Abstract
Bacterial cellulose is a biomaterial known for its physical and mechanical properties, including its high mechanical strength, water retention capacity, and biocompatibility. Its production from various carbohydrates has been widely studied, aiming to find more efficient and cost-effective culture media. This study investigated the production of bacterial cellulose from agroindustrial asparagus peel waste by Komagataeibacter rhaeticus QK23. A strain of QK23 was isolated and cultivated from a kombucha tea, identified based on morphological and molecular characteristics using the 16S rRNA gene. The waste was hydrolyzed and converted into fermentable sugars. Using the response surface methodology, the inoculum dose (1–20%) and incubation time (3–25 days) were evaluated concerning bacterial cellulose yield. The results demonstrated that with an optimal inoculum dose of 10.5% and an incubation time of 25 days, a production of 2.57 g/L was achieved. It was characterized as similar to type I cellulose, exhibiting a high degree of crystallinity (81.89%) and suitable morphological properties, evidenced by a fiber size of 178 nm and a surface roughness of 27.05 nm. Converting asparagus waste into bacterial cellulose is a sustainable and effective strategy that promotes the development of advanced biomaterials in biotechnology research.
1. Introduction
Recent technological advancements have facilitated the creation of innovative and multifunctional biomaterials that mimic natural ones, offering advanced properties for diverse applications, effectively replacing conventional materials and relying on sustainable resources [1]. Bacterial cellulose (BC) is a superior biomaterial with a unique nanofibrillar three-dimensional network and exceptional purity that provides new physical and mechanical properties [2]. BC stands out for its mechanical strength of up to 200 MPa, high water retention capacity of 100 times its weight, elevated crystallinity of 60–90% for increased durability, and excellent biocompatibility with minimal immune responses, which is particularly beneficial in wound healing [3]. These qualities have garnered significant interest in BC within scientific and commercial sectors.
BC is produced by various bacterial genera, with Gluconacetobacter (now Komagataeibacter) being the most widely studied due to its high efficiency in synthesizing cellulose nanofibers from glucose [4]. Biochemically, synthesis starts with the conversion of glucose to glucose 6-phosphate, followed by UDP-glucose and its subsequent polymerization into β-1,4 glucan by cellulose synthase [5]. BC has been obtained from a variety of carbohydrates, with fructose emerging as the most conducive substrate, particularly and synergistically with lactose, enhancing a substantial BC yield [6]. Additionally, the Hestrin–Schramm (HS) medium is the standard in BC culture, but its high costs arising from the need for supplements like glucose, yeast extract, and peptone limit its economical application in large-scale production [7]. Therefore, industrial BC production requires cheaper and more efficient culture media focused on low-cost carbon and nitrogen sources to increase yield [8].
The evaluation of BC yield generated from different waste substrates has been continuously investigated. Sugarcane residues [9], and hydrolysates from fruit and vegetable peels such as cucumber, tomato, apple, melon, and kiwi have been employed [10]. Additionally, starch industry waste [11] and hydrolysates from citrus peels (mandarin, lemon, grapefruit, and orange) have been fermented [12]. Consequently, industrial waste products are recognized as viable candidates for BC production; however, the feasibility of their use depends on the availability of these raw materials at a low cost, which is influenced by factors such as geographical location and climatic conditions [13]. Hence, agroindustrial by-products, generated in agricultural export zones, emerge as a promising option as substrates for BC production.
The high productivity of asparagus in Peru’s agro-exportation has demanded harvests of up to 229,522 tons, leading to an abundance of agricultural waste with energy potential, which have not been sufficiently exploited to date [14]. During asparagus processing, large volumes of organic residues such as trimmings and husks, containing high concentrations of cellulose (16–34.6%), hemicellulose (21.2%) and lignin (13.2%), and representing a total of 77.63% carbohydrates and 12.84% proteins, have been obtained [15,16]. Therefore, there have been no studies that have used hydrolysates of asparagus peel (Asparagus officinalis L.) as the sole carbon source for BC production.
The objective of this study is to optimize BC production using hydrolysates of agroindustrial asparagus peel waste as a carbon source, through fermentation by a newly isolated strain Komagataeibacter rhaeticus. The obtained BC was characterized using X-ray diffraction (XRD), atomic force microscopy (AFM), and Fourier-transform infrared spectroscopy (FTIR) to reveal its potential applications in fields such as the environmental field, the food industry, and health care.
2. Materials and Methods
2.1. Isolation and Characterization of Komagataeibacter rhaeticus
A SCOBY (symbiotic colony of bacteria and yeast) was obtained from kombucha tea at a local market in Lima, Peru. A total of 5 g of wet weight of the SCOBY membrane was enriched in 100 mL of HS medium containing: peptone 0.5%, yeast extract 0.5%, Na2HPO4 0.25%, citric acid 0.115%, and sucrose 4%; supplemented with 500 µL/100 mL of acetic acid (≥99.7%) and fluconazole (1 µg/mL) [17]. It was incubated for 5 days at 30 °C, followed by streak plating onto HS agar Petri dishes. The resulting colonies were provisionally corroborated and selected based on their macroscopic and microscopic morphological characteristics of the Komagataeibacter genus; then, they were subcultured in slanted HS agar bottles. These were stored at 5 °C for subsequent analysis.
The isolated culture was identified through molecular analysis using the polymerase chain reaction (PCR) method of the 16S rRNA gene following the methodology described by Cueva-Almendras et al. [18]. DNA was extracted from a pure 48-hour-old culture using the Quick-DNA™ Fungal/Bacterial Miniprep kit (Zymo Research, Irvine, CA, USA). Next, the full-length 16S rRNA gene was amplified via PCR using primers 27F and 1492R, employing Phusion High-Fidelity DNA Polymerase and a hybridization temperature of 56 °C. DNA sequencing was conducted by Macrogen Inc. (Seoul, Republic of Korea) and aligned with 16S rRNA sequences using a nucleotide BLAST (basic local alignment search tool) search for the closest homologous strains in the NCBI (National Center for Biotechnology Information) database [19]. A phylogenetic tree was constructed using rRNA sequences with the assistance of MEGA-X v11 software.
