Modelling Climate Using Leaves of Nothofagus cunninghamii—Overcoming Confounding Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
- The species and its close relatives are found in Quaternary deposits through southeastern Australia, where paleoclimates are currently unresolved. Thus, determining the responses of N. cunninghamii to current climates can assist in hindcasting paleoclimatic conditions and understanding the species response to past climate change.
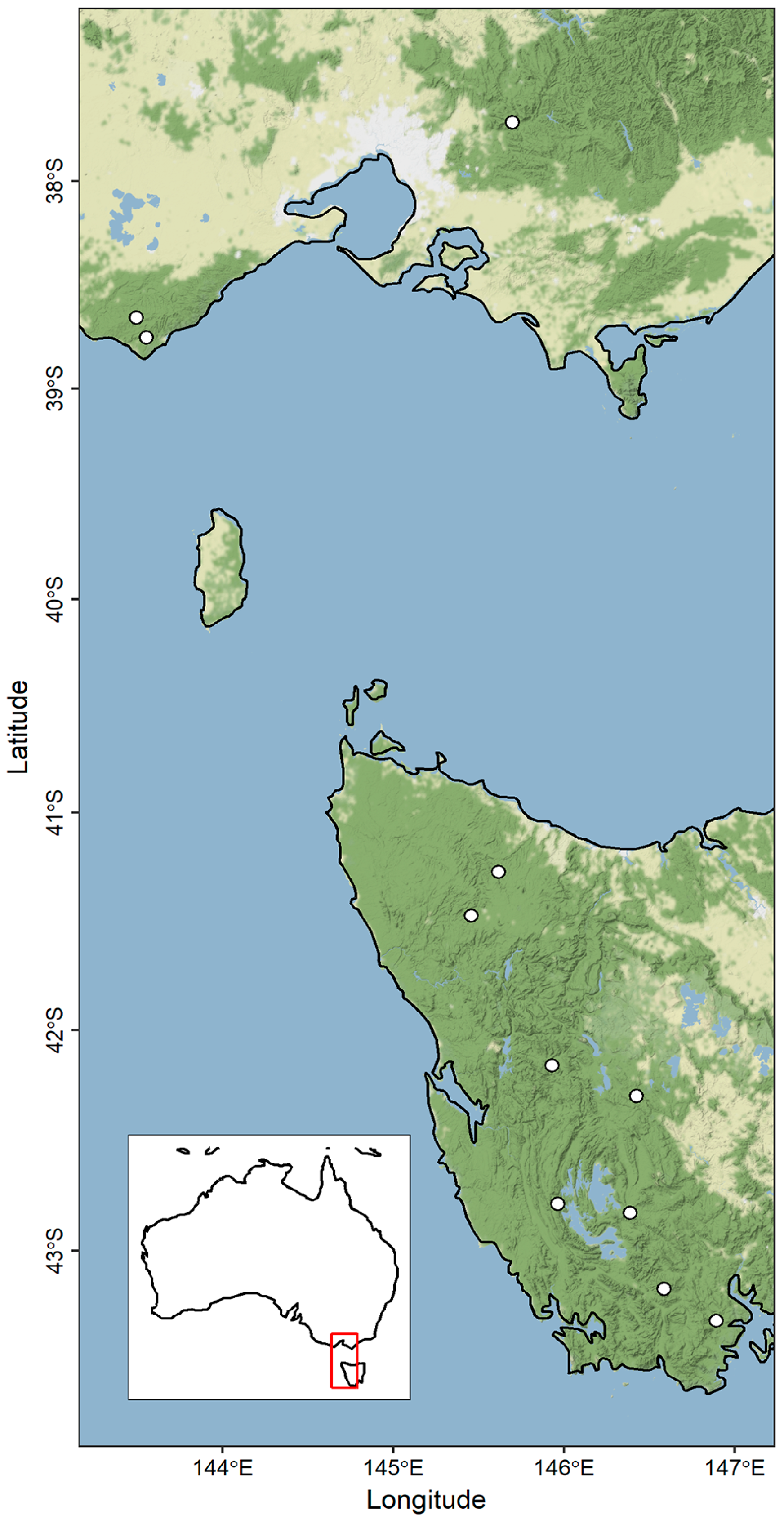
- Living Nothofagus cunninghamii occurs over a relatively large latitudinal and elevation gradient (Figure 1; elevation range = 112–1240 m), and the species has been found to be able to grow within a mean annual temperature range of 5 °C. This capacity to persist in a wide range of temperature environments makes N. cunninghamii ideal for modelling climate responses [14,15,16].
