Restoring a Degraded Riparian Forested Buffer While Balancing Phosphorus Remediation, Biodiversity, and Indigenous Land Access
Abstract
1. Introduction
2. Materials and Methods
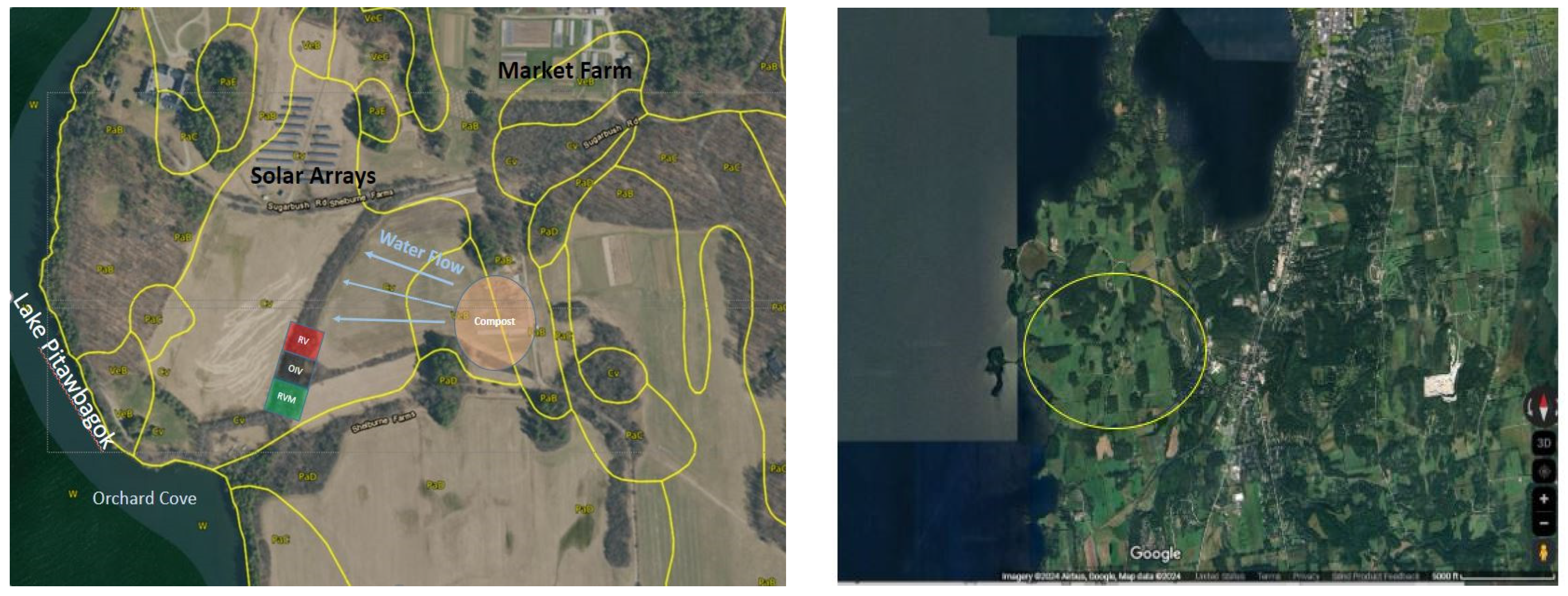
2.1. Study Site
2.2. Restoration Design
2.3. Implementation of Restoration
2.4. Sampling and Analysis
2.4.1. Soil, Water, and Plant Phosphorus Sampling
2.4.2. WEP-SRP Analysis
2.4.3. Total Phosphorus (TP) Analysis
2.4.4. Mehlich-3-Extractable P Analysis
2.4.5. Plant P Analysis
2.4.6. Plant Diversity Determination
2.4.7. Mycorrhizal Root Colonization Determination
2.5. Statistical Analysis
3. Results
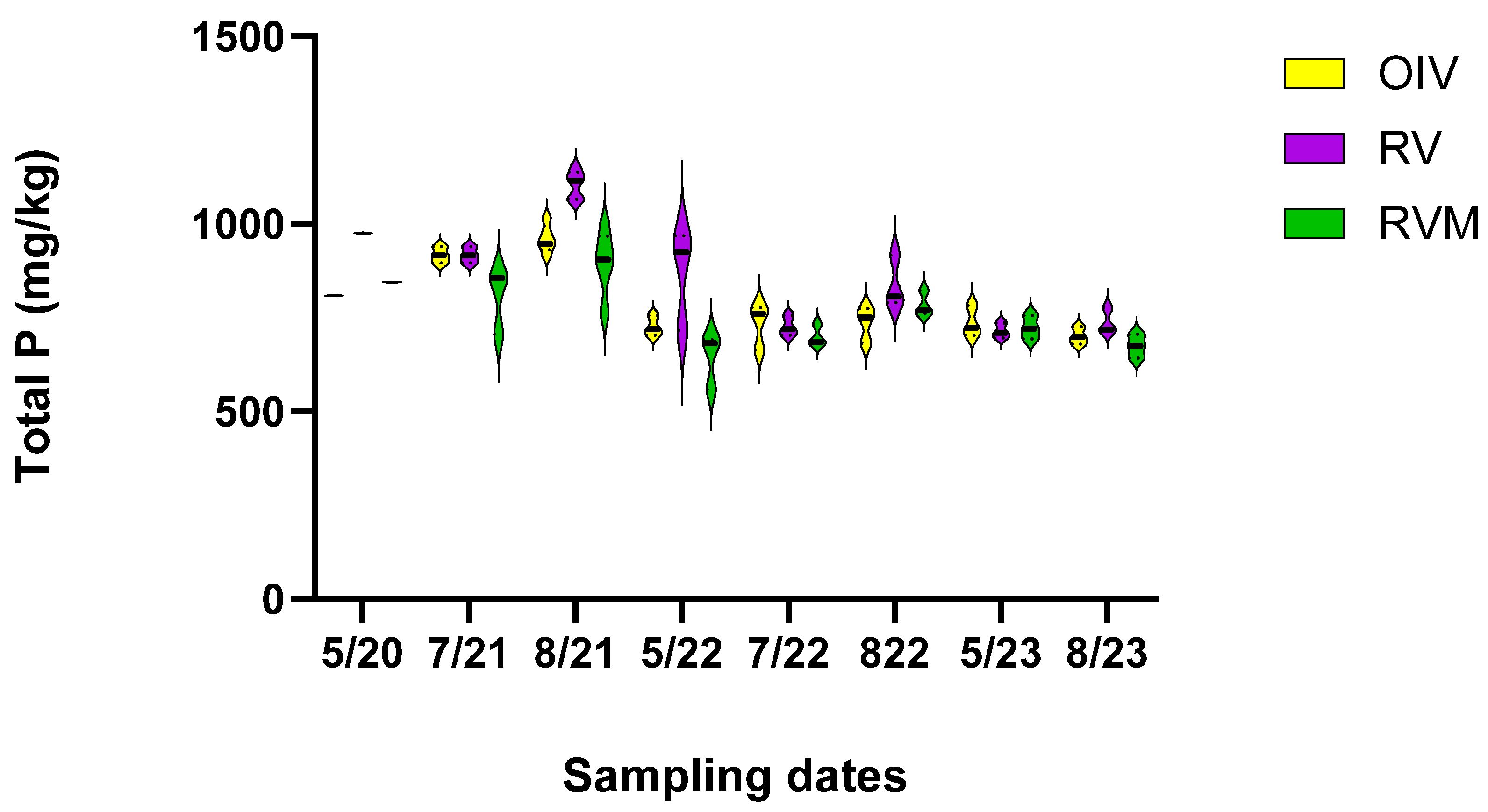
3.1. Phosphorus
3.2. P Mitigation Indicated through TP
3.3. Plant P Concentrations
3.4. Biomass—P
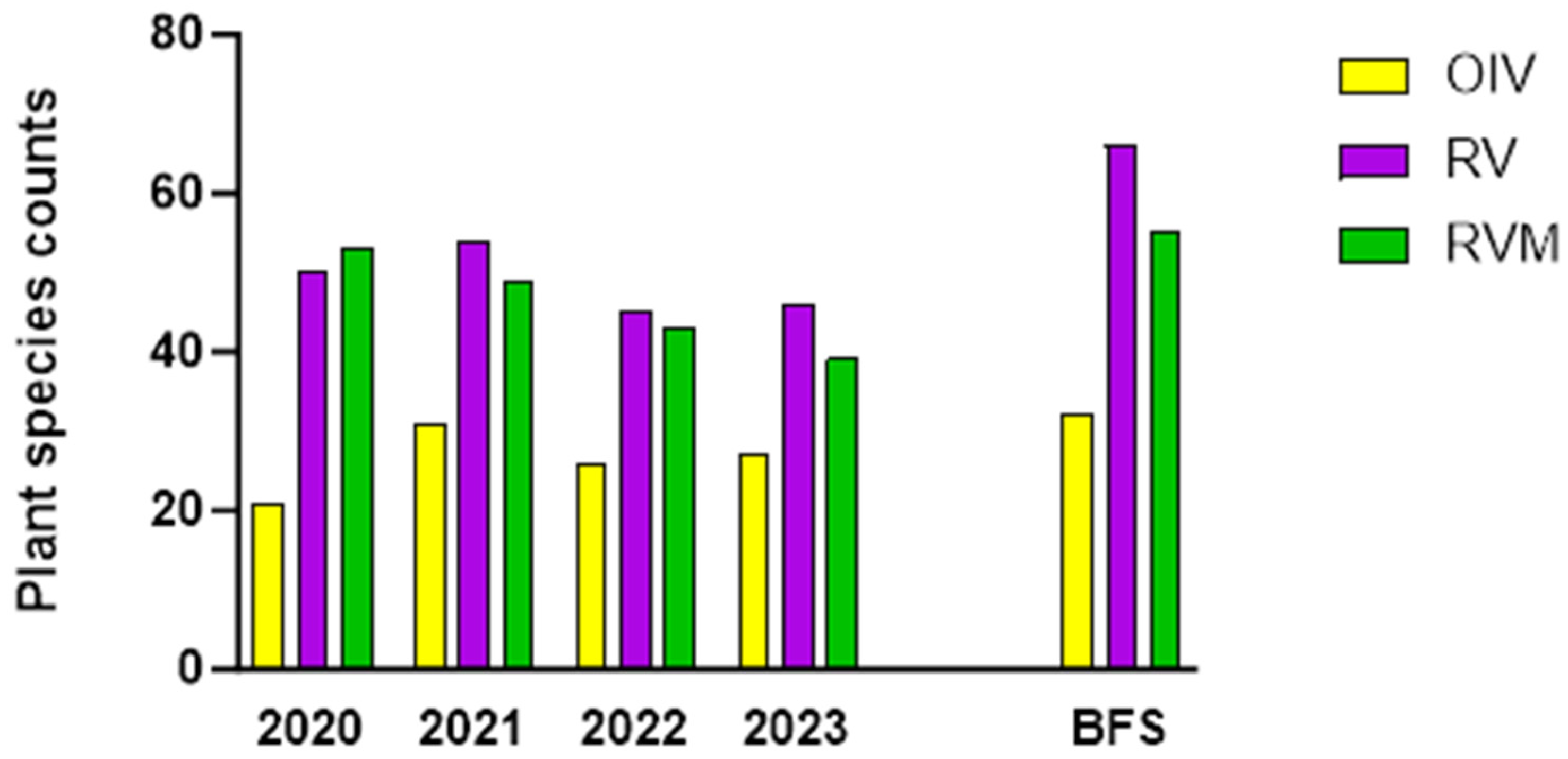
3.5. Plant Biodiversity
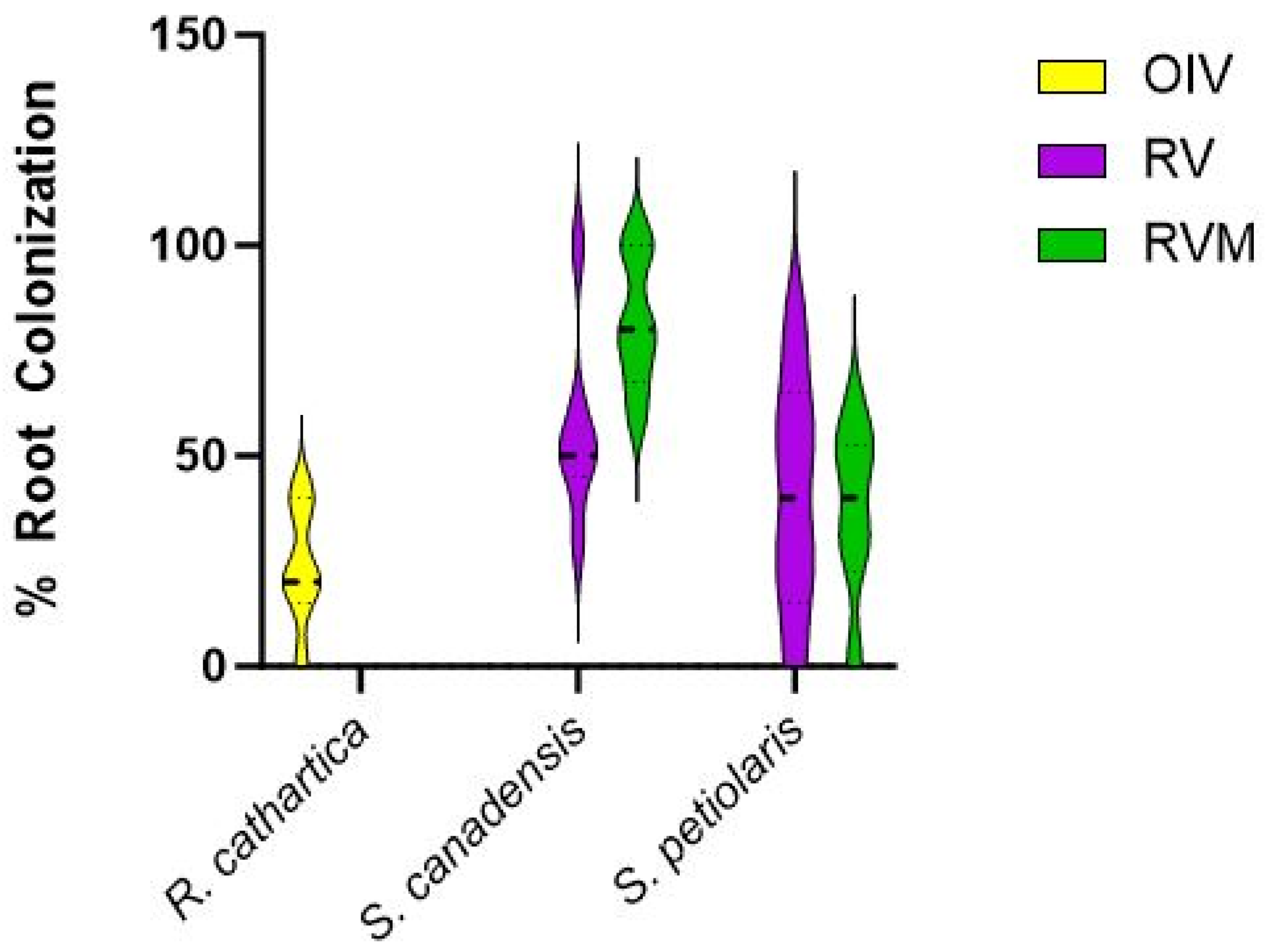
3.6. Mycorrhizal Colonization
4. Discussion
4.1. Phosphorus Mitigation
4.2. Succession, Pollinator Habitat, and Utility of the Plant Community to the Abenaki
4.3. Trade-Offs to Consider in Multi-Functional RFBs
4.4. Potential Solutions, Importance for Long Term Research, and Future Directions for This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Memmott, J.; Craze, P.G.; Waser, N.M.; Price, M.V. Global Warming and the Disruption of Plant–Pollinator Interactions. Ecol. Lett. 2007, 10, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D. The Insect Apocalypse, and Why It Matters. Curr. Biol. 2019, 29, R967–R971. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.L. Insect Declines in the Anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, M.T.H.; Jones, E.R.; Flörke, M.; Franssen, W.H.P.; Hanasaki, N.; Wada, Y.; Yearsley, J.R. Global Water Scarcity Including Surface Water Quality and Expansions of Clean Water Technologies. Environ. Res. Lett. 2021, 16, 024020. [Google Scholar] [CrossRef]
- Albert, J.S.; Destouni, G.; Duke-Sylvester, S.M.; Magurran, A.E.; Oberdorff, T.; Reis, R.E.; Winemiller, K.O.; Ripple, W.J. Scientists’ Warning to Humanity on the Freshwater Biodiversity Crisis. Ambio 2020, 50, 85–94. [Google Scholar] [CrossRef]
- Li, C.; Dong, Y.; Lei, Y.; Wu, D.; Xu, P. Removal of Low Concentration Nutrients in Hydroponic Wetlands Integrated with Zeolite and Calcium Silicate Hydrate Functional Substrates. Ecol. Eng. 2015, 82, 442–450. [Google Scholar] [CrossRef]
- Ojoawo, S.O.; Udayakumar, G.; Naik, P. Phytoremediation of Phosphorus and Nitrogen with Canna x Generalis Reeds in Domestic Wastewater through NMAMIT Constructed Wetland. Aquat. Procedia 2015, 4, 349–356. [Google Scholar] [CrossRef]
- Troy, A.; Wang, D.; Capen, D.; O’Neil-Dunne, J.; MacFaden, S. Updating the Lake Champlain Basin Land Use Data to Improve Prediction of Phosphorus Loading; Scientific Investigations Report; UVM: Burlington, VT, USA, 2017. [Google Scholar]