2.2. Collection and Conditioning of Agroindustrial Asparagus Waste
Asparagus peel waste (APW) originated from the production lines of agroindustrial companies in the La Libertad region (Peru). Residues showing fungal-initiated damages were discarded. A washing process with NaClO (100 ppm) for 10 min followed by three washes with distilled water were performed. Subsequently, 2 kg was dried at 60 °C for 6 h, then ground and sieved to a 0.45 mm pore size. Finally, samples were stored in polyethylene bags at room temperature [20].
2.3. Characterization of Agroindustrial Waste
Structural sugars in the residues were determined following the National Renewable Energy Laboratory (NREL) method [21]. High-performance liquid chromatography (HPLC) was utilized with a Thermo Scientific Ultimate 3000 system and a Corona Veo-RS charged aerosol detector, using Shodex SUGAR SP0810 and SH-G 6B columns. Monosaccharides were identified with high-purity reference standards (Sigma Aldrich, St. Louis, MO, USA), while maintaining the column temperature at 60 °C, and using a gradient of ultrapure water and acetonitrile as the mobile phase. A total of 300 mg of the APW sample was previously hydrolyzed with biphasic acid, first at 30 °C with 72% sulfuric acid and then the acid solution was diluted to 4%; then, this was autoclaved at 121 °C. Afterwards, it was centrifuged (5000 rpm for 10 min) and filtered to a pore size of 0.2 μm. Finally, monomeric sugars were quantified using HPLC, as delineated in the protocol.
2.4. Obtaining Fermentable Sugars from APW
Chemical hydrolysis was performed using 2.5% diluted sulfuric acid, adding 11.5% (w/v) APW to the solution at 80 °C for 60 min. The hydrolyzed liquid was filtered, and its pH was neutralized with a 2 N sodium hydroxide solution to pH 7. It was stored in a refrigerated environment at 4 °C until use [22].
2.5. Fermentative Production of BC
The effect of inoculum dose (1–20%, v/v) and incubation time (3–25 days) on the BC production yield in dry weight was evaluated using the response surface methodology (RSM) with a central composite rotational design (CCRD). This consisted of 13 runs based on a quadratic model with 4 factorial points, 5 repeated central points, and 4 axial points (Table 1). The experiments were conducted in 150 mL flasks with 20 mL of APW hydrolysate supplemented with: peptone 0.5%, yeast extract 0.5%, Na2HPO4 0.25%, and citric acid 0.115%, adding 500 µL/100 mL of acetic acid to reach pH 4.5. The initial inoculum was 0.8–1.0 OD600, obtained from a 48-hour-old culture. The samples were incubated at 30 °C under static conditions [23].

Table 1.
Values used according to the central composite rotatable design for two independent variables.
2.6. Harvesting and Purification of BC
The obtained BC underwent three wash cycles with distilled water and was then treated in a 2.5% aqueous solution of NaOH (J.T. Baker, Phillipsburg, NJ, USA) at 90 °C for 60 min. Subsequently, it was repeatedly rinsed with distilled water until a neutral pH was reached. As a final step, the sample was treated with 2.5% NaClO (The Clorox Company, Oakland, CA, USA) for 1 h [24]. Successive washes with distilled water were carried out to remove any residual NaClO, followed by drying at 45 °C for 24 h until a stable weight was reached in a drying oven (Memmert, UF260 PLUS, New York, NY, USA).
2.7. Analytical Measurements
Reducing sugars were determined using the dinitrosalicylic acid (DNS) method employing a UV–VIS spectrophotometer (SI Analytics—UviLine 9400, Mainz, Germany), preceded by a calibration curve with anhydrous glucose (Merck) [25]. The dry weight of the BC was measured using a precision balance (SHS GX600, Tokyo, Japan). Volumetric yield and consumption of reducing sugars were calculated at the end of fermentation as follows [26]:
2.8. BC Characterization
2.8.1. Infrared Spectrum
FTIR spectra were measured using a Nicolet IS50 instrument (Thermo Fisher, Waltham, MA, USA) in the attenuated total reflectance (ATR) mode with a diamond-type crystal. Analysis was conducted in the range of 4000–600 cm−1 with a resolution of 4 cm−1 [27].
2.8.2. XRD
The crystallinity index and crystalline area were obtained from X-ray diffraction analysis of the BC. They were subjected to an X-ray diffractometer (Rigaku Miniflex 600, Wilmington, MA, USA) with copper radiation at a voltage of 30 kV, a current of 20 mA, and a wavelength (λ) of 0.15406 nm [28]. A scanning range from 2θ = 5° to 50° at a scan speed of 2°/min was used. The crystalline fraction (CI) can be determined by integrating the areas under the diffraction patterns of crystalline Ac and amorphous Aa, giving us the percentage of crystallinity as expressed in the following equation:
The crystallite size was estimated using the Scherrer equation:
where (K) is the dimensionless Scherrer constant = 0.9, (λ) is the wavelength of X-rays, (β) is the total width of the peak at half its maximum intensity in radians, and (θ) is the diffraction angle in radians.
The Bragg equation was used to determine the atomic interplanar spacing (d) [27]:
where (n) is the order of the peak in the plane.
2.8.3. AFM
The morphology of BC membranes was examined using a Bruker atomic force microscope (Bruker, Dimension Edge, Bremen, Germany), employing the tapping mode to obtain images of a 30 × 30 µm area. Wet BC samples were cut into 1 × 1 cm squares, dried at 60 °C on a glass slide to form a thin dry film, and subjected to AFM readings [29]. The surface roughness of the membranes, known as RMS (Rq), and referring to the standard deviation of heights “z” in a specific area, was calculated using ImageJ 1.53k software (Bethesda, MD, USA), supplemented with the “SurfCharJ v1” plugin [30].
2.9. Statistical Analysis
For data processing, analysis of variance (ANOVA) was conducted. Results were presented in terms of mean ± standard deviation. For the analysis of resulting surfaces, Design Expert 13 software was employed, considering a value of p < 0.05 to determine statistical significance [31].