- The leaves of N. cunninghamii are evenly covered with stomata (including all veins except the mid-vein), and the stomata and epidermal cells are easily identified and counted, allowing their density to be measured relatively quickly and with accuracy. This is an important consideration, particularly when using fossil leaves for paleoclimatic reconstructions. In contrast, other species in the genus have stomata distributed in areoles that impact measures such as stomatal and epidermal cell density, especially in fragmentary fossil leaf material.
- The Cenozoic macrofossil record in eastern Australia contains many examples of leaves that appear to be intermediate in morphology between the living species N. cunninghamii and N. moorei, which is distributed further north in Australia, around the New South Wales and Queensland border [17,18]. This offers the potential to extend the range over which leaf anatomical data can be extended back into the Cenozoic.
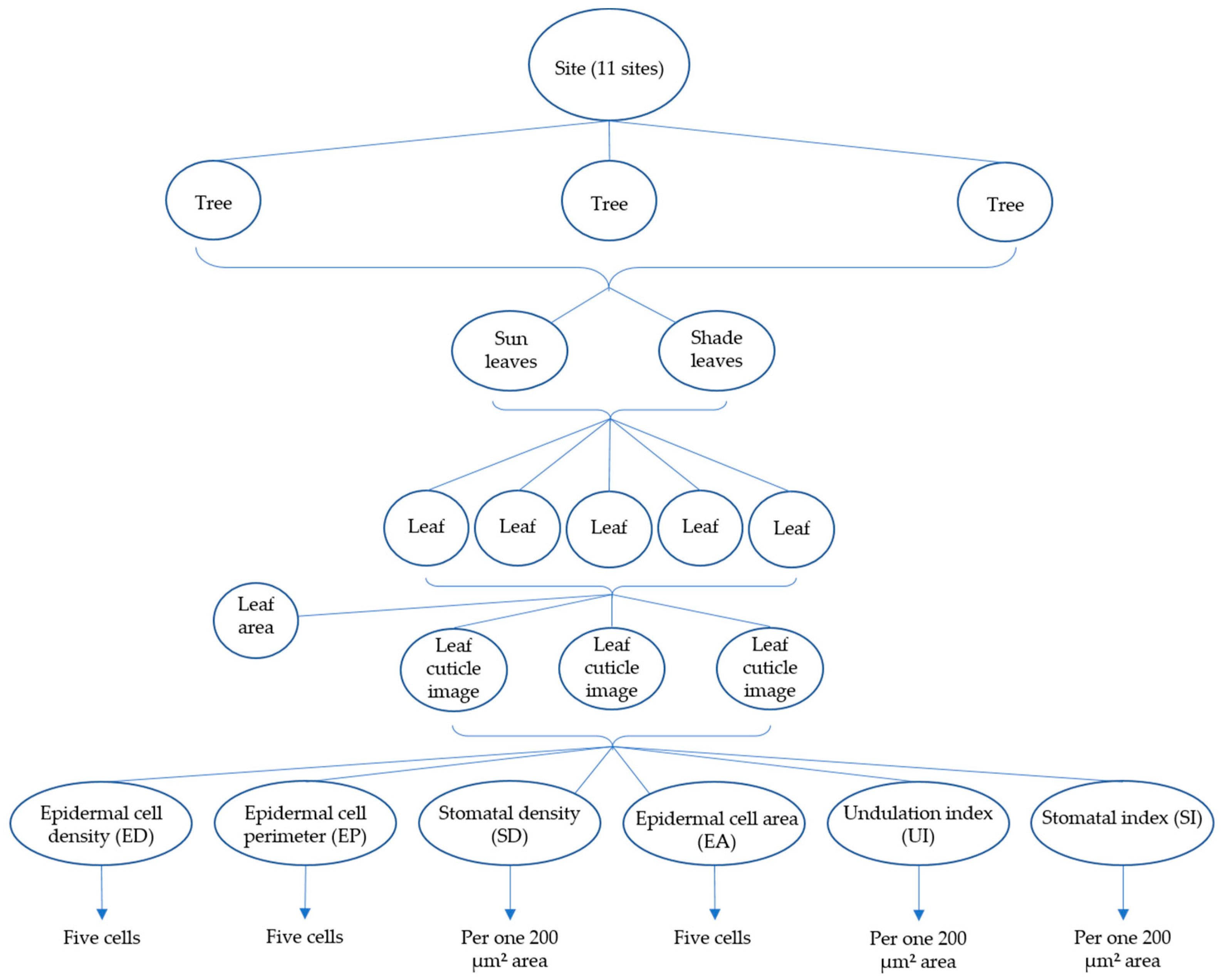
2.2. Measurements and Field Collection
2.3. Modelling
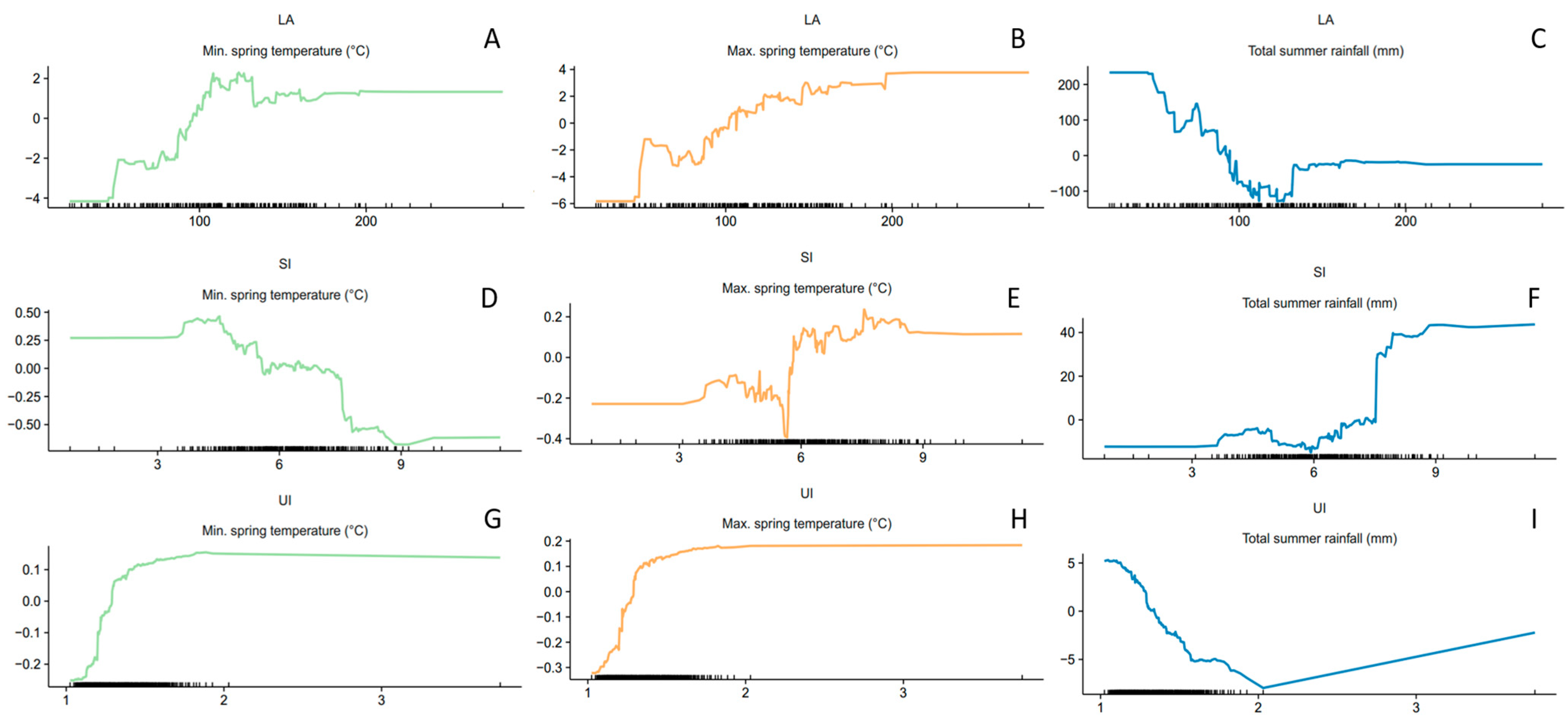
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peppe, D.J.; Royer, D.L.; Cariglino, B.; Oliver, S.Y.; Newman, S.; Leight, E.; Enikolopov, G.; Fernandez-Burgos, M.; Herrera, F.; Adams, J.M.; et al. Sensitivity of leaf size and shape to climate: Global patterns and paleoclimatic applications. New Phytol. 2011, 190, 724–739. [Google Scholar] [CrossRef]
- Diefendorf, A.F.; Mueller, K.E.; Wing, S.L.; Koch, P.L.; Freeman, K.H. Global patterns in leaf 13C discrimination and implications for studies of past and future climate. Proc. Natl. Acad. Sci. USA 2010, 107, 5738–5743. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.E.; Hill, R.S.; Watling, J.R. Pinnule and stomatal size and stomatal density of living and fossil Bowenia and Eobowenia specimens give insight into physiology during Cretaceous and Eocene paleoclimates. Int. J. Plant Sci. 2019, 180, 323–336. [Google Scholar] [CrossRef]