- Wang, H.; Wang, H. Mitigation of Lake Eutrophication: Loosen Nitrogen Control and Focus on Phosphorus Abatement. Prog. Nat. Sci. 2009, 19, 1445–1451. [Google Scholar] [CrossRef]
- Smith, D.R.; King, K.W.; Williams, M.R. What Is Causing the Harmful Algal Blooms in Lake Erie? J. Soil Water Conserv. 2015, 70, 27A–29A. [Google Scholar] [CrossRef]
- Cao, X.; Wang, Y.; He, J.; Luo, X.; Zheng, Z. Phosphorus Mobility among Sediments, Water and Cyanobacteria Enhanced by Cyanobacteria Blooms in Eutrophic Lake Dianchi. Environ. Pollut. 2016, 219, 580–587. [Google Scholar] [CrossRef]
- Hunter, P.D.; Tyler, A.N.; Gilvear, D.J.; Willby, N.J. Using Remote Sensing to Aid the Assessment of Human Health Risks from Blooms of Potentially Toxic Cyanobacteria. Environ. Sci. Technol. 2009, 43, 2627–2633. [Google Scholar] [CrossRef] [PubMed]
- Roy, E.D. Phosphorus Recovery and Recycling with Ecological Engineering: A Review. Ecol. Eng. 2017, 98, 213–227. [Google Scholar] [CrossRef]
- Weber, R. Lake Champlain Report Card: State Gets a D+ for Its Clean-Up Efforts; Conservation Law Foundation: Boston, MA, USA, 2018. [Google Scholar]
- Zaring, D. Agriculture, Nonpoint Source Pollution, and Regulatory Control: The Clean Water Act’s Bleak Present and Future. Harv. Environ. Law Rev. 1996, 20, 515. [Google Scholar]
- State of Vermont. Vermont Lake Champlain Phosphorus TMDL Phase 1 Implementation Plan; Vermont Department of Environmental Conservation: Montpelier, VT, USA, 2015; p. 152. [Google Scholar]
- Ishee, E.R.; Ross, D.S.; Garvey, K.M.; Bourgault, R.R.; Ford, C.R. Phosphorus Characterization and Contribution from Eroding Streambank Soils of Vermont’s Lake Champlain Basin. J. Environ. Qual. 2015, 44, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, N.; Brookes, P.C. Development of an Indicator for Risk of Phosphorus Leaching. J. Environ. Qual. 2000, 29, 105–110. [Google Scholar] [CrossRef]
- Meals, D.W.; Dressing, S.A.; Davenport, T.E. Lag Time in Water Quality Response to Best Management Practices: A Review. J. Environ. Qual. 2010, 39, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.K. Biogeochemical Time Lags May Delay Responses of Streams to Ecological Restoration. Freshw. Biol. 2012, 57, 43–57. [Google Scholar] [CrossRef]
- Kieta, K.A.; Owens, P.N.; Lobb, D.A.; Vanrobaeys, J.A.; Flaten, D.N. Phosphorus Dynamics in Vegetated Buffer Strips in Cold Climates: A Review. Environ. Rev. 2018, 26, 255–272. [Google Scholar] [CrossRef]
- Mason, R.E.; Merrill, S.C.; Görres, J.; Faulkner, J.; Niles, M.T. Agronomic and Environmental Performance of Dairy Farms in a Warmer, Wetter Climate. J. Soil Water Conserv. 2021, 76, 76–88. [Google Scholar] [CrossRef]
- Parkyn, S.M.; Davies-Colley, R.J.; Cooper, A.B.; Stroud, M.J. Predictions of Stream Nutrient and Sediment Yield Changes Following Restoration of Forested Riparian Buffers. Ecol. Eng. 2005, 24, 551–558. [Google Scholar] [CrossRef]
