Sustainability across the Medical Device Lifecycle: A Scoping Review
Abstract
1. Introduction
1.1. Global Emissions, Net Zero Policies, and the Healthcare Industry
1.2. Our Aim
- To systematically identify research studies addressing sustainability in medical design, development, manufacturing, distribution, utilisation, management, maintenance, and decommissioning.
- To map the included studies to the different stages of the medical lifecycle, from design to decommissioning.
- To identify strengths and gaps in the current research on the topic and highlight areas that require further inquiry and possible solutions.
1.3. Sustainability: A Catchall Term
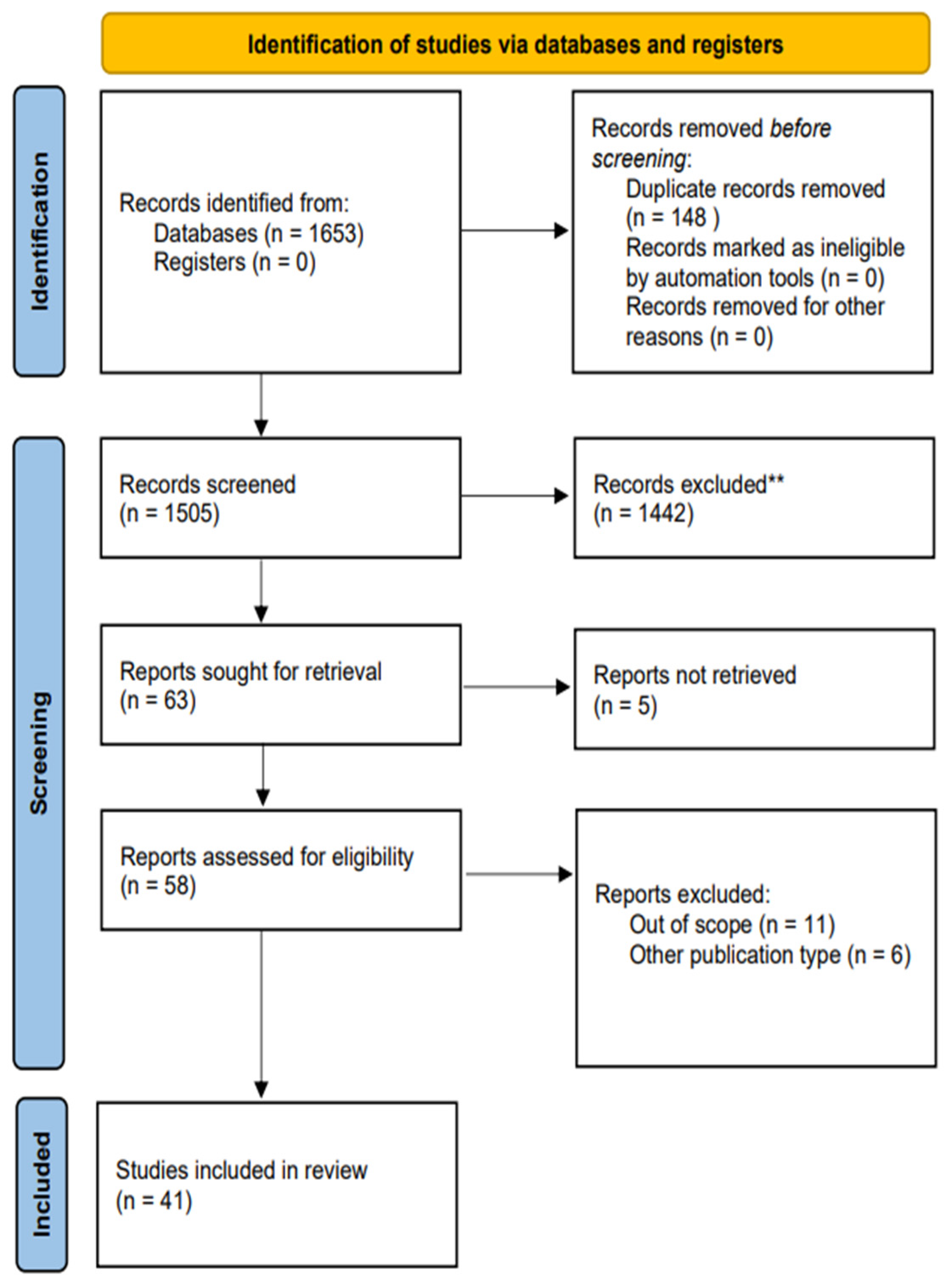
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
- P—MDs and equipment.
- C—sustainability (economic, social, and environmental) through the lifecycle of MDs.
- C—globally.
2.2. Data Extraction
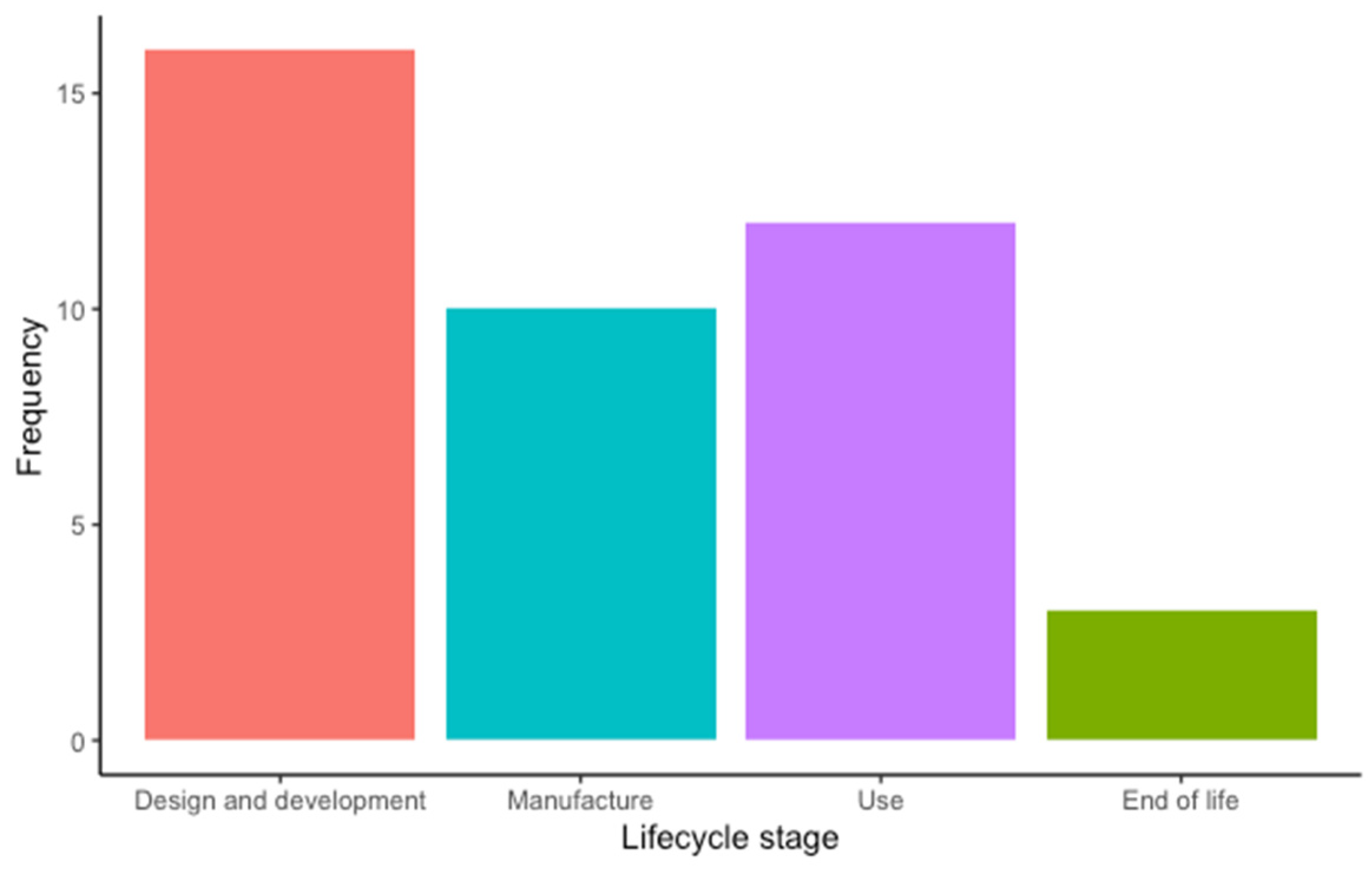
- Design and development. This stage includes the product design and development methodologies (e.g., ecodesign), material/product design and development, feasibility assessment, and prototyping.
