Potential Effects of Methane Metabolic Microbial Communities on the Glacial Methane Budget in the Northwestern Tibetan Plateau
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Sample Collection
2.2. Ice Decontamination
2.3. Direct Cell Count and HumM2 Gene Quantification
2.4. DNA Extraction and Microbial Community Studies
2.5. Estimation of Metabolic Rates of Methanogens and Methanotrophs at the Guliya Glacier Terminus
3. Results and Discussion
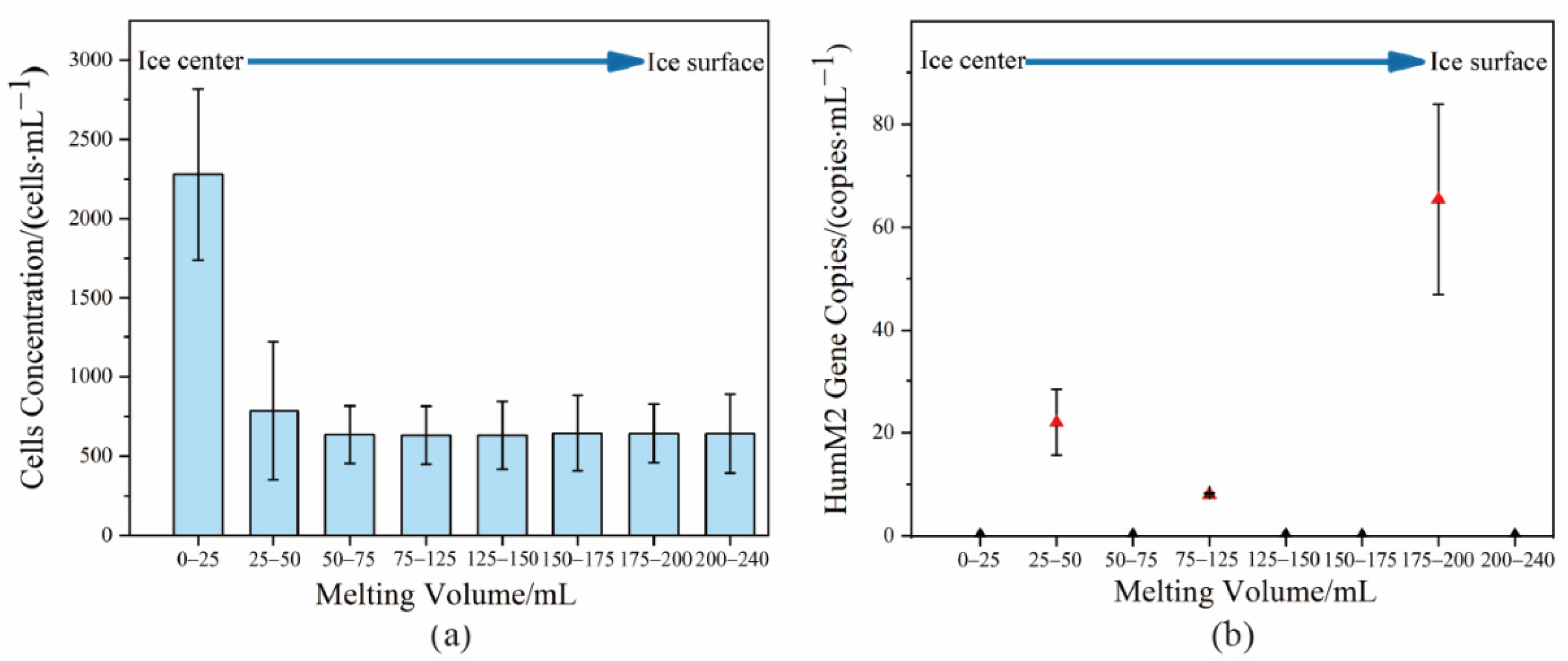
3.1. Evaluation of the Decontamination Effect of the Ice Samples
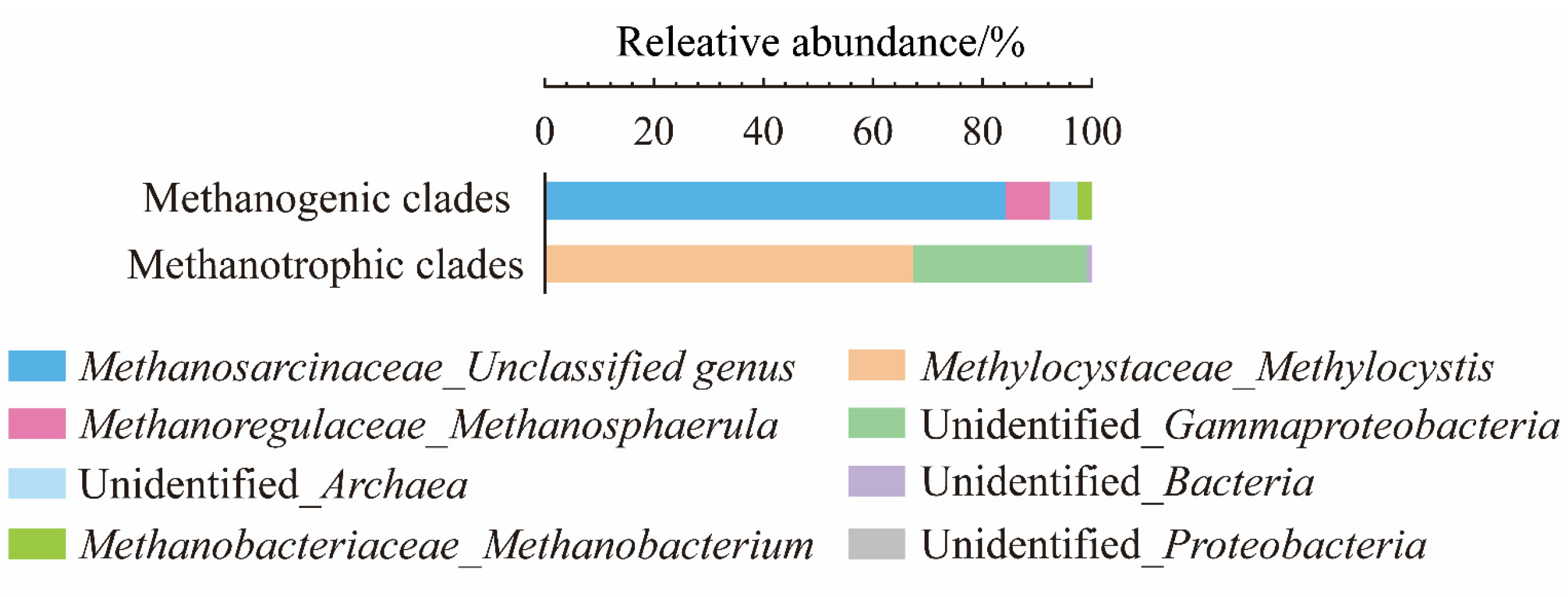
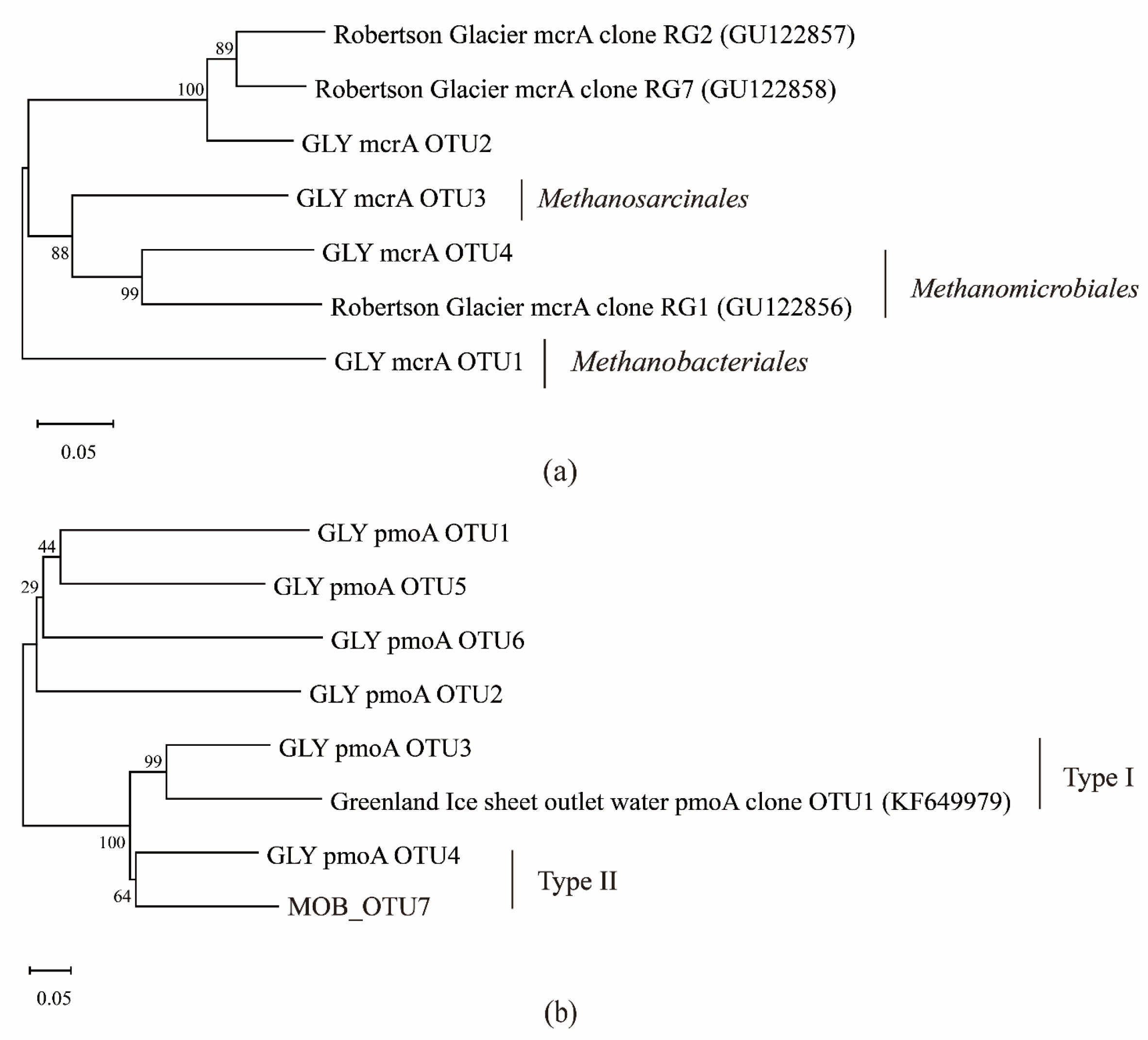
3.2. Analysis of the Microbial Community Composition of CH4-Metabolic Microbial Communities at the Guliya Glacier Terminus
3.3. Impact of CH4-Metabolic Microbial Communities on the Glacial CH4 Budget
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saunois, M.; Bousquet, P.; Poulter, B.; Peregon, A.; Ciais, P.; Canadell, J.G.; Dlugokencky, E.J.; Etiope, G.; Bastviken, D.; Houweling, S.; et al. The Global Methane Budget 2000–2012. Earth Syst. Sci. Data 2016, 8, 697–751. [Google Scholar]
- Kirschke, S.; Bousquet, P.; Ciais, P.; Saunois, M.; Canadell, J.G.; Dlugokencky, E.J.; Bergamaschi, P.; Bergmann, D.; Blake, D.R.; Bruhwiler, L.; et al. Three Decades of Global Methane Sources and Sinks. Nat. Geosci. 2013, 6, 813–823. [Google Scholar] [CrossRef]
- Conrad, R. The Global Methane Cycle: Recent Advances in Understanding the Microbial Processes Involved. Environ. Microbiol. Rep. 2009, 1, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Wadham, J.L.; Hawkings, J.R.; Tarasov, L.; Gregoire, L.J.; Spencer, R.G.M.; Gutjahr, M.; Ridgwell, A.; Kohfeld, K.E. Ice Sheets Matter for the Global Carbon Cycle. Nat. Commun. 2019, 10, 17. [Google Scholar]
- Wadham, J.L.; Arndt, S.; Tulaczyk, S.; Stibal, M.; Tranter, M.; Telling, J.; Lis, G.P.; Lawson, E.; Ridgwell, A.; Dubnick, A.; et al. Potential Methane Reservoirs Beneath Antarctica. Nature 2012, 488, 633–637. [Google Scholar] [CrossRef]
- Lamarche-Gagnon, G.; Wadham, J.L.; Lollar, B.S.; Arndt, S.; Fietzek, P.; Beaton, A.D.; Tedstone, A.J.; Telling, J.; Bagshaw, E.A.; Hawkings, J.R.; et al. Greenland Melt Drives Continuous Export of Methane from the Ice-Sheet Bed. Nature 2019, 565, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kang, S.; Wei, D.; Luo, X.; Wang, Z.; Gao, T. Sink or source? Methane and Carbon Dioxide Emissions from Cryoconite Holes, Subglacial Sediments, and Proglacial River Runoff During Intensive Glacier Melting on the Tibetan Plateau. Fundam. Res. 2021, 1, 232–239. [Google Scholar] [CrossRef]
