Simultaneous Removal of Arsenate and Fluoride Using Magnesium-Based Adsorbents
Abstract
1. Introduction
2. Materials and Methods
2.1. Mg-Based Adsorbents
2.2. Synthetic As(V)–F in Multi-Contaminated Water
2.3. As–F Simultaneous Removal Tests
2.4. Preparation of Samples for X-ray Diffraction (XRD) Analysis
3. Results
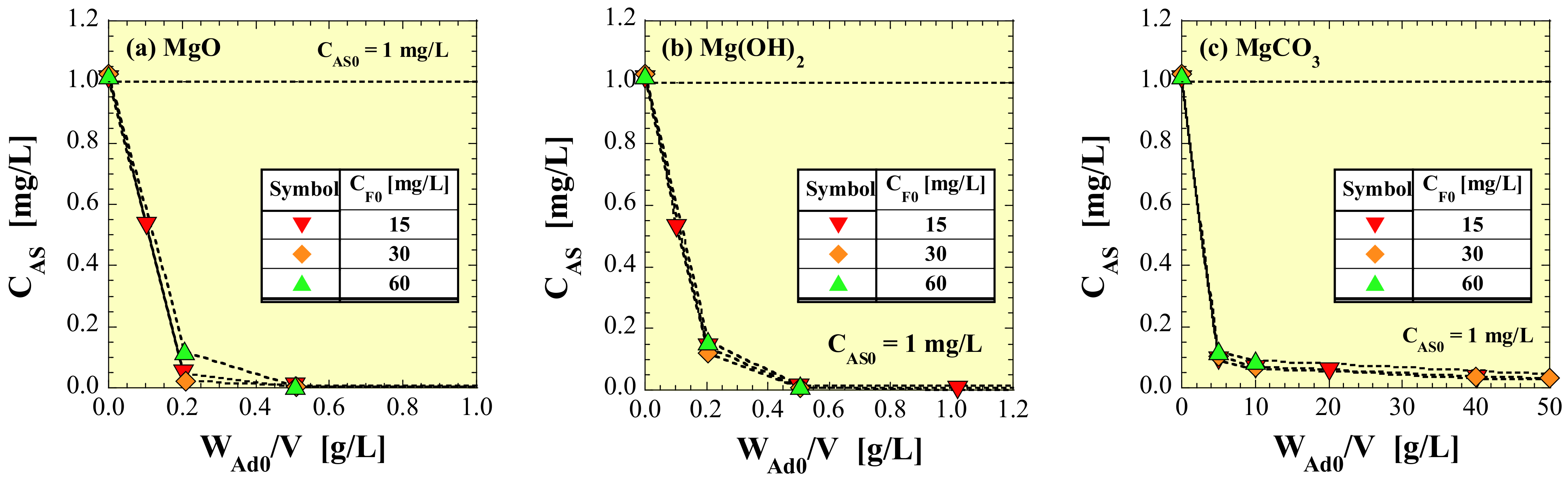
3.1. Residual as Concentration in Treated Water
3.2. Residual F Concentration in Treated Water
3.3. Leached Mg Concentration in Treated Water
3.4. pH of Treated Water
3.5. ORP of Treated Water
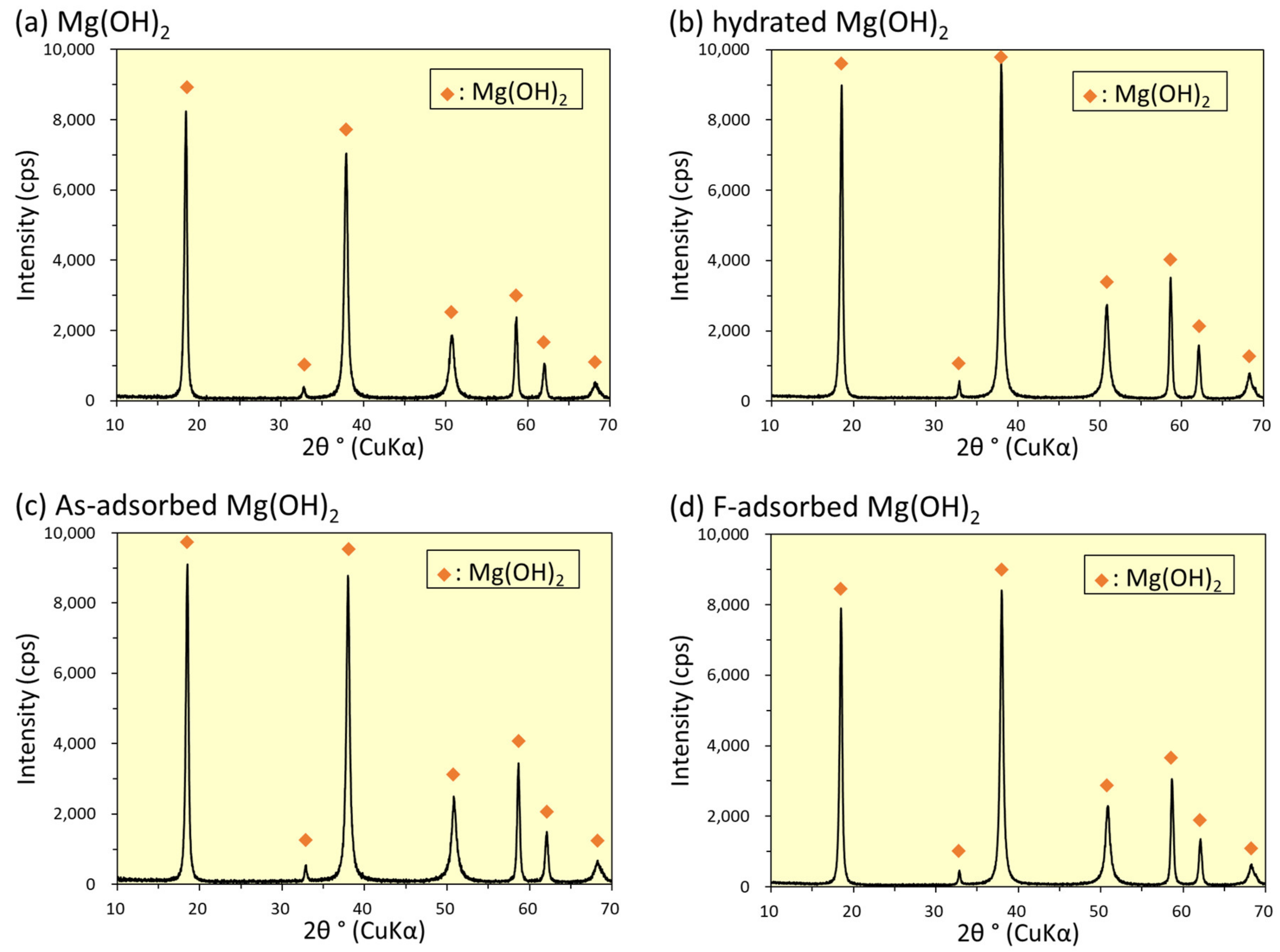
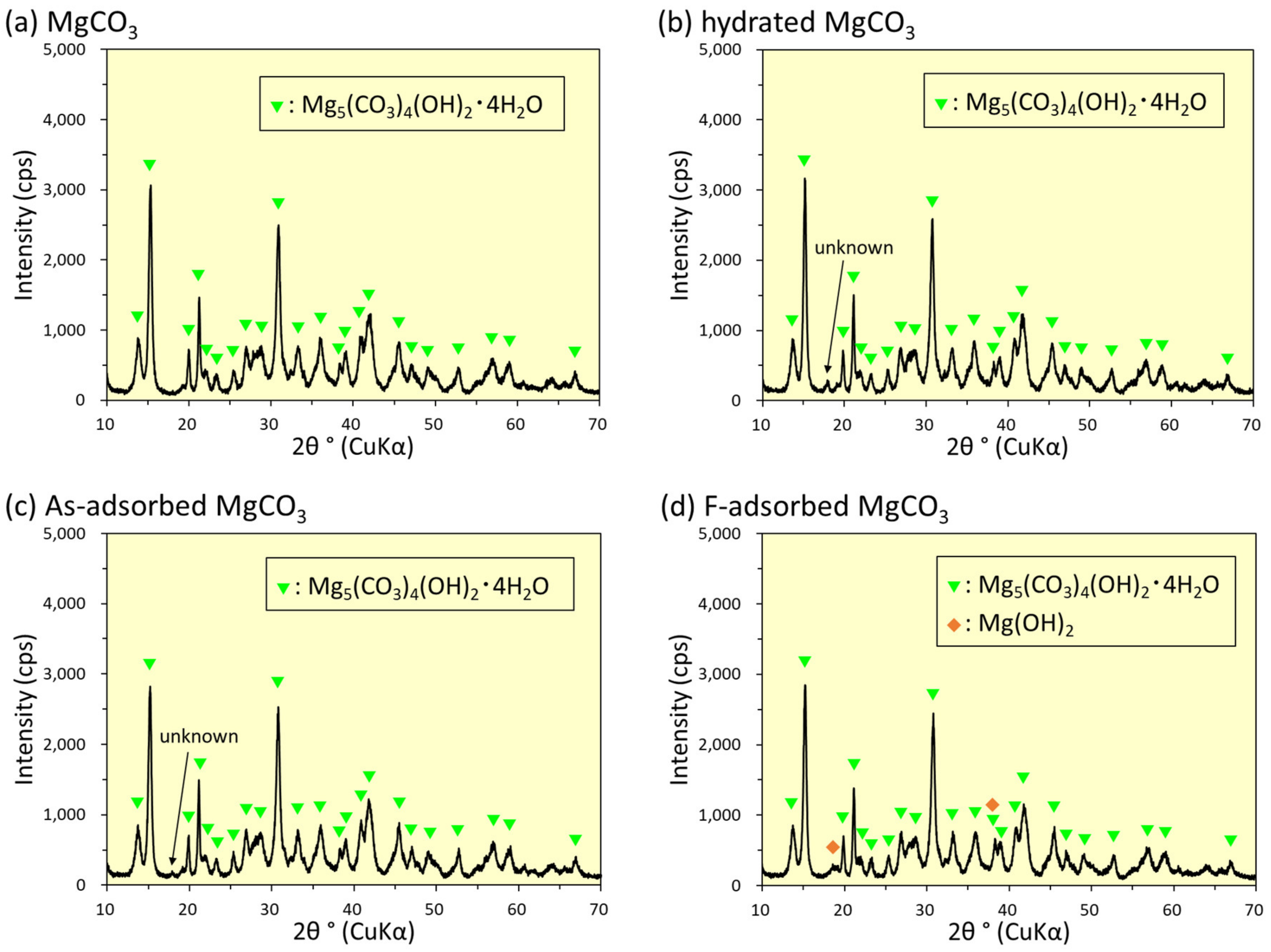