- Manufacture. This stage includes sourcing raw materials, supplier selection, production, and distribution.
- Use. This stage includes operation, reuse, and reprocessing.
- End of life. This stage includes decommissioning, disposal, and recycling.
2.3. Data Synthesis
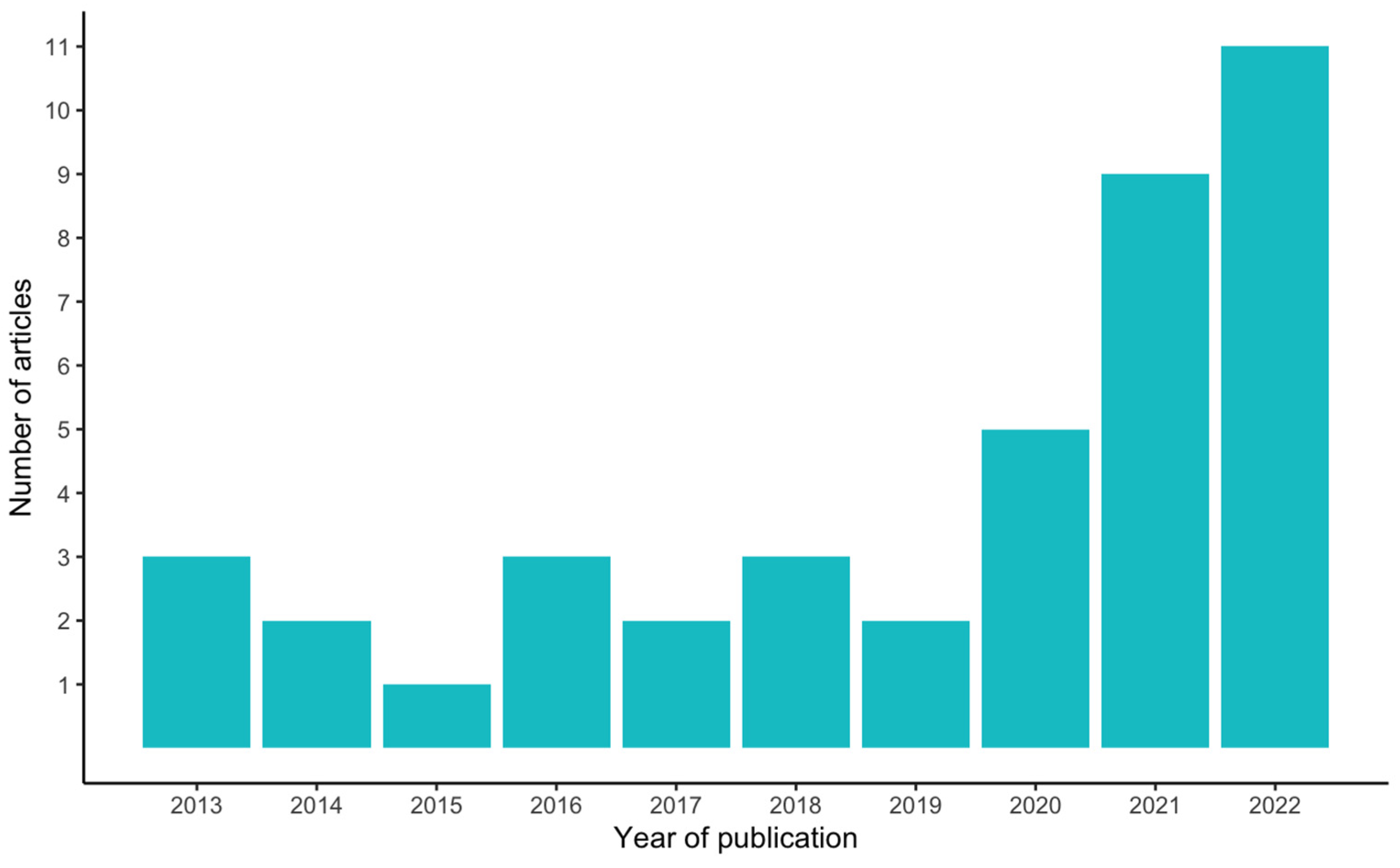
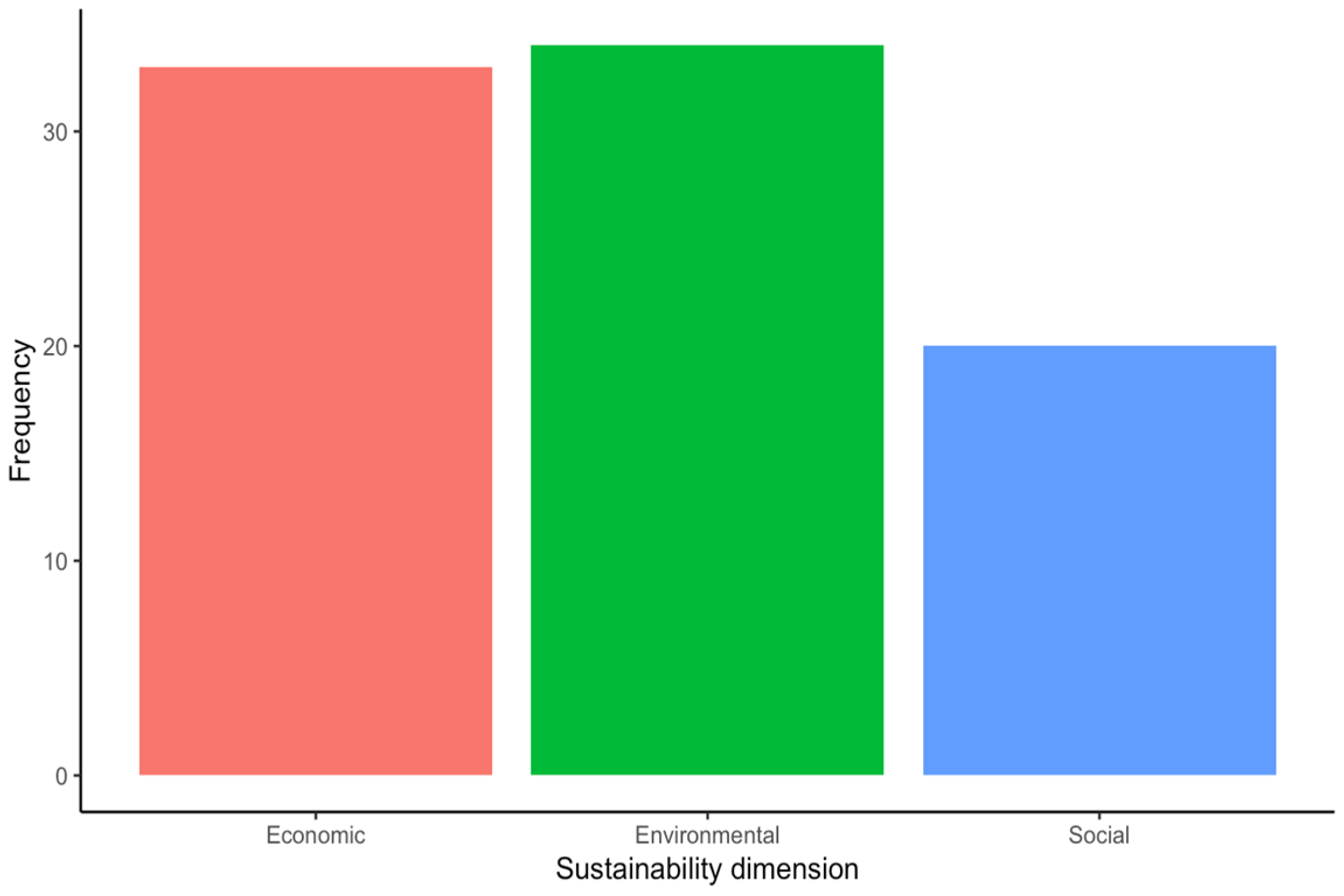
3. Results
3.1. Design and Development