- VT Agency of Natural Resources. Guidance for Agency Act 250 and Section 248 Comments Regarding Riparian Buffers; VT Agency of Natural Resources: Montpelier, VT, USA, 2005; p. 30. [Google Scholar]
- MacFarland, K.; Straight, R.; Dosskey, M. Riparian Forest Buffers: An Agroforestry Practice. Agrofor. Notes 2017, 49, 8. [Google Scholar]
- Benayas, J.M.R.; Bullock, J.M.; Newton, A.C. Creating Woodland Islets to Reconcile Ecological Restoration, Conservation, and Agricultural Land Use. Front. Ecol. Environ. 2008, 6, 329–336. [Google Scholar] [CrossRef]
- Carrasco-Rueda, F.; Loiselle, B.A. Do Riparian Forest Strips in Modified Forest Landscapes Aid in Conserving Bat Diversity? Ecol. Evol. 2019, 9, 4192–4209. [Google Scholar] [CrossRef]
- Cole, L.J.; Stockan, J.; Helliwell, R. Managing Riparian Buffer Strips to Optimise Ecosystem Services: A Review. Agric. Ecosyst. Environ. 2020, 296, 106891. [Google Scholar] [CrossRef]
- Fonseca, A.; Zina, V.; Duarte, G.; Aguiar, F.C.; Rodríguez-González, P.M.; Ferreira, M.T.; Fernandes, M.R. Riparian Ecological Infrastructures: Potential for Biodiversity-Related Ecosystem Services in Mediterranean Human-Dominated Landscapes. Sustainability 2021, 13, 10508. [Google Scholar] [CrossRef]
- Broadmeadow, S.; Nisbet, T.R. The Effects of Riparian Forest Management on the Freshwater Environment: A Literature Review of Best Management Practice. Hydrol. Earth Syst. Sci. Discuss. 2004, 8, 286–305. [Google Scholar] [CrossRef]
- Richardson, D.M.; Holmes, P.M.; Esler, K.J.; Galatowitsch, S.M.; Stromberg, J.C.; Kirkman, S.P.; Pyšek, P.; Hobbs, R.J. Riparian Vegetation: Degradation, Alien Plant Invasions, and Restoration Prospects. Divers. Distrib. 2007, 13, 126–139. [Google Scholar] [CrossRef]
- Johnson, O.W.; Han, J.Y.-C.; Knight, A.-L.; Mortensen, S.; Aung, M.T.; Boyland, M.; Resurrección, B.P. Intersectionality and Energy Transitions: A Review of Gender, Social Equity and Low-Carbon Energy. Energy Res. Soc. Sci. 2020, 70, 101774. [Google Scholar] [CrossRef]
- Maher Hasselquist, E.; Kuglerová, L.; Sjögren, J.; Hjältén, J.; Ring, E.; Sponseller, R.A.; Andersson, E.; Lundström, J.; Mancheva, I.; Nordin, A.; et al. Moving towards Multi-Layered, Mixed-Species Forests in Riparian Buffers Will Enhance Their Long-Term Function in Boreal Landscapes. For. Ecol. Manag. 2021, 493, 119254. [Google Scholar] [CrossRef]
- Bentrup, G. Conservation Buffers Design Guidelines for Buffers, Corridors, and Greenways; USDA: Asheville, NC, USA, 2008; p. 110. [Google Scholar]
- Rubin, J.A.; Görres, J.H. Potential for Mycorrhizae-Assisted Phytoremediation of Phosphorus for Improved Water Quality. Int. J. Environ. Res. Public Health 2021, 18, 7. [Google Scholar] [CrossRef]
- Rubin, J.A.; Görres, J.H. The Effects of Mycorrhizae on Phosphorus Mitigation and Pollinator Habitat Restoration within Riparian Buffers on Unceded Land. Restor. Ecol. 2022, 31, e13671. [Google Scholar] [CrossRef]
