Study on the Solubility of Industrial Lignin in Choline Chloride-Based Deep Eutectic Solvents
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of DES
2.3. Lignin Solubility Determination
2.4. Characterization of DES
2.5. Characterization of Lignin
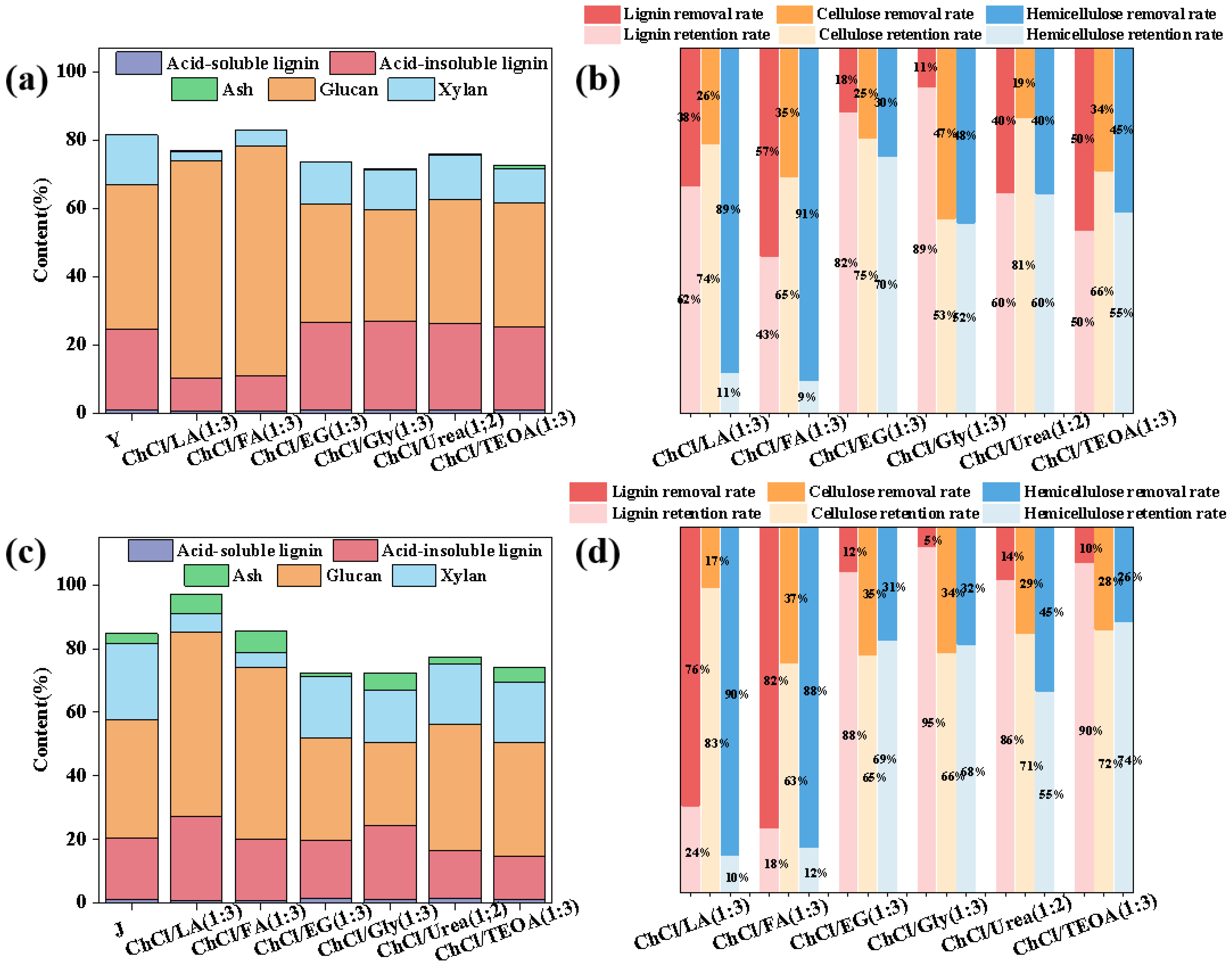
2.6. Pretreatment
2.7. Component Analysis
3. Results and Discussion
3.1. Initial Screening of DES
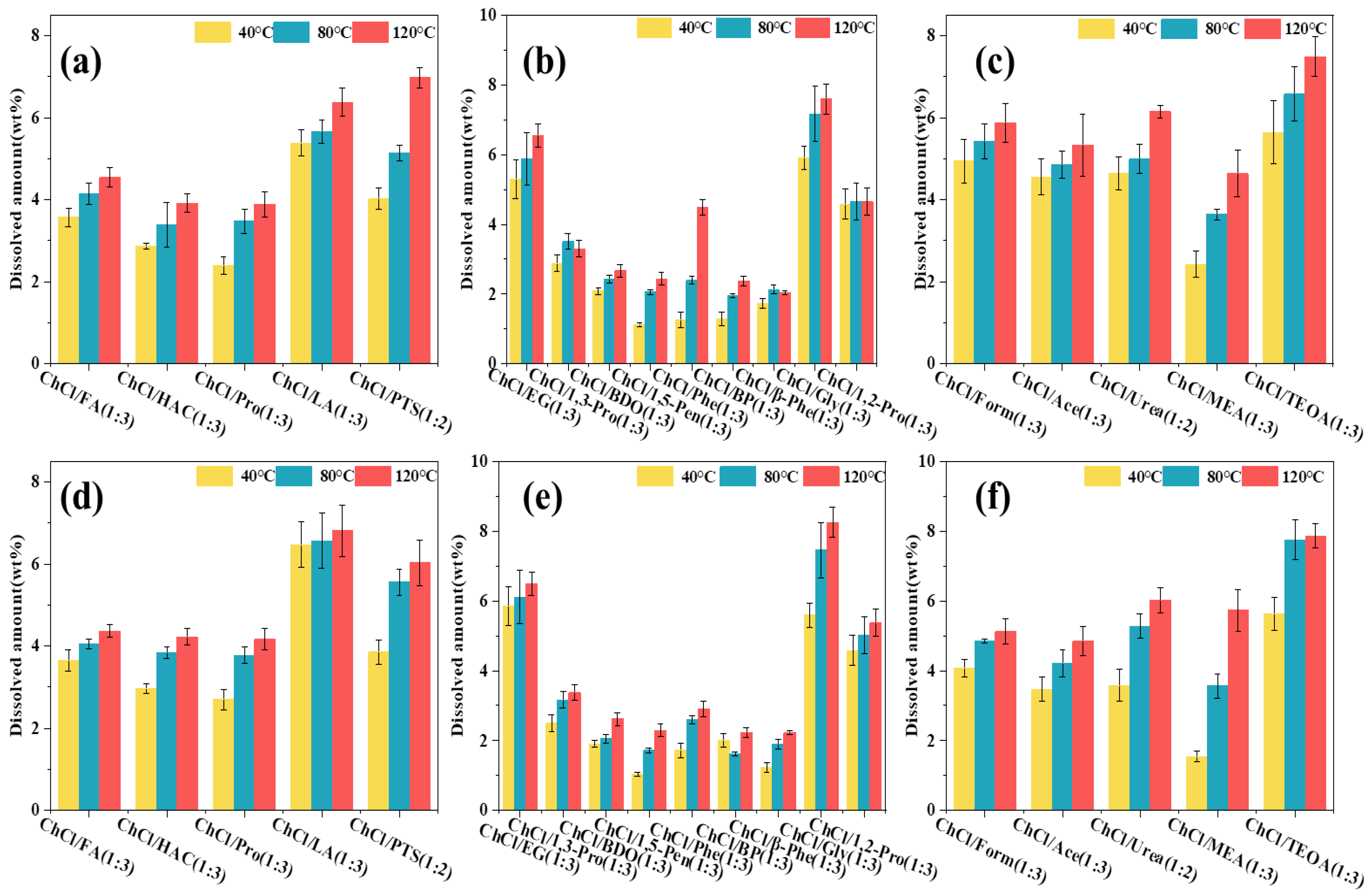
3.2. Solubility of Lignin in DES
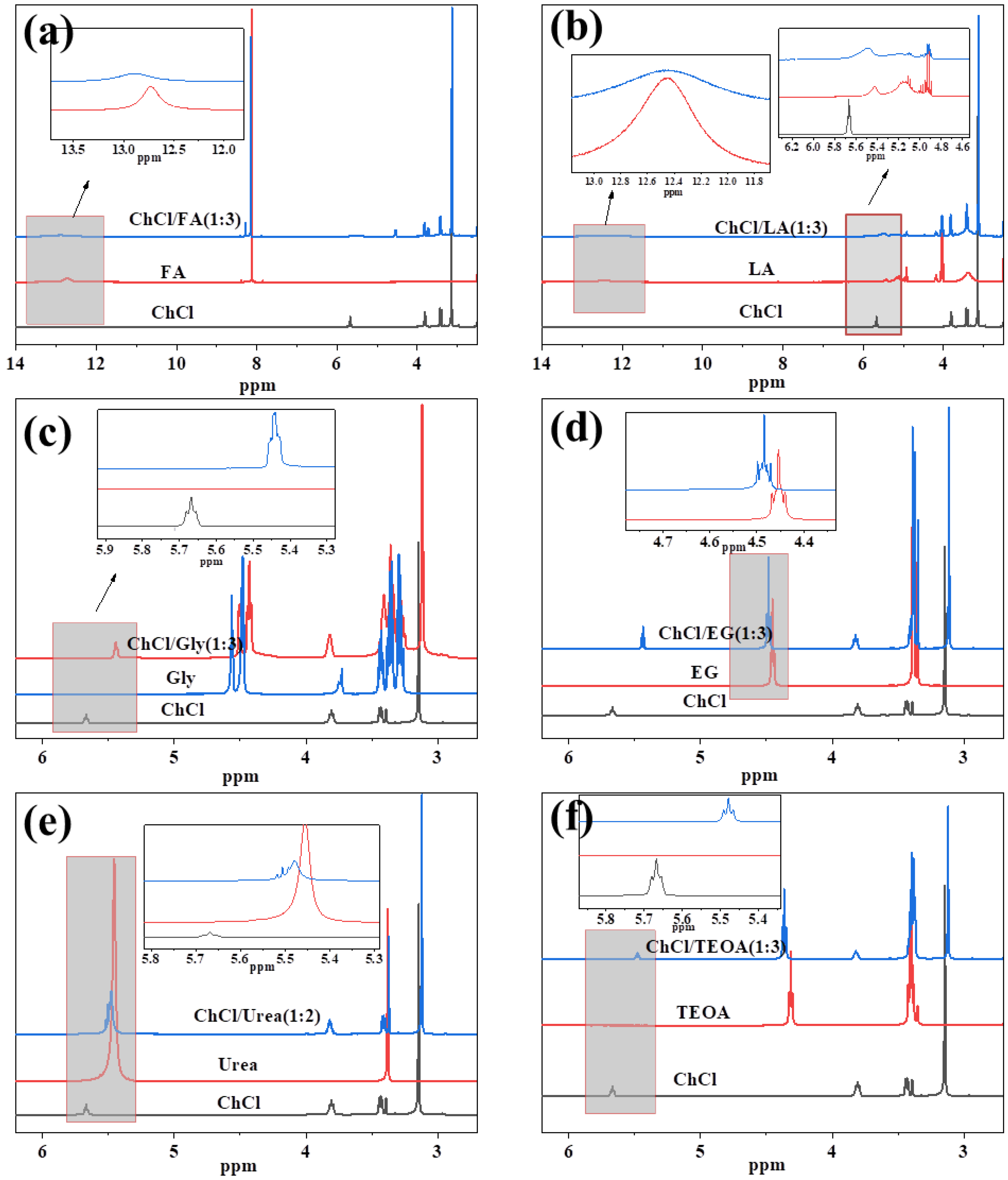
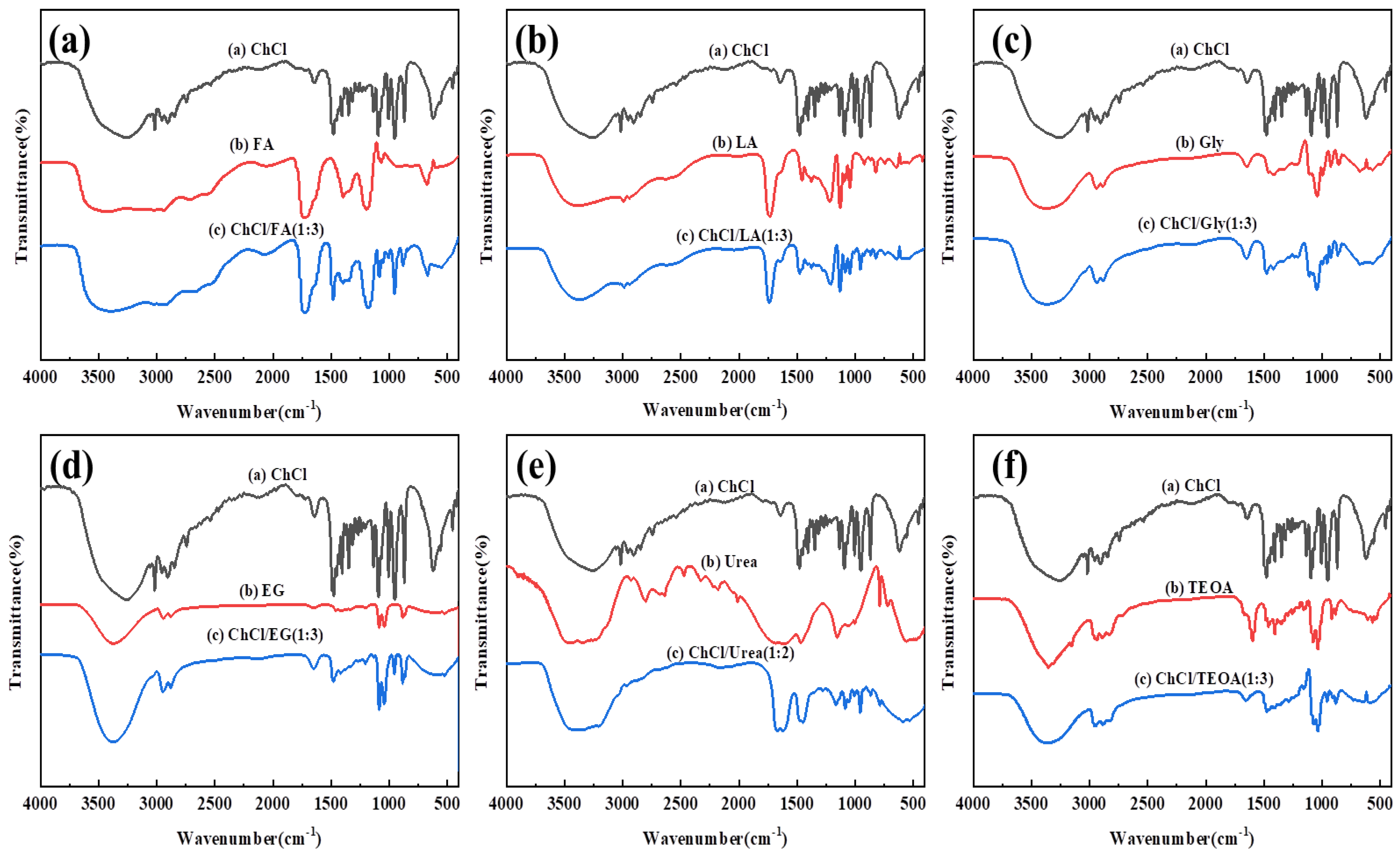
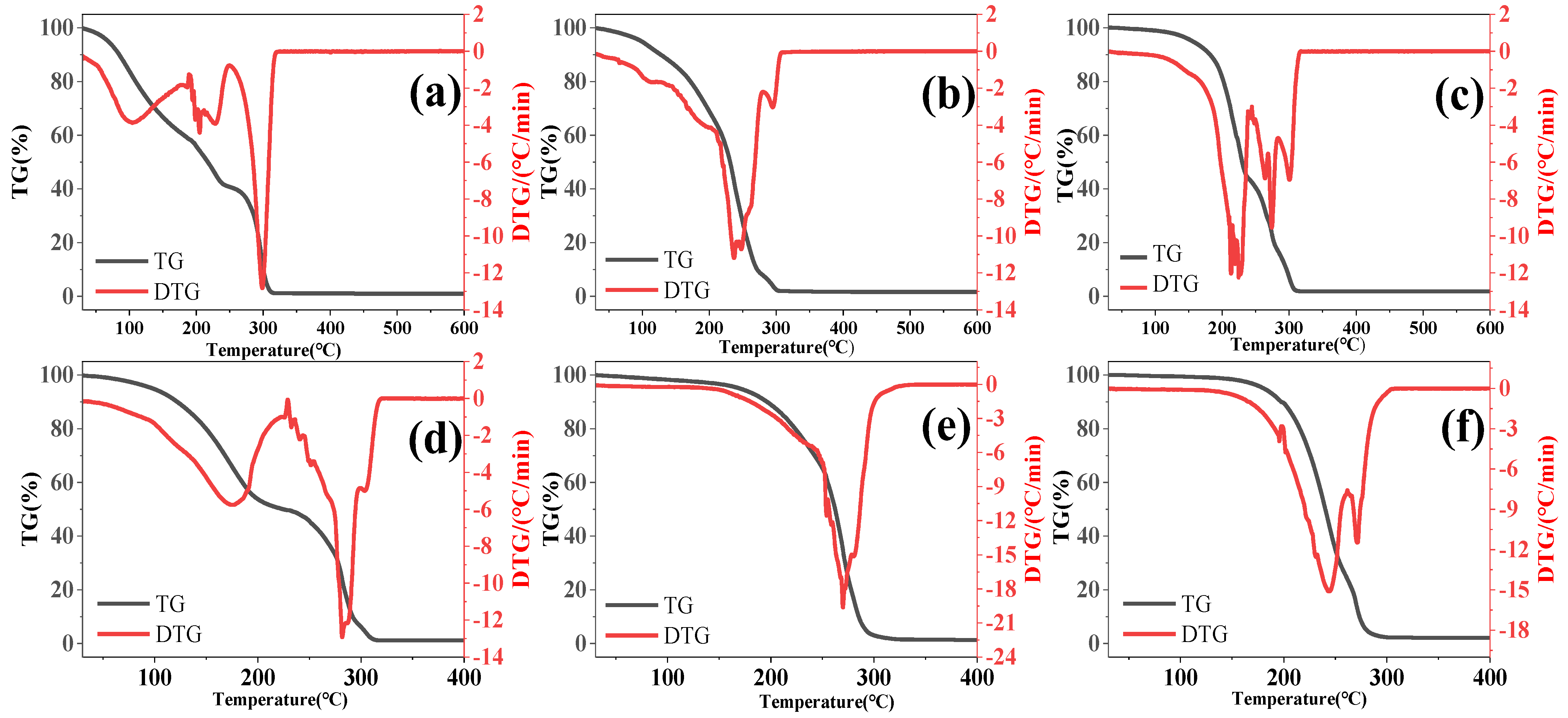
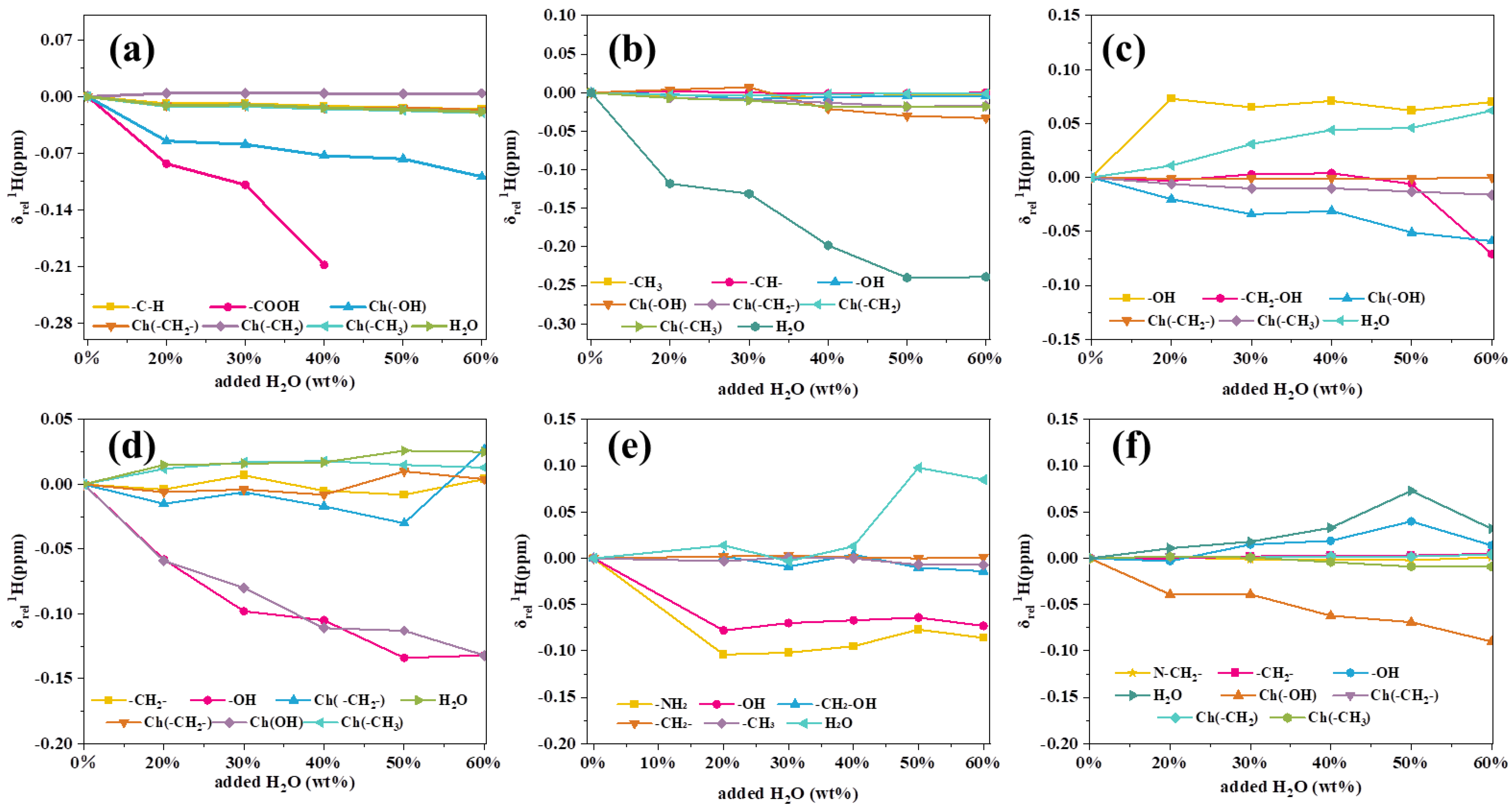
3.3. Characterization of DES
3.4. Structural Change of Lignin
3.5. The Separation of Lignin from Poplar and Corn Stover Using DES
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, H.; Cao, D.; Qiu, Z.; Li, C.-J. Palladium-Catalyzed Formal Cross-Coupling of Diaryl Ethers with Amines: Slicing the 4-O-5 Linkage in Lignin Models. Angew. Chem. Int. Ed. 2018, 57, 3752–3757. [Google Scholar] [CrossRef] [PubMed]
- Loow, Y.-L.; New, E.K.; Yang, G.H.; Ang, L.Y.; Foo, L.Y.W.; Wu, T.Y. Potential Use of Deep Eutectic Solvents to Facilitate Lignocellulosic Biomass Utilization and Conversion. Cellulose 2017, 249, 818–825. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, Q.; Pan, T.; Zuo, Y.; Fu, Y.; Guo, Q.-X. Depolymerization of Lignin by Catalytic Oxidation with Aqueous Polyoxometalates. Appl. Catal. A Gen. 2013, 467, 504–508. