Imbibition and Germination of Seeds with Economic and Ecological Interest: Physical and Biochemical Factors Involved
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Species and Processing
2.2. Seed Imbibition
2.3. Integument Hardness
2.4. Water Potential
2.5. Seed Respiration Rate
2.6. Germination Parameters
2.7. Seed Coat Ultrastructure
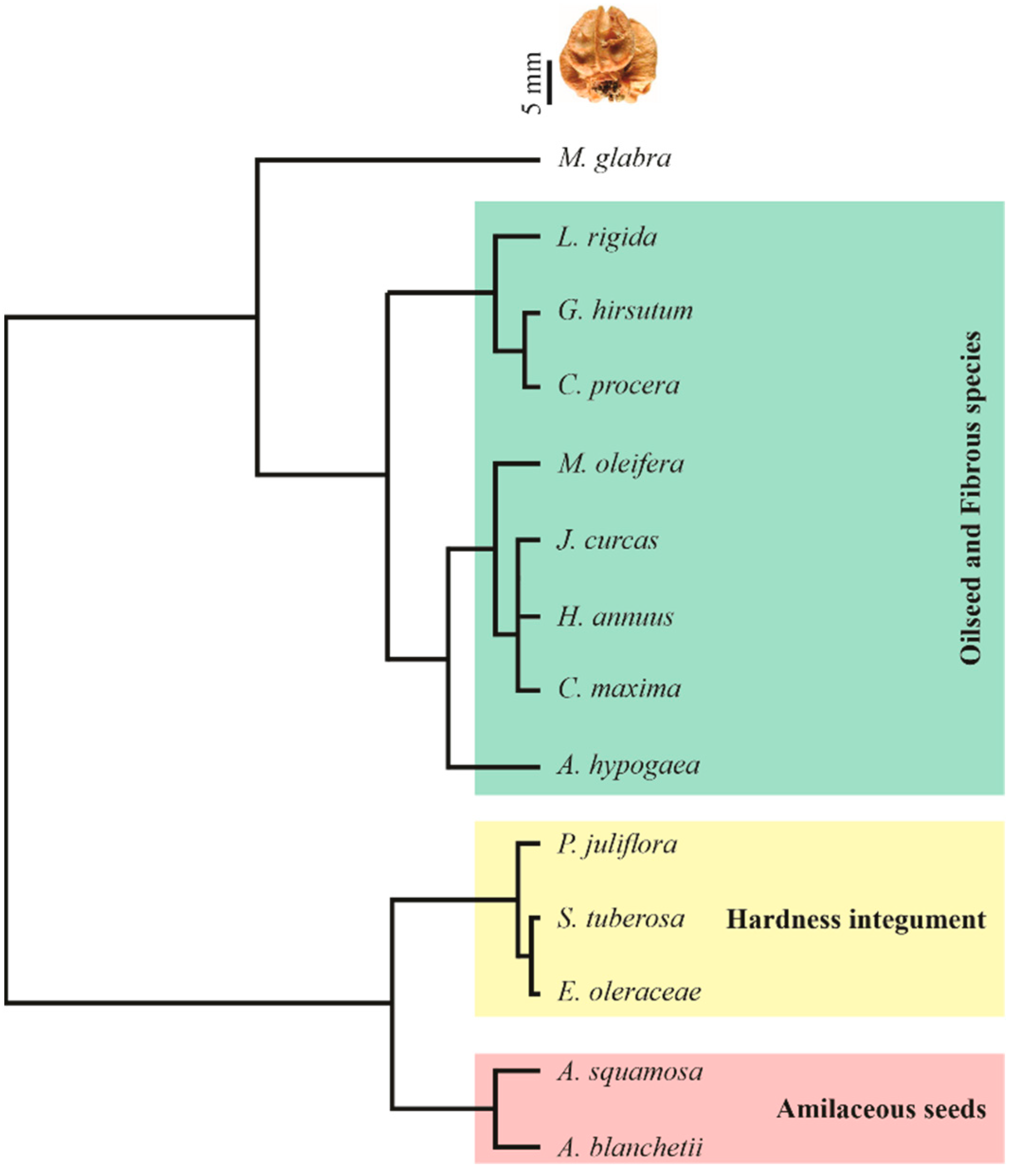
2.8. Dendrograms
2.9. Statistical Analysis
3. Results
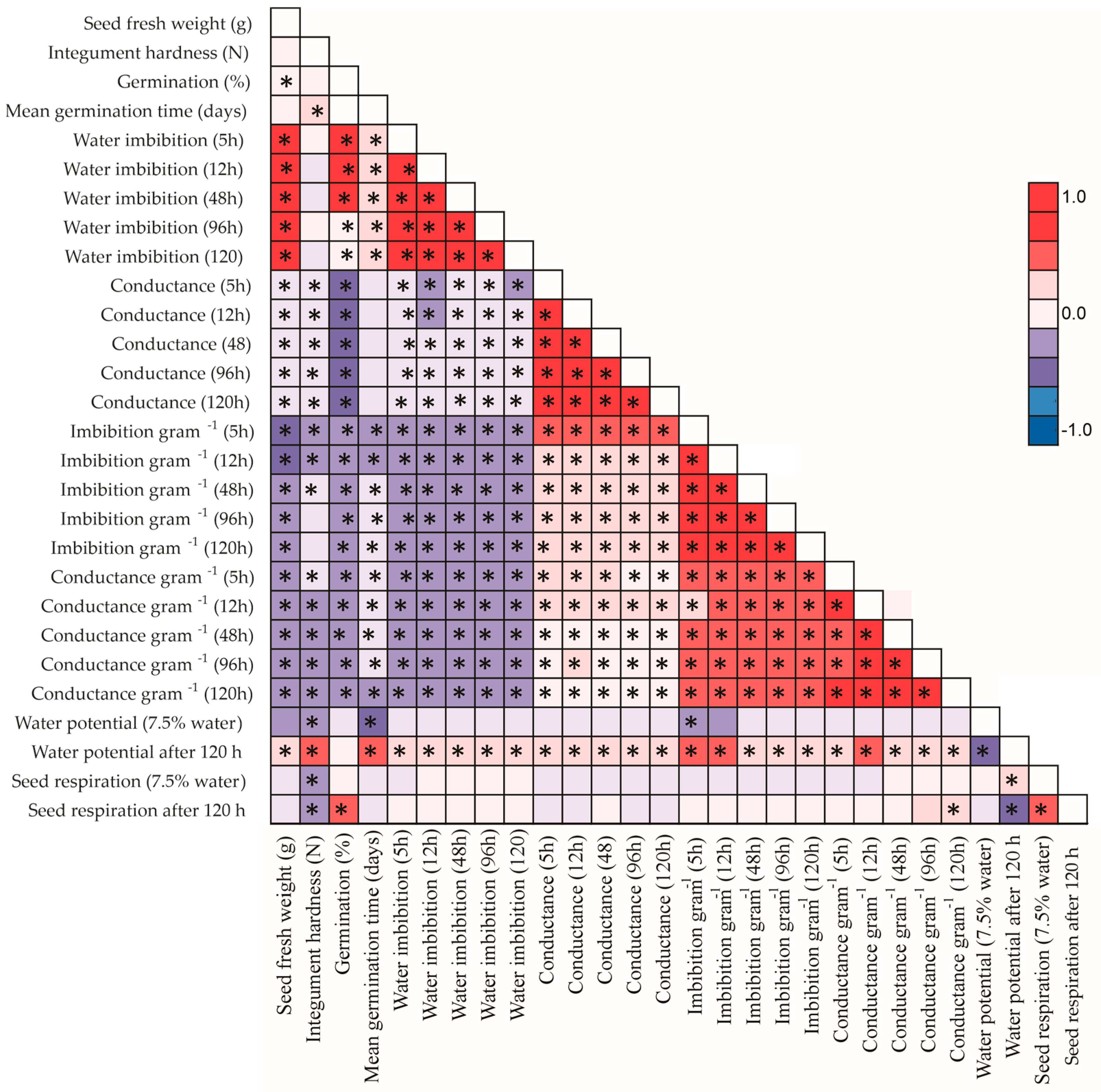
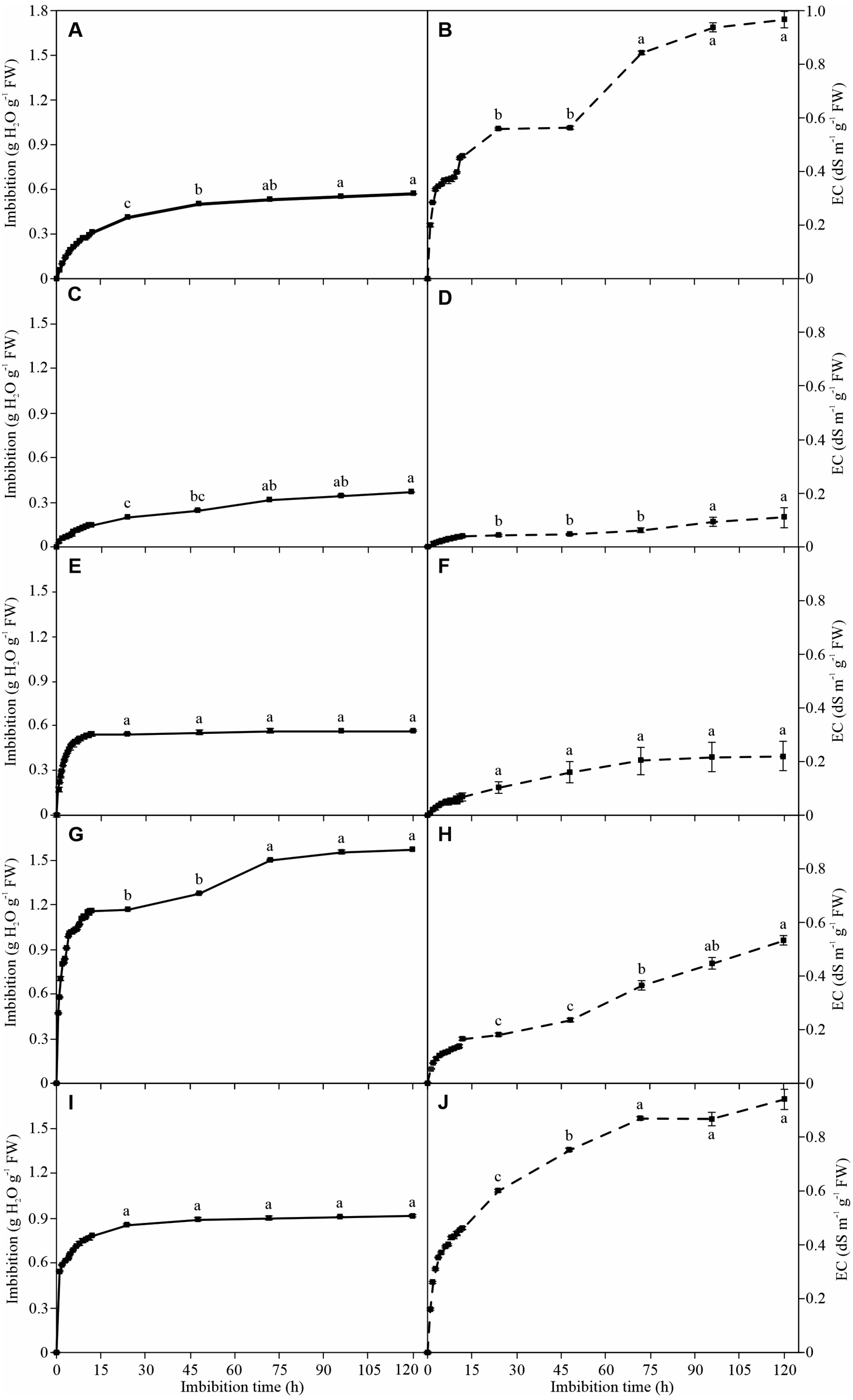
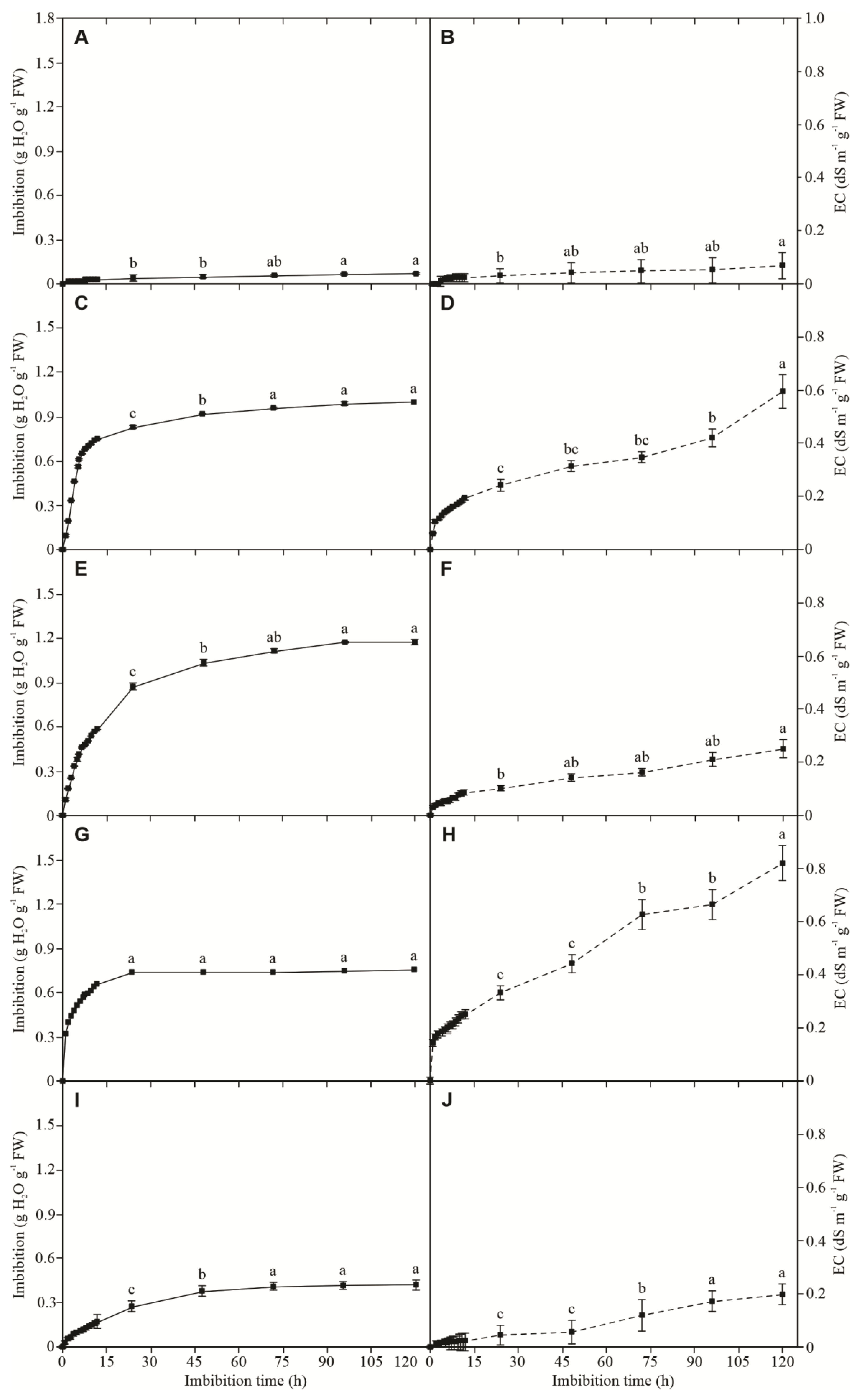
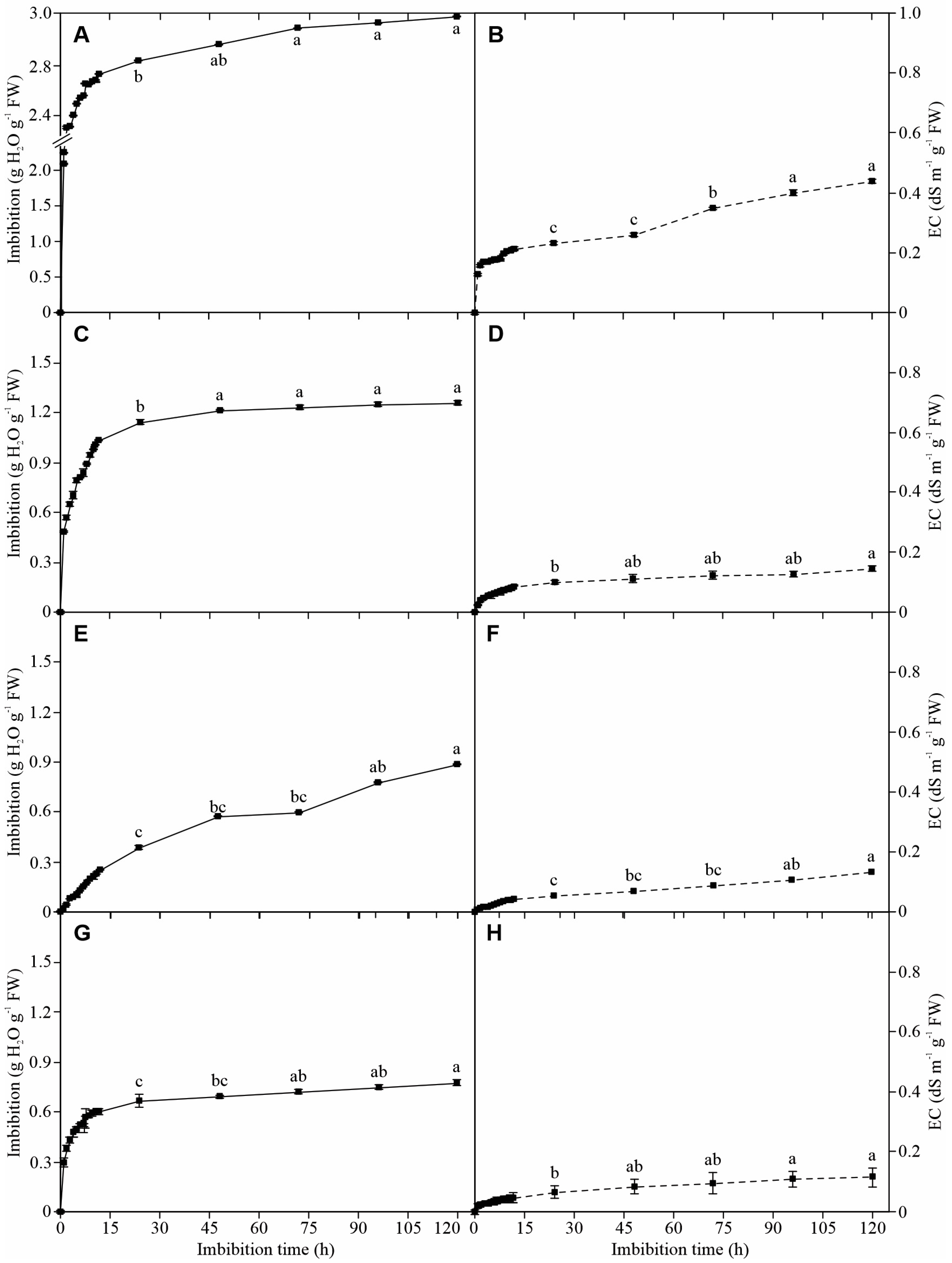
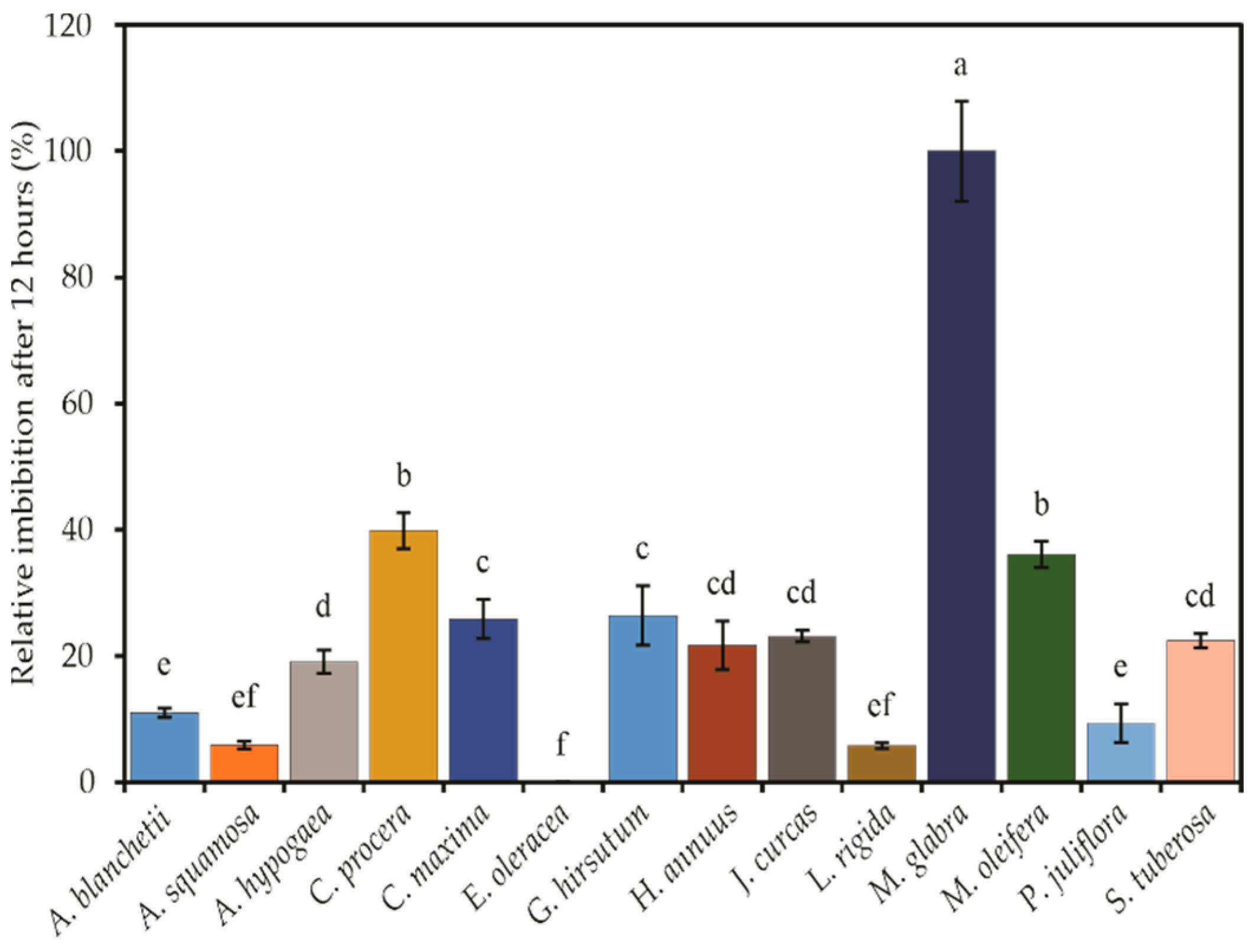
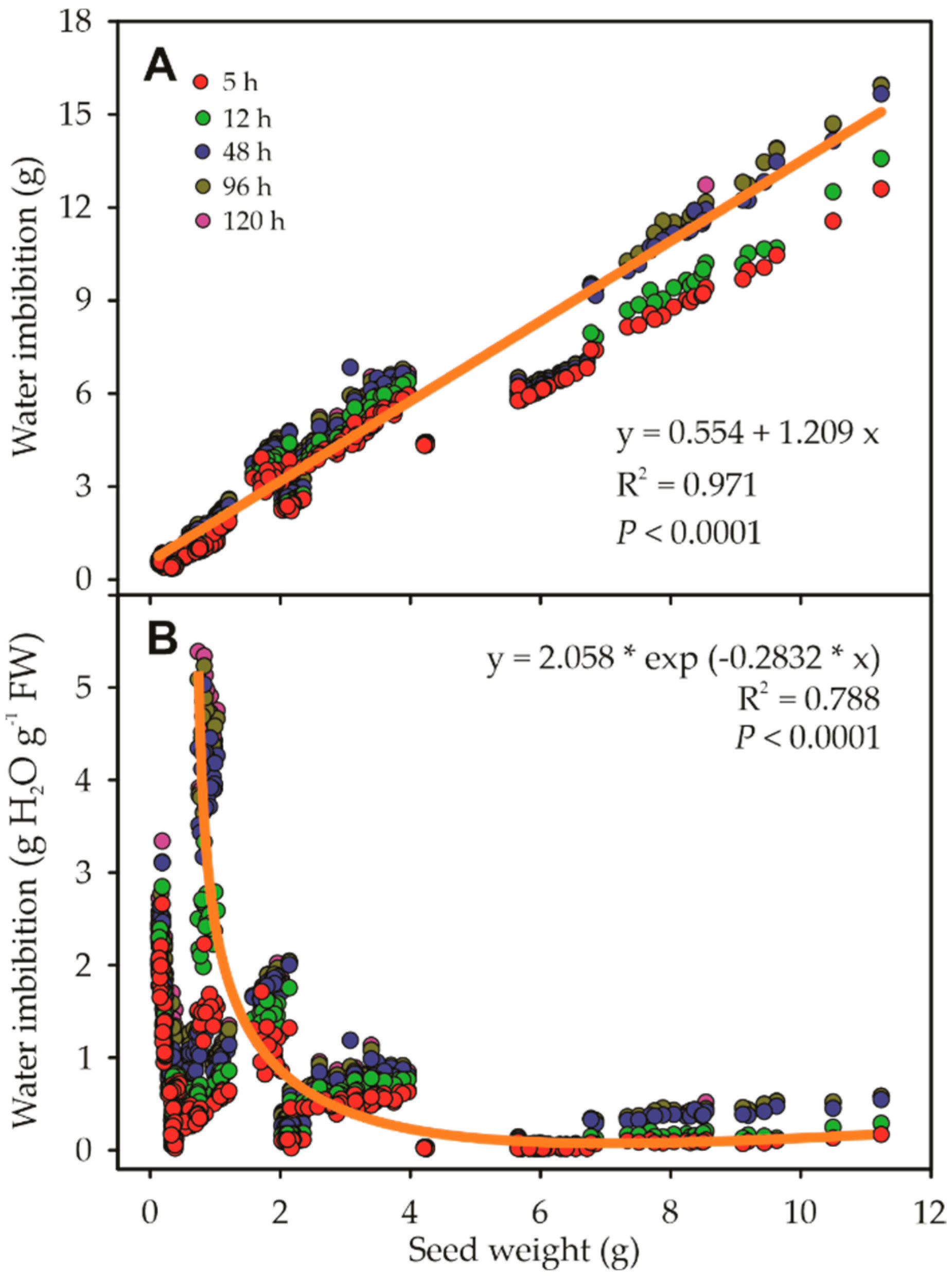
3.1. Seed Imbibition
3.2. Electrolyte Leakage
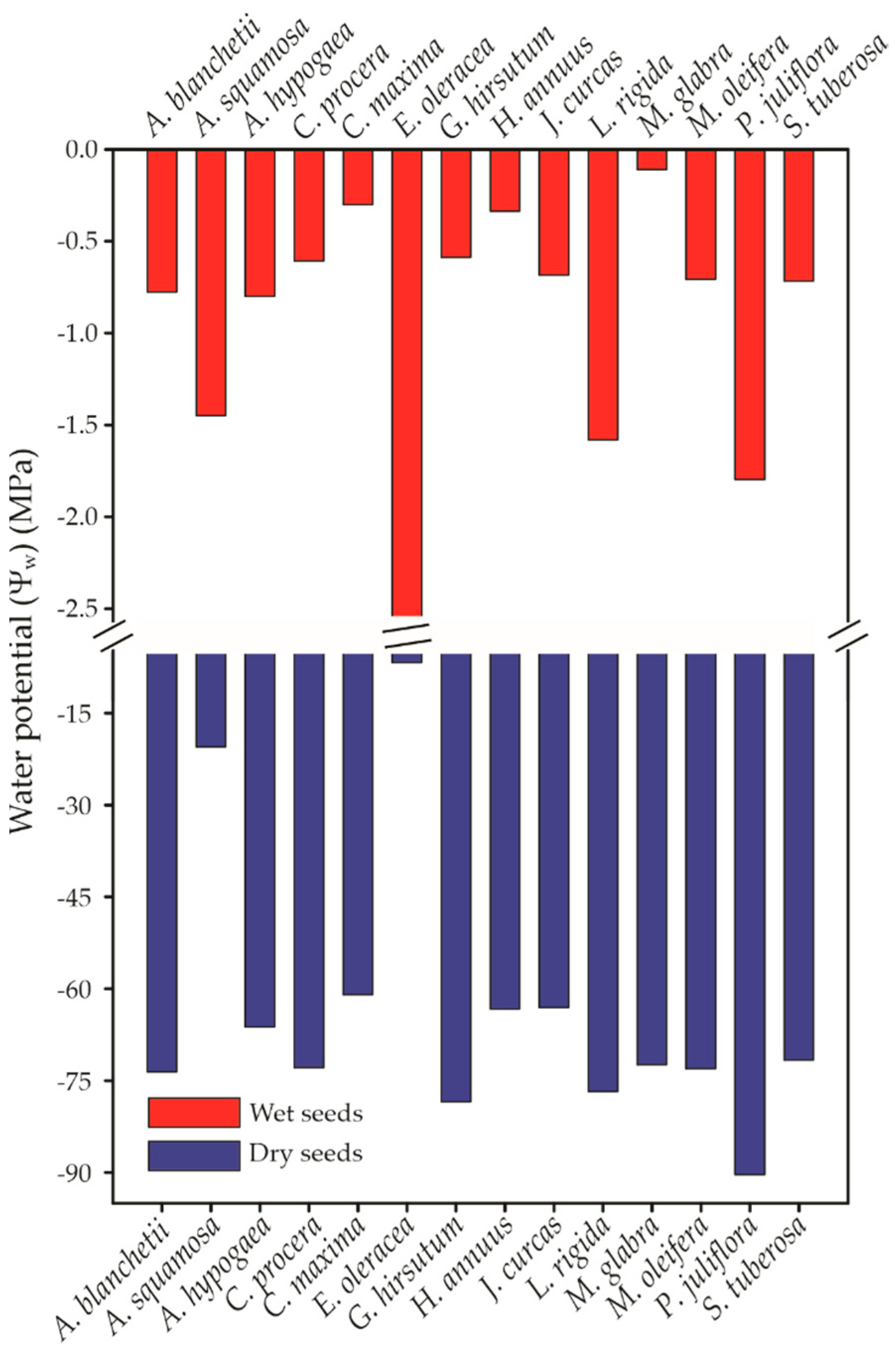
3.3. Water Potential
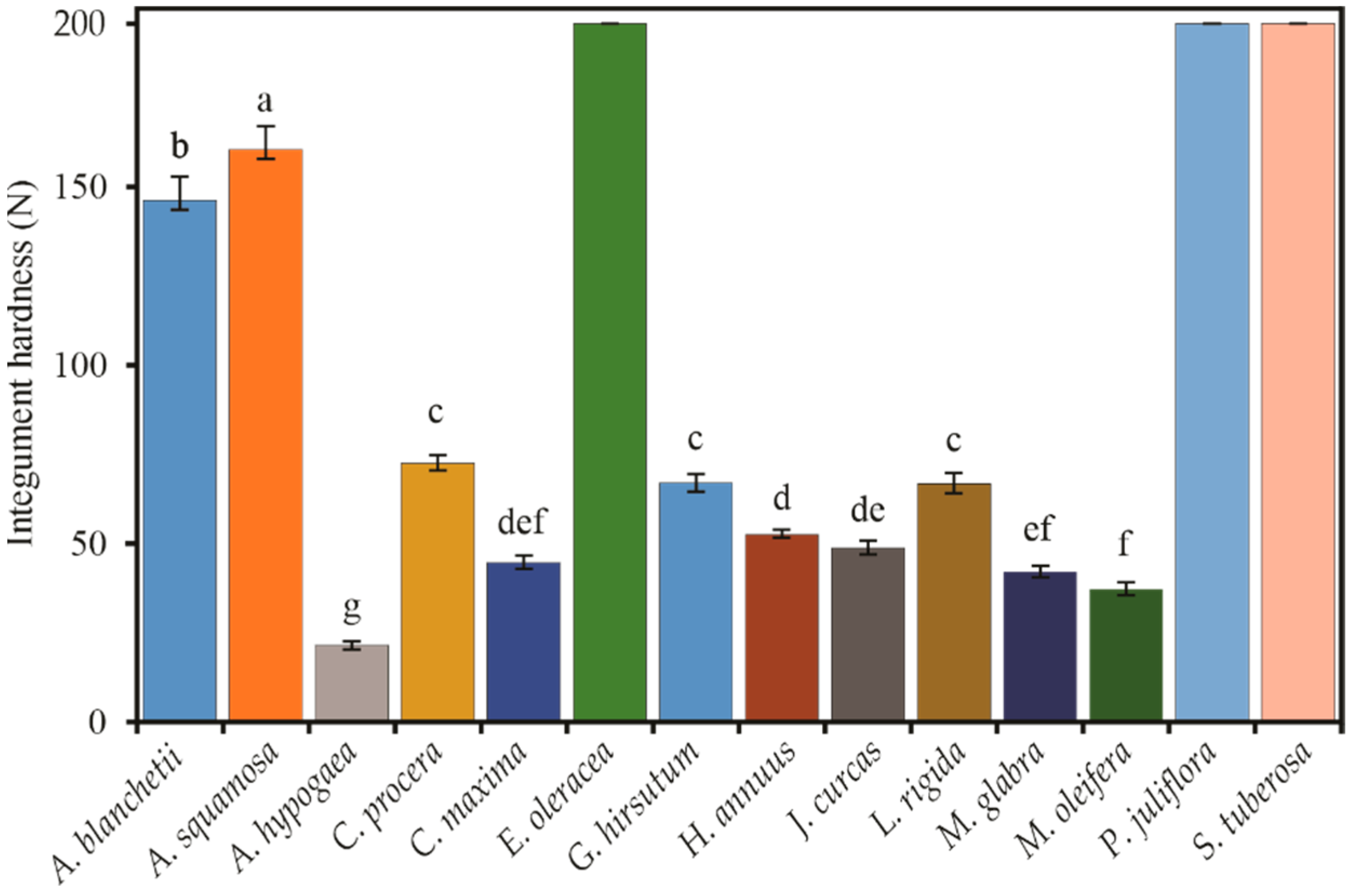
3.4. Integument Hardness
3.5. Germination Parameters
3.6. Seed Respiration and Viability
3.7. Seed Coat Ultrastructure
3.8. Dendrogram
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Variable | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | PC8 | PC9 | PC10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Seed fresh weight | −0.206 | −0.13 | 0.251 | −0.103 | 0.011 | 0.025 | 0.133 | −0.002 | 0.014 | −0.127 |
| Integument hardness | −0.096 | −0.157 | −0.09 | 0.376 | −0.121 | 0.1 | −0.255 | 0.169 | −0.018 | 0.115 |
| Water embebition after 5 h | −0.197 | −0.106 | 0.278 | −0.144 | −0.003 | 0.008 | 0.025 | −0.066 | 0.013 | 0.09 |
| Water embebition after 12 h | −0.189 | −0.09 | 0.306 | −0.134 | −0.031 | −0.001 | 0.004 | −0.045 | 0.033 | 0.098 |
| Water embebition after 48 h | −0.177 | −0.073 | 0.336 | −0.115 | −0.087 | 0.005 | 0.014 | 0.003 | 0.074 | 0.003 |
| Water embebition after 96 h | −0.175 | −0.072 | 0.339 | −0.106 | −0.099 | 0.004 | 0.015 | 0.015 | 0.094 | −0.01 |
