Plant Diversity and Species Composition in Relation to Soil Enzymatic Activity in the Novel Ecosystems of Urban–Industrial Landscapes
Abstract
1. Introduction
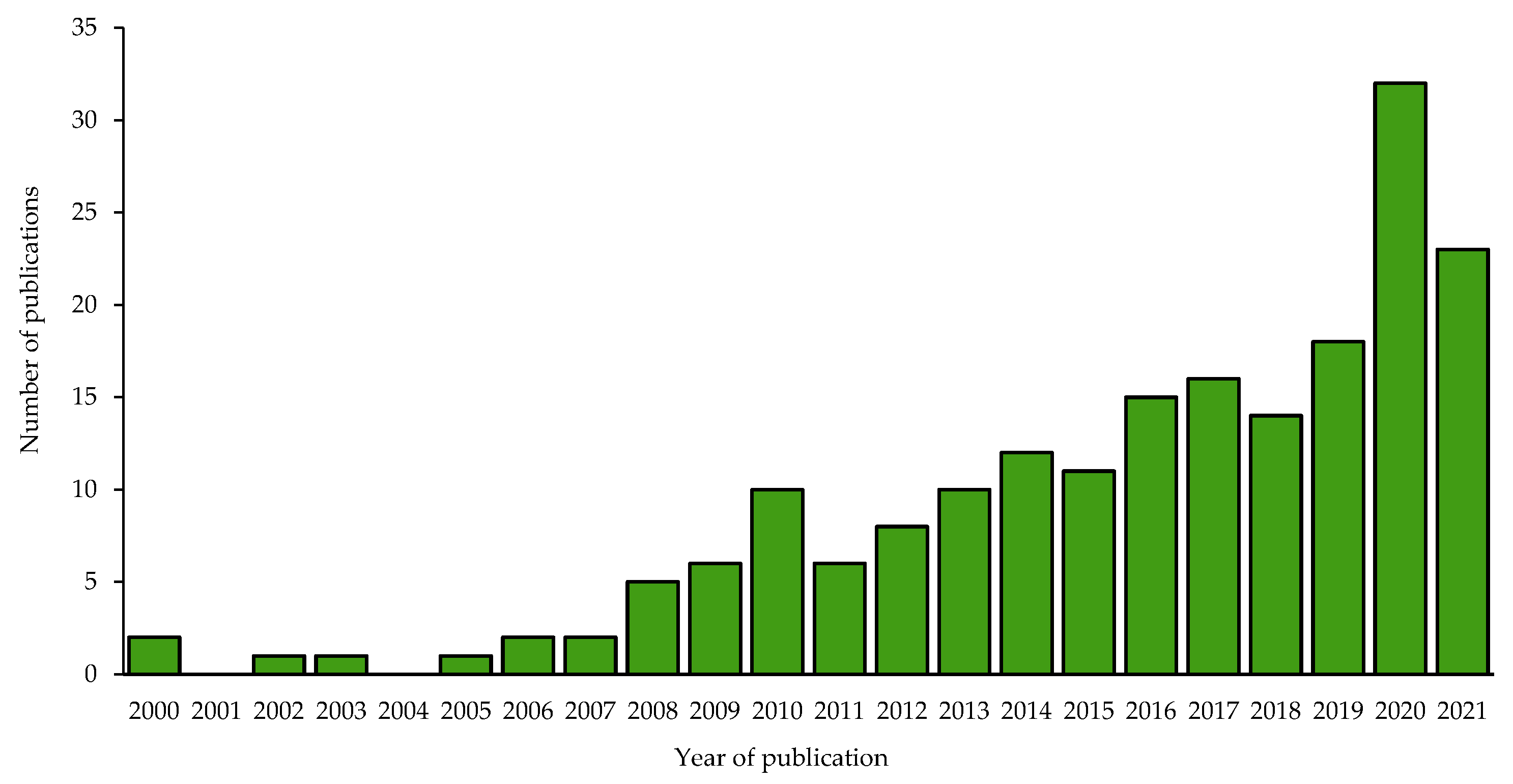
2. Procedure for Selecting the Bibliographic Portfolio and Data Collection
- (i)
- TITLE-ABSTRACT-KEYWORDS ({soil microbial activity} OR {soil enzymes} OR {soil enzyme activity} AND {novel ecosystems} AND LIMIT-TO (LANGUAGE, “English”) AND (LIMIT-TO (DOCTYPE, “article”) OR LIMIT-TO (DOCTYPE, “review”)).
- (ii)
- TITLE-ABSTRACT-KEYWORDS ({soil microbial activity} OR {soil enzymes} OR {soil enzyme activity} AND {urban area} OR {post industrial area} OR {spoil heaps} AND LIMIT-TO (LANGUAGE, “English”) AND (LIMIT-TO (DOCTYPE, “article”) OR LIMIT-TO (DOCTYPE, “review”)).
- (iii)
- TITLE-ABSTRACT-KEYWORDS ({soil microbial activity} OR {soil enzymes} OR {soil enzyme activity} AND {plant diversity}) AND LIMIT-TO (LANGUAGE, “English”) AND (LIMIT-TO (DOCTYPE, “article”) OR LIMIT-TO (DOCTYPE, “review”)).
