Vegetation Types Can Affect Soil Organic Carbon and δ13C by Influencing Plant Inputs in Topsoil and Microbial Residue Carbon Composition in Subsoil
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Experimental Design
2.3. Physical and Chemical Properties of Soil
2.4. Analysis of Amino Sugars and Lignin Phenols
2.5. δ13C in Soil
2.6. Statistical Analysis
3. Results
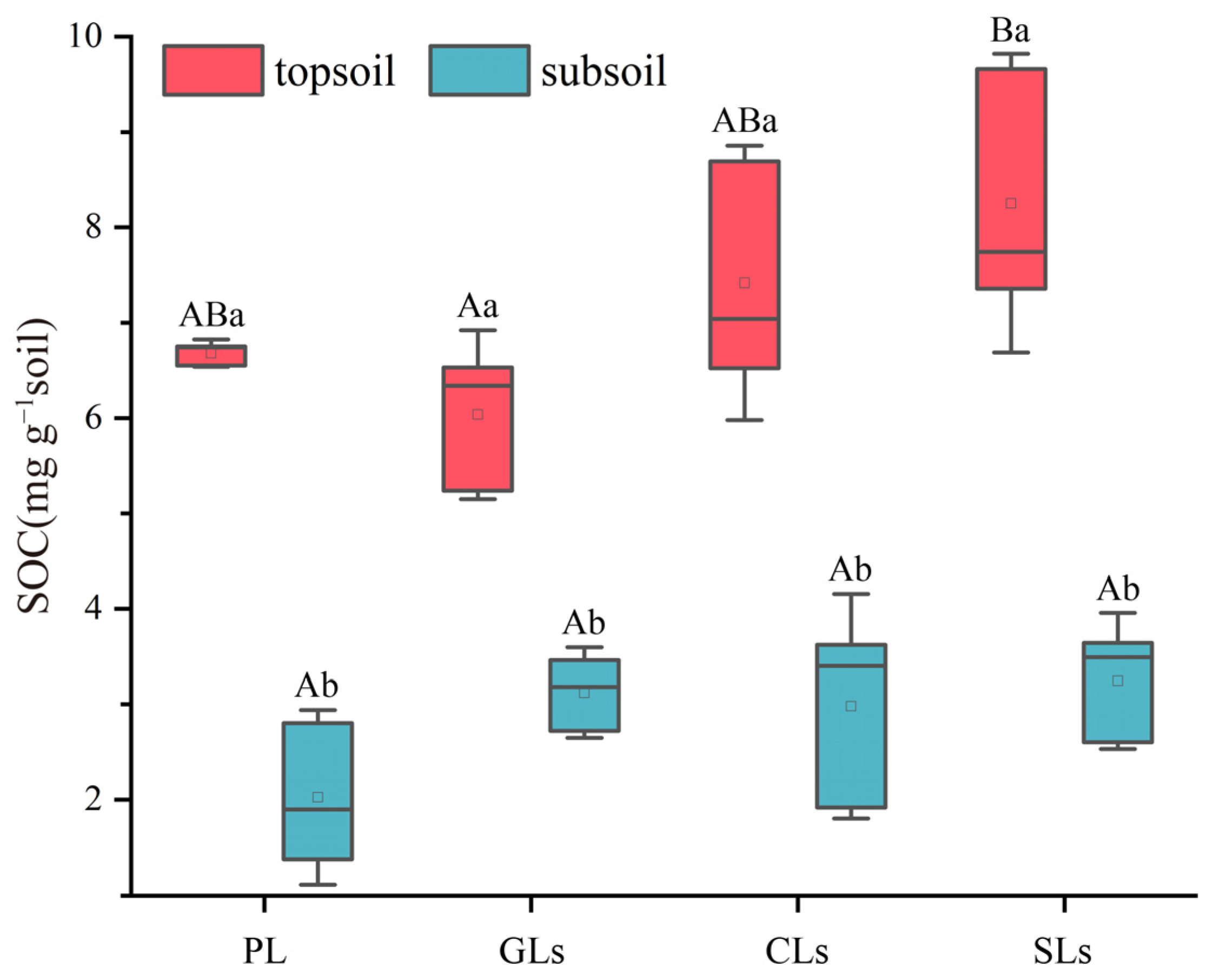
3.1. SOC Distribution Characteristics
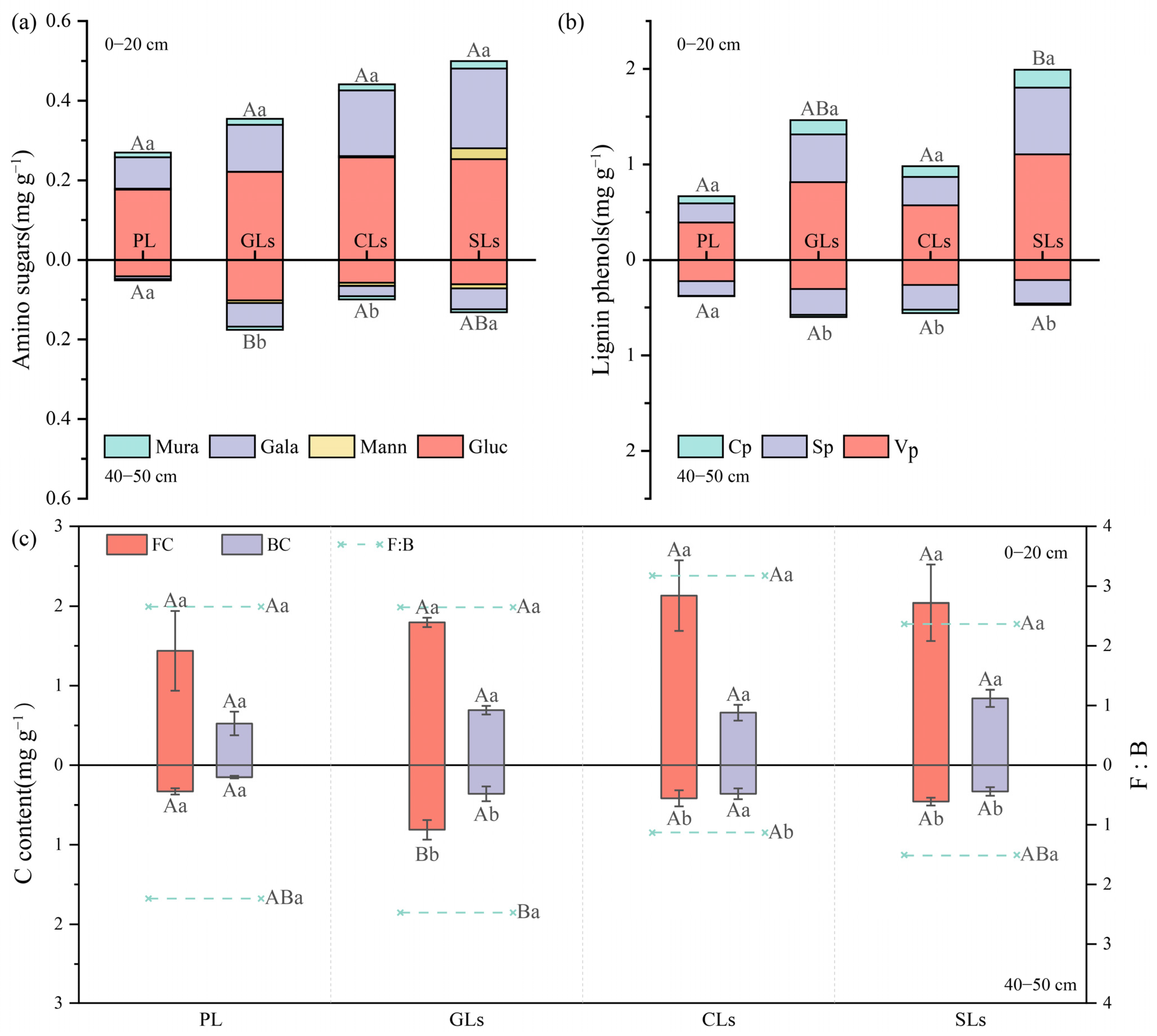
3.2. Amino Sugars, Lignin Phenols and Microbial Residue Carbon
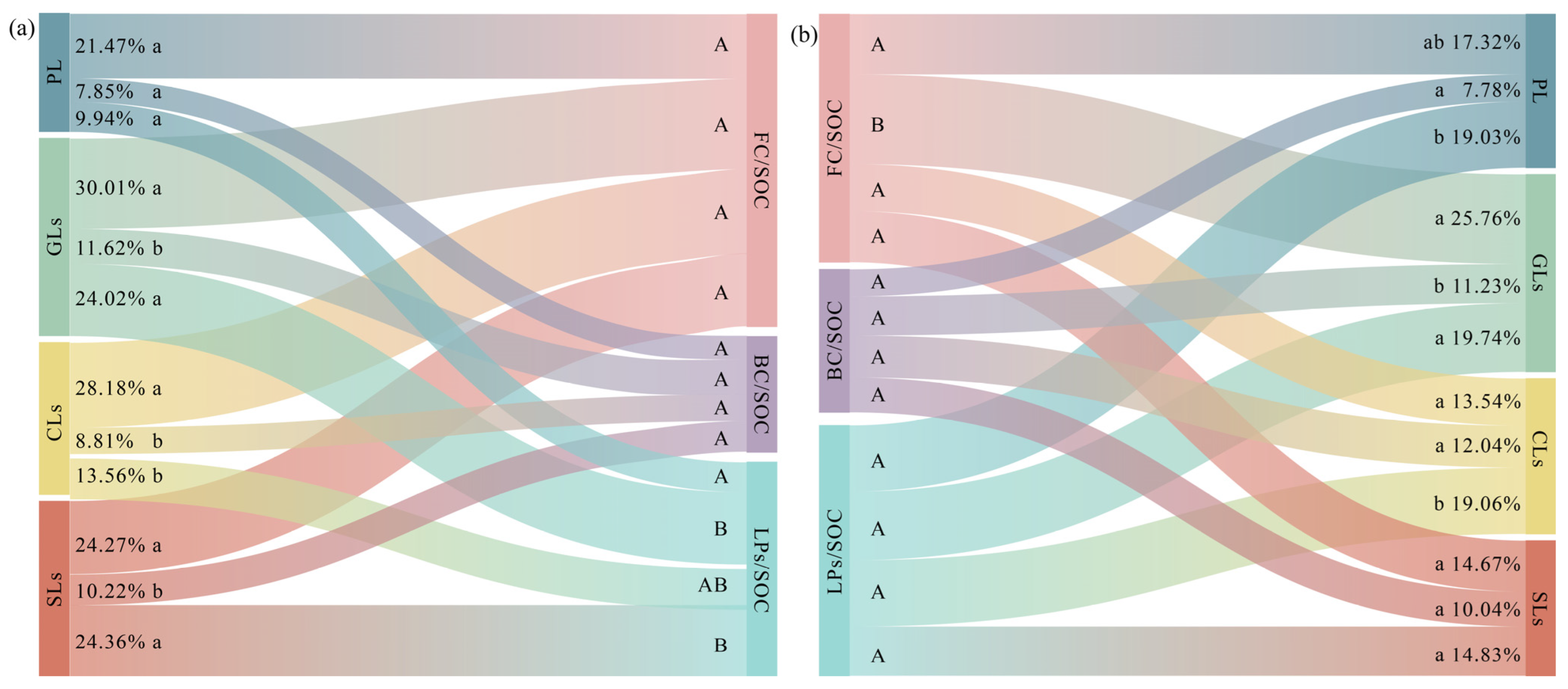
3.3. Contribution of Microbial Residue Carbon and Lignin Phenols to SOC
3.4. Distribution and Influencing Factors of δ13C
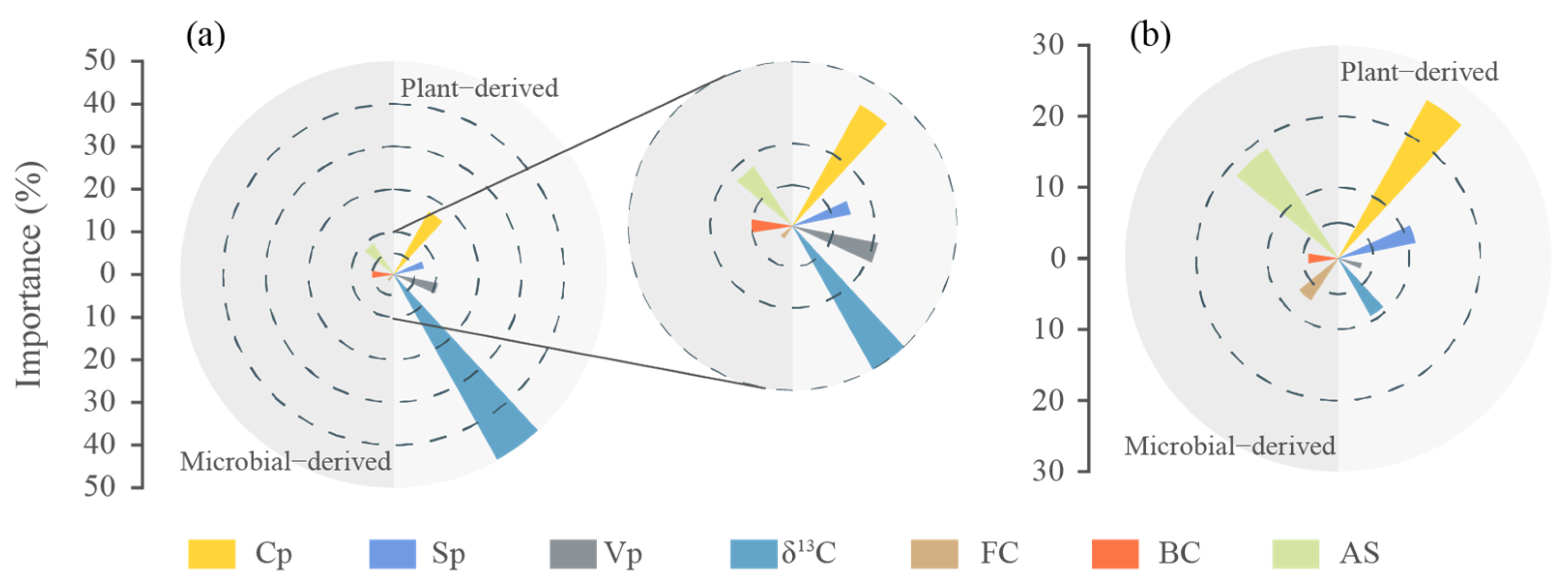
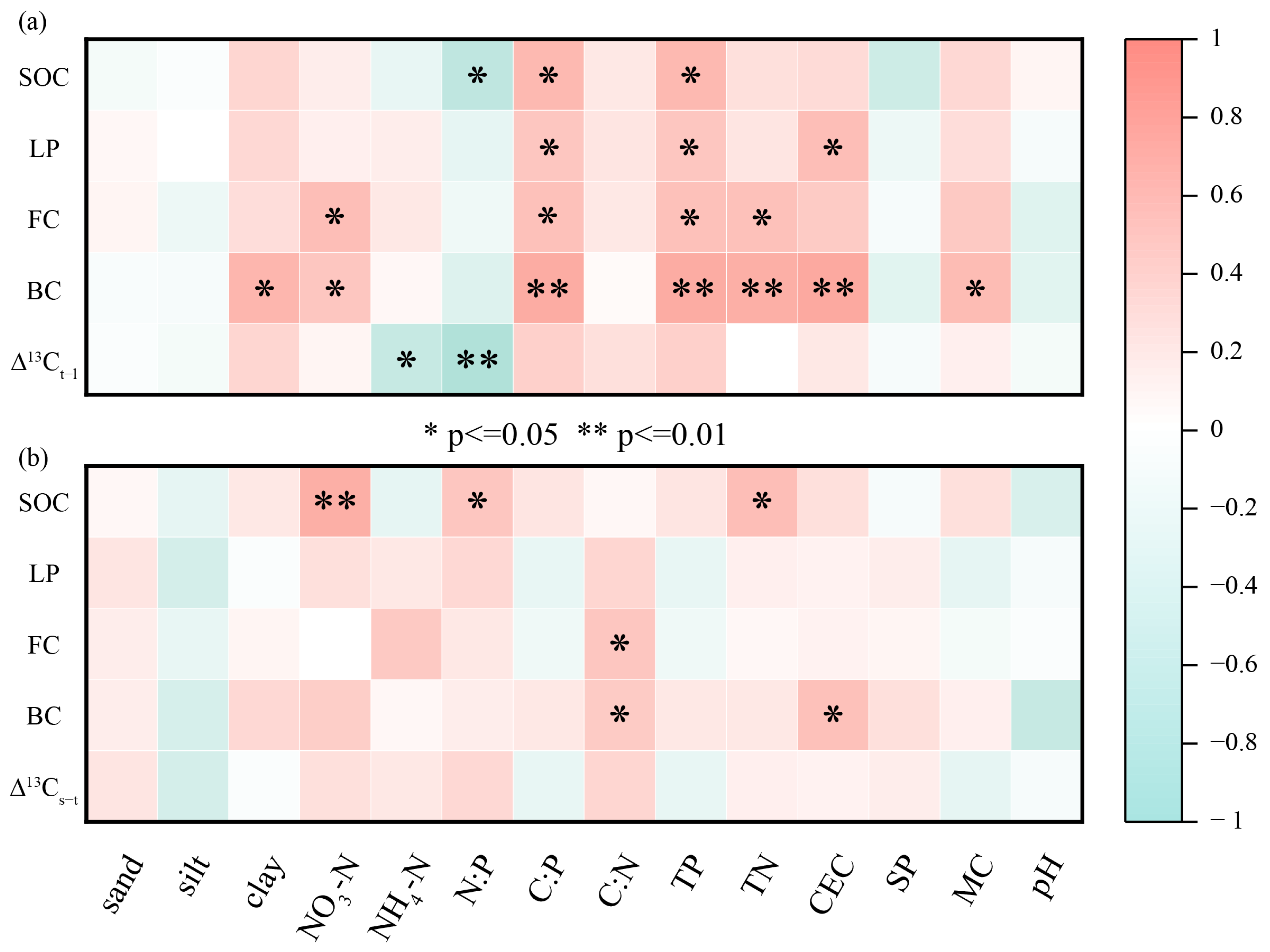
3.5. Influencing Factors of SOC, ∆13C, LPs, and Microbial Residue Carbon
4. Discussion
4.1. Distribution of SOC, LPs, and Microbial Residues
4.2. Stable Isotope Patterns
4.3. Environmental Factors That Control SOC Accumulation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pries, C.E.H.; Castanha, C.; Porras, R.C.; Torn, M.S. The whole-soil carbon flux in response to warming. Science 2017, 355, 1420–1422. [Google Scholar] [CrossRef] [PubMed]
- Wiesmeier, M.; Urbanski, L.; Hobley, E.; Lang, B.; von Luetzow, M.; Marin-Spiotta, E.; van Wesemael, B.; Rabot, E.; Liess, M.; Garcia-Franco, N.; et al. Soil organic carbon storage as a key function of soils—A review of drivers and indicators at various scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- Friggens, N.L.; Hester, A.J.; Mitchell, R.J.; Parker, T.C.; Subke, J.-A.; Wookey, P.A. Tree planting in organic soils does not result in net carbon sequestration on decadal timescales. Glob. Change Biol. 2020, 26, 5178–5188. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Qu, Q.; Lu, B.; Zhang, Y.; Liu, G.; Xue, S. Variation in soil organic carbon stability and driving factors after vegetation restoration in different vegetation zones on the Loess Plateau, China. Soil Tillage Res. 2020, 204, 104727. [Google Scholar] [CrossRef]
- LiuSui, Y.; Zhu, X.; Li, D.; Yan, C.; Sun, T.; Jia, H.; Zhao, X. Soil aggregate and intra-aggregate carbon fractions associated with vegetation succession in an alpine wetland of Northwest China. Catena 2019, 181, 104107. [Google Scholar] [CrossRef]
- Bach, E.M.; Williams, R.J.; Hargreaves, S.K.; Yang, F.; Hofmockel, K.S. Greatest soil microbial diversity found in micro-habitats. Soil Biol. Biochem. 2018, 118, 217–226. [Google Scholar] [CrossRef]
- Thevenot, M.; Dignac, M.-F.; Rumpel, C. Fate of lignins in soils: A review. Soil Biol. Biochem. 2010, 42, 1200–1211. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Koegel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Balser, T.C. Microbial production of recalcitrant organic matter in global soils: Implications for productivity and climate policy. Nat. Rev. Microbiol. 2011, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.J.; Simpson, M.J.; Smith, E.; Kelleher, B.P. Microbially derived inputs to soil organic matter: Are current estimates too low? Environ. Sci. Technol. 2007, 41, 8070–8076. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Zhu, X. The soil Microbial Carbon Pump as a new concept for terrestrial carbon sequestration. Sci. China Earth Sci. 2021, 64, 545–558. [Google Scholar] [CrossRef]
- Feng, J.; He, K.; Zhang, Q.; Han, M.; Zhu, B. Changes in plant inputs alter soil carbon and microbial communities in forest ecosystems. Glob. Change Biol. 2022, 28, 3426–3440. [Google Scholar] [CrossRef] [PubMed]