| Water embebition after 120 h | −0.175 | −0.072 | 0.34 | −0.103 | −0.102 | 0.005 | 0.01 | 0.018 | 0.096 | −0.005 |
| Conductivity of water after 5 h | 0.184 | −0.274 | −0.005 | −0.164 | 0.039 | 0.06 | 0.064 | 0.133 | −0.042 | −0.098 |
| Conductivity of water after 12 h | 0.184 | −0.274 | −0.004 | −0.165 | 0.041 | 0.06 | 0.063 | 0.134 | −0.044 | −0.093 |
| Conductivity of water after 48 h | 0.184 | −0.274 | −0.002 | −0.168 | 0.043 | 0.061 | 0.062 | 0.135 | −0.049 | −0.089 |
| Conductivity of water after 96 h | 0.183 | −0.274 | 0 | −0.17 | 0.041 | 0.063 | 0.064 | 0.137 | −0.048 | −0.092 |
| Conductivity of water after 120 h | 0.183 | −0.274 | 0 | −0.17 | 0.041 | 0.063 | 0.065 | 0.137 | −0.05 | −0.089 |
| Water embebition per seed gram after 5 h | 0.243 | −0.045 | 0.106 | −0.017 | −0.148 | −0.201 | 0.048 | −0.147 | −0.1 | 0.204 |
| Water embebition per seed gram after 12 h | 0.232 | 0.005 | 0.153 | 0.055 | −0.206 | −0.222 | −0.013 | −0.045 | −0.044 | 0.11 |
| Water embebition per seed gram after 48 h | 0.205 | 0.046 | 0.186 | 0.125 | −0.254 | −0.219 | −0.078 | 0.105 | 0.019 | −0.1 |
| Water embebition per seed gram after 96 h | 0.202 | 0.05 | 0.17 | 0.142 | −0.27 | −0.221 | −0.067 | 0.132 | −0.017 | −0.112 |
| Water embebition per seed gram after 120 h | 0.201 | 0.049 | 0.167 | 0.148 | −0.278 | −0.213 | −0.074 | 0.148 | −0.028 | −0.108 |
| Conductivity of water per seed gram after 5 h | 0.198 | 0.098 | 0.226 | 0.191 | 0.118 | 0.119 | 0.058 | 0.029 | 0.097 | −0.123 |
| Conductivity of water per seed gram after 12 h | 0.207 | 0.113 | 0.167 | 0.111 | 0.233 | 0.196 | 0.122 | −0.074 | 0.04 | −0.067 |
| Conductivity of water per seed gram after 48 h | 0.181 | 0.149 | 0.205 | 0.156 | 0.218 | 0.199 | 0.069 | −0.066 | 0.037 | −0.061 |