3.6. XRD Analysis
4. Discussion
4.1. As-Removal Ratio
4.2. F-Removal Ratio
4.3. Dissolved Forms of As(V) and F in Water
4.4. As- and F-Adsorption Amounts per Unit Mass of Adsorbent
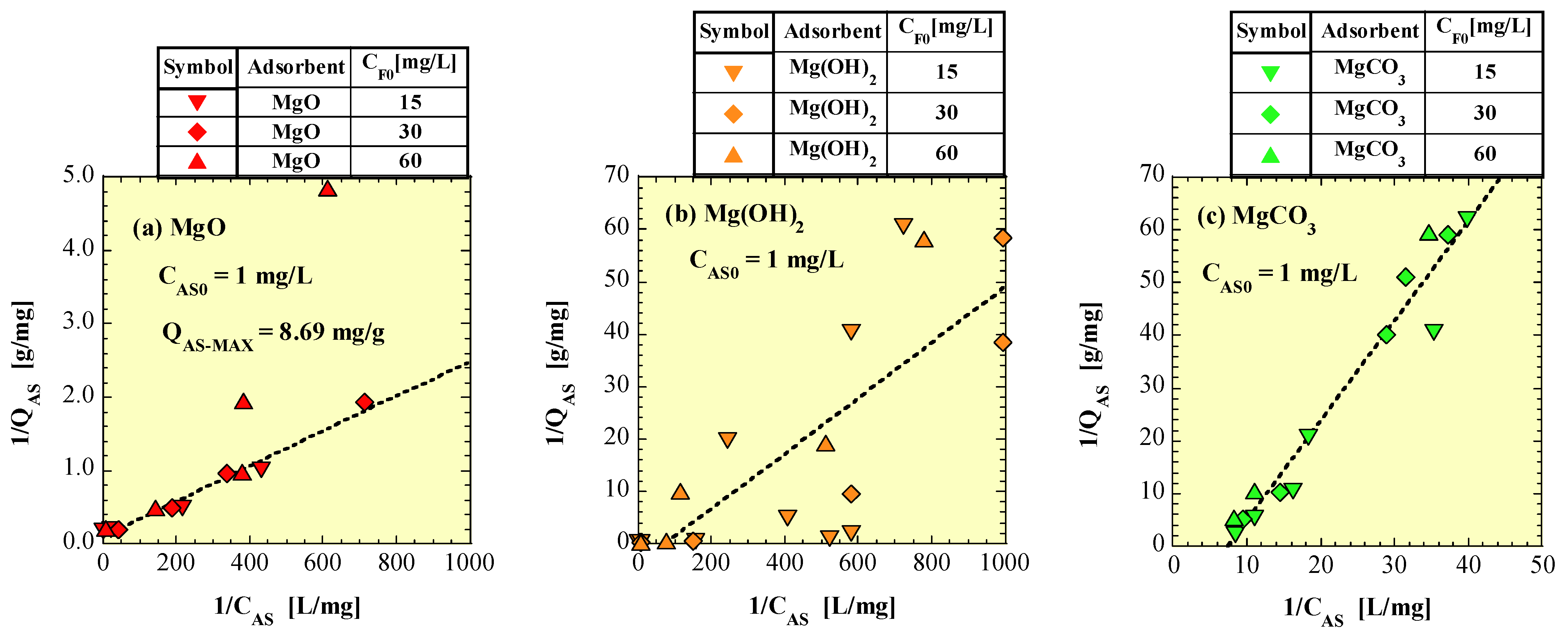
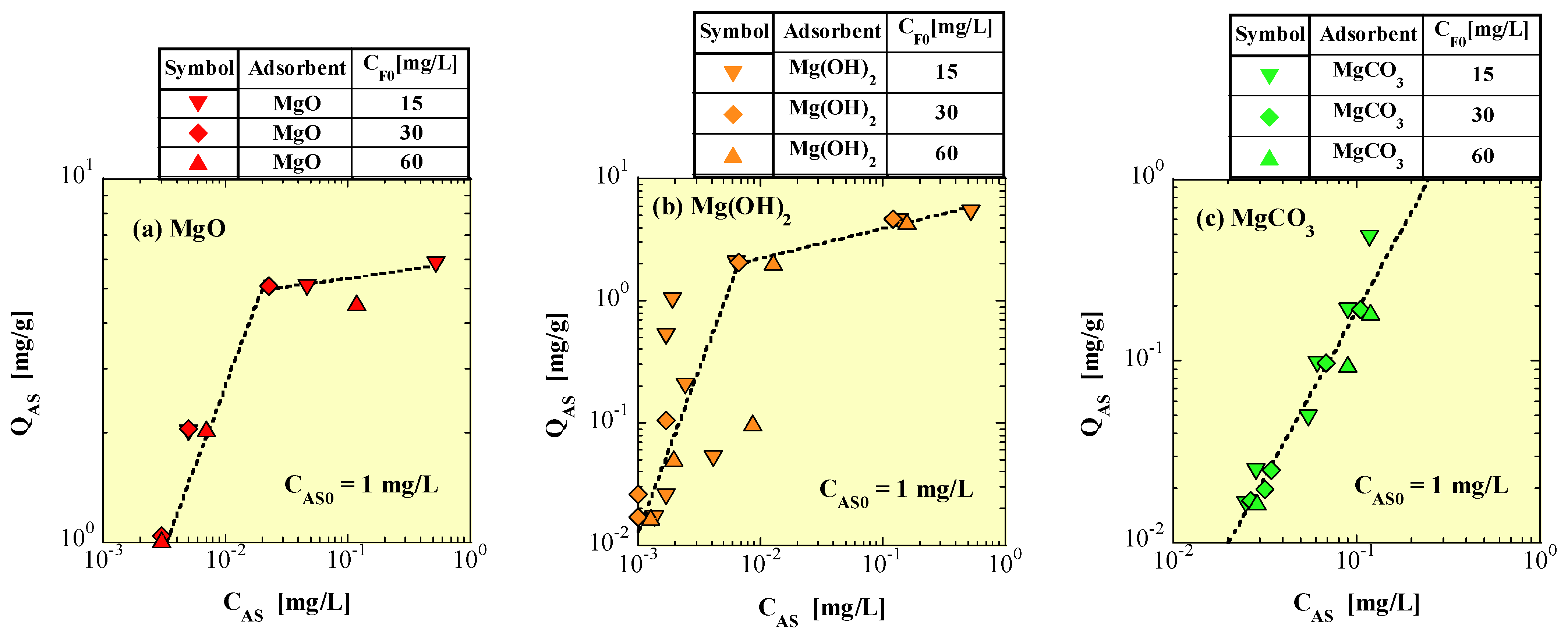
4.5. Langmuir Isotherm Model
4.6. Freundlich Isotherm Model
4.7. Correlation of pHf with CMg and ORPf
4.8. Removal Mechanisms of As(V) and F
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, C.; Bai, G.; Liu, X.; Li, Y. Screening high-fluoride and high-arsenic drinking waters and surveying endemic fluorosis and arsenism in Shaanxi province in western China. Water Res. 2006, 40, 3015–3022. [Google Scholar] [CrossRef] [PubMed]