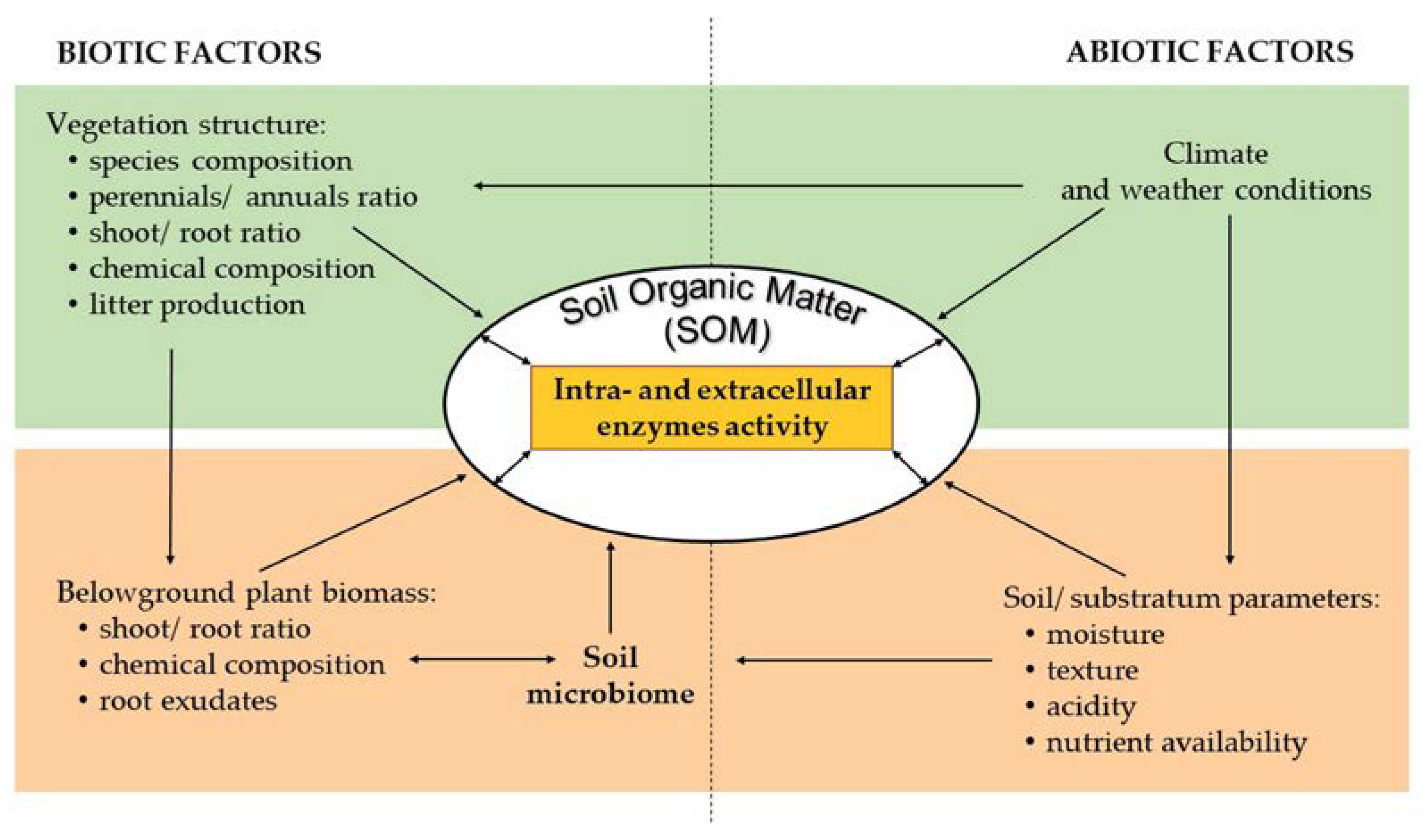
3. Soil Enzyme Activity—Basic Information
4. The Physicochemical Properties Filtering the Plant Diversity and the Species Composition
5. Soil Properties, Vegetation Composition, and Soil Enzyme Activity in Novel Ecosystems
5.1. Relations between Soil Physicochemical Properties and Soil Enzyme Activity in Novel Ecosystems
5.2. Relations between the Vegetation and the Soil Enzyme Activity in Novel Ecosystems
| Type of Site | Enzyme | Grasses | Herbs | Coniferous Trees | Deciduous Trees | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calamagrostis epigejos [61,136,137] | Poa compressa [61,136,137] | Digitaria eriantha + Cynodon dactylon + Eragrostis curvula [138] | Daucus carota [61,136,137] | Tussilago farfara [61,136,137] | Lantana camara [139] | Picea omorica + Picea pungens [129] | Pinus contorta + Pinus nigra [129] | Pinus sylvestris [130,132] | Larix decidua [129] | Quercus robur [129] | Betula pendula [130,132] | Alnus glutinosa + Alnus incana [129,130,132] | Tilia cordata [129] | Robinia pseudoacacia [130] | ||
| Coal mine heap | Dehydrogenase | 60–120 1 | 140–199 1 | 57–65 1 | 24–26 1 | 2.5 1 | ||||||||||
| Urease | 0.20–0.30 2 | 0.20–0.22 2 | 5 2 | 0.10–0.20 2 | 0.10–0.20 2 | |||||||||||
| Acid phosphatase | 1165–1754 3 | 899–1790 3 | 329–529 3 | 221–263 3 | ||||||||||||
| Alkaline phosphatase | 1445–3008 3 | 1966–2751 3 | 718–1057 3 | 546–1247 3 | ||||||||||||
| Brown coal heap | β-glucosidase | 1395 4 | 1500 4 | 2297 4 | 1500 4 | 2423 4 | 1668 4 | |||||||||
| Celobiohydrolase | 582 4 | 519 4 | 718 4 | 708 4 | 1253 4 | 949 4 | ||||||||||
| Xylosidase | 267 4 | 215 4 | 362 4 | 225 4 | 834 4 | 424 4 | ||||||||||
| Acid phosphatase | 213 3 | 234 3 | 291 3 | 322 3 | ||||||||||||
| Alkaline phosphatase | 218 3 | 275 3 | 284 3 | 248 3 | ||||||||||||
| Sulphur mining heap | Dehyrogenase | 100 1 | 690 1 | |||||||||||||
| Sand post mining site | Urease | 56 2 | 148 2 | 89 2 | ||||||||||||
| Acid phosphatase | 123 3 | 331 3 | 404 3 | |||||||||||||
| Alkaline phosphatase | 14 3 | 275 3 | 222 3 | |||||||||||||
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chapin, F.S., III; Zavaleta, E.S.; Eviner, V.T.; Naylor, R.L.; Vitousek, P.M.; Reynolds, H.L.; Hooper, D.U.; Lavorel, S.; Sala, O.E.; Hobbie, S.E.; et al. Consequences of changing biodiversity. Nature 2000, 405, 234–242. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Wright, J.P.; Cadotte, M.W.; Carroll, I.T.; Hector, A.; Srivastava, D.S.; Loreau, M.; Weis, J.J. Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc. Natl. Acad. Sci. USA 2007, 104, 18123–18128. [Google Scholar] [CrossRef] [PubMed]
- Adler, P.B.; Seabloom, E.W.; Borer, E.T.; Hillebrand, H.; Hautier, Y.; Hector, A.; Harpole, W.S.; O’Halloran, L.R.; Grace, J.B.; Anderson, T.M.; et al. Productivity is a poor predictor of plant species richness. Science 2011, 333, 1750–1753. [Google Scholar] [CrossRef] [PubMed]
- Naeem, S.; Loreau, M.; Inchausti, P. Biodiversity and ecosystem functioning: The emergence of a synthetic ecological framework. In Biodiversity and Ecosystem Functioning—Synthesis and Perspectives; Loreau, M., Naeem, S., Inchausti, P., Eds.; Oxford University Press: New York, NY, USA, 2002; pp. 3–11. [Google Scholar]
- Van der Putten, W.H.; Macel, M.; Visser, M.E. Predicting species distribution and abundance responses to climate change: Why it is essential to include biotic interactions across trophic levels. Philos. T. Roy Soc. B 2010, 365, 2025–2034. [Google Scholar] [CrossRef] [PubMed]
- Ravenek, J.M.; Bessler, H.; Engels, C.; Scherer-Lorenzen, M.; Gessler, A.; Gockele, A.; De Luca, E.; Temperton, V.M.; Ebeling, A.; Roscher, C.; et al. Long-term study of root biomass in a biodiversity experiment reveals shifts in diversity effects over time. Oikos 2014, 123, 1528–1536. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Lanoue, A.; Strecker, T.; Scheu, S.; Steinauer, K.; Thakur, M.P.; Mommer, L. Root biomass and exudates link plant diversity with soil bacterial and fungal biomass. Sci. Rep. UK 2017, 7, 44641. [Google Scholar] [CrossRef]
- Fontaine, S.; Mariotti, A.; Abbadie, L. The priming effect of organic matter: A question of microbial competition? Soil Biol. Biochem. 2003, 35, 837–843. [Google Scholar] [CrossRef]
- Levine, J.M.; Hille Ris Lambers, J. The importance of niches for the maintenance of species diversity. Nature 2009, 461, 254–257. [Google Scholar] [CrossRef]
- Frouz, J. Soil Biota and Ecosystem Development in Post Mining Sites; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 1–316. [Google Scholar]
- Witkamp, M.; Ausmus, B.S. Processes in decomposition and nutrient transport in forest systems. In The Role of Terrestrial and Aquatic Organisms in Decomposition Processes; Anderson, J.M., Macfadyen, A., Eds.; Blackwell Sci. Publ.: Hoboken, NJ, USA, 1976; pp. 375–396. [Google Scholar]
- Bardgett, R.D.; Bowman, W.D.; Kaufmann, R.; Schmidt, S.K. A temporal approach to linking aboveground and belowground ecology. Trends Ecol. Evol. 2005, 20, 634–641. [Google Scholar] [CrossRef]