| Conductivity of water per seed gram after 96 h | 0.186 | 0.144 | 0.241 | 0.12 | 0.189 | 0.162 | 0.067 | 0.035 | −0.049 | −0.018 |
| Conductivity of water per seed gram after 120 h | 0.183 | 0.161 | 0.223 | 0.102 | 0.202 | 0.167 | 0.112 | 0.011 | −0.14 | 0.065 |
| Relative water imbibition after 12 h | 0.217 | −0.153 | −0.052 | −0.214 | 0.008 | −0.041 | 0.066 | −0.202 | −0.145 | 0.143 |
| Water potential in non-imbibed seeds | −0.091 | −0.148 | 0.018 | 0.211 | 0.369 | −0.296 | 0.171 | 0.23 | 0.56 | 0.137 |
| Water potential in 120 h-imbibed seeds | 0.188 | 0.155 | 0.007 | −0.233 | 0.035 | 0.026 | −0.138 | −0.356 | 0.288 | 0.07 |
| Relative Water Content in non-imbibed seeds | −0.002 | −0.257 | −0.066 | 0.259 | 0.235 | −0.358 | 0.248 | 0.062 | −0.11 | 0.052 |
| Relative Water Content in 120 h-imbibed seeds | 0.19 | 0.003 | −0.03 | −0.169 | 0.128 | −0.372 | 0.05 | −0.414 | 0.185 | 0.111 |
| Seed germination in non-imbibed seeds | −0.164 | 0.249 | 0.038 | −0.078 | 0.097 | −0.122 | 0.309 | 0.028 | −0.548 | 0.097 |
| Seed germination in 120 h-imbibed seeds | −0.135 | 0.251 | −0.064 | −0.095 | −0.06 | −0.298 | 0.44 | 0.025 | 0.004 | −0.32 |
| Mean germination time in non-imbibed seeds | −0.106 | −0.251 | 0.09 | 0.223 | 0.117 | −0.061 | −0.298 | −0.44 | −0.155 | −0.158 |
| Mean germination time in 120 h-imbibed seeds | −0.1 | −0.251 | 0.132 | 0.236 | 0.229 | −0.157 | −0.101 | −0.222 | −0.308 | −0.157 |
| Respiration rate in non-imbibed seeds | −0.017 | 0.195 | −0.003 | −0.255 | 0.294 | −0.238 | −0.426 | 0.141 | −0.009 | −0.584 |
| Respiration rate in 120 h-imbibed seeds | 0.025 | 0.152 | 0.143 | −0.221 | 0.32 | −0.218 | −0.389 | 0.357 | −0.182 | 0.482 |
References