- Klink, S.; Keller, A.B.; Wild, A.J.; Baumert, V.L.; Gube, M.; Lehndorff, E.; Meyer, N.; Mueller, C.W.; Phillips, R.P.; Pausch, J. Stable isotopes reveal that fungal residues contribute more to mineral-associated organic matter pools than plant residues. Soil Biol. Biochem. 2022, 168, 108634. [Google Scholar] [CrossRef]
- Deng, L.; Wang, K.; Tang, Z.; Shangguan, Z. Soil organic carbon dynamics following natural vegetation restoration: Evidence from stable carbon isotopes (delta C-13). Agric. Ecosyst. Environ. 2016, 221, 235–244. [Google Scholar] [CrossRef]
- Diochon, A.; Kellman, L. Natural abundance measurements of 13C indicate increased deep soil carbon mineralization after forest disturbance. Geophys. Res. Lett. 2008, 35, 5. [Google Scholar] [CrossRef]
- Koesters, R.; Du Preez, C.C.; Amelung, W. Lignin dynamics in secondary pasture soils of the South African Highveld. Geoderma 2018, 319, 113–121. [Google Scholar] [CrossRef]
- Ruiz Pessenda, L.C.; Marques Gouveia, S.E.; Ribeiro, A.d.S.; De Oliveira, P.E.; Aravena, R. Late Pleistocene and Holocene vegetation changes in northeastern Brazil determined from carbon isotopes and charcoal records in soils. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 297, 597–608. [Google Scholar] [CrossRef]
- Jiang, Z.-Y.; Hu, Z.-M.; Lai, D.Y.F.; Han, D.-R.; Wang, M.; Liu, M.; Zhang, M.; Guo, M.-Y. Light grazing facilitates carbon accumulation in subsoil in Chinese grasslands: A meta-analysis. Glob. Change Biol. 2020, 26, 7186–7197. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhao, Y.; Wang, X.; Hao, Z.; Wang, K.; Jiang, S.; Liu, H.; Zhou, X. Effects of recovery models on organic carbon pathways: A method using C-13 natural abundance. Agric. Ecosyst. Environ. 2022, 328, 107851. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Jia, H.; Liu, Y.; Zhao, Y.; Shi, C.; Zhang, F.; Wang, K. Assessing the soil moisture effects of planted vegetation on slope stability in shallow landslide-prone areas. J. Soils Sediments 2021, 21, 2551–2565. [Google Scholar] [CrossRef]