- Jordan, G. Uncertainty in palaeoclimatic reconstructions based on leaf physiognomy. Aust. J. Bot. 1997, 45, 527–548. [Google Scholar] [CrossRef]
- Royer, D.L.; Wilf, P.; Janesko, D.A.; Kowalski, E.A.; Dilcher, D.L. Correlations of climate and plant ecology to leaf size and shape: Potential proxies for the fossil record. Am. J. Bot. 2005, 92, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.F.; Niinemets, Ü. Global leaf trait estimates biased due to plasticity in the shade. Nat. Plants 2016, 3, 16201. [Google Scholar] [CrossRef]
- Maslova, N.P.; Karasev, E.V.; Kodrul, T.M.; Spicer, R.A.; Volkova, L.D.; Spicer, T.E.V.; Jin, J.; Liu, X. Sun and shade leaf variability in Liquidambar chinensis and Liquidambar formosana (Altingiaceae): Implications for palaeobotany. Bot. J. Linn. Soc. 2018, 188, 296–315. [Google Scholar] [CrossRef]
- McMillen, G.G.; McClendon, J.H. Dependence of photosynthetic rates on leaf density thickness in deciduous woody plants grown in sun and shade. Plant Physiol. 1983, 72, 674–678. [Google Scholar] [CrossRef]
- Ashton, P.; Berlyn, G. Leaf adaptations of some Shorea species to sun and shade. New Phytol. 1992, 121, 587–596. [Google Scholar] [CrossRef]
- Abrams, M.D.; Kubiske, M.E. Leaf structural characteristics of 31 hardwood and conifer tree species in central Wisconsin: Influence of light regime and shade-tolerance rank. For. Ecol. Manag. 1990, 31, 245–253. [Google Scholar] [CrossRef]
- Kürschner, W.M.; van der Burgh, J.; Visscher, H.; Dilcher, D.L. Oak leaves as biosensors of late Neogene and early Pleistocene paleoatmospheric CO2 concentrations. Mar. Micropaleontol. 1996, 27, 299–312. [Google Scholar] [CrossRef]
- Poole, I.; Weyers, J.D.B.; Lawson, T.; Raven, J.A. Variations in stomatal density and index: Implications for palaeoclimatic reconstructions. Plant Cell Environ. 1996, 19, 705–712. [Google Scholar] [CrossRef]