- Rajjou, L.; Lovigny, Y.; Job, C.; Belghazi, M.; Groot, S.; Job, D. Seed quality and germination. In Seeds: Biology, Development and Ecology; Adkins, S., Ashmore, S., Navie, S.C., Eds.; CABI Pubishing: Wallingford, UK, 2007; pp. 324–332. [Google Scholar]
- Bewley, J.D.; Black, M. Seeds: Physiology of Development and Germination; Plenum Press: New York, NY, USA, 1994; p. 444. [Google Scholar]
- Bewley, J.D.; Black, M. Physiology and Biochemistry of Seeds Relation to Germination; Springer: Berlin/Heidelberg, Germany, 1985; p. 367. [Google Scholar]
- Gallardo, K.; Job, C.; Groot, S.P.C.; Puype, M.; Demol, H.; Vandekerckhove, J.; Job, D. Proteomic analysis of Arabidopsis seed germination and priming. Plant Physiol. 2001, 126, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, V.J.M. Germinação. In Fisiologia Vegetal, 2nd ed.; Kerbauy, G.B., Ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2008; pp. 384–408. [Google Scholar]
- MacCallum, K. Acesso Aberto: Uma Estrutura para Restauração Inteligente em Termos Climáticos. Available online: www.ser.org (accessed on 25 November 2022).
- Derero, A.; Coe, R.; Muthuri, C.; Hadgu, K.M.; Sinclair, F. Farmer-led approaches to increasing tree diversity in fields and farmed landscapes in Ethiopia. Agrofor. Syst. 2021, 95, 1309–1326. [Google Scholar] [CrossRef]
- Doran, J.C. Seed, nursery practice and establishment. In The Major Invertebrate Pests and Weeds of Agriculture and Plantation Forestry in the Southern and Western Pacific; Waterhouse, D.F., Ed.; The Australian Centre for International Agricultural Research: Canberra, ACT, Australia, 1997; pp. 1–29. [Google Scholar]
- Castro, J.A.; Morales-Rueda, F.; Alcaraz-Segura, D.; Tabik, S. Forest restoration is more than firing seeds from a drone. Restor. Ecol. 2023, 31, E13736. [Google Scholar] [CrossRef]