- Wadham, J.L.; Tranter, M.; Tulaczyk, S.; Sharp, M. Subglacial Methanogenesis: A Potential Climatic Amplifier? Glob. Biogeochem. Cycle 2008, 22, GB2021. [Google Scholar] [CrossRef]
- Skidmore, M.L.; Foght, J.M.; Sharp, M.J. Microbial Life Beneath a High Arctic Glacier. Appl. Environ. Microbiol. 2000, 66, 3214–3220. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, N.; Yuan, M.; Xiao, J.; Qin, Y.; Deng, Y.; Tu, Q.; Xue, K.; van Nostrand, J.D.; Wu, L.; et al. Warming Enhances Old Organic Carbon Decomposition Through Altering Functional Microbial Communities. ISME J. 2017, 11, 1825–1835. [Google Scholar] [CrossRef]
- Dean, J.F.; Middelburg, J.J.; Rockmann, T.; Aerts, R.; Blauw, L.G.; Egger, M.; Jetten, M.S.M.; de Jong, A.E.E.; Meisel, O.H.; Rasigraf, O.; et al. Methane Feedbacks to the Global Climate System in A Warmer World. Rev. Geophys. 2018, 56, 207–250. [Google Scholar]
- Du, Z.-H.; Wang, L.; Wei, Z.-Q.; Liu, J.-F.; Lin, P.-L.; Lin, J.-H.; Li, Y.-Z.; Jin, Z.-Z.; Chen, J.-Z.; Wang, X.-X.; et al. CH4 and CO2 Observations from A Melting High Mountain Glacier, Laohugou Glacier No. 12. Adv. Clim. Chang. Res. 2022, 13, 146–155. [Google Scholar] [CrossRef]
- Campen, R.K.; Sowers, T.; Alley, R.B. Evidence of Microbial Consortia Metabolizing Within a Low-Latitude Mountain Glacier. Geology 2003, 31, 231–234. [Google Scholar] [CrossRef]
- Anderson, I.; Ulrich, L.E.; Lupa, B.; Susanti, D.; Porat, I.; Hooper, S.D.; Lykidis, A.; Sieprawska-Lupa, M.; Dharmarajan, L.; Goltsman, E.; et al. Genomic Characterization of Methanomicrobiales Reveals Three Classes of Methanogens. PLoS ONE 2009, 4, e5759. [Google Scholar] [CrossRef]
- Whiticar, M.J. Carbon and Hydrogen Isotope Systematics of Bacterial Formation and Oxidation of Methane. Chem. Geol. 1999, 161, 291–314. [Google Scholar] [CrossRef]
- Op den Camp, H.J.M.; Islam, T.; Stott, M.B.; Harhangi, H.R.; Hynes, A.; Schouten, S.; Jetten, M.S.M.; Birkeland, N.K.; Pol, A.; Dunfield, P.F. Environmental, Genomic and Taxonomic Perspectives on Methanotrophic Verrucomicrobia. Environ. Microbiol. Rep. 2009, 1, 293–306. [Google Scholar] [CrossRef]
- Hanson, R.S.; Hanson, T.E. Methanotrophic Bacteria. Microbiol. Rev. 1996, 60, 439–471. [Google Scholar] [CrossRef]