- Thijs, S.; Sillen, W.; Rineau, F.; Weyens, N.; Vangronsveld, J. Towards an enhanced understanding of plant-microbiome interactions to improve phytoremediation: Engineering the metaorganism. Front. Microbiol. 2016, 7, 341. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Chen, F.; Li, J.; Wu, J.; Sun, Y.; Zhang, Y.; Deng, T.; Jiang, S.; Zhou, X.; et al. Soil organic matter enhances aboveground biomass in alpine grassland under drought. Geoderma 2023, 433, 116430. [Google Scholar] [CrossRef]
- Allison, V.J.; Condron, L.M.; Peltzer, D.A.; Richardson, S.J.; Turner, B.L. Changes in enzyme activities and soil microbial community composition along carbon and nutrient gradients at the Franz Josef chronosequence, New Zealand. Soil Biol. Biochem. 2007, 39, 1770–1781. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Jiang, S.; Xiao, J.; Li, J.; Zhou, X.; Liu, H.; Hao, Z.; Wang, K. Soil development mediates precipitation control on plant productivity and diversity in alpine grasslands. Geoderma 2022, 412, 115721. [Google Scholar] [CrossRef]
- Díaz, S.; Lavorel, S.; de Bello, F.; Quétier, F.; Grigulis, K.; Robson, T.M. Incorporating plant functional diversity effects in ecosystem service assessments. Proc. Natl. Acad. Sci. USA 2007, 104, 20684–20689. [Google Scholar] [CrossRef] [PubMed]
- De Deyn, G.B.; Quirk, H.; Yi, Z.; Oakley, S.; Ostle, N.J.; Bardgett, R.D. Vegetation composition promotes carbon and nitrogen storage in model grassland communities of contrasting soil fertility. J. Ecol. 2009, 97, 864–875. [Google Scholar] [CrossRef]
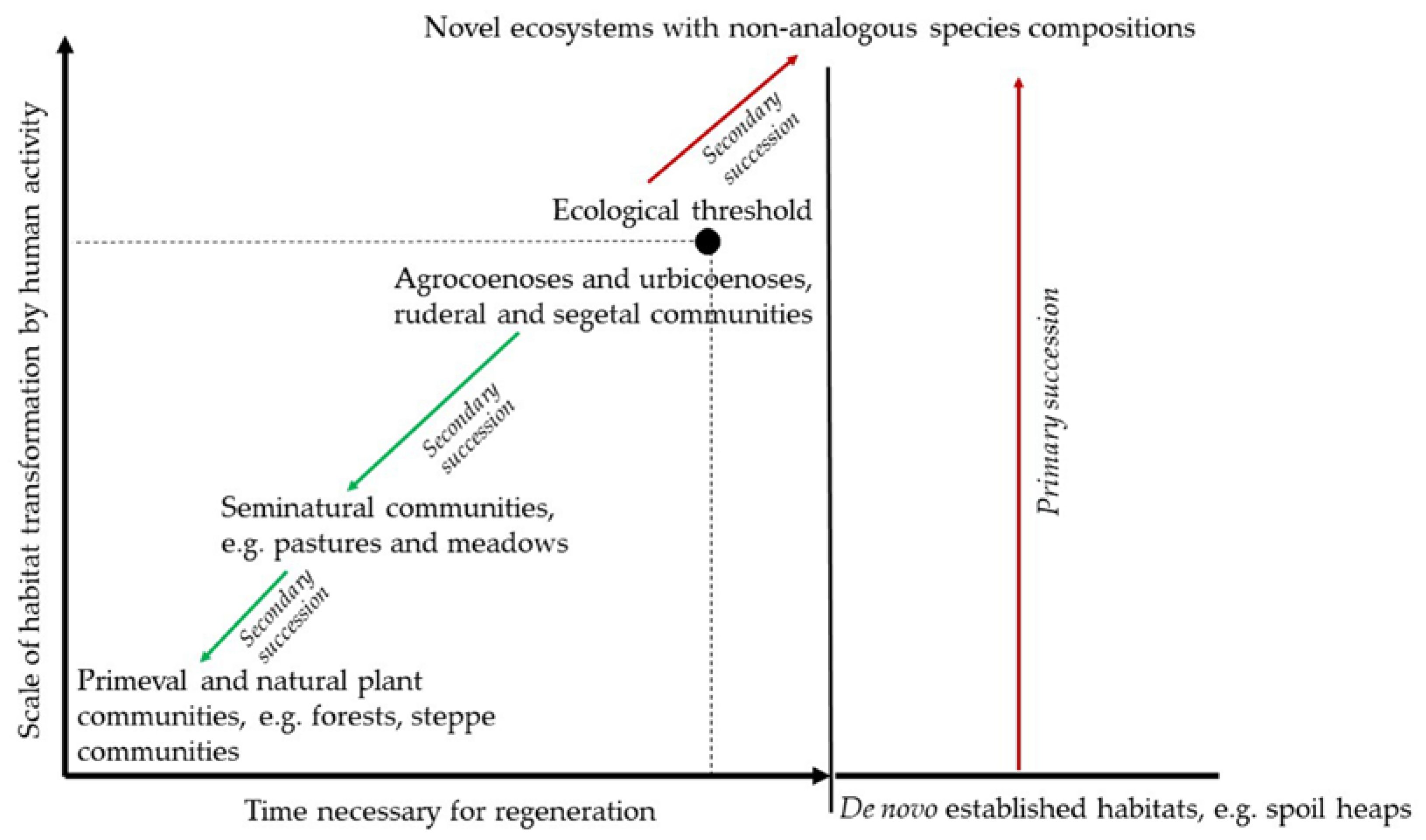
- Walker, L.R. The Biology of Disturbed Habitats; Oxford University Press: Oxford, UK, 2012; pp. 1–319. [Google Scholar]
- Hobbs, R.J.; Arico, S.; Aronson, J.; Baron, J.S.; Bridgewater, P.; Cramer, V.A.; Epstein, P.R.; Ewel, J.J.; Klink, C.A.; Lugo, A.E.; et al. Novel ecosystems: Theoretical and management aspects of the new ecological world order. Global Ecol. Biogeogr. 2006, 15, 1–7. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Higgs, E.S.; Harris, J.A.; Hall, C.M. Novel ecosystems: Implications for conservation and restoration. Trends Ecol. Evol. 2009, 24, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, R.J.; Higgs, E.S.; Hall, C.M. Novel Ecosystems: Intervening in the New Ecological World Order; John Wiley & Sons: Chichester, UK, 2013; pp. 1–384. [Google Scholar]
- Williams, J.W.; Jackson, S.T. Novel climates, no-analog communities and ecological surprises. Front. Ecol. Environ. 2007, 5, 475–482. [Google Scholar] [CrossRef]
- Keith, S.A.; Newton, A.S.; Herbert, R.J.H.; Morecroft, M.D.; Bealey, C.E. Non-analogous community formation in response to climate change. J. Nat. Conserv. 2009, 17, 228–235. [Google Scholar] [CrossRef]
- Morse, N.B.; Pellissier, P.A.; Cianciola, E.N.; Brereton, R.L.; Sullivan, M.M.; Shonka, N.K.; Wheeler, T.B.; McDowell, W.H. Novel ecosystems in the Anthropocene: A revision of the novel ecosystem concept for pragmatic applications. Ecol. Soc. 2014, 19, 12. [Google Scholar] [CrossRef]
- Rotherham, I.D. Recombinant Ecology—A Hybrid Future? Springer: Cham, Switzerland, 2017; pp. 1–85. [Google Scholar]
- Evers, C.R.; Wardropper, C.B.; Branoff, B.; Granek, E.F.; Hirsch, S.L.; Link, T.E.; Olivero-Lora, S.; Wilson, C. The ecosystem services and biodiversity of novel ecosystems: A literature review. Glob. Ecol. Conserv. 2018, 13, e00362. [Google Scholar] [CrossRef]
- Aon, M.A.; Colaneri, A.C., II. Temporal and spatial evolution of enzymatic activities and physico-chemical properties in an agricultural soil. Appl. Soil Ecol. 2001, 18, 255–270. [Google Scholar] [CrossRef]
- Baldrian, P.; Trögl, J.; Frouz, J.; Šnajdr, J.; Valášková, V.; Merahutová, V.; Cajthaml, T.; Herinková, J. Enzyme activities and microbial biomass in top soil layer during spontaneous succession in spoil heaps after brown coal mining. Soil Biol. Biochem. 2008, 40, 2107–2115. [Google Scholar] [CrossRef]
- Błońska, E.; Januszek, K. Usability of enzyme activity in the estimation of forest soil quality. Folia For. Pol. Ser. A 2013, 55, 18–26. [Google Scholar] [CrossRef]
- Li, J.J.; Zheng, Y.M.; Yan, J.X.; Li, H.J.; He, J.Z. Succession of plant and soil microbial communities with restoration of abandoned land in the Loess Plateau, China. J. Soil. Sediment. 2013, 13, 760–769. [Google Scholar] [CrossRef]
- Heděnec, P.; Vinduškov, O.; Kukla, J.; Šnajdr, J.; Baldrian, P.; Frouz, J. Enzyme activity of topsoil layer on reclaimed and unreclaimed post-mining sites. Biol. Commun. 2017, 62, 19–25. [Google Scholar] [CrossRef]
- Rohatgi, A. Web Plot Ditigizer 4.60. Available online: https://automeris.io/WebPlotDigitizer (accessed on 20 December 2022).
- Loreau, M. Linking biodiversity and ecosystems: Towards a unifying ecological theory. Philos. T. R. Soc. B 2010, 365, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Frouz, J.; Pižl, V.; Cienciala, E.; Kalčík, J. Carbon storage in post-mining forest soil, the role of tree biomass and soil bioturbation. Biogeochemistry 2009, 94, 111–121. [Google Scholar] [CrossRef]
- Frouz, J.; Pižl, V.; Tajovský, K. The effect of earthworms and other saprophagous macrofauna on soil microstructure in reclaimed and un-reclaimed post-mining sites in Central Europe. Eur. J. Soil Biol. 2007, 43, S184–S189. [Google Scholar] [CrossRef]