- Ngulube, M.R. Seed germination, seedling growth and biomass production of eight Central-American multipurpose trees under nursery conditions in Zomba, Malawi. For. Ecol. Manag. 1989, 27, 21–27. [Google Scholar] [CrossRef]
- Alouani, M.; Bani-Aameur, F. Argan (Argania spinosa (L.) Skeels) seed germination under nursery conditions: Effect of cold storage, gibberellic acid and mother-tree genotype. Ann. For. Sci. 2004, 61, 191–194. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Zhang, X.; Shi, S.; Cao, W. Effect of degradation and rebuilding of artificial grasslands on soil respiration and carbon and nitrogen pools on an alpine meadow of the Qinghai-Tibetan Plateau. Ecol. Eng. 2018, 111, 134–142. [Google Scholar] [CrossRef]
- Prach, K.; Jongepierová, I.; Řehounková, K.; Fajmon, K. Restoration of grasslands on ex-arable land using regional and commercial seed mixtures and spontaneous succession: Successional trajectories and changes in species richness. Agric. Ecosyst. Environ. 2014, 182, 131–136. [Google Scholar] [CrossRef]
- Kuusipalo, J.; Ådjers, G.; Jafarsidik, Y.; Otsamo, A.; Tuomela, K.; Vuokko, R. Restoration of natural vegetation in degraded Imperata cylindrica grassland: Understorey development in forest plantations. J. Veg. Sci. 1995, 6, 205–210. [Google Scholar] [CrossRef]
- Zipperer, W.C.; Sisinni, M.S.; Pouyat, R.V. Urban tree cover: An ecological perspective. Urban Ecosyst. 1997, 1, 229–246. [Google Scholar] [CrossRef]
- UNDP. UN Decade on Ecosystem Restoration: Empowering Stakeholders to Prevent, Halt, and Reverse the Degradation of Global Ecosystems. Available online: https://mptf.undp.org/fund/der00?utm_source=EN&utm_medium=GSR&utm_content=US_UNDP_PaidSearch_Brand_English&utm_campaign=CENTRAL&c_src=CENTRAL&c_src2=GSR&gclid=Cj0KCQiA8aOeBhCWARIsANRFrQHDO-EkuYjOYeRC2W2wmcm5bim0K62t3Zth_80Lxjk2oTx7CLIrdDcaAhb6EALw_wcB (accessed on 19 January 2023).