- Carins Murphy, M.R.; Jordan, G.J.; Brodribb, T.J. Differential leaf expansion can enable hydraulic acclimation to sun and shade. Plant Cell Environ. 2012, 35, 1407–1418. [Google Scholar] [CrossRef]
- Hovenden, M.J.; Vander Schoor, J.K. The response of leaf morphology to irradiance depends on altitude of origin in Nothofagus cunninghamii. New Phytol. 2006, 169, 291–297. [Google Scholar] [CrossRef]
- Worth, J.R.P.; Jordan, G.J.; McKinnon, G.E.; Vaillancourt, R.E. The major Australian cool temperate rainforest tree Nothofagus cunninghamii withstood Pleistocene glacial aridity within multiple regions: Evidence from the chloroplast. New Phytol. 2009, 182, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Hovenden, M.J. The influence of temperature and genotype on the growth and stomatal morphology of southern beech, Nothofagus cunninghamii (Nothofagaceae). Aust. J. Bot. 2001, 49, 427–434. [Google Scholar] [CrossRef]
- Hill, R. Evolution of Nothofagus cunninghamii and its relationship to N. moorei as inferred from Tasmanian macrofossils. Aust. J. Bot. 1983, 31, 453–465. [Google Scholar] [CrossRef]
- Hill, R.S. Biogeography, evolution and palaeoecology of Nothofagus (Nothofagaceae): The contribution of the fossil record. Aust. J. Bot. 2001, 49, 321–332. [Google Scholar] [CrossRef]
- Scriven, L.J.; Hill, R.S. Relationships among Tasmanian Tertiary Nothofagus (Nothofagaceae) populations. Bot. J. Linn. Soc. 1996, 121, 345–364. [Google Scholar] [CrossRef][Green Version]
- Kürschner, W.M. The anatomical diversity of recent and fossil leaves of the durmast oak (Quercus petraea Lieblein/Q. pseudocastanea Goeppert)—Implications for their use as biosensors of palaeoatmospheric CO2 levels. Rev. Palaeobot. Palynol. 1997, 96, 1–30. [Google Scholar] [CrossRef]