- Cameron, D.D. Arbuscular Mycorrhizal Fungi as (Agro)Ecosystem Engineers. Plant Soil 2010, 333, 1–5. [Google Scholar] [CrossRef]
- Manaut, N.; Sanguin, H.; Ouahmane, L.; Bressan, M.; Thioulouse, J.; Baudoin, E.; Galiana, A.; Hafidi, M.; Prin, Y.; Duponnois, R. Potentialities of Ecological Engineering Strategy Based on Native Arbuscular Mycorrhizal Community for Improving Afforestation Programs with Carob Trees in Degraded Environments. Ecol. Eng. 2015, 79, 113–119. [Google Scholar] [CrossRef]
- Chatterjee, A.; Khan, S.R.; Vaseem, H. Exploring the Role of Mycorrhizae as Soil Ecosystem Engineer. In Mycorrhizosphere and Pedogenesis; Varma, A., Choudhary, D.K., Eds.; Springer: Singapore, 2019; pp. 73–93. ISBN 9789811364808. [Google Scholar]
- Rodrigues, K.M.; Rodrigues, B.F. Chapter 12—Arbuscular Mycorrhizae: Natural Ecological Engineers for Agro-Ecosystem Sustainability. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, J.S., Singh, D.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 165–175. ISBN 978-0-444-64191-5. [Google Scholar]
- Wires, K.N.; LaRose, J. Sogorea Te’ Land Trust and Indigenous Food Sovereignty in the San Francisco Bay Area. J. Agric. Food Syst. Community Dev. 2019, 9, 31–34. [Google Scholar] [CrossRef]
- Ferreira, C.; Gaudet, J.C.; Loukes, K.A. Indigenous Women’s Worldview in Food-Related Research: Rematriating Food, Bodies and Lands. Appl. Physiol. Nutr. Metab. 2022, 47, 210–213. [Google Scholar] [CrossRef]
- Bunyard, B.A. Dual-Mycorrhizal Plants or Dueling Mycorrhizal Fungi: How Common Are Dual-Mycorrhizal Associations ? Fungi 2020, 13, 9. [Google Scholar]
- Newman, E.I.; Reddell, P. The Distribution of Mycorrhizas Among Families of Vascular Plants. New Phytol. 1987, 106, 745–751. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Kendrick, B. The mycorrhizal status, root anatomy, and phenology of plants in a sugar maple forest. Can. J. Bot. 1988, 66, 1153–1173. [Google Scholar] [CrossRef]
- Cooke, J.C.; Lefor, M.W. The Mycorrhizal Status of Selected Plant Species from Connecticut Wetlands and Transition Zones. Restor. Ecol. 1998, 6, 214–222. [Google Scholar] [CrossRef]
- Clark, R.B.; Zeto, S.K.; Zobel, R.W. Arbuscular Mycorrhizal Fungal Isolate Effectiveness on Growth and Root Colonization of Panicum Virgatum in Acidic Soil. Soil Biol. Biochem. 1999, 31, 1757–1763. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Dodd, J.; Castro, P. The Mycorrhizal Status of Phragmites Australis in Several Polluted Soils and Sediments of an Industrialised Region of Northern Portugal. Mycorrhiza 2001, 10, 241–247. [Google Scholar] [CrossRef]