- Zhou, Y.; Yue, D.; Guo, J.; Chao, Z.; Meng, X. Assessing the impact of land conversion and management measures on the net primary productivity in the Bailong River Basin, in China. Catena 2021, 207, 105672. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Wang, X.; Ou, Y.S.; Jia, H.X.; Li, J.; Shi, C.M.; Liu, Y. Variations in soil δ13C with alpine meadow degradation on the eastern Qinghai-Tibet Plateau. Geoderma 2019, 338, 178–186. [Google Scholar] [CrossRef]
- Aran, D.; Maul, A.; Masfaraud, J.-F. A spectrophotometric measurement of soil cation exchange capacity based on cobaltihexamine chloride absorbance. Comptes Rendus Geosci. 2008, 340, 865–871. [Google Scholar] [CrossRef]
- Zhang, X.D.; Amelung, W. Gas chromatographic determination of muramic acid, glucosamine, mannosamine, and galactosamine in soils. Soil Biol. Biochem. 1996, 28, 1201–1206. [Google Scholar] [CrossRef]
- Hedges, J.I.; Ertel, J.R. Characterization of lignin by gas capillary chromatography of cupric oxide oxidation products. Anal. Chem. 1982, 54, 174–178. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K.; Doran, J.W. Aggregation and soil organic matter accumulation in cultivated and native grassland soils. Soil Sci. Soc. Am. J. 1998, 62, 1367–1377. [Google Scholar] [CrossRef]
- Chen, X.B.; Xia, Y.H.; Hu, Y.J.; Gunina, A.; Ge, T.D.; Zhang, Z.H.; Wu, J.S.; Su, Y.R. Effect of nitrogen fertilization on the fate of rice residue-C in paddy soil depending on depth: 13C amino sugar analysis. Biol. Fertil. Soils 2018, 54, 523–531. [Google Scholar] [CrossRef]