3. Results and Discussion
An isolate was obtained from a commercial kombucha-derived SCOBY, and based on its morphology and Gram staining reaction, the isolated microorganism was determined to be Gram-negative (designated as QK23). This finding aligns with the characteristics of most acetic acid bacteria, to which the Komagataeibacter genus belongs, that are commonly found in these products [32]. The QK23 isolate exhibited convex colonies with regular edges, a smooth appearance, and a cream–yellow color (Figure 1). Microscopically, they appeared as rod-shaped bacteria, occurring singly or in pairs, and did not form spores. Additionally, cultivation in HS liquid medium showed biofilm formation after 48 h of growth; these characteristics correspond with reports by Fernández et al. [33], where they isolated and described various morphological features of the Komagataeibacter genus.
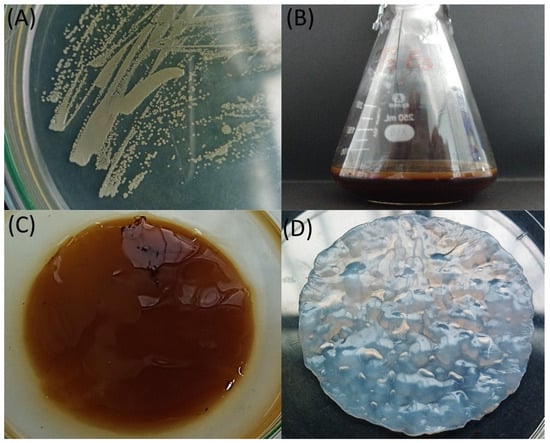
Figure 1.
Morphological characterization and BC production of the QK23 isolate: (A) colonies of the QK23 strain in HS agar medium after 48 h of growth; (B) BC production by QK23 in APW hydrolysate-based medium; (C) untreated BC from QK23, and (D) purified BC after fermentation.
The PCR amplification of the 16S rRNA gene produced a 1289 bp amplicon, and these sequences were compared using the GenBank database (BLAST). Sequence alignment revealed that the isolated strain exhibited 99% similarity to Komagataeibacter rhaeticus DSM 16663 (NR_118187). This strain was designated as Komagataeibacter rhaeticus QK23, and the nucleotide sequence was deposited with the accession number OR790884 in the NCBI database (https://www.ncbi.nlm.nih.gov/nuccore/OR790884, accessed on 10 December 2023). Additionally, phylogenetic analysis based on the 16S rRNA gene sequence placed the QK23 strain in close proximity to the Komagataeibacter rhaeticus group, with a branch length of 0.005 compared to the DSM 16663 strain. This indicates a high genetic similarity between QK23 and Komagataeibacter rhaeticus, demonstrating a recent common ancestor (Figure 2).
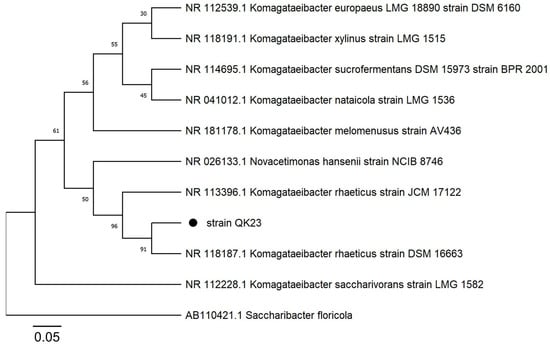
Figure 2.
The phylogenetic tree was constructed using the neighbor-joining method with a bootstrap of 1000 replicates. Evolutionary distances were determined using the Tamura 3-parameter model based on nucleotide replacement. Saccharibacter florícola AB110421 was used as an outgroup.
FTIR was used to identify the main lignocellulosic structures (lignin, hemicellulose, and cellulose) of APW. In Figure 3, IR peaks were observed at around 3380 cm−1, belonging to the region of OH group stretching vibration bands [34]. Peaks were found at around 1029 cm−1, representing the stretching band of C-OH, mainly from primary alcohols; 1152 cm−1 corresponded to C-O-C stretching bands of the glycosidic linkage, 1368 cm−1 represented C-H groups, and 866 cm−1 indicated the presence of β-glycosidic linkages [35]. The band at 2923 cm−1 was assigned to aliphatic axial deformation of -CH2 and -CH3 groups found in cellulose, lignin, and hemicellulose; there was also an absorption band close to 2854 cm−1 representing vibrations of the -OCH3 functional groups related to the aromatic group of lignin [36]. A peak was observed at 1724 cm−1, corresponding to the stretching vibration of unconjugated C=O in hemicellulose [37]. Other peaks, such as 1315 cm−1 were associated with C-N stretching of amide III groups related to proteins [38], and peaks between 1630 and 1600 cm−1 were associated with C-N stretching of amide I groups, related to the presence of proteins and lipids [39].
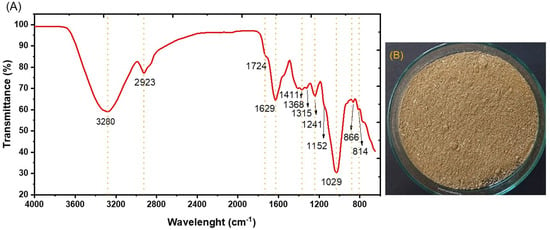
Figure 3.
Chemical analysis using: (A) infrared spectroscopy and (B) agroindustrial waste from asparagus husk conditioning.
On the other hand, the structural sugars were characterized by HPLC from total hydrolysis of the APH. In Figure 4, a higher content of glucose was found, reaching 20.17 ± 1.2%. This was attributed to the breakdown of cellulose, composed of extensive chains of D-glucose units connected by β-1,4-glycosidic linkages. The amount of glucose present was comparable to cellulose in agroindustrial waste, and lesser amounts were also detected in hemicelluloses, as per Salam et al. [40]. Glucose is crucial for fermentation, being the essential substrate for BC production. Additionally, low percentages of cellobiose were recorded, at around 10.8 ± 1.5%, suggesting the formation of byproducts derived exclusively from cellulose hydrolysis; high cellobiose percentages would indicate incomplete hydrolysis, as detailed by Eblagon et al. [41]. Although cellobiose is water soluble, Komagataeibacter bacteria cannot directly metabolize it to produce BC. On the other hand, small amounts of monomeric sugars such as arabinose, galactose, mannose, and xylose were identified, which are byproducts of hemicellulose hydrolysis, according to Lima et al. [42]. Previous studies, such as that by Chen et al. [43], have reported lower BC yields from these sugars. Moreover, 24.9 ± 2.6 g/L of initial RS was obtained from APW hydrolysates using chemical hydrolysis for the production of BC.