- Tung, H.C.; Bramall, N.E.; Price, P.B. Microbial Origin of Excess Methane in Glacial Ice and Implications for Life on Mars. Proc. Natl. Acad. Sci. USA 2005, 102, 18292–18296. [Google Scholar] [CrossRef]
- Tung, H.C.; Price, P.B.; Bramall, N.E.; Vrdoljak, G. Microorganisms Metabolizing on Clay Grains in 3-km-Deep Greenland Basal Ice. Astrobiology 2006, 6, 69–86. [Google Scholar] [CrossRef]
- Hou, S.; Chappellaz, J.; Raynaud, D.; Masson-Delmotte, V.; Jouzel, J.; Bousquet, P.; Hauglustaine, D. A New Himalayan Ice Core CH4 Record: Possible Hints at the Preindustrial Latitudinal Gradient. Clim. Past. 2013, 9, 2549–2554. [Google Scholar] [CrossRef]
- Dieser, M.; Broemsen, E.L.J.E.; Cameron, K.A.; King, G.M.; Achberger, A.; Choquette, K.; Hagedorn, B.; Sletten, R.; Junge, K.; Christner, B.C. Molecular and Biogeochemical Evidence for Methane Cycling Beneath the Western Margin of the Greenland Ice Sheet. ISME J. 2014, 8, 2305–2316. [Google Scholar] [CrossRef] [PubMed]
- Anesio, A.M.; Lutz, S.; Chrismas, N.A.M.; Benning, L.G. The Microbiome of Glaciers and Ice Sheets. NPJ Biofilms Microbomes 2017, 3, 10. [Google Scholar] [CrossRef]
- Boetius, A.; Anesio, A.M.; Deming, J.W.; Mikucki, J.A.; Rapp, J.Z. Microbial Ecology of the Cryosphere: Sea Ice and Glacial Habitats. Nat. Rev. Microbiol. 2015, 13, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Price, P.B. A Habitat for Psychrophiles in Deep Antarctic Ice. Proc. Natl. Acad. Sci. USA 2000, 97, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Rohde, R.A.; Price, P.B. Diffusion-Controlled Metabolism for Long-Term Survival of Single Isolated Microorganisms Trapped Within Ice Crystals. Proc. Natl. Acad. Sci. USA 2007, 104, 16592–16597. [Google Scholar] [CrossRef] [PubMed]
- Michaud, A.B.; Dore, J.E.; Achberger, A.M.; Christner, B.C.; Mitchell, A.C.; Skidmore, M.L.; Vick-Majors, T.J.; Priscu, J.C. Microbial Oxidation as A Methane Sink Beneath the West Antarctic Ice Sheet. Nat. Geosci. 2017, 10, 582–586. [Google Scholar] [CrossRef]
- Ma, H.; Yan, W.; Xiao, X.; Shi, G.; Li, Y.; Sun, B.; Dou, Y.; Zhang, Y. Ex Situ Culturing Experiments Revealed Psychrophilic Hydrogentrophic Methanogenesis Being the Potential Dominant Methane-Producing Pathway in Subglacial Sediment in Larsemann Hills, Antarctic. Front. Microbiol. 2018, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Thompson, L.; Yang, W.; Yu, W.; Gao, Y.; Guo, X.; Yang, X.; Duan, K.; Zhao, H.; Xu, B.; et al. Different Glacier Status with Atmospheric Circulations in Tibetan Plateau and Surroundings. Nat. Clim. Change 2012, 2, 663–667. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, M.; Yu, T.; Zaugg, J.; Anesio, A.M.; Zhang, Z.; Hu, S.; Hugenholtz, P.; Liu, K.; Liu, P.; et al. A Genome and Gene Catalog of Glacier Microbiomes. Nat. Biotechnol. 2022, 40, 1341–1348. [Google Scholar] [CrossRef]