3.1.1. Ecodesign, Sustainable Design, and Frugal Engineering
3.1.2. Energy Harvesting and Efficiency Management
3.1.3. Material Selection and Reduction
3.2. Manufacture
3.2.1. Production
3.2.2. Supply Chain
3.2.3. Supplier Selection
3.2.4. Distribution
3.3. Use
3.4. End of Life
4. Discussion
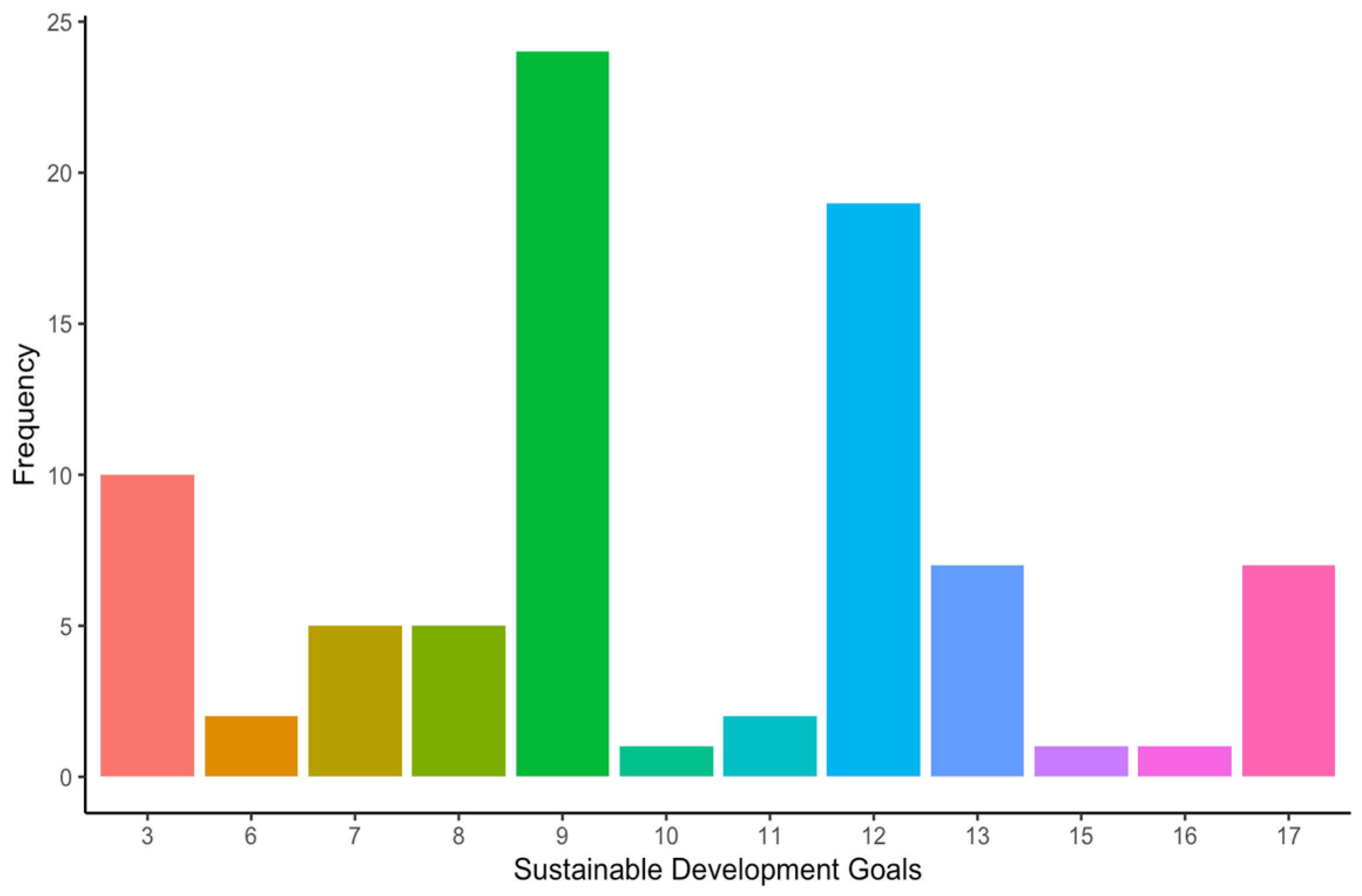
- SDG#9: industry, innovation, and infrastructure (28.6% of the occurrences).