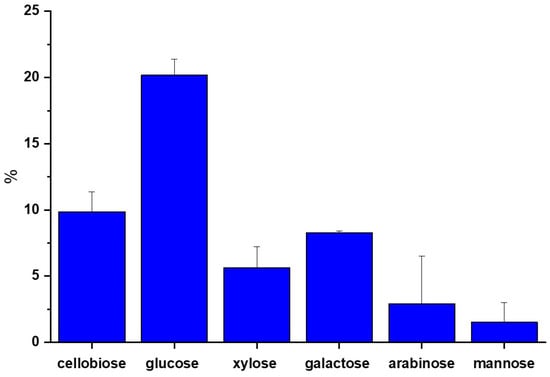
Figure 4.
Content of structural sugars by HPLC of APW.
In our study (Table 2), we investigated the impact of varying inoculum doses and incubation times on reducing sugar (RS) consumption, cell biomass growth (OD600), and BC yield on fermentation using APW hydrolysates as a sole carbon source (Figure 1). Our data revealed a nuanced relationship among these parameters. The inoculum dose showed a notable influence on the outcomes, ranging from 1% to 20%. Remarkably, higher inoculum doses (17.2% and 20%) tended to result in slightly greater RS consumption and higher BC yield. This was especially evident in runs 2, 4, and 6, suggesting that a higher initial bacterial load can enhance cellulose production. However, this was not uniformly the case, as seen in run 7 with a lower inoculum dose of 10.5%, displaying comparatively lower RS consumption and BC yield. Incubation time also played a critical role, ranging from 3 to 25 days. Longer incubation times, particularly 22 and 25 days (runs 3, 4, and 8), were associated with increased RS consumption and higher BC yields, indicating that prolonged incubation allows for more complete substrate utilization and improved cellulose production. This trend was particularly pronounced in run 8, which exhibited the highest RS consumption (79.01%) and the highest BC yield (2.57 g/L). On the other hand, with an inoculum dose of 3.8% and a 6-day incubation, the average BC/RS consumption stood at 0.12 g/g, while it slightly increased to 0.13 g/g for an inoculum dose of 10.5% and an incubation time of 14 days. These fluctuations imply that both the initial inoculum quantity and the duration of incubation significantly impact the optimization of bioconversion.

Table 2.
BC yield, cell biomass, consumed reducing sugars, and BC/RS consumed from APW hydrolysate fermentation at different inoculum doses and incubation times.
The results obtained from the maximum BC yield (2.57 g/L) from APW hydrolysate in our study surpass those reported by Akintunde et al. [44]. They obtained 1.6 g/L and 1.2 g/L of BC in dry weight from enzymatic hydrolysate of corn cob and sugarcane bagasse, respectively, at 30 °C for 10 days under agitated conditions using Komagataeibacter sp. The results also differ from those of Sundaram et al. [45], who achieved a maximum BC yield of 1.69 g/L with a hydrolyzed medium of sesame seed meal and glycerol using Komagataeibacter hansenii. Our findings align with the results from other residual sources like those of Nie et al. [46], who obtained 2.1 g/L from Nelumbo nucifera Loto rhizome byproducts at 30 °C for 14 days under static conditions using Acetobacter pasteurianus MGC-N8819. Nevertheless, the findings contrast with those of Cazón et al. [47], who achieved as much as 4.3 g/L of BC over 8 days at pH 3.7 and 30 °C, employing a bagasse–potato culture medium with Komagataeibacter xylinus.
The statistical analysis of the effects of inoculum dose and incubation time on bacterial cellulose (BC) production and reducing sugar (RS) consumption reveals significant values (Table 3). The overall model shows that, in general, these factors have a statistically significant influence on both responses, indicated by the low p-values. The inoculum dose significantly impacts BC production (F-value of 7.5 and p-value of 0.029), although its effect on RS consumption is not statistically significant (p-value of 0.7153). On the other hand, incubation time emerges as a determining factor for both BC production (F-value of 18.59 and p-value of 0.0035) and RS consumption (F-value of 373.6 and a low p-value). These findings suggest that, to optimize bacterial cellulose production and sugar utilization efficiency, incubation time is more crucial than the inoculum dose, providing valuable guidance for process design and improvement in biotechnology.

Table 3.
ANOVA data for BC (g/L) and RS consumed (%) in the fermentation of APW hydrolysates by Komagataeibacter rhaeticus QK23.
In Figure 5, contour maps display the combined effects of inoculum dose and incubation time on BC production and reducing sugar (RS) consumption. The warmer areas on the map (yellow to red colors) indicate higher BC yields, which are associated with longer incubation times and intermediate to high inoculum doses. The highest points in BC production seem to be within the inoculum dose region of approximately 10% to 17%, with incubation times of around 20 days. RS consumption also increases with longer incubation times, but its relationship with the inoculum dose is not as pronounced as that seen in BC production. The highest RS consumption does not seem to necessitate the highest inoculum dose; rather, a longer incubation time emerges as the more significant factor, which is consistent with the data points and the yellow to red areas.
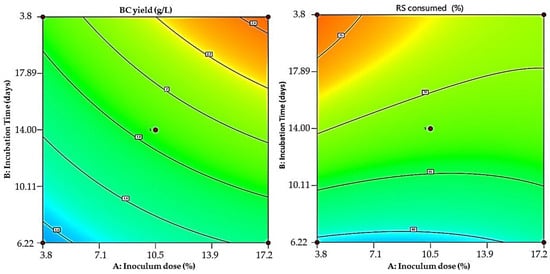
Figure 5.
Colored contour plots displaying the interaction between inoculum dose and incubation time for maximum BC production (left) and RS consumed (right).
These findings are consistent with those reported by Barshan et al. [48], who found that the maximum wet and dry weights of BC yield could be achieved at the longest incubation time from the vinasse fermentation using Komagataeibacter xylinus PTCC 1734. Similarly, Mardawati et al. [49] reported that a longer duration (5 to 15 days) gradually increased BC in the fermentation of pineapple byproducts. Inoculum density is a key factor in BC generation. It has been noted that BC production occurs at various inoculum size levels. However, incorporating an excess of inoculum into the fermentation medium causes competition among bacteria for nutrients, interfering with bacterial growth and BC production [50].