- Chen, D.; Xu, B.; Yao, T.; Guo, Z.; Cui, P.; Chen, F.; Zhang, R.; Zhang, X.; Zhang, Y.; Fan, J.; et al. Assessment of Past, Present and Future Environmental Changes on the Tibetan Plateau. Chin. Sci. Bull. 2015, 60, 3025–3035. [Google Scholar]
- Kang, S.; Xu, Y.; You, Q.; Flugel, W.-A.; Pepin, N.; Yao, T. Review of Climate and Cryospheric Change in the Tibetan Plateau. Environ. Res. Lett. 2010, 5, 015101. [Google Scholar] [CrossRef]
- Bolch, T.; Kulkarni, A.; Kaab, A.; Huggel, C.; Paul, F.; Cogley, J.G.; Frey, H.; Kargel, J.S.; Fujita, K.; Scheel, M. The State and Fate of Himalayan Glaciers. Science 2012, 336, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xia, X.; Liu, S.; Zhang, S.; Li, S.; Wang, J.; Wang, G.; Gao, H.; Zhang, Z.; Wang, Q.; et al. Significant Methane Ebullition from Alpine Permafrost Rivers on the East Qinghai-Tibet Plateau. Nat. Geosci. 2020, 13, 349–354. [Google Scholar] [CrossRef]
- Xing, T.; Liu, P.; Ji, M.; Deng, Y.; Liu, K.; Wang, W.; Liu, Y. Sink or Source: Alternative Roles of Glacier Foreland Meadow Soils in Methane Emission Is Regulated by Glacier Melting on the Tibetan Plateau. Front. Microbiol. 2022, 13, 862242. [Google Scholar] [CrossRef]
- Zhong, Z.-P.; Solonenko, N.E.; Gazitua, M.C.; Kenny, D.V.; Mosley-Thompson, E.; Rich, V.I.; van Etten, J.L.; Thompson, L.G.; Sullivan, M.B. Clean Low-Biomass Procedures and Their Application to Ancient Ice Core Microorganisms. Front. Microbiol. 2018, 9, 1094. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Ritterbusch, F.; Gu, J.-Q.; Hu, S.-M.; Jiang, W.; Lu, Z.-T.; Wang, D.; Yang, G.-M. Kr-81 Dating at the Guliya Ice Cap, Tibetan Plateau. Geophys. Res. Lett. 2019, 46, 6636–6643. [Google Scholar] [CrossRef]
- Rogers, S.O.; Theraisnathan, V.; Ma, L.J.; Zhao, Y.; Zhang, G.; Shin, S.-G.; Castello, J.D.; Starmer, W.T. Comparisons of Protocols for Decontamination of Environmental Ice Samples for Biological and Molecular Examinations. Appl. Environ. Microbiol. 2004, 70, 2540–2544. [Google Scholar] [CrossRef]