- SDG#12: responsible consumption and production (22.6% of the occurrences).
- SDG#3: good health and well-being (11.9% of the occurrences).
- (a)
- Design and development
- (i)
- Context-aware, lifecycle-aware design and development. This principle refers to incorporating sustainability considerations during the product design and development process using eco-, sustainable, and frugal design approaches. This principle loosely matches R1, which refers to rethinking a product to make it more sustainable by design.
- (ii)
- Energy considerations include eliminating the need for external supplies to power MDs by harvesting energy from the body or optimising energy usage through electronics design and microprogramming. This principle matches R2, which includes increasing efficiency in product manufacturing and use by consuming fewer resources.
- (iii)
- Material reduction and selection, including optimising the amount of materials in MDs and using more recyclable materials (e.g., less single-use plastics). The latter matches R8, which refers to processing materials to obtain high- or lower-grade materials, while the former matches R2, which also includes consuming fewer materials to manufacture a product.
- (b)
- Manufacture
- (i)
- Energy savings in production processes. While the included studies refer specifically to reducing energy usage during production, this principle can be extended to lowering all the required resources (e.g., energy and water). Hence, this principle aligns with R2, which includes increasing the efficiency of producing MDs by consuming fewer resources.
- (ii)
- Supply chain management, which considers optimising the entire supply chain to reduce the resources involved in product manufacturing, from supplying raw materials to distributing finished products. This principle also aligns with R2.
- (c)
- Use
- (i)
- Reusable vs. disposable. This principle refers to reusing MDs after proper sterilisation procedures whenever possible, which directly matches R3. Needless to say, scientific and clinical evidence must support the decision to reuse specific MDs to meet applicable safety standards.
- (ii)
- Adapting existing solutions to current needs and customer demands. This principle can align with R1, which considers using the same product for different functions, thus eliminating the need for a new product, or with R7, which considers using a discarded product (or its parts) in a new product with a different function.
- (iii)
- Repairing MDs based on decision-making tools, which extends the service life of MDs, reducing the need for new products. This principle aligns with R4 repair and R5 refurbish.
- (d)
- End of life
- (i)
- Repurposing. This principle aligns directly with R7 repurposing, which considers using a discarded product or its parts in a new product with a different function.
- (ii)
- Designing and implementing a take-back system for single-use MDs. Take-back systems aim to reduce MD manufacturers’ environmental impacts and increase efficiency and economic value by recycling or remanufacturing products or their materials. This principle aligns with R6 to R8, which includes remanufacturing, repurposing, and recycling.
- (iii)
- Improving waste management and communication between medical centres and waste centres. This principle aligns with R8 and R9 as it focuses on recovering the waste generated by MDs to recycle some of their materials or recovering energy from their incineration.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erb, T.; Perciasepe, B.; Radulovic, V.; Niland, M. Corporate Climate Commitments: The Trend Towards Net Zero. In Handbook of Climate Change Mitigation and Adaptation; Lackner, M., Sajjadi, B., Chen, W.-Y., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 2985–3018. ISBN 978-3-030-72578-5. [Google Scholar]
- Savaresi, A. The Paris Agreement: A New Beginning? J. Energy Nat. Resour. Law 2016, 34, 16–26. [Google Scholar] [CrossRef]
- Smil, V. Energy and Civilization: A History; The MIT Press: Cambridge, MA, USA, 2017; ISBN 978-0-262-03577-4. [Google Scholar]
- Della Vigna, M.; Stavrinou, Z.; Bhandari, N.; Neil, M. Brian Singer Carbonomics the Future of Energy in the Age of Climate Change; The Goldman Sachs Group, Inc.: New York, NY, USA, 2019. [Google Scholar]
- Hagens, N. Economics for the future—Beyond the superorganism. Ecol. Econ. 2020, 169, 106520. [Google Scholar] [CrossRef]
- Alvis, S.; Murphy, L.; Martínez, L.C.; Emden, J.; Jung, C. The End of Greenwashing—Driving Decarbonisation in the Real Economy; Institute for Public Policy Research: London, UK, 2023. [Google Scholar]
- Cui, R.Y.; Hultman, N.; Edwards, M.R.; He, L.; Sen, A.; Surana, K.; McJeon, H.; Iyer, G.; Patel, P.; Yu, S.; et al. Quantifying Operational Lifetimes for Coal Power Plants under the Paris Goals. Nat. Commun. 2019, 10, 4759. [Google Scholar] [CrossRef] [PubMed]
- Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.W.; Trisos, C.; Romero, J.; Aldunce, P.; Barrett, K.; Blanco, G.; et al. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2023. [Google Scholar]
- Statista Global CO2 Emissions by Year 1940–2023. Available online: https://www.statista.com/statistics/276629/global-co2-emissions/ (accessed on 31 January 2024).
- Lindsey, R. Climate Change: Atmospheric Carbon Dioxide. Available online: https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide (accessed on 31 January 2024).
- Ritchie, H.; Roser, M. CO₂ Emissions; Our World in Data: Oxford, UK, 2024. [Google Scholar]
- Kent, C. How Hospitals and the Medtech Sector Are Tackling the Climate Crisis. Medical Device Network 2021. Available online: https://www.medicaldevice-network.com/features/hospitals-medtech-environment/ (accessed on 15 January 2024).