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, Y.-C.; Hu, H.-Q.; Xie, F.-J.; Wei, X.-Y.; Fan, X. Structural Characterization of Lignin and Its Degradation Products with Spectroscopic Methods. J. Spectrosc. 2017, 2017, 8951658. [Google Scholar] [CrossRef]
- Xue, Z.; Zhao, X.; Sun, R.; Mu, T. Biomass-Derived γ-Valerolactone-Based Solvent Systems for Highly Efficient Dissolution of Various Lignins: Dissolution Behavior and Mechanism Study. ACS Sustain. Chem. Eng. 2016, 4, 3864–3870. [Google Scholar] [CrossRef]
- Sathitsuksanoh, N.; Holtman, K.M.; Yelle, D.J.; Morgan, T.; Stavila, V.; Pelton, J.; Blanch, H.; Simmons, B.A.; George, A. Lignin Fate and Characterization during Ionic Liquid Biomass Pretreatment for Renewable Chemicals and Fuels Production. Green Chem. 2013, 16, 1236–1247. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Zhou, Y.; Zhang, M.; Xia, Q.; Bi, W.; Chen, D.D.Y. Ecofriendly Mechanochemical Extraction of Bioactive Compounds from Plants with Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2017, 16, 1236–1247. [Google Scholar] [CrossRef]
- Oh, Y.; Park, S.; Yoo, E.; Jo, S.; Hong, J.; Kim, H.; Kim, K.; Oh, K.; Lee, S. Dihydrogen-bonding deep eutectic solvents as reaction media for lipase-catalyzed transesterification. Biochem. Eng. J. 2019, 142, 34–40. [Google Scholar] [CrossRef]