- Zhong, Z.-P.; Tian, F.; Roux, S.; Gazitua, M.C.; Solonenko, N.E.; Li, Y.-F.; Davis, M.E.; van Etten, J.L.; Mosley-Thompson, E.; Rich, V.I.; et al. Glacier Ice Archives Nearly 15,000-Year-Old Microbes and Phages. Microbiome 2021, 9, 160. [Google Scholar]
- Shanks, O.C.; Kelty, C.A.; Sivaganesan, M.; Varma, M.; Haugland, R.A. Quantitative PCR for Genetic Markers of Human Fecal Pollution. Appl. Environ. Microbiol. 2009, 75, 5507–5513. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global Patterns of 16S rRNA Diversity at A Depth of Millions of Sequences Per Sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Luton, P.E.; Wayne, J.M.; Sharp, R.J.; Riley, P.W. The mcrA Gene as An Alternative to 16S rRNA in the Phylogenetic Analysis of Methanogen Populations in Landfill. Microbiology 2002, 148, 3521–3530. [Google Scholar] [CrossRef] [PubMed]
- McDonald, I.R.; Bodrossy, L.; Chen, Y.; Murrell, J.C. Molecular Ecology Techniques for the Study of Aerobic Methanotrophs. Appl. Environ. Microbiol. 2008, 74, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Kolb, S.; Knief, C.; Stubner, S.; Conrad, R. Quantitative Detection of Methanotrophs in Soil by Novel pmoA-Targeted Real-Time PCR Assays. Appl. Environ. Microbiol. 2003, 69, 2423–2429. [Google Scholar] [CrossRef]
- Liu, C.; Li, H.; Zhang, Y.; Si, D.; Chen, Q. Evolution of Microbial Community Along with Increasing Solid Concentration During High-Solids Anaerobic Digestion of Sewage Sludge. Bioresour. Technol. 2016, 216, 87–94. [Google Scholar] [CrossRef]
- Xu, N.; Tan, G.; Wang, H.; Gai, X. Effect of Biochar Additions to Soil on Nitrogen Leaching, Microbial Biomass and Bacterial Community Structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Yang, S.; Liebner, S.; Alawi, M.; Ebenhoh, O.; Wagner, D. Taxonomic Database and Cut-Off Value for Processing mcrA Gene 454 Pyrosequencing Data by MOTHUR. J. Microbiol. Methods 2014, 103, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, L.M.; Regan, J.M. mcrA-Targeted Real-Time Quantitative PCR Method to Examine Methanogen Communities. Appl. Environ. Microbiol. 2009, 75, 4435–4442. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Fish, J.A.; Chai, B.L.; Wang, Q.; Sun, Y.; Brown, C.T.; Tiedje, J.M.; Cole, J.R. FunGene: The Functional Gene Pipeline and Repository. Front. Microbiol. 2013, 4, 291. [Google Scholar] [CrossRef]
- Boyd, E.S.; Skidmore, M.; Mitchell, A.C.; Bakermans, C.; Peters, J.W. Methanogenesis in Subglacial Sediments. Environ. Microbiol. Rep. 2010, 2, 685–692. [Google Scholar] [CrossRef]