- Piffer, P.R.; Calaboni, A.; Rosa, M.R.; Schwartz, N.B.; Tambosi, L.R.; Uriarte, M. Ephemeral forest regeneration limits carbon sequestration potential in the Brazilian Atlantic Forest. Glob. Chang. Biol. 2022, 28, 630–643. [Google Scholar] [CrossRef]
- Barreto, J.R.; Pardini, R.; Metzger, J.P.; Silva, F.A.B.; Nichols, E.S. When forest loss leads to biodiversity gain: Insights from the Brazilian Atlantic Forest. Biol. Conserv. 2023, 279, 109957. [Google Scholar] [CrossRef]
- Clement, C.R.; Higuchi, N. A Floresta Amazônica e o futuro do Brasil. Cienc. Cult. 2006, 58, 44–49. [Google Scholar]
- Caiado, N.; Guarnieri, P.; Xavier, L.H.; Chaves, G.L.D. A characterization of the Brazilian market of reverse logistic credits (RLC) and an analogy with the existing carbon credit markeT. Resour. Conserv. Recycl. 2017, 118, 47–59. [Google Scholar] [CrossRef]
- Moncaleano-Escandon, J.; Silva, B.C.F.; Silva, S.R.S.; Granja, J.A.; Alves, M.C.J.L.; Pompelli, M.F. Germination responses of Jatropha curcas L. seeds to storage and aging. Ind. Crop. Prod. 2013, 44, 684–690. [Google Scholar] [CrossRef]
- McDonald, M.B. Seed deterioration: Physiology, repair and assessment. Seed Sci. Technol. 1999, 27, 177–237. [Google Scholar]
- Brewer, H.E. Tetrazolium chloride as a test for damage in artificial cured peanuts. Science 1949, 110, 451–452. [Google Scholar] [CrossRef]
- Hailstones, M.D.; Smith, M.T. Soya bean seed invigoration by ferrous sulphate: Changes in lipid peroxidation, conductivity, tetrazolium reduction, DNA and protein synthesis. J. Plant. Physiol. 1991, 137, 307–311. [Google Scholar] [CrossRef]
- Lozano-Isla, F.; Campos, M.L.O.; Lauricio, E.; Pompelli, M.F. Effects of seed storage time and salt stress on the germination of Jatropha curcas L. Ind. Crop. Prod. 2018, 118, 214–224. [Google Scholar] [CrossRef]
- Fessel, S.A.; Vieira, R.D.; Cruz, M.C.P.; Paula, R.C.; Panobianco, M. Electrical conductivity testing of corn seeds as influenced by temperature and period of storage. Pesqui. Agropecu. Bras. 2006, 41, 1551–1559. [Google Scholar] [CrossRef]
- Hilst, P.C.; Dias, D.C.F.S.; Alvarenga, E.M.; Souza, B.L. Test of exudates color hues for evaluating the physiological potential of coffee (Coffea arabica L.) seeds. J. Seed Sci. 2012, 34, 212–217. [Google Scholar] [CrossRef]
- Demir, I.; Kenamoglu, B.B.; Özden, E. Seed vigour tests to estimate seedling emergence in cress (Lepidium sativum L.) seed lots. Not. Bot. Horti. Agrobot. 2019, 47, 881–886. [Google Scholar] [CrossRef]
- WFO. The Plant List Version 1.1. Snapshot of the Taxonomy. Available online: http://www.theplantlist.org/ (accessed on 1 January 2023).
- Campbell, E.C.; Campbell, G.S.; Barlow, W.K. A dewpoint hygrometer for water potential measurement. Agric. Meteorol. 1973, 12, 113–121. [Google Scholar] [CrossRef]
- Allen, E.; Alvarez, S. International Rules for Seed Testing 2020; The International Seed Testing Association: Bassersdorf, Switzerland, 2020. [Google Scholar]
- Lozano-Isla, F.; Alfaro, O.B.; Pompelli, M.F. GerminaR: An R package for germination analysis with the interactive web application ‘GerminaQuant for R’. Ecol. Res. 2019, 34, 339–346. [Google Scholar] [CrossRef]
- Karnovsky, M.J. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 1965, 27, 137–138. [Google Scholar]
- Pompelli, M.F.; Jarma-Orozco, A.; Rodríguez-Paéz, L.A. Screening of morphophysiological, anatomical, and ultrastructural traits to improve the elite genotype selection in sugarcane (Saccharum officinarum L.). Horticulturae 2022, 8, 1069. [Google Scholar] [CrossRef]
- Bhatt, A.; Daibes, L.F.; Gallacher, D.; Jarma-Orozco, A.; Pompelli, M.F. Water stress inhibits germination while maintaining embryo viability of subtropical wetland seeds: A functional approach with phylogenetic contrasts. Front. Plant. Sci. 2022, 13, 906771. [Google Scholar] [CrossRef]
- Bhatt, A.; Gallacher, D.; Jarma-Orozco, A.; Pompelli, M.F. Seed mass, dormancy and germinability variation among maternal plants of four Arabian halophytes. Seed Sci. Res. 2022, 32, 53–61. [Google Scholar] [CrossRef]
- Iqbal, W.; Afridi, M.Z.; Jamal, A.; Mihoub, A.; Saeed, M.F.; Székely, Á.; Zia, A.; Khan, M.A.; Jarma-Orozco, A.; Pompelli, M.F. Canola seed priming and its effect on gas exchange, chlorophyll photobleaching, and enzymatic activities in response to salt stress. Sustainability 2022, 14, 9377. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Allen, O.B.; Christie, B.R. Comparison of variety means using cluster analysis and dendrograms. Exp. Agric. 1989, 25, 259–269. [Google Scholar] [CrossRef]
- Forina, M.; Armanino, C.; Raggio, V. Clustering with dendrograms on interpretation variables. Anal. Chim. Acta 2002, 454, 13–19. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: London, UK, 2014. [Google Scholar]
- Foschi, M.L.; Juan, M.; Pascual, B.; Pascual-Seva, N. Water uptake and germination of caper (Capparis spinosa L.) seeds. Agronomy 2020, 10, 838. [Google Scholar] [CrossRef]
- Bewley, J.D.; Black, M. Physiology and Biochemistry of Seeds in Relation to Germination: Volume 2. Viability, Dormancy, and Environmental Control; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Zhu, Z.H.; Sami, A.; Chen, Z.P.; Fatima, M.; Zheng, W.Y.; Xu, Q.Q.; LeI, Y.H.; Jin, X.Z.; Zhang, H.; Li, Y.; et al. Effects of microscopic testa color and morphologyon the water uptake ability and drought tolerance of germination-stage rapeseed (Brassica napus L.). Bioengineered 2021, 12, 9341–9355. [Google Scholar] [CrossRef] [PubMed]
- Vertucci, C.W.; Leopold, A.C. Bound water in soybean seed and its relation to respiration and imbibitional damage. Plant Physiol. 1984, 75, 114–117. [Google Scholar] [CrossRef]
- Booth, D.T.; Sowa, S. Respiration in dormant and non-dormant bitterbrush seeds. J. Arid. Environ. 2001, 48, 35–39. [Google Scholar] [CrossRef]
- Phan, T.T.; Tyerman, S.D.; Schnell, N.; Tucker, M.; McGaughey, S.A.; Qiu, J.; Groszmann, M.; Byrt, C.S. Deciphering aquaporin regulation and roles in seed biology. J. Exp. Bot. 2020, 71, 1763–1773. [Google Scholar] [CrossRef]
- Tavecchio, N.E.M.; Vigliocco, A.E.; Terenti, O.A.; Wassner, D.; Reinoso, H.E.; Pedranzani, H.E. Jatropha curcas L. and J. macrocarpa Griseb: Seed morphology, viability, dormancy, germination and growth of seedlings. Am. J. Plant. Sci. 2018, 9, 1835–1854. [Google Scholar] [CrossRef]