- Schimann, H.; Petit-Jean, C.; Guitet, S.; Reis, T.; Domenach, A.M.; Roggy, J.C. Microbial bioindicators of soil functioning after disturbance: The case of gold mining in tropical rainforests of French Guiana. Ecol. Indic. 2012, 20, 34–41. [Google Scholar] [CrossRef]
- Boivin, M.-E.Y.; Breure, A.M.; Posthuma, L.; Rutgers, M. Determination of field effects of contaminants—Significance of pollution-induced community tolerance. Hum. Ecol. Risk Assess. 2002, 8, 1035–1055. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, G.; Fu, B.; Zheng, X. Relationship between plant species diversity and soil microbial functional diversity along a longitudinal gradient in temperate grasslands of Hulunbeir, Inner Mongolia, China. Ecol. Res. 2008, 23, 511–518. [Google Scholar] [CrossRef]
- Dassen, S.; Cortois, R.; Martens, H.; de Hollander, M.; Kowalchuk, G.A.; van der Putten, W.H.; De Deyn, G.B. Differential responses of soil bacteria, fungi, archaea and protists to plant species richness and plant functional group identity. Mol. Ecol. 2017, 26, 4085–4098. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Zak, D.R.; Reich, P.B.; Ellsworth, D.S. Plant species richness, elevated CO2, and atmospheric nitrogen deposition alter soil microbial community composition and function. Glob. Change Biol. 2007, 13, 980–989. [Google Scholar] [CrossRef]
- Waldrop, M.P.; Balser, T.C.; Firestone, M.K. Linking microbial community composition to function in a tropical soil. Soil Biol. Biochem. 2000, 32, 1837–1846. [Google Scholar] [CrossRef]
- Grayston, S.J.; Griffith, G.S.; Mawdsley, J.L.; Campbell, C.D.; Bardgett, R.D. Accounting for variability in soil microbial communities of temperate upland grassland ecosystems. Soil Biol. Biochem. 2001, 33, 533–551. [Google Scholar] [CrossRef]
- Porazinska, D.L.; Bardgett, R.D.; Postma-Blaauw, M.B.; Hunt, W.H.; Parsons, A.N.; Seastedt, T.R.; Wall, D.M. Relationships at the aboveground–belowground interface: Plants, soil biota, and soil processes. Ecol. Monogr. 2003, 73, 377–395. [Google Scholar] [CrossRef]
- Bartelt-Ryser, J.; Joshi, J.; Schmid, B.; Brandl, H.; Balser, T. Soil feedbacks of plant diversity on soil microbial communities and subsequent plant growth. Perspect. Plant Ecol. 2005, 7, 27–49. [Google Scholar] [CrossRef]
- Kao-Kniffin, J.; Balser, T.C. Elevated CO2 differentially alters belowground plant and soil microbial community structure in reed canary grass-invaded experimental wetlands. Soil Biol. Biochem. 2007, 39, 517–525. [Google Scholar] [CrossRef]
- Liang, C.; Fujinuma, R.; Wie, L.; Balser, T.C. Tree species-specific effects on soil microbial residues in an upper Michigan old-growth forest system. Forestry 2007, 80, 65–72. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Beßler, H.; Engels, C.; Gleixner, G.; Habekost, M.; Milcu, A.; Partsch, S.; Sabais, A.C.W.; Scherber, C.; Steinbeiss, S.; et al. Plant diversity effects on soil microorganisms support the singular hypothesis. Ecology 2010, 91, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Mariotte, P.; Vandenberghe, C.; Meugnier, C.; Ross, P.; Bardgett, R.D.; Buttler, A. Subordinate plant species impact on soil microbial communities and ecosystem functioning in grasslands: Findings from a removal experiment. Perspect. Plant Ecol. 2013, 15, 77–85. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J.; Modrzynski, J.; Mrozinski, P.; Hobbie, S.E.; Eissenstat, D.M.; Chorover, J.; Chadwick, O.A.; Hale, C.M.; Tjoelker, M.G. Linking litter calcium, earthworms and soil properties: A common garden test with 14 tree species. Ecol. Lett. 2005, 8, 811–818. [Google Scholar] [CrossRef]
- Wang, C.; Wang, G.; Liu, W.; Wu, P. The effects of plant-soil-enzyme interaction on plant composition, biomass, diversity of alpine meadows in the Qinghai-Tibetan Plateau. Int. J. Ecol. 2011, 2011, 180926. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, S.; Gao, Q.; Liu, S.; Ganjurjav, H.; Wang, X.; Su, X.; Wu, X. Soil bacterial and fungal diversity differently correlated with soil biochemistry in alpine grassland ecosystems in response to environmental changes. Sci. Rep. UK 2017, 7, 43077. [Google Scholar] [CrossRef]
- Franzluebbers, A.J. Soil organic carbon sequestration and agricultural greenhouse gas emissions in the southeastern USA. Soil Till. Res. 2005, 83, 120–147. [Google Scholar] [CrossRef]
- Coleman, M.D.; Dickson, R.E.; Isebrands, J.G. Contrasting fine-root production, survival and soil CO2 efflux in pine and poplar plantations. Plant Soil 2000, 225, 129–139. [Google Scholar] [CrossRef]
- Walela, C.; Daniel, H.; Wilson, B.; Lockwood, P.; Cowie, A.; Harden, S. The initial lignin: Nitrogen ratio of litter from above and below ground sources strongly and negatively influenced decay rates of slowly decomposing litter carbon pools. Soil Biol. Biochem. 2014, 77, 268–275. [Google Scholar] [CrossRef]