- Hong, S.; Shen, X.-J.; Pang, B.; Xue, Z.; Cao, X.-F.; Wen, J.-L.; Sun, Z.-H.; Lam, S.S.; Yuan, T.-Q.; Sun, R.-C. In-Depth Interpretation of the Structural Changes of Lignin and Formation of Diketones during Acidic Deep Eutectic Solvent Pretreatment. Green Chem. 2020, 22, 7219–7232. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, X.; Yu, D.; Yu, H.; Zhang, Y.; Xue, Z.; Mu, T. Novel Deep Eutectic Solvents with Different Functional Groups towards Highly Efficient Dissolution of Lignin†. Green Chem. 2019, 22, 1851–1858. [Google Scholar] [CrossRef]
- Feng, Y.; Yan, G.; Wang, T.; Jia, W.; Zeng, X.; Sperry, J.; Sun, Y.; Tang, X.; Lei, T.; Lin, L. Synthesis of MCM-41-Supported Metal Catalysts in Deep Eutectic Solvent for the Conversion of Carbohydrates into 5-Hydroxymethylfurfural. ChemSusChem 2019, 12, 978–982. [Google Scholar] [PubMed]
- Shen, X.-J.; Wen, J.-L.; Mei, Q.-Q.; Chen, X.; Sun, D.; Yuan, T.-Q.; Sun, R.-C. Facile Fractionation of Lignocelluloses by Biomass-Derived Deep Eutectic Solvent (DES) Pretreatment for Cellulose Enzymatic Hydrolysis and Lignin Valorization. Green Chem. 2019, 21, 275–283. [Google Scholar]
- Li, X.; Ning, C.; Li, L.; Liu, W.; Ren, Q.; Hou, Q. Fabricating Lignin-Containing Cellulose Nanofibrils with Unique Properties from Agricultural Residues with Assistance of Deep Eutectic Solvents. Carbohydr. Polym. 2021, 274, 118650. [Google Scholar] [PubMed]