- Sousa, A.C.; Veiga, A.; Maurício, A.C.; Lopes, M.A.; Santos, J.D.; Neto, B. Assessment of the environmental impacts of medical devices: A review. Environ. Dev. Sustain. 2021, 23, 9641–9666. [Google Scholar] [CrossRef]
- Karliner, J.; Slotterback, S.; Boyd, R.; Ashby, B.; Steele, K.; Wang, J. Health Care’s Climate Footprint: The Health Sector Contribution and Opportunities for Action; Health Care Without Harm: Washington, DC, USA, 2019. [Google Scholar]
- MacNeill, A.J.; Hopf, H.; Khanuja, A.; Alizamir, S.; Bilec, M.; Eckelman, M.J.; Hernandez, L.; McGain, F.; Simonsen, K.; Thiel, C.; et al. Transforming the Medical Device Industry: Road Map to a Circular Economy. Health Aff. 2020, 39, 2088–2097. [Google Scholar] [CrossRef]
- Belkhir, L.; Elmeligi, A. Carbon Footprint of the Global Pharmaceutical Industry and Relative Impact of Its Major Players. J. Clean. Prod. 2019, 214, 185–194. [Google Scholar] [CrossRef]
- Gavurova, B.; Rigelsky, M.; Ivankova, V. Greenhouse Gas Emissions and Health in the Countries of the European Union. Front. Public Health 2021, 9, 756652. [Google Scholar] [CrossRef]
- McAlister, S.; Morton, R.L.; Barratt, A. Incorporating Carbon into Health Care: Adding Carbon Emissions to Health Technology Assessments. Lancet Planet. Health 2022, 6, e993–e999. [Google Scholar] [CrossRef] [PubMed]
- Roostaie, S.; Nawari, N.; Kibert, C. Sustainability and Resilience: A Review of Definitions, Relationships, and Their Integration into a Combined Building Assessment Framework. Build. Environ. 2019, 154, 132–144. [Google Scholar] [CrossRef]
- Warde, P. The Invention of Sustainability: Nature and Destiny, c.1500–1870, 1st ed.; Cambridge University Press: Cambridge, UK, 2018; ISBN 978-1-316-58476-7. [Google Scholar]
- Brundtland, G.H. Our Common Future; World Commission on Environment and Development: London, UK, 1987. [Google Scholar]
- Jonas, H. The Imperative of Responsibility. In Search of an Ethics for the Technological Age; University of Chicago Press: Chicago, IL, USA, 1985; ISBN 978-0-226-40597-1. [Google Scholar]
- Elkington, J. Towards the Sustainable Corporation: Win-Win-Win Business Strategies for Sustainable Development. Calif. Manag. Rev. 1994, 36, 90–100. [Google Scholar] [CrossRef]
- Fankhauser, S.; Smith, S.M.; Allen, M.; Axelsson, K.; Hale, T.; Hepburn, C.; Kendall, J.M.; Khosla, R.; Lezaun, J.; Mitchell-Larson, E.; et al. The Meaning of Net Zero and How to Get It Right. Nat. Clim. Chang. 2022, 12, 15–21. [Google Scholar] [CrossRef]
- Lockwood, C.; dos Santos, K.B.; Pap, R. Practical Guidance for Knowledge Synthesis: Scoping Review Methods. Asian Nurs. Res. 2019, 13, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.; Sutton, A.; Papaioannou, D. Systematic Approaches to a Successful Literature Review, 2nd ed.; Sage: Los Angeles, LA, USA, 2016; ISBN 978-1-4739-1245-8. [Google Scholar]
- Popay, J.; Roberts, H.; Sowden, A.; Petticrew, M.; Arai, L.; Rodgers, M.; Britten, N. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. A Product from the ESRC Methods Programme Version. 2006. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=ed8b23836338f6fdea0cc55e161b0fc5805f9e27 (accessed on 15 January 2024).
- Hamner, S.R.; Narayan, V.G.; Donaldson, K.M. Designing for Scale: Development of the ReMotion Knee for Global Emerging Markets. Ann. Biomed. Eng. 2013, 41, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Hede, S.; Nunes, M.J.L.; Ferreira, P.F.V.; Rocha, L.A. Incorporating Sustainability in Decision-Making for Medical Device Development. Technol. Soc. 2013, 35, 276–293. [Google Scholar] [CrossRef]
- MacVittie, K.; Halámek, J.; Halámková, L.; Southcott, M.; Jemison, W.D.; Lobel, R.; Katz, E. From “Cyborg” Lobsters to a Pacemaker Powered by Implantable Biofuel Cells. Energy Environ. Sci. 2013, 6, 81–86. [Google Scholar] [CrossRef]
- Hamza, A.O.; Garma, F.B.B.; Eisa, B.I.B.; Almahdi, O.A.B.; Awadalla, A.M.B.; Dafaallah, A.S.B. Application of Solar Energy in Medical Instrumentation: Microscope. J. Clin. Eng. 2014, 39, 132–135. [Google Scholar] [CrossRef]
- Unger, S.R.; Landis, A.E. Comparative Life Cycle Assessment of Reused Versus Disposable Dental Burs. Int. J. Life Cycle Assess. 2014, 19, 1623–1631. [Google Scholar] [CrossRef]
- Gupta, R.; Patel, R.; Murty, N.; Panicker, R.; Chen, J. Developing Sustainable Global Health Technologies: Insight from an Initiative to Address Neonatal Hypothermia. J. Public Health Policy 2015, 36, 24–40. [Google Scholar] [CrossRef]
- Izadikhah, M.; Saen, R.F. Evaluating Sustainability of Supply Chains by Two-Stage Range Directional Measure in the Presence of Negative Data. Transp. Res. Part D Transp. Environ. 2016, 49, 110–126. [Google Scholar] [CrossRef]
- Moultrie, J.; Sutcliffe, L.; Maier, A. A Maturity Grid Assessment Tool for Environmentally Conscious Design in the Medical Device Industry. J. Clean. Prod. 2016, 122, 252–265. [Google Scholar] [CrossRef]
- Unger, S.; Landis, A. Assessing the Environmental, Human Health, and Economic Impacts of Reprocessed Medical Devices in a Phoenix Hospital’s Supply Chain. J. Clean. Prod. 2016, 112, 1995–2003. [Google Scholar] [CrossRef]
- Barbero, S.; Pereno, A.; Tamborrini, P. Systemic Innovation in Sustainable Design of Medical Devices. Des. J. 2017, 20, S2486–S2497. [Google Scholar] [CrossRef]
- Unger, S.R.; Hottle, T.A.; Hobbs, S.R.; Thiel, C.L.; Campion, N.; Bilec, M.M.; Landis, A.E. Do Single-Use Medical Devices Containing Biopolymers Reduce the Environmental Impacts of Surgical Procedures Compared with Their Plastic Equivalents? J. Health Serv. Res. Policy 2017, 22, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, J.; Littlewood, J.; Wilgeroth, P. Development of a Framework of Key Performance Indicators to Identify Reductions in Energy Consumption in a Medical Devices Production Facility. Int. J. Ambient. Energy 2018, 39, 202–210. [Google Scholar] [CrossRef]
- Ghadimi, P.; Ghassemi Toosi, F.; Heavey, C. A Multi-Agent Systems Approach for Sustainable Supplier Selection and Order Allocation in a Partnership Supply Chain. Eur. J. Oper. Res. 2018, 269, 286–301. [Google Scholar] [CrossRef]