- Finzi, A.C.; Canham, C.D. Non-additive effects of litter mixtures on net N mineralization in a southern New England forest. Forest Ecol. Manag. 1998, 105, 129–136. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Vitousek, P.M. The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol. Evol. 2000, 15, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Bierza, W. Soil Enzymes–a Tool to Monitor Soil-forming Processes in Coal Mine Spoil Heaps. In Green Scenarios: Mining Industry Responses to Environmental Challenges of the Anthropocene Epoch—International Mining Forum 2021; Dyczko, A., Jagodziński, A.M., Woźniak, G., Eds.; CRC Press Balkema, Taylor & Francis Group: Boca Raton, FL, USA, 2022; pp. 181–194. [Google Scholar]
- Naeem, S.; Hahn, D.R.; Schuurman, G. Producer–decomposer co-dependency influences biodiversity effects. Nature 2000, 403, 762–764. [Google Scholar] [CrossRef] [PubMed]
- Kompała-Bąba, A.; Bierza, W.; Sierka, E.; Błońska, A.; Besenyei, L.; Woźniak, G. The role of plants and soil properties in the enzyme activities of substrates on hard coal mine spoil heaps. Sci. Rep. 2021, 11, 5155. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Högberg, M.N.; Högberg, P.; Myrold, D.D. Is microbial community composition in boreal forest soils determined by pH, C-to-N ratio, the trees or all three? Oecologia 2007, 150, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lan, Y.; Zhang, H.; Ye, S. Research characteristics and hotspots of the relationship between soil microorganisms and vegetation: A bibliometric analysis. Ecol Indic. 2022, 141, 109145. [Google Scholar] [CrossRef]
- Balser, T.C.; Firestone, M.K. Linking microbial community composition and soil processes in a California annual grassland and mixed-conifer forest. Biogeochemistry 2005, 73, 395–415. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, W.; Liu, Y.; Yun, W.; Luo, B.; Chai, R.; Zhang, C.; Xiang, X.; Su, X. Changes in phosphorus mobilization and community assembly of bacterial and fungal communities in rice rhizosphere under phosphate deficiency. Front. Microbiol. 2022, 13, 953340. [Google Scholar] [CrossRef]
- Dhungana, I.; Kantar, M.B.; Nguyen, N.H. Root exudate composition from different plant species influences the growth of rhizosphere bacteria. Rhizosphere 2023, 25, 100645. [Google Scholar] [CrossRef]
- Sandnes, A.; Eldhuset, T.D.; Wollebæk, G. Organic acids in root exudates and soil solution of Norway spruce and silver birch. Soil Biol. Biochem. 2005, 37, 259–269. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [PubMed]
- Weisskopf, L.; Le Bayon, R.-C.; Kohler, F.; Page, V.; Jossi, M.; Gobat, J.-M.; Martinoia, E.; Aragno, M. Spatio-temporal dynamics of bacterial communities associated with two plant species differing in organic acid secretion: A one-year microcosm study on lupin and wheat. Soil Biol. Biochem. 2008, 40, 1772–1780. [Google Scholar] [CrossRef]
- Kuiper, I.; Lagendijk, E.L.; Bloemberg, G.V.; Lugtenberg, B.J.J. Rhizoremediation: A beneficial plant– microbe interaction. Mol. Plant Microbe Interact. 2004, 17, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Kraft, N.J.B.; Adler, P.B.; Godoy, O.; James, E.C.; Fuller, S.; Levine, J.M. Community phylogenetics and ecosystem functioning: Community assembly, coexistence and the environmental filtering metaphor. Funct. Ecol. 2015, 29, 592–599. [Google Scholar] [CrossRef]
- Nobel, I.R.; Slatyer, R.O. Post-fire succession of plants in Mediterranean ecosystems. In Proceedings of the Symposium on the Environmental Consequences of Fire and Fuel Management in Mediterranean Ecosystems, Palo Alto, CA, USA, 1–5 August 1977; Mooney, H.A., Conrad, C.E., Eds.; Forest Service: Washington, DC, USA, 1977; pp. 27–36. [Google Scholar]
- Van der Valk, A.G. Succession in wetlands—A Gleasonian approach. Ecology 1981, 62, 688–696. [Google Scholar] [CrossRef]
- Bazzaz, F.A. Habitat selection in plants. Am. Nat. 1991, 137, S116–S130. [Google Scholar] [CrossRef]
- Woodward, F.I.; Diament, A.D. Functional approaches to predicting the ecological effects of global change. Funct. Ecol. 1991, 5, 202–212. [Google Scholar] [CrossRef]
- Weiher, E.; Clarke, G.D.P.; Keddy, P.A. Community assembly rules, morphological dispersion, and the coexistence of plant species. Oikos 1998, 81, 309–322. [Google Scholar] [CrossRef]
- Webb, C.O. Exploring the phylogenetic structure of ecological communities: An example for rain forest trees. Am. Nat. 2000, 156, 145–155. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Schwilk, D.W.; Ackerly, D.D. A trait-based test for habitat filtering: Convex hull volume. Ecology 2006, 87, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Whitfeld, T.J.S.; Kress, W.J.; Erickson, D.L.; Weiblen, G.D. Change in community phylogenetic structure during tropical forest succession: Evidence from New Guinea. Ecography 2011, 35, 821–830. [Google Scholar] [CrossRef]
- Swenson, N.G.; Enquist, B.J.; Pither, J.; Kerkhoff, A.J.; Boyle, B.; Weiser, M.D.; Elser, J.J.; Fagan, W.F.; Forero, J.; Fyllas, N.M.; et al. The biogeography and filtering of woody plant functional diversity in North and South America. Global Ecol. Biogeogr. 2012, 21, 798–808. [Google Scholar] [CrossRef]
- Whittaker, R.H. Vegetation of the Siskiyou mountains, Oregon and California. Ecol. Monogr. 1960, 30, 279–338. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Ackerly, D. Community assembly and shifts in the distribution of functional trait values across an environmental gradient in coastal California. Ecol. Monogr. 2009, 79, 109–126. [Google Scholar] [CrossRef]