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Change Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Dai, G.H.; Zhu, S.S.; Chen, D.M.; Chen, L.T.; Lü, X.T.; Wang, X.B.; Zhu, J.T.; Zhang, Y.J.; Ma, W.H.; et al. Distribution and Preservation of Root- and Shoot-Derived Carbon Components in Soils Across the Chinese-Mongolian Grasslands. J. Geophys. Res.-Biogeosci. 2019, 124, 420–431. [Google Scholar] [CrossRef]
- Wang, B.R.; An, S.S.; Liang, C.; Liu, Y.; Kuzyakov, Y. Microbial necromass as the source of soil organic carbon in global ecosystems. Soil Biol. Biochem. 2021, 162, 13. [Google Scholar] [CrossRef]
- Pries, C.E.H.; Sulman, B.N.; West, C.; O’Neill, C.; Poppleton, E.; Porras, R.C.; Castanha, C.; Zhu, B.; Wiedemeier, D.B.; Torn, M.S. Root litter decomposition slows with soil depth. Soil Biol. Biochem. 2018, 125, 103–114. [Google Scholar] [CrossRef]
- Gu, X.; Fang, X.; Xiang, W.; Zeng, Y.; Zhang, S.; Lie, P.; Peng, C.; Kuzyakov, Y. Vegetation restoration stimulates soil carbon sequestration and stabilization in a subtropical area of southern China. Catena 2019, 181, 104098. [Google Scholar] [CrossRef]
- He, M.; Fang, K.; Chen, L.; Feng, X.; Qin, S.; Kou, D.; He, H.; Liang, C.; Yang, Y. Depth-dependent drivers of soil microbial necromass carbon across Tibetan alpine grasslands. Glob. Change Biol. 2021, 28, 936–949. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Maestre, F.T.; Gallardol, A.; Bowker, M.A.; Wallenstein, M.D.; Quero, J.L.; Ochoa, V.; Gozalo, B.; Garcia-Gomez, M.; Soliveres, S.; et al. Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature 2013, 502, 672–676. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Li, J.; Xiao, J.; Hao, Z.; Wang, K.; Jiang, S.; Zhou, X.; Liu, H. Variation of δ13C and soil organic carbon under different precipitation gradients in alpine grassland on the Qinghai–Tibetan Plateau. J. Soils Sediments 2022, 22, 2219–2228. [Google Scholar] [CrossRef]