- Singh, P.; Singh, K.R.B.; Singh, J.; Das, S.N.; Singh, R.P. Tunable electrochemistry and efficient antibacterial activity of plant-mediated copper oxide nanoparticles synthesized by Annona squamosa seed extract for agricultural utility. RSC Adv. 2021, 11, 18050. [Google Scholar] [CrossRef] [PubMed]
- Bayer, C.; Appel, O. Ocurrence and taxonomic significance of ruminate endosperm. Bot. Rev. 1996, 62, 301–310. [Google Scholar] [CrossRef]
- Svoma, E. Studies on the embryology and gynoecium structures in Drimys winteri (Winteraceae) and some Annonaceae. Plant Syst. Evol. 1998, 209, 205–229. [Google Scholar] [CrossRef]
- Svoma, E. Seed development and function in Artabotrys hexapetalus (Annonaceae). Plant Syst. Evol. 1997, 207, 205–223. [Google Scholar] [CrossRef]
- Corte-Real, N.; Endres, L.; Santos, K.P.O.; Figueirêdo, R.C.B.; Arruda, E.C.P.; Ulisses, C.; Pompelli, M.F. Morphoanatomy and ontogeny of the fruit and seeds of Jatropha curcas L.: A promising biofuel plant. In The Promising Future of Jatropha curcas: Proprieties and Potential Applications; Segura-Campos, M.R., Betancur-Ancova, D., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2016; pp. 141–158. [Google Scholar]
- Gopinathan, M.C.; Babu, C.R. Structural diversity and its adaptive significance in seeds of Vigna minima (Roxb.) Ohwi and Ohashi and its Allies (Leguminosae-Papilionoideae). Ann. Bot. 1985, 56, 723–732. [Google Scholar]
- Dong Ping, W.; Jian Feng, W.; Xuan Ke, W.; Xue Qin, H.; Jiang Ru, H.; Huan Yu, L. Study on seed morphology and seedling growth of different ranks of Jatropha curcas L. J. Henan Agric. Sci. 2015, 44, 131–135. [Google Scholar]
- Jordaan, A. Ultrastructure and histochemistry of seeds of Colophospermum mopane during imbibition. S. Afr. J. Bot. 2008, 74, 591–597. [Google Scholar] [CrossRef]
- Jordaan, A.; Wessels, D.C.J. The aril of Colophospermum mopane. Its role during seed germination and fruit opening. S. Afr. J. Bot. 1999, 65, 392–397. [Google Scholar] [CrossRef]
- McDonald, M.B., Jr.; Vertucci, C.W.; Roos, E.E. Soybean seed imbibition: Water absorption by seed parts. Crop. Sci. 1988, 28, 993–997. [Google Scholar] [CrossRef]
- Keim, P.; Diers, B.W.; Shoemaker, R.C. Genetic analysis of soybean hard seededness with molecular markers. Theor. Appl. Genet. 1990, 79, 465–469. [Google Scholar] [CrossRef]
- Miranda, R.Q.; Oliveira, M.T.P.; Correia, R.M.; Almeida-Cortez, J.S.; Pompelli, M.F. Germination of Prosopis juliflora (Sw) DC seeds after scarification treatments. Plant Species Biol. 2011, 26, 186–192. [Google Scholar] [CrossRef]
- Vilela, A.E.; Ravetta, D.A. The effect of radiation on seedling growth and physiology in four species of Prosopis L. (Mimosaceae). J. Arid. Environ. 2000, 44, 415–423. [Google Scholar] [CrossRef]
- Rahoui, S.; Chaoui, A.; El Ferjani, E. Membrane damage and solute leakage from germinating pea seed under cadmium stress. J. Hazard. Mater. 2010, 178, 1128–1131. [Google Scholar] [CrossRef]
- Olanlokun, J.O.; Oyebode, O.T.; Popoola, D.; Bodede, O.; Idowu, T.O.; Moodley, R.; Olorunsogo, O.O. In vitro effects of 2-methyl-3-propylbutane-1,4-diol purified from Alstonia boonei on erythrocyte membrane stabilization and mitochondrial membrane permeabilization. Chem. Biol. Drug Des. 2023, 101, 678–689. [Google Scholar] [CrossRef]
- Simões, L.A.; Fernandes, N.A.T.; dos Santos Junior, N.A.; Dias, D.R. Biosurfactants and their benefits for seeds. In Advancements in Biosurfactants Research; Aslam, R., Mobin, M., Aslam, J., Zehra, S., Eds.; Springer: London, UK, 2023. [Google Scholar]
- Thakur, P.S.; Tiwari, B.; Kumar, A.; Gedam, B.; Bhatia, V.; Krejcar, O.; Dobrovolny, M.; Nebhen, J.; Prakash, S. Deep transfer learning based photonics sensor for assessment of seed-quality. Comput. Electron. Agric. 2022, 196, 106891. [Google Scholar] [CrossRef]
- Diakaki, M.; Van der Heijden, L.; Lopez-Reyes, J.; Van Nieuwenhoven, A.; Notten, M.; Storcken, M.; Butterbach, P.; Köhl, J.; Boer, W.; Postma, J. Beetroot and spinach seed microbiomes can suppress Pythium ultimum infection: Results from a large-scale screening. Seed Sci. Res. 2022, 32, 274–282. [Google Scholar] [CrossRef]
- Thompson, G.A. Mechanisms of membrane response to environmental stress. In Frontiers of Membrane Research in Agriculture; John, J.B.S., Berlin, E., Jackson, P.C., Eds.; Rowman & Allanheld: Ottawa, ON, Canada, 1985; p. 512. [Google Scholar]
- Quinn, P.L. Models for adaptive changes in cell membranes. Biochem. Soc. Trans. 1983, 11, 329–331. [Google Scholar] [CrossRef]
- Gagné, J.; Stamatatos, L.; Diacovo, T.; Hui, S.W.; Yeagle, P.L.; Silvius, J.R. Physical properties and surface interactions of bilayer membrane containing N-methyl- ated phosphatidylethanolamine. Biochemistry 1985, 24, 4400–4408. [Google Scholar] [CrossRef]
- Chen, Y.; Burris, J.S. Effect of seed treatment emulsifiers on seed quality and membrane function in maize. Postharvest Biol. Technol. 1993, 2, 231–239. [Google Scholar] [CrossRef]
- Williams, W.A.; Elliot, J.R. Ecological significance of seed coat impermeability to moisture in crimson, subterranean and rose clovers in Mediterranean-type climate. Ecology 1960, 41, 733–742. [Google Scholar] [CrossRef]
- Souza, F.H.D.; Marcos-Filho, J. The seed coat as a modulator of seed-environment relationships in Fabaceae. Rev. Bras. Bot. 2001, 24, 365–375. [Google Scholar] [CrossRef]
- Aguirre, M.; Kiegle, E.; Leo, G.; Ezquer, I. Carbohydrate reserves and seed development: An overview. Plant Reprod. 2018, 31, 263–290. [Google Scholar] [CrossRef]
- Orozco-Almanza, M.S.; Lóon-García, L.P.; Grether, R.; García-Moya, E. Germination of four species of the genus Mimosa (leguminosae) in a semi-arid zone of Central Mexico. J. Arid. Environ. 2003, 55, 75–92. [Google Scholar] [CrossRef]
- Swanson, B.G.; Hughes, J.S.; Rasmussen, H.P. Seed microstructure: Review of water imbibition in legumes. Food Microstruct. 1985, 4, 115–124. [Google Scholar]
- Oguchi, R.; Hikosaka, K.; Hirose, T. Leaf anatomy as a constraint for photosynthetic acclimation: Differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees. Plant Cell. Environ. 2005, 28, 916–927. [Google Scholar] [CrossRef]
- Lozano-Isla, F.; Miranda, P.V.; Pompelli, M.F. Germination behavior of Jatropha curcas L. after different imbibition times. Per. J. Agron. 2017, 1, 32–38. [Google Scholar] [CrossRef]
- Mendes, C.R.; De Moraes, D.M.; Lima, M.G.S.; Lopes, N.F. Respiratory activity for the differentiation of vigor on soybean seeds lot. Rev. Bras. Sementes 2009, 31, 171–176. [Google Scholar] [CrossRef]