- Malanson, G.P.; Westman, W.E.; Yan, Y.L. Realized versus fundamental niche functions in a model of chaparral response to climatic-change. Ecol. Model. 1992, 64, 261–277. [Google Scholar] [CrossRef]
- McGill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Mayfield, M.M.; Levine, J.M. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 2010, 13, 1085–1093. [Google Scholar] [CrossRef]
- Hille Ris Lambers, J.; Adler, P.B.; Harpole, W.S.; Levine, J.M.; Mayfield, M.M. Rethinking community assembly through the lens of coexistence theory. Annu. Rev. Ecol. Evol. S. 2012, 43, 227–248. [Google Scholar] [CrossRef]
- Narwani, A.; Alexandrou, M.A.; Oakley, T.H.; Carroll, I.T.; Cardinale, B.J. Experimental evidence that evolutionary relatedness does not affect the ecological mechanisms of coexistence in freshwater green algae. Ecol. Lett. 2013, 16, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Godoy, O.; Kraft, N.J.B.; Levine, J.M. Phylogenetic relatedness and the determinants of competitive outcomes. Ecol. Lett. 2014, 17, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.O.; Ackerly, D.D.; McPeek, M.A.; Donoghue, M.J. Phylogenies and community ecology. Ann. Rev. Ecol. Syst. 2002, 33, 475–505. [Google Scholar] [CrossRef]
- Stubbs, W.J.; Wilson, J.B. Evidence for limiting similarity in a sand dune community. J. Ecol. 2004, 92, 557–567. [Google Scholar] [CrossRef]
- Balmford, A.; Bruner, A.; Cooper, P.; Costanza, R.; Farber, S.; Green, R.E.; Jenkins, M.; Jefferiss, P.; Jessamy, V.; Madden, J.; et al. Economic reasons for conserving wild nature. Science 2002, 297, 950–953. [Google Scholar] [CrossRef]
- Crutzen, P.J.; Stoermer, E.F. The “Anthropocene” (2000). In Paul J. Crutzen and the Anthropocene: A New Epoch in Earth’s History; Benner, S., Lax, G., Crutzen, P.J., Pöschl, U., Lelieveld, J., Brauch, H.G., Eds.; The Anthropocene: Politik—Economics—Society—Science; Springer: Cham, Switzerland, 2021; Volume 1, pp. 19–21. [Google Scholar]
- Wolińska, A.; Stępniewska, Z. Dehydrogenase activity in the soil environment. In Dehydrogenases; Canuto, R.A., Ed.; IntechOpen: London, UK, 2012; pp. 183–210. [Google Scholar]
- Wyszkowska, J.; Wyszkowski, M. Effect of cadmium and magnesium on enzymatic activity in soil. Pol. J. Environ. Stud. 2003, 12, 473–480. [Google Scholar]
- Woźniak, G.; Malicka, M.; Kasztowski, J.; Radosz, Ł.; Czarnecka, J.; Vangronsveld, J.; Prostański, D. How important are the relations between vegetation diversity and bacterial functional diversity for the functioning of novel ecosystems? Sustainability 2023, 15, 678. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Soler-Rovira, P.; Polo, A.; Díaz-Raviña, M.; Arias-Estévez, M.; Plaza, C. Enzyme activities in vineyard soils long-term treated with copper-based fungicides. Soil Biol. Biochem. 2010, 42, 2119–2127. [Google Scholar] [CrossRef]
- Abakumov, E.; Frouz, J. Humus accumulation and humification during soil development in post-mining soil. In Soil Biota and Ecosystem Development in Post Mining Sites; Frouz, J., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2013; pp. 19–37. [Google Scholar]
- Zhang, W.Y.; Yao, D.X.; Zhang, Z.G.; Yang, Q.; Zhao, K.; An, S.K. Characteristics of soil enzymes and the dominant species of repair trees in the reclamation of coal mine area. J. Coal Sci. Eng. 2013, 19, 256–261. [Google Scholar] [CrossRef]
- Markowicz, A.; Woźniak, G.; Borymski, S.; Piotrowska-Seget, Z.; Chmura, D. Links in the functional diversity between soil microorganisms and plant communities during natural succession in coal mine spoil heaps. Ecol. Res. 2015, 30, 1005–1014. [Google Scholar] [CrossRef]
- Stefanowicz, A.M.; Kapusta, P.; Błońska, A.; Kompała-Bąba, A.; Woźniak, G. Effects of Calamagrostis epigejos, Chamaenerion palustre and Tussilago farfara on nutrient availability and microbial activity in the surface layer of spoil heaps after hard coal mining. Ecol. Eng. 2015, 83, 328–337. [Google Scholar] [CrossRef]
- Jaworska, H.; Lemanowicz, J. Heavy metal contents and enzymatic activity in soils exposed to the impact of road traffic. Sci. Rep. 2019, 9, 19981. [Google Scholar] [CrossRef] [PubMed]
- Kompała-Bąba, A.; Bierza, W.; Błońska, A.; Sierka, E.; Magurno, F.; Chmura, D.; Besenyei, L.; Radosz, Ł.; Woźniak, G. Vegetation diversity on coal mine spoil heaps—How important is the texture of the soil substrate? Biologia 2019, 74, 419–436. [Google Scholar] [CrossRef]
- Orwin, K.H.; Wardle, D.A.; Greenfeld, L.G. Context-dependent changes in the resistance and resilience of soil microbes to an experimental disturbance for three primary plant chronosequences. Oikos 2006, 112, 196–208. [Google Scholar] [CrossRef]
- Rodriguez-Loinaz, G.; Onaindia, M.; Amezaga, I.; Mijangos, I.; Garbisu, C. Relationship between vegetation diversity and soil functional diversity in native mixed-oak forests. Soil Biol. Biochem. 2008, 40, 49–60. [Google Scholar] [CrossRef]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community composition at the continental scale. Appl. Environ. Microb. 2009, 75, 5111–5120. [Google Scholar] [CrossRef]