- Mateos-Rivera, A.; Ovreas, L.; Wilson, B.; Yde, J.C.; Finster, K.W. Activity and Diversity of Methane-Oxidizing Bacteria Along a Norwegian Sub-Arctic Glacier Forefield. FEMS Microbiol. Ecol. 2018, 94, fiy059. [Google Scholar] [CrossRef]
- Miteva, V.; Teacher, C.; Sowers, T.; Brenchley, J. Comparison of the Microbial Diversity at Different Depths of the GISP2 Greenland Ice Core in Relationship to Deposition Climates. Environ. Microbiol. 2009, 11, 640–656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Tian, J.; Jiang, N.; Guo, X.; Wang, Y.; Dong, X. Methanogen Community in Zoige Wetland of Tibetan Plateau and Phenotypic Characterization of A Dominant Uncultured Methanogen Cluster ZC-I. Environ. Microbiol. 2008, 10, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Li, L.; Chen, L.; Xing, T.; Liu, Y.; Dong, X. Methanogen Communities and Predominant Methanogenic Pathways in Three Saline-Alkaline Lakes on the Tibetan Plateau. Acta Microbiol. Sin. 2020, 60, 161–171. [Google Scholar]
- Eme, L.; Spang, A.; Lombard, J.; Stairs, C.W.; Ettema, T.J.G. Archaea and the Origin of Eukaryotes. Nat. Rev. Microbiol. 2017, 15, 711–723. [Google Scholar]
- Conrad, R. Contribution of Hydrogen to Methane Production and Control of Hydrogen Concentrations in Methanogenic Soils and Sediments. FEMS Microbiol. Ecol. 1999, 28, 193–202. [Google Scholar] [CrossRef]
- Yun, J.; Ma, A.; Li, Y.; Zhuang, G.; Wang, Y.; Zhang, H. Diversity of Methanotrophs in Zoige Wetland Soils Under Both Anaerobic and Aerobic Conditions. J. Environ. Sci. 2010, 22, 1232–1238. [Google Scholar] [CrossRef]
- Mo, Y.; Qi, X.; Li, A.; Zhang, X.; Jia, Z. Active Methanotrophs in Suboxic Alpine Swamp Soils of the Qinghai-Tibetan Plateau. Front. Microbiol. 2020, 11, 580866. [Google Scholar] [CrossRef]
- Chowdhury, T.R.; Dick, R.P. Ecology of Aerobic Methanotrophs in Controlling Methane Fluxes from Wetlands. Appl. Soil Ecol. 2013, 65, 8–22. [Google Scholar]
- Macalady, J.L.; McMillan, A.M.S.; Dickens, A.F.; Tyler, S.C.; Scow, K.M. Population Dynamics of Type I and II Methanotrophic Bacteria in Rice Soils. Environ. Microbiol. 2002, 4, 148–157. [Google Scholar] [CrossRef]
- Jia, Z.; Cai, Y.; Yun, J.; Du, W. Comparison of Soil Microbiome by Single Cell Technology, Classical Microscope Methods and High-Throughput MiSeq Sequencing. Acta Microbiol. Sin. 2017, 57, 899–919. [Google Scholar]