- Armienta, M.A.; Segovia, N. Arsenic and fluoride in the groundwater of Mexico. Environ. Geochem. Health 2008, 30, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.; Masuda, H.; Siddiqui, R.; Naseem, M. Sources of Arsenic and Fluoride in Highly Contaminated Soils Causing Groundwater Contamination in Punjab, Pakistan. Arch. Environ. Contam. Toxicol. 2009, 56, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Ingallinella, A.M.; Pacini, V.A.; Fernandez, R.G.; Vidoni, R.M.; Sanguinetti, G. Simultaneous removal of arsenic and fluoride from groundwater by coagulation-adsorption with polyaluminum chloride. J. Environ. Sci. Health A 2011, 46, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Herrera, M.T.; Bundschuh, J.; Nath, B.; Nicollid, H.B.; Gutierrez, M.; Reyes-Gomez, V.M.; Nunez, D.; Martín-Dominguez, I.R.; Sracek, O. Co-occurrence of arsenic and fluoride in groundwater of semi-arid regions in Latin America: Genesis, mobility and remediation. J. Hazard. Mater. 2013, 262, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Navarro, O.; González, J.; Júnez-Ferreira, H.E.; Bautista, C.-F.; Cardona, A. Correlation of Arsenic and Fluoride in the groundwater for human consumption in a semiarid region of Mexico. Procedia Eng. 2017, 186, 333–340. [Google Scholar] [CrossRef]
- Jha, P.K.; Tripathi, P. Arsenic and fluoride contamination in groundwater: A review of global scenarios with special reference to India. Groundw. Sustain. Dev. 2021, 13, 10057. [Google Scholar] [CrossRef]
- The World Health Organization (WHO). Guidelines for Drinking-Water Quality, 4th ed.; Arsenic, 315-318; WHO: Tarxien, Malta, 2011; ISBN 978-92-4-154815-1. Available online: https://apps.who.int/iris/bitstream/handle/10665/44584/9789241548151_eng.pdf (accessed on 10 December 2023).