- Lou, R.; Ma, R.; Lin, K.; Ahamed, A.; Zhang, X. Facile Extraction of Wheat Straw by Deep Eutectic Solvent (DES) to Produce Lignin Nanoparticles. ACS Sustain. Chem. Eng. 2019, 7, 10248–10256. [Google Scholar]
- Loow, Y.-L.; Wu, T.Y.; Yang, G.H.; Ang, L.Y.; New, E.K.; Siow, L.F.; Md Jahim, J.; Mohammad, A.W.; Teoh, W.H. Deep Eutectic Solvent and Inorganic Salt Pretreatment of Lignocellulosic Biomass for Improving Xylose Recovery. Bioresour. Technol. 2017, 249, 818–825. [Google Scholar] [PubMed]
- Chen, Z.; Bai, X.; Zhang, H.; Wan, C. Insights into Structural Changes of Lignin toward Tailored Properties during Deep Eutectic Solvent Pretreatment. ACS Sustain. Chem. Eng. 2020, 8, 9783–9793. [Google Scholar]
- da Costa Lopes, A.M.; Gomes, J.R.B.; Coutinho, J.A.P.; Silvestre, A.J.D. Novel Insights into Biomass Delignification with Acidic Deep Eutectic Solvents: A Mechanistic Study of β-O-4 Ether Bond Cleavage and the Role of the Halide Counterion in the Catalytic Performance. Green Chem. 2020, 22, 2474–2487. [Google Scholar]
- Hong, S.; Shen, X.-J.; Xue, Z.; Sun, Z.; Yuan, T.-Q. Structure–Function Relationships of Deep Eutectic Solvents for Lignin Extraction and Chemical Transformation. Green Chem. 2020, 22, 7219–7232. [Google Scholar]
- Alvarez-Vasco, C.; Ma, R.; Quintero, M.; Guo, M.; Geleynse, S.; Ramasamy, K.K.; Wolcott, M.; Zhang, X. Unique Low-Molecular-Weight Lignin with High Purity Extracted from Wood by Deep Eutectic Solvents (DES): A Source of Lignin for Valorization. Green Chem. 2016, 97, 321–329. [Google Scholar]