- Tang, Q.; Xue, X.; Wang, H.; Xing, P. New Knowledge of Methanogens and Methanotrophs in Lake Ecosystems. Hupo Kexue 2018, 30, 597–610. [Google Scholar]
- Mikucki, J.A.; Lee, P.A.; Ghosh, D.; Purcell, A.M.; Mitchell, A.C.; Mankoff, K.D.; Fisher, A.T.; Tulaczyk, S.; Carter, S.; Siegfried, M.R. Subglacial Lake Whillans Microbial Biogeochemistry: A Synthesis of Current Knowledge. Philos. Trans. R. Soc. A-Math. Phys. Eng. Sci. 2016, 374, 20140290. [Google Scholar] [CrossRef]
- Stibal, M.; Wadham, J.L.; Lis, G.P.; Telling, J.; Pancost, R.D.; Dubnick, A.; Sharp, M.J.; Lawson, E.C.; Butler, C.E.H.; Hasan, F.; et al. Methanogenic Potential of Arctic and Antarctic Subglacial Environments with Contrasting Organic Carbon Sources. Glob. Change Biol. 2012, 18, 3332–3345. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, G.; Song, Y.; Kou, D.; Wang, G.; Zhang, D.; Qin, S.; Mao, C.; Feng, X.; Yang, Y. Magnitude and Drivers of Potential Methane Oxidation and Production across the Tibetan Alpine Permafrost Region. Environ. Sci. Technol. 2020, 53, 14243–14252. [Google Scholar] [CrossRef] [PubMed]
- Fontana, D.; Conti, S.; Grillenzoni, C.; Mecozzi, S.; Petrucci, F.; Turco, E. Evidence of Climatic Control on Hydrocarbon Seepage in the Miocene of the Northern Apennines: The Case Study of the Vicchio Marls. Mar. Pet. Geol. 2013, 48, 90–99. [Google Scholar] [CrossRef]

| Target Groups | Primer Name | Sequence (5′→3′) | Application | References |
|---|---|---|---|---|
| Prokaryotic 16S rRNA V4 | 515F | GTGCCAGCMGCCGCGGTAA | PCR | [40] |
| 806R | GGACTACHVGGGTWTCTAAT | |||
| Methanogens mcrA gene | MLfF | GGTGGTGTMGGATTCACACARTAYGCWACAGC | PCR | [41] |
| MLrR | TTCATTGCRTAGTTWGGRTAGTT | |||
| Methanotrophs pmoA gene | A189F | GGNGACTGGGACTTCTGG | PCR | [42,43] |
| mb661R | CCGGMGCAACGTCYTTACC | |||
| Archara 16S rRNA V4 + V5 | 524F10extF | TGYCAGCCGCCGCGGTAA | PCR, qPCR | [44] |
| Arch958RmodR | YCCGGCGTTGAVTCCAATT | |||
| Bacteria 16S rRNA V3 + V4 | 338F | ACTCCTACGGGAGGCAGCAG | qPCR | [45] |
| 806R | GGACTACHVGGGTWTCTAAT | |||
| HumM2 gene | Hum2F | CGTCAGGTTTGTTTCGGTATTG | qPCR | [39] |
| Hum2R | TCATCACGTAACTTATTTATATGCATTAGC |
| Site Location | Sampling Environment | Dominant Methanogens | Methanogens Types | Dominant Methanotrophs | Methanotrophs Types | References |
|---|---|---|---|---|---|---|
| Guliya Ice Cap, TP | Terminal ice samples (35°12′ N, 81°31′ E) | Methanosarcinaceae | Acetoclastic | Methylocystis | Type II | This study |