- Jadhav, S.V.; Bringas, E.; Yadav, G.D.; Rathod, V.K.; Ortiz, I.; Marathe, K.V. Arsenic and fluoride contaminated groundwaters: A review of current technologies for contaminants removal. J. Environ. Manag. 2015, 162, 306–325. [Google Scholar] [CrossRef]
- Sarkar, A.; Paul, B. The global menace of arsenic and its conventional remediation—A critical review. Chemosphere 2016, 158, 37–49. [Google Scholar] [CrossRef]
- Ghosh, S.; Debsarkar, A.; Dutta, A. Technology alter-natives for decontamination of arsenic-rich groundwater—A critical review. Environ. Technol. Innov. 2019, 13, 277–303. [Google Scholar] [CrossRef]
- Kumar, R.; Patel, M.; Singh, P.; Bundschuh, J.; Pittman, C.U., Jr.; Trakal, L.; Mohan, D. Emerging technologies for arsenic removal from drinking water in rural and peri-urban areas: Methods, experience from, and options for Latin America. Sci. Total Environ. 2019, 694, 133427. [Google Scholar] [CrossRef]
- Yu, X.Y.; Luo, T.; Jia, Y.; Zhang, Y.X.; Liu, J.H.; Huang, X.J. Porous hierarchically micro-/nanostructured MgO: Morphology control and their excellent performance in As(III) and As(V) removal. J. Phys. Chem. C 2011, 115, 22242–22250. [Google Scholar] [CrossRef]
- Lin, Q.; Chen, W.; Lin, F.; Zhu, H.; Wang, X. Exaggerated arsenic removal efficiency and pH adaptability by adsorption using monodispersed porous pinecone-like magnesium hydroxide. AQUA 2023, 72, 969–982. [Google Scholar] [CrossRef]
- Mohapatra, M.; Anand, S.; Mishra, B.K.; Giles, D.E.; Singh, P. Review of fluoride removal from drinking water. J. Environ. Manag. 2009, 91, 67–77. [Google Scholar] [CrossRef]
- He, J.; Yang, Y.; Wu, Z.; Xie, C.; Zhang, K.; Kong, L.; Liu, J. Review of fluoride removal from water environment by adsorption. J. Environ. Chem. Eng. 2020, 8, 104516. [Google Scholar] [CrossRef]
- Gai, W.-Z.; Deng, Z.-Y. A comprehensive review of adsorbents for fluoride removal from water: Performance, water quality assessment and mechanism. Environ. Sci. Water Res. Technol. 2021, 7, 1362–1386. [Google Scholar] [CrossRef]
- Pillai, P.; Dharaskar, S.; Pandian, S.; Panchal, H. Overview of fluoride removal from water using separation techniques. Environ. Technol. Innov. 2021, 21, 101246. [Google Scholar] [CrossRef]
- Jin, Z.; Jia, Y.; Zhang, K.-S.; Kong, L.-T.; Sun, B.; Shen, W.; Meng, F.-L.; Liu, J.-H. Effective removal of fluoride by porous MgO nanoplates and its adsorption mechanism. Appl. Surf. Sci. 2015, 357, 1080–1088. [Google Scholar] [CrossRef]
- Wallace, A.R.; Su, C.; Sun, W. Adsorptive removal of fluoride from water using nanomaterials of ferrihydrite, apatite, and brucite: Batch and column studies. Environ. Eng. Sci. 2019, 36, 634–642. [Google Scholar] [CrossRef]
- Lopez-Guzman, M.; Alarcon-Herrera, M.T.; Irigoyen-Campuzano, J.R.; Torres-Castanon, L.A.; Reynoso-Cuevas, L. Simultaneous removal of fluoride and arsenic from well water by electrocoagulation. Sci. Total Environ. 2019, 678, 181–187. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Trikkaliotis, D.G.; Kyzas, G.Z.; Katsoyiannis, I.A.; Deliyanni, E.A. Simultaneous Removal of As(III) and Fluoride Ions from Water Using Manganese Oxide Supported on Graphene Nanostructures (GO-MnO2). Sustainability 2023, 15, 1179. [Google Scholar] [CrossRef]