- Bauer, C.R.; Kellogg, C.H.; Bridgham, S.D.; Lamberti, G.A. Mycorrhizal Colonization across Hydrologic Gradients in Restored and Reference Freshwater Wetlands. Wetlands 2003, 23, 8. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Ridgway, K.P.; Watson, I.J.; Fitter, A.H.; Young, J.P.W. Co-Existing Grass Species Have Distinctive Arbuscular Mycorrhizal Communities. Mol. Ecol. 2003, 12, 3085–3095. [Google Scholar] [CrossRef] [PubMed]
- Scagel, C.F. Enhanced Rooting of Kinnikinnick Cuttings Using Mycorrhizal Fungi in Rooting Substrate. HortTechnology 2004, 14, 355–363. [Google Scholar] [CrossRef]
- Wang, B.; Qiu, Y.-L. Phylogenetic Distribution and Evolution of Mycorrhizas in Land Plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef] [PubMed]
- Weishampel, P.A.; Bedford, B.L. Wetland Dicots and Monocots Differ in Colonization by Arbuscular Mycorrhizal Fungi and Dark Septate Endophytes. Mycorrhiza 2006, 16, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, B.E.; Weishampel, P.A.; Klironomos, J.N. Arbuscular Mycorrhizal Fungi and Water Table Affect Wetland Plant Community Composition. J. Ecol. 2006, 94, 905–914. [Google Scholar] [CrossRef]
- Brundrett, M.C. Mycorrhizal Associations and Other Means of Nutrition of Vascular Plants: Understanding the Global Diversity of Host Plants by Resolving Conflicting Information and Developing Reliable Means of Diagnosis. Plant Soil 2009, 320, 37–77. [Google Scholar] [CrossRef]
- Rudgers, J.A.; Swafford, A.L. Benefits of a Fungal Endophyte in Elymus Virginicus Decline under Drought Stress. Basic Appl. Ecol. 2009, 10, 43–51. [Google Scholar] [CrossRef]
- Comas, L.H.; Callahan, H.S.; Midford, P.E. Patterns in Root Traits of Woody Species Hosting Arbuscular and Ectomycorrhizas: Implications for the Evolution of Belowground Strategies. Ecol. Evol. 2014, 4, 2979–2990. [Google Scholar] [CrossRef]
- Lady Bird Johnson Wildlife Center. 2021. Available online: https://www.wildflower.org/ (accessed on 5 February 2024).
- National Wildlife Federation—Native Plant Finder. Available online: https://www.nwf.org/nativeplantfinder (accessed on 1 December 2021).
- Senier, S. “All This/Is Abenaki Country”: Cheryl Savageau’s Poetic Awikhiganak. Stud. Am. Indian Lit. 2010, 22, 1–25. [Google Scholar] [CrossRef]
- USDA Official Series Description—COVINGTON Series. Available online: https://soilseries.sc.egov.usda.gov/OSD_Docs/C/COVINGTON.html (accessed on 5 May 2020).
- Chapdelaine, C. Late Pleistocene Archaeology and Ecology in the Far Northeast; Texas A&M University Press: College Station, TX, USA, 2012; ISBN 978-1-60344-790-4. [Google Scholar]
- USDA NRCS. Hydrologic Soil Group; Web Soil Survey National Cooperative Soil Survey; USDA NRCS: Washington, DC, USA, 2015; pp. 1–7. [Google Scholar]
- VT ANR & DEC. Vermont Water Quality Standards Environmental Protection Rule; VT ANR & DEC: Montpelier, VT, USA, 2017; Chapter 29A; pp. 19–20. [Google Scholar]
- Brundrett, M. Mycorrhizal Associations: Methods for Examining Mycorrhizas. Available online: https://mycorrhizas.info/method.html (accessed on 24 December 2019).