| Zoige Wetland, TP | Soil samples (33°56′ N, 102°52′ E) | Methanosarcinaceae | Acetoclastic | Methylobacter Methylococcus | Type I | [53,57] |
| Gongzhu Co, TP | Sediment samples (30°37′ N, 82°06′ E) | Methanosaetaceae | Acetoclastic | − | − | [54] |
| Maqu Swamp, TP | Soil samples (33°39′ N, 101°52′ E) | − | − | Methylobacter | Type I | [58] |
| Greenland Ice Sheet | GISP2 ice core (72°36′ N, 38°30′ W) | Methanococcoides | Methylotrophic | − | − | [52] |
| SLW, West Antarctic Ice Sheet | Sediment samples (84°14′ S, 153°41′ W) | Methanohalophilus | Methylotrophic | Methylobacter | Type I | [26] |
| Site Location | Sampling Location | Methanogens Abundance /(Cells·mL−1) | Methanotrophs Abundance /(Cells·mL−1) | Method | Reference |
|---|---|---|---|---|---|
| Guliya Ice Cap, TP | Terminal ice samples (35°12′ N, 81°31′ E) | (0.71 ± 0.29) × 102 | (7.66 ± 1.64) × 102 | qPCR | This study |
| Greenland Ice Sheet | GISP2 ice core (72°36′ N, 38°30′ W) | (3.95 ± 3.87) × 102 | − | Direct cell counts | [18] |
| SLW, West Antarctic Ice Sheet | Water samples (84°14′ S, 153°41′ W) | (1.30 ± 0.40) × 102 | (6.87 ± 2.12) × 103 | Direct cell counts | [63] |
| Types of CH4 Metabolic Microbial Communities | Cell-Specific Metabolic Rates /(fmol·cell−1·d−1) | Microbial Abundance /(cells·mL−1) | Methanogenesis Rates (+)/ CH4 Oxidation Rates (−) /(pmol·mL−1·d−1) |
|---|---|---|---|
| Methanogens | 2.4 × 10−1 ① | (0.71 ± 0.29) × 102 | +(1.7 ± 0.7) × 10−2 |
| Methanotrophs | 1.85 × 103 ② | (7.66 ± 1.64) × 102 | −(1.42 ± 0.30) × 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Zhang, S. Potential Effects of Methane Metabolic Microbial Communities on the Glacial Methane Budget in the Northwestern Tibetan Plateau. Sustainability 2023, 15, 7352. https://doi.org/10.3390/su15097352
Guo Y, Zhang S. Potential Effects of Methane Metabolic Microbial Communities on the Glacial Methane Budget in the Northwestern Tibetan Plateau. Sustainability. 2023; 15(9):7352. https://doi.org/10.3390/su15097352
Chicago/Turabian StyleGuo, Yuchan, and Shuhong Zhang. 2023. "Potential Effects of Methane Metabolic Microbial Communities on the Glacial Methane Budget in the Northwestern Tibetan Plateau" Sustainability 15, no. 9: 7352. https://doi.org/10.3390/su15097352
APA StyleGuo, Y., & Zhang, S. (2023). Potential Effects of Methane Metabolic Microbial Communities on the Glacial Methane Budget in the Northwestern Tibetan Plateau. Sustainability, 15(9), 7352. https://doi.org/10.3390/su15097352