- Sugita, H.; Oguma, T.; Zhang, M.; Hara, J.; Takahashi, S. Environmental stability of spent magnesium-based and calcium-based arsenic adsorbents-Effects of soils. J. Jpn. Soc. Civ. Eng. Ser. G Environ. Res. 2016, 72, 437–448. [Google Scholar] [CrossRef]
- Rojo, J.M.; Mesa, J.L.; Pizarro, J.L.; Lezama, L.; Arriortua, M.I.; Rojo, T. Spectroscopic and Magnetic Study of the (Mg,M)3(AsO4)2·8H2O (M = Ni2+, Co2+) Arsenates. Mater. Res. Bull. 1996, 31, 925–934. [Google Scholar] [CrossRef]
- Ferraris, G.; Franchini-Angela, M. Hydrogen Bonding in the Crystalline State. Crystal Structure of MgHAsO4·7H2O, Roesslerite. Acta. Crystallogr. B 1973, 29, 286–292. [Google Scholar] [CrossRef]
- Baur, W.H.; Khan, A.A. Rutile-type Compounds. IV. SiO2, GeO2 and a Comparison with Other Rutile-type Structures. Acta Crystallogr. B 1971, 27, 2133–2139. [Google Scholar] [CrossRef]
- Crichton, W.A.; Parise, J.B.; Muller, H.; Breger, J.; Marshall, W.G.; Welch, M.D. Synthesis and Structure of Magnesium Hydroxide Fluoride, Mg(OH)F: A Topological Intermediate Between Brucite- and Rutile-type Structures. Mineral. Mag. 2012, 76, 25–36. [Google Scholar] [CrossRef]
- The Chemical Society of Japan (CSJ). Kagaku Binran (Handbook of Chemistry), Pure Chemistry II, 5th ed.; Maruzen: Tokyo, Japan, 2004; p. 332. [Google Scholar]











| No. | Adsorbent | αMg (%) | P (%) | Dp50 (μm) | SBET (m2/g) |
|---|---|---|---|---|---|
| (1) 1 | MgO | 59.1 | 98.0 | 1.54 | 4.3 |
| (2) 1 | Mg(OH)2 | 40.6 | 97.3 | 4.13 | 22.0 |
| (3) 1 | MgCO3 | 24.8 | 86.1 | 15.0 | 26.0 |
| Figure No. | Adsorbent | QAS-MAX (mg/g) | KL | r |
|---|---|---|---|---|
| Figure 11a | MgO | 8.69 * | 48.7 * | 0.988 * |
| Figure 11b | Mg(OH)2 | –0.284 ** | –66.9 ** | 0.791 |
| Figure 11c | MgCO3 | –14.0 ** | –7.40 ** | 0.976 |
| Figure No. | Adsorbent | QF-MAX (mg/g) | KL | r |
|---|---|---|---|---|
| Figure 12a | MgO | 33.1 * | 3.62 * | 0.991 * |
| Figure 12b | Mg(OH)2 | 5.84 | 0.161 | 0.991 |
| Figure 12c | MgCO3 | 1.74 | 0.0586 | 0.964 |
| Figure No. | Adsorbent | KF | n | r |
|---|---|---|---|---|
| Figure 13a | MgO | 162 | 1.12 | 0.965 |
| Figure 13b | Mg(OH)2 | 6.86 * | 4.10 * | 0.964 * |
| Figure 13c | MgCO3 | 12.6 | 0.547 | 0.856 |
| Figure No. | Adsorbent | KF | n | r |
|---|---|---|---|---|
| Figure 14a | MgO | 18.0 * | 4.89 * | 0.942 * |
| Figure 14b | Mg(OH)2 | 0.746 | 1.45 | 0.846 |
| Figure 14c | MgCO3 | 0.108 | 1.31 | 0.973 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugita, H.; Morimoto, K.; Saito, T.; Hara, J. Simultaneous Removal of Arsenate and Fluoride Using Magnesium-Based Adsorbents. Sustainability 2024, 16, 1774. https://doi.org/10.3390/su16051774
Sugita H, Morimoto K, Saito T, Hara J. Simultaneous Removal of Arsenate and Fluoride Using Magnesium-Based Adsorbents. Sustainability. 2024; 16(5):1774. https://doi.org/10.3390/su16051774
Chicago/Turabian StyleSugita, Hajime, Kazuya Morimoto, Takeshi Saito, and Junko Hara. 2024. "Simultaneous Removal of Arsenate and Fluoride Using Magnesium-Based Adsorbents" Sustainability 16, no. 5: 1774. https://doi.org/10.3390/su16051774
APA StyleSugita, H., Morimoto, K., Saito, T., & Hara, J. (2024). Simultaneous Removal of Arsenate and Fluoride Using Magnesium-Based Adsorbents. Sustainability, 16(5), 1774. https://doi.org/10.3390/su16051774