- Kohl, L.; Laganière, J.; Edwards, K.A.; Billings, S.A.; Morrill, P.L.; Van Biesen, G.; Ziegler, S.E. Distinct fungal and bacterial δ13C signatures as potential drivers of increasing δ13C of soil organic matter with depth. Biogeochemistry 2015, 124, 13–26. [Google Scholar] [CrossRef]
- Castro, J.; Fernandez-Ondono, E.; Rodriguez, C.; Lallena, A.M.; Sierra, M.; Aguilar, J. Effects of different olive-grove management systems on the organic carbon and nitrogen content of the soil in Jaen (Spain). Soil Tillage Res. 2008, 98, 56–67. [Google Scholar] [CrossRef]
- Repullo, M.A.; Carbonell, R.; Hidalgo, J.; Rodriguez-Lizana, A.; Ordonez, R. Using olive pruning residues to cover soil and improve fertility. Soil Tillage Res. 2012, 124, 36–46. [Google Scholar] [CrossRef]
- Iversen, C.M.; Keller, J.K.; Garten, C.T.; Norby, R.J. Soil carbon and nitrogen cycling and storage throughout the soil profile in a sweetgum plantation after 11 years of CO2-enrichment. Glob. Change Biol. 2012, 18, 1684–1697. [Google Scholar] [CrossRef]
- Peri, P.L.; Ladd, B.; Pepper, D.A.; Bonser, S.P.; Laffan, S.W.; Amelung, W. Carbon (delta C-13) and nitrogen (delta N-15) stable isotope composition in plant and soil in Southern Patagonia’s native forests. Glob. Change Biol. 2012, 18, 311–321. [Google Scholar] [CrossRef]
- Krüger, N.; Finn, D.R.; Don, A. Soil depth gradients of organic carbon-13—A review on drivers and processes. Plant Soil 2023, 495, 113–136. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Buchmann, N.; Flanagan, L.B. Carbon isotope ratios in belowground carbon cycle processes. Ecol. Appl. 2000, 10, 412–422. [Google Scholar] [CrossRef]