- Wang, A.S.; Angle, J.S.; Chaney, R.L.; Delorme, T.A.; McIntosh, M. Changes in soil biological activities under reduced soil pH during Thlaspi caerulescens phytoextraction. Soil Biol. Biochem. 2006, 38, 1451–1461. [Google Scholar] [CrossRef]
- Lee, S.S.; Lim, J.E.; Abd El-Azeem, S.A.M.; Choi, B.; Oh, S.E.; Moon, D.H.; Ok, Y.S. Heavy metal immobilization in soil near abandoned mines using eggshell waste and rapeseed residue. Environ. Sci. Pollut. R. 2013, 20, 1719–1726. [Google Scholar] [CrossRef]
- Mocek-Płóciniak, A. Wykorzystanie aktywności enzymatycznej do oceny wpływu antropogenicznych zmian wywołanych przez metale ciężkie w środowisku glebowym. Nauka Przyr. Technol. 2010, 4, 86. (In Polish) [Google Scholar]
- Chodak, M.; Niklińska, M. Effect of texture and tree species on microbial properties of mine soils. Appl. Soil Ecol. 2010, 46, 268–275. [Google Scholar] [CrossRef]
- Schinner, F.; Öhlinger, R.; Kandeler, E.; Margesin, R. Methods in Soil Biology; Springer: Berlin/Heidelberg, Germany, 1996; pp. 1–426. [Google Scholar]
- Olander, L.P.; Vitousek, P.M. Regulation of soil phosphatase and chitinase activity by N and P availability. Biogeochemistry 2000, 49, 175–190. [Google Scholar] [CrossRef]
- Woźniak, G. Zróżnicowanie Roślinności na Zwałach Pogórniczych Górnego Śląska; Instytut Botaniki im. W. Szafera PAN: Kraków, Poland, 2010; pp. 1–320. (In Polish) [Google Scholar]
- Tscherko, D.; Hammesfahr, U.; Marx, M.C.; Kandeler, E. Shifts in rhizosphere microbial communities and enzyme activity of Poa alpina across an alpine chronosequence. Soil Biol. Biochem. 2004, 36, 1685–1698. [Google Scholar] [CrossRef]
- Frouz, J.; Nováková, A. Development of soil microbial properties in topsoil layer during spontaneous succession in heaps after brown coal mining in relation to humus microstructure development. Geoderma 2005, 129, 54–64. [Google Scholar] [CrossRef]
- Šourková, M.; Frouz, J.; Fettweis, U.; Bens, O.; Hüttl, R.F.; Šantrůčková, H. Soil development and properties of microbial biomass succession in reclaimed postmining sites near Sokolov (Czech Republic) and near Cottbus (Germany). Geoderma 2005, 129, 73–80. [Google Scholar] [CrossRef]
- Elhottová, D.; Krištůlek, V.; Malý, S.; Frouz, J. Rhizosphere effect of colonizer plant species on the development of soil microbial community during primary succession on postmining sites. Commun. Soil Sci. Plan. 2009, 40, 758–770. [Google Scholar] [CrossRef]
- Finkenbein, P.; Kretschmer, K.; Kuka, K.; Klotz, S.; Heilmeier, H. Soil enzymes activities as bioindicators for substrate quality in revegetation of a subtropical coal mining dump. Soil Biol. Biochem. 2013, 56, 87–89. [Google Scholar] [CrossRef]
- Kuziemska, B. Enzymatic activity of nickel-contaminated soil. Teka Kom. Ochr. I Kształtowania Sr. Przyr. 2014, 11, 77–89. [Google Scholar]
- Tischer, A.; Blagodatskaya, E.; Harmer, U. Extracellular enzyme activities in a tropical mountain rainforest region of southern Ecuador affected by low soil P status and land-use change. Appl. Soil Ecol. 2014, 74, 1–11. [Google Scholar] [CrossRef]
- Orwin, K.H.; Buckland, S.M.; Johnson, D.; Turner, B.L.; Smart, S.; Oakley, S.; Bardgett, R.D. Linkages of plant traits to soil properties and the functioning of temperate grassland. J. Ecol. 2010, 98, 1074–1083. [Google Scholar] [CrossRef]
- Kara, O.; Babur, E.; Altun, L.; Seyis, M. Effects of afforestation on microbial biomass C and respiration in eroded soils of Turkey. J. Sustain. For. 2016, 35, 385–396. [Google Scholar] [CrossRef]
- Kang, H.; Gao, H.; Yu, W.; Yi, Y.; Wang, Y.; Ning, M. Changes in soil microbial community structure and function after afforestation depend on species and age: Case study in a sub-tropical alluvial island. Sci. Total Environ. 2018, 625, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Han, X.M.; Wang, R.Q.; Liu, J.; Wang, M.C.; Zhou, J.; Guo, W.H. Effects of vegetation type on soil microbial community structure and catabolic diversity assessed by polyphasic methods in North China. J. Environ. Sci. 2007, 19, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Wright, I.J.; Pereira, C.G.; Bellingham, P.J.; Bentley, L.P.; Boonman, A.; Cernusak, L.A.; Foulds, W.; Gleason, S.M.; Gray, E.F.; et al. Leaf manganese concentrations as a tool to assess belowground plant functioning in phosphorus-impoverished environments. Plant Soil 2021, 461, 43–61. [Google Scholar] [CrossRef]
- Mueller, K.E.; Tilman, D.; Fornara, D.A.; Hobbie, S.E. Root depth distribution and the diversity-productivity relationship in a long-term grassland experiment. Ecology 2013, 94, 787–793. [Google Scholar] [CrossRef]
- Šnajdr, J.; Dobiášová, P.; Urbanová, M.; Petránková, M.; Cajthaml, T.; Frouz, J.; Baldrian, P. Dominant trees affect microbial community composition and activity in post-mining afforested soils. Soil Biol. Biochem. 2013, 56, 105–115. [Google Scholar] [CrossRef]
- Chodak, M.; Sroka, K.; Pietrzykowski, M. Activity of phosphatases and microbial phosphorus under various tree species growing on reclaimed technosols. Geoderma 2021, 401, 115320. [Google Scholar] [CrossRef]