- Mann, H.; Mann, I.; Gullaiya, N. A Case in Medical Equipment Design for Strategic Sustainability. South Asian J. Bus. Manag. Cases 2018, 7, 111–119. [Google Scholar] [CrossRef]
- Ghadimi, P.; Wang, C.; Lim, M.K.; Heavey, C. Intelligent Sustainable Supplier Selection Using Multi-Agent Technology: Theory and Application for Industry 4.0 Supply Chains. Comput. Ind. Eng. 2019, 127, 588–600. [Google Scholar] [CrossRef]
- Magno, M.; Salvatore, G.A.; Jokic, P.; Benini, L. Self-Sustainable Smart Ring for Long-Term Monitoring of Blood Oxygenation. IEEE Access 2019, 7, 115400–115408. [Google Scholar] [CrossRef]
- Donahue, L.M.; Hilton, S.; Bell, S.G.; Williams, B.C.; Keoleian, G.A. A Comparative Carbon Footprint Analysis of Disposable and Reusable Vaginal Specula. Am. J. Obstet. Gynecol. 2020, 223, 225.e1–225.e7. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xu, S.; Liu, Y.; Gao, Y.; Tong, T.; Qi, Y.; Zhang, C. Flexible Drug Release Device Powered by Triboelectric Nanogenerator. Adv. Funct. Mater. 2020, 30, 1909886. [Google Scholar] [CrossRef]
- Lyne, A.; Ashley, P.; Saget, S.; Costa, M.P.; Underwood, B.; Duane, B. Combining Evidence-Based Healthcare with Environmental Sustainability: Using the Toothbrush as a Model. Br. Dent. J. 2020, 229, 303–309. [Google Scholar] [CrossRef]
- Riutord-Sbert, P.; De Pedro Gómez, J.E.; Pereira, T.C.; López-Safont, N.; García-Mosquera, I.; Jiménez-Recaredo, J.; Alomar-Velasco, P.J.; Paublini-Oliveira, H.J.; Dominguez-Pérez, J.; González-Carrasco, D.; et al. An Innovative, Reusable and Sustainable Face-Seal Device to Improve Protection Efficacy of Surgical Masks against COVID-19. Med. Balear. 2020, 35, 74–77. [Google Scholar] [CrossRef]
- Xu, Z.; Jin, C.; Cabe, A.; Escobedo, D.; Hao, N.; Trase, I.; Closson, A.B.; Dong, L.; Nie, Y.; Elliott, J.; et al. Flexible Energy Harvester on a Pacemaker Lead Using Multibeam Piezoelectric Composite Thin Films. ACS Appl. Mater. Interfaces 2020, 12, 34170–34179. [Google Scholar] [CrossRef]
- Alrashoudi, A.A.; Albalawi, H.I.; Aldoukhi, A.H.; Moretti, M.; Bilalis, P.; Abedalthagafi, M.; Hauser, C.A.E. Fabrication of a Lateral Flow Assay for Rapid In-Field Detection of COVID-19 Antibodies Using Additive Manufacturing Printing Technologies. Int. J. Bioprint. 2021, 7, 399. [Google Scholar] [CrossRef]
- Bayrak, T.; Soylu, S.I. Reprocessing of Single Use Medical Devices: A New Proposal for a Regulation. Health Policy Technol. 2021, 10, 100553. [Google Scholar] [CrossRef]
- Haber, N.; Fargnoli, M. Sustainable Product-Service Systems Customization: A Case Study Research in the Medical Equipment Sector. Sustainability 2021, 13, 6624. [Google Scholar] [CrossRef]
- Hanani, Z.; Izanzar, I.; Amjoud, M.; Mezzane, D.; Lahcini, M.; Uršič, H.; Prah, U.; Saadoune, I.; El Marssi, M.; Luk’Yanchuk, I.A.; et al. Lead-Free Nanocomposite Piezoelectric Nanogenerator Film for Biomechanical Energy Harvesting. Nano Energy 2021, 81, 105661. [Google Scholar] [CrossRef]
- Hasani, A.; Mokhtari, H.; Fattahi, M. A Multi-Objective Optimization Approach for Green and Resilient Supply Chain Network Design: A Real-Life Case Study. J. Clean. Prod. 2021, 278, 123199. [Google Scholar] [CrossRef]
- Liao, H.-Y.; Cade, W.; Behdad, S. Markov Chain Optimization of Repair and Replacement Decisions of Medical Equipment. Resour. Conserv. Recycl. 2021, 171, 105609. [Google Scholar] [CrossRef]
- Piaggio, D.; Namm, G.; Melillo, P.; Simonelli, F.; Iadanza, E.; Pecchia, L. Pupillometry via smartphone for low-resource settings. Biocybern. Biomed. Eng. 2021, 41, 891–902. [Google Scholar] [CrossRef]
- Szmelter-Jarosz, A.; Ghahremani-Nahr, J.; Nozari, H. A Neutrosophic Fuzzy Optimisation Model for Optimal Sustainable Closed-Loop Supply Chain Network during COVID-19. J. Risk Financ. Manag. 2021, 14, 519. [Google Scholar] [CrossRef]
- van Straten, B.; Ligtelijn, S.; Droog, L.; Putman, E.; Dankelman, J.; Weiland, N.H.S.; Horeman, T. A Life Cycle Assessment of Reprocessing Face Masks during the COVID-19 Pandemic. Sci. Rep. 2021, 11, 17680. [Google Scholar] [CrossRef]
- Benedettini, O. Green Servitization in the Single-Use Medical Device Industry: How Device OEMs Create Supply Chain Circularity through Reprocessing. Sustainability 2022, 14, 12670. [Google Scholar] [CrossRef]
- Epelle, E.I.; Macfarlane, A.; Cusack, M.; Burns, A.; Mackay, W.G.; Rateb, M.E.; Yaseen, M. Application of Ultraviolet-C Radiation and Gaseous Ozone for Microbial Inactivation on Different Materials. ACS Omega 2022, 7, 43006–43021. [Google Scholar] [CrossRef] [PubMed]
- Fargnoli, M.; Haber, N.; Tronci, M. Case Study Research to Foster the Optimization of Supply Chain Management through the PSS Approach. Sustainability 2022, 14, 2235. [Google Scholar] [CrossRef]
- Goli, A.; Sadeghi, P. Evaluation on the Use of COVID-19 Single-Use Face Masks to Improve the Properties of Hot Mix Asphalt. Road Mater. Pavement Des. 2022, 24, 1371–1388. [Google Scholar] [CrossRef]
- Le, H.T.; Le Quoc, K.; Nguyen, T.A.; Dang, K.T.; Vo, H.K.; Luong, H.H.; Le Van, H.; Gia, K.H.; Phu, L.V.C.; Quoc, D.N.T.; et al. Medical-Waste Chain: A Medical Waste Collection, Classification and Treatment Management by Blockchain Technology. Computers 2022, 11, 113. [Google Scholar] [CrossRef]
- Mallick, P.K.; Salling, K.B.; Pigosso, D.C.A.; McAloone, T.C. Designing Take-Back for Single Use Medical Devices: The Case of ReturpenTM. J. Diabetes Sci. Technol. 2022, 16, 1363–1369. [Google Scholar] [CrossRef]
- Nayeri, S.; Sazvar, Z.; Heydari, J. A Global-Responsive Supply Chain Considering Sustainability and Resiliency: Application in the Medical Devices Industry. Socio-Econ. Plan. Sci. 2022, 82, 101303. [Google Scholar] [CrossRef]
- Rostami, O.; Tavakoli, M.; Tajally, A.; GhanavatiNejad, M. A Goal Programming-Based Fuzzy Best–Worst Method for the Viable Supplier Selection Problem: A Case Study. Soft Comput. 2022, 27, 2827–2852. [Google Scholar] [CrossRef]
- Rouvière, N.; Chkair, S.; Auger, F.; Alovisetti, C.; Bernard, M.; Cuvillon, P.; Kinowski, J.-M.; Leguelinel-Blache, G.; Chasseigne, V. Ecoresponsible actions in Operating Rooms: A Health Ecological and Economic Evaluation. Int. J. Surg. 2022, 101, 106637. [Google Scholar] [CrossRef]