- Menichetti, L.; Houot, S.; van Oort, F.; Kätterer, T.; Christensen, B.T.; Chenu, C.; Barré, P.; Vasilyeva, N.A.; Ekblad, A. Increase in soil stable carbon isotope ratio relates to loss of organic carbon: Results from five long-term bare fallow experiments. Oecologia 2015, 177, 811–821. [Google Scholar] [CrossRef] [PubMed]
- McHugh, T.A.; Compson, Z.; van Gestel, N.; Hayer, M.; Ballard, L.; Haverty, M.; Hines, J.; Irvine, N.; Krassner, D.; Lyons, T.; et al. Climate controls prokaryotic community composition in desert soils of the southwestern United States. FEMS Microbiol. Ecol. 2017, 93, 13. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Feng, X.J.; He, J.S.; He, H.B.; Lin, L.; Liu, Z.G. Comparing microbial carbon sequestration and priming in the subsoil versus topsoil of a Qinghai-Tibetan alpine grassland. Soil Biol. Biochem. 2017, 104, 141–151. [Google Scholar] [CrossRef]
- Shao, P.S.; Lynch, L.; Xie, H.T.; Bao, X.L.; Liang, C. Tradeoffs among microbial life history strategies influence the fate of microbial residues in subtropical forest soils. Soil Biol. Biochem. 2021, 153, 7. [Google Scholar] [CrossRef]
- Ni, X.Y.; Liao, S.; Tan, S.Y.; Wang, D.Y.; Peng, Y.; Yue, K.; Wu, F.Z.; Yang, Y.S. A quantitative assessment of amino sugars in soil profiles. Soil Biol. Biochem. 2020, 143, 10. [Google Scholar] [CrossRef]
- Castellano, M.J.; Mueller, K.E.; Olk, D.C.; Sawyer, J.E.; Six, J. Integrating plant litter quality, soil organic matter stabilization, and the carbon saturation concept. Glob. Change Biol. 2015, 21, 3200–3209. [Google Scholar] [CrossRef] [PubMed]
- Samson, M.E.; Chantigny, M.H.; Vanasse, A.; Menasseri-Aubry, S.; Royer, I.; Angers, D.A. Management practices differently affect particulate and mineral-associated organic matter and their precursors in arable soils. Soil Biol. Biochem. 2020, 148, 9. [Google Scholar] [CrossRef]
