 | 0.92 | 0.92 | 0.75 | 0.63 | −0.70 | −0.80 | |
| (3) Fresh fruit weight (g) | 0.94 | 0.96 | 0.85 | −0.09 | −0.41 | −0.41 | 0.02 | 0.95 | 0.95 | 0.83 | 0.71 | −0.78 | −0.87 | |
| (4) fruit dry weight (g) | 0.80 | 0.68 | 0.83 | −0.18 | −0.73 | −0.65 | 0.47 | 0.83 | 0.83 | 0.98 | 0.96 | −0.96 | −0.97 | |
| (5) Vitamin C (%) | −0.52 | −0.40 | −0.59 | −0.77 | 0.66 | 0.80 | −0.12 | −0.13 | −0.13 | −0.16 | −0.16 | 0.13 | 0.14 | |
| (6) Total acidity (%) | −0.19 | −0.24 | −0.33 | −0.29 | 0.76 | 0.94 | −0.54 | −0.31 | −0.31 | −0.75 | −0.80 | 0.77 | 0.68 | |
| (7) Total soluble solids (%) | −0.32 | −0.30 | −0.44 | −0.56 | 0.91 | 0.90 | −0.45 | −0.27 | −0.27 | −0.66 | −0.69 | 0.66 | 0.64 | |
| (8) Fruit number (m−2) | 0.28 | 0.08 | 0.23 | 0.63 | −0.51 | −0.07 | −0.35 | 0.11 | 0.11 | 0.40 | 0.58 | −0.44 | −0.34 | |
| (9) Yield (m−2) | 0.88 | 0.90 | 0.96 | 0.89 | −0.63 | −0.28 | −0.48 | 0.39 | 1.00 | 0.81 | 0.72 | −0.77 | −0.84 | |
| (10) WP (kg m−2) | 0.88 | 0.90 | 0.96 | 0.89 | −0.63 | −0.28 | −0.48 | 0.39 | 1.00 | 0.81 | 0.72 | −0.77 | −0.84 | |
| (11) Ca meq/100 g DW | 0.77 | 0.63 | 0.80 | 0.98 | −0.78 | −0.30 | −0.58 | 0.60 | 0.85 | 0.85 | 0.97 | −0.99 | −0.98 | |
| (12) K meq/100 g DW | 0.72 | 0.54 | 0.70 | 0.95 | −0.75 | −0.21 | −0.52 | 0.64 | 0.78 | 0.78 | 0.98 | −0.98 | −0.94 | |
| (13) Cl meq/100 g DW | −0.82 | −0.72 | −0.84 | −0.98 | 0.66 | 0.14 | 0.42 | −0.54 | −0.89 | −0.89 | −0.97 | −0.94 | 0.97 | |
| (14) Na meq/100 g DW | −0.83 | −0.69 | −0.84 | −0.96 | 0.81 | 0.36 | 0.61 | −0.50 | −0.88 | −0.88 | −0.97 | −0.94 | 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqardaeai, T.; Alharbi, A.; Alenazi, M.; Alomran, A.; Alghamdi, A.; Obadi, A.; Elfeky, A.; Osman, M. Effectiveness of Grafting in Enhancing Salinity Tolerance of Tomato (Solanum lycopersicum L.) Using Novel and Commercial Rootstocks in Soilless Systems. Sustainability 2025, 17, 4333. https://doi.org/10.3390/su17104333
Alqardaeai T, Alharbi A, Alenazi M, Alomran A, Alghamdi A, Obadi A, Elfeky A, Osman M. Effectiveness of Grafting in Enhancing Salinity Tolerance of Tomato (Solanum lycopersicum L.) Using Novel and Commercial Rootstocks in Soilless Systems. Sustainability. 2025; 17(10):4333. https://doi.org/10.3390/su17104333
Chicago/Turabian StyleAlqardaeai, Thabit, Abdulaziz Alharbi, Mekhled Alenazi, Abdulrasoul Alomran, Abdulaziz Alghamdi, Abdullah Obadi, Ahmed Elfeky, and Mohamed Osman. 2025. "Effectiveness of Grafting in Enhancing Salinity Tolerance of Tomato (Solanum lycopersicum L.) Using Novel and Commercial Rootstocks in Soilless Systems" Sustainability 17, no. 10: 4333. https://doi.org/10.3390/su17104333
APA StyleAlqardaeai, T., Alharbi, A., Alenazi, M., Alomran, A., Alghamdi, A., Obadi, A., Elfeky, A., & Osman, M. (2025). Effectiveness of Grafting in Enhancing Salinity Tolerance of Tomato (Solanum lycopersicum L.) Using Novel and Commercial Rootstocks in Soilless Systems. Sustainability, 17(10), 4333. https://doi.org/10.3390/su17104333