- Muscolo, A.; Panuccio, M.R.; Sidari, M. The effect of phenols on respiratory enzymes in seed germination, respiratory enzyme activities during germination of Pinus laricio seeds treated with phenols extracted from different forest soils. Plant Growth Regul. 2001, 35, 31–35. [Google Scholar] [CrossRef]
- Yentur, S.; Leopold, A.C. Respiratory transition during seed germination. Plant Physiol. 1976, 57, 274–276. [Google Scholar] [CrossRef]
- Pompelli, M.F.; Ferreira, D.T.R.G.; Cavalcante, P.P.G.S.; Salvador, T.L.; Hsie, B.S.; Endres, L. Environmental influence on the physico-chemical and physiological properties of Jatropha curcas L. seeds. Aust. J. Bot. 2010, 58, 421–427. [Google Scholar] [CrossRef]
- Rao, R.G.S.; Singh, P.M.; Rai, M. Storability of onion seeds and effects of packaging and storage conditions on viability and vigour. Sci. Hortic. 2006, 110, 1–6. [Google Scholar] [CrossRef]
- Takayanagi, K.; Harrington, J.F. Enhancement of germination rate of aged seeds by ethylene. Plant Physiol. 1971, 47, 521–524. [Google Scholar] [CrossRef]


| Scientific Name | Common Name | Family | N * | Sampled ** |
|---|---|---|---|---|
| Allamanda blanchetii A.DC. | Purple allamanda | Apocynaceae | 8 | September, 2022. |
| Annona squamosa L. | Sugar-apple | Annonaceae | 8 | April, 2022. |
| Arachis hypogaea L. | Peanut | Leguminosae | 5 | April, 2022. |
| Calotropis procera (Aiton) Dryand. | Milkweed | Asclepiadaceae | 30 | July, 2022. |
| Cucurbita maxima subsp. Maxima | Winter squash | Cucurbitaceae | 8 | April, 2022. |
| Euterpe oleracea Mart. | Açaí palm | Arecaceae | 5 | September, 2022. |
| Gossypium hirsutum L. | Cotton | Malvaceae | 10 | June, 2022. |
| Helianthus annuus L. | Sunflower | Compositae | 30 | April, 2022. |
| Jatropha curcas L. | Purging nut | Euphorbiaceae | 5 | June, 2022. |
| Licania rigida Benth. | Oiticica | Chrysobalanaceae | 3 | August, 2022. |
| Malpighia glabra L. | Acerola | Malpighiaceae | 5 | September, 2022. |
| Moringa oleifera Lam. | Drumstick tree | Moringaceae | 5 | July, 2022. |
| Prosopis juliflora (Sw.) DC. | Mesquite | Leguminosae | 10 | September, 2022. |
| Spondias tuberosa Arruda | Umbu plant | Anacardiaceae | 2 | April, 2022. |
| Group | Species | Water Imbibition * (mg H2O g−1 Seed) |
|---|---|---|
| Group 1 | E. olareaceae | 64.82 ± 5.03 j |
| A. squamosa | 367.86 ± 29.27 i | |
| L. rigida | 413.21 ± 8.03 i | |
| Group 2 | A. hypogaea | 614.89 ± 45.55 h |
| A. blanchetti | 567.76 ± 12.92 h | |
| J. curcas | 749.62 ± 11.86 g | |
| S. tuberosa | 770.29 ± 20.19 g | |
| P. juliflora | 880.50 ± 67.62 f | |
| C. maxima | 888.63 ± 25.42 f | |
| G. hirsutum | 997.04 ± 15.80 e | |
| Group 3 | H. annus | 1155.10 ± 27.31 d |
| M. oleifera | 1251.96 ± 11.82 c | |
| Group 4 | C. procera | 1568.67 ± 63.97 b |
| Group 5 | M. glabra | 3173.31 ± 61.96 a |
| Group | Species | ECi | ECf | Seed Mass (g FW) | EC (dS m−1 g−1 Seed) |
|---|---|---|---|---|---|
| Group 1 | E. oleracea | 0.01 ± 0.01 c | 0.36 ± 0.12 d | 5.88 ± 0.11 b | 0.06 ± 0.02 e |
| M. oleifera | 0.02 ± 0.01 bc | 0.14 ± 0.02 d | 1.29 ± 0.15 g | 0.11 ± 0.01 de | |
| S. tuberosa | 0.05 ± 0.01 bc | 0.31 ± 0.06 d | 2.76 ± 0.15 e | 0.11 ± 0.01 de | |
| A. squamosa | 0.02 ± 0.01 bc | 0.25 ± 0.06 d | 2.20 ± 0.01 f | 0.11 ± 0.03 de | |
| P. juliflora | 0.01 ± 0.01 c | 0.05 ± 0.01 d | 0.38 ± 0.01 j | 0.13 ± 0.02 de | |
| L. rigida | 0.06 ± 0.01 b | 1.60 ± 0.26 b | 8.07 ± 0.01 a | 0.20 ± 0.03 de | |
| A. hypogaea | 0.03 ± 0.01 bc | 0.74 ± 0.24 c | 3.02 ± 0.01 d | 0.25 ± 0.08 d | |
| H. annuus | 0.02 ± 0.01 bc | 0.17 ± 0.07 d | 0.69 ± 0.01 i | 0.25 ± 0.10 d | |
| Group 2 | M. glabra | 0.03 ± 0.01 bc | 0.09 ± 0.01 d | 0.20 ± 0.01 l | 0.46 ± 0.03 c |
| C. procera | 0.01 ± 0.01 bc | 0.10 ± 0.03 d | 0.19 ± 0.01 m | 0.56 ± 0.19 c | |
| G. hirsutum | 0.06 ± 0.01 b | 0.61 ± 0.13 c | 1.02 ± 0.01 h | 0.59 ± 0.12 c | |
| Group 3 | J. curcas | 0.46 ± 0.07 a | 2.59 ± 0.33 a | 3.16 ± 0.01 c | 0.82 ± 0.10 b |
| Group 4 | C. maxima | 0.06 ± 0.01 b | 0.35 ± 0.01 d | 0.37 ± 0.01 k | 0.94 ± 0.04 a |
| A. blanchetti | 0.02 ± 0.01 bc | 0.11 ± 0.01 d | 0.11 ± 0.01 n | 0.97 ± 0.13 a |
| Species | Germination (%) | Mean Germination Time (Days) | ||
|---|---|---|---|---|
| Non-Imbibed | 120 h-Imbibed | Non-Imbibed | 120 h-Imbibed | |
| A. blanchetii | 43.87 ± 1.82 b | 57.11 ± 0.19 a | 12.55 ± 0.28 a | 8.45 ± 0.11 b |
| A. squamosa | 53.14 ± 0.91 b | 80.67 ± 0.38 a | 3.38 ± 0.05 a | 2.82 ± 0.03 b |
| A. hypogaea | 69.05 ± 0.13 b | 85.02 ± 0.17 a | 8.52 ± 0.04 a | 5.06 ± 0.05 b |
| C. procera | 81.01 ± 0.16 b | 99.54 ± 0.09 a | 5.69 ± 0.23 a | 4.57 ± 0.11 b |
| C. maxima | 57.59 ± 1.10 b | 66.00 ± 0.80 a | 8.34 ± 0.16 a | 4.69 ± 0.21 b |
| E. oleracea | 17.87 ± 2.44 a | 22.85 ± 0.74 a | 24.09 ± 0.35 b | 44.60 ± 0.51 a |
| G. hirsutum | 75.34 ± 0.49 b | 89.95 ± 0.21 a | 1.97 ± 0.08 a | 1.44 ± 0.13 b |
| H. annuus | 66.02 ± 0.32 b | 97.98 ± 0.08 a | 4.11 ± 0.22 a | 1.79 ± 0.03 b |
| J. curcas | 84.85 ± 0.50 a | 63.75 ± 0.59 b | 8.65 ± 0.04 a | 7.77 ± 0.05 b |
| L. rigida | 80.92 ± 0.37 b | 96.17 ± 0.30 a | 10.86 ± 0.31 a | 6.15 ± 0.04 b |
| M. glabra | 10.25 ± 0.24 b | 19.10 ± 0.62 a | 8.47 ± 0.32 a | 5.92 ± 0.25 b |
| M. oleifera | 59.73 ± 0.35 b | 88.02 ± 0.41 a | 6.04 ± 0.14 a | 2.10 ± 0.04 b |
| P. juliflora | 37.10 ± 1.23 a | 42.62 ± 0.42 a | 6.92 ± 0.12 a | 2.93 ± 0.19 b |
| S. tuberosa | 13.77 ± 0.95 a | 24.70 ± 0.62 a | 38.45 ± 0.62 a | 14.07 ± 0.33 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pompelli, M.F.; Jarma-Orozco, A.; Rodriguez-Páez, L.A. Imbibition and Germination of Seeds with Economic and Ecological Interest: Physical and Biochemical Factors Involved. Sustainability 2023, 15, 5394. https://doi.org/10.3390/su15065394
Pompelli MF, Jarma-Orozco A, Rodriguez-Páez LA. Imbibition and Germination of Seeds with Economic and Ecological Interest: Physical and Biochemical Factors Involved. Sustainability. 2023; 15(6):5394. https://doi.org/10.3390/su15065394
Chicago/Turabian StylePompelli, Marcelo F., Alfredo Jarma-Orozco, and Luis Alfonso Rodriguez-Páez. 2023. "Imbibition and Germination of Seeds with Economic and Ecological Interest: Physical and Biochemical Factors Involved" Sustainability 15, no. 6: 5394. https://doi.org/10.3390/su15065394
APA StylePompelli, M. F., Jarma-Orozco, A., & Rodriguez-Páez, L. A. (2023). Imbibition and Germination of Seeds with Economic and Ecological Interest: Physical and Biochemical Factors Involved. Sustainability, 15(6), 5394. https://doi.org/10.3390/su15065394