- Mycorrhizal Applications. Mycorrhizal Status of Plant Families and Genera; Mycorrhizal Applications: Grants Pass, OR, USA, 2020. [Google Scholar]
- Soudzilovskaia, N.A.; Vaessen, S.; Barcelo, M.; He, J.; Rahimlou, S.; Abarenkov, K.; Brundrett, M.C.; Gomes, S.I.F.; Merckx, V.; Tedersoo, L. FungalRoot: Global Online Database of Plant Mycorrhizal Associations. New Phytol. 2020, 227, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Jicha, T.M. EPA Standard Operating Procedures; U.S. Environmental Protection Agency: Washington, DC, USA, 2015. [Google Scholar]
- Wolf, A.; Beegle, D. Recommended Soil Tests for Macro and Micronutrients. In Recommended Soil Testing Procedures for the Northeastern United States; Cooperative Bulletin; Northeastern Regional Publication: Bangor, ME, UAS, 2009; p. 48. [Google Scholar]
- Deguchi, S.; Matsuda, Y.; Takenaka, C.; Sugiura, Y.; Ozawa, H.; Ogata, Y. Proposal of a New Estimation Method of Colonization Rate of Arbuscular Mycorrhizal Fungi in the Roots of Chengiopanax sciadophylloides. Mycobiology 2017, 45, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Gaston, L.A.; Eilers, R.T.L.; Kovar, J.L.; Cooper, D.; Robinson, D.L. Greenhouse and Field Studies on Hay Harvest to Remediate High Phosphorus Soil. Commun. Soil Sci. Plant Anal. 2006, 34, 2085–2097. [Google Scholar] [CrossRef]
- Kimmerer, R. Braiding Sweetgrass: Indigenous Wisdom, Scientific Knowledge and the Teachings of Plants; Milkweed Editions: Minneapolis, MN, USA, 2013; ISBN 978-1-57131-871-8. [Google Scholar]
- Johnson, N.C.; Graham, J.H.; Smith, F.A. Functioning of Mycorrhizal Associations along the Mutualism–Parasitism Continuum. New Phytol. 1997, 135, 575–585. [Google Scholar] [CrossRef]
- Johnson, N.C.; Graham, J.H. The Continuum Concept Remains a Useful Framework for Studying Mycorrhizal Functioning. Plant Soil 2013, 363, 411–419. [Google Scholar] [CrossRef]
- Johnson, N.C. Resource Stoichiometry Elucidates the Structure and Function of Arbuscular Mycorrhizas across Scales. New Phytol. 2010, 185, 631–647. [Google Scholar] [CrossRef]
- Buil, P.A.; Jansa, J.; Blažková, A.; Holubík, O.; Duffková, R.; Rozmoš, M.; Püschel, D.; Kotianová, M.; Janoušková, M. Infectivity and Symbiotic Efficiency of Native Arbuscular Mycorrhizal Fungi from High-Input Arable Soils. Plant Soil 2022, 482, 627–645. [Google Scholar] [CrossRef]
- Ryan, M.H.; van Herwaarden, A.F.; Angus, J.F.; Kirkegaard, J.A. Reduced Growth of Autumn-Sown Wheat in a Low-P Soil Is Associated with High Colonisation by Arbuscular Mycorrhizal Fungi. Plant Soil 2005, 270, 275–286. [Google Scholar] [CrossRef]
- Volf, M.; Rosolem, C. Soil P Diffusion and Availability Modified by Controlled-Release P Fertilizers. J. Soil Sci. Plant Nutr. 2020, 21, 162–172. [Google Scholar] [CrossRef]
- Catling, P.M.; Mitrow, G. Major Invasive Alien Plats of Natural Habitats in Canada. CBA/ABC Bull. 2012, 45, 70–77. [Google Scholar]
- Tomlinson, S.; Webber, B.L.; Bradshaw, S.D.; Dixon, K.W.; Renton, M. Incorporating Biophysical Ecology into High-Resolution Restoration Targets: Insect Pollinator Habitat Suitability Models. Restor. Ecol. 2018, 26, 338–347. [Google Scholar] [CrossRef]
- Kremen, C.; M’Gonigle, L.K.; Ponisio, L.C. Pollinator Community Assembly Tracks Changes in Floral Resources as Restored Hedgerows Mature in Agricultural Landscapes. Front. Ecol. Evol. 2018, 6, 170. [Google Scholar] [CrossRef]
- Lybbert, A.H.; Cusser, S.J.; Hung, K.-L.J.; Goodell, K. Ten-Year Trends Reveal Declining Quality of Seeded Pollinator Habitat on Reclaimed Mines Regardless of Seed Mix Diversity. Ecol. Appl. 2022, 32, e02467. [Google Scholar] [CrossRef] [PubMed]
- Kohler, F.; Verhulst, J.; Van Klink, R.; Kleijn, D. At What Spatial Scale Do High-Quality Habitats Enhance the Diversity of Forbs and Pollinators in Intensively Farmed Landscapes? J. Appl. Ecol. 2008, 45, 753–762. [Google Scholar] [CrossRef]
- Joseph, L.; Turner, N.J. “The Old Foods Are the New Foods!”: Erosion and Revitalization of Indigenous Food Systems in Northwestern North America. Front. Sustain. Food Syst. 2020, 4, 596237. [Google Scholar] [CrossRef]
- Adegbidi, H.G.; Briggs, R.D.; Volk, T.A.; White, E.H.; Abrahamson, L.P. Biomass and Nutrient Removal by Willow Clones in Experimental Bioenergy Plantations in New York State. Biomass Bioenergy 2001, 20, 399–411. [Google Scholar] [CrossRef]
- Irons, C. Vermont Abenakis, A Brief History of the People of the Dawnland, Vermont’s First Human Inhabitants; L. Brown and Sons: Barre, VT, USA, 2021. [Google Scholar]
- Jacke, D. Eric Toensmeier Edible Forest Gardens’ Ecological Vision and Theory for Temperate Climate Permaculture; Chelsea Green Publishing: Chelsea, VT, USA, 2005; Volume 1–2. [Google Scholar]
- Tallamy, D.W. Nature’s Best Hope: A New Approach to Conservation That Starts in Your Yard; Timber Press: Portland, OR, USA, 2019. [Google Scholar]
- Uprety, Y.; Asselin, H.; Bergeron, Y.; Doyon, F.; Boucher, J.-F. Contribution of Traditional Knowledge to Ecological Restoration: Practices and Applications. Écoscience 2012, 19, 225–237. [Google Scholar] [CrossRef]
- Lopez-Hernandez, D.; Brossard, M.; Frossard, E. P-Isotopic Exchange Values in Relation to Po Mineralisation in Soils with Very Low P-Sorbing Capacities. Soil Biol. Biochem. 1998, 30, 1663–1670. [Google Scholar] [CrossRef]
- Paré, L.L.L.-J. COP15 and Its Impacts on Indigenous and Environmental Law. Available online: https://www.lexology.com/library/detail.aspx?g=7acc98e3-9045-4a79-9504-e44cca941a40 (accessed on 23 October 2023).