To analyze the properties of BC produced by Komagataeibacter rhaeticus QK23, three different techniques were employed: ATR-FTIR, XRD, and AFM. As is shown in Figure 6A, the ATR-FTIR spectra of BC displayed a strong absorption peak at 3335 cm−1, which was assigned to O-H stretching, while the peak around 2800–2900 cm−1 was related to C-H stretching [51]. Water bending vibrations were observed at around 1640–1630 cm−1; additionally, peaks between 1050 and 1020 cm−1 correspond to C-O stretching in C3, C-C stretching, and C-O stretching in C6. Moreover, the peak around 996 cm−1 exhibits C-O stretching [52]. A band at 898 cm−1 was identified as C-O-C stretching in the β-(1,4) glycosidic linkage [53]. An absorption band detected at 1430 cm−1 is attributed to symmetric CH2 group bending vibrations, associated with the cellulose crystallinity level. This band is known as the cellulose crystallinity band, according to Reiniati et al. [54]. The FTIR spectrum obtained did not demonstrate similarities with cellulose type II characteristics, and the peaks obtained entirely matched the cellulose type I profile indicated by Oh et al. [55], suggesting that the washing treatment mode for purification is gentle enough to not modify cellulose type I into type II. Hence, Figure 6A reveals that the functional groups of BC obtained from APH hydrolysate fermentation matched those reported by Abol-Fotouh et al. [28], who used the Hestrin–Schramm (HS) medium in their study.
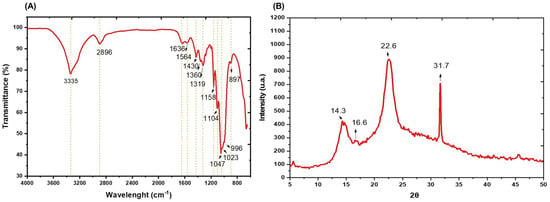
Figure 6.
Characterization of BC obtained after fermentation of APW hydrolysates by Komagataeibacter rhaeticus QK23: (A) infrared spectrum and (B) X-ray diffraction.
The structure of BC fibers was analyzed by X-ray diffraction, and the obtained patterns are shown in Figure 6B. The presence of three broad diffraction peaks around 14.3, 16.6, and 22.6° in APW-derived BC was observed; these peaks correspond to characteristic crystallographic planes and interplanar distances of the cellulose I α and I β phases [56]. Furthermore, these pairs of main peaks (14.3 and 22.6°) represent the cellulose type I structure, evident in the XRD patterns [57]. As us shown in Figure 6B, the peak at around 22.6° in the BC XRD pattern demonstrates the high crystallinity index of cellulose I with a value of 81.89%, a crystallite size of 3.7 nm, and an atomic interplanar spacing of 0.7 nm. These properties align with BC obtained by Ye et al. [58] from HS medium with values of 14.8, 16.8, and 22.8°, and a CI of 86%. Likewise, the CI is higher than the values obtained by El-Gendi et al. [59] who reported 64% from prickly pear peels.
Atomic force microscopy (AFM) in tapping mode was used to examine the topography and estimate the width of the obtained BC fibers, as illustrated in Figure 7. The width of AWH-derived BC fibers was 178 nm and these dimensions were consistent with the findings of Jia et al. [29]; additionally, a surface roughness (Rq) of 27.05 nm was observed. This value is higher than the 14–21 nm reported by Lopes et al. [60]. Surface roughness is a crucial parameter for BC applications in areas such as electronic and biomedical devices, according to Gutiérrez-Hernández et al. [61].
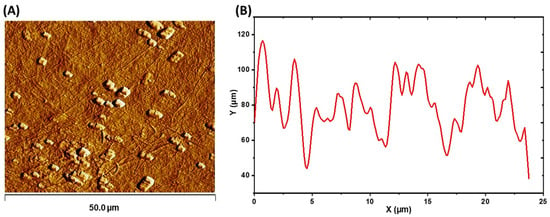
Figure 7.
AFM analysis of obtained BC: (A) dry BC topography and (B) texture profile plotted diagonally from the topographical image.
4. Conclusions
The results of this study demonstrated that hydrolysates from agroindustrial waste of asparagus peels can be used as a sole carbon source for BC production by Komagataeibacter rhaeticus QK23. An optimal inoculum dose (10.5%) and incubation time (25 days) were effective factors in enhancing BC production (2.57 g/L). FTIR spectra exhibited an identical chemical profile to type I cellulose. X-ray diffraction revealed a crystallinity index of around 81.89%, and AFM estimated a fiber size of 178.33 nm with a surface roughness (Rq) of 27.05 nm. Therefore, utilizing agroindustrial waste from asparagus peels as a carbon source for BC production by Komagataeibacter rhaeticus QK23 not only represents a sustainable and efficient alternative for agricultural waste management but also significantly contributes to the production of a high-quality biomaterial. This approach opens new research avenues for the development of more efficient and eco-friendly methods in producing valuable biopolymers, marking a significant milestone in applied biotechnology.