- Ding, X.L.; Liang, C.; Zhang, B.; Yuan, Y.R.; Han, X.Z. Higher rates of manure application lead to greater accumulation of both fungal and bacterial residues in macroaggregates of a clay soil. Soil Biol. Biochem. 2015, 84, 137–146. [Google Scholar] [CrossRef]


| Dominant Vegetation | Longitude | Latitude | Altitude | |
|---|---|---|---|---|
| PL | Olea europaea | 104°48′09″ | 33°26′15″ | 1149 |
| GLs | Setaria viridis | 104°48′19″ | 33°26′20″ | 1105 |
| CLs | Zea mays | 104°48′25″ | 33°26′14″ | 1092 |
| SLs | Lycium chinense Mill | 104°48′21″ | 33°26′17″ | 1106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wang, X.; Zhang, Y.; Duan, W.; Xia, J.; Wu, J.; Deng, T. Vegetation Types Can Affect Soil Organic Carbon and δ13C by Influencing Plant Inputs in Topsoil and Microbial Residue Carbon Composition in Subsoil. Sustainability 2024, 16, 4538. https://doi.org/10.3390/su16114538
Sun Y, Wang X, Zhang Y, Duan W, Xia J, Wu J, Deng T. Vegetation Types Can Affect Soil Organic Carbon and δ13C by Influencing Plant Inputs in Topsoil and Microbial Residue Carbon Composition in Subsoil. Sustainability. 2024; 16(11):4538. https://doi.org/10.3390/su16114538
Chicago/Turabian StyleSun, Yuxin, Xia Wang, Yuanye Zhang, Wenhui Duan, Jieyi Xia, Jinhong Wu, and Tao Deng. 2024. "Vegetation Types Can Affect Soil Organic Carbon and δ13C by Influencing Plant Inputs in Topsoil and Microbial Residue Carbon Composition in Subsoil" Sustainability 16, no. 11: 4538. https://doi.org/10.3390/su16114538
APA StyleSun, Y., Wang, X., Zhang, Y., Duan, W., Xia, J., Wu, J., & Deng, T. (2024). Vegetation Types Can Affect Soil Organic Carbon and δ13C by Influencing Plant Inputs in Topsoil and Microbial Residue Carbon Composition in Subsoil. Sustainability, 16(11), 4538. https://doi.org/10.3390/su16114538