- Jordan, W.R.; Lubick, G.M. Making Nature Whole: A History of Ecological Restoration; Island Press: Washington, DC, USA, 2011; ISBN 978-1-61091-042-2. [Google Scholar]
- Graham, N.; Bartel, R. Farmscapes: Property, Ecological Restoration and the Reconciliation of Human and Nature in Australian Agriculture. Griffith Law Rev. 2017, 26, 221–247. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Hoeksema, J.D.; Gehring, C.A.; Johnson, N.C.; Klironomos, J.N.; Abbott, L.K.; Pringle, A. The Promise and the Potential Consequences of the Global Transport of Mycorrhizal Fungal Inoculum. Ecol. Lett. 2006, 9, 501–515. [Google Scholar] [CrossRef]
- Vosátka, M.; Látr, A.; Gianinazzi, S.; Albrechtová, J. Development of Arbuscular Mycorrhizal Biotechnology and Industry: Current Achievements and Bottlenecks. Symbiosis 2012, 58, 29–37. [Google Scholar] [CrossRef]
- Faye, A.; Dalpé, Y.; Ndung’u-Magiroi, K.; Jefwa, J.; Ndoye, I.; Diouf, M.; Lesueur, D. Evaluation of Commercial Arbuscular Mycorrhizal Inoculants. Can. J. Plant Sci. 2013, 93, 1201–1208. [Google Scholar] [CrossRef]
- Maltz, M.R.; Treseder, K.K. Sources of Inocula Influence Mycorrhizal Colonization of Plants in Restoration Projects: A Meta-Analysis. Restor. Ecol. 2015, 23, 625–634. [Google Scholar] [CrossRef]
- Kolba, L.; Rubin, J.; Görres, J. Growing Local Mycorrhizal Inoculum, A Guide and Insights from a Field Trial; NESARE: Burlington, VT, USA, 2022; p. 14. [Google Scholar]
- Aoyama, L.; Shoemaker, L.G.; Gilbert, B.; Collinge, S.K.; Faist, A.M.; Shackelford, N.; Temperton, V.M.; Barabás, G.; Larios, L.; Ladouceur, E.; et al. Application of Modern Coexistence Theory to Rare Plant Restoration Provides Early Indication of Restoration Trajectories. Ecol. Appl. 2022, 32, e2649. [Google Scholar] [CrossRef] [PubMed]
- Corntassel, J. Re-Envisioning Resurgence: Indigenous Pathways to Decolonization and Sustainable Self-Determination. University of Victoria. Faculty Publications. UVicSPACE: Research & Learning Repository. 2012. Available online: https://jps.library.utoronto.ca/index.php/des/article/view/18627/15550 (accessed on 5 February 2024).
- Hutchens, A.R. A Handbook of Native American Herbs: The Pocket Guide to 125 Medicinal Plants and Their Uses; Shambhala Publications: Boulder, CO, USA, 1992; ISBN 978-0-8348-2422-5. [Google Scholar]
- Moerman, D.E. Native American Ethnobotany; Timber Press: Portland, OR, USA, 1998. [Google Scholar]
- Webmaster, D.R. (Kingdom) Plants-Montana Field Guide. Available online: https://fieldguide.mt.gov/displayPhyDiv.aspx?Kingdom=Plantae (accessed on 15 December 2023).
- Rhamnus cathartica, R. davurica. Available online: https://www.fs.usda.gov/database/feis/plants/shrub/rhaspp/all.html (accessed on 15 December 2023).
| Plant Palette for Myco-Phytoremediation Ecological Restoration Research Pilot Project at Shelburne Farms | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flora | Scientiific Name | Common Name | #/Plot | Abenaki Uses | Feb | Mar | Apr | May | Jun | Jul | Aug | Sept | Oct | Nov | Hosts |
| Trees | |||||||||||||||
| Acer rubrum | Red Maple | 1 | e,m | Native & honey bees, Cecropia moths, other moth larvae, birds | |||||||||||
| Acer saccharinum | Silver Maple | 1 | e,m | Birds, Cecropia moth | |||||||||||
| Alnus incana | Speckled Alder | 10 | m,c | Songbirds, waterbirds, mammals | |||||||||||
| Carya ovata | Shagbark Hickory | 2 | e,a | Insectivorous birds | |||||||||||
| Cornus Sericea | Red Osier Dogwood | 19 | m,a | Waterfowl, marsh & shore birds, butterflies, Spring Azure | |||||||||||
| Quercus bicolor | Swamp White Oak | 1 | e | Songbirds, ground birds, water birds, mammals | |||||||||||
| Salix nigra | Black Willow | 1 | m | Songbirds, water fowl, Mourning Cloak, Viceroy, Red Spotted Purple, Tiger Swallowtail | |||||||||||
| Salix petiolaris | Meadow Willow | 8 | a | Native bees, bumblees, honeybees, Mourning Cloak, Viceroy | |||||||||||
| Tilia americana | Basswood | 1 | e,a,u | Native & honey bees, birds | |||||||||||
| Ulmus americana | American Elm | 10 | a,m | Birds, Mourning Cloak, Columbia Silkmoth, Question Mark, Painted Lady, Comma Butterfly | |||||||||||
| Shrubs | |||||||||||||||
| Cephalanthus occidentalis | Buttonbush | 9 | m | Native bumblebees, honey bees, birds, butterflies, Titan Sphinx, Hydrangea Sphinx | |||||||||||
| Ilex verticillata | Winterberry | 4 | m | Honey bees, birds, butterflies, Elf Larval Host | |||||||||||
| Sambucus canadensis | Elderberry | 8 | m | Native, bumble and honey bees, birds, butterflies, Titan Sphinx, Hydrangea Sphinx | |||||||||||
| Viburnum dentatum | Arrowood | 4 | a,u | Native bees, bumblebees, birds, butterflies, Spring Azure | |||||||||||
| Viburnum lentago | Nannyberry | 4 | e,c,m | Birds, butterflies, Spring Azure | |||||||||||
| Perennials | |||||||||||||||
| Asarum canadense | Wild Ginger | 9 | m | Butterflies, Pipeline Swallowtail | |||||||||||