- Li, P.; Lu, Y.; Li, X.; Ren, J.; Jiang, Z.; Jiang, B.; Wu, W. Comparison of the Degradation Performance of Seven Different Choline Chloride-Based DES Systems on Alkaline Lignin. Polymers 2022, 14, 5100. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. Anal. Bioanal. Chem. 2008, 1617, 1–16. [Google Scholar]
- Zhang, C.-W.; Xia, S.-Q.; Ma, P.-S. Facile Pretreatment of Lignocellulosic Biomass Using Deep Eutectic Solvents. Bioresour. Technol. 2016, 219, 1–5. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Yu, J.; Lu, Y.; Jiang, B.; Fan, Y.; Wang, Z. High-Purity Lignin Isolated from Poplar Wood Meal through Dissolving Treatment with Deep Eutectic Solvents. Royal Soc. Open Sci. 2019, 6, 181757. [Google Scholar] [CrossRef] [PubMed]
- Al-Dawsari, J.N.; Bessadok-Jemai, A.; Wazeer, I.; Mokraoui, S.; AlMansour, M.A.; Hadj-Kali, M.K. Fitting of Experimental Viscosity to Temperature Data for Deep Eutectic Solvents. J. Mol. Liq. 2020, 310, 113127. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring Properties of Natural Deep Eutectic Solvents with Water to Facilitate Their Applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.K.; Parikh, B.S.; Pravakar, M. Natural Deep Eutectic Solvent Mediated Pretreatment of Rice Straw: Bioanalytical Characterization of Lignin Extract and Enzymatic Hydrolysis of Pretreated Biomass Residue. Environ. Sci. Pollut. Res. 2015, 23, 9265–9275. [Google Scholar] [CrossRef]