- Kanerva, S.; Kitunen, V.; Loponen, J.; Smolander, A. Phenolic compounds and terpenes in soil organic horizon layers under silver birch, Norway spruce and Scots pine. Biol. Fert. Soils 2008, 44, 547–556. [Google Scholar] [CrossRef]
- Chodak, M.; Sroka, K.; Woś, B.; Pietrzykowski, M. Chemical and microbial properties of post-mining and post-fire soils afforested with different tree species. Appl. Soil Ecol. 2022, 171, 104321. [Google Scholar] [CrossRef]
- Houlton, B.Z.; Wang, Y.P.; Vitousek, P.M.; Field, C.B. A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature 2008, 454, 327–330. [Google Scholar] [CrossRef]
- Margalef, O.; Sardans, J.; Fernández-Martínez, M.; Molowny-Horas, R.; Janssens, I.A.; Ciais, P.; Goll, D.; Richter, A.; Obersteiner, M.; Asensio, D.; et al. Global patterns of phosphatase activity in natural soils. Sci. Rep. 2017, 7, 1337. [Google Scholar] [CrossRef]
- Marklein, A.R.; Houlton, B.Z. Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol. 2012, 193, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Błońska, A.; Kompała-Bąba, A.; Sierka, E.; Besenyei, L.; Magurno, F.; Bierza, W.; Frydecka, K.; Woźniak, G. Impact of selected plant species on enzymatic activity of soil substratum on post-mining heaps. J. Ecol. Eng. 2019, 20, 138–144. [Google Scholar] [CrossRef]
- Kompała-Bąba, A.; Sierka, E.; Dyderski, M.K.; Bierza, W.; Magurno, F.; Besenyei, L.; Błońska, A.; Ryś, K.; Jagodziński, A.M.; Woźniak, G. Do the dominant plant species impact the substrate and vegetation composition of post-coal mining spoil heaps? Ecol. Eng. 2020, 143, 105685. [Google Scholar] [CrossRef]
- Ezeokoli, O.T.; Mashigo, S.K.; Paterson, D.G.; Bezuidenhout, C.C.; Adeleke, R.A. Microbial community structure and relationship with physicochemical properties of soil stockpiles in selected South African opencast coal mines. Soil Sci. Plant Nutr. 2019, 65, 332–341. [Google Scholar] [CrossRef]
- Ghosh, D.; Maiti, S.K. Effect of invasive weed biochar amendment on soil enzymatic activity and respiration of coal mine spoil: A laboratory experiment study. Biochar 2021, 3, 519–533. [Google Scholar] [CrossRef]
- Zak, D.R.; Holmes, W.E.; White, D.C.; Peacock, A.D.; Tilman, D. Plant diversity, soil microbial communities, and ecosystem function: Are there any links? Ecology 2003, 84, 2042–2050. [Google Scholar] [CrossRef]
- Lange, M.; Habekost, M.; Eisenhauer, N.; Roscher, C.; Bessler, H.; Engels, C.; Oelmann, Y.; Scheu, S.; Wilcke, W.; Schulze, E.D.; et al. Biotic and abiotic properties mediating plant diversity effects on soil microbial communities in an experimental grassland. PLoS ONE 2014, 9, e96182. [Google Scholar] [CrossRef]
- Kourtev, P.S.; Ehrenfeld, J.G.; Haggblom, M. Exotic plant species alter the microbial community structure and function in the soil. Ecology 2002, 83, 3152–3166. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Li, G.; Ma, W.; Wu, J.; Gong, Y.; Xu, G. Vegetation degradation impacts soil nutrients and enzyme activities in wet meadow on the Qinghai-Tibet Plateau. Sci. Rep. 2020, 10, 21271. [Google Scholar] [CrossRef]
- Pei, S.; Fu, H.; Wan, C. Changes in soil properties and vegetation following exclosure and grazing in degraded Alxa desert steppe of Inner Mongolia, China. Agric. Ecosyst. Environ. 2008, 124, 33–39. [Google Scholar] [CrossRef]
- Tuan, N.T.; Rodríguez-Hernández, D.I.; Tuan, V.C.; Van Quy, N.; Obiakara, M.C.; Hufton, J. Effects of tree diversity and stand structure on above-ground carbon storage in evergreen broad-leaved and deciduous forests in Southeast Vietnam. Dendrobiology 2022, 88, 38–55. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bierza, W.; Czarnecka, J.; Błońska, A.; Kompała-Bąba, A.; Hutniczak, A.; Jendrzejek, B.; Bakr, J.; Jagodziński, A.M.; Prostański, D.; Woźniak, G. Plant Diversity and Species Composition in Relation to Soil Enzymatic Activity in the Novel Ecosystems of Urban–Industrial Landscapes. Sustainability 2023, 15, 7284. https://doi.org/10.3390/su15097284
Bierza W, Czarnecka J, Błońska A, Kompała-Bąba A, Hutniczak A, Jendrzejek B, Bakr J, Jagodziński AM, Prostański D, Woźniak G. Plant Diversity and Species Composition in Relation to Soil Enzymatic Activity in the Novel Ecosystems of Urban–Industrial Landscapes. Sustainability. 2023; 15(9):7284. https://doi.org/10.3390/su15097284
Chicago/Turabian StyleBierza, Wojciech, Joanna Czarnecka, Agnieszka Błońska, Agnieszka Kompała-Bąba, Agnieszka Hutniczak, Bartosz Jendrzejek, Jawdat Bakr, Andrzej M. Jagodziński, Dariusz Prostański, and Gabriela Woźniak. 2023. "Plant Diversity and Species Composition in Relation to Soil Enzymatic Activity in the Novel Ecosystems of Urban–Industrial Landscapes" Sustainability 15, no. 9: 7284. https://doi.org/10.3390/su15097284
APA StyleBierza, W., Czarnecka, J., Błońska, A., Kompała-Bąba, A., Hutniczak, A., Jendrzejek, B., Bakr, J., Jagodziński, A. M., Prostański, D., & Woźniak, G. (2023). Plant Diversity and Species Composition in Relation to Soil Enzymatic Activity in the Novel Ecosystems of Urban–Industrial Landscapes. Sustainability, 15(9), 7284. https://doi.org/10.3390/su15097284