- Salisbury, E.J. On the causes and ecological significance of stomatal frequency, with special reference to the woodland flora. Philos. Trans. R. Soc. Lond. Ser. B Contain. Pap. A Biol. Character 1927, 216, 1–65. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Hill, K.E.; Guerin, G.R.; Hill, R.S.; Watling, J.R. Temperature influences stomatal density and maximum potential water loss through stomata of Dodonaea viscosa subsp. angustissima along a latitude gradient in southern Australia. Aust. J. Bot. 2015, 62, 657–665. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Meng, Y.A.; Yu, Y.; Cupples, L.A.; Farrer, L.A.; Lunetta, K.L. Performance of random forest when SNPs are in linkage disequilibrium. BMC Bioinform. 2009, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Strobl, C.; Boulesteix, A.-L.; Zeileis, A.; Hothorn, T. Bias in random forest variable importance measures: Illustrations, sources and a solution. BMC Bioinform. 2007, 8, 25. [Google Scholar] [CrossRef]
- Breiman, L.; Friedman, J.; Olshen, R.; Stone, C. Classification and Regression Trees, 1st ed.; Routledge: New York, NY, USA, 1984. [Google Scholar]
- Apley, D.W. Visualizing the effects of predictor variables in black box supervised learning models. arXiv 2016, arXiv:1612.08468. [Google Scholar] [CrossRef]
- Jordan, G.J. A new Early Pleistocene species of Nothofagus and the climatic implications of co-occurring Nothofagus fossils. Aust. Syst. Bot. 1999, 12, 757–765. [Google Scholar] [CrossRef]
- Körner, C. When meta-analysis fails: A case about stomata. Glob. Chang. Biol. 2017, 23, 2533–2534. [Google Scholar] [CrossRef] [PubMed]
- Scriven, L.J.; McLoughlin, S.; Hill, R.S. Nothofagus plicata (Nothofagaceae), a new deciduous Eocene macrofossil species, from southern continental Australia. Rev. Palaeobot. Palynol. 1995, 86, 199–209. [Google Scholar] [CrossRef]
- Busby, J.R. A biogeoclimatic analysis of Nothofagus cunninghamii (Hook.) Oerst. in southeastern Australia. Aust. J. Ecol. 1986, 11, 1–7. [Google Scholar] [CrossRef]
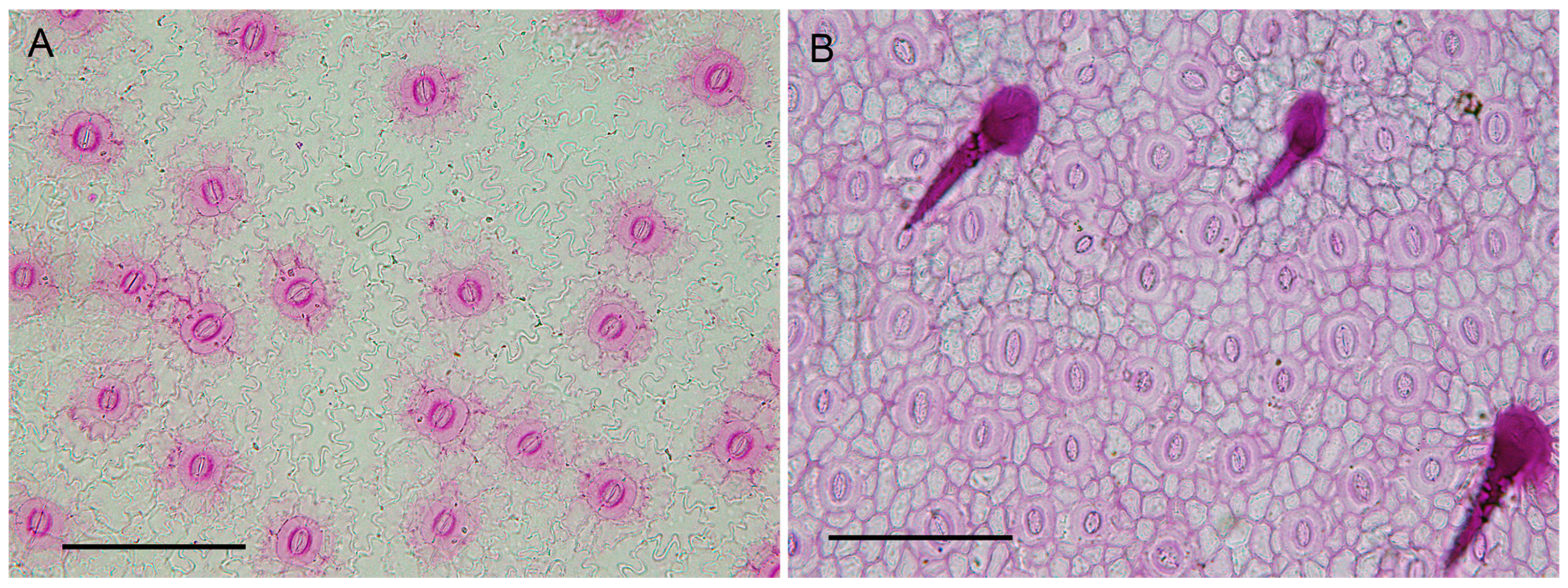