Author Contributions
Conceptualization, G.B.-J., C.Q.-C. and J.C.R.-S.; methodology, J.A.C.-M.; software, F.H.-B. and F.S.-M.; validation, D.S.-F.; formal analysis, C.Q.-C. and J.A.C.-M.; investigation, D.H.-C., F.S.-M., J.H.-C. and S.V.-M.; data curation, F.S.-M. and W.U.-L.; writing—original draft preparation, W.U.-L., F.H.-B., D.S.-F., J.H.-C., M.C.Q. and J.C.R.-S.; writing—review and editing, C.Q.-C.; visualization, D.H.-C. and S.V.-M.; supervision, M.C.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FONDECYT/CONCYTEC (N°06-2018-Fondecyt-BM) via the project “Design of bio-nanocomposites with potential application in the food industry from polyhydroxyalkanoates, bacterial cellulose and starch, biotechnological products from agroindustrial waste; applying an emerging technology: “Electrospinning/Electrospray””.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors acknowledge the infrastructure and support of the Laboratorio de Biotecnología e Ingeniería Genética of the Facultad de Ciencias Biológicas of the Universidad Nacional de Trujillo.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Popa, L.; Ghica, M.V.; Tudoroiu, E.E.; Ionescu, D.G.; Dinu-Pîrvu, C.E. Bacterial Cellulose—A Remarkable Polymer as a Source for Biomaterials Tailoring. Materials 2022, 15, 1054. [Google Scholar] [CrossRef] [PubMed]
- Swingler, S.; Gupta, A.; Gibson, H.; Kowalczuk, M.; Heaselgrave, W.; Radecka, I. Recent advances and applications of bacterial cellulose in biomedicine. Polymers 2021, 13, 412. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, D.; Nag, M.; Dutta, B.; Dey, A.; Sarkar, T.; Pati, S.; Edinur, H.A.; Kari, Z.A.; Noor, N.H.; Ray, R.R. Bacterial cellulose: Production, characterization and application as antimicrobial agent. Int. J. Mol. Sci. 2021, 22, 12984. [Google Scholar] [CrossRef]
- Esa, F.; Tasirin, S.M.; Rahman, N.A. Overview of Bacterial Cellulose Production and Application. Agric. Agric. Sci. Procedia 2014, 2, 113–119. [Google Scholar] [CrossRef]
- Zhong, C. Industrial-Scale Production and Applications of Bacterial Cellulose. Front. Bioeng. Biotechnol. 2020, 8, 605374. [Google Scholar] [CrossRef] [PubMed]
- Hungund, B.; Prabhu, S.; Shetty, C.; Acharya, S.; Prabhu, V.; Gupta, S.G. Production of bacterial cellulose from Gluconacetobacter persimmonis GH-2 using dual and cheaper carbon sources. J. Microb. Biochem. Technol. 2013, 5, 31–33. [Google Scholar] [CrossRef]
- Costa, A.F.; Almeida, F.C.; Vinhas, G.M.; Sarubbo, L.A. Production of bacterial cellulose by Gluconacetobacter hansenii using corn steep liquor as nutrient sources. Front. Microbiol. 2017, 8, 2027. [Google Scholar] [CrossRef]
- Urbina, L.; Corcuera, M.Á.; Gabilondo, N.; Eceiza, A.; Retegi, A. A review of bacterial cellulose: Sustainable production from agricultural waste and applications in various fields. Cellulose 2021, 28, 8229–8253. [Google Scholar] [CrossRef]
- Castro, C.; Zuluaga, R.; Putaux, J.L.; Caro, G.; Mondragon, I.; Gañán, P. Structural characterization of bacterial cellulose produced by Gluconacetobacter swingsii sp. from Colombian agroindustrial wastes. Carbohydr. Polym. 2011, 84, 96–102. [Google Scholar] [CrossRef]
- Güzel, M.; Akpınar, Ö. Preparation and characterization of bacterial cellulose produced from fruit and vegetable peels by Komagataeibacter hansenii GA2016. Int. J. Biol. Macromol. 2020, 162, 1597–1604. [Google Scholar] [CrossRef]
- Ciecholewska-juśko, D.; Broda, M.; Żywicka, A.; Styburski, D.; Sobolewski, P.; Gorący, K.; Migdał, P.; Junka, A.; Fijałkowski, K. Potato juice, a starch industry waste, as a cost-effective medium for the biosynthesis of bacterial cellulose. Int. J. Mol. Sci. 2021, 22, 10807. [Google Scholar] [CrossRef] [PubMed]
- Güzel, M.; Akpınar, Ö. Production and Characterization of bacterial cellulose from citrus peels. Waste Biomass Valoriz. 2019, 10, 2165–2175. [Google Scholar] [CrossRef]
- Kadier, A.; Ilyas, R.A.; Huzaifah, M.R.; Harihastuti, N.; Sapuan, S.M.; Harussani, M.M.; Azlin, M.N.; Yuliasni, R.; Ibrahim, R.; Atikah, M.S.; et al. Use of industrial wastes as sustainable nutrient sources for bacterial cellulose (BC) production: Mechanism, advances, and future perspectives. Polymers 2021, 13, 3365. [Google Scholar] [CrossRef]
- Ramirez-Hernandez, A.; Galagarza, O.A.; Álvarez-Rodriguez, M.V.; Pachari-Vera, E.; Valdez-Ortiz, M.d.C.; Deering, A.J.; Oliver, H.F. Food safety in Peru: A review of fresh produce production and challenges in the public health system. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3323–3342. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gu, Y.; Zhou, X.; Zhang, Y. Asparagus stem as a new lignocellulosic biomass feedstock for anaerobic digestion: Increasing hydrolysis rate, methane production and biodegradability by alkaline pretreatment. Bioresour. Technol. 2014, 164, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Tirado, J.P.; Vejarano, R.; Tapia-Blácido, D.R.; Angelats-Silva, L.M.; Siche, R. The addition of sugarcane bagasse and asparagus peel enhances the properties of sweet potato starch foams. Packag. Technol. Sci. 2019, 32, 227–237. [Google Scholar] [CrossRef]
- Angela, C.; Young, J.; Kordayanti, S.; Devanthi, P.V.P. Isolation and Screening of Microbial Isolates from Kombucha Culture for Bacterial Cellulose Production in Sugarcane Molasses Medium. KnE Life Sci. 2020, 2020, 111–127. [Google Scholar] [CrossRef]