- Al-Murshedi, A.Y.M.; Hartley, J.M.; Abbott, A.P.; Ryder, K.S. Effect of Water on the Electrodeposition of Copper on Nickel in Deep Eutectic Solvents. Trans. IMF 2019, 97, 321–329. [Google Scholar] [CrossRef]
- Soares, B.; Tavares, D.J.; Amaral, J.L.; Silvestre, A.J.; Freire, C.S.; Coutinho, J.A. Enhanced Solubility of Lignin Monomeric Model Compounds and Technical Lignins in Aqueous Solutions of Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2017, 5, 4056–4065. [Google Scholar] [CrossRef]
- Sumer, Z.; Van Lehn, R.C. Data-Centric Development of Lignin Structure–Solubility Relationships in Deep Eutectic Solvents Using Molecular Simulations. ACS Sustain. Chem. Eng. 2022, 10, 10144–10156. [Google Scholar] [CrossRef]
- Ma, C.; Laaksonen, A.; Liu, C.; Lu, X.; Ji, X. The Peculiar Effect of Water on Ionic Liquids and Deep Eutectic Solvents. Chem. Soc. Rev. 2018, 97, 321–329. [Google Scholar] [CrossRef]
- Cao, D.; Liu, Q.; Jing, W.; Tian, H.; Yan, H.; Bi, W.; Jiang, Y.; Chen, D.D.Y. Insight into the Deep Eutectic Solvent Extraction Mechanism of Flavonoids from Natural Plant. ACS Sustain. Chem. Eng. 2020, 8, 19169–19177. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, H.; Liu, J.; Dong, W.; Cao, Z.; Hu, X.; Zhou, Z. Acidic Deep Eutectic Solvents with Long Carbon Chains as Catalysts and Reaction Media for Biodiesel Production. Renew. Energy 2020, 162, 1842–1853. [Google Scholar] [CrossRef]