- Williams, E.; Piaggio, D.; Andellini, M.; Pecchia, L. 3D-Printed Activated Charcoal Inlet Filters for Oxygen Concentrators: A Circular Economy Approach. Dev. Eng. 2022, 7, 100094. [Google Scholar] [CrossRef]
- Abad, A.R.K.K.; Barzinpour, F.; Pasandideh, S.H.R. A Novel Separate Chance-Constrained Programming Model to Design a Sustainable Medical Ventilator Supply Chain Network during the COVID-19 Pandemic. J. Ind. Manag. Optim. 2023, 19, 1395. [Google Scholar] [CrossRef]
- Weyrauch, T.; Herstatt, C. What is Frugal Innovation? Three Defining Criteria. J. Frugal Innov. 2017, 2, 1. [Google Scholar] [CrossRef]
- Jain, V.; Wadhwa, S.; Deshmukh, S. Select Supplier-Related Issues in Modelling a Dynamic Supply Chain: Potential, Challenges and Direction for Future Research. Int. J. Prod. Res. 2009, 47, 3013–3039. [Google Scholar] [CrossRef]
- Ivanov, D.; Dolgui, A. Viability of Intertwined Supply Networks: Extending the Supply Chain Resilience Angles Towards Survivability. A Position Paper Motivated by COVID-19 Outbreak. Int. J. Prod. Res. 2020, 58, 2904–2915. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.; Trivedi, A.; Trivedi, A. Sustainable Green Supply Chain Management: Trends and Current Practices. Compet. Rev. Int. Bus. J. 2016, 26, 265–288. [Google Scholar] [CrossRef]
- Henry, M.; van Ardenne-van der Hoeven, A.; Demmers, M.; Dykstra, E.; Frissen, L.; de Graeff, J.J.; Hooimeijer, P.; Koeman, N.; van Lier Lels, M.; Meester, G. Circular Economy. From Wish to Practice. 2015. Available online: https://en.rli.nl/sites/default/files/advice_rli_circular_economy_interactive_def.pdf (accessed on 15 January 2024).
- United Nations Climate Action Fast Facts. Available online: https://www.un.org/en/climatechange/science/key-findings (accessed on 31 January 2024).
- Busalim, A.; Fox, G.; Lynn, T. Consumer Behavior in Sustainable Fashion: A Systematic Literature Review and Future Research Agenda. Int. J. Consum. Stud. 2022, 46, 1804–1828. [Google Scholar] [CrossRef]
- Puspita, H.; Chae, H. An explorative Study and Comparison between Companies’ and Customers’ Perspectives in the Sustainable Fashion Industry. J. Glob. Fash. Mark. 2021, 12, 133–145. [Google Scholar] [CrossRef]
- Sherman, J.D.; Thiel, C.; MacNeill, A.; Eckelman, M.J.; Dubrow, R.; Hopf, H.; Lagasse, R.; Bialowitz, J.; Costello, A.; Forbes, M.; et al. The Green Print: Advancement of Environmental Sustainability in Healthcare. Resour. Conserv. Recycl. 2020, 161, 104882. [Google Scholar] [CrossRef]
- Brightman, A.O.; Beever, J.; Hiles, M.C. Next-Generation Ethical Development of Medical Devices: Considering Harms, Benefits, Fairness, and Freedom. In Next-Generation Ethics; Abbas, A.E., Ed.; Cambridge University Press: Cambridge, UK, 2019; pp. 387–410. ISBN 978-1-108-61618-8. [Google Scholar]
- Di Pietro, L.; Piaggio, D.; Oronti, I.; Maccaro, A.; Houessouvo, R.C.; Medenou, D.; De Maria, C.; Pecchia, L.; Ahluwalia, A. A Framework for Assessing Healthcare Facilities in Low-Resource Settings: Field Studies in Benin and Uganda. J. Med. Biol. Eng. 2020, 40, 526–534. [Google Scholar] [CrossRef]
- Piaggio, D.; Castaldo, R.; Cinelli, M.; Cinelli, S.; Maccaro, A.; Pecchia, L. A Framework for Designing Medical Devices Resilient to Low-Resource Settings. Glob. Health 2021, 17, 64. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Okita, T.; Enzo, A.; Asai, A. The Ethics of the Reuse of Disposable Medical Supplies. Asian Bioeth. Rev. 2020, 12, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Cornock, M. Legal definitions of Responsibility, Accountability and Liability: Marc Cornock Clarifies the Use of Terms That Are Sometimes Used Interchangeably but Have Distinct Ramifications in Law. Nurs. Child. Young- People 2011, 23, 25–26. [Google Scholar] [CrossRef]
- Khan, M. Polluter-Pays-Principle: The Cardinal Instrument for Addressing Climate Change. Laws 2015, 4, 638–653. [Google Scholar] [CrossRef]
- Pratt, B. Sustainable Global Health Practice: An Ethical Imperative? Bioethics 2022, 36, 874–882. [Google Scholar] [CrossRef]
- Sun, J.; Freeman, B.D.; Natanson, C. Meta-Analysis of Clinical Trials. In Principles and Practice of Clinical Research; Elsevier: Amsterdam, The Netherlands, 2018; pp. 317–327. ISBN 978-0-12-849905-4. [Google Scholar]

| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Year of Publication | |
| 2013–2022 | 2012 and earlier; 2023-Present |
| Article Type | |
| Journal articles | Conference Papers, Reviews, Book Chapters, Books, Editorials, Letters, Notes |
| Language | |
| English | Any other language |
| Demographic origin | |
| Any region in the world | - |
| Medical technologies | |
| Any other medical technology (digital health, bioimaging…) | Chemical or biological action (e.g., drugs, vaccines) |
| Approaches | |
| Use of strategies, interventions, and/or frameworks assessing sustainability | Lack of strategies, interventions, and/or frameworks assessing sustainability |
| Study | Medical Technology (If Applicable) | Lifecycle Stage | Approach | Impact Type | SDGs |
|---|---|---|---|---|---|
| Hamner et al., 2013 [29] | Prosthetic knee | Design and development | Frugal innovation | Economic; social | 3, 9 |
| Hede et al., 2013 [30] | The proposed framework was assessed by four companies in Europe engaged in class I and II medical device development | Design and development | Sustainable design | Economic; environmental; social | 9, 12 |
| MacVittie et al., 2013 [31] | Any implantable electromedical equipment (e.g., pacemaker) | Design and development | Body energy harvesting | Economic; environmental | 7 |
| Hamza et al., 2014 [32] | Microscope | Use | Operation | Environmental; social | 7, 17 |
| Unger and Landis, 2014 [33] | Dental bur | Use | Reprocessing | Environmental | 3, 8, 9, 12 |
| Gupta et al., 2015 [34] | A medical device to treat neonatal hypothermia | Design and development | Frugal innovation; global health technologies | Economic; social | Not available |
| Izadikhah and Farzipoor Saen, 2016 [35] | Twenty-nine Iranian supply chains producing equipment of expendable medical devices | Manufacture | Supply chain management | Economic; environmental; social | 9, 12, 17 |