- Cueva-almendras, L.C.; Alva, J.; Fuentes-Olivera, A.; Llontop-Bernabé, K.; Quiñones, C.; Rodriguez-Soto, J.; Cruz-Monzón, J.; Quezada, M. Production of Polyhydroxyalkanoate by Bacillus thuringiensis Isolated from Agricultural Soils of Cascas-Peru. Braz. Arch. Biol. Techn. 2022, 65, 1–10. [Google Scholar] [CrossRef]
- Wang, B.; Rutherfurd-Markwick, K.; Zhang, X.X.; Mutukumira, A.N. Isolation and characterization of dominant acetic acid bacteria and yeast isolated from Kombucha samples at point of sale in New Zealand. Curr. Res. Food Sci. 2022, 5, 835–844. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Akter, S.; Zabed, H.M. Bioethanol production from water-soluble and structural carbohydrates of normal and high sugary corn stovers harvested at three growth stages. Energy Convers. Manag. 2020, 221, 113104. [Google Scholar] [CrossRef]
- Giraldo, L.; Martínez Correa, H.; Betancourt-Gutíerrez, J.; Castrillón-Castáño, C. Aprovechamiento del residuo agroindustrial del mango común (Mangifera indica L.) en la obtención de azúcares fermentables. Ing. Cienc. 2007, 3, 41–62. [Google Scholar]
- Aswini, K.; Gopal, N.O.; Uthandi, S. Optimized culture conditions for bacterial cellulose production by Acetobacter senegalensis MA1. BMC Biotechnol. 2020, 20, 46. [Google Scholar] [CrossRef]
- Feng, X.; Ullah, N.; Wang, X.; Sun, X.; Li, C.; Bai, Y.; Chen, L.; Li, Z. Characterization of Bacterial Cellulose by Gluconacetobacter hansenii CGMCC 3917. J. Food Sci. 2015, 80, E2217–E2227. [Google Scholar] [CrossRef]
- Marsden, W.L.; Gray, P.P.; Nippard, G.J.; Quinlan, M.R. Evaluation of the DNS method for analysing lignocellulosic hydrolysates. J. Chem. Technol. Biotechnol. 2007, 32, 1016–1022. [Google Scholar] [CrossRef]
- Volova, T.G.; Prudnikova, S.V.; Sukovatyi, A.G.; Shishatskaya, E.I. Production and properties of bacterial cellulose by the strain Komagataeibacter xylinus B-12068. Appl. Microbiol. Biot. 2018, 102, 7417–7428. [Google Scholar] [CrossRef] [PubMed]
- Trilokesh, C.; Uppuluri, K.B. Isolation and characterization of cellulose nanocrystals from jackfruit peel. Sci. Rep. 2019, 9, 16709. [Google Scholar] [CrossRef]
- Abol-Fotouh, D.; Hassan, M.A.; Shokry, H.; Roig, A.; Azab, M.S.; Kashyout, A.E. Bacterial nanocellulose from agro-industrial wastes: Low-cost and enhanced production by Komagataeibacter saccharivorans MD1. Sci. Rep. 2020, 10, 3491. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, X.; Huo, M.; Zhai, X.; Li, F.; Zhong, C. Preparation and characterization of a novel bacterial cellulose/chitosan bio-hydrogel. Nanomater. Nanotechnol. 2017, 7, 1847980417707172. [Google Scholar] [CrossRef]
- Chinga, G.; Johnsen, P.O.; Dougherty, R.; Berli, E.L.; Walter, J. Quantification of the 3D microstructure of SC surfaces. J. Microsc. 2007, 227, 254–265. [Google Scholar] [CrossRef]
- Terrones, N.; Quiñones-Cerna, C.E.; Robles, M.; Cruz-Monzon, J.A.; Butrón, F.; Rodríguez, J.C. Optimization of Total Carotenoid Production by Rhodotorula mucilaginosa from Artichoke Agroindustrial Waste Using Response Surface Methodology. Environ. Res. Eng. Manag. 2023, 79, 111–121. [Google Scholar] [CrossRef]
- Semjonovs, P.; Ruklisha, M.; Paegle, L.; Saka, M.; Treimane, R.; Skute, M.; Rozenberga, L.; Vikele, L.; Sabovics, M.; Cleenwerck, I. Cellulose synthesis by Komagataeibacter rhaeticus strain P 1463 isolated from Kombucha. Appl. Microbiol. Biot. 2017, 101, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.; Morena, A.G.; Valenzuela, S.V.; Pastor, F.I.; Díaz, P.; Martínez, J. Microbial Cellulose from a Komagataeibacter intermedius Strain isolated from commercial wine vinegar. J. Polym. Environ. 2019, 27, 956–967. [Google Scholar] [CrossRef]
- Liu, Y. Recent progress in Fourier transforms infrared (FTIR) spectroscopy study of compositional, structural and physical attributes of developmental cotton fibers. Materials 2013, 6, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Cunha, F.M.; Vasconcellos, V.M.; Florencio, C.; Badino, A.C.; Farinas, C.S. On-Site Production of Enzymatic Cocktails Using a Non-conventional Fermentation Method with Agro-Industrial Residues as Renewable Feedstocks. Waste Biomass Valorization 2017, 8, 517–526. [Google Scholar] [CrossRef]
- Rodríguez-Zúñiga, U.F.; Neto, V.B.; Couri, S.; Crestana, S.; Farinas, C.S. Use of spectroscopic and imaging techniques to evaluate pretreated sugarcane bagasse as a substrate for cellulase production under solid-state fermentation. Appl. Biochem. Biotechnol. 2014, 172, 2348–2362. [Google Scholar] [CrossRef] [PubMed]
- Bekiaris, G.; Triolo, J.M.; Peltre, C.; Pedersen, L.; Jensen, L.S.; Bruun, S. Rapid estimation of the biochemical methane potential of plant biomasses using Fourier transform mid-infrared photoacoustic spectroscopy. Bioresour. Technol. 2015, 197, 475–481. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.; Oktiani, R.; Ragadhita, R. How to read and interpret FTIR spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Fasoli, M.; Dell’Anna, R.; Dal-Santo, S.; Balestrini, R.; Sanson, A.; Pezzotti, M.; Monti, F.; Zenoni, S. Pectins, Hemicelluloses and Celluloses Show Specific Dynamics in the Internal and External Surfaces of Grape Berry Skin during Ripening. Plant Cell Physiol. 2016, 57, 1332–1349. [Google Scholar] [CrossRef]