- Yu, X.; Li, M.; Yagoub, A.E.A.; Chen, L.; Zhou, C.; Yan, D. Switchable (PH Driven) Aqueous Two-Phase Systems Formed by Deep Eutectic Solvents as Integrated Platforms for Production-Separation 5-HMF. J. Mol. Liq. 2020, 325, 115158. [Google Scholar] [CrossRef]
- Di Pietro, M.E.; Tortora, M.; Bottari, C.; Colombo Dugoni, G.; Pivato, R.V.; Rossi, B.; Paolantoni, M.; Mele, A. In Competition for Water: Hydrated Choline Chloride: Urea vs Choline Acetate: Urea Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2021, 9, 12262–12273. [Google Scholar] [CrossRef]
- Gabriele, F.; Chiarini, M.; Germani, R.; Tiecco, M.; Spreti, N. Effect of Water Addition on Choline Chloride/Glycol Deep Eutectic Solvents: Characterization of Their Structural and Physicochemical Properties. J. Mol. Liq. 2019, 291, 111301. [Google Scholar] [CrossRef]
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. The Effect of Water upon Deep Eutectic Solvent Nanostructure: An Unusual Transition from Ionic Mixture to Aqueous Solution. Angew. Chem. Int. Ed. 2017, 56, 9782–9785. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Ferrer, M.L.; Mateo, C.R.; del Monte, F. Freeze-Drying of Aqueous Solutions of Deep Eutectic Solvents: A Suitable Approach to Deep Eutectic Suspensions of Self-Assembled Structures. Langmuir 2009, 25, 5509–5515. [Google Scholar] [CrossRef]