| Variable | Sun | Shade |
|---|---|---|
| Stomatal density (stomata mm−2) | 396 ± 1 | 281 ± 9 |
| Epidermal cell density (epidermal cells mm−2) | 5788.6 ± 1255.4 | 4537.6 ± 1036.8 |
| Epidermal cell area (µm2) | 197.5 ± 94.8 | 286.1 ± 142.0 |
| Epidermal cell perimeter (µm) | 63.3 ± 22.7 | 86.8 ± 32.7 |
| Undulation index | 1.3 ± 0.2 | 1.5 ± 0.2 |
| Leaf area (mm2) | 108.6 ± 43.3 | 114.5 ± 42.2 |
| Stomatal index (%) | 6.4 ± 1.4 | 5.8 ± 1.4 |
| Variable | Maximum Spring Temperature (°C) | Minimum Spring Temperature (°C) | Total Summer Rainfall (mm) |
|---|---|---|---|
| Leaf area (mm2) | 1 | 1 | 1 |
| Epidermal cell density (epidermal cells mm−2) | 0.29 | 0.31 | 0.16 |
| Epidermal cell area (µm2) | 0.29 | 0.26 | 0.13 |
| Stomatal index (%) | 0.20 | 0.31 | 0.21 |
| Stomatal density (stomata mm−2) | 0.16 | 0.15 | 0.11 |
| Sun or shade leaf | 0.14 | 0.08 | 0.32 |
| Undulation index | 0.06 | 0.09 | 0.07 |
| Epidermal cell perimeter (µm) | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hill, K.E.; Brown, S.C.; Jones, A.; Fordham, D.; Hill, R.S. Modelling Climate Using Leaves of Nothofagus cunninghamii—Overcoming Confounding Factors. Sustainability 2023, 15, 7603. https://doi.org/10.3390/su15097603
Hill KE, Brown SC, Jones A, Fordham D, Hill RS. Modelling Climate Using Leaves of Nothofagus cunninghamii—Overcoming Confounding Factors. Sustainability. 2023; 15(9):7603. https://doi.org/10.3390/su15097603
Chicago/Turabian StyleHill, Kathryn E., Stuart C. Brown, Alice Jones, Damien Fordham, and Robert S. Hill. 2023. "Modelling Climate Using Leaves of Nothofagus cunninghamii—Overcoming Confounding Factors" Sustainability 15, no. 9: 7603. https://doi.org/10.3390/su15097603
APA StyleHill, K. E., Brown, S. C., Jones, A., Fordham, D., & Hill, R. S. (2023). Modelling Climate Using Leaves of Nothofagus cunninghamii—Overcoming Confounding Factors. Sustainability, 15(9), 7603. https://doi.org/10.3390/su15097603