- Salam, M.; Pondith, P.C.; Islam, A.; Khan, M.R.; Uddin, M.R.; Islam, M. Conversion of Cellulosic waste into fermentable sugar: Process optimization. J. Chem. Eng. 2014, 28, 27–31. [Google Scholar] [CrossRef]
- Eblagon, K.M.; Malaika, A.; Pereira, M.F.; Figueiredo, J.L. Cutting the Green Waste. Structure-Performance Relationship in Functionalized Carbon Xerogels for Hydrolysis of Cellobiose. ChemCatChem 2018, 10, 4948–4960. [Google Scholar] [CrossRef]
- Lima, C.S.; Conceição, M.M.; Silva, F.L.; Lima, E.E.; Conrado, L.S.; Leão, D.A. Characterization of acid hydrolysis of sisal. Appl. Energy 2013, 102, 254–259. [Google Scholar] [CrossRef]
- Chen, G.; Wu, G.; Chen, L.; Wang, W.; Hong, F.F.; Jönsson, L.J. Comparison of productivity and quality of bacterial nanocellulose synthesized using culture media based on seven sugars from biomass. Microb. Biotechnol. 2019, 12, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Akintunde, M.O.; Adebayo-Tayo, B.C.; Ishola, M.M.; Zamani, A.; Horváth, I.S. Bacterial Cellulose Production from agricultural Residues by two Komagataeibacter sp. strains. Bioengineered 2022, 13, 10010–10025. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, K.; Ganesh, N.; Reddy, S.R.; Katsuno, N.; Nishizu, T.; Senthilkumar, S. Bacterial cellulose production by Komagataeibacter hansenii utilizing agro-industrial residues and its application in coffee milk stabilization. Biomass Conv. Bioref. 2023, 13, 7971–7981. [Google Scholar] [CrossRef]
- Nie, W.; Zheng, X.; Feng, W.; Liu, Y.; Li, Y.; Liang, X. Characterization of bacterial cellulose produced by Acetobacter pasteurianus MGC-N8819 utilizing lotus rhizome. LWT 2022, 165, 113763. [Google Scholar] [CrossRef]
- Cazón, P.; Puertas, G.; Vázquez, M. Production and Characterization of Active Bacterial Cellulose Films Obtained from the Fermentation of Wine Bagasse and Discarded Potatoes by Komagateibacter xylinus. Polymers 2022, 14, 5194. [Google Scholar] [CrossRef]
- Barshan, S.; Rezazadeh-Bari, M.; Almasi, H.; Amiri, S. Optimization and characterization of bacterial cellulose produced by Komagatacibacter xylinus PTCC 1734 using vinasse as a cheap cultivation medium. Int. J. Biol. Macromol. 2019, 136, 1188–1195. [Google Scholar] [CrossRef]
- Mardawati, E.; Rahmah, D.M.; Rachmadona, N.; Saharina, E.; Pertiwi, T.Y.; Zahrad, S.A.; Ramdhani, W.; Srikandace, Y.; Ratnaningrum, D.; Endah, E.S.; et al. Pineapple core from the canning industrial waste for bacterial cellulose production by Komagataeibacter xylinus. Heliyon 2023, 9, e22010. [Google Scholar] [CrossRef]
- Yanti, N.A.; Ahmad, S.W.; Muhiddin, N.H. Evaluation of inoculum size and fermentation period for bacterial cellulose production from sago liquid waste. J. Phys. Conf. Ser. 2018, 1116, 052076. [Google Scholar] [CrossRef]
- Revin, V.; Liyaskina, E.; Nazarkina, M.; Bogatyreva, A.; Shchankin, M. Cost-effective production of bacterial cellulose using acidic food industry by-products. Braz. J. Microbiol. 2018, 49, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Irham, W.H.; Tamrin; Marpaung, L.; Marpongahtun. Characterization of bacterial cellulose from coconut water supplemented curcuma longa linn and Ziziphus mauritiana extract. AIP Conf. Proc. 2020, 2267, 020056. [Google Scholar]
- Avcioglu, N.H.; Birben, M.; Seyis-Bilkay, I. Optimization and physicochemical characterization of enhanced microbial cellulose production with a new Kombucha consortium. Process Biochem. 2021, 108, 60–68. [Google Scholar] [CrossRef]
- Reiniati, I.; Hrymak, A.N.; Margaritis, A. Kinetics of cell growth and crystalline nanocellulose production by Komagataeibacter xylinus. Biochem. Eng. J. 2017, 127, 21–31. [Google Scholar] [CrossRef]
- Oh, S.Y.; Dong, I.Y.; Shin, Y.; Hwan, C.K.; Hak, Y.K.; Yong, S.C.; Won, H.P.; Ji, H.Y. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.S.M.; Misnon, M.I.; Fadil, F. The effect of sodium hydroxide concentration on yield and properties of Bacterial Cellulose membranes. IOP Conf. Ser. Mater. Sci. Eng. 2020, 732, 012064. [Google Scholar] [CrossRef]
- Suryanto, H.; Muhajir, M.; Zakia, N.; Yanuhar, U.; Aminnudin, A.; Aji-Pradana, Y.R. Effect of drying methods on the structure of bacterial cellulose from pineapple peel extract. Key Eng. Mater. 2020, 851, 79–85. [Google Scholar] [CrossRef]
- Ye, J.; Zheng, S.; Zhang, Z.; Yang, F.; Ma, K.; Feng, Y.; Zheng, J.; Mao, D.; Yang, X. Bacterial cellulose production by Acetobacter xylinum ATCC 23767 using tobacco waste extract as culture medium. Bioresour. Technol. 2019, 274, 518–524. [Google Scholar] [CrossRef]
- El-Gendi, H.; Salama, A.; El-Fakharany, E.M.; Saleh, A.K. Optimization of bacterial cellulose production from prickly pear peels and its ex situ impregnation with fruit byproducts for antimicrobial and strawberry packaging applications. Carbohydr. Polym. 2023, 302, 120383. [Google Scholar] [CrossRef]
- Lopes, T.D.; Riegel-Vidotti, I.C.; Grein, A.; Tischer, C.A.; Faria-Tischer, P.C. Bacterial cellulose and hyaluronic acid hybrid membranes: Production and characterization. Int. J. Biol. Macromol. 2014, 67, 401–408. [Google Scholar] [CrossRef]
- Gutiérrez-Hernández, J.M.; Escobar-García, D.M.; Escalante, A.; Flores, H.; González, F.J.; Gatenholm, P.; Toriz, G. In vitro evaluation of osteoblastic cells on bacterial cellulose modified with multi-walled carbon nanotubes as scaffold for bone regeneration. Mater. Sci. Eng. C 2017, 75, 445–453. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).