- Xu, H.; Kong, Y.; Peng, J.; Wang, W.; Li, B. Mechanism of Deep Eutectic Solvent Delignification: Insights from Molecular Dynamics Simulations. ACS Sustain. Chem. Eng. 2021, 9, 7101–7111. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Wang, A.; Yan, C.; Liu, S.; Li, L.; Wu, Q.; Liu, Y.; Liu, Y.; Nie, G.; Nie, S.; et al. Study on the Solubility of Industrial Lignin in Choline Chloride-Based Deep Eutectic Solvents. Sustainability 2023, 15, 7118. https://doi.org/10.3390/su15097118
Chen H, Wang A, Yan C, Liu S, Li L, Wu Q, Liu Y, Liu Y, Nie G, Nie S, et al. Study on the Solubility of Industrial Lignin in Choline Chloride-Based Deep Eutectic Solvents. Sustainability. 2023; 15(9):7118. https://doi.org/10.3390/su15097118
Chicago/Turabian StyleChen, Haiyu, Ailin Wang, Cancan Yan, Shiwei Liu, Lu Li, Qiong Wu, Yue Liu, Yuxiang Liu, Genkuo Nie, Shuangxi Nie, and et al. 2023. "Study on the Solubility of Industrial Lignin in Choline Chloride-Based Deep Eutectic Solvents" Sustainability 15, no. 9: 7118. https://doi.org/10.3390/su15097118
APA StyleChen, H., Wang, A., Yan, C., Liu, S., Li, L., Wu, Q., Liu, Y., Liu, Y., Nie, G., Nie, S., Yao, S., & Yu, H. (2023). Study on the Solubility of Industrial Lignin in Choline Chloride-Based Deep Eutectic Solvents. Sustainability, 15(9), 7118. https://doi.org/10.3390/su15097118