| Carex comosa | Longhair Sedge | 18 | Nesting for insects and birds | ||||||||||||
| Chelone glabra | Turtlehead | 20 | m | Hummingbirds, butterflies, Baltimore Checkerspot | |||||||||||
| Eupatorium perfoliatum | Boneset | 14 | m | Native bees, birds, butterflies | |||||||||||
| Eutrochium purpureum | Joe Pye Weed | 21 | m | Native bees, birds, butterflies | |||||||||||
| Iris versicolor | Blue fllag Iris | 18 | m | Birds, hummingbirds | |||||||||||
| Symphyotrichum novae-angliae | NE Aster | 9 | m,e | Birds, butterflies | |||||||||||
| Wild Seed Mix | |||||||||||||||
| Panicum virgatum | Switch Grass | seed mix | Birds, butterflies, Delaware & Dotted Skipper | ||||||||||||
| Elymus virginicus | Virginia Wild Rye | seed mix | Birds, butterflies, Branded Skippers and Satyr Larval Hosts | ||||||||||||
| Festuca rubra | Red Fescue | seed mix | Birds | ||||||||||||
| Carex vulpinoidea | Fox Sedge | seed mix | Birds | ||||||||||||
| Scirpus cyperinus | Wool Grass | seed mix | Birds, Dion Skipper | ||||||||||||
| Scirpus atrovirens | Green Bullgrass | seed mix | Birds, waterfowl, songbirds, shorebirds | ||||||||||||
| Bidens cernua | Nodding Bur-Marigold | seed mix | m | Birds, native bees | |||||||||||
| Eupatorium perfoliatum | Common Boneset | seed mix | m | Native bees, butterflies, moths, birds | |||||||||||
| Eupatoriadelphus maculatus | Joe Pye Weed | seed mix | m | Butterflies, Moth caterpillars, deer, rabbit | |||||||||||
| Juncus effusus | Soft Rush | seed mix | a | Birds | |||||||||||
| Onaclea sensibilis | Sensitive Fern | seed mix | m | Birds, salamanders, frogs | |||||||||||
| Verbena hastata | Blue Vervain | seed mix | Native bees | ||||||||||||
| Symphyotrichum novae-angliae | NE Aster | seed mix | m,e | Native bees, bumblebees, honey bees, Pearl Crescent Larval Host | |||||||||||
| Ectomycorrhizal (ECM) | Endomycorrhizae (AMF) |
|---|---|
| * Rhizopogon villosulus, R. luteolus, R. amylopogon, R. fulvigleba | Glomus intraradices, G. mosseae, G. aggregatum, G. etunicatum, G. deserticola, G. monosporum, G. clarum |
| * Pisolithus tinctorius | Paraglomus brasilianum |
| * Suillus granulatus | Gigaspora margarita |
| * Laccaria bicolor, L. laccata | |
| Scleroderma cepa, S. citrinum |
| Sampling Dates | # Replicate/Treatment | Parameters Measured |
|---|---|---|
| 5/21/2020 | 1 composite of 5 | TP |
| 5/2020–9/2020 | 6 storm events | SRP (via lysimeters) ** |
| 5/2020–10/2020 monthly | 1 | Plant richness |
| 7/3/2021 | 3 | TP |
| 7/3/2021 | 3 | WEP-SRP ** |
| 7/3/2021 | 3 | Mehlich-3-extractable P ** |
| 8/10/2021 | 3 of each species | Plant P |
| 5/2021–10/2021 monthly | 1 | Plant richness |
| 5/18/22, 7/19/22, 8/10/2022 | 3 | TP |
| 5/18/22, 7/19/22, 8/10/2022 | 3 | WEP-SRP ** |
| 5/18/22, 7/19/22, 8/10/2022 | 3 | Mehlich-3-extractable P ** |
| 7/19/2022 | 3 of each species | % Mycorrhizal root colonization |
| 8/16/2022 | 4 of 4 species, 3 of 2 species * | Plant P |
| 5/2022–10/2022 monthly | 1 | Plant richness |
| 5/18/2023, 8/15/2023 | 2 | TP |
| 5/18/2023, 7/17/2023, 8/15/2023 | 3 | WEP-SRP ** |
| 8/15/2023 | 3 | Mehlich-3-extractable P ** |
| 7/17/2023 | 5 from 3 species | % Mycorrhizal root colonization |
| 8/15/2023 | 3 from 5 species * | Plant P |
| 5/2023–10/2023 alternate months | 1 | Plant richness |
| Plant Species | Mean P Concentrations (mg/kg) | % Difference between the Restored Plots for P Concentrations | Mean Biomass P (mg) | % Difference between the Restored Plots for Biomass-P |
|---|---|---|---|---|
| R. carthartica | 1489 | NA | 9.50 | NA |
| S. petiolaris | 1700 | +12.74 | 10.36 | +5.77 |
| S. canadensis | 3274 | −3.10 | 11.43 | −48.84 |
| C. sericea | 1530 | +21.31 | 10.46 | +10.46 |
| V. dentatum | 1018 | +7.66 | 7.92 | −6.89 |
| R. leucodermis | 2126 | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubin, J.; McGranaghan, C.; Kolba, L.; Görres, J. Restoring a Degraded Riparian Forested Buffer While Balancing Phosphorus Remediation, Biodiversity, and Indigenous Land Access. Sustainability 2024, 16, 3366. https://doi.org/10.3390/su16083366
Rubin J, McGranaghan C, Kolba L, Görres J. Restoring a Degraded Riparian Forested Buffer While Balancing Phosphorus Remediation, Biodiversity, and Indigenous Land Access. Sustainability. 2024; 16(8):3366. https://doi.org/10.3390/su16083366
Chicago/Turabian StyleRubin, Jessica, Carol McGranaghan, Luca Kolba, and Josef Görres. 2024. "Restoring a Degraded Riparian Forested Buffer While Balancing Phosphorus Remediation, Biodiversity, and Indigenous Land Access" Sustainability 16, no. 8: 3366. https://doi.org/10.3390/su16083366
APA StyleRubin, J., McGranaghan, C., Kolba, L., & Görres, J. (2024). Restoring a Degraded Riparian Forested Buffer While Balancing Phosphorus Remediation, Biodiversity, and Indigenous Land Access. Sustainability, 16(8), 3366. https://doi.org/10.3390/su16083366