| Moultrie et al., 2016 [36] | All types of medical devices | Design and development | Ecodesign | Economic; environmental | 7, 9, 12 |
| Unger and Landis, 2016 [37] | Seven devices, including the deep vein thrombosis (DVT) compression sleeve, pulse oximeter, ligasure, harmonic scalpel, endoscopic trocar, arthroscopic shaver, and scissor tip | Use | Reprocessing | Economic; environmental; social | 3, 8, 9, 12, 13 |
| Barbero et al., 2017 [38] | Dialysis equipment for chronic haemodialysis | Design and development | Ecodesign; systemic design | Economic; environmental; social | 9 |
| Unger et al., 2017 [39] | Various | Design and development | Material selection | Environmental; social | 3, 9 |
| Cosgrove et al., 2018 [40] | Not applicable | Manufacture | Production | Economic; environmental | 7, 9, 13 |
| Ghadimi et al., 2018 [41] | Diabetes monitoring solution | Manufacture | Supplier selection | Economic; environmental | 9, 12 |
| Mann et al., 2018 [42] | External fracture fixation device | Design and development | Material selection | Economic; environmental | 9 |
| Ghadimi et al., 2019 [43] | Not applicable | Manufacture | Supplier selection | Economic; environmental | 9, 12, 16 |
| Magno et al., 2019 [44] | Monitoring of blood oxygenation | Design and development | Efficient power management; solar energy harvesting; ultralow power processing | Economic; environmental | 7 |
| Donahue et al., 2020 [45] | Vaginal specula | Use | Reprocessing; procurement | Economic; environmental | 11, 12, 13 |
| Liu et al., 2020 [46] | Drug release device | Design and development | Body energy harvesting | Economic; social | Not available |
| Lyne et al., 2020 [47] | Toothbrush | Use | Procurement | Environmental; social | 9, 12, 13 |
| Riutord-Sbert at al., 2020 [48] | Surgical masks | Design and development | Material selection | Economic; social | 3, 9 |
| Xu et al., 2020 [49] | Pacemaker | Design and development | Body energy harvesting | Economic; environmental; social | Not available |
| Alrashoudi et al., 2021 [50] | Lateral flow immunoassay | Design and development | Reducing the amount of materials | Environmental | 9 |
| Bayrak and Soylu, 2021 [51] | Any single-use medical device | Use | Reprocessing | Economic | 3 |
| Haber and Fargnoli, 2021 [52] | Not applicable | Use | Product–service customisation | Economic; environmental | 9, 12 |
| Hanani et al., 2021 [53] | Any implantable electromedical equipment (e.g., pacemaker) | Design and development | Body energy harvesting | Environmental | Not available |
| Hasani et al., 2021 [54] | Unspecified for confidentiality reasons | Manufacture | Supply chain configuration | Economic; environmental | Not available |
| Liao et al., 2021 [55] | Various | Use | Repair/replacement decision making | Economic | Not available |
| Piaggio et al., 2021 [56] | Pupillometer | Design and development | Frugal innovation | Economic; environmental; social | 8, 12, 17 |
| Szmelter-Jarosz et al., 2021 [57] | Any medical equipment | Manufacture | Distribution | Economic; social | 3, 9 |
| van Straten et al., 2021 [58] | Face masks | Use | Reprocessing | Economic; environmental; social | 9, 12, 13, 17 |
| Benedettini, 2022 [59] | Any medical device that can be reprocessed | Use | Reprocessing | Economic; environmental | 9, 12 |
| Epelle et al., 2022 [60] | Any that need sterilisation | Use | Sterilisation | Economic; environmental | 6, 9 |
| Fargnoli et al., 2022 [61] | Not applicable | Manufacture | Supply chain management | Economic; environmental | Not available |
| Goli and Sadeghi, 2022 [62] | Medical mask | End of life | Repurpose | Economic; environmental | 3 |
| Le et al., 2022 [63] | Not applicable | End of life | Recycling; disposal | Economic; environmental | 9, 11, 12, 17 |
| Mallick et al., 2022 [64] | Disposable insulin pens | End of life | Recycling; remanufacturing | Environmental; social | 3, 17 |
| Nayeri et al., 2022 [65] | Blood bank refrigerator | Manufacture | Supply chain configuration | Environmental; social | 8, 9, 12, 17 |
| Rostami et al., 2022 [66] | Oxygen concentrator | Manufacture | Supplier selection | Economic; environmental | 12 |
| Rouvière et al., 2022 [67] | Medical devices in hospitals (mainly operating rooms) | Use | Waste reduction; waste sorting; procurement | Economic; environmental; social | 6, 8, 9, 12, 13 |
| Williams et al., 2022 [68] | Oxygen concentrator | Design and development | Frugal innovation; 3D printing; material selection | Economic; environmental; social | 3, 9, 10, 12, 15 |
| Abad et al., 2023 [69] | Medical ventilator | Manufacture | Supply chain management; distribution | Economic; environmental; social | 12,13 |
| Strategy | Action | Studies |
|---|---|---|
| Manufacturing and utilisation of a product | R1—rethink | [39,42,59] |
| R2—reduce | [30,33,38,44,54,58,61,63,65,67] | |
| Extended lifespan of product and its components | R3—reuse | [45,48] |
| R4—repair | [55] | |
| R5—refurbish | [51] | |
| R6—remanufacture | [68] | |
| R7—repurpose | [51] | |
| Effective (re)utilisation of materials | R8—recycle | [62,64] |
| R9—recover | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montesinos, L.; Checa Rifá, P.; Rifá Fabregat, M.; Maldonado-Romo, J.; Capacci, S.; Maccaro, A.; Piaggio, D. Sustainability across the Medical Device Lifecycle: A Scoping Review. Sustainability 2024, 16, 1433. https://doi.org/10.3390/su16041433
Montesinos L, Checa Rifá P, Rifá Fabregat M, Maldonado-Romo J, Capacci S, Maccaro A, Piaggio D. Sustainability across the Medical Device Lifecycle: A Scoping Review. Sustainability. 2024; 16(4):1433. https://doi.org/10.3390/su16041433
Chicago/Turabian StyleMontesinos, Luis, Pedro Checa Rifá, Mireya Rifá Fabregat, Javier Maldonado-Romo, Stefano Capacci, Alessia Maccaro, and Davide Piaggio. 2024. "Sustainability across the Medical Device Lifecycle: A Scoping Review" Sustainability 16, no. 4: 1433. https://doi.org/10.3390/su16041433
APA StyleMontesinos, L., Checa Rifá, P., Rifá Fabregat, M., Maldonado-Romo, J., Capacci, S., Maccaro, A., & Piaggio, D. (2024). Sustainability across the Medical Device Lifecycle: A Scoping Review. Sustainability, 16(4), 1433. https://doi.org/10.3390/su16041433










